Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia and kidney injury caused by a dysregulation of the alternative complement pathway.
Methods
We conducted a multicenter nonregistry study aimed at collecting clinical, laboratory and genetic information of patients with aHUS in Brazil. Demographic data, genetic findings, treatments and outcomes are presented.
Results
Thirty-four patients were included, 62% were female and 67% were Caucasian. Half of the patients had the first manifestation of aHUS before the age of 18 years (pediatric group). Among the 17 patients who had the first manifestation after the age of 18 years (adult group), 6 were kidney transplant patients. Overall, 22 patients (65%) received plasma exchange/plasma infusion (PE/PI) and 31 patients (91%) received eculizumab. Eculizumab was started later in the adult group compared with the pediatric group. Two patients stopped dialysis after PE/PI and 19 patients stopped dialysis after eculizumab despite a late start. A pathogenic/likely pathogenic variant was found in 24.3% of patients. A coexisting condition or trigger was present in 59% of patients (infections, pregnancy, hypertension, autoimmune disease and transplant), especially in the adult group. There was a 30% relapse rate after stopping eculizumab, irrespective of genetic status.
Conclusion
This is the largest case series of aHUS in Brazil involving a wide range of patients for which eculizumab was the main treatment. Although eculizumab was started later than advised in the guidelines, most patients were able to stop dialysis at variable intervals. Discontinuation of eculizumab was associated with a 30% relapse of aHUS.
Keywords: atypical hemolytic uremic syndrome, eculizumab, plasma exchange, thrombotic microangiopathy
INTRODUCTION
Atypical hemolytic uremic syndrome (aHUS) is a rare disease caused by a dysregulation of the alternative complement pathway and characterized clinically by the thrombotic microangiopathy (TMA) triad: microangiopathic hemolytic anemia, thrombocytopenia and organ injury in variable degrees [1]. Since there is still not a gold standard diagnostic test for aHUS, it is important to rule out other more common causes of TMA [2], which is a challenge in a disease with high morbidity and mortality [3].
In addition to multicountry registry data [4–6], regional data of aHUS have been published from Japan [7], France [8], Korea [9], China [10], Eastern Europe [11] and India [12] with variable numbers of patients and genetic findings. This highlights the importance of knowing the genetic background and correlation with the phenotype of distinct regional populations.
In Brazil, a case series published in 2017 [13] included seven patients with aHUS who underwent kidney transplantation and for whom eculizumab was the first-line treatment. The Brazilian Society of Nephrology, through its Committee for Rare Disease (COMDORA), is launching an aHUS registry, but so far a population analysis of aHUS cases in Brazil is still not available.
MATERIALS AND METHODS
On October 2017, the Brazilian Thrombotic Microangiopathy and Atypical Hemolytic Uremic Syndrome Study Group (aHUSBrazil) was created and the Evaluation of a Case Series of Atypical Hemolytic Uremic Syndrome in Brazil project was approved by the ethics committee and registered in the national research platform, Plataforma Brasil (www.plataformabrasil.saude.gov.br; number CAAE 78121617.6.1001.5253). This is a nonregistry, retrospective analysis of clinical, laboratory and genetic information of patients with aHUS from different regions in Brazil. Initial centers to enroll patients were Hospital Federal de Bonsucesso (Rio de Janeiro), State University of Campinas (Campinas), Clínica do Rim e Hipertensão (Campinas), Hospital Moinhos de Vento (Porto Alegre), Hospital de Clínicas de Porto Alegre (Porto Alegre), Hospital Materno-Infantil de Goiania (Goiania) and Hospital Barão de Lucena (Recife), which encompasses patients from the five regions of the country. All patients or legal caregivers gave written informed consent.
The diagnosis of aHUS was based on the triad of microangiopathic hemolytic anemia, thrombocytopenia and kidney injury [1]. ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) activity was >10% in all included patients. Stool culture or polymerase chain reaction for Shiga toxin in stools was collected for patients with diarrhea and all specimens were negative. If the patient presented with a secondary cause or coexisting condition but TMA persisted despite treatment of the underlying condition, then the diagnosis of aHUS was made. When the secondary cause was a drug or infection, an interval of 3–7 days after removal or treatment of the underlying cause was used to characterize persistent TMA. In the case of pregnancy, TMA that persisted 72 h after delivery, especially in the setting of severe kidney injury, was diagnosed as aHUS. In cases of systemic diseases, we considered the diagnosis of absence of improvement of TMA as well as worsening of chronicity on biopsy after 2 months of treatment [14]. In summary, patients with persistent TMA despite trigger removal or treatment of coexisting conditions (varying from 7 days to 2 months according to the underlying disease) were diagnosed as having aHUS. The clinical diagnosis of aHUS in this setting aimed at preventing death or kidney impairment by considering complement blockade as a treatment. In this cohort, patients with secondary TMA—here defined as TMA remission after treatment of the underlying cause—were not included.
Age of onset, gender, hematological parameters and kidney function, need and length of dialysis, treatment and number of sessions of plasma exchange or plasma infusion (PE/PI), use of eculizumab, kidney and patient survival and genetic data were collected until 31 December 2018. Estimated glomerular filtration rate [eGFR; determined by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation] at the last follow-up was obtained for patients who either stopped or did not need dialysis.
The genetic analyses were performed in six laboratories according to the physicians’ preferences and were reported according to American College of Medical Genetics and Genomics (ACMG) guidelines [15]:
CENTOGENE Lab (Schillingasse 68,18057 Rostock/Germany), 21 patients: For the aHUS panel, the entire coding region of the ADAMTS13, complement component 3 (C3), membrane cofactor protein (MCP or CD46), complement factor B (CFB), complement factor H (CFH), complement factor H–related proteins (CFHR1, CFHR2, CFHR3, CFHR5), complement factor I (CFI), diacylglycerol kinase epsilon (DGKE), phosphatidylinostol glycan anchor biosynthesis class A (PIGA) and thrombomodulin (THBD) genes, including 10 bp of intronic flanking sequences, were amplified and sequenced. Raw sequence data analysis, including base calling, demultiplexing, alignment to the hg19 human reference genome (Genome Reference Consortium GRCh37) and variant calling was performed using validated in-house software. All identified variants were evaluated regarding their pathogenicity and causality. All variants except benign or likely benign variants are reported. Analysis does not include copy number variations or large deletions/duplications. Multiplex ligation-dependent probe amplification (MLPA) analyses were performed using SALSA® MLPA probemix P236-A3 provided by MRC Holland (Amsterdam, The Netherlands) to test for deletions or duplications within or including the CFH, CFHR1, CFHR2, CFHR3 and CFHR5 genes. Variants were reported as pathogenic, probably pathogenic and variant of unknown significance according to ACMG guidelines.
