Abstract
Background
Sclerostin and Dickkopf-related protein-1 (Dkk-1) proteins are inhibitors of the canonical Wnt/β-catenin bone pathway. Sclerostin but not Dkk-1 is associated with increased arterial stiffness. This study examined the prognostic significance of sclerostin and Dkk-1 levels for cardiovascular outcomes and mortality in haemodialysis (HD) patients.
Methods
Serum sclerostin and Dkk-1 levels were measured with enzyme-linked immunosorbent assay in 80 HD patients that were followed-up for a median of 45 months. Factors that could interfere with the association of sclerostin and Dkk-1 with outcomes [including carotid–femoral pulse wave velocity (PWV), parathyroid hormone (PTH), calcium–phosphate product and others] were assessed at baseline. The primary endpoint was a combination of all-cause death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, hospitalization for decompensated heart failure and new-onset atrial fibrillation. Secondary endpoints included cardiovascular and all-cause mortality.
Results
Cumulative freedom from the primary endpoint was significantly lower for higher tertiles of sclerostin (77.8, 69.2 and 40.7%; Tertiles 1–3, respectively; log-rank P = 0.004). The risk for the primary outcome gradually increased for higher sclerostin tertiles [Tertile 3: hazard ratio (HR) = 3.847, 95% confidence interval (CI) 1.502–9.851]. No significant association was evident between sclerostin and all-cause mortality, whereas higher sclerostin levels presented a trend towards higher risk for cardiovascular mortality. Dkk-1 levels exhibited no association with the risk of the primary or secondary endpoints. In stepwise Cox regression modelled analysis, sclerostin levels were associated with the primary outcome, independently of PTH, calcium–phosphate product, serum albumin, C-reactive protein and PWV levels (HR = 2.921, 95% CI 1.401–6.090; P = 0.004).
Conclusions
High sclerostin levels are associated with lower cumulative freedom and higher risk for a composite endpoint of cardiovascular events and mortality. Dkk-1 exhibited no association with the future risk of adverse outcomes.
Keywords: arterial stiffness, cardiovascular events, haemodialysis, mortality, pulse wave velocity, sclerostin
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality in patients with end-stage renal disease (ESRD) undergoing haemodialysis (HD), accounting for >50% of deaths with known causes [1]. Increased rates of cardiovascular events can only partially be explained by traditional risk factors, such as age, hypertension, diabetes and others, in these patients and several other factors related to kidney failure and the chronic inflammatory state of ‘uraemia’ may be involved [2]. Premature arterial stiffening, mainly due to disturbed calcium–phosphate homeostasis, is an important mediator of these associations [2, 3]. Pulse wave velocity (PWV), the optimal measure of arterial stiffness, is an established independent determinant of cardiovascular events and mortality in HD patients [4].
Sclerostin is a glycoprotein secreted by osteocytes, which binds to transmembrane receptors of osteoblasts low-density lipoprotein receptor-related proteins 5 and 6 (LPR5/6) and inhibits the Wnt/β-catenin signalling pathway, suppressing osteoblast proliferation, maturation, differentiation and bone formation [5]. Sclerostin levels gradually increase with renal function deterioration and are significantly associated with bone biomarkers [intact parathyroid hormone (iPTH), fibroblast growth factor-23 and bone-specific alkaline phosphatase] within the frame of the chronic kidney disease-mineral bone disorder (CKD-MBD) [6, 7]. In parallel, sclerostin participates in the pathogenesis of extraskeletal calcification with potential impact on arteriosclerotic vascular damage, as its expression is upregulated by osteoblast-like cells in calcified vessels [8]. The mechanistic background of this process is controversial; some studies suggest that sclerostin has a potential defensive role, whereas others suggest the opposite [9]. Dickkopf-related protein-1 (Dkk-1) also interacts with the complex of receptors LPR5/6 and inhibits the Wnt signalling pathway of the osteoblasts, thereby regulating bone cell differentiation; Dkk-1 is also produced by other cell types, such as vascular smooth muscle cells (VSMCs), platelets and endothelial cells [5, 10], whereas pilot studies suggest an association of Dkk-1 with ischaemic heart and cerebrovascular disease and coronary artery calcification [11, 12]. In a previous cross-sectional study, we observed that serum sclerostin levels were associated with PWV independently of routine markers of CKD-MBD in HD patients, whereas in contrast, Dkk-1 exhibited no association with arterial stiffness [13]. Over the past years, a few studies with relatively small samples and short follow-up periods evaluated the association between sclerostin and outcomes in HD patients, producing highly controversial results. A recent meta-analysis included nine studies on the association of sclerostin levels with cardiovascular events and cardiovascular or all-cause mortality [14], but could not perform a combined analysis of all studies due to significant heterogeneity; pooling of three relevant studies suggested that sclerostin was not associated with all-cause mortality, and of two studies that it was not associated with cardiovascular mortality [14]. Furthermore, no study so far has examined the association of Dkk-1 with outcomes in HD. Therefore, the present study aimed to examine the long-term prognostic significance of serum sclerostin and Dkk-1 protein levels for major cardiovascular outcomes, cardiovascular and all-cause mortality in patients undergoing HD.
