Abstract
Background
The use of kidneys from elderly controlled donation after circulatory death (cDCD) donors has increased significantly in recent years. Concerns about outcomes achieved with these elderly cDCD kidneys have arisen. We aimed to compare outcomes from elderly cDCD kidney transplant recipients (KTrs) and elderly donation after brain death donors (DBDs) in KTrs.
Methods
We conducted a single-centre retrospective study including 87 cDCD-KTrs (46 from donors ≥65 years of age and 41 from <65 years) and 126 DBD-KTrs from donors ≥65 years of age from 2013 through 2017). Young cDCD-KTrs were used as controls. The median follow-up was 27.1 months for all cDCD-KTrs and 29.7 months for DBD-KTrs ≥65 years of age.
Results
Donors >65 years of age represented more than half of our global cDCD cohort (52.9%). KTs from elderly cDCDs had similar rates of delayed graft function, primary non-function and vascular complications compared with young cDCD-KTrs and elderly DBD-KTrs. Short and medium-term graft survival from elderly cDCD kidneys are excellent and are comparable to those from young cDCD and elderly DBD kidneys (90% young cDCD versus 88% elderly cDCD versus 80% elderly DBD at 36 months, P = 0.962 and 0.180, respectively). Although recipients from cDCDs ≥65 years of age showed lower 3-year patient survival (78% versus 87% in elderly DBD-KTrs; P = 0.01), recipient age was the only determinant of patient survival [hazard ratio 1.10 (95% confidence interval 1.02–1.17); P < 0.01], without any influence of donor characteristics.
Conclusions
The use of kidneys from elderly cDCDs is increasing in Spain. Short- and medium-term graft outcomes are similar when comparing kidneys from elderly cDCDs and DBDs. Recipient age is the only determinant of patient survival. Additional studies are needed to assess long-term outcomes.
Keywords: brain death donors, clinical outcomes, donors after circulatory death, elderly donors, kidney transplantation
INTRODUCTION
In Spain, kidney transplant (KT) activity has continuously increased in recent years [1]. This increase, driven by the growing number of patients on the waiting list, has been accomplished through the acceptance of new donor profiles: (i) the emergence of controlled donation after circulatory death (cDCD) as a viable source of organs in 2012 [2] and (ii) the extended use of increasingly older deceased donors [3].
Kidneys from cDCDs offer a valuable extension of the donor pool, but not without controversy. Unlike those from donation after brain death (DBD), cDCD kidneys are subject to warm ischaemic injury that increases the risk of primary non-function (PNF) and delayed graft function (DGF) and may compromise long-term graft survival [4–7]. Consequently, many cDCD kidneys are discarded due to concerns about their outcomes, particularly when they come from expanded criteria donors (ECDs), and prolonged ischaemia times [3, 8, 9]. In contrast, because of the risks associated with DGF, patients receiving cDCD kidneys usually receive induction immunosuppression with polyclonal antibodies in order to delay the use of tacrolimus or cyclosporine and reduce the rate of DGF. This increase in global immunosuppression might compromise the recipient’s outcome.
Transplant physicians are evolving to more open-minded attitude towards donor acceptance criteria, because evidence supporting these strategies has emerged in recent years [10–12]. Several studies of KTs in elderly recipients and the use of advanced-age kidneys, adopting an old-for-old allocation policy, have shown favourable results, demonstrating improved survival compared with waitlisted patients remaining on dialysis [10–14]. However, the experience with cDCD elderly kidneys allocated to elderly recipients has not been that optimistic so far, and two cohorts have recently shown that survival in these recipients might be poorer than remaining on dialysis [15, 16]. In this context, although some series have shown similar short-term patient and graft outcomes between cDCD-ECDs, cDCD standard criteria donors (SCDs) and DBD-ECDs [3, 15, 17, 18], outcomes with KTs from elderly cDCDs need to be carefully studied in order to avoid poor patient and graft outcomes.
In Spain, the rate of KTs reached 70.8 transplants per million population (pmp) in 2018 (>100 in Catalonia) [19], the highest worldwide. Kidneys from cDCDs represented 26% of all deceased donor KT activity, with a 22% increase since 2012 [1]. A study from the GEODAS working group including 19 transplant centres (including ours) in Spain recently found that 24% of cDCD kidneys transplanted between January 2012 and January 2017 were from donors >65 years of age. Recipients from cDCDs showed similar short-term outcomes regardless of donor age [18]. So far, there are no available data comparing graft and patient outcomes between elderly cDCDs and DBDs in Spain.
In view of the increasingly important contribution of cDCDs to KTs and waitlisted outcomes, we aimed to compare short- and medium-term graft and patient outcomes between elderly cDCD-KTs and elderly DBD-KTs. Young cDCD-KTs were evaluated as controls.
MATERIALS AND METHODS
Patients
We conducted a retrospective cohort study among all cDCDs (Maastricht Type III) and DBD-KTs ≥65 years of age performed in Hospital del Mar, Barcelona, from January 2013 to December 2017. Follow-up was until July 2019. Clinical and epidemiological information were collected from our local transplant database. The final cohort consisted of 213 KTs (87 cDCD-KTs: 46 from donors ≥65 years of age and 41 from donors <65 years of age and 126 DBDs ≥65 years of age). The median time to follow-up was 27.1 months [interquartile range (IQR) 21.7–42.3] for cDCDs and 29.7 months (IQR 14.7–50.3) for DBDs ≥65 years of age.
Study variables
We defined donors and recipients <65 years of age as young and donors and recipients ≥65 years of age as elderly. Recorded baseline data included donor and recipient characteristics [age, sex, ethnicity, body mass index, body surface area, kidney donor profile index (KDPI)], Remuzzi histological score, cause of end-stage kidney disease, time on dialysis before transplant, type of renal replacement therapy (RRT), comorbidities (diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease) and transplant-related factors such as cold ischaemia time (CIT), calculated panel reactive antibodies (cPRAs) and immunosuppressive therapy.
