Abstract
Clinical trials have shown that antiretroviral drugs are highly effective in preventing HIV acquisition. Such pre-exposure prophylaxis (PrEP), including in sub-Saharan Africa, has almost exclusively focused on certain priority groups, particularly female sex workers, men having sex with men, pregnant women, serodiscordant couples, and young women. As part of a demonstration project of PrEP for the general population at six primary healthcare facilities in Eswatini (formerly Swaziland), we conducted a randomized trial of a healthcare facility-based PrEP promotion package to increase PrEP uptake. Over the 18-month study period, 33.6% (517/1,538) of adults identified by healthcare workers as being at risk of acquiring HIV took up PrEP and 30.0% of these individuals attended all scheduled appointments during the first six months after initiation of PrEP. The PrEP promotion package was associated with a 55% (95% CI: 15% to 110%, p=0.036) relative increase in the number of individuals taking up PrEP, with an absolute increase of 2.2 individuals per month per healthcare facility. When asked how PrEP uptake could be improved in 212 accompanying in-depth qualitative interviews, interviewees perceived an expansion of PrEP promotion activities from healthcare facilities to communities to be essential. Although a healthcare facility-based promotion package improved PrEP uptake, both uptake and retention remained low. Expanding promotion activities to the community is likely needed to achieve greater PrEP coverage among adults at risk of HIV infection in Eswatini and similar settings.
One-sentence summary:
While clinic-based promotion of pre-exposure prophylaxis (PrEP) for HIV led to a small increase in PrEP uptake, only a third of at-risk adults at primary healthcare facilities in Eswatini took up PrEP.
Introduction
Oral pre-exposure prophylaxis (PrEP) for HIV has been shown to be effective in preventing HIV infection in clinical trials(1–5). This has led the World Health Organization to recommend PrEP as an additional prevention choice for all people at substantial risk of HIV acquisition, provisionally described as populations with an HIV incidence of three per 100 person-years or higher in the absence of PrEP(6). In most countries, such a high HIV incidence is only reached in key populations, such as sex workers, men who have sex with men (MSM), or people who inject drugs(6, 7). However, in Southern Africa – the region hardest hit by the HIV epidemic(7) – annual HIV incidence in many sub-groups of the general population (young women, for example) is well above three percent(8–13). In combination with increasing coverage of antiretroviral therapy (ART) among people living with HIV, a population-wide scale-up of PrEP thus has the potential to substantially alter the trajectory of the HIV epidemic in the Southern African region. Unlike programs focused on key populations at the very highest risk of HIV acquisition, such as sex workers, a population-wide scale-up could target everyone who has at least a minimal risk of HIV acquisition. Such a “PrEP for all” approach may also be desirable in settings with a lower HIV incidence, because focusing PrEP on key populations only may engender stigma around PrEP, hampering uptake among those who would benefit most from this HIV prevention method.(14, 15) In addition, promoting and offering PrEP widely may be logistically simpler and more efficient than attempting to reach key populations only, who are often highly marginalized.
Although there is some evidence on PrEP uptake by MSM populations in the United States(16–18), by adolescent women and female sex workers in South Africa(19, 20), and after population-based HIV testing in rural Kenya and Uganda(21), there has been little study of uptake when PrEP is offered at primary healthcare facilities to any HIV-negative adult in the general population who requests it. In addition to reaching a high level of uptake, developing and evaluating strategies to achieve high adherence to PrEP during patients’ periods of risk will equally be important. However, because uptake is the first step in the PrEP care cascade and currently thought to be the step at which the largest number of patients are lost in the care pathway,(21) the primary focus of this study was on PrEP uptake..
This study was part of a PrEP demonstration project by Eswatini’s Ministry of Health, in which PrEP was made available at six public-sector primary healthcare facilities. Healthcare workers at these facilities actively offered PrEP to individuals 16 years and older who were at risk of acquiring HIV (as determined by the answers to six screening questions) and PrEP was also provided to any HIV-negative adult who requested it. Using both quantitative and qualitative research methods, our aims were to (i) determine the level of PrEP uptake and how uptake varied according to individuals’ socio-demographic characteristics, (ii) establish the impact of a healthcare facility-based PrEP Promotion Package (PPP) on the number of clients who took up PrEP, and (iii) characterize the views of PrEP users, PrEP decliners, healthcare workers, and policymakers on how PrEP uptake could be further increased.
Results
Study setting
Eswatini is a landlocked country in Southern Africa with an estimated population of 1.3 million in 2016(22). The country has the world’s highest HIV prevalence at 27.0% among those aged ≥15 years(23). Despite falling by nearly half between 2011 and 2016(8, 24), annual HIV incidence among adults in Eswatini was still 1.4% in 2016 (1.0% in men and 1.7% in women)(23). This study took place at six public-sector primary healthcare facilities in the Hhohho Region in Northwestern Eswatini (Figure S1). The four most Northern healthcare facilities (Horo, Ndvwabangeni Nazarene, Ndzingeni Nazarene, and Ntfonjeni) were located in relatively remote rural areas while the other two healthcare facilities were located in peri-urban areas 30km (Hhukwini) and 15km (Siphocosini) from the country’s capital, Mbabane. All sites were middle- to high-volume healthcare facilities (Table S1), nurse-led, and provided HIV services, including ART, free of charge and other services for a nominal fee. These six healthcare facilities were selected by the Ministry of Health for the PrEP demonstration project, and thus for this research, because of their previous involvement in a “test and treat” trial (the MaxART trial(25)) and adequate staffing levels.
Overview of study components
The qualitative component of this study consisted of 212 in-depth qualitative interviews with PrEP users, clients who declined PrEP, healthcare workers, and policymakers. The quantitative component of this study was a stepped-wedge randomized trial conducted at the six primary healthcare facilities (“clusters” in Figure 1) that were selected for the PrEP demonstration project. The trial tested the effect of a healthcare facility-based PPP on PrEP uptake. The PPP consisted of a PrEP promotion video played in the waiting room, a t-shirt promoting PrEP that healthcare workers were encouraged to wear, a flipchart to guide healthcare workers’ PrEP counseling, a booklet on PrEP for clients, and an assessment form that clients could fill out to assess their risk of acquiring HIV. Three of the six healthcare facilities were randomized to sequence 1 (implementing the PPP starting on February 1st 2018) with the remaining three being allocated to sequence 2 (implementing the PPP starting on May 1st 2018). This trial, therefore, had three periods (August 1 2017 to January 31 2018, during which none of the healthcare facilities were implementing the PPP; February 1 2018 to April 30 2018, during which three healthcare facilities were implementing the PPP and three were not; and May 1 2018 to January 2019, during which all six healthcare facilities were implementing the PPP). Thus, three healthcare facilities implemented the PPP from February 1 2018 to January 2019 and three from May 1 2018 to January 2019. All healthcare facilities completed all three periods of the study. Throughout the entire study period, healthcare workers at the study healthcare facilities were asked to offer PrEP to any HIV-negative client who requested it and to actively screen clients for their risk of HIV acquisition (using six questions detailed in the Materials and Methods section) to then offer PrEP to those at risk.
Figure 1.
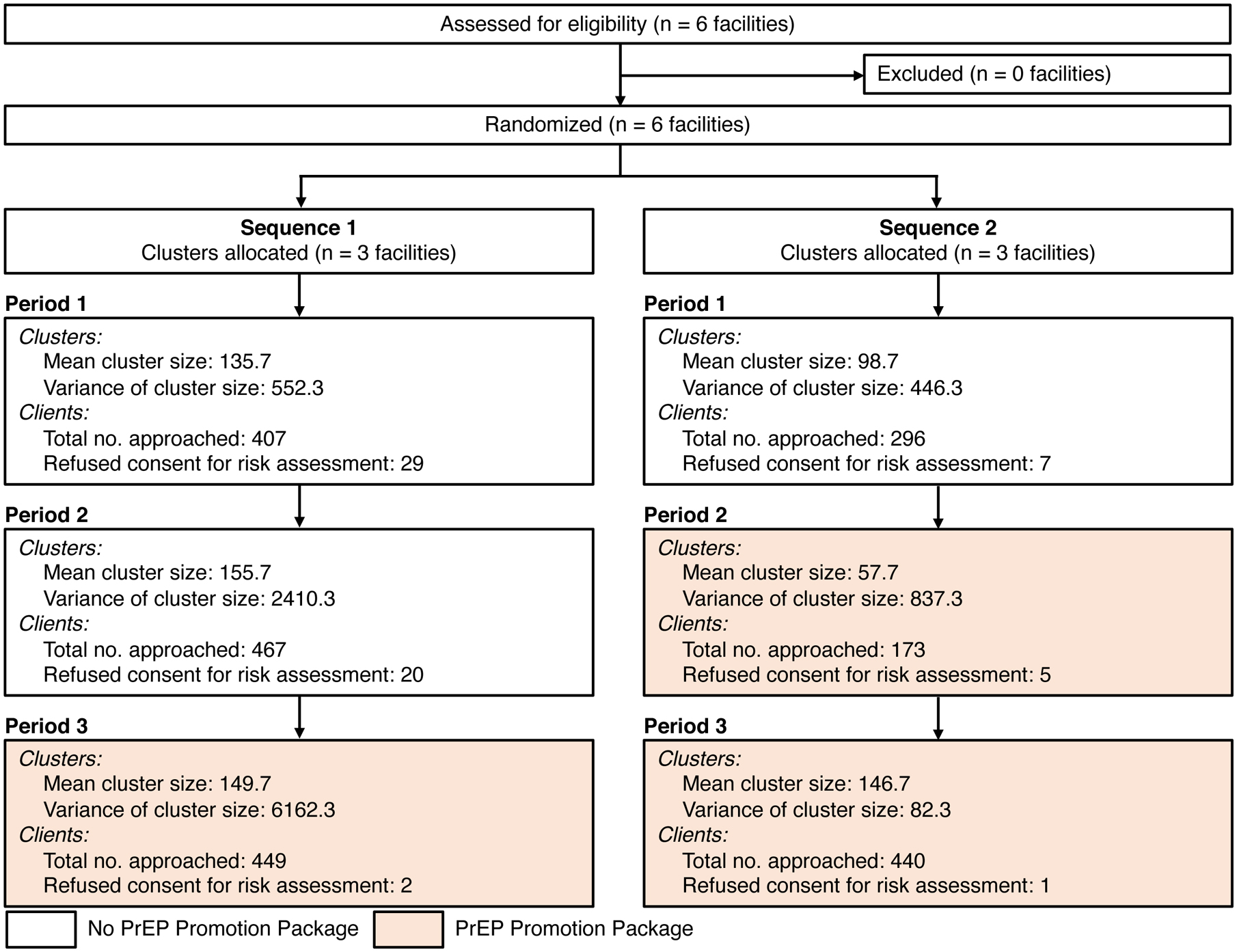
Flowchart of healthcare facilities (“clusters”) through the trial.
