Abstract
Background
Despite high contagiousness and rapid spread, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to heterogeneous outcomes across affected nations. Within Europe (EU), the United Kingdom (UK) is the most severely affected country, with a death toll in excess of 100,000 as of January 2021. We aimed to compare the national impact of coronavirus disease 2019 (COVID-19) on the risk of death in UK patients with cancer versus those in continental EU.
Methods
We performed a retrospective analysis of the OnCovid study database, a European registry of patients with cancer consecutively diagnosed with COVID-19 in 27 centres from 27th February to 10th September 2020. We analysed case fatality rates and risk of death at 30 days and 6 months stratified by region of origin (UK versus EU). We compared patient characteristics at baseline including oncological and COVID-19–specific therapy across UK and EU cohorts and evaluated the association of these factors with the risk of adverse outcomes in multivariable Cox regression models.
Findings
Compared with EU (n = 924), UK patients (n = 468) were characterised by higher case fatality rates (40.38% versus 26.5%, p < 0.0001) and higher risk of death at 30 days (hazard ratio [HR], 1.64 [95% confidence interval {CI}, 1.36–1.99]) and 6 months after COVID-19 diagnosis (47.64% versus 33.33%; p < 0.0001; HR, 1.59 [95% CI, 1.33–1.88]). UK patients were more often men, were of older age and have more comorbidities than EU counterparts (p < 0.01). Receipt of anticancer therapy was lower in UK than in EU patients (p < 0.001). Despite equal proportions of complicated COVID-19, rates of intensive care admission and use of mechanical ventilation, UK patients with cancer were less likely to receive anti–COVID-19 therapies including corticosteroids, antivirals and interleukin-6 antagonists (p < 0.0001). Multivariable analyses adjusted for imbalanced prognostic factors confirmed the UK cohort to be characterised by worse risk of death at 30 days and 6 months, independent of the patient's age, gender, tumour stage and status; number of comorbidities; COVID-19 severity and receipt of anticancer and anti–COVID-19 therapy. Rates of permanent cessation of anticancer therapy after COVID-19 were similar in the UK and EU cohorts.
Interpretation
UK patients with cancer have been more severely impacted by the unfolding of the COVID-19 pandemic despite societal risk mitigation factors and rapid deferral of anticancer therapy. The increased frailty of UK patients with cancer highlights high-risk groups that should be prioritised for anti–SARS-CoV-2 vaccination. Continued evaluation of long-term outcomes is warranted.
Keywords: COVID-19, SARS-CoV-2, Cancer, UK, Europe, Mortality
1. Introduction
Coronavirus disease 2019 (COVID-19) has, since its emergence in late 2019 [1], claimed the life of nearly 2 million people worldwide as of January 2021. The response of healthcare services to the escalating threat posed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to significant changes in the practice of medicine including reorganisation and redeployment of the workforce, modification to emergency and elective services and expansion of community and in-hospital SARS-CoV-2 testing to facilitate early recognition of the disease and reduce risk of mortality in patients and healthcare workers.
In over a year of rapidly accumulating observational evidence, it is now clear that COVID-19 disproportionally affects the elderly and those with comorbidities [[2], [3], [4], [5]]. Patients with cancer are inherently susceptible to severe SARS-CoV-2, and determinants of mortality such as age, comorbid burden and presence of active malignancy have been reproducibly documented as drivers of an adverse disease course across studies [[6], [7], [8], [9], [10]].
Despite some evidence regarding the negative role of previous chemotherapy [11,12], anticancer therapy does not appear to worsen the prognosis from COVID-19. However, the immunosuppressive nature of most systemic anticancer therapies (SACTs), the requirement for regular hospital attendance and the risk of morbidity and hospitalisation from treatment-related adverse events have induced a more cautious delivery of oncological therapies in an attempt to prevent harm and avoid SARS-CoV-2 exposure.
Despite national lockdowns, social distancing measures, broad-reaching precautionary attempts and early dissemination of clinical practice guidelines, the United Kingdom (UK) has registered the highest number of SARS-CoV-2–related deaths in Europe (EU), with a death toll in excess of 100,000 patients as of January 2021 [13].
It is unknown whether the higher mortality observed in the general UK population translates into worse outcomes from COVID-19–infected patients with cancer. Previous results from the OnCovid study have revealed a higher case fatality rate in the UK (44.4%) than in Italy (33.2%) and Spain (29.6%) [6]. Understanding whether there is regional variation in the natural course of COVID-19 is of utmost importance in the context of a still-unresolved healthcare crisis. Such effort not only helps portraying the healthcare system response to COVID-19 but also can aid characterisation of geographical heterogeneity in the clinical characteristics underlying the vulnerability of patients with cancer to SARS-CoV-2 infection.
In addition to regional differences in case fatality rates from COVID-19, it is important to understand whether deferral and discontinuation of SACT recommended at the onset of the pandemic [14] might have impacted the overall survival (OS) of patients with cancer in the UK, a population that is already characterised by poorer 5-year survival outcomes in a number of solid tumours [15].
In an attempt to prevent indiscriminate deferral of therapy, which is known to affect oncological outcomes in cancer [[16], [17], [18]], in March 2020, the UK National Health Service identified 6 priority levels for SACT based on treatment intent and expected efficacy so that treatment can proceed for those in whom benefits clearly outweigh risks [19].
In this ad hoc analysis of the OnCovid registry, we aimed to compare and contrast the risk of death after diagnosis of COVID-19 in patients with cancer diagnosed in the UK versus those diagnosed in continental EU.
