Abstract
Background
Bedside experience and studies of critically ill patients with coronavirus disease 2019 (COVID-19) indicate COVID-19 to be a devastating multisystem disease. We aim to describe the incidence, associated variables, and outcomes of rhabdomyolysis in critically ill COVID-19 patients.
Materials and Methods
Data for all critically ill adult patients (≥18 years old) admitted to the ICU at a large academic medical center with confirmed COVID-19 between March 13, 2020 and April 18, 2020 were prospectively collected. Patients with serum creatine kinase (CK) concentrations greater than 1000 U/L were diagnosed with rhabdomyolysis. Patients were further stratified as having moderate (serum CK concentration 1000-4999 U/L) or severe (serum CK concentration ≥5000 U/L) rhabdomyolysis. Univariate and multivariate analyses were performed to identify outcomes and variables associated with the development of rhabdomyolysis.
Results
Of 235 critically ill COVID-19 patients, 114 (48.5%) met diagnostic criteria for rhabdomyolysis. Patients with rhabdomyolysis more often required mechanical ventilation (P < 0.001), prone positioning (P < 0.001), pharmacological paralysis (P < 0.001), renal replacement therapy (P = 0.010), and extracorporeal membrane oxygenation (ECMO) (P = 0.025). They also had longer median ICU length of stay (LOS) (P < 0.001) and hospital LOS (P < 0.001). No difference in mortality was observed. Male sex, patients with morbid obesity, SOFA score, and prone positioning were independently associated with rhabdomyolysis.
Conclusions
Nearly half of critically ill COVID-19 patients in our cohort met diagnostic criteria for rhabdomyolysis. Male sex, morbid obesity, SOFA score, and prone position were independently associated with rhabdomyolysis.
Keywords: COVID-19, SARS-CoV-2, Rhabdomyolysis, Critical-illness
Background
Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2) is an enveloped RNA Betacoronavirus that was first observed in China in December 2019.1 , 2 The majority of patients with confirmed SARS-CoV-2 only experience minor symptoms, but a subset of COVID-19 patients can progress to life threatening critical illness.3, 4, 5, 6 While pulmonary manifestations of the disease are prevalent, front line clinicians are increasingly reporting bedside findings suggestive of systemic manifestations of COVID-19 infection, including neurologic, cardiac, gastrointestinal, hematologic, and renal complications.7, 8, 9, 10, 11, 12, 13
Rhabdomyolysis is a complex disease ranging from asymptomatic illness with mild elevations in serum creatine kinase (CK) concentration to a severe illness characterized by electrolyte imbalances and acute kidney injury (AKI).14 , 15 A serum CK greater than 1000 U/L is diagnostic, and serum CK concentrations in the severe range (>5000 U/L) have been associated with increased risk for AKI.15, 16, 17, 18 The incidence of AKI in patients with rhabdomyolysis is estimated to range between 10 and 50%.17 , 19, 20, 21, 22 Although rare, viral etiologies of rhabdomyolysis have been described.23, 24, 25, 26 A case report characterized rhabdomyolysis associated with a previous Severe Acute Respiratory Syndrome (SARS) outbreak.27 In addition, case reports describe atypical presentations of SARS-CoV-2 associated rhabdomyolysis in elderly patients.26 , 28, 29, 30, 31, 32 In this study, we report our experience with a high incidence of, and adverse outcomes for rhabdomyolysis in critically ill COVID-19 patients during the pandemic surge in Massachusetts, and investigate potential factors independently associated with developing rhabdomyolysis in this patient population.
Methods
Study population
All adult (≥18 years old) patients with confirmed SARS-CoV-2 infection via reverse transcriptase polymerase chain reaction (RT-PCR) testing of nasopharyngeal swabs and who were admitted to any of 13 pre-existent and surge Intensive Care Units (ICUs) at the largest academic medical center in New England, USA between March 13, 2020 and April 18, 2020 were included. All patients were prospectively followed until July 2, 2020.
Study variables
A systematic medical record review was performed to collect patient demographics (e.g. age, sex, race, ethnicity, insurance status), presenting symptoms (e.g. fever, shortness of breath, cough, diarrhea), comorbidities (e.g. hypertension, diabetes mellitus, chronic kidney disease) and severity of illness at ICU admission (e.g. Sequential Organ Failure Assessment or SOFA Score). Rhabdomyolysis was defined as a serum CK concentration above 1000 U/L. The severity of rhabdomyolysis was categorized by serum CK concentration, with severe rhabdomyolysis defined as serum CK concentrations ≥5000 U/L.14 , 15 , 22 The hospital course and outcome variables included were: the need for dialysis, the need for mechanical ventilation, total number of days on mechanical ventilation, prone positioning, pharmacologic paralysis, extracorporeal membrane oxygenation (ECMO), ICU length of stay (LOS), hospital LOS, systemic complications (e.g. thromboembolic, pulmonary, cardiac, gastronintestinal, renal, neurologic) and mortality. We also collected data on the specific treatment modalities for COVID-19 (e.g. hydroxychlroquine, azithromycin, steroids) and rhabdomyolysis (e.g. statin avoidance or discontinuation, fluid resuscitation, diuresis).