Healthcare facilities’ and participants’ characteristics
2,232 participants were approached for an HIV risk assessment at the six healthcare facilities over the 18-month study period such that the mean number of participants approached at each facility during a period varied from 57.7 participants per facility in Sequence 2 facilities in Period 2 to 155.7 participants per facility in Sequence 1 facilities in Period 2 (Figure 1). In total, 64 (out of 2,232) participants refused to undergo an assessment for their risk of HIV acquisition.
Those who were approached by a healthcare worker for a risk assessment (or who actively approached a healthcare worker for PrEP) were predominantly (76.6%) female (Table 1 and Table S2). During the in-depth qualitative interviews, all respondent groups described women as more ‘able’ to access the clinics and more likely to be ‘convinced’ of PrEP, because women would (regardless of whether the healthcare visit concerned PrEP) ‘talk more’ and engage more actively with the healthcare workers. The mean age of participants who underwent a risk assessment was 29.0 years, with only 5.5% being older than 45 years. 26.2% had not had any secondary school education and 89.0% reported that they had one partner at the time of the risk assessment. While patients did not have to be members of a target group to access PrEP, the Eswatini government defined “priority populations” for whom it considered achieving high PrEP coverage to be particularly important. These priority populations were women between 16 and 25 years of age, those in a relationship with an HIV-positive partner, sex workers, men having sex with men (MSM), those with a current sexually transmitted infection, pregnant women, and lactating women. 54.6% of participants were in at least one of these priority populations. One participant self-identified as being a sex worker and no participant as being MSM. The characteristics of interviewees for the in-depth qualitative interviews are shown in Table 2.
Table 1.
Characteristics of clients who underwent the HIV risk assessment1
| Total | Sequence 1 | Sequence 2 | |||
|---|---|---|---|---|---|
| No PPP | PPP | No PPP | PPP | ||
| n | 2168 | 378 | 894 | 457 | 439 |
| Female, n (%) | 1650 (76.6) | 328 (86.8) | 632 (71.6) | 364 (79.6) | 326 (74.8) |
| Missing, n (%) | 14 (0.6) | 0 (0.0) | 11 (1.2) | 0 (0.0) | 3 (0.7) |
| Age, mean (SD) | 29.0 (10.4) | 29.2 (8.5) | 29.0 (9.6) | 28.0 (7.9) | 29.6 (14.9) |
| Age group, n (%) | |||||
| 16–25 years | 863 (40.4) | 138 (37.1) | 349 (39.5) | 192 (42.2) | 184 (43.0) |
| 26–35 years | 874 (40.9) | 158 (42.5) | 352 (39.9) | 189 (41.5) | 175 (40.9) |
| 36–45 years | 284 (13.3) | 53 (14.2) | 136 (15.4) | 54 (11.9) | 41 (9.6) |
| >45 years | 117 (5.5) | 23 (6.2) | 46 (5.2) | 20 (4.4) | 28 (6.5) |
| Missing, n (%) | 30 (1.4) | 6 (1.6) | 11 (1.2) | 2 (0.4) | 11 (2.5) |
| Education, n (%) | |||||
| No formal schooling | 69 (3.9) | 24 (6.4) | 24 (3.8) | 11 (2.5) | 10 (3.1) |
| Some or completed primary school | 395 (22.3) | 92 (24.6) | 124 (19.9) | 117 (26.1) | 62 (19.3) |
| Some or completed secondary school | 1195 (67.6) | 238 (63.6) | 427 (68.4) | 304 (67.9) | 226 (70.2) |
| Some or completed tertiary education | 109 (6.2) | 20 (5.3) | 49 (7.9) | 16 (3.6) | 24 (7.5) |
| Missing, n (%) | 400 (18.5) | 4 (1.1) | 270 (30.2) | 9 (2.0) | 117 (26.7) |
| Relationship status, n (%) | |||||
| Multiple partners | 135 (7.5) | 10 (2.6) | 48 (7.5) | 46 (10.1) | 31 (9.2) |
| One partner, living together | 688 (38.1) | 185 (48.9) | 225 (35.4) | 153 (33.6) | 125 (37.2) |
| One partner, not living together | 919 (50.9) | 171 (45.2) | 339 (53.3) | 245 (53.8) | 164 (48.8) |
| Single, no relationship | 63 (3.5) | 12 (3.2) | 24 (3.8) | 11 (2.4) | 16 (4.8) |
| Missing, n (%) | 363 (16.7) | 0 (0.0) | 258 (28.9) | 2 (0.4) | 103 (23.5) |
| Member of a priority population, n (%) | |||||
| Any priority population2 | 1183 (54.6) | 255 (67.5) | 409 (45.7) | 303 (66.3) | 216 (49.5) |
| Women aged 16 to 25 years | 683 (31.5) | 139 (36.8) | 233 (26.1) | 182 (39.8) | 129 (29.4) |
| Pregnant women | 283 (13.1) | 63 (16.7) | 86 (9.6) | 68 (14.9) | 66 (15.0) |
| Women who are breastfeeding | 281 (13.0) | 79 (20.9) | 67 (7.5) | 77 (16.8) | 58 (13.2) |
| Relationship with an HIV-positive partner | 275 (12.7) | 78 (20.6) | 87 (9.7) | 67 (14.7) | 43 (9.8) |
| Sex worker | 1 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Men having sex with men | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Current STI | 133 (6.1) | 10 (2.6) | 79 (8.8) | 25 (5.5) | 19 (4.3) |
| Attended specifically for PrEP | 108 (6.0) | 27 (7.1) | 34 (5.3) | 30 (6.6) | 17 (5.0) |
Abbreviations: PPP=Pre-exposure prophylaxis Promotion Package; SD=standard deviation
Percentages shown have been calculated excluding those with a missing value for that variable.
Priority populations were women between 16 and 25 years of age, those in a relationship with an HIV-positive partner, sex workers, men having sex with men, those with a current sexually transmitted infection, pregnant women, and lactating women.
Table 2.
Sample characteristics of interviewees in the in-depth qualitative interviews
| Characteristic | Number (%) |
|---|---|
| n | 217 (100.0) |
| Female | 158 (72.8) |
| Age group | |
| 16–19 years | 8 (3.7) |
| 20–29 years | 82 (37.8) |
| 30–39 years | 78 (35.9) |
| 40–49 years | 16 (7.4) |
| 50–59 years | 14 (6.5) |
| Not disclosed | 19 (8.8) |
| Interviewee type | |
| Client | 132 (60.8) |
| Started PrEP on day of interview | 38 (17.5) |
| Declined PrEP | 29 (13.4) |
| Attended all follow-up visits until the day of the interview | 38 (17.5) |
| Discontinued PrEP prior to the interview | 26 (12.0) |
| Deferred PrEP uptake | 1 (0.5) |
| Healthcare worker | 55 (25.3) |
| Nurse | 31 (14.3) |
| Nursing assistant | 2 (0.9) |
| HIV testing counselor | 7 (3.2) |
| HIV expert client | 7 (3.2) |
| Mother-to-mother mentor | 4 (1.8) |
| Other | 4 (1.8) |
| Stakeholder | 30 (13.8) |
| Ministry of Health official | 9 (4.1) |
| Member of non-governmental organization | 14 (6.5) |
| Member of United Nations or foreign government agency | 3 (1.4) |
| Member of a parastatal organization | 1 (0.5) |
| Community activist | 3 (1.4) |
PrEP=HIV pre-exposure prophylaxis.
