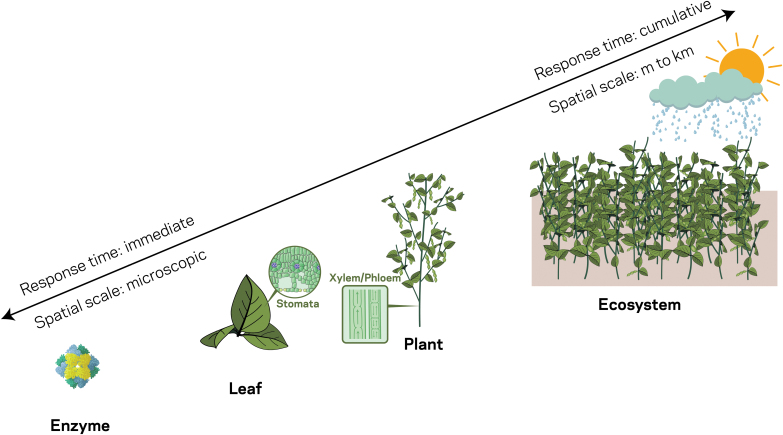
This review explores current understanding in crop photosynthesis and temperature across scales, linking enzyme processes within the leaf to stomata, plant transport systems, individual plants, and ecosystem-scale responses.
Keywords: Cropping system, gross primary productivity, heat stress, resilience, Rubisco, stomata, vapour pressure deficit
Abstract
As global land surface temperature continues to rise and heatwave events increase in frequency, duration, and/or intensity, our key food and fuel cropping systems will likely face increased heat-related stress. A large volume of literature exists on exploring measured and modelled impacts of rising temperature on crop photosynthesis, from enzymatic responses within the leaf up to larger ecosystem-scale responses that reflect seasonal and interannual crop responses to heat. This review discusses (i) how crop photosynthesis changes with temperature at the enzymatic scale within the leaf; (ii) how stomata and plant transport systems are affected by temperature; (iii) what features make a plant susceptible or tolerant to elevated temperature and heat stress; and (iv) how these temperature and heat effects compound at the ecosystem scale to affect crop yields. Throughout the review, we identify current advancements and future research trajectories that are needed to make our cropping systems more resilient to rising temperature and heat stress, which are both projected to occur due to current global fossil fuel emissions.
Introduction
Global land surface temperatures are increasing due to rising atmospheric CO2 from anthropogenic emissions that are causing climate change, and with this comes the challenge of meeting food and fuel supply demands under more stressful crop growing conditions. Despite a drop in emissions associated with the coronavirus pandemic of 2020 (COVID-19; Le Quéré et al., 2020), global emissions are currently tracking the worst-case ‘business as usual’ emissions scenario (RCP8.5) that will very likely equate to unprecedented warming from pre-industrial (1850–1990) levels of 3–5 °C by 2100 (IPCC, 2014). A recent IPCC report indicated, with medium confidence, that crop yields will experience ‘severe and widespread impacts’ if global warming exceeds 1.5 °C above pre-industrial levels, but that these impacts can be managed below this warming threshold (IPCC, 2018). Coupled with rising mean global temperature is a projected increase in the frequency, intensity, and duration of extreme heatwave events that have the potential to cripple crop yields (Battisti and Naylor, 2009; Perkins et al., 2012; Hatfield and Prueger, 2015; Hoegh-Guldberg et al., 2018). Additionally, some cropping areas, such as temperate, high-latitude regions, will likely face even greater warming than tropical regions of the world (Hoegh-Guldberg et al., 2018). Therefore, there is an urgent need, first and foremost, for mitigation strategies to reduce fossil fuel emissions to cap warming at 1.5 °C (IPCC, 2018), but also for development of our major cropping systems to be more resilient to hotter growing seasons and extreme temperature events that seem inevitable in the coming century.
Global yield losses in key crops, such as maize and wheat, have been attributed to higher growing season temperatures (Lobell et al., 2011; Lobell and Gourdji, 2012; Asseng et al., 2015). Without crop improvement strategies, including genetic engineering and adaptation under carbon dioxide (CO2) fertilization, substantial yield declines per °C of warming have been projected for the major cropping systems of maize (7.4%), wheat (6.0%), rice (3.2%), and soybean (3.1%) (C. Zhao et al., 2017). Yet, to keep pace with supplying food and fuel to the growing human population, agricultural production will need to double (based on average yield in 2005) over this century to meet increased caloric demand (Long and Ort, 2010; Ray et al., 2013). Additionally, the full theoretical extent of the CO2 fertilization effect is unlikely to be realized due to the impact of rising temperature (Long et al., 2006a; Ainsworth and Long, 2020). Thus, improving crop resilience to temperature stress is a vital step towards ensuring global food and fuel demands are met.