Serum creatinine, estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease-4 formula and proteinuria were recorded at 1, 3, 6, 12 and 36 months. Clinical events, such as DGF, PNF, poor kidney function, vascular complications, surgical complications, fluid complications, acute rejection (AR), de novo donor-specific antibodies (DSAs), cytomegalovirus infection/disease, BK virus infection, number of hospital admissions during the first year after KT, graft loss and patient death were recorded.
The KDPI was calculated from donor variables using the method described by the Organ Procurement Transplant Network [20]. Pre-transplant histological assessment with Remuzzi score, although recommended, was not a routine technique in our standard practice. Thus it was only performed on 28 elderly DCD-KTs and 22 elderly DBD-KTs. DGF was defined as the need for dialysis during the first week after KT, not secondary to PNF or graft thrombosis, and followed by recovery of allograft function. PNF was defined as no dialysis independence after transplantation, excluding those causes of graft loss due to hyperacute rejection, thrombosis or surgical complication. Poor kidney function at 12 months was defined as an eGFR <30 mL/min. AR was identified on biopsy and classified according to the Banff 2013 classification and its subsequent updates [21, 22].
Immunosuppression treatment
Induction immunosuppression therapy was based on the patient’s immunological risk, and high immunological risk patients were those with a pre-transplant cPRA >50%, with the presence of DSA at the moment of transplant or those with two or more previous KTs. For low immunological risk patients, basiliximab was administered at a dose of 20 mg on the day of the operation and on postoperative Day 4. For high immunological risk patients, rabbit antithymocyte globulin was administered at a total dose of 2.5–5 mg/kg. Standard maintenance immunosuppression included tacrolimus (trough target levels 5–8 ng/mL after Month 6 post-transplantation), mycophenolic acid and prednisone. All patients received 250 mg of methylprednisolone on Day 0 and 125 mg on Day 1. Prednisone dosage was then tapered from 20 to 5 mg/day within 42 days after KT. Some low immunological risk patients received an alternative maintenance immunosuppression including low-dose tacrolimus (target levels 2–4 ng/mL after Month 6), everolimus (target levels 3–8 ng/mL) and prednisone.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) or median and IQR according to their distribution. Categorical data are expressed as percentages. Comparisons of baseline characteristics between groups were made using chi-square or Fisher’s exact tests to analyse categorical variables, Student’s t-test for continuous variables with normal distribution and Mann–Whitney test for non-parametric variables.
Patient and graft survival was estimated using Kaplan–Meier curves, applying the log-rank test. Death-censored graft loss was considered from the transplant date to the beginning of an alternative RRT (return to dialysis or retransplantation).
Logistic regression was used to estimate the odds ratio (OR) for DGF and early patient mortality. Univariate and multivariate Cox regressions were performed to estimate the hazard ratio (HR) for survival. The following variables were included in the univariate analysis: recipient age and comorbidity, CIT, donor/recipient body surface index, dialysis vintage, human leucocyte antigen mismatches, cPRA >30%, donor type, Remuzzi score, induction and maintenance therapy, biopsy-proven AR, vascular complications, surgical complications, fluid complications and number of hospital admissions during the first year after KT. These variables were chosen based on their clinical relevance and previous results obtained from our group [23].
Statistical analysis was performed using SPSS version 21 software (IBM, Armonk, NY, USA). P-values <0.05 were considered statistically significant.
RESULTS
Characteristics according to donor type
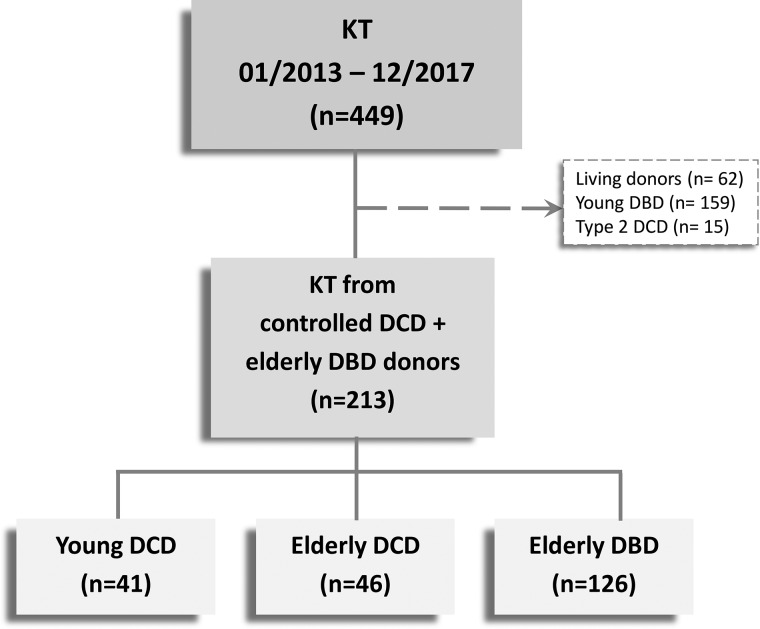
A total of 213 KTs were included in the study. Of them, 41 received a KT from a cDCD <65 years of age, 46 from acDCD ≥65 years of age and 126 from a DBD ≥65 years of age (Figure 1). Details of baseline recipient, donor and transplant-related characteristics are shown in Table 1.
FIGURE 1:
Patients flow chart. n, number of cases.
Table 1.