PrEP uptake and retention
Over the study duration of 18 months, a total of 2,168 clients underwent a risk assessment for HIV (a mean of 20 clients per month per healthcare facility). For comparison, in the 14 months prior to study start, the mean monthly number of visits (double counting patients who visited the healthcare facility several times in a month, and including patients who are not suitable for PrEP, such as children and HIV patients) per healthcare facility at the six study healthcare facilities varied from 521 to 1,884, with a facility-level mean of 1,207 visits (Table S1). 20 (0.9%) of the clients who underwent a risk assessment tested HIV-positive on the day of the risk assessment and were all connected to services providing antiretroviral therapy (ART). For 18 (0.8%) clients who underwent a risk assessment, the data recorded in the client file was insufficient to allow us to determine whether the client was at risk for acquiring HIV. 1,538 (72.2%) of the remaining 2,130 clients were identified as being at risk and thus were offered PrEP. Out of 108 clients who stated that they had attended the healthcare facility specifically to learn about PrEP or take up PrEP, all underwent a risk assessment and all were considered at risk of acquiring HIV by this assessment. 100 out of these 108 (92.6%) clients took up PrEP. Overall 33.6% (517/1,538) of clients identified as being at risk took up PrEP, averaging 4.8 clients per facility per month. In in-depth interviews, the most prominent reasons for declining to take up PrEP included a fear of oral daily medication and the side-effects that may occur, adherence concerns, insufficient time and information to make a decision regarding PrEP use, and the fear that disclosing PrEP use to family and friends would result in stigmatization, arguments, abandonment, and violence (Table 3).
Table 3.
Illustrating quotes on reasons to decline PrEP initiation
| Reason for PrEP decline | Example quotation |
|---|---|
| PrEP perceived as too similar to ART | “They told me that I will be taking the pill every day. And I thought that I do not like taking a pill and that it will be like I am taking the AIDS pill; that I would take the pill every day and every day. ” (Female, 20, control phase) |
| Perceived inability to adhere | “I think it’s going to take me a long time to consider taking it. The nurse told me that this a pill that is taken daily for it to be effective and since I have a busy kind of lifestyle, I don’t think I’ll manage. I am an alcoholic and I club a lot. This might lead me into forgetting to take the pill at the stipulated time I would have chosen for myself. ” (Male, 30, PPP phase) |
| Knowledge of PrEP not sufficient to make a decision on the day | “I can’t make a decision now on this day. I need more time to read and understand this information. ” (Female, 26, PPP phase) |
| Need to consult with partners before they can begin PrEP | “I thought that even myself I can love to take them for prevention, but I may want to start to talk to my partner; then return to take the pill. Otherwise it’s a good one. ” (Female, 21, control phase) |
| Fear of stigmatization and arguments | “Then I also mentioned PrEP at home to my partner, and he said ‘No, it’s not like that. The nurses are lying. This pill is for AIDS, not for prevention. There’s no such thing as a pill for AIDS prevention. This pill is for AIDS’. And then I considered the fact that if I decide to take it, then he’ll say I’m taking AIDS pills and I don’t tell him. We’d get into a heated fight because he might even just say it’s actually the AIDS pill and that I’m hiding the fact that I’m taking HIV pills. You see that? ” (Female, 31, control phase) |
| “I told the father of my child that these are the pills they gave me at the clinic to protect myself from getting infected, and he asked me some questions that since I’m taking these pills, do I not trust myself? And I told him there are no secretive things that I do but I just want to keep my life healthy. We argued and it was bad; and he continued to say that there are things that make me not trust myself. And I told him I did not do anything secretive. So we continued arguing that I was being unfaithful to him.” (Female, age not disclosed, PPP phase). | |
| Use of other prevention methods | “I think it’s because when I - most of the time - when maybe I’m having sex, we use a condom.” (Female, 20, PPP phase) |
PrEP=Pre Exposure Prophylaxis; ART=antiretroviral therapy; PPP=PrEP Promotion Package
35.7% (180/504) of clients who took up PrEP at least one month prior to the study end date (and were thus expected to have made at least one follow-up visit prior to study closure) did not attend any follow-up visit. 30.0% (126/420) of clients who took up PrEP at least six months prior to the end date of this study attended all scheduled follow-up visits within seven days of their scheduled appointment during the first 180 days after PrEP initiation. Only one client who took up PrEP during the study period tested HIV-positive after initiating PrEP. This client took up PrEP while the healthcare facility was in the control phase and attended the first follow-up visit (at which she tested HIV-negative) but was six weeks late for the second follow-up visit (at which time she tested HIV-positive). Out of the 100 clients who initiated PrEP and reported that they had attended the healthcare facility specifically to learn about PrEP or take up PrEP, 79 took up PrEP at least six months prior to the end of the study, of whom 35 (44.3%) attended all scheduled follow-up visits within seven days of their scheduled appointment during the first 180 days after PrEP initiation.
Effects of the healthcare facility-based PrEP Promotion Package on uptake
Both the number of clients who underwent a risk assessment and the number who were identified as being at risk increased within the first month after implementation of the healthcare facility-based PPP but tended to decrease over the subsequent months (Figure 2). This pattern, however, appeared to differ somewhat between healthcare facilities. Our qualitative findings highlighted several differences in the way the PPP was used and viewed across facilities, which may explain some of this variation in PrEP uptake patterns between facilities. Healthcare workers from the Siphocosini clinic, for example, mentioned that they were participants in the production of the video and were therefore ‘proud’ to ‘play the video very often.’ Clients at the Siphocosini clinic said they liked that they could recognize the people in the video as this made the information more relatable, interesting, and friendly. Healthcare workers from Horo and Hhukwini, however, frequently remarked that there were not enough nurses or healthcare workers in their clinics that could initiate clients on PrEP, or meet the patient demands generally. These clinics were two of the most rural clinics, and healthcare workers and clients described limited resources and assistance from the government or development partners. Our observational notes described the self-completion risk assessment form to be visible and available in clinic waiting rooms at Siphocosini and Ntfonjeni, but to a lesser extent in the other four clinics.
Figure 2.
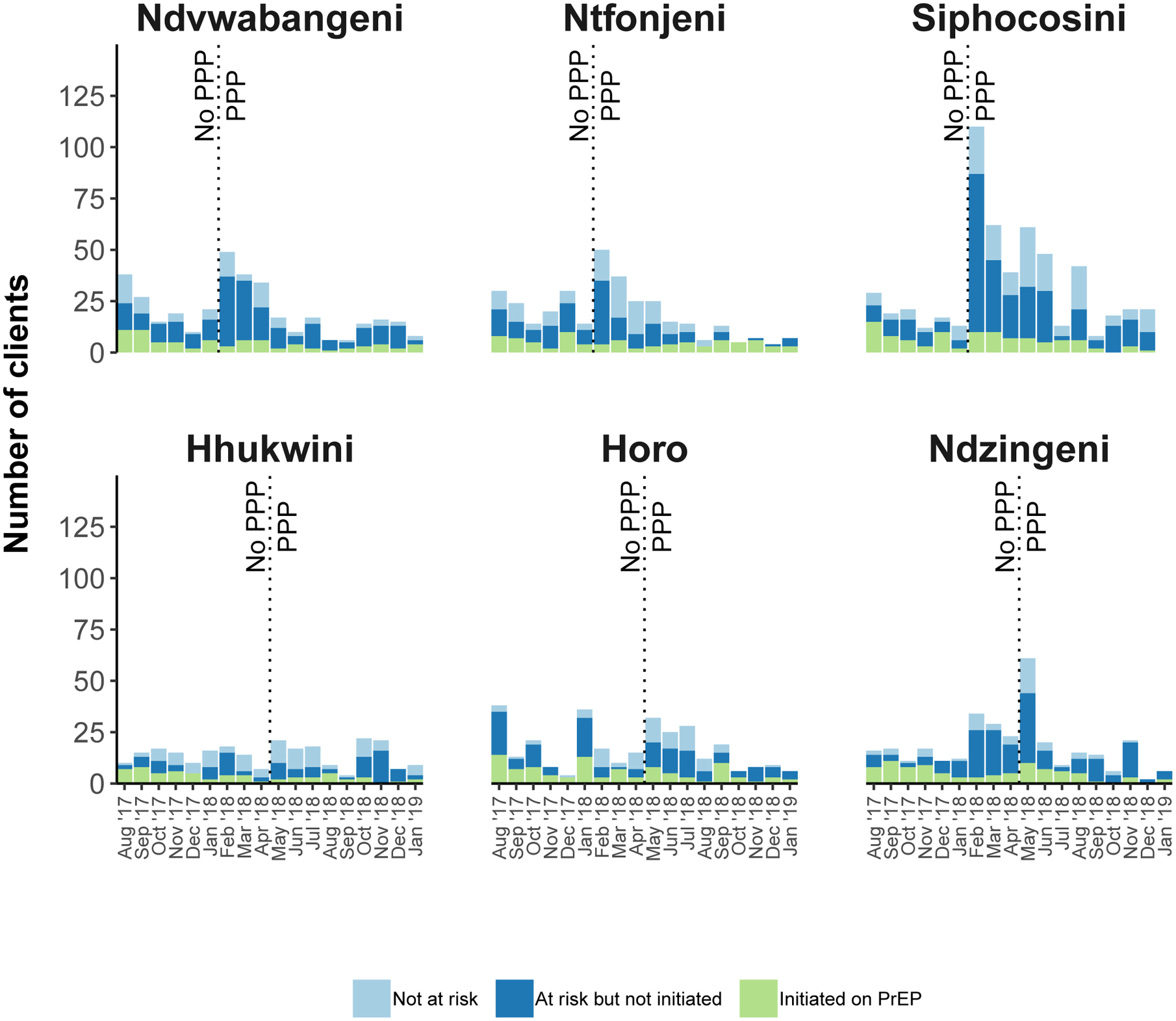
Number of clients who underwent a risk assessment, number identified as being at risk, and number who initiated PrEP, by healthcare facility and month
“Not at risk” refers to clients who underwent an assessment of their risk of acquiring HIV but for whom the risk assessment concluded that they were not at risk of acquiring HIV. “At risk but not initiated” refers to clients who underwent a risk assessment and for whom the risk assessment concluded that they were at risk of acquiring HIV but who were not initiated on PrEP. “Initiated on PrEP” refers to clients who underwent a risk assessment, for whom the risk assessment concluded they were at risk, and who were initiated on PreEP. PPP=PrEP Promotion Package; PrEP=Pre Exposure Prophylaxis
Being in the intervention phase of the PPP was associated with a 55% (risk ratio [RR]: 1.55, 95% confidence interval [CI]: 1.15 – 2.10, p=0.036) increase in the number of clients taking up PrEP at a healthcare facility (Table 4). The average marginal effect was 2.2 additional clients per month per healthcare facility (from an average adjusted prediction of 4.0 clients per month per facility in the control phase). The intracluster correlation coefficient (the between-cluster variance divided by the sum of the within-cluster and between-cluster variance) for the primary endpoint was 0.0036. The PPP intervention phase was also associated with an increase in both the number of clients at a healthcare facility who were identified as being at risk of acquiring HIV and the number of clients who underwent a risk assessment – RR of 2.80 (95% CI: 1.91 – 4.10, p=0.033) and RR of 2.91 (95% CI: 1.98 – 4.26, p<0.001), respectively. The PPP was negatively associated (although not significantly at the p<0.05 level) with the percentage of those at risk who took up PrEP (RR: 0.63; 95% CI: 0.49 – 0.81; p=0.066). There was no significant association between the PPP and retention in the PrEP program at six months. When testing for an interaction of the PPP’s effect by gender for each of the individual-level outcomes (whether or not a client who was approached was at risk, whether or not a client who was at risk took up PrEP, and whether or not a client who took up PrEP was retained at six months), none of the interaction effects were of a meaningful effect size nor were they statistically significant.