Temperature is a critical meteorological determinant of crop development and function. Temperature alters enzyme function within a leaf (Bernacchi et al., 2001; Walker et al., 2013; Florian et al., 2014; Kumarathunge et al., 2019; Timm et al., 2019) and triggers changes in developmental growth stage that are tightly coupled with crop yield (Ruiz-Vera et al., 2018; Zhu et al., 2018). Furthermore, the amount of water vapour in air at saturation increases exponentially with temperature, raising the vapour pressure deficit (VPD), and driving more potential water loss from plants (Novick et al., 2016; Grossiord et al., 2020). The result of these broad crop physiological responses to temperature means that any shifts in long-term mean annual temperature and extreme temperature events will be likely to have significant impacts on crop production from the key food and fuel growing regions of the world.
Improvements in how crops function from the enzyme to ecosystem scale are required to maintain historic increases in crop yields into the future, whilst ensuring cropping systems remain resilient to rising temperatures (Long and Ort, 2010). Engineering improvements to photosynthesis, including its resilience to perform under hotter temperatures at the leaf, plant, and canopy levels, is an emergent strategy that may help boost yields (Long et al., 2006b; Ainsworth and Ort, 2010; Ort et al., 2015; Betti et al., 2016; Kromdijk and Long, 2016; Kubis and Bar-Even, 2019; Posch et al., 2019; Simkin et al., 2019; Wu et al., 2019; Furbank et al., 2020). Developing better warning systems, such as early detection of crop ecosystem stress, will also improve targeted management approaches that reduce resource use (i.e. water and pesticides), expenditure, and time (Guanter et al., 2014; Chlingaryan et al., 2018; Camino et al., 2019).
Realizing the full impact of temperature increase on crop photosynthesis across scales is an area of ongoing investigation, particularly given the complex interactions of water availability, increasing atmospheric CO2 concentrations ([CO2]), nutrient availability, and the increased frequency and/or intensity of extreme climate events that feed back to alter annual crop photosynthesis and productivity. There have been several seminal reviews on the effect of rising temperature on crop photosynthetic performance (Ainsworth and Ort, 2010), photosynthetic enzyme function (Slattery and Ort, 2019), plant carbon metabolism (Dusenge et al., 2019), and plant development (Wang et al., 2012), as well as global assessments of how crop yield is likely to change as temperatures rise (Lobell and Gourdji, 2012; C. Zhao et al., 2017). Yet, reviews that address all these scales in one are limited.
This review focuses on synthesizing current advances in understanding the effects of temperature on cropping systems from the enzyme to ecosystem scale (Fig. 1) to provide a comprehensive assessment of how crop photosynthesis changes as temperature increases. Beginning at the enzyme scale, we discuss (i) within-leaf responses to temperature, followed by (ii) stomata and plant transport system responses to heat; (iii) temperature effects on whole plants and their development; and (iv) how each of these factors scales to the crop ecosystem to impact photosynthesis and annual yield (Fig. 1). Key abbreviations used throughout the review are listed and expanded in Table 1. For each scale discussed, we identify areas for research development that are needed to ensure the major crops that feed and fuel the world are more resilient to the impacts of rising temperature that will occur without implementation of climate mitigation strategies.
Fig. 1.
The spatial scale and temporal response time of photosynthetic processes in cropping systems from the enzyme to ecosystem scale.
Table 1.
Nomenclature and explanation of terms used across different scales
| Abbreviation | Long name | Description |
|---|---|---|
| [CO2] | CO2 concentration | The concentration of carbon dioxide in the atmosphere, or within the leaf if specified as such |
| A | Assimilation | Net carbon assimilation during photosynthesis |
| Ea | Activation energy | The input energy required to result in a chemical reaction |
| ER | Ecosystem respiration | Carbon consumed in an ecosystem by plants (autotrophic) or animals/microbes/fungi (heterotrophic) |
| ET | Evapotranspiration | Water loss through the processes of evaporation from surfaces and transpiration from leaves |
| FACE | Free air CO2 enrichment | An open-air experimental design that raises atmospheric [CO2] above ambient conditions experienced by plants at the ecosystem scale |
| FSPM | Functional and structural plant modelling | Models developed to simulate morphology and growth of single plants as they interact with their environment. |
| GPP | Gross primary productivity | Photosynthesis of all leaves and other photosynthetic plant parts represented at the ecosystem scale |
| gs | Stomatal conductance | A measure of the capacity for gaseous exchange of CO2 entering and water vapour leaving a leaf, measured as a molar flux on an area basis (mol m–2 s–1) |