DLE (Diagnostico Laboratorial Especializado) Medicina Laboratorial (Rua Pedro de Toledo 164, 04039-000 São Paulo/Brazil), 1 patient: Performed Next Generation Sequencing of 10 fragments with >97% coverage of the genes ADAMTS13, CD46, CFH, CFI, THBD, C3, CFB, CFHR1, CFHR2, CFHR3, CFHR4, CFHR5 and DGKe (diacylglycerol kinase epsilon). Variants were reported as pathogenic, probably pathogenic and variant of unknown significance according to ACMG guidelines.
Machaon Diagnostics (3023 Summit St., Ste 100, Oakland, CA 94609, USA), 1 patient: As per October 2015 (when analysis was performed), 12 genes have been sequenced and analyzed as part of the aHUS panel, including CFH, MCP (CD46), CFI, C3, CFB, CFHR1, CFHR3, CFHR4, CFHR5, THBD, plasminogen (PLG) and DGKe. The sequences have been compared with the reference human genome (Hg19) sequence. Machaon Diagnostics maintains a database of >230 aHUS-associated mutations, disease-associated polymorphisms, benign polymorphisms and other known variants of undetermined significance. This database is actively updated as new mutations are identified or reported in public databases and the published literature. Machaon Diagnostics employs a targeted next-generation sequencing technique (patent pending) that allows for detection of >230 mutations, deletions and polymorphisms that have been previously reported as associated with complement-mediated TMAs. That assay may also discover deleterious variants not previously reported that were reported as such.
Mendelics Análise Genômica S.A. (Rua Cubatão 86, 04013-000 São Paulo/Brazil), 2 patients: Performed whole-exome sequencing by next-generation sequencing with the Illumina HiSeq 2500 (Illumina, San Diego, CA, USA). Variant identification was made through bioinformatics using the Genome Reference Consortium GRCh37. The percentage of target bases with at least 10 readings was 96.7% and each base was read 103 times. In silico models were used for pathogenicity prediction and all variants (from pathogenic to benign) were reported. The aHUS panel comprises six genes: C3, CD46, CFB, CFH, CFI and THBD.
Secugen, S.L. (Ramiro de Maetzu, 9 28040 Madrid/Spain), 1 patient: aHUS panel sequencing involving 14 genes (CFH, MCP, CFI, CFB, C3, THBD, DGKE, CFHR1, CFHR2, CFHR3, CFHR4, CFHR5, CFP and ADAMTS13) using next-generation sequencing with the Illumina MiSeq (mean capture 1500×, minimum capture 20×) with confirmation by Sanger sequencing of variants but not polymorphisms.
Hospital Georges Pompidou (Paris/France), 1 patient: Performed direct sequencing analysis for CFH, MCP and CFI and MLPA for genomic disorders affecting CFH and CFHRs (nine genes in total).
In the ACMG guidelines, variants are classified as pathogenic, likely pathogenic, variants of uncertain significance (VUS), benign or likely benign. The term likely pathogenic or likely benign is applied when there is >90% certainty of the gene’s pathogenicity. When the pathogenicity cannot be adequately predicted, the variant is called a VUS and current recommendations suggest that these variants be reclassified at given intervals. In our study, when patients presented with more than one genetic variant with different classifications, we considered the most pathogenic as the main one. The pathogenicity of the variants was presented according to the respective laboratory report. The only exception was regarding the CFHR1–CFHR3 deletion, which was considered a VUS in all cases since current evidence [16] does not support its pathogenicity in the absence of anti-factor H antibodies, which were not available for this study.
Eculizumab was approved by the US Food and Drug Administration (FDA) for use in patients with aHUS on September 2011. In Brazil, eculizumab was registered with the National Sanitary Vigilance Agency (Agência Nacional de Vigilância Sanitária) of the Ministry of Health on March 2017. Nevertheless, it is still not available for immediate use and the importation process may take 4–6 weeks. Therefore, in patients diagnosed with aHUS, standard treatment includes supportive measures and PE or PI until eculizumab is available. The mean turnaround time for an ADAMTS13 result is 10 days and for stool culture/Shiga toxin it is 5 days. When aHUS is diagnosed, eculizumab is requested and genetic testing is not required to start treatment.
RESULTS
Thirty-four patients were included in this cohort. Twenty-one patients were female, 23 were Caucasian and the mean age at diagnosis was 16.2 ± 13.6 years (ranging from 6 months to 43 years). Half of the patients presented the first manifestation of aHUS before the age of 18 years (pediatric group) and the remaining presented the first manifestation after the age of 18 years (adult group; Table 1). The mean follow-up from the first aHUS manifestation until 31 December 2018 was 92 ± 78 months for the adult group and 59 ± 46 months for the pediatric group.
Table 1.
Demographic characteristics of the 34 patients included in the first Brazilian cohort of aHUS
| Characteristics | Total | Adult group | Pediatric group |
|---|---|---|---|
| (n = 34) | (n = 17) | (n = 17) | |
| Age at diagnosis (years), median (IQR) | 18 (1.83–30) | 30 (22–35) | 1.83 (0.83–7) |
| Female, n (%) | 21 (62) | 17 (100) | 4 (23.5) |
| Genetic analysis (patients), n | 27 | 16 | 11 |
| Genetic variants, na | 37 | 20 | 17 |
| Pathogenic/likely pathogenic, n (%) | 9 (24.3) | 8 (40) | 2 (11.7) |
| VUS, n (%) | 23 (62) | 9 (45) | 14 (82) |
| Benign/likely benign, n (%) | 2 (5.4) | 2 (10) | 0 |
| Negative (%) [5] | 3 (8.1) | 2 (10) | 1 (5.8) |
| Not performed, n | 7 | 1 | 6 |
| Dialysis at diagnosis, n (%) | 30 (88.2) | 16 (94) | 14 (82) |
| Kidney transplant, n | 6 | 6 | 0 |
| PE/PI, n (%) | 22 (65) | 14 (82) | 7 (41) |
| Eculizumab, n (%) | 31 (91) | 16 (94) | 15 (88) |
| Time from first manifestation to first dose of eculizumab (days), median (range) | 61 (17–7792) | 260 (51–7792) | 31 (17–5790) |
| Stopped dialysisb, n (%) | 22 (65) | 9 (53) | 13 (76) |
| eGFR at last follow-up for patients who stopped dialysisb (mL/min/1.73 m2) | 69.4 ± 29.2 | 67.5 ± 33.8 | 76.5 ± 25.2 |
| eGFR at last follow-up for patients who did not receive dialysisb (mL/min/1.73 m2) | 76.7 ± 21.6 | 68.5 ± 21.6 | 85 ± 21.2 |
Thirty-seven genetic variants were found in 24 patients (14 adult cases and 10 pediatric cases).
