Abstract
Background
Bisphenol S (BPS) is a structural analogue of bisphenol A (BPA) that is found in the environment. BPS may accumulate in anuric patients due to decreased urinary excretion. The toxicity and health effects of BPS are poorly characterized.
Methods
A cross-over study was performed using polynephron (PN) or polysulphone (PS) dialysers for a short (1 week each, 14 patients) or long (3 months each, 20 patients) period on each dialyser. Plasma BPA, BPS and hippuric acid were assessed by SRM mass spectrometry (SRM-MS). The biological significance of the BPS concentrations found was explored in cultured kidney tubular cells.
Results
In haemodiafiltration (HDF) patients, plasma BPS was 10-fold higher than in healthy subjects (0.53 ± 0.52 versus 0.05 ± 0.01 ng/mL; P = 0.0015), while BPA levels were 35-fold higher (13.23 ± 14.65 versus 0.37 ± 0.12 ng/mL; P = 0.007). Plasma hippuric acid decreased after an HDF session, while BPS and BPA did not. After 3 months of HDF with the same membranes, the BPS concentration was 1.01 ± 0.87 ng/mL for PN users and 0.62 ± 0.21 ng/mL for PS users (P non-statistically significant). In vitro, BPS and BPA leaked from dialysers containing them. In cultured tubular cells, no biological impact (cytotoxicity, inflammatory and oxidative stress gene expression) was observed for BPS up to 200 µM, while BPA was toxic at concentrations ≥100 µM.
Conclusions
BPS may be released from dialysis membranes, and dialysis patients display high BPS concentrations. However, BPS concentrations are lower than BPA concentrations and no BPS toxicity was observed at concentrations found in patient plasma.
Keywords: bisphenol A, bisphenol S, chronic kidney disease, haemodialysis, haemodiafiltration, toxins, xenobiotics
INTRODUCTION
Bisphenol A (BPA) is a chemical component of polycarbonate plastics and epoxy resins used to manufacture consumer products such as coatings and packaging of food cans, which represent the main source of exposure, baby bottles and toys, dental materials, personal care products and paper products such as paper bills and cashier receipts [1, 2]. In recent years, many studies have demonstrated adverse effects of BPA on perinatal, childhood and adult health including diabetes, reproductive disorders, cardiovascular disease and kidney disease, among others [3, 4]. The health risk is particularly high for kidney patients due to the urinary excretion of BPA. In this regard, serum BPA levels are increased in haemodialysis (HD) patients [4, 5] and in 2015, the EU SCENIHR experts report on ‘The safety of the use of bisphenol A in medical devices’ recommended the elimination of BPA from medical materials used in dialysis patients [6]. As a result, in the 2000s, industries started replacing BPA with analogues such as bisphenol S (BPS) or bisphenol F. BPS is a structural analogue of BPA where the central quaternary carbon has been replaced by a sulphone and two hydroxyl groups, rendering it stronger in terms of resistance to acidity, heat and sunlight than BPA. Due to these similarities, it has replaced BPA for many uses [7]. In fact, an association has been described between the urinary excretion of BPS and BPA [8].
Exposure to BPS occurs on a daily basis through ingestion, inhalation and dermal contact, but urine concentrations are lower than for BPA, probably reflecting a lower industrial use as compared with BPA [9]. Although BPS metabolism is not fully understood, in in vitro (human cells) and in vivo studies in mice and zebrafish, BPS is metabolized mainly by Phase II reactions, resulting in BPS sulphate and BPS glucuronide. BPS excretion is mainly via the urine, as BPS glucuronide (∼97%), although BPS has also been found in faeces [10].
The possible toxicity and health effects of BPS are less well known than those of BPA. However, different in vitro and in vivo exposure studies suggest that BPS and its metabolites could act as endocrine disruptors, having weak oestrogenic receptor agonistic [7] or antiandrogenic activity [3, 11], and interfering with thyroid function [12]. BPS also decreased the viability of proliferating adipose stromal cells [13] and testosterone secretion by human testes [14, 15], and disturbed the developing nervous system in zebrafish larvae [16]. In addition, BPS exposure was associated with oxidative stress in cultured hepatocytes and erythrocytes and rats [7, 17, 18]. This may be related to the induction of mitochondrial dysfunction [19]. Genotoxic damage and mutagenesis have also been observed [20]. BPS may also stimulate liver glycogenolysis and/or gluconeogenesis [21] and contribute to the progression of breast cancer, as it induced epigenetic and transcriptional changes in the human breast cancer cell line MCF-7 [22, 23]. A 2015 review characterized BPS as an endocrine disruptor potentially having the same health hazards as BPA [3]. However, many of these studies have been criticized because BPS concentrations were orders of magnitude higher than those occurring in the environment [24]. In this regard, elevated urinary BPA was associated with oxidative stress markers, but this was not the case for urinary BPS [25]. In any case, little or nothing is known about BPS effects on humans. Specifically, little is known about circulating BPS concentrations in humans with chronic kidney disease (CKD), a condition in which not only is urinary BPS excretion compromised, but also patients may be exposed to BPS intravenously. BPS is a component of certain dialysis membranes, in which it forms a copolymer with BPA in the synthesis of polysulphone (PS), currently the most commonly used dialysis membrane. Additionally, BPS is also a major component in alternative dialysis membranes such as polyethersulphone, also known as polynephron (PN) or polyester polymer alloy . Thus, by analogy to BPA, we hypothesized that HD patients may be exposed parenterally to this compound during the dialysis session as a result of its release from dialysis membranes and that this, associated with anuria, may increase circulating BPS to potentially toxic levels in these patients. In this regard, BPS and BPA are partially hydrophobic and are usually bound to plasma proteins, making it more difficult to eliminate during dialysis.