| NEE | Net ecosystem exchange | A measure of the net flux of carbon between the land surface and the atmosphere |
| NSCs | Non-structural carbohydrates | Soluble sugars and starch that provide energy for plant growth and metabolism |
| PSII | Photosystem II | The first link in the electron transport chain of photosynthesis |
| QTLs | Quantitative trail loci | Sections of DNA (loci) that relate to a quantitative trait in the phenotype of an organism |
| Rca | Rubisco activase | An accessory protein that activates Rubisco |
| RA | Autotrophic respiration | Carbon consumed in an ecosystem by plants for growth and maintenance |
| RH | Heterotrophic respiration | Carbon consumed in an ecosystem by non-photosynthetic organisms |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/ oxygenase | Enzyme that all plants use to fix carbon dioxide as an entry point to the photosynthetic carbon reduction cycle. Rubisco also catalyses a reaction with oxygen, which is the first step in photorespiration |
| RuBP | Ribulose-1,5-bisphosphate | Five-carbon molecule that is used, along with CO2, as a substrate in photosynthesis in a reaction catalysed by Rubisco. RuBP will also bind with oxygen to initiate the process of photorespiration, also catalysed by Rubisco. |
| Sc/o | Rubisco specificity | The specificity of Rubisco for binding CO2 compared with O2 |
| SD | Stomatal density | The number of stomata per unit of leaf area |
| SIF | Sun-induced chlorophyll fluorescence | The emission of red light by plants during the process of sunlit photosynthesis |
| T opt | Thermal optimum | Describes an optimal temperature for driving a particular process |
| VPD | Vapour pressure deficit | A measure of the difference between the amount of moisture in the air and how much moisture air can hold before it becomes saturated. |
Temperature response of photosynthesis within the leaf: the critical role of enzyme function
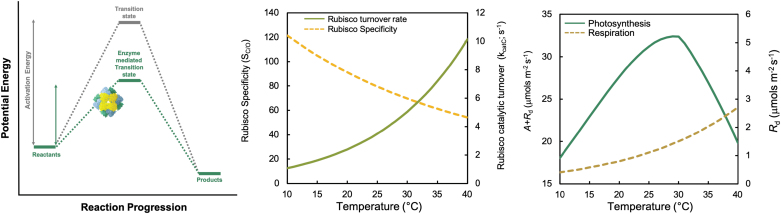
Temperature regulation is foundational in biological systems, as chemical reaction rates are a function of the tissue temperature and the energy required to initiate the reaction—the activation energy (Ea) (Fig. 2A). Enzymes lower this Ea barrier, enhancing the rate of enzyme-catalysed reactions driving biological metabolism (Wolfenden and Snider, 2001). In theory, reaction rates are predicted to increase exponentially with temperature. In reality, most biological temperature responses increase exponentially with temperature until reaching a thermal optimum (Topt), after which rates decline due to enzyme deactivation and denaturation at increasingly high temperatures (Fig. 2B, C).
Fig. 2.
Temperature effects on enzyme-driven processes of photosynthesis. (A) Schematic energy profile of an exergonic chemical reaction. Enzymes, such as Rubisco, facilitate biochemical reaction progression by lowering the activation energy requirements of the transition state between reactants and product formation, though in the case of Rubisco this is simplified as the enzyme facilitates a multistep catalysis (Flamholz et al., 2019). (B) Modelled temperature responses of tobacco Rubisco carboxylation catalytic turnover rate (green solid) and specificity for CO2 over O2 (yellow dashed line), using parameters from Orr et al. (2016) and temperature responses from Bernacchi et al. (2001). (C) Temperature response of gross photosynthesis (carbon assimilation A+mitochondrial respiration Rd, green solid line) and of mitochondrial respiration (Rd, gold dotted line) for an idealized C3 species. Data were modelled using the leaf model of photosynthesis (Farquhar et al., 1980) with temperature adjustments (Bernacchi et al., 2001).
The photosynthetic machinery within a leaf is a logical place to begin when considering the effects of temperature on crop photosynthesis, as many component processes of photosynthetic metabolism are highly temperature sensitive. At a biochemical level, net photosynthetic carbon assimilation (A) is largely determined by Rubisco efficiency and activation, and ribulose bisphosphate (RuBP) regeneration (Table 1) (Farquhar et al., 1980). The predominant determinant varies with chloroplastic [CO2]; RuBP regeneration limits A at elevated [CO2], but Rubisco performance limits A at ambient and subambient [CO2]. Enzyme degradation at elevated temperatures can impede the function of PSII, decrease electron transport rates, inhibit Rubisco activase (Rca), and decrease chlorophyll content (Salvucci et al., 2001; Guo et al., 2006; Allakhverdiev et al., 2008; Prasad and Djanaguiraman, 2011). Elevated temperature can also induce membrane permeability, leading to direct damage of the chloroplast thylakoid membranes, which further inhibits light harvesting, electron transport rates, and ATP generation (Schrader et al., 2004; Prasad et al., 2008; Djanaguiraman et al., 2013; Pokharel et al., 2020). However, thermal lability of enzymes directly involved in A remains the major cause of photosynthetic inhibition of C3 and C4 crops grown under elevated temperatures (Crafts-Brandner and Salvucci, 2000; Schrader et al., 2004; Sage and Kubien, 2007; Perdomo et al., 2016; Slattery and Ort, 2019).