Baseline characteristics among KT recipients
| Demographic parameter | <65-year cDCDs (n = 41) | ≥65-year cDCDs (n = 46) | ≥65-year DBDs (n = 126) | P-value | P-value |
|---|---|---|---|---|---|
| Recipient characteristics | |||||
| Age (years), mean ± SD | 52.6 ± 10.8 | 66.4 ± 6.5 | 69.7 ± 6.9 | <0.001 | 0.01 |
| Age ≥65 years, n (%) | 8 (21.6) | 29 (63) | 126 (100) | <0.001 | <0.001 |
| Female gender, n (%) | 18 (58.1) | 13 (41.9) | 52 (41.3) | 0.13 | 0.12 |
| Caucasian race, n (%) | 38 (92.7) | 45 (97.8) | 120 (95.2) | 0.25 | 0.45 |
| Hypertension, n (%) | 37 (90.2) | 43 (93.5) | 115 (91.3) | 0.58 | 0.64 |
| Diabetes mellitus, n (%) | 10 (25) | 18 (39.1) | 46 (36.5) | 0.16 | 0.75 |
| Body mass index (kg/m2), mean ± SD | 26.3 ± 5.0 | 28.4 ± 5.4 | 27.6 ± 4.6 | 0.15 | 0.35 |
| Previous cardiovascular event, n (%) | |||||
| Ischaemic heart disease | 3 (7.3) | 11 (24.4) | 23 (18.3) | 0.03 | 0.37 |
| Stroke | 2 (5) | 3 (6.5) | 13 (10.3) | 0.76 | 0.45 |
| Peripheral vascular disease | 3 (7.3) | 5 (10.9) | 15 (11.9) | 0.57 | 0.85 |
| Cause of end-stage renal disease, n (%) | |||||
| Hypertensive nephropathy | 0 (0) | 1 (2.2) | 18 (14.3) | 0.61 | 0.32 |
| Diabetic nephropathy | 7 (17.1) | 9 (19.6) | 24 (19) | ||
| Glomerulonephritis | 13 (31.7) | 7 (15.2) | 14 (11.1) | ||
| Interstitial | 3 (7.3) | 6 (13) | 15 (11.9) | ||
| Polycystic | 2 (4.9) | 2 (4.3) | 11 (8.7) | ||
| Others | 2 (4.9) | 3 (6.5) | 5 (4) | ||
| Unknown | 14 (34.1) | 18 (39.1) | 39 (31) | ||
| Previous RRT, n (%) | |||||
| Haemodialysis | 30 (73.2) | 39 (84.8) | 99 (78.6) | 0.19 | 0.65 |
| Peritoneal dialysis | 11 (26.8) | 6 (13) | 22 (17.5) | ||
| Preemptive KT | 0 (0) | 1 (2.2) | 5 (4) | ||
| Dialysis vintage (months), median (IQR) | 20.0 (12.2–46.5) | 23.5 (15.1–44.9) | 19.7 (10.9–37.5) | 0.08 | 0.44 |
| Patients with previous KT, n (%) | 7 (17.1) | 2 (4.3) | 10 (7.9) | 0.05 | 0.41 |
| Donor characteristics | |||||
| Age (years), mean ± SD | 51.7 ± 5.4 | 72.5 ± 5.6 | 74.5 ± 6.0 | <0.001 | 0.05 |
| Female gender, n (%) | 17 (41.5) | 22 (47.8) | 74 (58.7) | 0.55 | 0.20 |
| Donor/recipient body surface index, mean ± SD | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.77 | 0.04 |
| Expanded criteria donors, n (%) | 12 (29.3) | 46 (100) | 126 (100) | <0.001 | NA |
| Cause of death, n (%) | |||||
| Stroke | 19 (46.3) | 27 (58.7) | 96 (76.2) | 0.51 | 0.05 |
| Anoxia | 16 (39) | 11 (23.9) | 19 (15.1) | ||
| Trauma | 3 (7.3) | 4 (8.7) | 9 (7.1) | ||
| Other | 3 (7.3) | 4 (8.7) | 2 (1.6) | ||
| KDPI (%), mean ± SD | 64.2 ± 17.7 | 95.2 ± 5.9 | 96.4 ± 5.6 | <0.001 | 0.26 |
| Remuzzi score, median (IQR)a | – | 2.9 (1–4) | 3.8 (3–4.3) | NA | 0.04 |
| Transplant characteristics | |||||
| HLA mismatches, median (IQR), n | 5 (4–5) | 4 (3–5) | 4 (3–5) | 0.01 | 0.14 |
| Peak PRA >30%, n (%) | 9 (22) | 6 (13) | 12 (9.5) | 0.27 | 0.50 |
| Cold ischaemia time (h), median (IQR) | 11.2 (6.0–15.0) | 9.0 (5.0–14.3) | 16.5 (13.0–20.5) | 0.66 | 0.15 |
| Warm ischaemia time (min), median (IQR) | 12.0 (8.0–20.0) | 16.0 (13.0–24.5) | – | 0.03 | NA |
| Initial immunosuppression, n (%) | |||||
| Thymoglobulin induction | 9 (22) | 3 (6.5) | 13 (10.3) | 0.04 | 0.45 |
| Tacrolimus | 41 (100) | 46 (100) | 126 (100) | NA | NA |
| mTORi | 13 (31.7) | 12 (26.1) | 24 (19.2) | 0.56 | 0.33 |
| Mycophenolic acid derivatives | 28 (68.3) | 34 (73.9) | 101 (80.8) | 0.56 | 0.33 |
| 1 year after KT immunosuppression, n (%) | |||||
| Tacrolimus use, n (%) | 36 (100) | 37 (100) | 96 (100) | NA | NA |
| mTORi use, n (%) | 11 (30.6) | 6 (17.1) | 21 (21.9) | 0.17 | 0.55 |
| Mycophenolic acid derivatives use, n (%) | 23 (63.9) | 22 (62.9) | 67 (69.8) | 0.81 | 0.45 |
NA, not applicable; mTORi, mammalian target of rapamycin inhibitor. aRemuzzi score was only performed on 28 elderly DCD-KTs and 22 elderly DBD-KTs.
*Comparison between young and elderly cDCD KTs.
**Comparison between elderly DBD and cDCD KTs.
Comparison between young and elderly cDCD KTs
As expected, recipients of <65-year cDCD grafts were younger and donor KDPI values were lower compared with the elderly cDCD group. The prevalence of comorbidities such as hypertension, diabetes, stroke or peripheral vascular disease was similar between groups, but recipients of elderly cDCD grafts presented with a considerably higher rate of ischaemic heart disease before transplantation (7.3 versus 24.4%; P = 0.03). The retransplantation rate was greater in the younger group, with an increased use of thymoglobulin as induction therapy. Both groups presented similar CITs, but warm ischaemia time was shorter in the younger group.
Comparison between elderly DBD and cDCD KTs
Recipients of DBD grafts were older than recipients of cDCD-KTs. Although they received older kidneys, KDPI values were similar. Stroke was the first cause of death in both groups and was more frequent in the DBD group (76.2 versus 58.7%; P = 0.05).