Table 4.
Effect of the PPP on the trial endpoints
| RR (95% CI)1 | P2 | Average adjusted prediction | Average marginal effect | ||
|---|---|---|---|---|---|
| No PPP | PPP | ||||
| Primary endpoint: | |||||
| Number taking up PrEP | 1.55 (1.15 – 2.10) | 0.036 | 4.0 clients per month per facility | 6.2 clients per month per facility | 2.2 clients per month per facility |
| Secondary endpoints: | |||||
| Number identified at risk | 2.80 (1.91 – 4.09) | 0.033 | 8.9 clients per month per facility | 24.9 clients per month per facility | 16.0 clients per month per facility |
| % of those at risk who took up PrEP | 0.63 (0.49 – 0.81) | 0.066 | 42.7 percent | 26.9 percent | −15.8 percentage points |
| Number approached | 2.91 (1.98 – 4.26) | <0.0001 | 12.1 clients per month per facility | 35.1 clients per month per facility | 23.0 clients per month per facility |
| % of those approached who were at risk | 1.04 (0.93 −1.15) | 0.513 | 70.8 percent | 73.3 percent | 2.5 percentage points |
| Number taking up PrEP or linked to ART | 1.58 (1.17 – 2.12) | <0.0002 | 4.1 clients per month per facility | 6.5 clients per month per facility | 2.4 clients per month per facility |
| % of those taking up PrEP retained at six months3 | 0.74 (0.40 – 1.37) | 0.103 | 33.1 percent | 24.6 percent | −8.5 percentage points |
PPP=HIV pre-exposure prophylaxis package; PrEP=pre-exposure prophylaxis; RR=relative risk; CI=confidence interval; ART=antiretroviral therapy
The 95% confidence interval was taken from a model that did not adjust for intracluster correlation and may thus be too narrow.
The p-value was calculated using a permutation test – the swpermute package in Stata(38) – that adjusts for intracluster correlation non-parametrically.
defined as attending all follow-up visits during the first six months after PrEP initiation not more than seven days after the scheduled appointment date.
Predictors of being at risk for HIV and PrEP uptake
Among those who were identified as being at risk of acquiring HIV, participants in the age groups 36–45 and >45 years were more likely to take up PrEP than those aged 16–25 years in both unadjusted and adjusted regressions (Table 5). There was no clear pattern to the associations of taking up PrEP with education and relationship status. Those in at least one priority population were more likely to take up PrEP – conditional on being at risk – than those who were not in a priority population, but this association was lower in magnitude and non-significant in the covariate-adjusted regression. The reasons for which participants were categorized as being at risk are shown in Figure S2.
Table 5.
Negative binomial regressions of PrEP uptake onto clients’ socio-demographic characteristics1
| Covariate-unadjusted2 | Covariate-adjusted3 | |||
|---|---|---|---|---|
| RR (95% CI) | P4 | RR (95% CI) | P5 | |
| Female | 0.85 (0.69 – 1.04) | 0.175 | 0.90 (0.72 – 1.13) | 0.398 |
| Age group | ||||
| 16–25 years | Ref. | Ref. | ||
| 26–35 years | 1.29 (1.01 – 1.67) | 0.083 | 1.27 (1.01 – 1.59) | 0.088 |
| 36–45 years | 1.65 (1.29 – 2.12) | 0.010 | 1.51 (1.20 – 1.89) | 0.011 |
| >45 years | 1.48 (1.18 – 1.87) | 0.031 | 1.37 (1.11 – 1.69) | 0.032 |
| Education | ||||
| No formal schooling | Ref. | Ref. | ||
| Some or completed primary school | 0.88 (0.62 – 1.25) | 0.582 | 0.98 (0.73 – 1.30) | 0.804 |
| Some or completed secondary school | 0.70 (0.51 – 0.97) | 0.039 | 0.83 (0.61 – 1.12) | 0.255 |
| Some or completed tertiary education | 0.80 (0.50 – 1.29) | 0.432 | 0.92 (0.60 – 1.41) | 0.741 |
| Relationship status | ||||
| Multiple partners | Ref. | Ref. | ||
| One partner, living together | 0.78 (0.61 – 1.00) | 0.099 | 0.77 (0.58 – 1.03) | 0.195 |
| One partner, not living together | 0.66 (0.52 – 0.83) | 0.062 | 0.73 (0.55 – 0.95) | 0.148 |
| Single, no relationship | 0.53 (0.27 – 1.02) | 0.079 | 0.57 (0.25 – 1.31) | 0.198 |
| Member of a priority population | 1.61 (1.07 – 2.41) | 0.018 | 1.19 (1.00 – 1.40) | 0.177 |
PrEP = HIV pre-exposure prophylaxis; RR=relative risk; CI=confidence interval
All regressions were negative binomial regression models with a binary variable (‘fixed effect’) for each healthcare facility. The outcome was a binary variable indicating whether the participant took up PrEP. The sample was restricted to those 1,543 participants who were considered at risk of acquiring HIV. P-values were computed using the score bootstrap procedure with Webb weights and 1,000 replications.(42)
These regressions only had one independent variable.
These regressions had all variables shown in the table as independent variables, i.e., ten-year age group (categorical), education (categorical), relationship status (categorical), and whether the participant was in at least one priority population (binary).
The p-values for the chi-square test testing the null hypothesis that all categories of the categorical variables are equal to zero was 0.0001 for age group, 0.0085 for education, and 0.0018 for relationship status.
The p-values for the chi-square test testing the null hypothesis that all categories of the categorical variables are equal to zero was 0.0026 for age group, 0.1237 for education, and 0.1320 for relationship status.
Views on the healthcare facility-based PrEP Promotion Package
Healthcare workers generally expressed a high degree of motivation to provide PrEP during both rounds of interviews (Table S3). Interviewees’ views on the PrEP promotion materials implemented during the control phase of the study are shown in Table S4. When asked about their views on each PPP component during interviews conducted in the intervention phase, all interviewee groups provided the most positive feedback about the booklet, which they deemed ‘practical’ and ‘informative.’ The PrEP T-shirts and the video also received positive feedback and respondents tended to expect they would increase PrEP uptake. The self-risk assessment form elicited mixed responses, as while it was thought to speed up the process of initiation, healthcare workers felt it could not be used with illiterate clients and was also largely unnecessary because in Eswatini ‘everyone is at risk.’ The flipchart received the most negative feedback, with many healthcare workers commenting that it was too long and not viable to use in a ‘normal’ counselling session. Several clients said they had ‘never seen’ the flipchart. These views, along with illustrating quotes, are summarized in Table S5.