The optimal temperature of RuBP regeneration is generally higher than that of Rubisco carboxylation (Hikosaka et al., 2006); therefore, under current atmospheric [CO2] and saturating light, the temperature dependence of photosynthesis is well explained by Rubisco biochemistry (Sage and Kubien, 2007). As temperatures increase, the fraction of enzyme able to meet or exceed the Ea required for catalysis increases, and so Rubisco carboxylation activity increases (Fig. 2B). However, Rubisco is a bi-functional enzyme, also catalysing the oxygenation of RuBP (Ogren and Bowes, 1971; Tcherkez, 2016; Bathellier et al., 2020; von Caemmerer, 2020). The specificity of Rubisco for CO2 versus O2 (SC/O) declines as temperatures increase, decreasing the ratio of carboxylation to oxygenation in vivo (Fig. 2B). This increased propensity for Rubisco oxygenation at elevated temperatures produces more 2-phosphoglycolate, which must be cycled through the photorespiratory pathway, resulting in a loss of previously fixed carbon at an energetic expense (Walker et al., 2016).
In C4 photosynthesis, CO2 is concentrated around Rubisco in bundle sheath chloroplasts. Thus, stimulation of photorespiration by elevated temperatures is minimal, and A in C4 plants has a higher Topt than in C3 plants (Sage and Kubien, 2007). Above the Topt, C4 photosynthesis may also be limited through inactivation of Rubisco (Crafts-Brandner and Salvucci, 2002), or by rates of other C4 bundle sheath enzymes (Boyd et al., 2015), which show species-specific temperature responses (Sonawane et al., 2017). This impact is evident in field-grown maize, where leaf-level A and yield decline with elevated temperature, even under elevated CO2 conditions (Ruiz-Vera et al., 2015).
The duration and intensity of future warming events are both projected to change (Hoegh-Guldberg et al., 2018), resulting in significant impacts on any potential thermal acclimation of A (Kattge and Knorr, 2007; Vico et al., 2019). In sunlit leaves near the top of the canopy, photosynthetic acclimation through increased electron transport capacity, differential expression of Rca isoforms, and heat shock protein expression can occur with long-term growth at warmer temperatures (Yamori et al., 2014). However, short-term temperature increases can increase leaf respiration, resulting in lower A compared with those at ambient temperature, and a strong and relatively rapid acclimation response that reduces the effect as higher temperatures persist (Way and Yamori, 2014; Kumarathunge et al., 2019). During heatwaves or acute heat stress, defined by sudden increases in temperature (Smith and Dukes, 2017) with significant but reversible effects on photosynthesis (Siebers et al., 2015, 2017; Thomey et al., 2019), the acclimation responses may be too slow or small to confer a measurable benefit. In these situations, energy balances will shift as rates of photosynthesis decline above the Topt and respiration rates increase (Fig. 2C). Thus, most opportunities for improving crop productivity in a warmer world focus on improving photosynthetic carbon gain above Topt.
Recent advances made at the leaf level to improve understanding on temperature effects
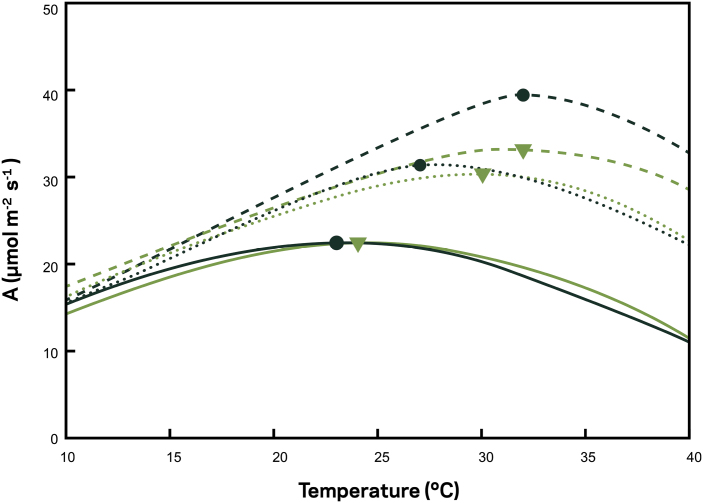
The response of A to a wide range of environmental conditions is well understood based on the leaf model of photosynthesis (Farquhar et al., 1980; Long, 1991). Despite the mechanistic understanding of modelled predictions, there remain significant uncertainties. For example, the leaf photosynthesis model (Farquhar et al., 1980) was recently parameterized using values measured from C3 plants grown under field conditions exposed to supplemental heating (Bagley et al., 2015). The results demonstrate that growth at higher temperatures does not translate to a higher Topt but does lower photosynthetic rates at all temperatures. An interaction between warmer temperature and elevated [CO2] was observed; however, acclimation of photosynthetic enzymatic activity to higher temperature negatively impacted the benefit of higher CO2 (Fig. 3) (Bagley et al., 2015). These results demonstrate the challenges associated with temperature, namely that short- and long-term responses of photosynthesis are complex and are complicated by other environmental variables.
Fig. 3.
Temperature response of C3 leaf photosynthesis (μmol m–2 s–1) modelled at atmospheric [CO2] of 400 (solid lines), 600 (dotted lined), and 800 (dashed lines) μmol mol-–1. Model parameters were taken from Bernacchi et al. (2001, 2003, black circles) and Long (1991, green triangles), with the symbol location on the curve representing the temperature optimum for each photosynthetic response curve. The figure has been redrawn from Bagley et al. (2015), with permission.