2. Study design and outcomes
OnCovid (NCT04393974) is an active European registry study that has collected, since the beginning of the pandemic, consecutive patients fulfilling the following inclusion criteria: (1) age ≥18 years, (2) diagnosis of SARS-CoV-2 infection confirmed by Reverse transcription polymerase chain reaction (RT-PCR) of a nasopharyngeal swab [20] and (3) history of solid or haematologic malignancy, at any time during the patient's past medical history, either active or in remission at the time of COVID-19 diagnosis. Patients with a history of non-invasive/premalignant lesions or with low malignant potential (i.e. basal cell carcinoma of the skin, non-invasive carcinoma in situ of the cervix and ductal carcinoma in situ) were excluded. For haematologic malignancies, only patients with a history of oncologic diseases with defined malignant behaviour (lymphoma, leukaemia and multiple myeloma) were included.
As a primary end-point of our study, we elected the all-cause 30-day risk of death, a measure that mirrors end-points used in clinical trials of COVID-19 therapeutics [21]. In view of the extended length of follow-up of our cohort compared with earlier studies reporting case fatality rates censored at 14 days of observation [[6], [7], [8], [9],22,23], we reported, as an additional study end-point, all-cause risk of death at 6 months after COVID-19 diagnosis. The choice of this additional end-point allowed us to preliminarily investigate determinants of longer-term prognosis in COVID-19 survivors [[24], [25], [26]].
In comparing outcomes from UK and EU patients, we evaluated the distribution of baseline characteristics already known to be major determinants of mortality [[6], [7], [8],22,23]. These included gender, age, number of comorbidities, smoking history, tumour type (clustered as breast, gastrointestinal, gynaecological/genitourinary, haematological, thoracic and others) [[7], [8], [9],27], tumour stage (defined as advanced versus non-advanced as per disease-specific criteria), tumour status (presence of active versus non-measurable disease), receipt of anticancer or anti–COVID-19 therapy and occurrence of complicated COVID-19 as described before [6]. The role of each determinant of mortality was explored across the two cohorts using univariable analysis. Accounting for their unbalanced distribution across cohorts, a fixed multivariable regression analysis model was adopted to verify their independent prognostic role.
The differential distribution across UK and EU patients of other characteristics of interest including hospitalisation and intensive care unit (ICU) admission rates, need for supplemental oxygen therapy and assisted ventilation, emergence of COVID-19–related complications and receipt of COVID-19–specific therapy was also reported as described previously [6,23]. In addition, we reported rates of permanent discontinuation of anticancer therapy among those patients who were listed as receiving anticancer therapy at COVID-19 diagnosis, including only patients alive after 30 days since COVID-19 diagnosis.
3. Study procedures
OnCovid was granted central approval by the UK Health Research Authority (20/HRA/1608) and by the corresponding research ethics committees at each participating institution outside the UK. Core study data were collated from electronic medical records into a case report form designed using Research Electronic Data Capture software (REDCap; Vanderbilt University, Nashville, TN, United States of America [USA]). Multisite access and data curation was coordinated by the Medical Statistics Unit in Novara, Italy. A list of participating centres is provided in Supplementary Table 1. Six institutions were from the UK, and 21 institutions were from continental EU. The data cut-off for the present analysis was 1st November 2020.
4. Statistical analysis
Key baseline characteristics were summarised as categorical variables and reported as counts and percentages. Associations between categorical variables were tested using the Pearson χ2 test. OS and all-cause 30-day and 6-month survival curves for the two cohorts of interest were also reported as per the Kaplan-Meier method and compared using the log-rank test. OS was defined as the survival interval from COVID-19 diagnosis to death and/or last follow-up. Univariable and multivariable Cox proportional hazards models were used to assess the impact of the factors and the geographical area (the UK vs EU) on risk of death from all causes at 30-day and 6-month landmark time points. All the explored baseline characteristics have been included in the multivariable model, in view of their strong linkage with mortality within the study population [6,23] and because of their differential distribution across the UK and EU cohorts. The results of Cox regression analysis were presented as hazard ratios (HRs) and the corresponding 95% confidence intervals (95% CIs). A p-value of <0.05 was considered statistically significant. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA), and SPSS, version 25 (IBM Inc.).
5. Results
5.1. Demographic features of UK and EU patients with cancer and COVID-19
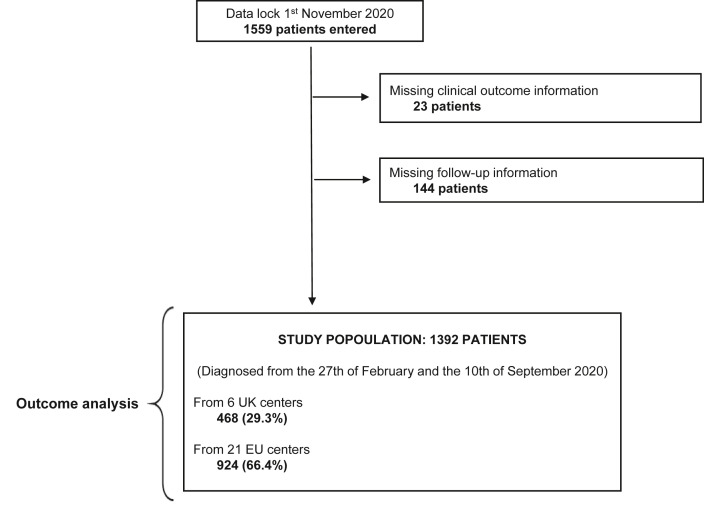
At database lock, the registry included 1559 patients consecutively diagnosed with COVID-19. A total of 167 patients were excluded because of missing outcome data (n = 23) or loss to follow-up (n = 144). The final population consisted of 1392 patients accrued from 27 institutions across 6 countries (UK, Italy, Spain, France, Belgium and Germany) and diagnosed with COVID-19 between 27th February and 10th September 2020 (Fig. 1 ). Patient distribution across the participating centres is provided in Supplementary Table 1.
Fig. 1.
Study diagram. UK, United Kingdom; EU, Europe.
The UK cohort included 468 patients (33.6%), whereas the continental EU cohort included 924 patients (66.4%). The distribution of baseline patient characteristics across cohorts is summarised in Table 1 .