Thromboembolic complications for this study include central or arterial line thrombosis, pulmonary embolism (PE) and deep vein thrombosis (DVT). Pulmonary complications included acute respiratory distress syndrome (ARDS) (As per the Berlin Definition), pneumonia, upper airway edema, and pneumothorax. Cardiac complications included new onset arrhythmia, myocardial infarction (MI), cardiac arrest, myocarditis, cardiomyopathy, and congestive heart failure (CHF). Gastrointestinal complications included transaminitis, ogilvie syndrome, ileus, Clostridium difficile infection, gastrointestinal bleeding (GIB), and mesenteric ischemia. Renal complications included AKI and urinary tract infections (UTIs). AKI was defined via the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (i.e. increase in serum creatinine ≥1.5 times the baseline).33 Chart review was conducted to identify those patients with AKI with secondary oliguria or anuria. Neurologic complications included stroke, delirium, and seizures.
Statistical analysis
Descriptive statistics were performed to compare baseline characteristics of rhabdomyolysis and non-rhabdomyolysis patients. Median and inter-quartile ranges (IQR) were recorded for continuous data, while categorical data were summarized using the incidence (actual number) (n) and percentages (cumulative incidence). Continuous variables were compared using the Wilcoxon rank-sum test and categorical variables were compared using Pearson's chi-squared or Fisher's exact test. Univariate and multivariable analyses were performed to identify variables associated with the development of rhabdomyolysis and variables associated with developing AKI in patients with rhabdomyolysis. All covariates with P values less than 0.2 in the univariate analyses were included in the logistic regression model. Odds ratios (OR) and 95% confidence intervals (CI) following multivariable analyses are reported. Statistical analyses were performed using StataCorp 2017 (Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and GraphPad Prism 8 software (GraphPad Software, La Jolla California USA). Figures were generated with Python 3.7 using the Pandas and Matplotlib packages and StataCorp 2017 (Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Sub-analysis using multinomial (polytomous) logistic regression
A sub-analysis was performed in order to determine factors associated with moderate and severe rhabdomyolysis. This was accomplished by first stratifying patients by severity of rhabdomyolysis using serum CK concentration as a surrogate marker for disease severity. Those individuals with a serum CK<1000 U/L were defined as not having rhabdomyolysis, patients with a serum CK concentration between 1000 U/L and 4999 U/L were defined as the “moderate” rhabdomyolysis cohort, and those patients with a serum CK concentration ≥5000 U/L were defined as the “severe” rhabdomyolysis cohort. An analysis of variance (ANOVA) was performed to characterize the demographic and in-hospital characteristics of these three groups. A multinomial (polytomous) logistic regression was performed to characterize variables independently associated with moderate and/or severe rhabdomyolysis. Calculations of the relative risk ratios (RRR) were performed to estimate the strength of association between independent and dependent variables in this model.
Ethical oversight
A detailed review of this project was conducted by the institutional review board (IRB). This study was granted a waiver for consent/authorization. Following an expedited review, this study was granted IRB approval.
Results
Patient characteristics
Out of a total of 235 critically ill COVID-19 patients, 114 (48.5%) developed rhabdomyolysis, including 90 (79.0%) with moderate (1000-4999 U/L) peak serum CK concentrations, and 24 (21.0%) with severely elevated (>5000 U/L) peak serum CK concentrations. As of the last day of the study, 58 patients had died (24.7%) and 175 (74.5%) were discharged from the hospital; only 2 (0.9%) remained in the hospital on the floor.
Table 1 compares the demographic characteristics of patients with and without rhabdomyolysis. Patients with rhabdomyolysis were younger, more often male, and had a higher body mass index (BMI kg/m2). There was no significant difference between the two groups in terms of race, ethnicity, insurance status, smoking history, symptoms on presentation, or SOFA Scores. Patients with rhabdomyolysis also had similar comorbidities to those without rhabdomyolysis, except that they were less likely to have hypertension. Table 1 contains a summary of patient presenting symptoms, comorbid conditions, and median SOFA Score.