Early and medium-term transplant outcomes
Table 2 shows the observed incidence of transplant outcomes for each patient group. No differences were found regarding early clinical events (early patient mortality, PNF, DGF, days until creatinine decrease and inpatient days) between groups. Nor did we find any difference in de novo DSA, biopsy-proven AR, cytomegalovirus and BK virus infection, number of hospital admissions during the first year after KT, vascular and surgical complications or lymphocele rates. When analysing potential risk factors for DGF and early patient mortality in the elderly cohort through logistic regression models, dialysis vintage and haemodialysis as RRT were found to be risk factors for DGF [OR 1.02 (95% CI 1.00–1.04), P = 0.02 and OR 7.22 (95% CI 1.61–32.29), P = 0.01, respectively; Table 3), while recipient age and surgical complications conditioned early patient mortality [OR 1.12 (95% CI 1.02–1.22), P = 0.02 and OR 4.23 (95% CI 1.49–12.01), P = 0.01, respectively; Table 4).
Table 2.
Patient and transplant outcomes among KTrs
| Outcome | <65-year cDCD (n = 41) | ≥65-year cDCD (n = 46) | ≥65-year DBD (n = 126) | P-value | P-value |
|---|---|---|---|---|---|
| Early outcomes | |||||
| Early patient mortality, n (%) | 2 (4.9) | 6 (13) | 13 (10.3) | 0.19 | 0.61 |
| Primary non-function, n (%) | 1 (2.4) | 1 (2.2) | 5 (4) | 0.93 | 0.57 |
| DGF, n (%) | 11 (28.9) | 18 (41.9) | 36 (31.9) | 0.22 | 0.24 |
| Days until creatinine decrease, median (IQR) | 5 (1.8–10.3) | 8 (3–13) | 5 (2–9) | 0.41 | 0.15 |
| Inpatient days, median (IQR) | 8 (7–12) | 11 (7–16) | 12.8 (7–13) | 0.11 | 0.55 |
| Graft function | |||||
| Creatinine at 12 months (mg/dL), mean ± SD | 1.7 ± 0.7 | 1.9 ± 0.7 | 1.9 ± 0.6 | 0.28 | 0.99 |
| eGFR at 12 months (mL/min), mean ± SD | 48.2 ± 22.3 | 40.9 ± 13.5 | 38.4 ± 14.7 | 0.10 | 0.38 |
| eGFR <30 mL/min at 12 months, n (%) | 8 (22.2) | 9 (24.3) | 31 (32.3) | 0.83 | 0.37 |
| Proteinuria at 12 months (mg/g), median (IQR) | 213.6 (115.3–390.1) | 227 (151.5–608.2) | 274.5(170.6–489.3) | 0.14 | 0.10 |
| Creatinine at 36 months (mg/dL), mean ± SD | 1.7 ± 0.8 | 1.8 ± 0.9 | 2.1 ± 0.7 | 0.62 | 0.24 |
| eGFR at 36 months (mL/min), mean ± SD | 48.0 ± 24.1 | 41.2 ± 15.0 | 34.0 ± 14.7 | 0.36 | 0.10 |
| eGFR <30 mL/min at 36 months, n (%) | 2 (13.3) | 3 (20) | 21 (42) | 0.62 | 0.12 |
| Proteinuria at 36 months (mg/g), median (IQR) | 169.0 (68.8–200.6) | 221 (127.4–564.0) | 194.2 (131.5–436.7) | 0.04 | 0.89 |
| De novo DSA, n (%) | 4 (10.8) | 3 (7.1) | 7 (6.7) | 0.57 | 0.92 |
| Biopsy-proven acute rejection, n (%) | 5 (12.8) | 2 (4.7) | 14 (12.4) | 0.19 | 0.16 |
| Other outcomes | |||||
| Cytomegalovirus prophylaxis, n (%) | 11 (28.9) | 7 (16.3) | 22 (20) | 0.17 | 0.60 |
| Cytomegalovirus, n (%) | |||||
| No | 26 (68.4) | 22 (51.2) | 57 (52.3) | 0.22 | 0.67 |
| Infection | 12 (31.6) | 20 (46.5) | 46 (42.2) | ||
| Disease | 0 (0) | 1 (2.3) | 6 (5.5) | ||
| BK virus infection, n (%) | 5 (13.2) | 7 (16.3) | 25 (22.7) | 0.70 | 0.38 |
| Number of hospital admissions during first year after ≥2 KTs, n (%) | 5 (12.8) | 11 (25.6) | 36 (36.1) | 0.15 | 0.22 |
| Vascular complications, n (%) | 4 (9.8) | 5 (10.9) | 29 (23) | 0.87 | 0.08 |
| Surgical complications, n (%) | 2 (4.9) | 5 (11.1) | 28 (22.2) | 0.29 | 0.11 |
| Fluid collections, n (%) | 5 (12.2) | 13 (28.9) | 42 (33.3) | 0.06 | 0.58 |
*Comparison between young and elderly cDCD KTs.
**Comparison between elderly DBD and cDCD KTs.
Table 3.
Risk factors for DGF with Poisson regression analysis
| Risk factor | DGF |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Recipient age | 1.03 (0.98–1.08) | 0.28 | – | – |
| Ischaemic cardiopathy | 1.23 (0.55–2.78) | 0.61 | – | – |
| Dialysis vintage | 1.02 (1.00–1.04) | 0.01 | 1.02 (1.00–1.03) | 0.02 |
| Haemodialysis as RRT | 10.33 (2.36–45.21) | <0.01 | 7.22 (1.61–32.29) | 0.01 |
| KDPI | 1.00 (0.94–1.06) | 0.88 | – | – |
| Donor/recipient body surface index | 0.17 (0.13–2.18) | 0.17 | – | – |
| Remuzzi score | 1.24 (0.85–1.82) | 0.26 | – | – |
| Number of HLA mismatches | 1.15 (0.87–1.51) | 0.32 | – | – |
| Peak PRA >30% | 1.45 (0.48–4.48) | 0.50 | – | – |
| Cold ischaemia time | 1.00 (0.96–1.05) | 0.74 | – | – |
| mTORi as maintenance treatment | 0.66 (0.29–1.51) | 0.33 | – | – |
| Thymoglobulin induction | 2.38 (0.76–7.45) | 0.14 | – | – |
mTORi, mammalian target of rapamycin inhibitor.