Table 1.
Patient characteristics of the cohorts of interest.
| EU cohort |
UK cohort |
χ2 test |
|
|---|---|---|---|
| N = 924 (%) | N = 468 (%) | p-value | |
| Gender | |||
| Male | 451 (48.86) | 287 (61.72) | <0.0001 |
| Female | 472 (51.14) | 1778 (38.28) | |
| Missing | 4 | ||
| Age | |||
| <65 years | 382 (41.75) | 151 (32.26) | 0.0006 |
| ≥65 years | 533 (58.25) | 317 (67.74) | |
| Missing | 9 | ||
| Number of comorbidities | |||
| 0–1 | 420 (45.45) | 175 (37.39) | 0.0041 |
| ≥2 | 504 (54.55) | 293 (62.61) | |
| Missing | 0 | ||
| Smoking history | |||
| Never-smokers | 407 (44.97) | 192 (41.29) | 0.8355 |
| Former/current smokers | 383 (42.32) | 176 (37.85) | |
| Missing | 234 | ||
| Cancer site | |||
| Breast | 219 (23.70) | 58 (12.39) | <0.0001 |
| Gastrointestinal | 167 (18.07) | 92 (19.66) | |
| Gynaecological/genitourinary | 132 (14.29) | 146 (31.20) | |
| Haematological | 172 (18.61) | 53 (11.32) | |
| Lung | 118 (12.77) | 58 (12.64) | |
| Other | 116 (12.55) | 61 (13.03) | |
| Missing | 0 | ||
| Tumour stage | |||
| Local/locoregional | 390 (42.21) | 294 (62.82) | <0.0001 |
| Advanced | 380 (41.13) | 151 (32.26) | |
| Missing | 177 | ||
| Tumour status | |||
| Remission/non-measurable disease | 299 (32.97) | 150 (32.97) | 0.9996 |
| Active malignancy | 608 (67.03) | 305 (67.03) | |
| Missing | 30 | ||
| Anticancer therapy at COVID-19 diagnosis | |||
| No | 371 (40.55) | 278 (61.64) | <0.0001 |
| Yes | 544 (59.55) | 173 (38.36) | |
| Missing | 26 | ||
| COVID-19 therapy (any) | |||
| No | 224 (25.31) | 163 (39.47) | <0.0001 |
| Yes | 661 (74.69) | 250 (60.53) | |
| Missing | 94 | ||
| Complicated COVID-19 | |||
| No | 367 (39.72) | 179 (8.25) | 0.5955 |
| Yes | 557 (60.28) | 289 (61.75) | |
| Missing | 0 | ||
COVID-19, coronavirus disease 2019; EU, Europe; UK, United Kingdom.
The distribution of primary tumours between the two cohorts was significantly different across cohorts (p < 0.0001): the UK cohort had a lower proportion of patients with breast cancer (12.39% vs 23.70%) and haematological malignancies (11.32% vs 18.61%) and a higher proportion of patients with gynaecological/genitourinary cancer (31.20% vs 14.29%) than the continental EU cohort.
Compared with the rest of EU, the UK cohort included a significantly higher proportion of patients with adverse baseline features with respect to COVID-19–related outcomes, including male gender (61.72% vs 48.86%, p < 0.0001), age ≥65 years (67.74% vs 58.25%, p = 0.0006) and ≥2 comorbidities (62.61% vs 54.5%, p = 0.0041). Conversely, UK patients were less likely to have advanced-stage cancer (32.26% vs 41.13%, p < 0.0001) and to be receiving active anticancer therapy within 4 weeks before COVID-19 diagnosis (60.53% vs 74.69%, p < 0.0001). No difference between the cohorts was found with respect to smoking status (former/current smokers: 37.85% vs 42.32%, p = 0.8355), the presence of active malignancy (67.03% vs 67.03%, p = 0.9996) and rates of complicated COVID-19 (61.75% vs 60.28%, p = 0.5955). However, UK patients were less likely to have received COVID-19–specific therapies of any kind (60.53% vs 74.69%, p < 0.0001).
Supplementary Table 2 provides the detailed distribution across the cohorts of patients' comorbidities, specific anticancer therapy, COVID-19 symptoms at diagnosis, complications and provision of COVID-19–specific therapy. It also summarises hospitalisation and ICU admission rates across the cohorts. Although a higher proportion of hospitalisations were reported for the UK cohort (87.39% vs 82.47%, p = 0.0175), there was no significant difference regarding ICU admission rates (14.14% vs 13.77%, p = 0.6919), requirement for oxygen therapy (57.91% vs 57.86%, p = 0.9868) and mechanical ventilation (12.29% vs 10.30%, p = 0.8630). However, a higher proportion of patients requiring non-invasive ventilation were reported in the UK cohort (71.15% vs 36.78%, p < 0.0001). Among patients who were on anticancer therapy at the moment of COVID-19 diagnosis and were alive at 30 days (n = 406), no significant difference was found in the rates of permanent cessation of anticancer therapy after COVID-19 between the UK (n = 10 of 94, 10.6%) and the EU cohorts (n = 32 of 312, 10.3%, p = 0.9152).