With or without eculizumab.
Overall, 22 patients received PE/PI ranging from 2 to 56 sessions (median 19 sessions) and 31 patients (91%) received eculizumab, which was started 17–7792 days after the first manifestation. In the adult group, six patients received a kidney transplant and this group was analyzed separately. There were no kidney transplant patients in the pediatric group. The mean follow-up of patients on eculizumab was 34 ± 21 months in the adult group and 45 ± 17 months in the pediatric group.
From the 30 patients who required dialysis, 22 patients were able to stop this procedure. At the last follow-up, the eGFR (CKD-EPI) for patients who stopped dialysis was 69.4 ± 29.2 mL/min/1.73 m2 and for the patients who never received dialysis it was 76.7 ± 21.6 mL/min/1.73 m2.
Adult group with native kidneys
From the 11 patients who never received a kidney transplant (Table 2), the mean age was 29.4 ± 7.6 years (range 18–43) and all were female. Ten patients were treated with eculizumab and nine patients were treated with PE/PI before eculizumab was started.
Table 2.
Demographic data, treatment, need for dialysis and presence of coexisting conditions/triggers in the 17 patients from the aHUS adult group (first manifestation at age ≥18 years)
| Patient | Group | Gender | Ethnicity | Age (years) | Tecu (days) | Stop ecu | Stop (days) | Relapse | PE/PI | Dialysis | Dialysis stop | Coexisting condition trigger | Variant | ACMG class |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Native kidney | Female | Caucasian | 22 | 92 | No | Yes | Yes | Yes | No | Heterozygous variant CFI c.559C>T p.(Arg187a), heterozygous variant C3 c.193A>C p.(Lys65Gln) | Pathogenic, VUS | ||
| 2 | Native kidney | Female | Afro-Brazilian | 23 | 2184 | Yes | 215 | No | Yes | Yes | Yes | Infection | Heterozygous variant CFHR1 c.614C>T p.(Thr205Met), heterozygous variant C3 c.3100T>C p.(Trp1034Arg), heterozygous deletion CFHR1–CFHR3 | VUS, VUS, VUS |
| 3 | Native kidney | Female | Caucasian | 24 | 129 | No | Yes | Yes | Yes | Infection | Heterozygous variant hybrid gene CFH/CFHR1 (exons 1–20 derived from CFH and 22/23 from CFHR1) | Pathogenic | ||
| 4 | Native kidney | Female | Caucasian | 28 | 5936 | Yes | 200 | No | Yes | Yes | No | Pregnancy | Homozygous variant CFH c.1756C>T p.(Gln586a) | Likely pathogenic |
| 5 | Native kidney | Female | Caucasian | 30 | 69 | No | No | Yes | Yes | No | Heterozygous variant CFH c.1422del p.(Lys474Asnfsa 6) and heterozygous variant in ADAMTS13 | Pathogenic, VUS | ||
| 6 | Native kidney | Female | Afro-Brazilian | 30 | 391 | No | Yes | Yes | Yes | No | Heterozygous variant CFHR4 c.275T>C p. (Val92Ala), CFP c.716C>T p. (Pro239Leu) | Likely benign, likely benign | ||
| 7 | Native kidney | Female | Caucasian | 30 | 31 | Yes | 124 | No | Yes | Yes | Yes | No | Not performed | Not performed |
| 8 | Native kidney | Female | Caucasian | 35 | No | NA | Yes | Yes | Yes | Pregnancy | Heterozygous variant C3 c.193A>C p. (Lys65GIn) | Pathogenic | ||
| 9 | Native kidney | Female | Caucasian | 40 | 17 | No | Yes | Yes | Yes | No | Negative | Negative | ||
| 10 | Native kidney | Female | Caucasian | 43 | 26 | Yes | 169 | No | No | No | Transplant/CMV | Heterozygous variant CFI c.608C>T p.(Thr203Ile) | VUS | |
| 11 | Native kidney | Female | Afro-Brazilian | 18 | 72 | Yes | 160 | Yes | Yes | yes | yes | Systemic lupus erythematosus | Large heterozygous deletion CFHR1–CFHR3 | VUS |
| 12 | Transplant | Female | Caucasian | 22 | 2721 | No | Yes | Yes pre-tx | No | Heterozygous variant C3 c.193A>C p. (Lys65GIn) | Pathogenic | |||
| 13 | Transplant | Female | Afro-Brazilian | 22 | 2772 | Yes | 415 | Yes/death | Yes | Yes pre-tx | Pregnancy | Negative | Negative | |
| 14 | Transplant | Female | Caucasian | 25 | 51 | Yes | 121 | No | No | Yes pre-tx | No | Heterozygous deletion in exon 6 of CFHR1 | VUS | |
| 15 | Transplant | Female | Caucasian | 30 | 944 | No | Yes | Yes | No | Heterozygous variant CFHR5 c.254-2_266dup p.(Ser88_Phe89insLeuGlyMetCysSer) and heterozygous duplication in exon 6 of CFHR1 | VUS, VUS | |||
| 16 | Transplant | Female | Caucasian | 30 | 7792 | Yes | 200 | No | Yes | Yes pre-tx | Pregnancy | Homozygous variant in CFH c.1756C>T p.(Gln586a) | Likely pathogenic | |
| 17 | Transplant | Female | Caucasian | 33 | 578 | Yes | 109 | Yes | Yes | Yes pre-tx | Severe hypertension | Heterozygous variant in CFH c.3572C>T p.(Ser1191Leu) | Pathogenic |
Patients 4 and 16 are sisters.
Tecu, time between first manifestation and first eculizumab infusion; Stop ecu, stopped eculizumab; Stop (days), length of eculizumab stop; NA, nonapplicable; tx, kidney transplant. CFHR1–CFHR3 deletions are only considered pathogenic if anti-CFH antibodies are present.