We have now explored the circulating levels of BPS in end-stage kidney disease (ESRD) patients on dialysis and the impact of commonly used dialysis membranes. Additionally, we explored the potential toxicity of clinically relevant BPS concentrations and compared them with BPA toxicity.
MATERIALS AND METHODS
The study was approved by the IIS-Fundación Jiménez Díaz Ethics Committee and was performed in accordance with the Declaration of Helsinki and the European Union Clinical Trial Directive (2001/20/EC). Patients were enrolled after providing written informed consent.
Study design
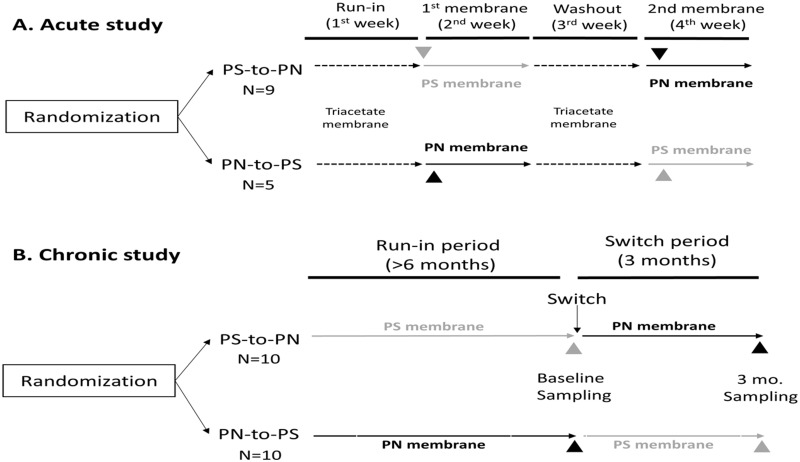
Two different experimental designs were explored. The acute study (Figure 1) assessed the effects of a single haemodiafiltration (HDF) session on BPS levels: 14 patients on HDF were recruited with the following inclusion criteria: arteriovenous fistula that guaranteed flows of 350–400 mL/mL, Kt/Vurea >1.2 and a diuresis <500 mL/day, and who provided signed informed consent. After a run-in period of 1 week dialysed with a cellulose triacetate membrane (three sessions), pre- and post-dialysis blood samples were drawn for the first HDF session with the study synthetic membrane (Membrane 1: PS or PN). After a week of HDF with this membrane, a washout period of 1 week on cellulose triacetate membrane was followed by 1 week in which the alternative synthetic membrane was used (Membrane 2: PN or PS). Again, pre- and post-dialysis blood samples were drawn for the first HDF session with the study synthetic membrane.
FIGURE 1.
Experimental design: impact of dialysis membrane on plasma BPA, BPS and hippuric acid concentration. (A) Acute study. After a 1 week run-in using a control membrane (triacetate), blood was drawn pre- and post-dialysis during the first session with the study membrane (PN or PS) to assess the impact of different membranes on plasma analyte concentration. Following 1 week of triacetate membrane washout, the study was repeated with a different membrane (PN or PS). (B) Chronic study. Blood was only drawn pre-dialysis following at least 6 months of run-in with the study membrane (PN or PS) and after 3 months of dialysis with alternative (switch) membrane.
In the chronic study (Figure 1B), the accumulation of BPA in patients on long-term online HDF (OL-HDF) was explored. Briefly, this was a previously described prospective 9-month crossover study in which we compared BPA-free and BPA-containing dialysers in 72 patients on OL-HDF [5]. Dialysers only differed in the BPA content in the dialysis membrane. Specifically, BPA-free high-flux PN membranes (Elisio, NiproCorp, Osaka, Japan) were compared with high-flux PS (Helixone®) dialysers that contain BPA (Fx80, Fresenius, Bad Homburg, Germany). Blood for BPS assessment was drawn at the start of the dialysis session (pre-dialysis) after a run-in period of at least 6 months with the study membrane as well as after 3 months with the switch (alternative) membrane. For BPS assessment, samples were available from 10 patients in each arm of the study (20), and 14 samples from the short-term study.
Table 1 summarizes patient characteristics for both studies. All patients used the same dialysis monitors (5008 model, Fresenius). This model is equipped with two PS ultrafilters to filter ultrapure water before the monitors make the dialysis fluid. The housing material is polypropylene, while the potting material is polyurethane. Before the study, patients were dialysed with Elisio 21 H or Fx80 membranes, which are the two membranes routinely available in our centre for patients without suspected hypersensitivity reactions. In the latter, cellulose triacetate is used.
Table 1.
Patient characteristics of both populations measured. Expressed as mean ± SD.
| Variable | Acute study (n = 14) | Chronic study (n = 20) |
|---|---|---|
| Age, years | 64 ± 14 | 65.8 ± 16.3 |
| Female sex, n (%) | 3/12 (25) | 6/14 (30) |
| Dialysis vintage, months | 74 ± 53 | 93 ± 38 |
| Qd, mL | 641 ± 20 | 620 ± 35 |
| Qb, mL | 390 ± 24 | 402 ± 28 |
| Kt/V | 1.76 ± 0.32 | 1.77 ± 0.25 |
| Residual diuresis | >200 | >200 |
| Infusion fluid, L | 19.90 ± 0.12 | 24.95 ± 2.85 |
Clinical and biochemical variables
Fasting blood samples were drawn from the arteriovenous fistula just prior to a midweek dialysis session and plasma was frozen at –80°C. Laboratory parameters were assessed by automated blood analysers (Advia 2400 chemistry system and Advia 2120 haematology system, Siemens).