MATERIALS AND METHODS
Study population
This was a prospective cohort study including 80 patients undergoing HD in two affiliated HD centres in Northern Greece. We included patients on a standard thrice-weekly HD schedule for ≥3 months, who provided informed written consent. Exclusion criteria were (i) chronic atrial fibrillation (AF) or other cardiac arrhythmias; (ii) non-functional arteriovenous fistula in the contralateral brachial arm area of the one used for vascular access that could interfere with the PWV measurement; (iii) dry weight and antihypertensive treatment modification during 1 month prior to study enrolment; (iv) Stages III and IV congestive heart failure, according to the New York Heart Association classification; (v) severe peripheral occlusive arterial disease; (vi) history of acute myocardial infarction (MI) or stroke 3 months before the beginning of the study; and (vii) history of parathyroidectomy, active malignancy, infection, treatment with antibiotics or immunosuppressive agents at the time of the study. The study protocol was approved by the Ethics Committee of School of Medicine, Aristotle University of Thessaloniki, and all protocol procedures were conducted in accordance with the Declaration of Helsinki (2008 Amendment).
Study procedures and data collection
Baseline evaluations were performed between May 2014 and February 2016, during a mid-week non-dialysis day and included recording of demographics, anthropometric characteristics, cause of ESRD, comorbidities, concomitant medications, routine laboratory tests and dialysis-related parameters. All subjects were instructed to refrain from smoking, heavy exercise, caffeine and alcohol consumption for at least 2 h before the examination. All measurements were performed by a single trained physician in the non-fistula arm in a quiet room with stable air temperature (∼22°C) after at least 10 min of rest and with patient in the supine position. Details of the baseline evaluation can be found in a previous report on the cross-sectional evaluations of sclerostin and Dkk-1 with arterial stiffness [13].
Study participants underwent assessment of arterial stiffness and arterial wave parameters with the Sphygmocor® device (AtCor, Sydney, Australia). Measurements of peripheral blood pressure (BP) for the calibration of the aortic pulse waveforms were performed in the brachial artery-level in the non-fistula or the non-dominant arm with a validated oscillometric device and a cuff of appropriate size. Each patient’s systolic BP (SBP) and diastolic BP (DBP) were the mean value of three consecutive measurements within 5 min [15]. Pulse pressure (SBP – DBP) and mean arterial pressure [(SBP + 2 × DBP)/3] were estimated. Carotid–femoral aortic PWV was measured by pulse waveforms recordings at the carotid and femoral arteries, as described elsewhere [16, 17]. Pulse waveforms were referenced to a concurrently recorded electrocardiogram, and pulse wave transit time between the subsequent recording sites was calculated from the Sphygmocor® software, according to the foot-to-foot time difference between carotid/femoral waveforms. Pulse waveforms were recorded over 10 consecutive heartbeats to cover a complete respiratory cycle. The device’s quality control criteria for optimal pressure waveform recording were applied; the first valid recording was used in the subsequent analysis.
Blood samples for each participant were drawn from a peripheral vein under fasting conditions in the morning of a midweek routine dialysis session. Serum samples were separated from clotted blood by immediate centrifugation (1500g for 10 min) and then aliquoted and stored at −70°C until the measurement. Serum levels of sclerostin and Dkk-1 were measured by an enzyme-linked immunosorbent assay (ELISA) using commercially available kits [human SOST Quantakine, human Dkk-1 Quantakine, Bio-techne (R&D Systems), detection limit 1.74 and 4.2 pg/mL, respectively]. iPTH levels were measured by radioimmunoassay. Serum C-reactive protein (CRP) levels were measured by nephelometry. Routine laboratory parameters were measured with an automated analyser (Olympus AU560, Hamburg, Germany). Levels of albumin were time-averaged for the last 6 months before inclusion in the study.
Study endpoints
All studied outcomes were prospectively recorded, and study patients were censored on the date of the first occurrence of the endpoints under study or on 30 June 2019. The primary endpoint was a combination of any of all-cause death, non-fatal MI, non-fatal stroke, coronary revascularization procedure, hospitalization for decompensated heart failure or new-onset AF at their first occurrence. Secondary endpoints included (i) all-cause mortality and (ii) cardiovascular mortality defined as fatal MI (death by any cardiovascular mechanism within 30 days after an MI related to the immediate consequences of the MI) or fatal stroke (death within 30 days after a stroke that is either a direct consequence of the stroke or a complication of the stroke) or sudden death.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences version 23.0 (SPSS Inc, Chicago, IL, USA). Values of P < 0.05 (two-tailed) were considered statistically significant for all comparisons. The Shapiro–Wilk or the Kolmogorov–Smirnov test was applied to examine the normality of distribution for continuous variables. Quantitative variables are presented as mean ± standard deviation or median with interquartile range according to the normality of distribution, and qualitative variables as absolute frequencies and percentages [n (%)]. To compare differences in occurrence of study endpoints among the different levels of each studied parameter, data were categorized in ascending order into tertiles of patients. Kaplan–Meier curves were created, and the log-rank test was applied to compare the differences among the tertiles in the occurrence or freedom from the studied endpoints during follow-up. The future risk for the occurrence of outcomes was evaluated with Cox regression analysis. In addition, the impact of various factors, such as age PTH, calcium–phosphate product, albumin, CRP and PWV levels, which could possibly interfere with the association between high sclerostin (higher tertile >263.50 pg/mL cut-off point) and the primary outcome, was evaluated with stepwise Cox regression modelled analysis (enter method). We report hazard ratios (HRs) with 95% confidence intervals (CIs).
