Abstract
The coronavirus disease 2019 (COVID-19) pandemic has significantly affected utilization of preventative health care, including vaccines. We aimed to assess HPV vaccination rates during the pandemic, and conduct a simulation model-based analysis to estimate the impact of current coverage and future pandemic recovery scenarios on disease outcomes. The model population included females and males of all ages in the US. The model compares pre-COVID vaccine uptake to 3 reduced coverage scenarios with varying recovery speed. Vaccine coverage was obtained from Truven Marketscan™. Substantially reduced coverage between March-August 2020 was observed compared to 2018–2019. The model predicted that 130,853 to 213,926 additional cases of genital warts; 22,503 to 48,157 cases of CIN1; 48,682 to 110,192 cases of CIN2/3; and 2,882 to 6,487 cases of cervical cancer will occur over the next 100 years, compared to status quo. Providers should plan efforts to recover HPV vaccination and minimize potential long-term consequences.
Keywords: COVID-19, 9-valent human papillomavirus (HPV) vaccine, United States, Genital warts, Cervical cancer
1. Introduction
Since the introduction of the first human papillomavirus (HPV) vaccine in 2006, the proportion of United States (US) adolescents 13–17 years old who have received at least one dose of the vaccine has climbed to 71% [1]. In the first 8 years following introduction of the vaccine, the prevalence of HPV infection caused by subtypes included in the vaccine fell by 71% in 14 to 19-year-old females and by 61% in 20- to 24-year old females [2]. In May 2018, the World Health Organization (WHO) invited participation in a global call to action aimed at eliminating cervical cancer, with a Global Strategy to Accelerate the Elimination of Cervical cancer launched in November 2020 [3]. A key goal of this initiative is for 90% of girls to be fully vaccinated with the HPV vaccine by the age of 15 years by the year 2030.
The pandemic caused by the SARS-CoV-2 virus (COVID-19) has significantly impacted the utilization of preventative health care services, including vaccines. For example, data from the Michigan Care Improvement Registry showed that the proportion of patients up-to-date with Advisory Committee on Immunization Practices (ACIP)-recommended vaccines was significantly lower in May 2020 compared to the previous 4 years [4]. Vaccine utilization via the Vaccines for Children Program showed that the number of vaccines administered in April 2020 as low as 42% of the previous year’s figures [5]. To the best of our knowledge, no data specific to the HPV vaccine are available. Due to concern for outbreaks of vaccine-preventable diseases, WHO issued guidance encouraging the prioritization of immunization as allowed under safe conditions without undue risk to patients, caregivers, and providers [6].
Given concern for delayed or missed HPV vaccination due to COVID-19 pandemic, it is important to understand how changes in vaccination behaviors might impact short- and long-term outcomes such as cases of genital warts and cervical diseases and cancers caused by HPV. Therefore, we aimed to assess HPV vaccination rates during the COVID-19 pandemic, and to conduct a model-based simulation to estimate the impact of vaccine coverage changes due to the pandemic and future recovery of coverage to pre-COVID rates in response to the end of the pandemic driven by the availability of a COVID-19 vaccine.
2. Material and methods
This is an application of a previously published compartmental dynamic transmission model that assessed the public health and economic impacts of the 9-valent HPV vaccine (HPV9; GARDASIL® 9) in various populations [7], [8], [9], [10], [11]. The model estimated the impact of different HPV coverage scenarios over a 100-year time horizon. The model includes the entire US population, estimated at 330 million [12], and assumes a constant population size and age distribution.
Changes in coverage for HPV vaccines were estimated from Truven Marketscan™ Commercial and Medicare Supplemental Database data from January 2018 through December 2019, and Truven Marketscan™ Early View data [13] from January 2020 through August 2020 (latest data release at the time the analysis was conducted). For each month of 2020 through August, the rate of vaccination was divided by the average of the rate in the corresponding month of 2018 and 2019 to create a raw ratio. The coverage reduction factor is finally obtained by normalizing the ratio such that the reduction factor for January 2020 is 1.0. The reduction factor was projected to corresponding months of subsequent years according to assumptions described below.