Table 1.
Demographic characterization, presenting symptomology, comorbidities, illness severity, and hospital outcomes of patients with and without rhabdomyolysis.
| No rhabdomyolysis (n = 121) | Rhabdomyolysis (n = 114) | P value | |
|---|---|---|---|
| Age, median (IQR) | 63 (51, 72) | 57 (45, 67) | 0.009 |
| Male, n (%) | 72 (59.5%) | 84 (73.7%) | 0.021 |
| BMI, median (IQR) | 29 (25.5, 33.0) | 32.3 (27.1, 36.9) | 0.002 |
| Race, n (%) | 0.58 | ||
| White | 46 (38.0%) | 32 (28.1%) | |
| Black or African American | 13 (10.7%) | 13 (11.4%) | |
| Asian | 7 (5.8%) | 6 (5.3%) | |
| American Indian/Alaska Native | 0 (0.0%) | 1 (0.9%) | |
| Other | 44 (36.4%) | 50 (43.9%) | |
| Unknown | 11 (9.1%) | 12 (10.5%) | |
| Ethnicity, n (%) | 0.77 | ||
| Hispanic | 49 (40.5%) | 469(43.0%) | |
| Non-Hispanic | 57 (47.1%) | 47 (41.2%) | |
| Other | 5 (4.1%) | 5 (4.4%) | |
| Unknown | 10 (8.3%) | 13 (11.4%) | |
| Insurance status, n (%) | 0.145 | ||
| Private | 44 (45.4%) | 53 (54.6%) | |
| Government (Medicare/Medicaid) | 77 (55.8%) | 61 (44.2%) | |
| History of smoking, n (%) | 28 (23.1%) | 29 (25.4%) | 0.68 |
| Presenting symptoms, n (%) | |||
| Fever | 94 (78%) | 83 (75%) | 0.54 |
| Cough | 93 (77.5%) | 84 (75.7%) | 0.758 |
| Hemoptysis | 2 (1.7%) | 2 (1.8%) | 1.00 |
| Productive cough | 7 (5.8%) | 11 (9.9%) | 0.327 |
| Myalgias | 43 (35.8%) | 43 (38.7%) | 0.684 |
| Fatigue | 54 (45.0%) | 42 (37.8%) | 0.287 |
| Diarrhea | 33 (27.5%) | 31 (27.9%) | 1.00 |
| Nausea/vomiting | 29 (24.2%) | 23 (20.7%) | 0.637 |
| Anosmia/dysgeusia | 11 (9.2%) | 8 (7.2%) | 0.638 |
| Shortness of breath | 84 (70.0%) | 81 (73.0%) | 0.663 |
| Chest pain | 10 (8.3%) | 11 (9.9%) | 0.820 |
| Headache | 12 (10.0%) | 13 (11.7%) | 0.679 |
| Sore throat | 20 (16.7%) | 15 (13.5%) | 0.583 |
| Comorbid conditions, n (%) | |||
| Hypertension | 68 (58.6%) | 48 (41.4%) | 0.049 |
| Diabetes | 56 (55.5%) | 45 (44.5 %) | 0.357 |
| Chronic kidney disease | 17 (54.8%) | 14 (45.2%) | 0.847 |
| Disseminated cancer | 4 (80%) | 1 (40%) | 0.371 |
| Coronary heart disease | 14 (66.7%) | 7 (33.3%) | 0.176 |
| CHF | 7 (63.6%) | 4 (36.4%) | 0.542 |
| COPD | 10 (62.5%) | 6 (37.5%) | 0.444 |
| Asthma | 14 (60.9%) | 9 (39.1%) | 0.389 |
| Prehospital dialysis | 5 (4.1%) | 1 (0.9%) | 0.11 |
| *SOFA score on presentation, median (IQR) | 5 (3, 7) | 6 (4, 8) | 0.052 |
| Days mechanically ventilated, median (IQR) | 14 (8, 19) | 16 (12, 27) | 0.006 |
| ICU LOS, median (IQR) | 12 (5, 21) | 20 (14, 29) | <0.001 |
| Hospital LOS, median (IQR) | 19 (10, 26) | 24 (18, 32) | <0.001 |
| Mortality, n (%) | 26 (21.5%) | 32 (28.1%) | 0.24 |
BMI = body mass index (kg/m2); CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; ICU = Intensive care unit; IQR = interquartile range; LOS = length of stay; NSAIDS = nonsteroidal anti-inflammatory drugs; SOFA = Sequential Organ Failure Assessment Score.