Two patients (Patients 2 and 4) presented the first manifestation of aHUS before eculizumab was FDA approved. These two patients started eculizumab 2184 and 5936 days, respectively, after the first manifestation and Patient 2 was able to stop dialysis 1 year after treatment with complement blockade.
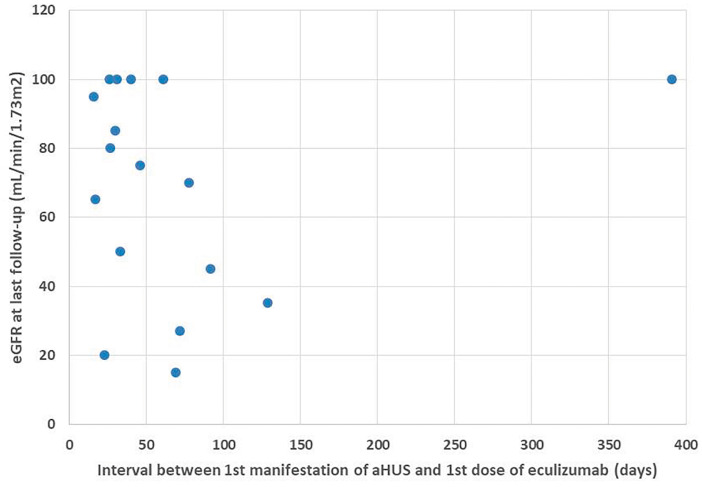
Nine patients presented the first manifestation of aHUS after eculizumab was FDA approved. One patient (Patient 8) responded to PE and did not receive eculizumab. Eight patients started eculizumab at a median of 103 [interquartile range (IQR) 26–129] days after the first aHUS manifestation—one patient (Patient 10) remained dialysis-free and seven patients were able to stop dialysis from 1 month to 1 year after eculizumab was introduced. At the last follow-up, the mean eGFR in the eculizumab-treated patients was 59.5 ± 37.4 mL/min/1.73 m2, with a trend for higher eGFR in patients who started treatment earlier (Figure 1).
FIGURE 1.
eGFR at the last follow-up of all patients treated with eculizumab who stopped dialysis (adult group, n = 7; pediatric group, n = 10). One adult patient started eculizumab 2184 days after the first manifestation and was able to stop dialysis 1 year later (not in the plot).
Six patients had a coexisting condition/trigger (pregnancy [2], upper respiratory infection [2], systemic lupus erythematosus [1, 14] and bone marrow transplant/CMV [1]), among whom a pathogenic/likely pathogenic genetic variant was found in 50% (Table 2).
Stopping eculizumab is not current practice in the centers that enrolled patients. Nevertheless, five patients stopped the infusions due to a lack of supply and one patient presented a relapse 30 days after stopping that responded to drug restart—this patient had a heterozygous deletion in the CFHR1–CFHR3 genes [16].
Adult group with kidney transplant
Six patients received a kidney transplant (Table 2); all of them were female with mean age of 27±4.6 years (range 22–33). Three of them lost a kidney graft in the pre-eculizumab era (Patients 12, 13 and 16) and started using eculizumab while on the waitlist for a second transplant. Two of these patients interrupted the use of eculizumab due to a lack of supply and one patient died of a severe aHUS relapse 30 days after stopping (negative genetic finding).
Three patients received a kidney transplant with prophylactic eculizumab (Patients 14, 15 and 17); two of them stopped due to a lack of supply and one had a relapse 40 days after stopping, which responded to eculizumab restart. All patients had functioning grafts at the last follow-up.
There were no adverse events that required eculizumab interruption in the adult group.
Pediatric group
Seventeen patients presented the first manifestation of aHUS before the age of 18 years (Table 3). The mean age at diagnosis was 4 ± 4 years (range 6 months–15 years), 4 patients were female and 11 patients were Caucasian. Two of the 11 patients who underwent genetic analysis had a pathogenic/likely pathogenic variant (18%). Two patients presented a homozygous deletion in the CFHR1–CFHR3 genes that were considered of uncertain significance once anti-factor H was not available. Four patients received PI (ranging from 5 to 24 days) and three patients underwent PE (6, 6 and 10 sessions, respectively). Fifteen patients received eculizumab, six of whom preceeded it with PI or PE.
Table 3.
Demographic data, treatment, need for dialysis and presence of coexisting conditions/triggers in the 17 patients from the aHUS pediatric group (first manifestation at age <18 years)
| Patient | Group | Gender | Ethnicity | Age (years) | Tecu (days) | Stop ecu | Stop (days) | Relapse | PE/PI | Dialysis | Dialysis stop | Coexisting condition/ trigger condition | Variants | ACMG Class |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 | Pediatric | Male | Caucasian | 0.5 | 5790 | Yes | 33 | No | Yes | Yes | Yes | Infection | Homozygous deletion DGKe c. 1068_1071 del p.(Asn356Lysfs*6) | Likely Pathogenic |
| 19 | Pediatric | Female | Caucasian | 0.5 | 78 | No | No | Yes | Yes | No | CFHR1 heterozygous duplication encompassing intron 4 to exon 6 | VUS | ||
| 20 | Pediatric | Male | Caucasian | 0.83 | 46 | No | No | Yes | Yes | No | Heterozygous deletion CFHR3 encompassing 5′-UTR and exon 1 CFHR3 | VUS | ||
| 21 | Pediatric | Male | Afro-Brazilian | 0.83 | 31 | No | No | No | No | Heterozygous variant CFH, heterozygous variant CFI and heterozygous deletion encompassing CFHR1–CFHR3 | VUS, VUS, VUS | |||
| 22 | Pediatric | Male | Afro-Brazilian | 0.83 | 27 | No | Yes | Yes | Yes | No | Heterozygous variant C3 c.3100T>C p.(Trp103Arg), heterozygous variant CFHR1 c.614C>T p.(Thr205Met) | VUS, VUS | ||
| 23 | Pediatric | Female | Afro-Brazilian | 0.91 | 31 | No | No | Yes | Yes | No | Not performed | Not performed | ||
| 24 | Pediatric | Male | Caucasian | 1.41 | 31 | No | No | Yes | Yes | No | Not performed | Not performed | ||
| 25 | Pediatric | Male | Afro-Brazilian | 1.83 | 61 | No | No | Yes | Yes | Infection | Heterozygous variant CFI c.251C>A p.(Thr84Lys), heterozygous variant CFI c.1270A>C p.(Ile424Leu), heterozygous deletion CFHR1–CFHR3 | VUS, VUS, VUS | ||
| 26 | Pediatric | Male | Caucasian | 1.83 | 17 | Yes | 200 | No | Yes | Yes | Yes | No | Not performed | Nor performed |
| 27 | Pediatric | Male | Caucasian | 4 | 31 | Yes | 122 | Yes | No | No | No | Homozygous deletion CFHR1–CFHR3 | VUS | |
| 28 | Pediatric | Male | Caucasian | 4 | 26 | No | Yes | Yes | Yes | Infection | Not performed | Not performed | ||
| 29 | Pediatric | Female | Caucasian | 6 | 29 | No | No | No | No | Homozygous deletion CFHR1–CFHR3, heterozygous variant in CFHR5 c.254-2_266dup p.(Ser88_Phe89insLeuGlyMetCysSer) | VUS, VUS | |||
| 30 | Pediatric | Male | Afro-Brazilian | 6 | No | NA | Yes | Yes | Yes | No | Not performed | Not performed | ||
| 31 | Pediatric | Male | Caucasian | 7 | 1095 | No | No | Yes | No | No | Heterozygous variant Plasminogen c.1431C>T p.Ser477Ser) | VUS | ||
| 32 | Pediatric | Male | Caucasian | 7 | 30 | Yes | 185 | No | Yes | Yes | Yes | Infection | Heterozygous variant C3 c.193A>C p.(Lys65Gln) e Heterozygous deletion CFHR1-CFHR3 | Pathogenic, VUS |
| 33 | Pediatric | Male | Afro-Brazilian | 9 | 23 | No | Yes | Yes | Yes | No | Not performed | Not performed | ||
| 34 | Pediatric | Female | Caucasian | 15 | No | NA | Yes | Yes | Yes | No | Negative | Negative |
CFHR1–CFHR3 deletions are only considered pathogenic if anti-CFH antibodies are present.