The model compares four scenarios with respect to HPV vaccine coverage among males and females 9–26 years old. The pre-COVID status quo scenario assumes age-specific coverage as shown in Table 1 . The status quo scenario assumes pre-COVID coverage remains constant over time beginning in January 2020. The other three scenarios assume that a COVID-19 vaccine will be available to the general public in early summer 2021 and will trigger a recovery in routine health care utilization including HPV vaccine coverage. Each of these scenarios starts with the coverage that results from multiplying status quo coverage by the reduction factor calculated above and assuming that the August 2020 coverage is maintained through May 2021 at which point we model different recovery scenarios. In Scenario 1, we assume that the coverage increases linearly from June 2021 through December 2021 and returns to previous coverage by January 2022. In Scenario 2, the coverage increases linearly from June 2021 through December 2022 and returns to previous coverage by January 2023. In Scenario 3, the recovery from June 2021 through December 2021 only reaches 85% of the status quo and continues to increase linearly to full recovery by January 2028. This scenario represents a future with greater vaccine hesitancy. We do not explicitly model a COVID-related HPV catch-up program, however, a substantial portion of those that miss vaccination in either primary or catch-up cohorts will be caught up in future years by virtue of our assumption of return to pre-COVID coverage for all cohorts.
Table 1.
Pre-COVID status quo annual HPV vaccine coverage by age.
| Age Groups (years) | 9–11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19–26 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-COVID Status Quo | Female | 0 | 68.4 | 36.0 | 22.3 | 4.1 | 5.3 | 16.9 | 16.9 | 3.4 |
| Male | 0 | 65.4 | 22.8 | 10.9 | 5.4 | 7.8 | 11.7 | 11.7 | 2.3 | |
These represent the percentage of people in each age group that are vaccinated annually with one or more doses of HPV vaccine. Note, the coverage percentages are applied only to the unvaccinated population in each age group at any point in time. Coverage is derived from the 2019 NIS-Teen Survey vaccine coverage data. Details of the assumptions used in the derivation are described in Daniels et al. [11].
2.1. Model inputs
Model inputs included US population size, vaccine efficacy, disease treatment patterns, sexual behavior, screening practices, and HPV and disease related natural history assumptions. Full details of the model inputs and references can be found in previous publications [7], [8], [9], [10], [11]. Unique to this analysis are the vaccine coverage assumptions outlined previously.
2.2. Model outputs
Key model outputs included the number of additional diagnosed cases of genital warts, cervical intraepithelial neoplasia (CIN) 1/2/3, and cervical cancer over 100 years resulting from reduced coverage in Scenarios 1–3 compared to status quo. All outcomes include only HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58-related disease. In addition, the model predicts when the peak incidence of these excess events would be expected to occur.
3. Results
3.1. Vaccine coverage
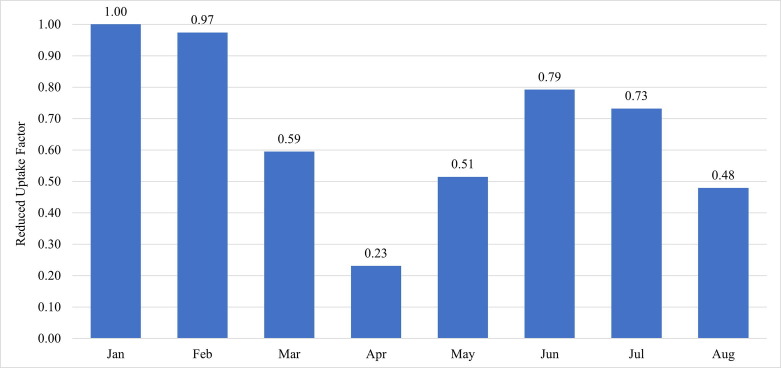
The analysis using Truven Marketscan™ data showed substantially reduced coverage beginning in March 2020 and continuing through August 2020 (Fig. 1 ), compared to the corresponding months from 2018 and 2019 (Table 1). Coverage decreased through March and April, reaching a low of 23% of the previous years’ rate. Coverage increased through May and June to a high of 79% of the previous rate and fell again in July and August.
Fig. 1.
Normalized HPV vaccine coverage factor by month in 2020 in comparison to 2018/2019. In all scenarios, we assume the August factor remains constant through June 2021.