SOFA Score was calculated at time of admission to the ICU
Analysis of serum CK concentration trends
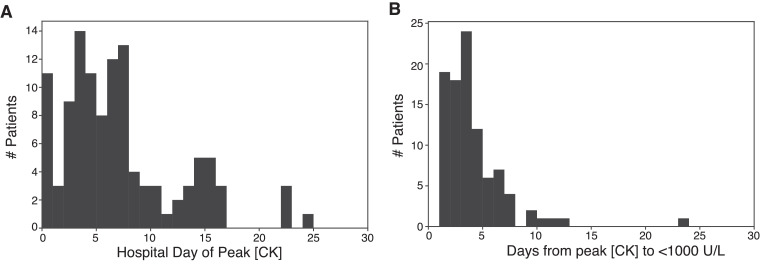
When examining admission CK levels, most rhabdomyolysis patients (81%) did not have a serum CK concentration that met minimum criteria for the diagnosis of rhabdomyolysis on admission to the hospital, but instead developed elevated serum CK concentrations later during their admission. The median admission serum CK concentration in patients who developed rhabdomyolysis was significantly higher than in those patients who did not develop rhabdomyolysis, and median peak serum CK concentrations were higher for patients with rhabdomyolysis. Table 2 summarizes admission and peak serum CK concentrations. The median hospital day that patients with rhabdomyolysis reached their peak serum CK concentration was hospital day 6 (3, 9) with the majority of patients reaching peak value by hospital day 7 (Table 2, Fig. 1 ). While many patients had a return to a serum CK level below 1000 U/L within three days of peak serum CK concentration, the range of days required to return to normal concentrations was 1 to 23 days.
Table 2.
Characterization of serum CK concentration and renal function in COVID-19 ICU patients.
| No rhabdomyolysis (n = 121) | Rhabdomyolysis (n = 114) | P value | |
|---|---|---|---|
| Admission CK, median (IQR) | 112 (61, 221) | 341 (138, 824) | <0.001 |
| Peak CK, median (IQR) | 289 (111, 570) | 2672 (1607, 4755) | <0.001 |
| Hospital day of peak serum CK concentration, median (IQR) |
– |
6 (3, 9) |
– |
| AKI, n (%) | 69 (57%) | 94 (82.5%) | <0.001 |
| Hospital day AKI, median (IQR) | 1 (0, 3) | 1 (0, 4) | 0.613 |
| Oliguria, n (%) | 57 (47.1%) | 80 (70.2%) | <0.001 |
| Anuria, n (%) | 14 (11.6%) | 21 (18.4%) | 0.098 |
AKI = acute kidney injury; CK = creatine kinase; IQR = Interquartile range.
Fig. 1.
Histograms characterizing hospital day of peak serum CK concentration and days required to return to below serum CK concentration of 1000 U/L in patients with eventual diagnosis of rhabdomyolysis. (A) Hospital day of peak serum CK concentration. (B) Number of days required for serum CK concentration to return to <1000 U/L.
Hospital course and outcomes
Tables 1 and 3 detail the hospital course and outcomes of patients with and without rhabdomyolysis. In summary, patients with rhabdomyolysis more often required mechanical ventilation, prone positioning, pharmacologic paralysis to optimize respiratory support, initiation of dialysis, and ECMO. Patients with rhabdomyolysis had longer total days ventilated, ICU LOS, and hospital LOS. The rates of mortality were statistically similar between those with and without rhabdomyolysis.
Table 3.
Implemented treatment strategies and hospital complications.