Tecu, time between first manifestation and first eculizumab infusion; Stop ecu, stopped eculizumab; Stop (days), lenghth of eculizumab stop; NA, not applicable; tx, kidney transplant.
Three patients (Patients 21, 27 and 29) presented with kidney injury with no need for renal replacement therapy and were treated with eculizumab starting 29–30 days after the first presentation. They remained dialysis-free and with normal kidney function until the last follow-up.
Fourteen patients required dialysis on the first manifestation:
One patient (Patient 18) had the first aHUS manifestation at 6 months of age, before eculizumab was FDA approved. He was treated with PI and was able to stop peritoneal dialysis after 6 months. This patient progressed to end-stage renal disease (ESRD) in adulthood, when eculizumab was started, with a lack of response. Later, a genetic analysis revealed a homozygous mutation in the DGKe gene [17] and eculizumab was discontinued.
Two patients did not receive eculizumab. One (Patient 34) is a 15-year-old girl who recovered completely with four sessions of PI/PE and dialysis and presented normal kidney function and hematological exams at the last follow-up. The other patient is a 6-year-old boy (Patient 30) who progressed to ESRD and the treating physician decided not to start eculizumab due to a lack of overt TMA. He is on the waitlist for a kidney transplant.
From the 11 patients who were on dialysis at the moment eculizumab was started, 10 were able to stop the procedure. The time between the first manifestation and first dose of eculizumab was a median of 31 (IQR 23–61) days (Figure 1) in this group. One patient (Patient 31) who remained on chronic dialysis had started eculizumab 1095 days after the first presentation.
Six (35%) patients presented the first aHUS manifestation in the first year of life, 10 (59%) patients between 1 and 12 years and 1 (6%) patient at the age of 15 years. Of the six patients presenting aHUS in the first year of life (age range 6–11-months), four were male, four presented with systemic arterial hypertension, three had gastrointestinal symptoms with negative Shiga toxin on stools and five patients needed dialysis. Two patients received PI, and all six received eculizumab ranging from 27 days to 15 years after initial symptoms (Table 3). The patient who started eculizumab 15 years after the first manifestation presents a homozygous mutation on DGKe and had eculizumab discontinued [17]. Among the remaining five patients in this subgroup, the mean time for eculizumab infusion was 42.6 days (range 27–78). All patients stopped dialysis and were using eculizumab until the last follow-up.
Ten patients presented the first manifestation of aHUS between 1 and 12 years of age. Six patients presented with gastrointestinal symptoms with negative Shiga toxin or stool culture. Eight children required dialysis, four patients received PI and nine patients started eculizumab (range 17 days–3 years). Six patients were able to stop dialysis. Five patients had genetic testing, of whom four have a pathogenic variant. Three patients discontinued eculizumab due to a lack of supply and one had a relapse that was controlled after restarting the drug.
The only adolescent in this cohort was a 15-year-old female (Patient 34) who presented with aHUS requiring dialysis, but she recovered completely after four sessions of plasmapheresis. Her genetic analysis was negative (although only six genes were analyzed) and she is being followed in the outpatient clinic with no relapses after 2 years of the first TMA manifestation.
There were no adverse events that required eculizumab interruption and there were no deaths in the pediatric group. The differences in age of onset, severity, kidney outcome, genetic findings and response to treatment are presented in Table 4.
Table 4.
Pediatric group: differences in outcome according to genetic findings
| Characteristics | DGKe (n = 1) | Complement (n = 8) | Plasminogen (n = 1) | Negative |
|---|---|---|---|---|
| Age of onset (years), median (IQR) | 0.5 | 1.33 (0.83–6) | 7 | 15 |
| Severity | Peritoneal dialysis for 6 months | Dialysis (n = 5) | Dialysis | Dialysis |
| Kidney outcome | Stopped dialysis, slow progression to ESRD | All stopped dialysis | Did not recover | Recovered completely |
| Response to PE/PI | Partial response | Two received PI with no response | Did not receive | Complete recovery with PE/PI |
| Time to eculizumab (days), median (IQR) | 5790 | 31 (27–60) | 1095 | Did not receive eculizumab |
Genetic analysis
From the 34 patients enrolled, 27 patients had genetic analysis for the aHUS panel (Tables 2 and 3). The genetic analyses were detailed previously and performed in six laboratories: Centogene (n = 21), DLE (n = 1), Machaon (n = −1), Mendelics (n = 2), Secugen (n = 1) and Georges Pompidou (n = 1). Although the number of genes analyzed in the respective panels varied from 6 to 14, the main genes involved in aHUS were included in all the panels and the findings were reported according to ACMG guidelines [15]. Only two patients (Patients 8 and 34) did not have the CFHRs analyzed (Mendelics Lab) and both recovered completely with PE/PI, had no relapses and did not receive eculizumab.