The ranking exercise conducted with 69 interviewees (21 healthcare workers and 48 clients) from June to September 2018 confirmed these qualitative findings. Booklets were the most popular PPP component among female and male clients and female healthcare workers (ranked in the top three PPP components among 58% of female clients, 73% of male clients, and 53% of female healthcare workers), whereas the most popular PPP component among male healthcare workers was the T-shirt (ranked in the top three by 83% of male healthcare workers). Table S6 shows each component’s popularity assessed among healthcare workers, opt-in clients (new, refill, or cycling), and opt-out clients (discontinuing or declining PrEP) regardless of gender. Table S7 shows the combination of PPP components that would likely appeal to most respondents by including in the set at least one of their top-three ranked choices. We note that even the least popular PPP components are included in these sets, indicating that the minority to whom they appealed might not favor some of the more broadly popular PPP components.
Qualitative research findings regarding measures to increase PrEP uptake
Interviewees offered several suggestions to improve the PPP and PrEP promotion more generally (Table 6). The most reoccurring theme across all interviewee groups was in relation to providing the PPP outside the clinic environment and in specific locations (such as at village meetings, dip tanks, barber shops, communal barbecues, and during household visits by community health workers) within the community. Long-term, consistent, community-based education was seen as reaching important groups, especially men, who would influence the ability of their partners to use PrEP, in addition to taking PrEP themselves. Without community-based access to the PPP and PrEP information and education in general, most respondents also felt that people would not have enough information and time to make an informed choice, and that PrEP would not reach those most at risk of acquiring HIV.
Table 6.
Findings from 132 in-depth qualitative interviews on healthcare workers’ and clients’ views on improving PrEP uptake
| Improvement | Rationale | Example quotation |
|---|---|---|
| Engage traditional community leaders |
|
“Have you spoken to the community leaders? You won’t move anything here unless they say that it is okay, that this should be in their community. ” (Stakeholder, Male, Control phase) |
| Reach men in communities |
|
“They don’t come back because it’s forbidden. When they reach their homes and tell their partners, it will be forbidden. These women cannot make this choice. Men make the choices for them. ” (Healthcare worker, Female, Intervention phase) |
| Distribute PPP in communities |
|
“This should not be the first place people see these materials. They need to be in the community. It’s too much to take in, especially if you have come for some other kind of treatment”. (Healthcare worker, Female, Control phase) |
| Establish PrEP ‘champions’ |
|
“You need to start by doing outreach activities, going straight into the communities, teach about PrEP. ” (Decline client, Female, Intervention phase) |
PrEP=HIV pre-exposure prophylaxis; PPP=PrEP promotion package
Discussion
When PrEP was made available at public-sector primary healthcare facilities in Eswatini to everyone above the age of 16 years who was at risk of acquiring HIV or who requested PrEP, a third of those who were offered PrEP agreed to take it up. 30% of those taking up PrEP attended all follow-up visits on time during the first six months after PrEP initiation. We found that a healthcare facility-based package of five PrEP promotion activities had a large relative, but only a small absolute, effect on the total number of clients who initiated PrEP. There was no meaningful, nor statistically significant, effect on the proportion of clients approached who were at risk of acquiring HIV, implying that the PPP did not affect the efficiency with which healthcare workers were able to identify clients at risk of acquiring HIV. However, since the PPP reduced the proportion of clients at risk of acquiring HIV who initiated PrEP, the PPP either led healthcare workers to approach clients who, on average, perceived themselves of being in less need of PrEP and/or the PPP performed less well than the standard of care in persuading clients at risk of acquiring HIV to take up PrEP. Clients, healthcare workers, policymakers, and implementation stakeholders all felt that PrEP promotion activities must be extended to the community to achieve high levels of PrEP uptake among the general population.
This study has several limitations. First, this research was conducted at only six primary healthcare facilities. While these facilities were similar to most public-sector healthcare facilities in Eswatini, they were selected by the Eswatini Ministry of Health to participate in an early access to antiretroviral therapy trial (the MaxART trial (25, 26)) prior to this study. It is likely that these healthcare facilities were (at least at the time of the design of the MaxART trial) deemed to be well-functioning healthcare facilities by the Ministry of Health. It is therefore possible that PrEP uptake and retention in the study healthcare facilities were higher than they would have been had the study been implemented at a random sample of healthcare facilities in Eswatini. Similarly, it is likely that the PPP was implemented with higher fidelity in the study healthcare facilities than may have been the case at a truly representative sample of healthcare facilities. Second, given that socio-cultural factors and health system structures likely have an important influence on PrEP uptake, our findings may not be generalizable to other countries. Third, the study period was only 18 months. As such, we are unable to comment on longer-term patterns of PrEP uptake and retention, and the effectiveness of the PPP over a longer time horizon. Fourth, the stepped-wedge trial had only two sequences (with three healthcare facilities in each sequence) and three periods. In a stepped-wedge trial, the probability that randomization eliminates confounding increases as the number of sequences and the number of clusters in each sequence grows. Despite the randomized design, it is thus possible that our effect estimates for the PPP were affected by time-varying confounders, such as changes in the number of patients attending the healthcare facilities (and, thus, the number who could be approached for an assessment of their risk of acquiring HIV) that differed across facilities over time. Fifth, we did not have data on the total number of patients who visited each healthcare facility during the study period and as such are unable to estimate the proportion of all patients at these facilities who took up PrEP. Sixth, while all PrEP clients underwent an HIV-test at each follow-up visit, we were unable to ascertain the HIV-status of those clients who were non-retained. It is thus possible that more clients seroconverted during the first six months after PrEP initiation than observed in this study. Lastly, the quantitative analysis of respondents’ ranking of the PPP components was based on the convenience samples used for the qualitative research, and thus may not generalize to all clients, healthcare workers, and policymakers.
This PrEP rollout at primary healthcare facilities in Eswatini did not reach men to the same degree as it reached women. Differential uptake by gender has also been observed by studies of ART programs in sub-Saharan Africa, which have noted that men are less likely to get tested for HIV(9), initiate ART when eligible(27), and be retained in ART care long term(28, 29). One important reason for the low number of men who underwent a risk assessment and took up PrEP in this study likely is that men in Eswatini, and sub-Saharan Africa more broadly, generally attend healthcare facilities less frequently than women(30). Prior to the study, we were hopeful that a substantial number of men would attend the healthcare facility specifically to ask for PrEP, which could have reduced the discrepancy in PrEP uptake by gender. However, only 8% (38% of whom were men) of those identified to be at risk of acquiring HIV in this study reported that they came to the healthcare facility specifically for PrEP. As such, the current PrEP rollout in Eswatini is largely reaching individuals who are already attending the healthcare facility for other reasons; that is, mainly women. While the 2016–2017 Swaziland HIV Incidence Measurement Survey reported a far higher HIV incidence among women than among men(23), identifying the most effective approaches to reach men at a high risk of acquiring HIV will nonetheless be important. Such approaches might include the promotion of PrEP at community gatherings, sports events, and through mass media channels (including social media), collaborating with shebeens (local bars) on PrEP promotion, and employing mobile clinics to provide PrEP.
More generally, extending PrEP promotion to the community is crucial to reach those population groups who do not regularly attend a healthcare facility, including many women. Importantly, implementing community-based PrEP promotion may not only increase PrEP uptake among men and those women who rarely attend primary care, but could also improve uptake and retention among women who already regularly visit healthcare facilities. There is evidence from sub-Saharan Africa that men’s reactions to their female partners using PrEP can range from active opposition to active support, including in settings with high gender inequity and a high prevalence of gender-based violence(31). Therefore sensitizing men to PrEP may increase support for their partner’s wish to take up PrEP, which many of our interviewees felt would be key for women’s ability to start and adhere to PrEP. Our in-depth interview participants emphasized the importance of promoting PrEP at community gatherings with the involvement of village chiefs, current PrEP users, people living with HIV, community police, and community health workers. How uptake and adherence for PrEP among men and communities in general can best be increased in various settings is a vital question for future research.
Beyond extending PrEP to community-based settings, our study also provides some insight into promising approaches to achieving high PrEP uptake and retention at healthcare facilities. The PPP’s booklet, video, and PrEP T-shirts were all well-liked by patients and healthcare workers. To our surprise, the self-risk assessment form received mixed feedback. We had hypothesized that the self-risk assessment forms would be liked by patients because it reduces the need to engage in a discussion about socially sensitive topics with the healthcare worker, and by healthcare workers because it speeds up the PrEP initiation process. One simple, but important, lesson from our qualitative research is that these forms must be short and simple to read. More broadly, an important finding of our analysis is that the number of clients who underwent a risk assessment and the number who were identified as being at risk of acquiring HIV tended to decrease over the first few months of the PrEP rollout, and then increased initially during the PPP phase to finally decline again over the subsequent months. Given that the mean monthly number of primary care visits at the six healthcare facilities during the 14 months prior to study start varied from only 521 at Ndzingeni clinic to 1,884 at Horo clinic, a ‘saturation’ effect may be at least partially responsible for this pattern. That is, the number of clients who had not yet undergone a risk assessment in the preceding months is likely to have declined over time. However, it is also possible that healthcare workers’ motivation to screen clients for PrEP eligibility declined with time and was given only a transient ‘boost’ by the PPP. Our qualitative findings suggest that healthcare workers in some of the study clinics felt overburdened by the increased workload that PrEP added and may not have been able to maintain PrEP activities in the longer term. Although this pattern of a short-lived boost after PPP implementation was less evident for the number of clients who took up PrEP (whereby clients who initiated, discontinued, and reinitiated PrEP were only counted once), it is possible that the effect of the PPP on PrEP uptake would have been smaller in magnitude had the study period been longer.