| No rhabdomyolysis (n = 121) | Rhabdomyolysis (n = 114) | P value | |
|---|---|---|---|
| COVID-19 treatment strategies, n (%) | |||
| Statin | 93 (78.8%) | 81 (72.3%) | 0.25 |
| Hydroxychloroquine | 82 (74.5%) | 80 (73.4%) | 0.85 |
| Azithromycin | 52 (69%) | 44 (62%) | 0.35 |
| Ceftriaxone | 60 (82%) | 63 (89%) | 0.27 |
| Steroids | 22 (18.5%) | 19 (17.0%) | 0.76 |
| NSAIDS | 10 (8.4%) | 7 (6.4%) | 0.56 |
| Blood Transfusion, n (%) | 15 (14.6%) | 20 (21.3%) | 0.22 |
| Mechanical ventilation, n (%) | 93 (76.9%) | 111 (97.4%) | <0.001 |
| Prone positioning, n (%) | 57 (47.1%) | 89 (78.1%) | <0.001 |
| Paralysis, n (%) | 43 (35.5%) | 74 (64.9%) | <0.001 |
| In-hospital Dialysis, n (%) | 19 (15.7%) | 34 (29.8%) | 0.010 |
| ECMO, n (%) | 1 (0.8%) | 7 (6.2%) | 0.025 |
| Rhabdomyolysis treatment strategies, n (%) | |||
| Statin discontinuation | – | 96 (84.2%) | |
| Fluid resuscitation | – | 24 (21.1%) | |
| Diuresis | – | 10 (8.8%) | |
| ICU Complications, n (%) | |||
| Thromboembolic complication | 23 (19.0%) | 39 (34.2%) | 0.008 |
| Pulmonary complication | 103 (85.1%) | 112(98.2%) | <0.001 |
| Cardiac complication | 25 (20.7%) | 32 (28.1%) | 0.19 |
| Gastrointestinal complication | 67 (55.4%) | 86 (75.4%) | 0.001 |
| Neurologic complication | 16 (13.2%) | 21 (18.4%) | 0.27 |
| Renal complication | 75 (62.0%) | 98 (86.0%) | <0.001 |
| Shock | 96 (80.0%) | 112 (98.2%) | <0.001 |
ECMO = extracorporeal membrane oxygenation; HSAIDs = non-steroidal anti-inflammatory drugs.
When examining systemic complications, patients with rhabdomyolysis more frequently experienced thrombotic complications, pulmonary complications, gastrointestinal complications, renal complications, and shock requiring vasopressors. Table 3 characterizes and compares the systemic complications described for patients with and without rhabdomyolysis.
Rhabdomyolysis and AKI
Table 2 describes renal characteristics of patients with and without rhabdomyolysis. In summary, of 235 patients in our cohort, 163 had their ICU course complicated by AKI. No statistically significant difference in the rate of pre-existent chronic kidney disease (CKD) was observed between those with and without rhabdomyolysis. Of these 163 patients with AKI, 94 had concomitant rhabdomyolysis. Of those diagnosed with rhabdomyolysis, 82.5% had their ICU course complicated by AKI. Of all patients diagnosed with AKI, 33 (20.1%) were diagnosed following peaking of serum CK concentrations. The median hospital day of AKI diagnosis was not statistically different when comparing those with and without rhabdomyolysis. We performed additional analysis comparing median ICU admission serum creatinine values in patients with rhabdomyolysis and AKI vs. those without an eventual diagnosis of rhabdomyolysis and AKI. Median ICU admission serum creatinine was 0.84 for those with an eventual diagnosis of rhabdomyolysis and AKI compared to an admission serum creatinine value of 0.71 for those without an eventual diagnosis of rhabdomyolysis and AKI. Of those diagnosed with rhabdomyolysis, 70% developed significant oliguria and 18.4% became anuric.
Rhabdomyolysis treatment
In the majority of rhabdomyolysis patients, the most common treatment or mitigation strategy was statin avoidance or discontinuation. Of 114 patients with rhabdomyolysis, 24 (21.1%) patients were never initiated on a statin, and 72 (63.2%) patients had their statin discontinued as a result of elevated serum CK concentrations. Fluid resuscitation was provided to 24 (21.1%) patients with rhabdomyolysis, and diuresis was initiated to maintain adequate urine output for 10 (8.8%) rhabdomyolysis patients. Specific treatment strategies for our COVID-19 rhabdomyolysis patient cohort are summarized in Table 3.
Variables associated with rhabdomyolysis
Table 4 shows the results of the multivariable analysis identifying variables independently associated with rhabdomyolysis. In summary, male sex, patients with morbid obesity, SOFA score, and prone positioning were independently associated with rhabdomyolysis. Out of 29 patients who were male, morbidly obese and proned, 26 (89.7%) developed rhabdomyolysis. Supplemental Table 1 indicates all covariates included in the multivariable logistic regression. The results of model diagnostics can be found in Supplemental Table 2 and Supplemental Fig. 1.
Table 4.
Adjusted multivariable analysis (logistic regression) for identification of variables independently associated with rhabdomyolysis and AKI.
| Covariates | OR (95% CI) | P value |
|---|---|---|
| *Variables associated with rhabdomyolysis | ||
| Male sex | 2.26 (1.15, 4.45) | 0.018 |
| Morbid obesity (BMI ≥35 kg/m2) (Ref: BMI <35 kg/m2) | 2.77 (1.30, 5.92) | 0.008 |
| SOFA Score | 1.15 (1.03, 1.29) | 0.016 |
| Prone positioning | 3.85 (1.82, 8.16) | <0.001 |
| †Variables associated with AKI among rhabdomyolysis patients | ||
| Male sex | 30.18 (1.27, 716.32) | 0.035 |
AKI = acute kidney injury; BMI = body mass index; SOFA = sequential organ failure assessment.