Among the 37 variants found in 27 patients, 10 (27%) variants were classified as pathogenic/likely pathogenic, 25 (67.5%) as a VUS and two (5%) as benign/likely benign. Three of the 27 patients (11%) had negative genetic findings.
Pathogenic/likely pathogenic variants were detected in 10 adults and 2 children: CFH, CFI, C3, CFH/CFHR1 and DGKe. No variants in MCP were identified in our population. Anti-factor H antibody assay was not available, therefore CFHR1–CFHR3 deletions that were identified in six patients were considered as VUS and not as pathogenic genetic findings. Jozsi et al. [16] identified 16 children with positive anti-factor H antibodies among 147 patients with aHUS, and anti-factor H antibodies were absent in 100 healthy individuals, thus defining the novel subgroup of aHUS termed deficiency of CFHR proteins and CFH autoantibody positive (DEAP) HUS.
In the patients with pathogenic/likely pathogenic variants (n = 12), the median age at first presentation was 23 (IQR 7–28) years and all patients received eculizumab starting at a median of 354 (IQR 31–5936) months. Of the nine patients who needed dialysis at presentation, four were able to stop (ranging from 27 days to 1 year after first eculizumab dose) and eGFR at the last follow-up was a mean of 46 ± 25.6 mL/min/1.73 m2. Six patients had nonprotocol eculizumab stop in this group, and one patient presented a relapse of aHUS 122 days after stopping.
Among the patients with no pathogenic variants (n = 17), the median age was 18 (IQR 1.83–30) years and 15 patients received eculizumab starting at a median of 54 (IQR 29–1095) days after first presentation. Twelve patients were on dialysis initially and eight were able to stop (ranging from 13 to 15 days after the first eculizumab dose). eGFR at the last follow-up for this group was a mean of 77 ± 23.8 mL/min/1.73 m2. Five patients stopped eculizumab and there were two relapses—one at 160 days that resolved with restart of the drug and one relapse 415 days after stopping that ended with the patient’s death.
A coexisting condition was identified in 13 patients during the first manifestation of aHUS (Tables 2 and 3): 4 children (all with infection) and 9 adults (4 pregnancies, 2 infections, 1 bone marrow transplant, 1 systemic disease and 1 severe hypertension). Seven of the 13 patients (53.8%) carried a pathogenic/likely pathogenic variant, mainly in the adult group.
Regarding ethnicity, of the 34 patients in this cohort, 24 patients were Caucasian (70.6%) and 10 patients were Afro-Brazilian (29.4%). In the Caucasian group, 10 of the 20 patients who had genetic testing (50%) carried a pathogenic/likely pathogenic variant. Among the seven Afro-Brazilian patients with a genetic test, none presented a pathogenic/likely pathogenic variant.
DISCUSSION
This is the first report of a case series of aHUS in Brazil involving a broad range of patients. Half of the patients presented the first manifestation as adults, confirming that aHUS is not an exclusively pediatric disease.
Following the milestone article of Bell et al. in 1991 [18], for two decades the treatment of choice for thrombotic thrombocytopenic purpura/HUS was PE. The initial management for an adult presenting with TMA still broadly relies on plasma-based therapies. In our series, 82% of the adults enrolled received PE/PI for initial management. Nevertheless, only one patient responded completely; the remaining 16 patients needed to switch or initiate the complement blocker eculizumab. Besides understanding the pathophysiology of aHUS, determining the underlying defect in the alternative complement pathway has therapeutic implications. In September 2011, eculizumab (Soliris, Alexion) was approved by the FDA as the first terminal complement blocker for patients with aHUS. The outcomes of patients with aHUS treated with eculizumab have been published in pivotal trials both in adults [19–21] and children [22] showing excellent efficacy and safety.
The median time for the use of eculizumab in the adult group was 8.6 months, reflecting the challenge of precision diagnosis and access to newer drugs in a developing country. When considering the eight patients with native kidneys who had the first manifestation in the eculizumab era, the median time for eculizumab decreased to 71 days, still a large interval when compared with consensus document recommendations [23, 24]. Despite this longer interval for treatment, all patients who had needed dialysis were able to stop it.
Of all the patients who were on dialysis at the moment eculizumab was started, 69% were able to stop dialysis. There was a trend towards higher eGFRs in patients with earlier introduction of eculizumab, as previously reported [25, 26]. Children recovered earlier and presented better kidney function than adults, probably due to earlier initiation of eculizumab. Nevertheless, one adult patient was able to stop dialysis 1 year after starting complement blockade, which is an encouraging finding since diagnostic tools and access to eculizumab vary worldwide. In our series, patients with likely pathogenic variants presented lower eGFRs after stopping dialysis than patients without pathogenic variants. The former group started eculizumab later than the latter (median 92 versus 51 days, respectively), which may have accounted for the worse kidney function. The fact that most patients with pathogenic findings presented with a coexisting disease may explain the delay in starting eculizumab once the persistence of TMA despite treatment of the underlying cause was the criterion used to diagnose aHUS.
The six patients who were on the kidney transplant subgroup received eculizumab much later after initial presentation and three of them had previously lost a kidney graft. Eculizumab used prophylatically allowed the patients to undergo a successful kidney transplant, and recent publications reinforce the benefit of prophylactic eculizumab in the setting of kidney transplantation in patients with aHUS [27, 28].
In children, the most common cause of TMA is the Shiga toxin–associated HUS [29, 30]. In our series, five patients presented with diarrhea with negative Shiga toxin or stool cultures. Diarrhea may be a manifestation of aHUS [6] and collecting stool specimens early on TMA presentation (preferably in the emergency room) is very important for the differential diagnosis, as late specimen retrieval or the use of antibiotics may return a negative stool test for Shiga toxin [31]. The absence of pathogenic stool agents as well as severe hypertension, recurrent patterns or family history of TMA favored strongly the diagnosis of aHUS, and treatment with eculizumab was recommended early in our cohort. Fewer children had access to genetic testing than adults in our cohort, and only two patients carried a pathogenic variant. The good outcomes with eculizumab in the pediatric group may explain the lower pursuit of genetic testing by physicians or families. The lower incidence of pathogenic variants in the pediatric population may be due to the small cohort tested, and a nationwide registry has been started that will help us understand the genetic background in a broader population from Brazil.