Sample and standard preparation
Plasma samples were prepared to assess the total plasma concentration of BPA, BPS and hippuric acid by liquid–liquid extraction with methanol (1:5) on 100 µL plasma. Samples were treated with glucuronidase/sulphatase in ammonium acetate 1 M pH 7 with 20% methanol overnight, as described by Völkel et al. [26] with modifications. BPA, BPS and hippuric acid standards were analytical standard grade (99%) from Supelco/Sigma-Aldrich Química, S.L. (Madrid, Spain).
Total BPA, BPS and hippuric acid plasma measurements by SRM-MS
Plasma BPA, BPS and hippuric acid concentrations were assessed using high-performance liquid chromatography with tandem mass spectrometry (MS) on a Shimadzu triple quadrupole LC-MS/MS system (LCMS-8060; Shimadzu, Japan) equipped with an electrospray ionization source working in the negative multiple reaction mode. Conditions for combined assessment were optimized at the UCM MS facility, injecting 10 µL of treated samples on a reverse-phase column (Phenomenex Gemini 5 u C18 110 A 150 × 2 mm, Torrance, CA, USA) using a gradient of water/methanol at 0.3 mL/min for analyte separation. The observed transitions were for BPA 227.2 > 212.05 CE = +18 V and 227.2 > 133.10 CE = +25 V; for BPS 249.3 > 108.1 CE = +27 V and 249.3 > 92.05 CE = +35 V; and for hippuric acid 178.0 > 134.1 CE = +18 V and 178.0 > 77.15 CE = +25 V (Supplementary data, Figure S1).
Limits of detections were 0.05 ng/mL (ppb) for BPA and BPS and 3 ng/mL (ppb) for hippuric acid, while the limits of quantifications were 0.16 ng/mL (ppb) for BPS and BPA and 10 ng/mL (ppb) for hippuric acid.
Nuclear magnetic resonance characterization of BPS content in dialyser fibres
Dialyser fibres were characterized using monodimensional experiments of 1H, 13C normal and 13C edited (DEPT-135) and two-dimensional homo- and heteronuclear experiments (1H-1H COSY, 1H-1H TOCSY, 1H-13C HMQC, 1H-13C HSQC edited and 1H-13C HMBC) of nuclear magnetic resonance using chloroform and deuterated dimethyl sulphoxide (DMSO) according to the sample under study. For Fx80 dialyser fibres, 8.0 mg were dissolved in 500 µL deuterated chloroform and for Elisio-19H dialyser fibres, 7.4 mg were dissolved in 500 µL deuterated DMSO and 200 µL deuterated chloroform. Analyses were performed on a Bruker Avance III HD 500 MHz spectrometer equipped with an indirect triple TBI 1H/31P/BB probe.
In vitro studies
Cell culture and reagents
Human renal proximal tubular epithelial cells (HK-2 cell line, ATCC CRL-2190) were cultured in RPMI 1640 (Sigma-Aldrich), with 10% heat-inactivated foetal bovine serum, 2 mM glutamine, 100 µg/mL streptomycin, 100 U/mL penicillin, insulin transferrin selenite (5 µg/mL) and hydrocortisone (36 ng/mL) in 5% CO2 at 37°C. BPA and BPS were added to cell culture media and dose–response (200 nM, 500 nM, 1 µM, 10, 25, 50, 100 and 200 µM), and time-course experiments were performed to optimize experimental conditions. In some experiments, 20 mM bovine serum albumin was added to the culture media. All chemicals were of the highest grade and purchased from Sigma Chemical Co. (St Louis, MO, USA) unless otherwise noted. Stimuli were solubilized in DMSO. A steroid-free medium containing DMSO (0.5% v/v) was used as the control.
Cell viability was determined using the MTT assay (Sigma M2128) in 24-well plates (3 × 104 cells per well).
Real-time PCR
Total RNA was obtained using the Tripure isolation reagent (Roche Diagnostic GmbH, Mannheim, Germany). The quantity and purity of extracted RNA were assessed by measuring absorbance at 260 and 280 nm in a UV spectrophotometer (NanoDrop Inc., Wilmington, DE, USA). Only samples with an Abs 260:Abs 280 ratio up to 1.8 were used for reverse transcription and real-time PCR. Multiplex real-time PCR was performed using Applied Biosystems expression assays for Nrf-2 (Hs0097596_m1), heme oxygenase (HO-1) (Hs01110250_m1), NQO-1 (Hs00168547_m1), interleukin (IL)-6 (Hs00174131_m1) and Tumour necrosis factor-α (Hs00174128_m1). Data were normalized with GAPDH VIC (Hs02786624_g1).
Statistical analysis
The distribution of continuous variables was assessed by the Shapiro–Wilk test. Variables were expressed as absolute and relative frequencies, mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Comparisons between values were performed using Wilcoxon signed-rank test or Kruskal–Wallis test. All comparisons used the bilateral hypothesis test and a significance level of 0.05. One-way ANOVA was performed to compare pre- and post-dialysis values at baseline and after 3 months. Data analysis was performed in R (version 3.5.2, https://cran.r-project.org/).