Despite the complex interaction between temperature and photosynthesis, promising strategies have been identified to increase photosynthetic A at higher temperatures by either enhancing RuBP carboxylation or improving energy efficiency of photorespiration. The limitations imposed by Rubisco include a slow catalytic rate, competitive inhibition by O2, and activation requirement via heat-sensitive Rca. Strategies to improve our understanding of Rubisco are needed to overcome these temperature impacts.
Rubisco has long been a target for modification to improve its catalytic rate and substrate specificity (Somerville and Ogren, 1982; Zhu et al., 2004; Sharwood, 2017). For example, an apparent trade-off between catalytic rate and specificity hinders progress for exploitation (Tcherkez et al., 2006; Savir et al., 2010; Flamholz et al., 2019). Recently, a systematic survey of prokaryotic Rubisco has identified the fastest version of the enzyme measured to date (22 s–1), but it still displays characteristically poor substrate specificity (Davidi et al., 2020). Screening for natural variation in Rubisco performance has uncovered kinetic diversity among land plants that would confer a predicted benefit to crop A, particularly at elevated temperatures (Galmés et al., 2015; Orr et al., 2016; Sharwood et al., 2016). Unique combinations of Rubisco small and large subunits from different species also provide an opportunity to optimize kinetic performance at higher temperatures (Lin et al., 2020; Martin-Avila et al., 2020; Sakoda et al., 2020). Finally, the newfound ability to assemble plant Rubisco in a bacterial host will enable both structure–function comparisons and directed evolution studies to identify novel mutations to improve Rubisco performance (Aigner et al., 2017; Zhou and Whitney, 2019).
Rca regulates Rubisco activity by displacing inhibitory sugar phosphates from the catalytic site of Rubisco. Although Rubisco remains active up to 50 °C in vitro, Rca activity declines well below this temperature (Salvucci and Crafts-Brandner, 2004; Galmés et al., 2016), and thus can limit photosynthesis at high temperatures. The production of inhibitory catalytic misfire products increases with temperature, implying that the role of Rca also becomes increasingly important. However, when measured in vitro, the rate of spontaneous release of these inhibitors also increases at elevated temperatures, resulting in less inhibition of Rubisco activity, which contradicts this assumption (Schrader et al., 2006; Carmo-Silva et al., 2015; Bracher et al., 2017). Despite this, manipulating Rca thermostability has improved photosynthetic thermotolerance in Arabidopsis (Kurek et al., 2007; Kumar et al., 2009) and rice (Wang et al., 2010; Scafaro et al., 2016, 2018; Shivhare and Mueller-Cajar, 2017), motivating research efforts to enhance the thermotolerance of Rca in other crops. Exploiting temperature-induced differential expression of Rca is a potential strategy to accomplish this objective. In many crops, Rca consists of multiple protein isoforms with differing heat sensitivity (Crafts-Brandner et al., 1997; Law et al., 2001; Law and Crafts-Brandner, 2001; Carmo-Silva et al., 2015; Scafaro et al., 2019; Kim et al., 2020). In bread wheat, altered thermal tolerance between Rca isoforms is conferred by a single amino acid substitution that acts as a thermal and regulatory switch, providing a compelling target for future genome editing efforts (Scafaro et al., 2019; Degen et al., 2020).
The photorespiratory pathway recycles the inhibitory by-products of Rubisco oxygenation, which releases previously fixed carbon and ammonium that is energetically costly to re-fix. Photorespiratory CO2 loss limits productivity in C3 plants, reducing crop yields by >20% in soy and wheat (Walker et al., 2016). Engineering carbon-concentrating mechanisms (CCMs) to directly increase the [CO2] at the site of Rubisco represents one strategy for stimulating carboxylation over oxygenation (Long et al., 2018; Atkinson et al., 2020). This can be accomplished via the introduction of a biophysical CCM, such as those found in cyanobacteria and algae (Hennacy and Jonikas, 2020), or via the conversion of C3 photosynthesis to C4 or C2 types. Researchers have recently established a functioning C4 pathway in rice by transformation with a single construct harbouring coding sequences for five enzymes, although expression will require optimization before any benefit is realized (Ermakova et al., 2020). Engineering C2 photosynthesis, a simple CCM that captures, concentrates, and re-assimilates photorespired CO2, is a promising approach currently in its infancy. An advantage of C2 photosynthesis is the ability to exploit native genes and alter only their regulation and expression, as all required genes are present in C3 species (Lundgren, 2020). Finally, direct manipulations of the photorespiratory pathway can lower the cost of photorespiration. Overexpression of native photorespiratory genes can enhance A and growth, probably altering the balance between photosynthesis and photorespiration (Timm et al., 2012, 2015, 2018; Flügel et al., 2017; López-Calcagno et al., 2019). Synthetic glycolate metabolic pathways using enzymes from other organisms in combination with RNAi to limit glycolate flux through the native pathway increase tobacco biomass under field-grown conditions (South et al., 2019). Similarly, an alternative photorespiratory pathway introduced into rice using three rice enzymes improved A, leading to increased aboveground biomass, but displayed inconsistent improvements in yield (Shen et al., 2019). Further carbon-conserving glycolate metabolic pathways have also been designed and tested in vitro (Trudeau et al., 2018; Ross et al., 2020). While these and the previous strategies to enhance photosynthetic performance above the Topt hold potential to improve crop performance, testing in food and fuel crops over diverse environmental ranges will provide the key validation of their efficacy.