Fifteen children received eculizumab at a median interval of 31 days (the earliest was 17 days), significantly shorter than the adult population, but still far from the guideline recommendation to start in the first 24 h of presentation [32] or in the first week [26]. Despite the longer interval in our cohort, overall 85% of the patients who required dialysis were able to stop it. When our data are analyzed together with published cohorts, both prospective [20] and case reports [33], we may conclude that eculizumab should be started as soon as aHUS is diagnosed, even if later in the course of the disease.
The first association between aHUS and the alternative complement defect was described in 1981 [34], when siblings with aHUS and consanguineous parents were found to have decreased blood levels of CFH. In 1998, researchers [35] unraveled the association between aHUS and chromosome 1q32, which contains the genes for complement regulators. In the following years, defects in other components of complement regulators of the alternative pathway have been found. More recently, mutations in genes of the coagulation pathway (PLG [36] and DGKe [37]) have been detected in patients with aHUS, and both of them were found in our series. In a Chinese cohort of 10 pediatric patients with aHUS [38] who had whole-exome sequencing performed, new variants were found in terminal complement genes (C8B, C9), lectin pathway genes [mannan-binding lectin-associated serine protease 1 (MASP1)] and in coagulation genes (von Willebrand factor and CD36), which may act as causative or modifier genes.
The role of anti-factor H antibodies in the pathogenesis of aHUS [39], especially in children, was also demonstrated, and its detection may have an impact on treatment since PE and immunosuppression, including rituximab, may be an option [24], but this test was not available in our cohort. It is widely accepted that the homozygous CFHR1–CFHR3 deletion is relevant only in the context of anti-factor H–associated aHUS. Thus, in the absence of anti-factor H autoantibodies analyses, the homozygous CFHR1–CFHR3 deletion is not considered a pathogenic variant. Only two children in our series presented homozygous deletions of CFHR1–CFHR3. Both patients had kidney injury that did not require dialysis and were treated with eculizumab as a first-line treatment 1 month after the first manifestation. One patient is a 4-year-old boy who presented a TMA relapse after stopping eculizumab and the other patient is a 6-year-old girl who presented hypertension and proteinuria before increasing the eculizumab dose for weight adjustment; she improved with the increased dose (personal communication). In both cases, eculizumab was effective, and the switch to immunosupression [40] in case the anti-factor H test is available and positive should be debated.
In our series, 80% of the patients had genetic analyses, which were performed in six laboratories according to the patient’s access and physician preferences, as this was not a prospective cohort. Although the number of genes analyzed varied from 6 to 14 according to each laboratory, the main genes involved in aHUS were included in all panels and all findings were reported according to ACMG guidelines [15]. In addition, 80% of the patients had the genetic test performed in a single laboratory with the most complete gene panel.
Overall, 24.3% of variants were classified as pathogenic/likely pathogenic according to ACMG guidelines [15] (40% in the adult group and 11.7% in the pediatric group). The finding of pathogenic variants in different populations and cohorts varies from 18.2% in India [12] to 80% in Japan [7], with the proportion of disease-associated findings varying according to pathogenicity prediction models in association with population genetic background. The fact that in our cohort deletions in the CFHR1–CFHR3 genes were the most prevalent and that no patient presented MCP polymorphisms also differs from the literature. Some initiatives, such as the Brazilian aHUS Registry supported by the Brazilian Society of Nephrology and a Brazilian Study Group of TMAs, may yield insights into the genetic background and genotype–phenotype correlation in our country. In the pediatric group, a larger proportion of patients presented a VUS, which could be pathogenic if anti-factor H assays were available. Most importantly, other causes of TMA were ruled out and the patients benefited from complement blockade.
Distinguishing secondary TMAs from aHUS is one of the main challenges in the differential diagnosis of TMA. In our cohort, 50% of the patients with a coexisting condition carried a pathogenic variant and 30% carried a VUS. This finding is distinct from the recent data from Le Clech et al. [41], in which patients with secondary TMA had the same genetic findings as the general population—the patients with severe kidney involvement were treated with eculizumab and a significant percentage recovered renal function. Therefore the role of complement blockade in secondary forms of HUS with no genetic findings is still a matter to be studied. We conclude that in our cohort, the coexisting condition unmasked aHUS instead of being secondary TMA.
Almost a decade after the first published use of eculizumab for aHUS [42], the question is whether all patients need lifelong treatment and, if not, who is eligible to stop [43]. In both the pediatric and adult group in our series, the relapse rate after eculizumab discontinuation was ~30%, similar to previously published data [43, 44]. There was no correlation with genetic findings in our series.
In conclusion, this is the first report of a case series of aHUS in Brazil involving a broad range of patients. Eculizumab was the treatment of choice in the majority of patients and was started earlier in children than in adults. Most patients were able to stop dialysis. There was a trend towards better kidney function after stopping dialysis when eculizumab was started earlier. Eculizumab interruption was associated with a 30% relapse of aHUS. Although 80% of patients had genetic testing, a genotype–phenotype correlation was not possible due to the small number of patients, which may be elucidated by the aHUS Registry and TMA Study Groups who have already started to enroll patients.
ACKNOWLEDGEMENTS
The authors thank Alexion Brazil, for providing diagnostic tests to investigate TMA and for partially sponsoring the genetic testing, and Prof. Craig B. Langman for the critical review.
AUTHORS’ CONTRIBUTIONS
L.M.P.P. and M.I.H. collected data and wrote drafts of the manuscript. G.C.D., M.K.S.T. and R.G.E. collected patient data and reviewed the manuscript.
CONFLICT OF INTEREST STATEMENT
All authors have received honoraria from Alexion Pharmaceuticals for speaker’s fees. L.M.P.P. has received honoraria for advisory board participation. None of the authors received honoraria to write this manuscript. Neither the Brazilian TMA or aHUS Study Group nor the health facilities where patients are followed have received honoraria or grants.