RESULTS
High plasma BPS levels in patients with impaired renal function
Plasma total BPS (free, conjugated with sulphate or glucuronate, or bound to proteins) was assessed in 10 healthy subjects (blood donors) and in 14 patients with ESRD treated with HDF who had been dialysed for 1 week with cellulose triacetate membranes (Figure 1A). Most (n = 7) healthy controls had plasma BPS below the detection limit of the assay (0.05 ng/mL, 1 ppb) being the range of those that could be measured 0.05–0.07 ng/mL, while in HDF patients, pre-dialysis BPS was 0.54 ± 0.52 ng/mL [median (IQR) 0.47 (0.69)] (P = 0.0015 versus healthy controls) (Figure 2). In contrast, BPA concentrations were 13.23 ± 14.65 ng/mL [median (IQR) 5.51 (21.32)] in HDF patients and 0.37 ± 0.12 ng/mL [median (IQR) 0.4 (0.075)] in controls (P = 0.007) (Figure 2). BPS values were significantly lower than BPA values both in healthy individuals (P = 0.0006) and in HDF patients (P = 7.9E-11).
FIGURE 2.
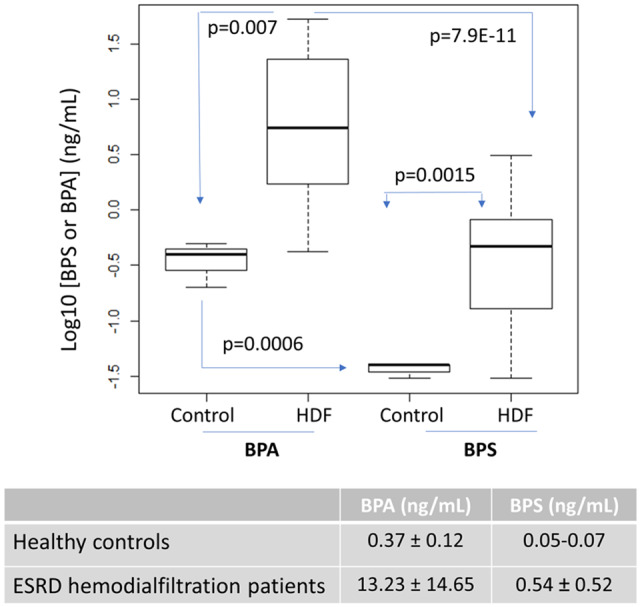
Acute study. Plasma BPA and BPS in HDF patients and healthy controls (Experimental design 1 in Figure 1A). A boxplot of logarithmic representation of BPA and BPS values. The table shows mean and SD.
Plasma BPS concentration does not change while hippuric acid decreases during a single HDF session
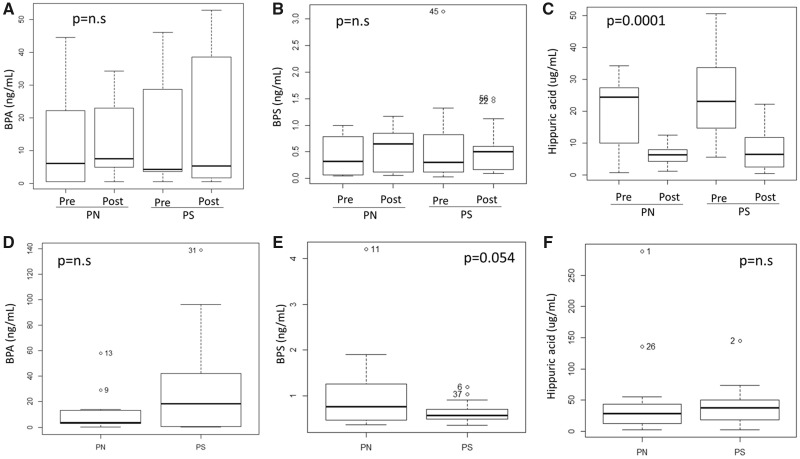
Plasma BPS concentration was assessed at the start (pre-dialysis) and at the end (post-dialysis) of the HDF session using different dialysis membranes, following 1 week on the same membrane, and was compared with BPA and with a uraemic toxin of similar molecular weight (179 g/mol versus 228 g/mol) and closer hydrophobicity characteristics than other uraemic toxins (hippuric acid). PN membranes are BPA-free but contain BPS, while PS contains both BPA and BPS. Neither BPA (Table 2 and Figure 3A) nor BPS (Table 3 and Figure 3B) concentrations changed significantly during the HDF session.
Table 2.
Plasma BPA concentration before and after single HDF session with different membranes following 1 week on the same membrane
| BPA (ng/mL) |
||
|---|---|---|
| Mean ± SD | Median (IQR) | |
| PN pre-dialysis | 11.51 ± 13.55 | 6.12 (19.86) |
| PN post-dialysis | 12.42 ± 11.10 | 7.54 (15.98) |
| PS pre-dialysis | 13.17 ± 14.81 | 4.27 (23.41) |
| PS post-dialysis | 15.83 ± 19.25 | 5.31 (30.68) |
No significant differences were observed when comparing pre- versus post-HDF values.
FIGURE 3.