5.2. Clinical outcomes
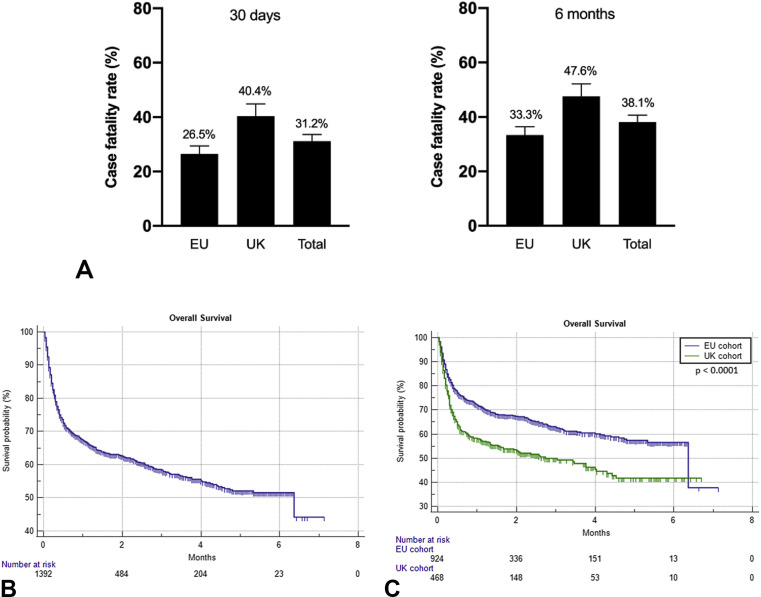
The median follow-up interval for the entire population was 2.2 months (95% CI: 2.1–7.1) and similar for the UK (2.2 months [95% CI: 2.1–6.7]) and EU cohort (2.2 months [95% CI: 2.0–7.1]). When considering the entire population (n = 1392), the overall all-cause case fatality rates at 30 days and 6 months were 31.17% (434 events) and 38.14% (531 events), respectively. As shown in Fig. 2 A, case fatality rates were higher in UK versus EU patients both at 30 days (40.38%, 189 events versus 26.5%, 245 events; p < 0.0001) and at 6 months (47.64%, 223 events versus 33.33%, 308 events; p < 0.0001).
Fig. 2.
(A) Histograms illustrating the case fatality rates at 30 days and 6 months for the cohorts of interest. Kaplan-Meier survival curves. (B) Overall survival for the entire study population: 6.3 months (95% CI: 4.4–6.3; 532 events). (C) Overall survival for the cohorts of interest: UK cohort, 2.7 months (95% CI: 1.5–4.3; 223 events); EU cohort, 6.3 months (95% CI: 6.3–6.3; 309 events). Log-rank: p < 0.0001. CI, confidence interval; UK, United Kingdom; EU, Europe.
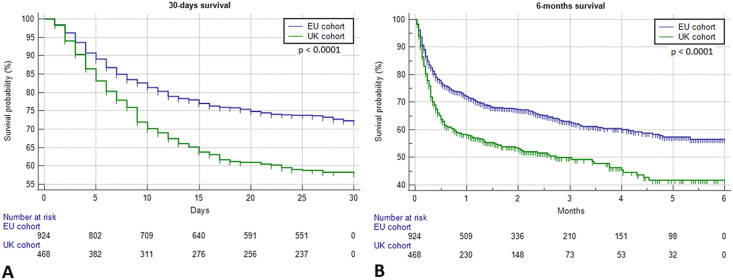
At the time of censoring, the median survival time of the overall OnCovid population was 6.3 months (95% CI: 4.4–6.3), with 532 recorded deaths. Fig. 2B and C illustrate the Kaplan-Meier estimation of OS for the entire population and after stratification into UK and EU cohorts. Univariable analyses revealed patients from the UK cohort to have experienced a significantly higher risk of death at 30 days (HR = 1.64 [95% CI: 1.36–1.99]) and 6 months (HR = 1.58 [95% CI: 1.33–1.88]) than patients from the EU cohort. Fig. 3 A and B illustrate the significant difference in patients' OS at 30-day and 6-month landmark time points for UK versus EU patients.
Fig. 3.
Kaplan-Meier survival curves. (A) Thirty-day survival for the cohorts of interest: UK cohort, not reached (189 events); EU cohort, not reached (245 events). Log-rank: p < 0.0001. (B) Six-month survival for the cohorts of interest: UK cohort, 2.7 months (95% CI: 1.5–4.3; 223 events); EU cohort, not reached (208 events). Log-rank: p < 0.0001. CI, confidence interval; UK, United Kingdom; EU, Europe.
5.3. Risk factors of outcomes in UK versus EU patients with cancer andCOVID-19
To evaluate clinical determinants of worse outcomes in UK patients with cancer and COVID-19, we initially performed univariable analyses to identify the factors associated with the risk of death at 30 days and 6 months in the whole population (Table 2 ). Alongside a significant increase in the risk of death at 30 days (HR = 1.64, 95% CI: 1.36–1.99) and 6 months (HR = 1.58, 95% CI: 1.333–1.881) documented for UK patients, we confirmed patients' gender, age, number of comorbidities, smoking status, tumour stage, status and occurrence of complicated COVID-19 were to be significantly associated with an increased risk of death at 30 days and 6 months, in line with previously published reports [6,22,23]. Receipt of anticancer therapy at COVID-19 diagnosis was significantly associated with improved risk of death at both the 30-day and 6-month landmarks, a finding that mirrors previously published evidence from the OnCovid study [6]. With the exception of patients with breast cancer and those in the other malignancy subgroup, who were characterised by a decreased risk of death at 30 days and at 6 months compared with patients with lung cancer, no other significant differences were found with respect to clinical outcomes regarding primary tumour subgroups.
Table 2.