RESULTS
Baseline characteristics and study outcomes
Table 1 depicts demographic characteristics, routine laboratory data, dialysis-related parameters and drug treatment of study participants. A total of 45 men and 35 women with a mean age of 60.91 ± 13.67 years, being on standard HD therapy for a median of 36.50 (54.75) months, were followed-up for a median 45.17 (14.83) months. At baseline, 71.3% of patients had hypertension, 21.3% had diabetes mellitus and 22.5% had a history of CVD, while 20% were smokers. Table 2 depicts the frequencies of outcomes under study. During follow-up, the primary endpoint occurred in 30 (37.5%) patients. A total of 21 (26.3%) of the participants died, 11 (13.8%) due to cardiovascular and 10 (12.5%) due to non-cardiovascular causes. Moreover, three (3.8%) patients had a non-fatal MI, three (3.8%) had a non-fatal stroke, three (3.8%) underwent coronary revascularization procedure, six (7.5%) patients were hospitalized for decompensated heart failure and nine (11.3%) for new-onset AF.
Table 1.
Baseline demographic, anthropometric, clinical and routine laboratory characteristics of the study participants
| Baseline characteristics | Total population, n = 80 |
|---|---|
| Male, n (%) | 45 (56.3) |
| Age, years | 60.91 ± 13.67 |
| Weight, kg | 71.17 ± 14.25 |
| ΒΜΙ, kg/m2 | 25.31 ± 4.62 |
| Dialysis vintage, months | 36.50 (54.75) |
| Hypertension, n (%) | 57 (71.3) |
| Diabetes, n (%) | 17 (21.3) |
| CVD history, n (%) | 18 (22.5) |
| Smokers, n (%) | 16 (20) |
| SBP, mmHg | 136.29 ± 19.11 |
| DBP, mmHg | 79.49 (17.00) |
| PWV, m/s | 9.50 (4.00) |
| Sclerostin | 201.00 (170.41) |
| Dkk-1 | 401.40 (248.13) |
| Kt/V | 1.49 ± 0.31 |
| Dialysis modality, n (%) | |
| Standard HD | 53 (66.3) |
| Haemodiafiltration | 37 (33.7) |
| Haemoglobin, g/dL | 11.50 (0.80) |
| Creatinine, mg/dL | 7.61 ± 2.12 |
| Urea, mg/dL | 130.00 (29.75) |
| Albumin, g/dL | 4.06 ± 0.34 |
| Cholesterol, mg/dL | 153.28 ± 33.63 |
| Triglycerides, mg/dL | 115.50 (72.00) |
| LDL-cholesterol, mg/dL | 86.25 ± 33.46 |
| HDL-cholesterol, mg/dL | 40.00 (14.75) |
| Serum calcium, mg/dL | 8.88 ± 0.56 |
| Serum phosphate, mg/dL | 4.79 ± 1.12 |
| Ca×P, mg2/dL2 | 42.62 ± 10.60 |
| PTH, pmol/L | 323.00 (213.50) |
| Sclerostin, pg/mL | 201.00 (170.41) |
| Dkk-1, pg/mL | 339.25 (244.06) |
| Alkaline phosphatase, U/L | 153.50 (118.75) |
| CRP, mg/L | 3.49 (1.46) |
Quantitative variables are presented as mean ± SD or median with interquartile range according to the normality of distribution, and qualitative variables as absolute frequencies and percentages [n (%)]; BMI, body mass index; Ca×P, calcium × phosphorus product; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Hypertension is defined as predialysis SBP ≥140 mmHg or DBP ≥90 mmHg or use of antihypertensive drugs. Smoker status is defined as regular tobacco use or smoking cessation within the previous year. CVD history includes the coronary artery disease, ischaemic or haemorrhagic stroke or peripheral occlusive arterial disease defined as the presence of aortic aneurysm or intermittent claudication or previous peripheral angioplasty.
Table 2.