Acute and chronic studies. Plasma analyte concentration in HDF patients on different membranes. (A–C) Acute study (Experimental design in Figure 1A). Plasma concentrations of BPA (A), BPS (B) and hippuric acid (C) pre- and post-HDF session using different membranes: PN and PS. (D–F) Chronic study (Experimental design in Figure 1B). Pre-dialysis plasma concentrations for BPA (D), BPS (E) and hippuric acid (F) after at least 3 months using the same membrane for HDF. Data correspond to the average of baseline and 3-month sampling for each dialysis membrane. Please note the different scales for different analytes.
Table 3.
Plasma BPS concentrations before and after single HDF session with different membranes following 1 week on the same membrane
| BPS (ng/mL) |
||
|---|---|---|
| Mean ± SD | Median (IQR) | |
| PN pre-dialysis | 0.42 ± 0.35 | 0.32 (0.65) |
| PN post-dialysis | 0.56 ± 0.36 | 0.64 (0.68) |
| PS pre-dialysis | 0.59 ± 0.82 | 0.30 (0.61) |
| PS post-dialysis | 0.58 ± 0.47 | 0.51 (0.37) |
No significant differences were observed when comparing pre- versus post-HDF values.
In contrast, the concentration of hippuric acid significantly decreased during the HDF session with either membrane (Figure 3C).
Bioaccumulation of BPS in ESRD patients
To assess the potential bioaccumulation of BPS during long-term HDF, we designed a chronic study (Figure 1B). This study used pre-dialysis plasma concentrations after 3–6 months using the same dialysis membrane as a marker of BPA or BPS accumulation.
In this population (n = 20), MS analysis did not disclose statistically significant differences between plasma BPA concentration in patients on long-term PS compared with those on long-term PN dialysers [26.55 ± 35.01 ng/mL, median (IQR) 13.94 (28.62) versus 8.74 ± 12.08 ng/mL, median (IQR) 2.85 (7.54), NS; Figure 3D], although consistent with prior results in a larger sample (n = 72) using ELISA to assess pre-dialysis BPA levels after the long-term use of BPA-containing membranes in either HD [4] or HDF [5], values on PS were numerically higher.
For BPS concentration, no significant differences were observed between patients on long-term PS and those on long-term PN dialysers [0.62 ± 0.21 ng/mL, median (IQR) 0.56 (0.21) versus 1.01 ± 0.87 ng/mL, median (IQR) 0.76 (0.78), P = NS] (Figure 3E).
There were no differences in hippuric acid concentrations [PS 39.64 ± 31.60 µg/mL, median (IQR) 37.88 (26.86), versus PN 44.24 ± 64.47 µg/mL, median (IQR) 28.24 (29.77), NS] (Figure 3F).
Leaking of BPA and BPS from dialyser membranes
The source of bisphenols is expected to be the release from plastics that compose the dialysis system. A first analysis did not disclose the presence of measurable free BPS or BPA in the dialyser PN or PS fibres. However, incubation of dialyser fibres under physiological conditions (37°C in culture media) for 24 h allowed the detection of quantifiable amounts of BPS and/or BPA monomers for both PN (BPS 100 ng/mL from 10 mg PN fibres) and PS (BPS 1960 ng/mL and BPA 8060 ng/mL, from 10 mg PS fibres).
In vitro cytotoxicity of BPS and BPA
To assess the potential clinical significance of BPS found in ESRD patient plasma, we compared BPS toxicity with that of equimolar concentrations of BPA, a well-characterized toxin for, among others, kidney tubular cells. In the chronic study, the mean plasma BPA values of the whole group, combining both dialyser membranes, was 17.7 ng/mL (77.5 µM), while the highest concentration was 81.1 ng/mL (350 µM). Respective figures for BPS were 1.01 ng/mL (3.99 µM) and 4.21 ng/mL (16 µM).
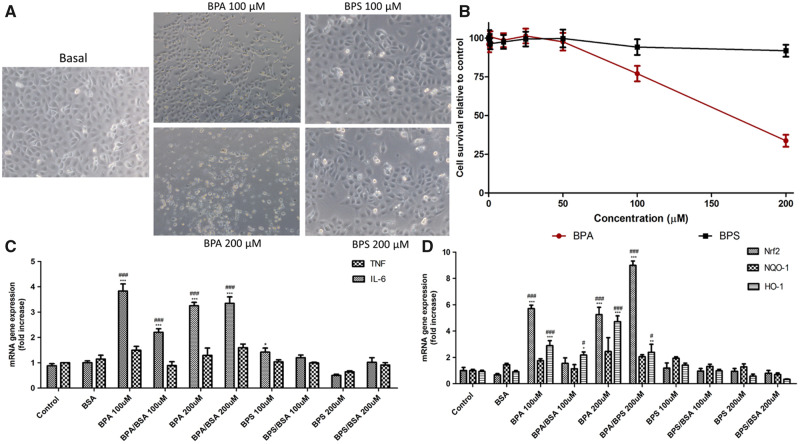
Thus, we explored the impact of a concentration range of 200 nM to 200 µM free BPA or BPS over the MMT-assessed survival of cultured human tubular epithelial cells cultured in presence of albumin and survival factors contained in FCS. BPA was cytotoxic at concentrations of 100 µM or higher, while BPS was not toxic within this clinically relevant concentration range, consistent with contrast phase microscopy results (Figure 4A and B ).
FIGURE 4.
Comparison of the cytotoxicity and pro-inflammatory and pro-oxidants effects in BPS and to BPA in renal tubular cells (HK-2). (A) Representative contrast phase micrographs after 24 h of stimulation. Dead cells display birefringence. (B) Dose–response cell survival at 24 h assessed by the MTT assay. (C) Expression of inflammatory gene mRNA in response to BPA or BPS stimulation for 24 h in presence or absence of albumin (BSA). (D) Gene expression of Nrf2, NQO-1 and HO-1 mRNA under the same experimental conditions as in C.