Rhabdomyolysis multivariable analysis was performed on 235 patients to identify variables independently associated with rhabdomyolysis in this population.
AKI multivariable analysis was performed on 33 patients diagnosed with AKI following peaking of serum CK (U/L) concentration.
Moderate and severe rhabdomyolysis were defined via serum CK cutoffs of 1000 U/L to 4999 U/L and greater than or equal to 5000 U/L, respectively. Overall, 90 patients were included in the “moderate” rhabdomyolysis cohort, and 24 patients met criteria for “severe” rhabdomyolysis. Following a multinomial logistic regression analysis, male sex, race, a BMI ≥35 kg/m2, SOFA score, and prone positioning were identified as variables statistically associated with moderate rhabdomyolysis. This sub-analysis also indicated that patients with a race of “other” (non-white/Asian/Black or African American/American Indian/Alaskan Native and unknown), a BMI ≥35 kg/m2, and an elevated SOFA score were independently associated with severe rhabdomyolysis. Prone positioning trended towards significance for being independently associated with severe rhabdomyolysis.
Variables associated with AKI in patients with rhabdomyolysis
Table 4 shows results of the multivariable analysis identifying variables independently associated with developing AKI in all patients diagnosed with AKI following peaking of their serum CK concentration. A sub-analysis of these 33 patients identified male sex as highly associated with AKI in this subpopulation. Supplemental Table 1 indicates all covariates included in the multivariable logistic regression.
Discussion
To our knowledge, this is the first comprehensive study reporting on rhabdomyolysis in a large prospective cohort of critically ill COVID-19 patients. Nearly half of our patient population developed rhabdomyolysis. Male patients with morbid obesity and who were placed into prone positioning were significantly more likely to develop rhabdomyolysis. Whether rhabdomyolysis in our study was the result of critical illness per se, or was directly related to the COVID-19 infection itself remains unclear, warranting further studies. We also recognize that an additional diagnostic consideration in patients with elevated serum CK concentrations is viral myositis, which by itself is a known etiology of rhabdomyolysis. However, as the pandemic continues to dramatically unfold in many countries across the world and across many US states, we believe that sharing our experience could be helpful to front line health care providers taking care of critically ill COVID-19 patients.
Prone positioning is an important strategy used to increase alveolar recruitment and promote increased gas exchange and oxygenation in patients with ARDS.34 Prone positioning is associated with decreased mortality in patients with severe ARDS, and as such early prone positioning is recommended for COVID-19 patients with ARDS and worsening hypoxemia.35 , 36 While our data does not examine the risks and benefits of prone positioning in this vulnerable patient population, it does suggest the need for close monitoring of CK levels in mechanically ventilated and proned COVID-19 patients, especially when other variables characterized as being independently associated with rhabdomyolysis are present, such as male sex and/or morbid obesity. This will allow for early identification and intervention for patients with rising CK levels. The interplay between sex, obesity, prone positioning and rhabdomyolysis warrants further investigation.
The most common etiologies of rhabdomyolysis, such as crush injuries, drug toxicity, extreme physical exertion, metabolic myopathies, crush syndromes (traumatic or from immobilization), viral infection, and electrolyte disorders, are often easily identifiable.15 , 19 , 21 , 37 , 38 However, critically ill patients with confirmed COVID-19 have a wide spectrum of sequelae secondary to viral infection, including but not limited to coagulopathy, thrombotic events, hematologic complications, cardiac dysfunction, sepsis, shock, liver dysfunction, mesenteric ischemia, severe renal dysfunction, pulmonary dysfunction, and encephalopathy, that could have resulted in rhabdomyolysis.7 , 10 , 39, 40, 41, 42, 43 The vast majority of patients critically ill with COVID-19 in the ICU were administered Propofol during their ICU admission, and although unlikely, Propofol Infusion Syndrome (PRIS) is a potential precipitant of rhabdomyolysis in our patient population. Critical illness with numerous concomitant systemic manifestations, multiple medication administration, and high rates of pharmacologic paralysis make identification of a primary etiology for rhabdomyolysis in our cohort challenging; in fact, the etiology is most likely multifactorial. Additionally, fluid balance and the potential for inadequate resuscitation may be another complicating factor in the development of rhabdomyolysis in our cohort. However, the pathophysiology of COVID-19 remains incompletely elucidated, and as such, a direct impact of the SARS-CoV-2 virus on the musculoskeletal system cannot be definitely ruled out. To aid in the early identification, management, and mitigation of rhabdomyolysis, we recommend trending serum CK concentrations throughout ICU admission, particularly for patients with any one of the aforementioned variables found to be associated with rhabdomyolysis in our cohort of critically ill COVID-19 patients.