Table 4.
Risk factors for early patient mortality with Poisson regression analysis
| Risk factor | Early patient mortality |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR multivariate (95% CI) | P-value | |
| Recipient age | 1.12 (1.03–1.23) | 0.01 | 1.12 (1.02–1.22) | 0.02 |
| Ischaemic heart disease | 1.52 (0.51–4.54) | 0.44 | – | – |
| Dialysis vintage | 1.00 (0.99–1.02) | 0.65 | – | – |
| Haemodialysis as RRT | 1.36 (0.37–4.95) | 0.23 | – | – |
| Previous KT | 3.00 (0.74–12.23) | 0.13 | – | – |
| Elderly cDCD (versus elderly DBD) | 1.30 (0.46–3.66) | 0.61 | – | – |
| Thymoglobulin induction | 3.13 (0.90–10.94) | 0.07 | 2.56 (0.67–9.82) | 0.17 |
| Number hospital admissions during first year after ≥2 KTs | 1.49 (0.45–4.96) | 0.51 | – | – |
| Vascular complications | 1.53 (9.51–4.58) | 0.45 | – | – |
| Surgical complications | 4.80 (1.77–13.06) | <0.01 | 4.23 (1.49–12.01) | 0.01 |
| Fluid collections | 2.07 (0.79–5.44) | 0.14 | – | – |
In terms of graft function, recipients of elderly DBD grafts experienced a trend towards a higher rate of poor renal function (eGFR <30 mL/min) at 36 months compared with elderly cDCD-KTs, although the difference was not statistically significant (42 versus 20%, respectively; P = 0.12) (Table 2). Proteinuria at 36 months was slightly increased in elderly cDCD-KTs compared with young cDCD-KTs (221 versus 169 mg/g; P = 0.04).
Survival analysis
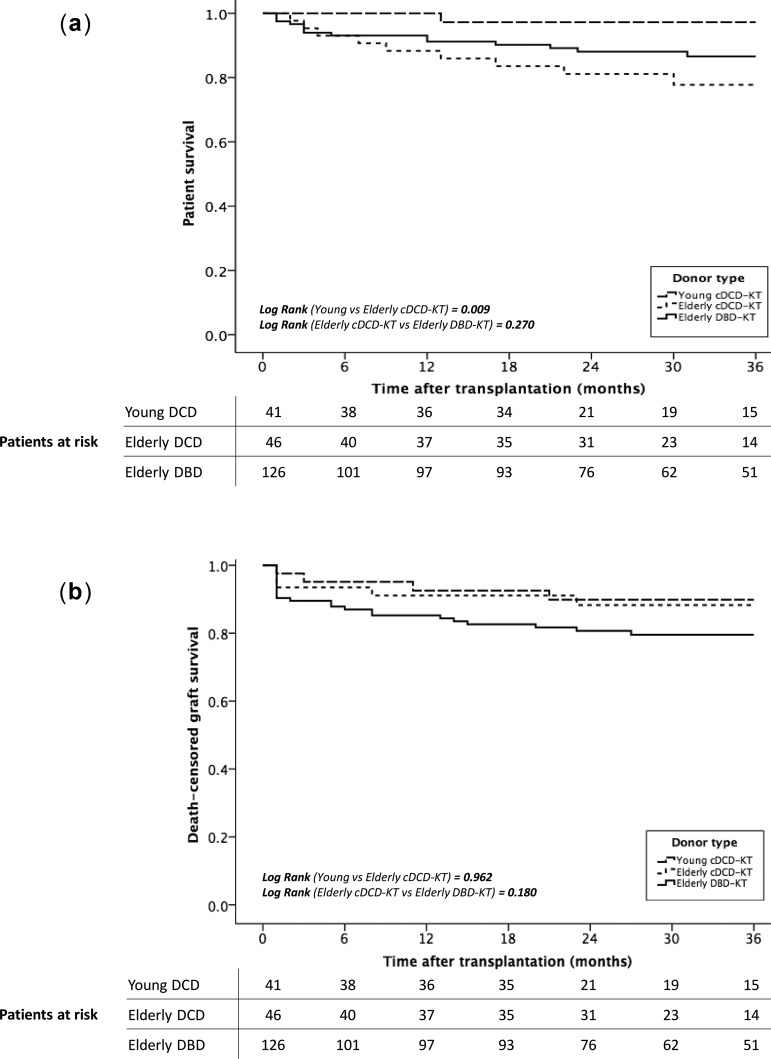
Survival analyses (estimated by the Kaplan–Meier method) showed a lower patient survival at 36 months after transplantation in those recipients of cDCD ≥65-year grafts (97% young cDCDs versus 78% elderly DCDs versus 87% elderly DBDs; P = 0.01 and 0.27, respectively; Figure 2A). When analysing risk factors for mortality in recipients from ≥65-year donor grafts, the logistic regression model adjusted by recipient age and comorbidities showed that recipient age was the only risk factor related to patient death, conferring a 10% excess risk for mortality per each recipient’s year [HR 1.10 (95% CI 1.02–1.17), P < 0.01; Table 5).
FIGURE 2:
(A) Patient and (B) death-censored graft survival after KT. (A) Kaplan–Meier curve shows 3-year mortality rates of KT patients from young-cDCDs, elderly cDCDs and elderly DBDs. (B) Kaplan–Meier curve shows 3-year death-censored graft survival of KT patients from young-cDCDs, elderly cDCDs and elderly DBDs.
Table 5.