Despite a modest effect size on the absolute scale, our facility-based PPP appears to have been effective in improving PrEP uptake and could thus – with adaptations for other settings – accompany the rollout of PrEP at primary healthcare facilities in similar settings. However, to reach population groups who only rarely attend primary care, particularly men, it will be critical for PrEP promotion to be conducted in community-based settings, including through the involvement of traditional community leaders, current PrEP users, and community health workers. Future research should investigate what the most effective community-based intervention designs are to increase PrEP uptake and adherence.
Materials and Methods
Study design
Set in Eswatini, the overall goal of this study was to determine the level of PrEP uptake and retention when PrEP is offered to anyone aged 16 years and older who is at risk of acquiring HIV or who requests PrEP, test the impact of a healthcare facility-based PPP on PrEP uptake, and characterize stakeholders’ views on how PrEP uptake could be further increased. Our main hypothesis was that PrEP uptake in this setting would be low but that, if designed well, a healthcare facility-based PPP could achieve at least modest improvements in uptake.
This was a stepped-wedge randomized study with two sequences (each consisting of three clusters) and three periods (Table 7). We chose a stepped-wedge instead of a cross-sectional randomized design for the evaluation of the PPP because it is statistically more efficient when the outcome is observed at the cluster rather than the individual level(33). A cluster was a healthcare facility.
Table 7.
The stepped-wedge randomized design of this study
| Healthcare facility | Aug 2017 | Sep 2017 | Oct 2017 | Nov 2017 | Dec 2017 | Jan 2018 | Feb 2018 | Mar 2018 | Apr 2018 | May 2018 | Jun 2018 | Jul 2018 | Aug 2018 | Sep 2018 | Oct 2018 | Nov 2018 | Dec 2018 | Jan 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ndvwabangeni | C | C | C | C | C | C | I | I | I | I | I | I | I | I | I | I | I | I |
| Ntfonjcni | C | C | C | C | C | C | I | I | I | I | I | I | I | I | I | I | I | I |
| Siphocosini | C | C | C | C | C | C | I | I | I | I | I | I | I | I | I | I | I | I |
| Hhukwini | C | C | C | C | C | C | C | C | C | I | I | I | I | I | I | I | I | I |
| Horo | C | C | C | C | C | C | C | C | C | I | I | I | I | I | I | I | I | I |
| Ndzingeni | C | C | C | C | C | C | C | C | C | I | I | I | I | I | I | I | I | I |
C=Control (i.e., no implementation of the PrEP Promotion Package); I=Intervention (i.e., the PrEP Promotion Package was implemented); PrEP = HIV pre-exposure prophylaxis
Three of the six healthcare facilities were randomized to sequence 1 (implementing the PPP starting on February 1st 2018) with the remaining three being allocated to sequence 2 (implementing the PPP starting on May 1st 2018). The randomization was done by SK using Stata version 15.1. No stratification was used in the randomization. The length of each period was set prior to the trial based on discussions with the Eswatini Ministry of Health. For feasibility reasons, the research team, healthcare workers, and participants were not blinded to the intervention. However, healthcare facilities were not informed as to when they were expected to start implementing the PPP until two weeks prior to the start of the intervention phase to reduce bias from healthcare workers anticipating the intervention phase. This trial was registered on clinicaltrials.gov (NCT03254550). We reported our results following the guidance by the CONSORT checklist for stepped-wedge randomized trials (Table S8).
The study was approved by the Ministry of Health’s National Health Research Review Board (MH/599C/IRB 0009688/NHRRB538/17) in Eswatini and the Chesapeake Institutional Review Board in the United States of America (Pro00021864). We received an exemption for ethical review from the Ethics Committee of the Faculty of Medicine at Heidelberg University in Germany.
Training of healthcare providers for the provision of PrEP
Healthcare workers who staffed the healthcare facilities included in this study consisted of nurses, nursing assistants, and support staff including expert clients, HIV testing counselors, and mentor mothers. Expert clients are adults living with HIV who provide counseling to HIV patients on leading a healthy lifestyle, measures to prevent transmission of HIV, and ART adherence. Mentor mothers are mothers living with HIV who provide such counseling for women living with HIV during pregnancy and the postpartum period.
These healthcare workers at all study healthcare facilities participated in two training sessions (each lasting two to three hours), which aimed to educate healthcare workers about PrEP in general, the assessment of patients’ risk of acquiring HIV, the PrEP initiation process, monitoring of PrEP patients at follow-up visits, as well as the aims, design, and procedures of this study. During the study period, mentors (who were nurses with multiple years of experience working in the Eswatini health system) visited healthcare facilities on a monthly basis (twice per month during the first month of the study period) to provide advice and encouragement to healthcare workers and monitor the number and quality of PrEP client files. In addition, the study team conducted an on-site feedback session every four months, which was used to review progress, discuss challenges with study implementation and possible solutions, and reiterate some of the information provided during the initial training. In the week prior to the start of the intervention phase at a healthcare facility, the research team carried out a two-hour onsite training for healthcare workers in the use of the PPP.
Control phase:
None of the healthcare facilities had ever provided PrEP prior to study start. At the beginning of the study period, all healthcare facilities began offering PrEP without the use of the PPP. PrEP promotion in the control phase consisted of each clinic displaying posters advertising PrEP (Figure S3), one-page pamphlets (Figure S4), and palm cards (Figure S5) for patients to take from the clinic. In addition, healthcare workers in Eswatini’s primary healthcare facilities routinely provided a brief health education session to clients waiting at the healthcare facility in the morning. In addition to continuing to cover the topics that were discussed in these sessions prior to the implementation of this trial (e.g., the importance of regular HIV testing and advice on leading a healthy lifestyle), healthcare workers were asked to inform clients during these health education sessions of the availability of PrEP at the healthcare facility and encourage those who reported to be HIV-negative or did not know their HIV status to undergo an HIV test.
Healthcare workers administered a standard HIV prevention package to those clients who tested HIV-negative, which included counselling on reducing one’s risk of acquiring HIV, condoms, symptomatic screening for sexually transmitted infections when indicated, and basic information about PrEP. Those expressing an interest in using PrEP after this session underwent an assessment of their risk of acquiring HIV according to the form shown in Figure S6. Specifically, the risk assessment consisted of six questions about behavior in the past six months: i) have you had unprotected (condom-less) sex?; ii) have you had sex with partners who are HIV-positive or whose HIV-status you did not know?; iii) have you had a sexually transmitted infection?; iv) have you been using post-exposure prophylaxis (PEP)?; v) have you had sex under the influence of alcohol and/or drugs?; and vi) have you experienced or do you expect any situations which you consider to be risky for acquiring HIV? If a client responded with “yes” to any of these six questions or expressed a desire to start PrEP, the healthcare worker would discuss with the client the risk of acquiring HIV and the benefits and limitations of PrEP in order to make a joint decision as to whether PrEP should be initiated. In the control phase, the risk assessment was performed by the healthcare worker, while in the intervention phase (as detailed below) a self-risk assessment form was made available in the waiting room, which clients could fill out to then hand to the healthcare worker during the consultation. The risk assessment did not ask about whether a client had a new sexual partner or whether the client had concerns about their partner having other sexual partners.
Those accepting the offer to start PrEP were informed about the study and asked to provide written informed consent. Exclusion criteria for enrolling in the study were being unable to provide written informed consent (thumb prints instead of signatures were allowed for illiterate participants), being younger than 16 years, having a suspected acute HIV infection, or having contraindications to Tenofovir disoproxil fumarate (TDF) or Lamivudine (3TC) as specified in the 2018 Eswatini Integrated HIV Management guidelines(34). In addition, clients who stated that they would be unable to attend a follow-up visit at around one month after PrEP initiation were asked to return for PrEP initiation at a time when they would be able to return for their first follow-up visit. This exclusion criterion existed because the Eswatini Ministry of Health deemed it essential that PrEP clients were able to attend their scheduled follow-up visits, which were also used to refill a client’s PrEP supply. Being pregnant was neither an exclusion criterion for participating in the study nor for initiating PrEP. Enrolled participants then underwent a confirmatory HIV-test (unless the participant had already had an HIV test on the day of PrEP initiation)(34), a point-of-care rapid test for Hepatitis B surface Antigen (HBsAg), and a laboratory-based serum creatinine count. Depending on the healthcare facility, the creatinine count result required three to seven calendar days to return to the healthcare facility. Those testing positive for HBsAg also underwent a liver function test prior to PrEP initiation, the result of which would take another three to seven calendar days to return to the healthcare facility. Participants with a contraindication to starting PrEP based on these tests were contacted by phone and advised to discontinue PrEP. Same-day PrEP initiation was the norm. That is, PrEP – consisting of a daily combined oral pill of 300mg of TDF and 300mg of 3TC – was prescribed at the same visit during which the risk assessment was conducted if the participant expressed readiness to start PrEP and had no contraindications. Clients taking up PrEP were asked to return for follow-up visits at one month and three months after PrEP initiation, and every three months thereafter. At each of these visits, clients received a supply of PrEP pills calculated to last until the next scheduled appointment. The recommended procedures during each of the follow-up visits are detailed in Table S9.