REFERENCES
- 1. Laurence J, Haller H, Mannucci PM. et al. Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol 2016; 14(Suppl 11): 2–15 [PubMed] [Google Scholar]
- 2. George JN, Nester CM.. Syndromes of thrombotic microangiopathy. N Engl J Med 2014; 371: 1847–1848 [DOI] [PubMed] [Google Scholar]
- 3. Noris M, Remuzzi G.. Atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361: 1676–1687 [DOI] [PubMed] [Google Scholar]
- 4. Noris M, Caprioli J, Bresin E. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010; 5: 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bresin E, Rurali E, Caprioli J. et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol 2013; 24: 475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaefer F, Ardissino G, Ariceta G. et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 7. Fan X, Yoshida Y, Honda S. et al. Analysis of genetic and predisposing factors in Japanese patients with atypical hemolytic uremic syndrome. Mol Immunol 2013; 54: 238–246 [DOI] [PubMed] [Google Scholar]
- 8. Fremeaux-Bacchi V, Fakhouri F, Garnier A. et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013; 8: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JM, Park YS, Lee JH. et al. Atypical hemolytic uremic syndrome: Korean pediatric series. Pediatr Int 2015; 57: 431–438 [DOI] [PubMed] [Google Scholar]
- 10. Zhang T, Lu J, Liang S. et al. Comprehensive analysis of complement genes in patients with atypical hemolytic uremic syndrome. Am J Nephrol 2016; 43: 160–169 [DOI] [PubMed] [Google Scholar]
- 11. Szarvas N, Szilagyi A, Csuka D. et al. Genetic analysis and functional characterization of novel mutations in a series of patients with atypical hemolytic uremic syndrome. Mol Immunol 2016; 71: 10–22 [DOI] [PubMed] [Google Scholar]
- 12. Thergaonkar RW, Narang A, Gurjar BS. et al. Targeted exome sequencing in anti-factor H antibody negative HUS reveals multiple variations. Clin Exp Nephrol 2018; 22: 653–660 [DOI] [PubMed] [Google Scholar]
- 13. de Andrade LGM, Contti MM, Nga HS. et al. Long-term outcomes of the atypical hemolytic uremic syndrome after kidney transplantation treated with eculizumab as first choice. PLoS One 2017; 12: e0188155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Holanda MI, Porto LC, Wagner T. et al. Use of eculizumab in a systemic lupus erythemathosus patient presenting thrombotic microangiopathy and heterozygous deletion in CFHR1-CFHR3. A case report and systematic review. Clin Rheumatol 2017; 36: 2859–2867 [DOI] [PubMed] [Google Scholar]
- 15. Richards S, Aziz N, Bale S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jozsi M, Licht C, Strobel S. et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111: 1512–1514 [DOI] [PubMed] [Google Scholar]
- 17. de Holanda MI, Gomes CP, Araujo SA. et al. Diacylglycerol kinase epsilon nephropathy: late diagnosis and therapeutic implications. Clin Kidney J 2019; 12: 641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell WR, Braine HG, Ness PM. et al. Improved survival in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome—Clinical experience in 108 patients. N Engl J Med 1991; 325: 398–403 [DOI] [PubMed] [Google Scholar]
- 19. Legendre CM, Licht C, Muus P. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013; 368: 2169–2181 [DOI] [PubMed] [Google Scholar]
- 20. Licht C, Greenbaum LA, Muus P. et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 2015; 87: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fakhouri F, Hourmant M, Campistol JM. et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis 2016; 68: 84–93 [DOI] [PubMed] [Google Scholar]
- 22. Greenbaum LA, Fila M, Ardissino G. et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 2016; 89: 701–711 [DOI] [PubMed] [Google Scholar]
- 23. Campistol JM, Arias M, Ariceta G. et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia 2015; 35: 421–447 [DOI] [PubMed] [Google Scholar]
- 24. Goodship TH, Cook HT, Fakhouri F. et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017; 91: 539–551 [DOI] [PubMed] [Google Scholar]
- 25. Zuber J, Fakhouri F, Roumenina LT. et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 2012; 8: 643–657 [DOI] [PubMed] [Google Scholar]
- 26. Walle JV, Delmas Y, Ardissino G. et al. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J Nephrol 2017; 30: 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez Suarez ML, Thongprayoon C, Mao MA. et al. Outcomes of kidney transplant patients with atypical hemolytic uremic syndrome treated with eculizumab: a systematic review and meta-analysis. J Clin Med 2019; 8:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zuber J, Frimat M, Caillard S. et al. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am Soc Nephrol 2019; 30: 2449–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joly BS, Zheng XL, Veyradier A.. Understanding thrombotic microangiopathies in children. Intensive Care Med 2018; 44: 1536–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keir LS. Shiga toxin associated hemolytic uremic syndrome. Hematol Oncol Clin North Am 2015; 29: 525–539 [DOI] [PubMed] [Google Scholar]
- 31. Menne J, Nitschke M, Stingele R. et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104: H4 induced haemolytic uraemic syndrome: case-control study. BMJ 2012; 345: e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loirat C, Fakhouri F, Ariceta G. et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 2016; 31: 15–39 [DOI] [PubMed] [Google Scholar]
- 33. Palma LM, Langman CB.. Critical appraisal of eculizumab for atypical hemolytic uremic syndrome. J Blood Med 2016; 7: 39–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson RA, Winterborn MH.. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol 1981; 46: 110–119 [PMC free article] [PubMed] [Google Scholar]
- 35. Warwicker P, Goodship TH, Donne RL. et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 1998; 53: 836–844 [DOI] [PubMed] [Google Scholar]
- 36. Bu F, Maga T, Meyer NC. et al. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol 2014; 25: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lemaire M, Fremeaux-Bacchi V, Schaefer F. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 2013; 45: 531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tseng MH, Tsai JD, Tsai IJ. et al. Whole-exome sequencing detects mutations in pediatric patients with atypical hemolytic uremic syndrome in Taiwan. Clin Chim Acta 2019; 494: 143–150 [DOI] [PubMed] [Google Scholar]
- 39. Dragon-Durey MA, Loirat C, Cloarec S. et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 2005; 16: 555–563 [DOI] [PubMed] [Google Scholar]
- 40. Bagga A, Khandelwal P, Mishra K. et al. Hemolytic uremic syndrome in a developing country: consensus guidelines. Pediatr Nephrol 2019; 34: 1465–1482 [DOI] [PubMed] [Google Scholar]
- 41. Le Clech A, Simon-Tillaux N, Provot F. et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int 2019; 95: 1443–1452 [DOI] [PubMed] [Google Scholar]
- 42. Mache CJ, Acham-Roschitz B, Fremeaux-Bacchi V. et al. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2009; 4: 1312–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fakhouri F, Fila M, Provot F. et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 2017; 12: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ardissino G, Possenti I, Tel F. et al. Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 2015; 66: 172–173 [DOI] [PubMed] [Google Scholar]