Risk factors for patient survival estimated with Cox regression analysis
| Risk factor | Patient survival |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR multivariate (95% CI) | P-value | |
| Recipient age | 1.09 (1.02–1.17) | 0.01 | 1.10 (1.02–1.17) | <0.01 |
| Diabetes mellitus | 0.94 (0.44–2.00) | 0.87 | ||
| Ischaemic cardiopathy | 2.25 (1.04–4.84) | 0.04 | 1.99 (0.90–4.42) | 0.09 |
| Dialysis vintage | 1.01 (0.99–1.02) | 0.44 | – | – |
| Elderly cDCD (versus elderly DBD) | 1.54 (0.71–3.33) | 0.27 | – | – |
| KDPI | 1.03 (0.95–1.12) | 0.46 | – | – |
| Thymoglobulin induction | 3.01 (1.13–8.00) | 0.03 | 2.42 (0.88 - 6.67) | 0.09 |
| Number hospital admissions during first year after ≥2 KTs | 1.79 (0.78–4.09) | 0.17 | – | – |
On the other hand, death-censored graft survival at 36 months was similar among groups (90 versus 88 versus 80%, P = 0.962 and 0.180, respectively) (Figure 2B). Independent risk factors for graft loss in KT from elderly donors were DGF (HR = 4.27, 95% CI 1.56–11.67; P = 0.01) and biopsy-proven AR (HR = 3.43, 95% CI 1.19–9.84; P = 0.02, Table 6).
Table 6.
Risk factors for death-censored graft survival estimated with Cox regression analysis
| Risk factor | Death-censored graft survival |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR multivariate (95% CI) | P-value | |
| Recipient age | 1.00 (0.97–1.02) | 0.89 | – | – |
| Elderly cDCD (versus elderly DBD) | 0.53 (0.20–1.37) | 0.19 | – | – |
| KDPI | 1.08 (0.98–1.19) | 0.13 | – | – |
| Donor/recipient body surface index | 0.56 (0.03–7.25) | 0.56 | – | – |
| Number of HLA mismatches | 0.98 (0.74–1.30) | 0.88 | – | – |
| Peak PRA >30% | 1.96 (0.75–5.15) | 0.17 | – | – |
| Cold ischaemia time/h | 1.03 (0.97–1.09) | 0.38 | – | – |
| DGF | 4.67 (0.72–12.67) | <0.01 | 4.27 (1.56–11.67) | 0.01 |
| mTORi as maintenance treatment | 0.56 (0.20–1.59) | 0.28 | – | – |
| Thymoglobulin induction | 1.77 (0.62–5.10) | 0.29 | – | – |
| Biopsy-proven acute rejection | 4.09 (1.44–11.63) | 0.01 | 3.43 (1.19–9.84) | 0.02 |
mTORi, mammalian target of rapamycin inhibitor.
DISCUSSION
We report the first comparative study between elderly cDCDs and DBDs in Spain. Our preliminary results confirm similar rates of DGF, PNF and vascular complications between elderly cDCD grafts, young cDCD-KTs and elderly DBD-KTs. Short- and medium-term graft survival from elderly cDCD kidneys are excellent and are comparable to those from young cDCD and elderly DBD kidneys. We found lower 3-year patient survival among KT recipients from elderly cDCDs, but related to recipient age. More importantly, our findings noted that donor type itself did not have any impact on patient or graft survival in the short and medium term.
In Spain, DCD programmes have experienced unprecedented growth since 2012, reaching a worldwide maximum of 18.4 DCD-KTs pmp in 2018 [24]. Other European countries also have successful DCD programmes [24]. The increase in the availability of cDCD organs is mainly due to the use of older donors [9, 18, 25], which may result either from a better evaluation of potential donors in this age group or a greater utilization of these grafts. In fact, elderly donors constitute nearly 25% of cDCDs in Spain [18, 26] and represented up to 52.9% of all cDCD-KTs in our cohort. So far, age-matching allocation policies have shown favourable results [10–13]. However, as kidneys affected by age-related changes and other comorbidities may be more sensitive to the warm ischaemic injury of cDCD grafts, a variable proportion of these kidneys are being discarded, particularly those with longer warm ischaemia time, high KDPIs or those not treated with machine perfusion. Pre-implantation biopsy showed contradictory results [27, 28].
Several retrospective studies have evaluated outcomes from combined ECD–DCD kidneys using the classic ECD definition [17, 29–33]. Locke et al. [29] analysed the effect of age and donor type on the risk of graft failure in KTs from the United Network for Organ Sharing database between 1993 and 2005. Donor age was associated with an increased risk of graft failure, although graft survival was similar between ECD-DBD and >50-year DCD kidneys. A large registry analysis from the USA including 562 ECD–DCD KTs showed a slightly increased graft loss in DCD-KTs compared with non-DCD recipients. The increased risk of total graft failure in DCD recipients was not significantly modified by ECD status [30]. Overall, ECD–DCDs have shown poorer patient and graft outcomes compared with SCD–DCDs, but not inferior to ECD–DBDs [6, 17, 29–31, 34]. The 3-year death-censored graft survival for ECD–DCD kidneys is 70–90% [6, 30, 31, 35]. In our study, elderly cDCD kidneys showed good medium-term graft survival (88%), comparable to that from young cDCD and elderly DBD kidneys (90 and 80%, respectively). On the other hand, elderly cDCD recipients showed a lower 3-year patient survival (78%), but similar to that previously reported [16, 30, 35].
It is worth noting that those studies that evaluate outcomes from elderly cDCDs using the classic ECD definition have a mean donor age ranging between 56 and 64 years, much lower than the one in our elderly cohort, with a mean age of almost 75 years. Donor age has been associated with inferior outcomes after KT due to lower kidney functional reserve and increased vasculopathy, which presumably reduce their capability to respond to injuries [36, 37]. Nonetheless, only a few studies have focused on analysing results from elderly cDCDs [15, 16, 18, 35]. The first experiences were not very positive and described no benefit in terms of survival using kidneys from old DCDs (>65 years) compared with those who remained waitlisted on dialysis [15, 16]. Since then, other studies have supported good death-censored graft survival and acceptable graft function in >65-year cDCD grafts, comparable to results from elderly DBDs [18, 35]. Khalid et al. [35] showed an overall death-censored graft survival of 90 and 80% at 3 and 5 y, respectively. Outcomes from >70-year cDCDs did not differ from the age group of 60–69 years (P = 0.79). The same happened with the median eGFR at 3 and 5 years. However, in terms of patient survival, the eldest group showed a significant increased mortality. In fact, a Spanish study recently showed that recipient age was the only determinant of patient mortality in their cDCD cohort [18], similar to our results. Interestingly, in none of these studies did donor type itself have any impact on patient or graft survival in the short and medium term.