Intervention phase:
During the intervention phase, all healthcare facilities continued to offer PrEP according to the same procedures as during the control phase. PrEP promotion included those materials provided in the control phase (posters, pamphlets, and palm cards) plus five additions. These five additions constituted the PPP. First, each healthcare facility played a five-minute PrEP promotion video (available from https://www.youtube.com/watch?v=iQ-ZwRm3kHw) on repeat – alternating between an English and a Siswati version – in the waiting room. Second, all healthcare workers were provided with a T-shirt (Figure S7) – either in English or in Siswati – to wear during the intervention phase. The T-shirt said “What if there was a pill that could prevent HIV?” on the front and “Actually there is: Pre-Exposure Prophylaxis. Ask me for more information about PrEP” on the back. Third, healthcare workers were asked to use a detailed flipchart (Figure S8) to guide their counselling about PrEP with all clients who were at risk for acquiring HIV and expressed an interest in possibly using PrEP. Fourth, clients at risk and interested in PrEP were given a booklet (Figure S9) for information. Fifth, a self-risk assessment form (Figure S10) was displayed in the waiting room for clients to fill out and hand to the healthcare worker. This self-risk assessment form asked the same six questions to ascertain risk for acquiring HIV as the form used by the healthcare workers.
Endpoints:
The primary endpoint of this trial was the monthly number of clients who took up PrEP. PrEP uptake was defined as being prescribed PrEP and being given the first one-month’s supply of PrEP pills. Clients who initiated, discontinued, and then re-initiated PrEP during the study period were only counted once (when they were first initiated). We chose the number of clients taking up PrEP as the primary endpoint rather than a proportion (e.g., the proportion of those identified as being at risk of acquiring HIV who initiated PrEP) because the PPP may affect both the numerator (the number who initiate PrEP) and the denominator (the total number of clients who undergo an HIV risk assessment and/or who are identified as being at risk of acquiring HIV) of such a proportion. In addition, the Eswatini Ministry of Health was primarily concerned with the total number of clients who take up PrEP (regardless of how many risk assessments were required to achieve this number) rather than the efficiency with which individuals who are likely to take up PrEP were identified (which would be better represented by a proportion). However, we did analyze as secondary endpoints i) the probability that those identified as being at risk of acquiring HIV (i.e., those who answered at least one of the six questions of the risk assessment with “yes”) took up PrEP; and ii) the probability that those who were approached for an HIV risk assessment were determined to be at risk of acquiring HIV. Additional secondary endpoints were i) the monthly number of clients who were identified as being at risk of acquiring HIV; ii) the monthly number of clients who underwent a risk assessment; iii) the monthly number of clients who either took up PrEP (if HIV negative) or were linked to antiretroviral therapy (if HIV-positive); iv) the probability that clients initiated on PrEP seroconverted during the first six months after taking up PrEP; and v) the probability that clients who took up PrEP were retained at six months after PrEP initiation. The retention outcome was defined as attending all follow-up visits during the first 180 days after PrEP initiation not more than seven days after the scheduled appointment.
Quantitative data collection:
The Eswatini Ministry of Health introduced a PrEP clinical register and individual client PrEP files to record healthcare facility visits (Figure S11 and S12). A team of two research assistants visited the study healthcare facilities for four days a week to enter all data from the client PrEP files into tablets.
Details on the qualitative data collection and design of the PrEP Promotion Package are shown in Text S1.
Statistical power:
Prior to the start of the trial, we estimated power for the primary endpoint of the trial using simulation. With 1,000 simulations, an intracluster correlation coefficient (ICC) of 0.05, no time effect, and a within-facility standard deviation of two, we had 84% power at the α<0.05 level to detect a relative risk (RR) of 1.4 comparing the intervention to the control phase.
Statistical analysis:
We did not conduct any interim analyses and there were no stopping rules for this trial. To analyze the primary endpoint of this trial, we used negative binomial regression to regress the number of clients taking up PrEP in a given month at a healthcare facility onto a binary indicator for whether the healthcare facility was in the intervention phase, study month as a continuous variable, and a binary indicator for each healthcare facility. To avoid linearity assumptions about the relationship between month and the outcomes, we used fractional polynomials for month (with a maximum degree of two) searching the powers −2, −1, −0.5, zero, 0.5, one, two, and three. The fractional polynomial routine, therefore, fit 44 different functional forms for month and then selected (based on the deviance metric and using a significance level of 0.05) the best-fitting form for inclusion in the regression.(37) We tested for the statistical significance of the point estimate for the intervention phase using a permutation test – with 5,000 repetitions – as implemented with the swpermute package in Stata(38). We chose a permutation test because it does not rely on asymptotic properties to account for intra-cluster correlations that may not hold in randomized trials with few clusters(39). We adjusted non-parametrically for clustering of the endpoint within healthcare facilities over time. All secondary endpoints were analyzed using the same model with the exception that when examining the probability that those identified as being at risk of acquiring HIV took up PrEP, that those who were approached were determined to be at risk of acquiring HIV, and that those who took up PrEP were retained at six months after PrEP initiation, we i) analyzed the data at the individual rather than the month-facility level, and ii) used a Poisson model with a robust error structure.(40) The probability that clients initiated on PrEP seroconverted during the first six months after taking up PrEP was not analyzed in a regression model because only one client in the study was recorded as having seroconverted after PrEP initiation.
To determine predictors of PrEP uptake among clients who were identified as being at risk of acquiring HIV, we used negative binomial regression to regress whether or not a client took up PrEP on sex, ten-year age group, educational attainment, relationship status, whether or not a client was in at least one priority population, and a binary indicator for each healthcare facility. Priority populations were women between 16 and 25 years of age, those in a relationship with an HIV-positive partner, sex workers, men having sex with men (MSM), those with a current sexually transmitted infection, pregnant women, and lactating women. We used all variables as predictors that we hypothesized may be associated with PrEP uptake. The p-value for the association between each independent variable and the outcome was obtained using a score bootstrap procedure with Webb weights and 1,000 replications.
Qualitative analysis began in the field via debriefings, wherein highly salient themes were discussed and triangulated among the research team(35), and a draft codebook was written. The codebook was then refined and applied to transcripts. As analysis progressed, new codes were added and then applied across the full dataset. We approached the data using a general inductive analytical strategy(41). This allowed us to condense the large amounts of data into a concise summary format, and to draw links between our summaries and the research objectives.
The ranking data were analyzed by categorizing the PPP components ranked in the top three of eight components by each respondent as ‘preferred’ components for that respondent. We then assessed for each PPP component the proportion of respondents for whom that component was a preferred component, and we determined what combination of PPP components would include at least one preferred component for 95% or more of the sample.
Supplementary Material
Acknowledgements
We would like to thank all participants and healthcare providers at the study’s healthcare facilities for making this research possible.
Funding:
Support for this project was provided by the World Health Organization (TB; grant number: H9/181/R) through the United States Agency for International Development (USAID) under the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). The study was also supported by the Bob L. Herd Foundation (TB) and the Alexander von Humboldt Foundation (TB). PG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR003143. CEO was supported in part by a Research to Prevent Blindness Career Development Award. TB was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award, funded by the Federal Ministry of Education and Research; as well as by NICHD of NIH (R01-HD084233), NIA of NIH (P01-AG041710), NIAID of NIH (R01-AI124389 and R01-AI112339), and FIC of NIH (D43-TW009775). The contents in this article are the sole responsibility of the authors, and do not necessarily reflect the views of WHO, USAID, PEPFAR, the United States Government, the Bob L. Herd Foundation, NIH, or the Alexander von Humboldt Foundation. Other than the contribution of SD as an author, the funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Competing interests: The authors declare no competing interests, and no relevant paid or unpaid consulting relationships.
Data and materials availability: The de-identified dataset and all cleaning and analysis code are available on the Stanford Digital Repository. All data associated with this study are present in the paper or supplementary materials.