Outcomes of interest and study end points during follow-up in the total population
| Parameter | Value, n (%) |
|---|---|
| Non-fatal MI | 3 (3.8) |
| Non-fatal stroke | 3 (3.8) |
| Coronary revascularization procedure | 3 (3.8) |
| Hospitalization for acute decompensated heart failure | 6 (7.5) |
| AF | 9 (11.3) |
| Cardiovascular death | 11 (13.8) |
| Non-cardiovascular death | 10 (12.5) |
| All-cause death | 21 (26.3) |
| All-cause death or non-fatal MI or non-fatal stroke | 24 (30.0) |
| Cardiovascular death or non-fatal MI or non-fatal stroke | 14 (17.5) |
| Cardiovascular death or non-fatal MI or non-fatal stroke or coronary revascularization or hospitalization for heart failure or AF | 20 (25) |
| All cause death or non-fatal MI or non-fatal stroke or coronary revascularization or hospitalization for heart failure or AF | 30 (37.5) |
Association of serum sclerostin with the primary and secondary endpoints
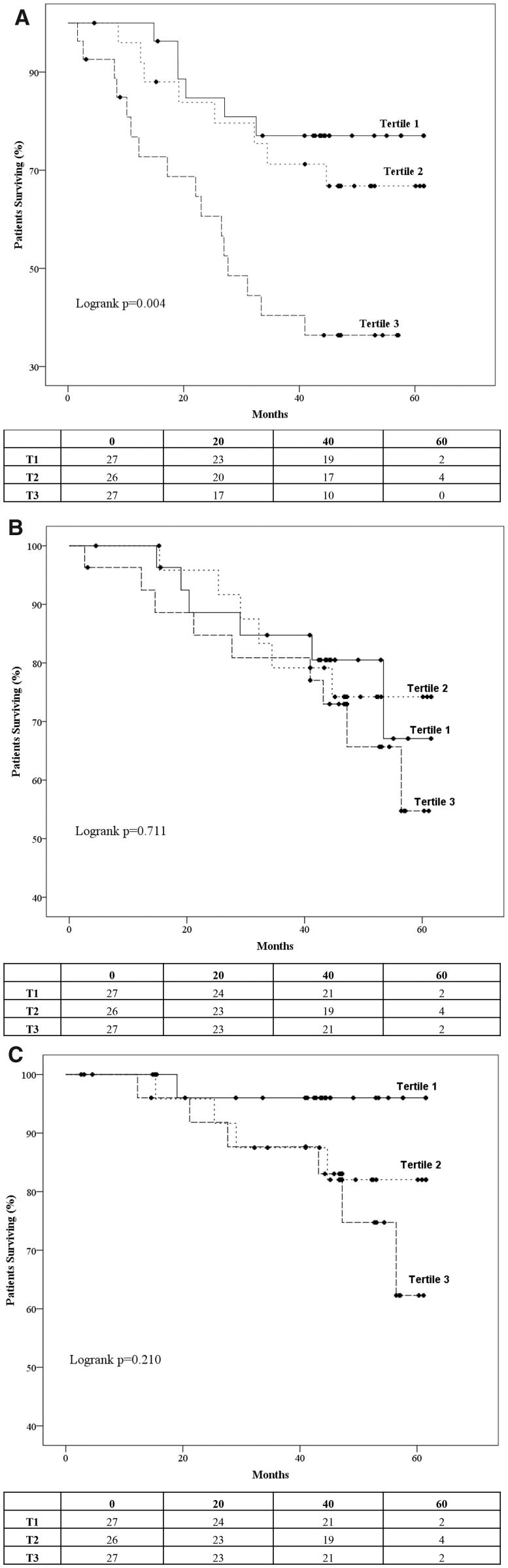
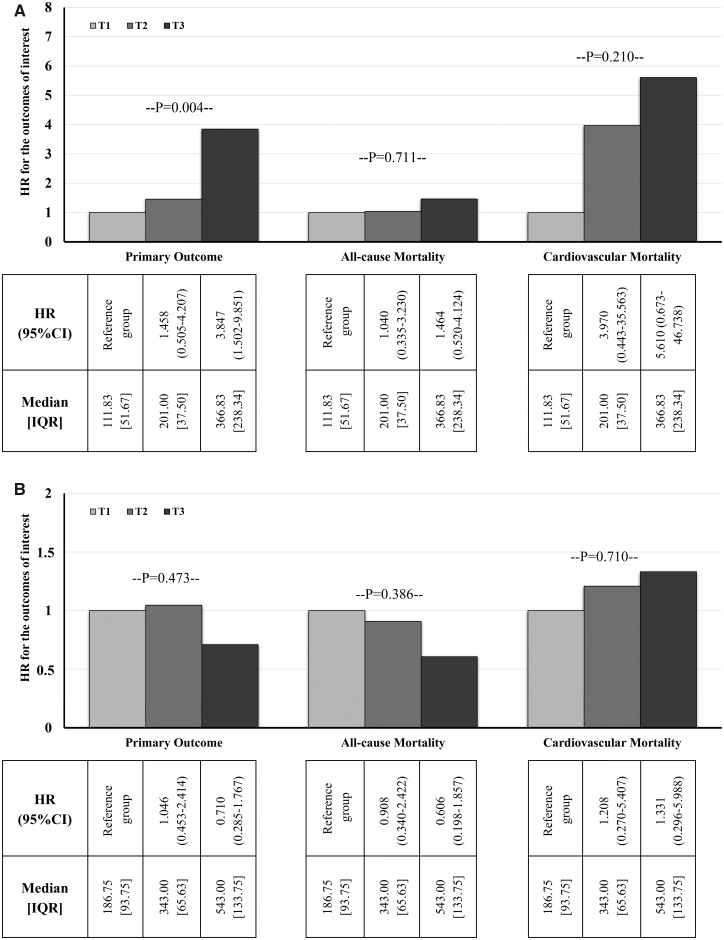
Figure 1 depicts the Kaplan–Meier curves and the life tables for the occurrence of the primary and the secondary endpoints in the tertiles of sclerostin levels. Cumulative freedom from the primary endpoint was significantly lower for higher tertiles of sclerostin (77.8, 69.2 and 40.7% for Tertiles 1–3, respectively; log-rank P = 0.004; Figure 1A). Cumulative survival was similar for the tertiles of sclerostin (77.8, 76.9 and 66.7% for Tertiles 1–3, respectively; log-rank P = 0.711; Figure 1B), whereas cumulative freedom from cardiovascular mortality was slightly lower for higher tertiles of sclerostin but without significant differences between them (96.3, 84.6 and 77.8% for Tertiles 1–3, respectively; log-rank P = 0.210; Figure 1C). The corresponding HRs of the primary and secondary endpoints for tertiles of sclerostin levels are presented in Figure 2A. As depicted in the figure, future risk of the composite primary endpoint was gradually increasing for higher tertiles of sclerostin (Tertile 3: HR = 3.847, 95% CI 1.502–9.851). Future risk of death was no different among the tertiles sclerostin (Tertile 3: HR = 1.464, 95% CI 0.520–4.124), and cardiovascular mortality risk was insignificantly higher for higher sclerostin values (Tertile 3: HR = 5.610, 95% CI 0.673–46.738).
FIGURE 1.