Temperature impacts on stomata and plant transport systems
Scaling the response of plant photosynthesis, from the chloroplast to leaf or whole plant, involves CO2 diffusion to the site of the chloroplast, as well as subsequent photosynthate transport throughout the plant. To reach the site of carboxylation within chloroplasts, CO2 must first diffuse from the atmosphere to the substomatal cavities, then through the intercellular airspaces to the chloroplast. This gaseous diffusion imposes a restriction on CO2 availability in the chloroplast that depends on the CO2 conductance through the leaf boundary layer, stomata, and intercellular environment (i.e. mesophyll conductance). The temperature response of mesophyll conductance varies between species, and can impose a limitation on carbon fixation, which has been well reviewed (Niinemets et al., 2009; Flexas et al., 2012, 2014; von Caemmerer and Evans, 2015). In this section, we discuss temperature impacts on stomata, as well as the plant transport systems that move photosynthate from leaves to other parts of the plant for growth, maintenance, and storage.
How stomatal function links leaf to whole-plant photosynthesis
Stomata control the majority of gaseous exchange between the atmosphere and the leaf interior. Therefore, stomatal behaviour is critically important for CO2 uptake to meet photosynthetic demand and for controlling leaf water loss that impacts evaporative cooling, nutrient uptake, and plant water status (Lawson et al., 2010; Matthews and Lawson, 2019; Lawson and Matthews, 2020). Stomata open and close in response to various environmental signals and internal leaf conditions. In general, conditions of high or increasing light intensity, low (internal) [CO2], and low VPD open stomata, whilst closure is observed under opposite conditions (Matthews and Lawson, 2019). Stomatal conductance (gs) provides a measure of the capacity for gaseous exchange of water vapour leaving the leaf (Table 1), and is determined by the number of stomata per unit leaf area and the size of the pore aperture. Thus, alterations in both leaf morphological features and leaf functional responses to external meteorological forcing can influence gs, which in turn can impact photosynthesis and overall crop performance.
According to the optimization hypothesis, plants coordinate gs and A to maximize A whilst minimizing water loss (Cowan and Farquhar, 1977; Lawson et al., 2010; Buckley et al., 2017). However, this is not always the case, as a decoupling between gs and A has been reported (von Caemmerer and Evans, 2015; Urban et al., 2017), whereby stomata open to increase leaf cooling despite the suppression of A (Drake et al., 2018). A positive correlation between steady-state gs and yield has been observed in the field (Fischer et al., 1998; Fischer and Rebetzke, 2018), reflecting the control stomata exert on CO2 uptake for photosynthesis and on evaporative cooling. Temperature can severely limit stomatal performance and consequently yield, especially in temperature-sensitive crops such as wheat, where evaporative cooling to maintain Topt can be more important than removal of diffusional constraints for photosynthesis (Fischer et al., 1998; Lu et al., 1998). The same environmental cues that stimulate changes in stomatal aperture can also induce alterations to the stomatal density (SD) per unit leaf area and their distribution across the leaf (Weyers et al., 1997; Weyers and Lawson, 1997), which impacts gs with implications for A. Changes in one anatomical trait (i.e. SD) are often compensated for by modifications in another (i.e. stomatal size), with many studies reporting a strong negative correlation between SD and size (e.g. Drake et al., 2013). However, while this relationship appears in closely related species (Faralli et al., 2019), it does not hold across multiple diverse species (McAusland et al., 2016).
One of the most well-studied impacts of environment on stomatal numbers is atmospheric [CO2], which has been demonstrated to decrease SD with increasing [CO2] in a number of different species (Hetherington and Woodward, 2003), including several major cropping systems (Ainsworth and Rogers, 2007). Global warming associated with rising [CO2] has been shown to increase SD in several crop species (Rodrigues et al., 2016; Caine et al., 2019) including soybean (Jumrani et al., 2017), tobacco (Hu et al., 2014), and grape (Rogiers et al., 2011), often with concurrent decreases in stomatal size (Rodrigues et al., 2016), although no effect was reported for maize (Zheng et al., 2013). However, such changes in anatomy (i.e. SD or guard cell length) do not necessarily translate into differences in gs, and vice versa (Rodrigues et al., 2016; Kapadiya et al., 2017), illustrating the importance of considering both functional responses and anatomical alterations with growth temperature.