Univariable analysis of factors predictive for the risk of death at 30 days and 6 months.
| 30 days |
HR (95% CI) | 6 months |
HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Alive |
Death |
Alive |
Death |
|||
| N = 958 (%) | N = 434 (%) | N = 861 (%) | N = 531 (%) | |||
| Area | ||||||
| Other EU | 679 (70.88) | 245 (56.45) | 1 | 616 (71.54) | 308 (58.00) | 1 |
| UK | 279 (29.12) | 189 (43.55) | 1.64 (1.36–1.99) | 245 (28.46) | 223 (42.00) | 1.58 (1.333–1.881) |
| Gender | ||||||
| Male | 474 (49.63) | 264 (60.97) | 1 | 414 (48.25) | 324 (61.13) | 1 |
| Female | 481 (50.37) | 169 (39.03) | 0.68 (0.56–0.83) | 444 (51.75) | 206 (38.87) | 0.68 (0.573–0.813) |
| Missing | 4 | 4 | ||||
| Age | ||||||
| <65 years | 438 (46.01) | 95 (22.04) | 1 | 407 (47.49) | 126 (23.95) | 1 |
| ≥65 years | 514 (53.99) | 336 (77.96) | 2.53 (2.01–3.18) | 450 (52.51) | 400 (76.05) | 2.39 (1.963–2.932) |
| Missing | 9 | 9 | ||||
| Number of comorbidities | ||||||
| 0–1 | 457 (47.70) | 138 (31.80) | 1 | 428 (49.71) | 167 (31.45) | 1 |
| ≥2 | 501 (52.30) | 296 (68.20) | 1.78 (1.45–2.18) | 433 (50.29) | 364 (68.55) | 1.89 (1.574–2.272) |
| Missing | 0 | 0 | ||||
| Smoking history | ||||||
| Never-smokers | 431 (53.74) | 168 (47.19) | 1 | 395 (54.33) | 204 (47.33) | 1 |
| Former/current smokers | 371 (46.26) | 188 (52.81) | 1.25 (1.01–1.54) | 332 (45.67) | 227 (52.67) | 1.24 (1.031–1.504) |
| Missing | 234 | 234 | ||||
| Cancer site | ||||||
| Breast | 226 (23.90) | 48 (11.06) | 0.35 (0.25–0.51) | 216 (25.09) | 61 (11.49) | 0.39 (0.28–0.55) |
| Gastrointestinal | 172 (17.95) | 87 (20.05) | 0.76 (0.55–1.03) | 147 (17.07) | 112 (21.09) | 0.85 (0.64–1.1) |
| Gynaecological/genitourinary | 184 (19.21) | 94 (21.66) | 0.76 (0.56–1.03) | 166 (19.28) | 112 (21.09) | 0.80 (0.60–1.06) |
| Haematological | 142 (14.82) | 83 (19.12) | 0.78 (0.57–1.07) | 121 (14.05) | 104 (19.59) | 0.79 (0.59–1.06) |
| Lung | 102 (10.65) | 74 (17.05) | 1 | 92 (10.69) | 84 (15.82) | 1 |
| Other | 129 (13.47) | 48 (11.06) | 0.58 (0.40–0.84) | 119 (13.82) | 58 (10.92) | 0.59 (0.42–0.83) |
| Missing | 0 | 0 | ||||
| Tumour stage | ||||||
| Local/locoregional | 501 (59.71) | 183 (48.67) | 1 | 467 (61.94) | 217 (47.07) | 1 |
| Advanced | 338 (40.29) | 193 (51.33) | 1.42 (1.16–1.74) | 287 (38.06) | 244 (52.93) | 1.58 (1.32–1.90) |
| Missing | 177 | 177 | ||||
| Tumour status | ||||||
| Remission/non-measurable disease | 342 (36.62) | 107 (25.00) | 1 | 332 (38.57) | 117 (22.37) | 1 |
| Active malignancy | 592 (63.38) | 321 (75.00) | 1.55 (1.24–1.93) | 507 (60.43) | 406 (77.63) | 1.85 (1.51–2.28) |
| Missing | 30 | 30 | ||||
| Anticancer therapy at COVID-19 diagnosis | ||||||
| No | 419 (44.62) | 230 (53.86) | 1 | 371 (43.96) | 278 (53.26) | 1 |
| Yes | 520 (55.38) | 197 (46.14) | 0.72 (0.59–0.87) | 473 (56.04) | 244 (46.74) | 0.73 (0.61–0.87) |
| Missing | 26 | 26 | ||||
| COVID-19 therapy (any) | ||||||
| No | 283 (31.20) | 104 (26.60) | 1 | 258 (31.39) | 129 (27.10) | 1 |
| Yes | 624 (68.80) | 287 (73.40) | 1.16 (0.92–1.45) | 564 (68.61) | 347 (72.90) | 1.15 (0.94–1.41) |
| Missing | 94 | 94 | ||||
| Complicated COVID-19 | ||||||
| No | 488 (50.94) | 58 (13.36) | 1 | 445 (51.68) | 101 (19.02) | 1 |
| Yes | 470 (49.06) | 376 (86.64) | 5.10 (3.86–6.72) | 416 (48.32) | 430 (80.98) | 3.53 (2.84–4.38) |
| Missing | 0 | 0 | ||||
COVID-19, coronavirus disease 2019; EU, Europe; UK, United Kingdom; HR, hazard ratio; CI, confidence interval.
To evaluate whether UK origin was independently associated with outcomes, we designed a multivariable Cox regression model adjusted for all the prognostic covariates tested in univariable models. As shown in Table 3 , after adjustment for all the included covariates, patients from the UK cohort were confirmed to have a significantly higher risk of death at 30 days (HR = 1.52 [95% CI: 1.17–1.99]) and at 6 months (HR = 1.41 [95% CI: 1.10–1.80]) than patients from the rest of EU. Multivariable analysis confirmed receipt of anticancer therapy not to influence the risk of death at 30-day mortality but to exert a protective effect at 6 months (HR = 0.72 [95% CI: 0.57–0.92]). Exposure to any COVID-19–specific therapy was found to be associated with a decreased risk of death at 30 days (HR = 0.72 [95% CI: 0.59–0.87]) and at 6 months (HR = 0.73 [95% CI: 0.61–0.87]), whereas the occurrence of complicated COVID-19 was confirmed to be associated with an increased risk of death at both 30 days (HR = 5.10 [95% CI: 3.86–6.72]) and 6 months (HR = 3.53 [95% CI: 2.84–4.38]).