3.2. Model outcomes
Even under the most optimistic recovery assumptions (Scenario 1), more than 130,000 additional cases of genital warts, more than 22,000 cases of CIN1, and more than 48,000 cases of CIN2/3 were predicted to occur over the next 100 years, compared to the status quo (Fig. 2 ). Of these, over half of the cases of genital warts (67,198 cases; 51%), CIN1 (13,368 cases; 59%), and CIN2/3 (27,031 cases; 55%) were projected to occur within the first 25 years. Under the least optimistic of our assumptions (Scenario 3), over 169,000 additional cases of genital warts, 40,000 cases of CIN1, and 92,000 cases of CIN2/3 were predicted to occur over the next 50 years. Incidence of genital warts over time is shown in Fig. 3 A. Peak incidence of additional genital warts in the general population was predicted to occur in the year 2032 in Scenario 1, 2033 in Scenario 2, and 2034 in Scenario 3.
Fig. 2.
Additional cases of genital warts and cervical intraepithelial neoplasia (CIN) over 100 years compared to status quo. Scenario 1, Reduced coverage during the COVID-19 pandemic with rapid recovery to baseline coverage by January 2022; Scenario 2, Reduced coverage during the COVID-19 pandemic with slow recovery to baseline coverage by the end of December 2022; Scenario 3, Reduced coverage during the COVID-19 pandemic with partial recovery to 85% of previous years’ coverage by the end of December 2021 and full recovery by 2028.
Fig. 3.
Status quo incidence (right Y axis) and additional incidence for 3 scenarios (left Y axis) of genital warts (panel A) and cervical cancer (panel B).
The model estimated that over 100 years, an additional 2,882 cases of cervical cancer would occur in Scenario 1 compared to the status quo, an additional 3,884 cases in Scenario 2, and an additional 6,487 cases in Scenario 3. Of these, the percentage of cases occurring during the first 50 years was 66% in Scenarios 1 and 2 and 60% in Scenario 3. Incidence of cervical cancer over time is shown in Fig. 3B. Peak excess incidence of cervical cancer in the general female population was predicted to occur in the year 2054 in Scenario 1, 2055 in Scenario 2, and 2058 in Scenario 3.
4. Discussion
The model demonstrates how the impact of the disruption caused by COVID-19 reaches far beyond this disease, as its impact on HPV vaccination has the potential to slow progress made in recent years toward the goal of eliminating cervical cancer. The impact of missed vaccinations can be both early and prolonged, with excess cases of genital warts peaking in 2032 and peak incidence of excess cases of cervical cancer as late as 2058.
Globally, WHO estimates that achieving 90% vaccination rate by 2030 would reduce cervical cancer incidence by 42% by the year 2045 [3]. Our model shows how the decline in HPV vaccination rates in the United States are projected to slow the progress that is being made, but the global impact of the COVID-19 pandemic on our ability to meet WHO targets is not yet known.
To our knowledge this is the first report of vaccination rates for the HPV vaccine during the COVID-19 pandemic and confirms the reduction in vaccine coverage seen with other vaccines and populations. Our analysis showed that the HPV vaccination rate in April 2020 was approximately a quarter of the rate in April 2018 and 2019. The impact of the current “second wave” of COVID-19 could be expected to keep coverage low for the near future.
Catch-up vaccination of adolescents who missed vaccination during the pandemic would likely avert some of this disease, bringing future scenarios closer to the pre-COVID scenario. Post-pandemic efforts to improve HPV vaccination rates will be needed to reduce the potential impact of the pandemic on HPV-related disease, focusing on adolescents who would have been vaccinated during the pandemic. Healthcare systems and health providers should plan outreach to patients to encourage them to resume wellness visits as soon as it is safe to do so, and every health care visit should be viewed as an opportunity to review vaccination status and catch-up patients as needed.