Stomatal behavioural responses to elevated temperature
Whilst higher temperatures can disrupt a number of metabolic processes, including those that take place in the guard cells, stomatal response to high temperatures is often complicated by the fact that temperature also affects photosynthesis, VPD, transpiration, and plant water status, which all feed back on stomatal behaviour (Urban et al., 2017). Changes in temperature alter VPD (see Scaling from plants to ecosystem), which subsequently alters transpiration as stomata respond to the change in atmospheric dryness (e.g. Brodribb and McAdam, 2011; Merilo et al., 2018). Higher VPD increases the leaf–atmosphere diffusion gradient, driving greater water loss and triggering stomatal closure to maintain plant water status (Mott and Peak, 2013). The actual mechanisms for stomatal response to VPD are still not fully elucidated, except for a broad classification into two hydraulic responses: active and passive (Xie et al., 2006; Chater et al., 2011; Bauer et al., 2013; Franks, 2013; McAdam and Brodribb, 2014).
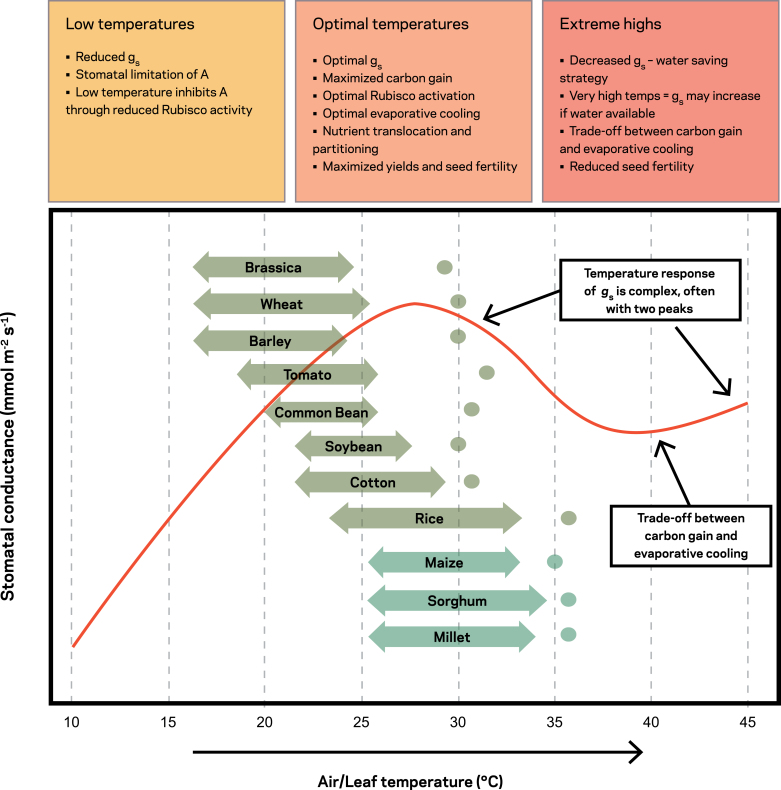
Studies examining stomatal responses specifically to temperature have received less attention than those focusing on other environmental factors (Way, 2011; Teskey et al., 2015), and the findings are highly variable between species (Sage and Kubien, 2007; Matthews and Lawson, 2019). gs has a mixed response with rising temperature across crop species (Schulze et al., 1975; Lu et al., 2000; von Caemmerer and Evans, 2015; Urban et al., 2017), with an increase in gs of 163% observed in maize (Zheng et al., 2013), yet a decrease (Sage and Sharkey, 1987; Raven et al., 2005) or no effect on gs at all with increased temperature reported in other crops (Sage and Sharkey, 1987; Aphalo and Jarvis, 1991; von Caemmerer and Evans, 2015). Generalizing stomatal response to changes in leaf temperature is complicated by interactions between temperature and VPD, but also by the non-linearity in responses, often described as bell shaped (Fig. 4) (Way, 2011; Matthews and Lawson, 2019). gs tends to increase with temperature up to a tipping point (Way, 2011; Tricker et al., 2018) before rapidly decreasing at greater temperatures (Šantrůcek and Sage, 1996), and can increase again if stomata reopen at very high temperatures (Fig. 4). The temperature where stomata commence closure in the bell-shaped response is species specific and dependent on the growth temperature conditions (Sage and Sharkey, 1987). It is likely that this variation can be explained by differences in hydraulic conductance and temperature effects on viscosity (Cochard et al., 2000), as well as photosynthetic demand (Šantrůcek and Sage, 1996).
Fig. 4.
Impact of temperature on changes in stomatal conductance and response in major cropping systems. Highlighted is a generic response of stomatal conductance (gs) across a temperature range (red line); optimal temperature ranges for major global crop types (two-headed arrows), including critical temperatures when biomass and yield are significantly reduced (dots). Reproduced with permission from Matthews and Lawson (2019).
Heat stress induces responses in gs that vary genotypically (Zhou et al., 2017; Ferguson et al., 2020); however, whether this variation in gs can be linked to heat sensitivity levels remains unclear. Plants can also acclimate to different growth temperatures, resulting in lower stomatal sensitivity to short-term (i.e. minutes) changes in ambient temperature (e.g. Šantrůcek and Sage, 1996). Under different growth temperatures, the gs response that plants exhibit can be a similar shape, though the magnitude can vary greatly (Yamori et al., 2006; Way, 2011).