Our results suggest that the majority of patients did not present to the ED with rhabdomyolysis, but rather developed rhabdomyolysis during their ICU admission. Many of these patients had concomitant AKI. Multiple theories to explain AKI as a complication of rhabdomyolysis exist, including a direct toxic effect of myoglobin on tubular cells, tubular obstruction by myoglobin, or changes in glomerular filtration rate secondary to ischemic changes to the kidneys resulting from the release of vasoconstrictive mediators.17 , 44 In addition, some reports suggest that SARS-CoV-2 may cause direct damage to kidneys. Postmortem pathologic examination of kidney damage in 26 patients with severe COVID-19 characterized extensive acute tubular and endothelial injury with evidence of direct parenchymal, tubular, epithelial, and podocyte viral infection.11 Another recent study by Naar et al reported that nearly 3 in 4 critically ill COVID-19 patients develop AKI during their ICU stay.45 Their analysis also identified a significantly higher mortality rate in patients with AKI. In our study, the median hospital day of peak serum CK concentration was day six, while the median hospital day of AKI diagnosis was on the first day of hospitalization, suggesting that rhabdomyolysis is unlikely to be the sole or even the primary etiology behind AKI for the majority of patients. Still, nearly one third of rhabdomyolysis patients developed AKI after peaking of serum CK concentrations, and as such, early identification and management of rhabdomyolysis in COVID-19 patients may help prevent further decline in renal function, and potentially decrease AKI-related mortality. Close monitoring of renal specific laboratory values should be made in the male patient, as male sex was independently associated with AKI following rhabdomyolysis.
This study has a few limitations. First, this is a single institutional experience with potentially limited generalizability. Second, the use of serum CK concentration alone without additional confirmatory tests (e.g. myoglobin in urine, muscle biopsy) to diagnose rhabdomyolysis may have led to overdiagnosis of rhabdomyolysis. Due to inconsistent and limited recordings of serum CK-MM (muscle breakdown) levels in this cohort, our capacity to confirm that elevated serum CK concentrations was secondary to skeletal muscle breakdown was not possible. Additionally, future investigations should focus on parsing out the potential role of fluid under-resuscitation in the development of rhabdomyolysis in critically ill COVID-19 patients. The occurrence of AKI in many patients prior to rhabdomyolysis limits our ability to clearly decipher the renal impairment that can be attributed to rhabdomyolysis. Future efforts should focus on characterizing the link between rhabdomyolysis and worsening renal impairment. Lastly, longer-term and post-ICU follow up for our cohort is a natural next-step, and we are planning to undertake additional chart review to capture these data to assess the long-term impact of rhabdomyolysis and AKI on renal function in critically-ill subjects who recover from COVID-19.
In this large cohort of critically ill COVID-19 patients, nearly half developed rhabdomyolysis. We found male patients who were obese and underwent prone positioning to have a particularly high incidence of rhabdomyolysis, warranting close observation and a low threshold for diagnostic consideration of rhabdomyolysis in this cohort. Additionally, we were able to characterize shared and unique variables associated with moderate and severe rhabdomyolysis in our cohort, including male sex, race, morbid obesity, SOFA score, and prone positioning. Further studies are warranted to confirm our findings, but healthcare providers on the front lines need to be aware of this complication in order to prevent it and mitigate its adverse effects.
Author Contributions
Ava K. Mokhtari was the primary author for this study, and contributed in a significant way towards study conception, study design, literature search, data collection, data analysis, data interpretation, manuscript writing, table and figure design, and critical revisions. Lydia R. Maurer contributed to the study conception, study design, data collection, data interpretation, manuscript writing, table and figure design, critical revisions, and helped with study supervision. Mathias A. Christensen helped with study design, data collection, data interpretation, manuscript writing, and critical revisions. Mohamad El Moheb helped with study design, data collection, data interpretation, manuscript writing, and critical revisions of the manuscript. Leon Naar was a significant contributor for data collection, data interpretation, and critical revisions of the manuscript. Osaid Alser helped with data collection, data interpretation, model diagnostics, and critical revisions of the manuscript. Apostolos Gaitanidis helped with data collection, data interpretation, and critical revisions of the manuscript. Kimberly Langeveld helped with data collection, data interpretation, and critical revisions of the manuscript. Carolijn Kapoen helped with data collection, data interpretation, and critical revisions of the manuscript. Kerry Breen helped with data collection, data interpretation, and critical revisions of the manuscript. George C. Velmahos helped guide this study with respect to the study design, data interpretation, manuscript writing, table and figure design, critical revisions, and study supervision. Lastly, Haytham M.A. Kaafarani was integral in helping generate the study concept, the design of the study, data interpretation, manuscript writing, table and figure design, critical revisions, and was the key figure providing study supervision.