Disparities have been found in other outcomes, such as DGF or PNF. While the Canadian group showed higher DGF and PNF rates in DCD kidneys compared with the DBD ones, regardless of ECD status [30], other groups found higher rates of both DGF and PNF and poorer kidney function in ECD–DCD kidneys compared with younger donors [6, 17]. However, an update of the UK Registry showed similar rates of PNF and similar 5-year graft survival between ECD–DCD and ECD–DBD KTs [3]. Similarly, the GEODAS group found an increased rate of DGF in elderly cDCDs compared with young cDCDs, but similar PNF rates (<4%) [18]. Unlike other studies, our cohort showed no differences regarding early clinical events (early patient mortality, PNF, DGF and vascular complications) or graft function between groups. Moreover, CIT in the elderly group (DBDs and cDCDs) was not a risk factor for DGF or graft survival. Nor did thymoglobulin induction have any effect on DGF, considering that in our clinical practice cDCD recipients receive tacrolimus starting the first day after transplantation and therefore thymoglobulin is only used in highly sensitized recipients.
Finally, we noticed that our elderly cDCDs were younger and less likely to have died of a stroke compared with elderly DBD-KTs. This suggests an unintended organ selection in the process of evaluating supposedly ‘higher risk’ donor organs for transplantation, similar to what has been observed in other studies [30]. In fact, we observed a slightly better kidney function at 36 months in elderly cDCDs compared to elderly DBDs, probably due to the difference in age and a greater Remuzzi score plus increased prevalence of AR in the elderly DBD group. In contrast, elderly cDCD recipients tended to be more comorbid: 40% of them had diabetes and 25% had previous cardiovascular events. This highlights the importance of careful recipient selection in order to avoid early patient mortality, which in our elderly cDCD cohort reached up to 13%. Although patient age was the only determinant of patient survival, the small number of events precluded multivariate assessment of early patient mortality.
Understanding the outcomes from KTs using kidneys from more marginal donors is a key to evaluating whether and how these kidneys should be used. This study suggests that elderly cDCD kidneys, when carefully selected, may be an appropriate strategy to increase the deceased donor pool. In line with other authors, we believe that the same criteria should be used to evaluate both elderly cDCDs and elderly DBDs [6, 17, 18, 30, 35].
The main limitations of the study are the small sample size and the relatively short-term follow-up. Low event rates precluded multivariate analysis of early patient survival or PNF. In addition, even with an appreciable sample size, the small number of graft losses could reduce the statistical power of the multivariate survival analysis. Moreover, this was a retrospective study and some relevant clinical information might be limited, such as organ extraction and preservation methods. However, because the cDCD KT programme began in our centre in 2013 and recipient age is increasing, we aimed to compare early and medium-term results from elderly cDCD versus DBD grafts. In fact, this is the first study that compares outcomes between these two cohorts in Spain.
In conclusion, donors ≥65 years of age represented more than half of our global cDCD cohort. KTs from elderly cDCDs had similar rates of DGF, PNF and vascular complications compared with young cDCD-KTs and elderly DBD-KTs. Short- and medium-term graft survival from elderly cDCD kidneys are excellent and comparable to those from young cDCD and elderly DBD kidneys. Although recipients from cDCDs ≥65 years of age showed lower 3-year patient survival, recipient age was the only determinant of patient survival without any influence of donor characteristics. Although careful pre-transplant evaluation should be performed for outcome improvement, these findings suggest that the use of kidneys from old cDCDs may be an appropriate strategy to expand the donor pool. Longer-term follow-up studies with larger sample sizes are needed in order to determine the long-term outcomes of these grafts and define which recipients might benefit the most.
ACKNOWLEDGEMENTS
We thank Adriana Sierra, Alfonso Califano, Sheila Bermejo, Ana Marina Granados, Anna Faura, Sara Alvarez, Maria Vera and Montserrat Folgueiras for their contribution to the development of the TRASMAR database. We thank Rosa Causadias and Aurora Sanchez for their technical help.
FUNDING
This study was performed with funding from projects PI16/00619 (Spanish Ministry of Health ISCIII FIS-FEDER) and RD16/0009/0013 (ISCIII FEDER REDinREN).
AUTHORS’ CONTRIBUTIONS
A.B. and G.V. participated in acquisition of data. A.B., C.A.C., J.P. and M.J.P.S. designed the study and performed the analysis and validation of the data. M.J.P.S., D.R.P., A.Z., C.B., M.M., M.C. and J.P. participated in critical revision of the manuscript for important intellectual content. A.B., C.A.C., M.J.P.S., D.R.P. and J.P. participated in preparation of the manuscript. A.B. was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.Organización Nacional de Trasplantes. Balance de Actividad de Donación y Trasplante Renal. 2018http://wwwontes/infesp/Memorias/Actividad de Donación%20y%20Trasplan te%20Renalpdf (18 December 2019, date last accessed)
- 2.Matesanz R, Coll E, Domíngez-Gi B et al. Donación en Asistolia en España: Situación actual y Recomendaciones. Documento de Consenso. 2012 http://wwwontes/infesp/Docum entosDeConsenso/DONACIÓN%20EN%20ASISTOLIA%20EN% 20ESPAÑA%20SITUACIÓN%20ACTUAL%20Y%20RECOMEND ACIONESpdf (3 December 2019, date last accessed )
- 3. Summers DM, Watson CJ, Pettigrew GJ. et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 2015; 88: 241–249 [DOI] [PubMed] [Google Scholar]
- 4. Snoeijs MG, Winkens B, Heemskerk MB. et al. Kidney transplantation from donors after cardiac death: a 25-year experience. Transplantation 2010; 90: 1106–1112 [DOI] [PubMed] [Google Scholar]
- 5. Summers DM, Johnson RJ, Allen J, Fuggle SV. et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010; 376: 1303–1311 [DOI] [PubMed] [Google Scholar]