Kaplan–Meier survival curves and life tables for the occurrence of the (A) primary endpoints (all-cause death, or non-fatal MI or non-fatal stroke or coronary revascularization or hospitalization for heart failure or AF) and the secondary endpoints, (B) all-cause mortality and (C) cardiovascular mortality in the tertiles of sclerostin levels.
FIGURE 2.
(A) HRs of the primary and secondary outcomes for tertiles of serum sclerostin. (B) HRs of the primary and secondary outcomes for tertiles of serum Dkk-1 protein. IQR, interquartile range; T, tertile.
Association of serum Dkk-1 protein with the primary and secondary endpoints
Regarding the association of Dkk-1 protein with outcomes, cumulative freedom from the primary endpoint was similar for tertiles of Dkk-1 (59.3, 57.7 and 70.4% for Tertiles 1–3, respectively; log-rank P = 0.473). Similarly, cumulative survival (70.4, 69.2 and 81.5% for Tertiles 1–3, respectively; log-rank P = 0.386) and cumulative freedom from cardiovascular mortality (88.9, 84.6 and 85.2% for Tertiles 1–3, respectively; log-rank P = 0.710) were similar for the tertiles of Dkk-1. Future risks for the primary endpoint (Tertile 3: HR = 0.710, 95% CI 0.285–1.767), overall mortality (Tertile 3: HR = 0.606, 95% CI 0.198–1.857) and cardiovascular mortality (Tertile 3: HR = 1.331, 95% CI 0.296–5.988) were similar between the tertiles of baseline serum Dkk-1 protein, as shown in Figure 2B.
Determinants of the association between serum sclerostin levels and the risk of the primary outcome levels
In Table 3, we present the stepwise Cox regression modelled analysis that was performed to elucidate the effect of possible confounders on the association between high sclerostin levels (Tertile 3; >263.5 pg/mL cut-off point) and the occurrence of the primary endpoint. High sclerostin levels were significantly associated with higher risk for the primary outcome in adjusted analysis (HR = 3.156, 95% CI 1.537–6.482; P = 0.002). This association was significant after stepwise adjustment for PTH (HR = 3.155, 95% CI 1.535–6.486; P = 0.002) and calcium–phosphate product levels (HR = 3.072, 95% CI 1.490–6.336; P = 0.002). Additional adjustment for serum albumin and CRP (HR = 3.052, 95% CI 1.452–6.415; P = 0.003) and then for PWV levels (HR = 2.921, 95% CI 1.401–6.090; P = 0.004) did not affect the degree or the significance of this association. As presented in the table, the association between sclerostin levels and the primary outcome was rendered insignificant only after additional adjustment for the age of study participants (HR = 1.799, 95% CI 0.839–3.856; P = 0.131).
Table 3.