Table 3.
Multivariable analysis of factors predictive of the risk of death at 30 days and 6 months.
| 30-day risk of death |
6-month risk of death |
|
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Area | ||
| Other EU | 1 | 1 |
| UK | 1.52 (1.17–1.99) | 1.41 (1.10–1.80) |
| Gender | ||
| Male | 1 | 1 |
| Female | 1.09 (0.82–1.45) | 1.12 (0.86–1.45) |
| Age | ||
| <65 years | 1 | 1 |
| ≥65 years | 1.76 (1.30–2.39) | 1.60 (1.23–2.10) |
| Number of comorbidities | ||
| 0–1 | 1 | 1 |
| ≥2 | 1.34 (1.02–1.76) | 1.50 (1.17–1.92) |
| Smoking history | ||
| Never-smokers | 1 | 1 |
| Current/former smokers | 1.18 (0.92–1.52) | 1.18 (0.93–1.48) |
| Cancer site | ||
| Breast | 0.84 (0.51–1.37) | 0.84 (0.54–1.32) |
| Gastrointestinal | 1.08 (0.74–1.57) | 1.22 (0.86–1.71) |
| Gynaecological/genitourinary | 0.89 (0.61–1.28) | 0.94 (0.67–1.33) |
| Haematological | 1.29 (0.82–2.04) | 1.20 (0.78–1.85) |
| Lung | 1 | 1 |
| Other | 0.86 (0.52–1.40) | 0.92 (0.59–1.43) |
| Tumour stage | ||
| Local/locoregional | 1 | 1 |
| Advanced | 1.56 (1.15–2.10) | 1.71 (1.30–2.25) |
| Tumour status | ||
| Remission/non-measurable disease | 1 | 1 |
| Active malignancy | 1.54 (1.09–2.17) | 1.82 (1.32–2.51) |
| Anticancer therapy at COVID-19 diagnosis | ||
| No | 1 | 1 |
| Yes | 0.81 (0.62–1.06) | 0.72 (0.57–0.92) |
| COVID-19 therapy (any) | ||
| No | 1 | 1 |
| Yes | 0.63 (0.48–0.84) | 0.72 (0.55–0.93) |
| Complicated COVID-19 | ||
| No | 1 | 1 |
| Yes | 6.99 (4.61–10.62) | 4.52 (3.26–6.26) |
COVID-19, coronavirus disease 2019; EU, Europe; UK, United Kingdom; HR, hazard ratio; CI, confidence interval.
6. Discussion
The high proportion of asymptomatic transmission has made SARS-CoV-2 a rapidly escalating global threat. However, mortality from COVID-19 is unevenly distributed across affected countries [28]. A number of factors play a role in determining this heterogeneity, including differences in infection control policies, healthcare systems, racial disparity and diverse distribution of age and comorbidities. Although a number of studies have evaluated severity of COVID-19 in patients with cancer versus patients without cancer [29], little effort has been dedicated to understanding whether the mortality of patients with cancer and COVID-19 is geographically influenced. The UK has reported one of the highest numbers of deaths per capita from COVID-19 in EU, and it detained the world primate before SARS-CoV-2 infections peaked in the Americas [28].
Our ad hoc analysis of the OnCovid registry confirms that UK patients with cancer were 1.5 times more likely to die from COVID-19 than patients enrolled from EU countries. In line with many other studies, our analysis confirms that exposure to anticancer therapy plays no role in the 30-day risk of death from COVID-19 [6,9,23,30]. Interestingly, UK patients were less likely to be receiving anticancer therapy at the moment of COVID-19 diagnosis. This is likely to reflect, at least in part, the rapid diffusion of the National Institute of Clinical Excellence guidelines on SACT prioritisation and deferral in the UK on 20th March 2020 [31].
Previously published evidence from the OnCovid registry had shown that patients on active anticancer therapy achieved better outcomes from COVID-19 as they were more likely younger, of female gender, with fewer comorbidities and with lower proportion of active disease [6]. Consistent with this view, in this updated analysis of the OnCovid registry data, recent exposure to anticancer therapy was protective for the risk of mortality at 6 months in the UK and EU cohorts, suggesting the survival disadvantage seen in UK patients to be independent from the delivery of anticancer therapy per se and reflect different degrees of patient fitness, for which candidacy to SACT may act as a proxy.
Interestingly, the significantly higher risk of death of UK patients was not restricted to estimates at 30 days after COVID-19 diagnosis but persisted in the evaluation of mortality at 6 months after infection. Although there are no high-quality data to characterise excess risk of long-term mortality attributable to COVID-19, recent studies have demonstrated the considerable long-term impact of SARS-CoV-2 on respiratory function, fatigue and psychological well-being in patients without cancer [32,33]. We hypothesised that an imbalance in the resumption of anticancer therapies in the UK versus EU cohort might be contributory to the differential risk of death. Our results, however, argue against that interpretation, given the rates of permanent discontinuation of therapy were similar across UK and EU cohorts.
Careful evaluation of baseline patient characteristics gives important insight as to the geographical difference in outcomes from COVID-19, highlighting a number of vulnerabilities that are typical of patients with cancer in the UK. In particular, the higher proportion of male, elderly patients with higher comorbid burden highlights a higher degree of frailty in UK patients.
The constellation of clinical features enriched in the UK cohort has been long time characterised as adverse prognostic traits in patients with cancer, capable of defining a state of intrinsic vulnerability and poor return to physiologic homoeostasis after a stressor event [34].
Recognition of these adverse prognostic factors from the patient's medical and oncological history should continue to inform the basis of an individualised risk assessment in planning hospital attendance, in delivery of cancer care and in prioritising the delivery of immunisation against SARS-CoV-2 in a context of scarce vaccinal resources [35].