Oxidative and inflammatory responses to BPS
We further explored the non-lethal effects of high BPS and BPA concentrations on the expression of genes coding for inflammatory cytokines or proteins of the oxidative stress response. BPS and BPA were studied in the absence or presence of albumin since they bind to albumin in the circulation. Free BPA or albumin-bound BPA increased the gene expression of IL-6 and the master regulator of oxidative stress-protective responses Nrf2 as well as its target HO-1; Figure 4C and D). Even the highest BPS concentrations tested did not elicit significant proinflammatory or oxidative stress responses (Figure 4C and D).
DISCUSSION
BPS is a molecule that is widely used to replace BPA, but due to the structural similarities between these two molecules, the question arises whether BPS can be considered a safe replacement. BPS safety is particularly important for CKD patients. We now provide three key pieces of clinically relevant information: BPS accumulates in anuric patients undergoing HDF and it may leak from dialysis membranes, providing a source for this xenobiotic in dialysis patients. However, plasma BPS levels are considerably lower than BPA levels and in the human cell culture system used it was less toxic than BPA, even at concentrations >10-fold higher than those found in patients and after assessing sensitive sublethal responses associated to cell stress.
Exposure to BPS in the general population is still small. The estimated daily intake in China was <0.25 ng/kgbody weight/day for each detected BPS, much lower than the tentative oral reference dose values for BPA (4μg/kgbw/day) recommended by the European Food Safety Authority [27]. In urine studies, BPA was detected in ≥85% of the spot samples, while BPS could be measured only in 13% of the samples [25]. Our results are in line with these observations since plasma BPS was undetectable or just above the detection limit in healthy subjects, a reflection of a lower environmental exposure than to BPA. However, despite the lower environmental exposure of general populations to this compound, in this study, it is observed for the first time that plasma BPS concentration is increased in patients with CKD in HD, suggesting accumulation in these patients. The mechanisms underlying the accumulation are likely the same as for BPA: lack of renal elimination being the main cause, together with the potential transfer from dialysis membranes [4]. Still, plasma BPS values were much lower than BPA values, which together with the lower observed cytotoxicity are reassuring. However, much remains to be understood about BPS in HD, such as whether it may accumulate in certain cell types or organs (selective deposition), what is the pharmacokinetics in patients in ESRD and whether long-term exposure may be toxic or whether it may have cell type-specific toxicity.
Our data are consistent with dialysers being a potential source of BPS for dialysis patients, although again, the lower leakage values of BPS versus BPA are reassuring. This aspect, as far as we know, has not been published despite manufacturers’ need to evaluate it to comply with sanitary device specifications in each country. The marginal percentage of free monomers, similar in both types of fibres, is expected in a device for medical use. Similarly, both fibres are quite stable under the conditions found in dialysis, with the quantities measured being minimal but appreciable in both cases.
Similar to previous findings in larger HD [4] and OL-HDF [5] studies for BPA, the chronic use of BPA-containing dialysers is associated with non-statistically significant, numerically higher plasma BPA levels than the use of BPA-free dialysers, suggesting BPA accumulation. The limited sample size may have precluded observing statistically significant differences described in prior larger studies. Regarding BPS, both dialysers contained this molecule, and plasma BPS was higher in HD patients than in healthy individuals. However, studies using BPS-free dialysers are required to further characterize the relative contribution of dialysers versus other environmental sources to BPS accumulation in ESRD patients, as BPS accumulation has been described in non-dialysis CKD patients. Thus, in a recent study from China, serum BPA and BPS were found to increase with decreasing kidney function and to be higher in HD than in PD patients, suggesting release from HD equipment, and thus supporting our findings [28, 29].
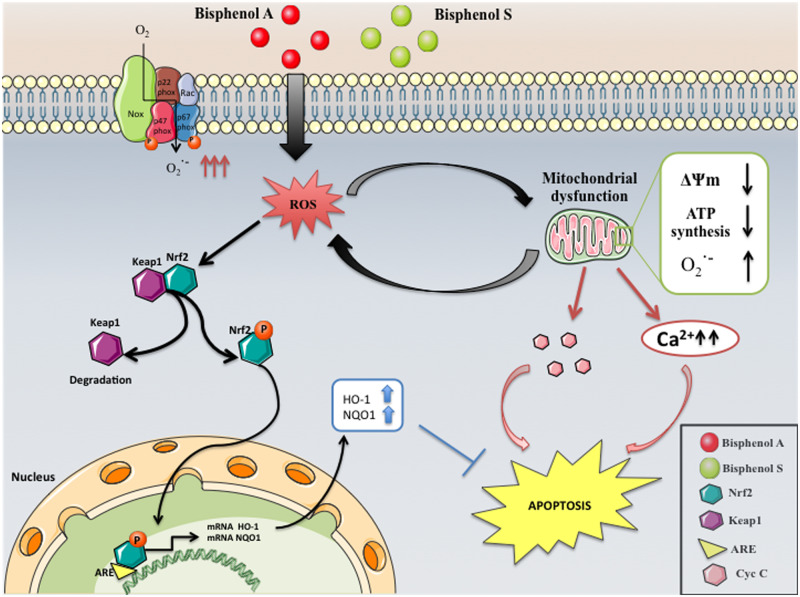
The other relevant question for the renal patient is the acute and chronic toxicity of BPS. There is an extensive literature on BPA toxicity through different mechanisms, including interference with mitochondrial function [30] (Figure 5). It can be hypothesized that BPS may also be toxic by recruiting similar mechanisms. However, the literature on BPS toxicity is scarce and frequently marred by the use of very high concentrations without clinical relevance [13, 31–34]. Additionally, the blood–placental barrier is more efficient in limiting foetal exposure to BPS than to BPA [35]. Thus, despite the cross-sectional association of urinary bisphenols, including BPA and BPS, with human diabetes mellitus, a nonlinear positive association between lower concentrations and diabetes mellitus was only observed for BPA [8]. Our results, testing clinically relevant concentrations of BPA and BPS concentrations >10-fold higher than the clinically relevant ones, are consistent with lower toxicity of BPS. It should be remembered that tubular cells may also be a source of systemic inflammation and may play important functions even in dialysis patients, by synthesizing proteins such as the anti-ageing factor Klotho and calcitriol [36, 37]. Since systemic inflammation downregulates tubular cell Klotho expression [36] and preservation of Klotho expression during AKI is nephroprotective [38], it may be speculated that the choice of membranes may have potential effects on recovery of renal function. However, this hypothesis should be tested experimentally.