Stepwise Cox regression modelled analysis for associations between high sclerostin levels (Tertile 3; >263.5 pg/mL cut-off point) and the occurrence of the primary endpoint (all cause death, or non-fatal MI or non-fatal stroke or coronary revascularization or hospitalization for heart failure or resuscitation after cardiac arrest, or AF)
| HR (95% CIs) for the high sclerostin group | P-value | |
|---|---|---|
| Model 1 | 3.156 (1.537–6.482) | 0.002 |
| Model 2 | 3.155 (1.535–6.486) | 0.002 |
| Model 3 | 3.072 (1.490–6.336) | 0.002 |
| Model 4 | 3.052 (1.452–6.415) | 0.003 |
| Model 5 | 2.921 (1.401–6.090) | 0.004 |
| Model 6 | 1.799 (0.839–3.856) | 0.131 |
Model 1: unadjusted; Model 2: adjusted for PTH; Model 3: adjusted for PTH and Ca×P; Model 4: adjusted for PTH, Ca×P, albumin and CRP; Model 5: adjusted for PTH, Ca×P, albumin, CRP and PWV; and Model 6: adjusted for PTH, Ca×P, albumin, CRP, PWV and age.
Ca×P, calcium × phosphorus product.
DISCUSSION
This prospective cohort study is the first to examine simultaneously the associations of serum sclerostin and Dkk-1 protein levels with cardiovascular outcomes and all-cause mortality in HD patients. We found that higher tertiles of serum sclerostin levels were associated with lower cumulative freedom from and higher future risk of the composite primary endpoint. No significant association was evident between high sclerostin levels and all-cause mortality, whereas higher tertiles of serum sclerostin levels presented insignificantly higher risk for cardiovascular mortality. Dkk-1 levels exhibited no association with the future risks of the primary outcome or secondary outcomes. In a stepwise Cox regression modelled analysis, sclerostin levels were significantly associated with the primary outcome, independently of PTH, calcium–phosphate product, serum albumin, CRP and PWV levels; this association rendered insignificant when age was added to the model.
Arterial stiffness is possibly the main factor leading to increased cardiovascular risk in HD patients [4]. The arteries of these individuals present specific remodelling and functional alterations, which include increased arterial diameter and intima-media thickness, reduced wall-to-lumen ratio, calcification of elastic lamellae, elastinolysis, increased collagen content and collagen cross-linking and rarefaction of VSMCs [18, 19]. Increased arterial stiffness results in opposition to left ventricular (LV) ejection and afterload increase, and thus in LV remodelling and failure, but also in transformation of cyclic high-flow and pressure oscillations in the aorta into continuous and low-pressure capillary flow, particularly in organs with decreased precapillary resistance, such as the brain [20]. The consequence of these pathophysiologic changes is high occurrence of LV hypertrophy, cerebrovascular events and cardiovascular death [2].
Available data suggest that Wnt/β-catenin pathway inhibitors, sclerostin and Dkk-1, could participate in the pathogenesis of extraskeletal calcification with potential impact on arteriosclerotic vascular damage and valvular calcification [21–23]. Sclerostin is a potent suppressor of bone formation by inhibiting osteoblast differentiation and inducing their apoptosis. In calcified vessels, sclerostin expression is upregulated by osteoblast-like cells as a potential defensive response against the progression of calcification. Circulating sclerostin increases progressively as renal function declines [6, 24]. We have previously shown that high sclerostin was significantly associated with increased PWV levels with the optimal measure of arterial stiffness in HD [13]. However, herein, the association between sclerostin and cardiovascular events remained significant after adjustment with PWV. Thus, whether sclerostin has a causal role in increased arterial stiffness, promotes cardiovascular events through other mechanisms (i.e. intima or valvular calcification) or represents a compensatory response to vascular calcification initiated by other mechanisms is currently not known [25]. On the other hand, Dkk-1 is involved in the development of focal intimal calcification in atherosclerotic disease [26], and in addition to osteogenic vascular cells, Dkk-1 is also expressed by endothelial cells, inflammatory cells and platelets, also within the atherosclerotic plaque. Thus, Dkk-1 may not have an important role in medial calcification, as also suggested by the lack of associations with PWV [13]. In our study, the absence of any association with Dkk-1 with outcomes suggests a less crucial role for this mediator in the pathogenesis of vascular damage.
Results from preliminary studies evaluating the association of sclerostin with outcomes are contradictory; the duration of follow-up may be an important factor affecting these associations [14]. The largest of these studies included 673 HD patients and included two different periods, a mid-term 18-month and a long-term 4-year period; high sclerostin was independently associated with lower risk for cardiovascular (HR = 0.29, 95% CI 0.13–0.62) and all-cause mortality (HR = 0.39, 95% CI 0.22–0.68) only during the shorter period [26]. Similarly, in a cohort study following 125 HD patients for 2 years, higher sclerostin was associated with slightly lower risk for cardiovascular events [27]. In a Japanese cohort, sclerostin levels were not associated with the risk for cardiovascular (HR = 1.16, 95% CI 0.42–3.34) or all-cause mortality (HR = 1.09, 95% CI 0.56–2.14; P = 0.7980) during 42 months [28]; this was also the case in another observational study in 164 HD patients with a mean follow-up of 2 years [29]. Results from another study in 97 HD patients and median follow-up of 27 months also suggested that sclerostin levels were not associated with the risk for fatal or non-fatal cardiovascular events [30].
