Abstract
Whether C5 blocking may improve the outcomes of patients developing chemotherapy-induced thrombotic microangiopathy (TMA) remains elusive. Lung fibrosis is a well-known complication of bleomycin, whereas TMAs are very rare (<20 cases described). Here, we report an exceptional case of a male patient that developed acute respiratory distress syndrome and TMA following administration of bleomycin, cisplatin and etoposide . Refractoriness to plasma exchanges prompted us to use eculizumab as salvage therapy. Eculizumab led to complete remission of the TMA before Day 2. However, the patient progressed towards refractory respiratory failure, suggesting that pathophysiological mechanisms of bleomycin-induced lung fibrosis and TMA differ.
Keywords: bleomycin, complement, eculizumab, lung fibrosis, thrombotic microangiopathy
BACKGROUND
Thrombotic microangiopathies (TMAs) are rare, life-threatening diseases, with frequent renal involvement. Causes of TMA are heterogeneous and include infections by Shiga toxin-producing Escherichia coli, inherited or acquired deficiency of the ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats-13) metalloprotease, pregnancy, autoimmune diseases, mutations in a gene coding for one of the complement system components or drugs [1].
The role of complement activation in the development of TMA has been emphasized in the last two decades, especially in patients developing the haemolytic and uraemic syndrome (HUS). However, whether complement blocking using the C5 inhibitor eculizumab (Alexion, Boston, MA, USA) should be used in patients with TMA not related to a genetic deficiency in the complement pathway remains a matter of debate. In 2013, we reported a case of refractory mitomycin-C-induced TMA that was successfully reversed with eculizumab [2], but its effects in gemcitabine-induced TMA are less conclusive [3], suggesting heterogeneous underlying pathophysiological mechanisms. Thus, reporting the response to eculizumab of rare causes of TMA is mandatory to better define its indication in this clinical setting.
CASE REPORT
We described a 56-year-old male patient that was referred to our intensive care unit (ICU) for TMA/HUS and acute respiratory failure. Two years before admission, he had upper-right lung lobectomy to treat a localized non-seminoma germ cell tumour. In the 2 months preceding the admission to the ICU, he received a chemotherapy regimen combining cisplatin, bleomycin and etoposide for relapse of the malignancy. On admission, serum creatinine was 620 μmol/L (77 μmol/L at baseline), urinary protein to creatinine ratio was 2.2 g/g and microscopic haematuria was identified. Haemolytic anaemia with schistocytes and thrombocytopenia was identified. Blood pressure was 180/100 mmHg. Search for Shiga toxin in stool, circular dichroism (CD) 46 deficiency or anti-factor H or anti-ADAMTS13 antibodies was negative. Complement componentsC3, C4 and CH50 were normal. Chest computed tomography scan showed interstitial lung disease suggestive of bleomycin-related lung fibrosis. Diagnosis of bleomycin-induced HUS was retained.
Daily plasma exchanges were started at Day 1 and dialysis at Day 2. Acute respiratory distress syndrome required mechanical ventilation at Day 1. Because TMA was still active, salvage therapy by eculizumab was started at Day 5 after anti-pneumococcal and anti-meningococcal vaccination (900 mg weekly, intravenously). Ceftriaxone was used as anti-bacterial prophylaxis. As shown in Figure 1, eculizumab administration was followed by rapid correction of haemolysis, normalization of haptoglobin levels and an increase in platelets to 100 000/mL. No relapse of the TMA occurred. However, no renal recovery was observed and lung fibrosis progressed despite protective ventilation and secondary introduction of steroids and tocilizumab. Worsening of the lung compliance ultimately led to fatal respiratory failure at Day 25.
FIGURE 1.
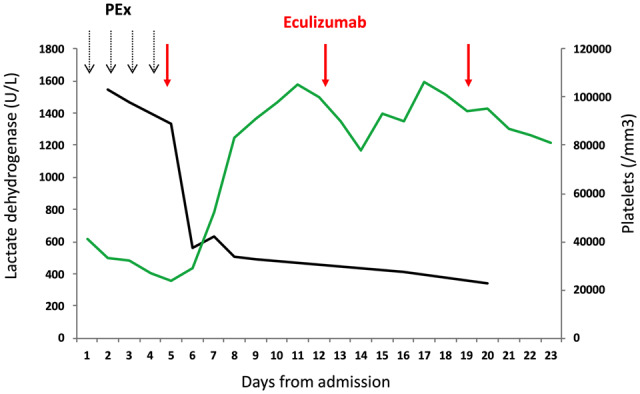
Platelets (green line) and lactate dehydrogenase (U/L; black line) after eculizumab in a patient with bleomycin-induced TMA. PEx, plasma exchanges.
DISCUSSION
Bleomycin is a very rare cause of TMA, with <20 cases described (Supplementary data, Table S1). Some data suggest that cisplatin may potentiate bleomycin-induced vascular injury and vasospasm [4] with subsequent release of a large amount of von Willebrand factor multimers, platelet aggregation and fibrin deposition. Patients also frequently developed TMA and acute lung fibrosis concomitantly, but whether endothelial injury is the culprit of both renal and pulmonary manifestations remains elusive. Moreover, apart from anecdotal cases of bleomycin-induced TMA successfully reversed with plasma transfusion and glucocorticoids, most patients develop refractory TMA and lung disease. Here, we showed that eculizumab can immediately stop TMA, suggesting that uncontrolled activation of the complement pathways promotes bleomycin-induced vascular toxicity. Whether earlier administration of eculizumab may prevent progression towards end-stage renal failure warrants further investigations.
On the contrary, C5 blocking did not prevent progression towards refractory lung fibrosis, whereas complement activation and beneficial effects of complement inhibition were previously reported in animal models of bleomycin-induced lung fibrosis [5]. Several hypotheses can be drawn to explain this finding, including alternative underlying mechanisms, late administration of eculizumab or the development of superimposed ventilation-induced lung injuries not targeted by eculizumab.
In summary, we reported here that C5 inhibition with eculizumab can treat bleomycin-induced TMA and should probably be administered early in the course of TMA to improve renal, and maybe lung, prognosis.
PATIENT CONSENT
The study was conducted according to the French law regarding the retrospective observational studies, and methodology was approved by the Institutional Review Board of the University Hospital of Toulouse, France, which waived the need to obtain a written informed consent.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Joost P. Schanstra for his review of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Fakhouri F, Zuber J, Frémeaux-Bacchi V. et al. Haemolytic uraemic syndrome. Lancet 2017; 390: 681–696 [DOI] [PubMed] [Google Scholar]
- 2. Faguer S, Huart A, Frémeaux-Bacchi V. et al. Eculizumab and drug-induced haemolytic-uraemic syndrome. Clin Kidney J 2013; 6: 484–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daviet F, Rouby F, Poullin P. et al. Thrombotic microangiopathy associated with gemcitabine use: Presentation and outcome in a national French retrospective cohort. Br J Clin Pharmacol 2019; 85: 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doll DC, List AF, Greco FA. et al. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med 1986; 105: 48–51 [DOI] [PubMed] [Google Scholar]
- 5. Gu H, Fisher AJ, Mickler EA. et al. Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis. FASEB J 2016; 30: 2336–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