FIGURE 5.
Graphical abstract of known and hypothesized mechanisms of BPA cytotoxicity and major pathways involved. BPS was not found to activate the same pathways at the concentrations studied.
Our study had several limitations. Thus, the sample size was relatively small, although large enough to observe significant differences for hippuric acid. Due to scarce information at the time of design of the study, the 1-week washout period was chosen empirically, and it may not have been the optimal washout period. In any case, patients were randomized to the initial test membrane and then crossover was performed. Furthermore, we did not study tissue levels of BPA or BPS, and the chronic study was relatively short (months) with respect to the potential decades’ survival on chronic HD. Thus, we cannot exclude the long-term consequences of tissue BPS accumulation. Finally, regarding the cell system to assess toxicity, we cannot exclude potential toxic effects of BPS to other readouts or cell systems upon longer exposure.
In conclusion, despite synthetic dialysis membranes being a potential source of the high BPS levels in HD patients, the toxicity profile of BPS is safer than for BPA, at least for the parameters measured in this study. However, the precautionary principle should prevail, and BPS cannot be considered harmless for patients with ESRD until detailed prospective studies confirm its long-term safety.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
The Renal, Vascular and Diabetes Laboratory is funded by Ministerio de Economia, Industria y competitividad: FIS ISCIII FEDER funds PI16/01298, PI15/00298, PI16/02057, PI16/01900, PI 17/01495, ISCIII-RETIC REDinREN RD12/0021 RD16/0009, CYTED IBERERC and Sociedad Madrileña de Nefrologia. This work was supported by a grant from Nipro corporation and Fundación Renal Íñigo Álvarez de Toledo (FRIAT), and A.R.-P. was funded by grant from the Fundación Conchita Rábago.
CONFLICT OF INTEREST STATEMENT
E.G.-P. is a recipient of a grant from Nipro corporation for the study of bisphenol A in kidney patients. The funders had no role in the design of the study, interpretation of the results or the writing of the manuscript.
Supplementary Material
REFERENCES
- 1. Dodds EC, Goldberg L, Lawson W. et al. Oestrogenic activity of certain synthetic compounds. Nature 1938; 141: 247–248 [Google Scholar]
- 2. Molina-Molina JM, Jiménez-Díaz I, Fernández MF. et al. Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ Res 2019; 170: 406–415 [DOI] [PubMed] [Google Scholar]
- 3. Rochester JR, Bolden AL.. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 2015; 123: 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosch-Panadero E, Mas S, Sanchez-Ospina D. et al. The choice of hemodialysis membrane affects serum bisphenol A levels. J Am Soc Nephrol 2016; 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mas S, Bosch-Panadero E, Abaigar P. et al. Influence of dialysis membrane composition on plasma bisphenol A levels during online hemodiafiltration. PLoS One 2018; 13: e0193288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scientific Committee on Emerging and Newly Identified Health Risks. The Safety of the Use of Bisphenol A in Medical Devices. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_040.pdf 2015
- 7. Wu LH, Zhang XM, Wang F. et al. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ 2018; 615: 87–98 [DOI] [PubMed] [Google Scholar]
- 8. Duan Y, Yao Y, Wang B. et al. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ Pollut 2018; 243: 1719–1726 [DOI] [PubMed] [Google Scholar]
- 9. Liao C, Liu F, Guo Y. et al. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 2012; 46: 9138–9145 [DOI] [PubMed] [Google Scholar]
- 10. Song Y, Xie P, Cai Z.. Metabolism of bisphenol S in mice after oral administration. Rapid Commun Mass Spectrom 2018; 32: 495–502 [DOI] [PubMed] [Google Scholar]
- 11. Kojima H, Takeuchi S, Sanoh S. et al. Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 2019; 413: 48–55 [DOI] [PubMed] [Google Scholar]
- 12. Zhang YF, Ren XM, Li YY. et al. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environ Pollut 2018; 237: 1072–1079 [DOI] [PubMed] [Google Scholar]
- 13. Berni M, Gigante P, Bussolati S. et al. Bisphenol S, a Bisphenol A alternative, impairs swine ovarian and adipose cell functions. Domest Anim Endocrinol 2019; 66: 48–56 [DOI] [PubMed] [Google Scholar]
- 14. Eladak S, Grisin T, Moison D. et al. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 2015; 103: 11–21 [DOI] [PubMed] [Google Scholar]