4.1. Limitations
This analysis has some limitations. The Marketscan™ Early View data consist of adjudicated claims for commercially-insured patients, which may be incomplete [13]. Coverage may change as additional claims are adjudicated, and the population may not be representative of the overall US population. The scenarios included in the model are based on assumptions about the timing of the availability of COVID-19 vaccine, and speculation about the how the population HPV vaccination-seeking behavior changes in response to having an end to the pandemic in sight. Actual recovery from the pandemic may differ from these scenarios. The model does not account for missed cervical cancer screenings during the pandemic, or any changes in sexual activity resulting from COVID-19 related stay-at-home orders. One study estimated that 40,000 cervical cancer screenings were missed between March 15 and June 16, 2020, representing a 67% reduction from the historical average, which may also negatively impact cervical cancer outcomes in the near term [14]. The model assumes homogeneous changes in HPV vaccination nationally, whereas there may be substantial variation sub-nationally due to various state vaccination policies and pandemic response and recovery efforts. The model does not account for potential changes in background cancer incidence that is independent of HPV vaccination.
5. Conclusion
Missed HPV vaccinations due to COVID-19 may result in preventable excess cases of genital warts, CIN1/2/3, and cervical cancer as well as other HPV-related diseases and cancers. Healthcare providers should plan for recovery efforts by seeking to immunize all of their eligible patients in the coming months and years in order to promptly recover HPV vaccination and to minimize potential long-term consequences.
Funding
This work was supported by Merck & Co., Inc.
Declaration of Competing Interest
Vincent Daniels, Kunal Saxena, Craig Roberts, Lixia Yao, and Smita Kothari are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may own Merck & Co., Inc., Kenilworth, NJ, USA restricted stock units and/or stock options. Shelby Corman is an employee of Pharmerit – an OPEN Health Company, which received consulting fees in conjunction with this work. Linda Niccolai is a Scientific Advisor for Merck and Scientific Advisory Board member for Moderna.
References
- 1.Centers for Disease Control and Prevention. 2019 Adolescent Human Papillomavirus (HPV) Vaccination Coverage Dashboard. Available at. Accessed November 23 2020. https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/hpv/dashboard/2019.html.
- 2.Oliver S.E., Unger E.R., Lewis R., McDaniel D., Gargano J.W., Steinau M., et al. Prevalence of Human Papillomavirus Among Females After Vaccine Introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216(5):594–603. doi: 10.1093/infdis/jix244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Available at: Accessed November 23 2020. https://apps.who.int/iris/bitstream/handle/10665/336583/9789240014107-eng.pdf.
- 4.Bramer C.A., Kimmins L.M., Swanson R., Kuo J., Vranesich P., Jacques-Carroll L.A., et al. Decline in Child Vaccination Coverage During the COVID-19 Pandemic - Michigan Care Improvement Registry, May 2016-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(20):630–631. doi: 10.15585/mmwr.mm6920e1. [DOI] [PubMed] [Google Scholar]
- 5.Santoli J.M., Lindley M.C., DeSilva M.B., Kharbanda E.O., Daley M.F., Galloway L., et al. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):591–593. doi: 10.15585/mmwr.mm6919e2. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guiding principles for immunization activities during the COVID-19 pandemic. Available at: https://apps.who.int/iris/handle/10665/331590. Accessed October 6 2020.
- 7.Elbasha E.H., Dasbach E.J. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Elbasha E.H., Dasbach E.J., Insinga R.P., Haupt R.M., Barr E. Age-based programs for vaccination against HPV. Value Health. 2009;12(5):697–707. doi: 10.1111/j.1524-4733.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Elbasha E.H., Dasbach E.J., Insinga R.P. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbasha E.H., Dasbach E.J., Insinga R.P. A multi-type HPV transmission model. Bull Math Biol. 2008;70(8):2126–2176. doi: 10.1007/s11538-008-9338-x. [DOI] [PubMed] [Google Scholar]
- 11.Daniels V, Prabhu VS, Palmer C, et al. Public health impact and cost-effectiveness of catch-up 9-valent HPV vaccination of individuals through age 45 years in the United States. Hum Vaccin Immunother. 2021:1-9. [DOI] [PMC free article] [PubMed]
- 12.Bureau USC. U.S. and World Population Clock. Available at: Accessed March 25 2021. https://www.census.gov/popclock/.
- 13.IBM Watson Health. IBM MarketScan Research Databases for Health Services Researchers. 2019; Available at. Accessed March 23 2021. https://www.ibm.com/downloads/cas/6KNYVVQ2.
- 14.Epic Health Research Network. Delayed Cancer Screenings—A Second Look. Available at. Accessed November 23 2020. https://www.ehrn.org/articles/delayed-cancer-screenings-a-second-look/.