Baseline patient features are not the sole determinants of outcomes to COVID-19, and despite the unfavourable imbalance in prognostic factors for UK patients, our multivariate analyses of survival were adjusted for all the available key confounders present at baseline and during the course of the observation including the emergence of COVID-19 complications and receipt of anti–COVID-19 therapy [6,7,9,22,23].
Interestingly, patients in the UK cohort were less likely to have received specific anti–COVID-19 therapy, a factor that emerged to be protective for 30-day and 6-month risk of death after adjustment for COVID-19 severity.
When considering anti–COVID-19 therapies in detail (Supplementary Table 2), it should be emphasised that most agents listed were used off-label or on compassionate grounds on the basis of the opinion of the treating physician. Although some agents including hydroxychloroquine were later on judged ineffective in reducing mortality [36], others such as interleukin-6 inhibitors, corticosteroids and remdesivir were subsequently shown to improve some COVID-19–related outcomes in different stages of disease [21,[37], [38], [39], [40]]. A direct cause-effect relationship between exposure to each agent and mortality from COVID-19 across UK and EU cohorts cannot be inferred because of the observational, retrospective nature of our study, wherein most patients were treated with varying combinations of agents and in response to different levels of severity of the disease. However, the lower level of exposure to anti–COVID-19 therapies that have been proven effective such as corticosteroids and tocilizumab cannot be discounted as a potential factor influencing the worse outcome of patients belonging to the UK cohort.
Another important aspect that should be considered in interpreting our results is hospital capacity, one of the determining factors for the overall COVID-19 mortality in the UK during the first wave [28]. In our study, we report a higher hospitalisation rate for UK patients than for EU patients, despite equal proportion of complicated COVID-19 and no differences with regard to the intensive care admission rates and mechanical ventilation. Although a registry study such as OnCovid cannot claim to be fully illustrative of the countrywide hospital capacity, the lack of difference in key measures of severity and treatment escalation aids us in addressing hospital capacity and escalation of treatment beyond ward-based care as important confounders in our estimates of mortality. To this end, we believe the higher hospitalisation rate of UK patients to be an imperfect indicator of capacity or severity of COVID-19, being more likely to reflect the scarcity of community testing observed at the early beginning of the pandemic in the UK, when SARS-CoV-2 PCR testing capacity was limited to hospitalised patients and to those with more severe forms of COVID-19.
Although many studies have described outcomes from SARS-CoV-2–infected patients with cancer in the UK [8,22], this is the first study to perform a comparative assessment of outcomes taking advantage of a large cohort of European patients. Our study is largely an account of the first wave of the pandemic and predates the widespread diffusion of the variant of concern B.1.1.7, for which increased lethality has been postulated [41], but not definitively proven. With increased physician experience, resilience of healthcare services and widespread use of active anti–COVID-19 therapies, infections diagnosed in the so-called ‘second wave’ might be characterised by improved outcomes: a hypothesis that we aim to test when clinical data from our registry are fully mature. Similarly, although our study relies on significantly longer follow-up time than that in earlier reports, more mature survival data will allow us to provide further insight into the topic of long-term outcomes from COVID-19.
Despite attempting to control for key clinicopathological factors, our analyses might still be affected by unmeasured bias. For instance, we lack data on quantitative estimation of the SARS-CoV-2 viral load, a parameter associated with disease severity and mortality from COVID-19 [42] and that might have given us insight into severity of community exposure or the underlying immune dysfunction in our study participants [[43], [44], [45]].
Notwithstanding the acknowledged limitations, this study provides a comprehensive, comparative assessment of the impact of the SARS-CoV-2 pandemic in UK patients with cancer, a population already characterised by intrinsically poorer survival outcomes from cancer compared with many other industrialised countries [15]. We highlight key areas of vulnerability to COVID-19 in UK patients with cancer, in particular higher comorbid burden and age, which, in a healthcare system characterised by the highest overall mortality from COVID-19 in EU, calls for the rapid implementation of protective strategies against SARS-CoV-2 in this exquisitely vulnerable patient cohort.
Rapid and widespread vaccination of patients with cancer should be advocated as a priority in UK patients with cancer. Second, clinical use of anti–COVID-19 therapies with proven benefit against SARS-CoV-2 should be facilitated in UK patients with cancer, a population that is under-represented in clinical trials of vaccines and therapeutics against SARS-CoV-2 [46]. Although the UK is at the forefront of drug development in COVID-19 [47], concerted efforts should continue to be aimed at maintaining the ever-so-delicate balance between protection from harm due to the pandemic and preservation of oncological outcomes in patients at risk of cancer relapse or progression.
Author contributions
A.C. and D.J.P. had full access to all data in the study and take responsibility for data integrity and analysis. D.J.P., A.C., L.S. and D.F. contributed to study concept and design. All authors contributed to acquisition of data. D.J.P., A.C., L.S. and D.F. contributed to analysis and interpretation of data. D.J.P. and A.C. contributed to drafting of the manuscript. All authors contributed to manuscript revision and input. L.S., A.C. and D.F. contributed to statistical analysis. D.J.P. obtained funding. D.J.P. contributed to study supervision.
Funding
OnCovid acknowledges infrastructural support from the Imperial College Biomedical Research Centre. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Consent for publication
Informed consent was waived by competent authorities because of anonymised nature of patient data and retrospective design of the study.
Availability of data and material
Study data made available upon reasonable request.