In contrast, two smaller cohort studies with average follow-up periods of 10 [31] and 5 years [32] showed that serum sclerostin is associated with higher risk for all-cause mortality, whereas a study on 130 HD patients followed-up for 7 years showed that serum sclerostin was independently associated with cardiovascular mortality (P = 0.008) but not with mortality due to non-cardiovascular causes (P = 0.346) [33]. Another study, including 106 HD patients, showed that higher sclerostin levels (>84 pmol/L) were significantly associated with increased risk for cardiovascular but not for all-cause mortality (HR = 2.577, 95% CI 1.0002–10.207; P = 0.04) and mortality due to infection over a mean follow-up of 5 years [34]. A recent meta-analysis of nine relevant studies including 1788 HD patients did not manage to perform a pooled analysis due to differences in homogeneity, blood sampling (serum or plasma), method of sclerostin measurement and different outcome definitions. It suggested that sclerostin was not associated with all-cause mortality (HR = 1.01, 95% CI 0.99–1.03) based on three studies with 503 patients, or with cardiovascular mortality (HR = 1.03, 95% CI 0.99–1.07) based on only two studies with 412 patients [14].
Our study adds to current knowledge, showing that higher sclerostin levels increased the risk of the primary composite cardiovascular outcome but not all-cause mortality in this population. Although the follow-up period was rather long (median of about 4 years) and the basic observations were clear in terms of statistical significance, the main limitation of this study is the small sample size. This is related to one of the main strengths of the study, i.e. that we opted to include baseline evaluation of several possibly confounding factors, including PWV measurements using the gold standard method of tonometry, in order to be able to perform appropriate multiple regression analyses. Another limitation may be that the primary endpoint included multiple outcomes; future studies with larger samples may need to use more restricted primary endpoints, such as a combination of MI, stroke and cardiovascular death. Considering that sclerostin is a dialysable molecule and its levels decrease during HD [35], evaluation of its levels in a standardized way (before a midweek routine dialysis session) and use of a valid ELISA method are other strengths of the study. Most importantly, this is the first study to simultaneously assess the possible association of Dkk-1 with outcomes, showing a clear difference between the two molecules.
In conclusion, the present study showed that high serum sclerostin levels are associated with lower cumulative freedom and higher risk for the primary composite endpoint of cardiovascular events and mortality. When mortality was studied separately, no association with sclerostin levels was noted. Adjustment for several factors involved in the pathophysiology of CKD-MBD and/or particularly driving cardiovascular events and survival in this population did not affect the association between sclerostin and the primary endpoint, with the exception of age. These results suggest that sclerostin may be involved in the pathogenesis of vascular damage and lead to elevated cardiovascular risk. Whether this is mediated through increased arterial stiffness or other pathways, and whether novel agents targeting sclerostin, such as romosozumab [36], could potentially improve outcomes in HD patients are areas for future research.
FUNDING
This article was not supported by any source and represents an original effort of the authors.
CONFLICT OF INTEREST STATEMENT
All authors disclose that they do not have any financial or other relationships that might lead to a conflict of interest.
REFERENCES
- 1. Saran R, Robinson B, Abbott KC. et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgianos PI, Sarafidis PA, Lasaridis AN.. Arterial stiffness: a novel cardiovascular risk factor in kidney disease patients. Curr Vasc Pharmacol 2015; 13: 229–238 [DOI] [PubMed] [Google Scholar]
- 3. Memmos E, Sarafidis P, Pateinakis P. et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol 2019; 20: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarafidis PA, Loutradis C, Karpetas A. et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension 2017; 70: 148–157 [DOI] [PubMed] [Google Scholar]
- 5. Monroe DG, McGee-Lawrence ME, Oursler MJ. et al. Update on Wnt signaling in bone cell biology and bone disease. Gene 2012; 492: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelletier S, Dubourg L, Carlier MC. et al. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol 2013; 8: 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyses RM, Schiavi SC.. Sclerostin, osteocytes, and chronic kidney disease - mineral bone disorder. Semin Dial 2015; 28: 578–586 [DOI] [PubMed] [Google Scholar]
- 8. Moe SM, Chen NX.. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 2008; 19: 213–216 [DOI] [PubMed] [Google Scholar]
- 9. Mill C, George SJ.. Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc Res 2012; 95: 233–240 [DOI] [PubMed] [Google Scholar]