References:
- 1.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367, 399–410 (2012); published online EpubAug 2 ( 10.1056/NEJMoa1108524). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363, 2587–2599 (2010); published online EpubDec 30 ( 10.1056/NEJMoa1011205). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT, Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367, 423–434 (2012); published online EpubAug 2 ( 10.1056/NEJMoa1110711). [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, Chiamwongpaet S, Kitisin P, Natrujirote P, Kittimunkong S, Chuachoowong R, Gvetadze RJ, McNicholl JM, Paxton LA, Curlin ME, Hendrix CW, Vanichseni S, Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381, 2083–2090 (2013); published online EpubJun 15 ( 10.1016/s0140-6736(13)61127-7). [DOI] [PubMed] [Google Scholar]
- 5.Fonner G, Grant R, Baggaley RC, “Oral pre-exposure prophylaxis (PrEP) for all populations: a systematic review and meta-analysis of effectiveness, safety, and sexual and reproductive health outcomes.,” (World Health Organization, Geneva, Switzerland, 2015). [Google Scholar]
- 6.World Health Organization, “Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection - Recommendations for a public health approach,” (World Health Organization, Geneva, 2016). [PubMed] [Google Scholar]
- 7.UNAIDS, “Miles to go - closing gaps, breaking barriers, righting injustices,” Global AIDS Update (UNAIDS, Geneva, Switzerland, 2018). [Google Scholar]
- 8.Justman J, Reed JB, Bicego G, Donnell D, Li K, Bock N, Koler A, Philip NM, Mlambo CK, Parekh BS, Duong YT, Ellenberger DL, El-Sadr WM, Nkambule R, Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. The lancet. HIV 4, e83–e92 (2017); published online EpubFeb ( 10.1016/s2352-3018(16)30190-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Zungu N, Labadarios D, O. D, “South African National HIV Prevalence, Incidence and Behaviour Survey, 2012,” (Human Sciences Research Council, Cape Town, South Africa, 2014). [Google Scholar]
- 10.Perez-Hoyos S, Naniche D, Macete E, Aponte JJ, Sacarlal J, Sigauque B, Bardaji A, Moraleda C, de Deus N, Alonso PL, Menendez C, Stabilization of HIV incidence in women of reproductive age in southern Mozambique. HIV Med 12, 500–505 (2011); published online EpubSep ( 10.1111/j.1468-1293.2010.00908.x). [DOI] [PubMed] [Google Scholar]
- 11.ICAP, “Lesotho Population-Based HIV Impact Assessment,” (Columbia University, Maseru, Lesotho, 2017). [Google Scholar]
- 12.Statistics Botswana, “Botswana AIDS Impact Survey IV,” (Ministry of Health of the Republic of Botswana, Gaborone,, 2013). [Google Scholar]
- 13.Ministry of Health and Child Care of the Republic of Zimbabwe, “Zimbabwe National HIV and AIDS Estimates,” (Government of the Republic of Zimbabwe, Harare, Zimbabwe, 2013). [Google Scholar]
- 14.Golub SA, PrEP Stigma: Implicit and Explicit Drivers of Disparity. Curr HIV/AIDS Rep 15, 190–197 (2018); published online EpubApr ( 10.1007/s11904-018-0385-0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivet Amico K, Bekker LG, Global PrEP roll-out: recommendations for programmatic success. The lancet. HIV 6, e137–e140 (2019); published online EpubFeb ( 10.1016/s2352-3018(19)30002-5). [DOI] [PubMed] [Google Scholar]
- 16.Parsons JT, Rendina HJ, Lassiter JM, Whitfield TH, Starks TJ, Grov C, Uptake of HIV Pre-Exposure Prophylaxis (PrEP) in a National Cohort of Gay and Bisexual Men in the United States. J Acquir Immune Defic Syndr 74, 285–292 (2017); published online EpubMar 1 ( 10.1097/qai.0000000000001251). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A, HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav 22, 1096–1099 (2018); published online EpubApr ( 10.1007/s10461-017-2009-x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton LA, Driffin DD, Bauermeister J, Smith H, Conway-Washington C, Minimal Awareness and Stalled Uptake of Pre-Exposure Prophylaxis (PrEP) Among at Risk, HIV-Negative, Black Men Who Have Sex with Men. AIDS patient care and STDs 29, 423–429 (2015); published online EpubAug ( 10.1089/apc.2014.0303). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekker LG, Gill K, Wallace M, Pre-exposure prophylaxis for South African adolescents: What evidence? S Afr Med J 105, 907–911 (2015); published online EpubNov ( 10.7196/SAMJ.2015.v105i11.10222). [DOI] [PubMed] [Google Scholar]
- 20.Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, Saayman E, Moorhouse M, Venter WDF, Rees H, HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project. PLoS Med 14, e1002444 (2017); published online EpubNov ( 10.1371/journal.pmed.1002444). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koss CA, Charlebois ED, Ayieko J, Kwarisiima D, Kabami J, Balzer LB, Atukunda M, Mwangwa F, Peng J, Mwinike Y, Owaraganise A, Chamie G, Jain V, Sang N, Olilo W, Brown LB, Marquez C, Zhang K, Ruel TD, Camlin CS, Rooney JF, Black D, Clark TD, Gandhi M, Cohen CR, Bukusi EA, Petersen ML, Kamya MR, Havlir DV, Uptake, engagement, and adherence to pre-exposure prophylaxis offered after population HIV testing in rural Kenya and Uganda: 72-week interim analysis of observational data from the SEARCH study. Lancet HIV 7, e249–e261 (2020); published online EpubApr ( 10.1016/s2352-3018(19)30433-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United Nations Population Division, “World Population Prospects: The 2017 Revision, Key Findings and Advance Tables,” Working Paper No. ESA/P/WP/248 (United Nations, New York, NY, 2017). [Google Scholar]
- 23.Government of the Kingdom of Eswatini, “Swaziland HIV Incidence Measurement Survey 2 (SHIMS2) 2016–2017. Final Report.,” (Government of the Kingdom of Eswatini, Mbabane, 2019). [Google Scholar]
- 24.Government of the Kingdom of Eswatini, “Swaziland HIV Incidence Measurement Survey 2: A population-based HIV impact assessment,” (ICAP, Mbabane, 2017). [Google Scholar]
- 25.Walsh FJ, Barnighausen T, Delva W, Fleming Y, Khumalo G, Lejeune CL, Mazibuko S, Mlambo CK, Reis R, Spiegelman D, Zwane M, Okello V, Impact of early initiation versus national standard of care of antiretroviral therapy in Swaziland’s public sector health system: study protocol for a stepped-wedge randomized trial. Trials 18, 383 (2017); published online EpubAug 18 ( 10.1186/s13063-017-2128-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogbuoji O, Geldsetzer P, Wong C, Khan S, Mafara E, Lejeune C, Walsh F, Okello V, Barnighausen T, Impact of immediate initiation of antiretroviral therapy on HIV patient satisfaction. Aids 34, 267–276 (2020); published online EpubFeb 1 ( 10.1097/qad.0000000000002392). [DOI] [PubMed] [Google Scholar]
- 27.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, Keiser O, Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Tropical medicine & international health : TM & IH 17, 1509–1520 (2012); published online EpubDec ( 10.1111/j.1365-3156.2012.03089.x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, Ewald T, Mugusi F, Mtasiwa D, Fawzi W, Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. Aids 25, 1189–1197 (2011); published online EpubJun 1 ( 10.1097/QAD.0b013e3283471deb). [DOI] [PubMed] [Google Scholar]
- 29.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, Maskew M, Prozesky H, Wood R, Johnson LF, Egger M, Boulle A, Myer L, Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med 9, e1001304 (2012) 10.1371/journal.pmed.1001304). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galdas PM, Cheater F, Marshall P, Men and health help-seeking behaviour: literature review. J Adv Nurs 49, 616–623 (2005); published online EpubMar ( 10.1111/j.1365-2648.2004.03331.x). [DOI] [PubMed] [Google Scholar]
- 31.Doggett EG, Lanham M, Wilcher R, Gafos M, Karim QA, Heise L, Optimizing HIV prevention for women: a review of evidence from microbicide studies and considerations for gender-sensitive microbicide introduction. J Int AIDS Soc 18, 20536 (2015) 10.7448/ias.18.1.20536). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T, Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS 28 Suppl 2, S187–204 (2014); published online EpubMar ( 10.1097/qad.0000000000000252). [DOI] [PubMed] [Google Scholar]
- 33.Spiegelman D, Evaluating Public Health Interventions: 2. Stepping Up to Routine Public Health Evaluation With the Stepped Wedge Design. Am J Public Health 106, 453–457 (2016); published online EpubMar ( 10.2105/ajph.2016.303068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ministry of Health, “Swaziland Integrated HIV Management Guidelines,” (Government of the Kingdom of Eswatini, Mbabane, 2018). [Google Scholar]
- 35.McMahon SW, P, Systematic Debriefing after Qualitative Encounters: An Essential Analysis Step in Applied Qualitative Research. BMJ Global Health, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandura A, Social Cognitive Theory of Mass Communication. Media Psychology 3, 265–299 (2001); published online Epub2001/08/01 ( 10.1207/S1532785XMEP0303_03). [DOI] [Google Scholar]
- 37.Royston P, Sauerbrei W, Multivariable Model - Building: A Pragmatic Approach to Regression Anaylsis based on Fractional Polynomials for Modelling Continuous Variables. (John Wiley & Sons Ltd, Chichester, England, 2008), vol. 1. [Google Scholar]
- 38.Thompson JA, in Statistical Software Components S458426. (Boston College Department of Economics, Boston, MA, 2017). [Google Scholar]
- 39.Athey S, Imbens G, The Econometrics of Randomized Experiments. 2016. (arXiv:1607.00698). [Google Scholar]
- 40.Zou G, A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology 159, 702–706 (2004); published online EpubApr 1 ( [DOI] [PubMed] [Google Scholar]
- 41.Thomas DR, A general inductive approach for qualitative data analysis. (2003). [Google Scholar]
- 42.Roodman D, Nielsen MØ, MacKinnon JG, Webb MD, Fast and wild: Bootstrap inference in Stata using boottest. The Stata Journal 19, 4–60 (2019) 10.1177/1536867x19830877). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