Conflict of interest statement
D.J.P. received lecture fees from ViiV Healthcare and Bayer Healthcare, travel expenses from BMS and Bayer Healthcare; consulting fees for MiNA Therapeutics, EISAI, Roche and AstraZeneca and research funding (to the institution) from MSD and BMS. A.P. has declared personal honoraria from Pfizer, Roche, MSD Oncology, Eli Lilly and Daiichi Sankyo; travel, accommodations and expenses paid by Daiichi Sankyo; research funding from Roche and Novartis and a consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol Myers Squibb. T.N-D. has declared a consulting/advisory role for Amgen, Bayer, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Otsuka, Pfizer, Roche and Takeda; speaker fees from AstraZeneca, MSD, Roche, Takeda and travel, accommodations and expenses paid by AstraZenca, BMS, Boehringer Ingelheim, Lilly, MSD, Otsuka, Roche and Takeda. J.B. has declared a consulting/advisory role for MSD and AstraZeneca. PPS has declared a consulting/advisory role for Sanofi and AbbVie. A.P. has declared a consulting/advisory role for Takeda and Sanofi. MP has declared a consulting/advisory role for Gilead and Bayer. A.G. has declared a consulting/advisory role for Roche, MSD, Eli Lilly, Pierre Fabre, EISAI and Daiichi Sankyo; is on the speaker's bureau for Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, AstraZeneca, Celgene and Daiichi Sankyo and declared research funds from EISAI, Eli Lilly and Roche. C.M.-V. has received travel grants and other honoraria from BMS, MSD, Novartis and Roche. L.R. received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi and Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche and Sanofi; travel expenses from Ipsen and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, FibroGen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche and Zymeworks. J.T. reports personal financial interest in form of a scientific consultancy role for Array BioPharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd., Genentech, Inc., HalioDX SAS, Ikena Oncology, IQVIA, Imedex, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, NeoPhore, Novartis, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Servier, Taiho, Tessa Therapeutics and TheraMyc. A.C. received consulting fees from MSD, BMS, AstraZeneca, Roche and speakers' fee from AstraZeneca, MSD, Novartis and Astellas. All the remaining authors have declared no conflicts of interest.
Acknowledgements
D.J.P. is supported by grant funding from The Wellcome Trust Institutional Strategic Support Fund (ISSF) (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Cancer Research UK Imperial Centre and the NIHR Imperial Biomedical Research Centre. G.G. is supported by the AIRC 5 × 1000 Grant no. 21198, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy. A.G. is supported by the AIRC IG Grant no. 14230, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy. A.G. and G.G. from the University of Piemonte Orientale (Novara, Italy) acknowledge support from the UPO Aging Project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.03.035.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pan A., Liu L., Wang C., et al. Association of public Health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020 May 19;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020 May 26;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020 Apr 28;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinato D.J., Zambelli A., Aguilar-Company J., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Canc Discov. 2020 Jul 31;10(10):1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini K.S., Tagliamento M., Lambertini M., et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Canc. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yekeduz E., Utkan G., Urun Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Canc. 2020;141:92–104. doi: 10.1016/j.ejca.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lievre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Canc. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UK Government Office of National Statistics . 2020. Number of Coronavirus (COVID-19) cases and risk in the UK. [Google Scholar]
- 14.Ueda M., Martins R., Hendrie P.C., et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. 2020:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 15.Arnold M., Rutherford M.J., Bardot A., et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raphael M.J., Biagi J.J., Kong W., Mates M., Booth C.M., Mackillop W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Canc Res Treat. 2016;160(1):17–28. doi: 10.1007/s10549-016-3960-3. [DOI] [PubMed] [Google Scholar]
- 17.Biagi J.J., Raphael M.J., Mackillop W.J., Kong W., King W.D., Booth C.M. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z., King W., Pearcey R., Kerba M., Mackillop W.J. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87(1):3–16. doi: 10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 19.National Health Service England Clinical guide for the management of non-coronavirus patients requiring acute treatment: Cancer. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf Published 2020. [Accessed]
- 20.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee L.Y., Cazier J.B., Angelis V., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinato D.J., Lee A.J.X., Biello F., et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12(7) doi: 10.3390/cancers12071841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellul M., Varatharaj A., Nicholson T.R., et al. Defining causality in COVID-19 and neurological disorders. J Neurol Neurosurg Psychiatry. 2020;91(8):811–812. doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistics OfN Measuring pre-existing health conditions in death certification – deaths involving COVID- 19: March 2020. A method for deciding which pre-existing condition mentioned on death certificates is the main pre-existing condition. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/measuringpreexistinghealthconditionsindeathcertificationdeathsinvolvingcovid19march2020 Published 2020.
- 27.de Joode K., Dumoulin D.W., Tol J., et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Canc. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephen Rocks O.I. Did hospital capacity affect mortality during the pandemic's first wave? https://www.health.org.uk/news-and-comment/charts-and-infographics/did-hospital-capacity-affect-mortality-during-the-pandemic Published 2020.
- 29.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Canc Discov. 2020 Jun;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brar G., Pinheiro L.C., Shusterman M., et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol. 2020;38(33):3914–3924. doi: 10.1200/JCO.20.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark J.J., Dwyer D., Pinwill N., Clark P., Johnson P., Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22(1):66–73. doi: 10.1016/S1470-2045(20)30619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellan M., Soddu D., Balbo P.E., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handforth C., Clegg A., Young C., et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 35.Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 36.Group R.C., Horby P., Mafham M., et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2021 Feb 25;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biran N., Ip A., Ahn J., et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomazini B.M., Maia I.S., Cavalcanti A.B., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. J Am Med Assoc. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020 Nov 5;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. Epub 2020 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iacobucci G. Covid-19: new UK variant may be linked to increased death rate, early data indicate. BMJ. 2021;372:n230. doi: 10.1136/bmj.n230. [DOI] [PubMed] [Google Scholar]
- 42.Fajnzylber J., Regan J., Coxen K., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu T., Chen C., Zhu Z., et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.To K.K., Tsang O.T., Leung W.S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiara Garassino Ng Marina, Grivas Petros, Jordan Karin, Lucibello Francesca, Mir Olivier, George Pentheroudakis, et al. Esmo statements for vaccination against COVID-19 IN patients with cancer. https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination Published 2021.
- 47.Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. GOV.UK. https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19#cev Published 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data made available upon reasonable request.