- 10. Ueland T, Otterdal K, Lekva T. et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Anterioscler Thromb Vasc Biol 2009; 29: 1228–1234 [DOI] [PubMed] [Google Scholar]
- 11. Goliasch G, Wiesbauer F, Kastl SP. et al. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 2012; 222: 251–256 [DOI] [PubMed] [Google Scholar]
- 12. Register TC, Hruska KA, Divers J. et al. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab 2013; 98: E60–E65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stavrinou E, Sarafidis PA, Koumaras C. et al. Increased sclerostin, but not Dickkopf-1 protein, is associated with elevated pulse wave velocity in hemodialysis subjects. Kidney Blood Press Res 2019; 44: 679–689 [DOI] [PubMed] [Google Scholar]
- 14. Kanbay M, Solak Y, Siriopol D. et al. Sclerostin, cardiovascular disease and mortality: a systematic review and meta-analysis. Int Urol Nephrol 2016; 48: 2029–2042 [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, Fagard R, Narkiewicz K. et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357 [DOI] [PubMed] [Google Scholar]
- 16. Sarafidis PA, Georgianos PI, Karpetas A. et al. Evaluation of a novel brachial cuff-based oscillometric method for estimating central systolic pressure in hemodialysis patients. Am J Nephrol 2014; 40: 242–250 [DOI] [PubMed] [Google Scholar]
- 17. Loutradis C, Papagianni A, Ekart R. et al. Excess volume removal following lung ultrasound evaluation decreases central blood pressure and pulse wave velocity in hemodialysis patients: a LUST sub-study. J Nephrol 2020. (In press) [DOI] [PubMed] [Google Scholar]
- 18. London GM. Mechanisms of arterial calcifications and consequences for cardiovascular function. Kidney Int Suppl (2011) 2013; 3: 442–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. London GM, Marchais SJ, Guerin AP. et al. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens 2004; 26: 689–699 [DOI] [PubMed] [Google Scholar]
- 20. Chirinos JA, Townsend RR.. Reducing arterial stiffness in CKD: revising the paradigms. Clin J Am Soc Nephrol 2015; 10: 547–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pickering TG, Hall JE, Appel LJ. et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111: 697–716 [DOI] [PubMed] [Google Scholar]
- 22. London GM. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif 2018; 45: 154–158 [DOI] [PubMed] [Google Scholar]
- 23. London GM, Safar ME, Pannier B.. Aortic aging in ESRD: structural, hemodynamic, and mortality implications. J Am Soc Nephrol 2016; 27: 1837–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asamiya Y, Tsuchiya K, Nitta K.. Role of sclerostin in the pathogenesis of chronic kidney disease-mineral bone disorder. Ren Replace Ther 2016; 2: 8 [Google Scholar]
- 25. Evrard S, Delanaye P, Kamel S. et al. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta 2015; 438: 401–414 [DOI] [PubMed] [Google Scholar]
- 26. Drechsler C, Evenepoel P, Vervloet MG. et al.; for the NECOSAD Study Group. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 2015; 30: 288–293 [DOI] [PubMed] [Google Scholar]
- 27. Yang CY, Chang ZF, Chau YP. et al. Circulating Wnt/beta-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol Dial Transplant 2015; 30: 1356–1363 [DOI] [PubMed] [Google Scholar]
- 28. Sato M, Hanafusa N, Kawaguchi H. et al. A prospective cohort study showing no association between serum sclerostin level and mortality in maintenance hemodialysis patients. Kidney Blood Press Res 2018; 43: 1023–1033 [DOI] [PubMed] [Google Scholar]
- 29. Delanaye P, Krzesinski JM, Warling X. et al. Clinical and biological determinants of sclerostin plasma concentration in hemodialysis patients. Nephron Clin Pract 2014; 128: 127–134 [DOI] [PubMed] [Google Scholar]
- 30. Kundakci Gelir G, Sengul S, Nergizoglu G. et al. Is sclerostin level associated with cardiovascular diseases in hemodialysis patients? Blood Purif 2018; 46: 118–125 [DOI] [PubMed] [Google Scholar]
- 31. Goncalves FL, Elias RM, dos Reis LM. et al. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol 2014; 15: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen A, Sun Y, Cui J. et al. Associations of sclerostin with carotid artery atherosclerosis and all-cause mortality in Chinese patients undergoing maintenance hemodialysis. BMC Nephrol 2018; 19: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novo-Rodriguez C, Garcia-Fontana B, Luna-Del Castillo JD. et al. Circulating levels of sclerostin are associated with cardiovascular mortality. PLoS One 2018; 13: e0199504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalousova M, Dusilova-Sulkova S, Kubena AA. et al. Sclerostin levels predict cardiovascular mortality in long-term hemodialysis patients: a prospective observational cohort study. Physiol Res 2019; 68: 547–558 [DOI] [PubMed] [Google Scholar]
- 35. Bielesz BO, Hempfing T, Kieweg H. et al. Sclerostin declines during hemodialysis and appears in dialysate. Blood Purif 2014; 38: 30–36 [DOI] [PubMed] [Google Scholar]
- 36. Tartaglione L, Pasquali M, Rotondi S. et al. Positioning novel biologicals in CKD-mineral and bone disorders. J Nephrol 2017; 30: 689–699 [DOI] [PubMed] [Google Scholar]