Disclosure
Dr. Kaafarani is a member of the Editorial Board of the Journal of Surgical Research; as such, he was excluded from the entire peer-review and editorial process for this manuscript.
Acknowledgments
No specific financial support was used for funding.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jss.2021.03.049.
Appendix. Supplementary materials
References
- 1.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L-L, Hsu C-W, Tian Y-C, Fang J-T. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome: severe acute respiratory syndrome. Int J Clin Pract. 2005;59:1162–1166. doi: 10.1111/j.1368-5031.2005.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaafarani HM, El Moheb M, Hwabejire JO, et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272:e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16:308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore HB, Barrett CD, Moore EE, et al. Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome? J Trauma Acute Care Surg. 2020;88:1–2. doi: 10.1097/TA.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20:135. doi: 10.1186/s13054-016-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58–69. [PMC free article] [PubMed] [Google Scholar]
- 16.Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749–756. doi: 10.1515/CCLM.2010.151. [DOI] [PubMed] [Google Scholar]
- 17.Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988;148:1553. [PubMed] [Google Scholar]
- 18.Brown CVR, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56:1191–1196. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 19.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 20.Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27:803–811. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 21.Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore) 2005;84:377–385. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 22.El-Abdellati E, Eyselbergs M, Sirimsi H, et al. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care. 2013;3:8. doi: 10.1186/2110-5820-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swaringen JC, Seiler JG, Bruce RW. Influenza A induced rhabdomyolysis resulting in extensive compartment syndrome. Clin Orthop Relat Res. 2000:243–249. doi: 10.1097/00003086-200006000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Paletta CE, Lynch R, Knutsen AP. Rhabdomyolysis and lower extremity compartment syndrome due to influenza B virus. Ann Plast Surg. 1993;30:272–273. doi: 10.1097/00000637-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Joshi MK, Liu HH. Acute rhabdomyolysis and renal failure in HIV-infected patients: risk factors, presentation, and pathophysiology. AIDS Patient Care STDS. 2000;14:541–548. doi: 10.1089/108729100750018308. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee A, Ghosh R, Aftab G. Rhabdomyolysis in a patient with coronavirus disease 2019. Cureus. 2020;12:e8956. doi: 10.7759/cureus.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J-L, Wang J-T, Yu C-J, et al. Rhabdomyolysis associated with probable SARS. Am J Med. 2003;115:421–422. doi: 10.1016/S0002-9343(03)00448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KH, Farouji I, Abu Hanoud A, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am J Emerg Med. 2020;38 doi: 10.1016/j.ajem.2020.05.015. 1548.e1-1548.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26:1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain R, Corcuera-Solano I, Dayan E, Jacobi AH, Huang M. Rhabdomyolysis as a manifestation of a severe case of COVID-19: a case report. RCR. 2020;15:1633–1637. doi: 10.1016/j.radcr.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valente-Acosta B, Moreno-Sanchez F, Fueyo-Rodriguez O, Palomar-Lever A. Rhabdomyolysis as an initial presentation in a patient diagnosed with COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivas-García S, Bernal J, Bachiller-Corral J. Rhabdomyolysis as the main manifestation of coronavirus disease 2019. Rheumatology. 2020;59:2174–2176. doi: 10.1093/rheumatology/keaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical practice guideline for acute kidney injury (AKI) 2012. Acute Kidney Injury (AKI). Accessed June 3, 2020. https://kdigo.org/guidelines/acute-kidney-injury/
- 34.Cornejo RA, Díaz JC, Tobar EA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188:440–448. doi: 10.1164/rccm.201207-1279OC. [DOI] [PubMed] [Google Scholar]
- 35.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 36.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis – an overview for clinicians. Crit Care. 2004;9:158. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12:e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Heptol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honda N. Acute renal failure and rhabdomyolysis. Kidney Int. 1983;23:888–898. doi: 10.1038/ki.1983.112. [DOI] [PubMed] [Google Scholar]
- 45.Naar L, Langeveld K, El Moheb M, et al. Acute kidney injury in critically-ill patients with COVID-19: a single-center experience of 206 consecutive patients. Ann Surg. 2020;272:e280–e281. doi: 10.1097/SLA.0000000000004319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.