- 15. Desdoits-Lethimonier C, Lesné L, Gaudriault P. et al. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum Reprod 2017; 32: 1465–1473 [DOI] [PubMed] [Google Scholar]
- 16. Gu J, Zhang J, Chen Y. et al. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio). Chemosphere 2019; 217: 629–635 [DOI] [PubMed] [Google Scholar]
- 17. Maćczak A, Cyrkler M, Bukowska B. et al. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol In Vitro 2017; 41: 143–149 [DOI] [PubMed] [Google Scholar]
- 18. Ullah H, Jahan S, Ain QU. et al. Effect of bisphenol S exposure on male reproductive system of rats: a histological and biochemical study. Chemosphere 2016; 152: 383–391 [DOI] [PubMed] [Google Scholar]
- 19. Nakagawa Y, Tayama S.. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol 2000; 74: 99–105 [DOI] [PubMed] [Google Scholar]
- 20. Fic A, Žegura B, Sollner Dolenc M. et al. Mutagenicity and DNA damage of bisphenol a and its structural analogues in hepg2 cells. Arch Ind Hyg Toxicol 2013; 64: 189–200 [DOI] [PubMed] [Google Scholar]
- 21. Rezg R, Abot A, Mornagui B. et al. Bisphenol S exposure affects gene expression related to intestinal glucose absorption and glucose metabolism in mice. Environ Sci Pollut Res 2019; 26: 3636–3642 [DOI] [PubMed] [Google Scholar]
- 22. Huang W, Zhao C, Zhong H. et al. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ Pollut 2019; 246: 697–703 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Dong T, Hu W. et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int 2019; 123: 325–336 [DOI] [PubMed] [Google Scholar]
- 24. Lee S, Kim C, Shin H. et al. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 2019; 221: 115–123 [DOI] [PubMed] [Google Scholar]
- 25. Wang YX, Liu C, Shen Y. et al. Urinary levels of bisphenol A, F and S and markers of oxidative stress among healthy adult men: variability and association analysis. Environ Int 2019; 123: 301–309 [DOI] [PubMed] [Google Scholar]
- 26. Völkel W, Colnot T, Csanády GA. et al. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol 2002; 15: 1281–1287 [DOI] [PubMed] [Google Scholar]
- 27. Zhang H, Zhang Y, Li J, Yang M.. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci Total Environ 2019; 655: 607–613 [DOI] [PubMed] [Google Scholar]
- 28. Kuo CC, Huang JK, Chou CT. et al. Effect of bisphenol A on Ca(2+) fluxes and viability in Madin-Darby canine renal tubular cells. Drug Chem Toxicol 2011; 34: 454–461 [DOI] [PubMed] [Google Scholar]
- 29. Shen Y, Liu T, Shi Y. et al. Bisphenol A analogs in patients with chronic kidney disease and dialysis therapy. Ecotoxicol Environ Saf 2019; 185: 109684. [DOI] [PubMed] [Google Scholar]
- 30. Bosch-Panadero E, Mas S, Civantos E. et al. Bisphenol A is an exogenous toxin that promotes mitochondrial injury and death in tubular cells. Environ Toxicol 2018; 33: 325–332 [DOI] [PubMed] [Google Scholar]
- 31. Wang W, Zhang X, Qin J. et al. Long-term bisphenol S exposure induces fat accumulation in liver of adult male zebrafish (Danio rerio) and slows yolk lipid consumption in F1 offspring. Chemosphere 2019; 221: 500–510 [DOI] [PubMed] [Google Scholar]
- 32. Speidel JT, Xu M, Abdel-Rahman SZ.. Bisphenol A (BPA) and bisphenol S (BPS) alter the promoter activity of the ABCB1 gene encoding P-glycoprotein in the human placenta in a haplotype-dependent manner. Toxicol Appl Pharmacol 2018; 359: 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berto-Júnior C, Santos-Silva AP, Ferreira ACF. et al. Unraveling molecular targets of bisphenol A and S in the thyroid gland. Environ Sci Pollut Res Int 2018; 25: 26916–26926 [DOI] [PubMed] [Google Scholar]
- 34. Lin Z, Zhang X, Zhao F, Ru S.. Bisphenol S promotes the cell cycle progression and cell proliferation through ERα-cyclin D-CDK4/6-pRb pathway in MCF-7 breast cancer cells. Toxicol Appl Pharmacol 2019; 366: 75–82 [DOI] [PubMed] [Google Scholar]
- 35. Grandin FC, Lacroix MZ, Gayrard V. et al. Is bisphenol S a safer alternative to bisphenol A in terms of potential fetal exposure? Placental transfer across the perfused human placenta. Chemosphere 2019; 221: 471–478 [DOI] [PubMed] [Google Scholar]
- 36. Moreno JA, Izquierdo MC, Sanchez-Niño MD. et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 2011; 22: 1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandez-Fernandez B, Izquierdo MC, Valiño-Rivas L. et al. Albumin downregulates Klotho in tubular cells. Nephrol Dial Transplant 2018; 33: 1712–1722 [DOI] [PubMed] [Google Scholar]
- 38. Liao HK, Hatanaka F, Araoka T. et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell 2017; 171: 1495–1507.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.