- 6. Summers DM, Johnson RJ, Hudson A. et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 2013; 381: 727–734 [DOI] [PubMed] [Google Scholar]
- 7. Kokkinos C, Antcliffe D, Nanidis T. et al. Outcome of kidney transplantation from nonheart-beating versus heart-beating cadaveric donors. Transplantation 2007; 83: 1193–1199 [DOI] [PubMed] [Google Scholar]
- 8. Rao PS, Ojo A.. The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol 2009; 4: 1827–1831 [DOI] [PubMed] [Google Scholar]
- 9. McDonald S, Clayton P.. DCD ECD kidneys—can you make a silk purse from a sow’s ear? Am J Transplant 2013; 13: 249–250 [DOI] [PubMed] [Google Scholar]
- 10. Lloveras J, Arcos E, Comas J. et al. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years. Transplantation 2015; 99: 991–996 [DOI] [PubMed] [Google Scholar]
- 11. Perez-Saez MJ, Arcos E, Comas J. et al. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis. Am J Transplant 2016; 16: 2724–2733 [DOI] [PubMed] [Google Scholar]
- 12. Arcos E, Pérez-Sáez M, Comas J. et al. Assessing the limits in kidney transplantation: use of extremely elderly donors and outcomes in elderly recipients. Transplantation 2020; 104: 176–183 [DOI] [PubMed] [Google Scholar]
- 13. Ojo A, Hanson J, Meier-Kriesche H. et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 2001; 12: 589–597 [DOI] [PubMed] [Google Scholar]
- 14. Perez-Saez MJ, Montero N, Redondo-Pachon D. et al. Strategies for an expanded use of kidneys from elderly donors. Transplantation 2017; 101: 727–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mirshekar-Syahkal B, Summers D, Bradbury LL. et al. Local expansion of donation after circulatory death kidney transplant activity improves waitlisted outcomes and addresses inequities of access to transplantation. Am J Transplant 2017; 17: 390–400 [DOI] [PubMed] [Google Scholar]
- 16. Peters-Sengers H, Berger SP, Heemskerk MBA. et al. Stretching the limits of renal transplantation in elderly recipients of grafts from elderly deceased donors. J Am Soc Nephrol 2017; 28: 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagaraja P, Roberts GW, Stephens M. et al. Impact of expanded criteria variables on outcomes of kidney transplantation from donors after cardiac death. Transplantation 2015; 99: 226–231 [DOI] [PubMed] [Google Scholar]
- 18. Perez-Saez MJ, Lafuente Covarrubias O. et al. Early outcomes of kidney transplantation from elderly donors after circulatory death (GEODAS study). BMC Nephrol 2019; 20: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catalan Renal Registry. Statistical Report. Barcelona, Spain: Catalan Transplant Organization, Health Department 2019. 2018
- 20.Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting the Kidney Donor Profle Index (KDPI). https://optn.transplant.hrsa.gov/resources/guidance/kidney-donor-profile-index-kdpi-guide-for-clinicians/ (18 December 2019, date last accessed)
- 21. Haas M, Sis B, Racusen LC. et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14: 272–283 [DOI] [PubMed] [Google Scholar]
- 22. Haas M, Loupy A, Lefaucheur C. et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018; 18: 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arias-Cabrales C, Pérez-Sáez MJ, Redondo-Pachón D. et al. Usefulness of the KDPI in Spain: a comparison with donor age and definition of standard/expanded criteria donor. Nefrología 2018; 38: 503–513 [DOI] [PubMed] [Google Scholar]
- 24. Domínguez-Gil B. International Figures on Donation and Transplantation—2018. Newsletter Transplant. 2019: 24
- 25. Chang GJ, Mahanty HD, Ascher NL. et al. Expanding the donor pool: can the Spanish model work in the United States? Am J Transplant 2003; 3: 1259–1263 [DOI] [PubMed] [Google Scholar]
- 26. Portolés JM, Pérez-Sáez MJ, López-Sánchez P. et al. Trasplante renal con órganos procedentes de donación tras parada circulatoria controlada: resultados del estudio multicéntrico GEODAS-3. Nefrología 2019; 39: 151–159 [DOI] [PubMed] [Google Scholar]
- 27. Gill J, Rose C, Lesage J. et al. Use and outcomes of kidneys from donation after circulatory death donors in the United States. J Am Soc Nephrol 2017; 28: 3647–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh SK, Kim SJ.. Epidemiology of kidney discard from expanded criteria donors undergoing donation after circulatory death. Clin J Am Soc Nephrol 2016; 11: 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Locke JE, Segev DL, Warren DS. et al. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant 2007; 7: 1797–1807 [DOI] [PubMed] [Google Scholar]
- 30. Singh SK, Kim SJ.. Does expanded criteria donor status modify the outcomes of kidney transplantation from donors after cardiac death? Am J Transplant 2013; 13: 329–336 [DOI] [PubMed] [Google Scholar]
- 31. Favi E, Puliatti C, Iesari S. et al. Impact of donor age on clinical outcomes of primary single kidney transplantation from Maastricht category-III donors after circulatory death. Transplant Direct 2018; 4: e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomita Y, Tojimbara T, Iwadoh K. et al. Long-term outcomes in kidney transplantation from expanded-criteria donors after circulatory death. Transplant Proc 2017; 49: 45–48 [DOI] [PubMed] [Google Scholar]
- 33. Saidi RF, Elias N, Kawai T. et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant 2007; 7: 2769–2774 [DOI] [PubMed] [Google Scholar]
- 34. Doshi MD, Hunsicker LG.. Short- and long-term outcomes with the use of kidneys and livers donated after cardiac death. Am J Transplant 2007; 7: 122–129 [DOI] [PubMed] [Google Scholar]
- 35. Khalid U, Jameel M, Sabah T. et al. Older donation after circulatory death kidneys for older recipients: a single-center experience. Transplant Proc 2019; 51: 701–706 [DOI] [PubMed] [Google Scholar]
- 36. Watson CJ, Johnson RJ, Birch R. et al. A simplified donor risk index for predicting outcome after deceased donor kidney transplantation. Transplantation 2012; 93: 314–318 [DOI] [PubMed] [Google Scholar]
- 37. Moreso F, Seron D, Gil-Vernet S. et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant 1999; 14: 930–935 [DOI] [PubMed] [Google Scholar]