Increased gs values at higher temperatures will benefit plant performance by removing diffusional constraints on CO2 diffusion into the leaf, and the resulting increase in intercellular CO2 will help to reduce the negative impact of increased photorespiration at higher leaf temperatures (see previous section). Additionally, higher gs will facilitate enhanced transpiration and evaporative cooling, which will support the maintenance of leaf temperature closer to the Topt for photosynthesis, further reducing photorespiratory processes (Urban et al., 2017). However, the increased water loss through higher gs can compromise plant water status (Matthews and Lawson, 2019) which, depending on the degree of water stress, could be detrimental to plant performance and growth. Furthermore, high atmospheric temperatures often occur in conjunction with reduced water availability, so stomatal temperature responses are linked closely not only with VPD but also with drought and water potential (Urban et al., 2017). Stomata close when water becomes limiting to avoid catastrophic water loss, even when demands for photosynthesis are high, demonstrating the hierarchal response of one signal over-riding others. As gs decreases with rising temperature and/or limited water availability, leaf temperature will further increase due to reduced evaporative cooling, leading to metabolic disruptions (Tezara et al., 1999; Perdomo et al., 2017), and lower photosynthesis from restricted CO2 diffusion (Chaves et al., 2003).
Advancements needed to improve stomatal resilience to heat stress
Manipulation of stomatal anatomy and metabolism has been suggested as a potential mechanism for crop improvement under adverse environmental conditions. SD has been altered via manipulating the stomatal development pathway, which can be achieved by focusing on the epidermal patterning factor family of transcription factors (EPFs). Many studies suggest that decreasing SD will reduce water loss and improve water use efficiency (Hughes et al., 2017; Caine et al., 2019), but this could also increase leaf temperatures. However, rice with reduced SD (due to increased expression of osEPF1) showed reduced water use that resulted in lower leaf temperature relative to wild-type controls under drought stress (Caine et al., 2019). Conversely, overexpression of EPF9/Stomagen results in increased gs and A, but at the expense of water use efficiency (Tanaka et al., 2013). Masle et al. (2005) demonstrated in Arabidopsis that the ERECTA gene not only influenced SD (and subsequently gs), but also the coordination between A and gs, which offers the potential to manipulate transpiration efficiency. Thus, it would be interesting to explore the potential of these mutants under different water, temperature, and VPD stress conditions (Lawson et al., 2014).
Manipulating guard cell metabolism or signalling pathways is an alternative and mostly unexplored avenue for future consideration (Lawson and Blatt, 2014; Lawson et al., 2014). For example, Hettenhausen et al. (2012) manipulated a mitogen-activated protein kinase, MPK4, in tobacco that results in increased gs, whilst overexpression of aquaporins in rice and grapevine increases gs and A under both stress and non-stress conditions (Hanba et al., 2004; Sade et al., 2010). There are many other examples where components of guard cell osmoregulation and/or mesophyll metabolism have altered stomatal function (see table 1 in Matthews and Lawson, 2019) that provide a mostly unexploited genetic reservoir of material to explore for manipulating stomatal behaviour to cope with global warming. Altogether, these studies suggest that manipulation of stomatal anatomy and function could be a promising path to increase evaporative cooling as a strategy to cope with future climate conditions, but this may increase water requirements as a consequence.
The detrimental effect of elevated temperature is often associated with impacts on leaf biochemistry; however, for some crops, the main cause of decreased yield is due to high temperature during the reproductive stage of growth (Akter and Islam, 2017). Therefore, manipulating SD and stomatal function in non-foliar tissue may also be an important and overlooked route for reducing temperature stress at key times (Simkin et al., 2020). Furthermore, the function of stomata in both foliar and non-foliar tissue and the role they play in translocation of photosynthate from source to sink tissues, including grain yield, is often ignored, as bulk flow within the phloem requires bulk flow of water in the xylem, which is a direct result of transpirational water loss that is ultimately controlled by stomata. Additionally, coordination between SD and minor vein density, which is a principle determinant of leaf hydraulic capacity (Brodribb et al., 2007), has been observed in many species contributing to the balance between leaf water supply and demand (W.-L. Zhao et al., 2017). The effect of rising temperature on this relationship requires further investigation, since trends differ across species (Hu et al., 2014; Yang et al., 2020).
Temperature impacts on source to sink allocation and phloem transport
Carbohydrate translocation from photosynthetic source tissues (sources) to non-photosynthetic sink tissues (sinks) via the phloem is critical for vegetative and reproductive development, and ultimately crop yield. Alterations in plant source–sink balances, often induced by environmental stress such as high temperature, can impair carbohydrate allocation and negatively impact photosynthetic capacity and yield. Generally, heat stress decreases photosynthetic efficiency while increasing respiration and photorespiration rates (see earlier) and can affect reproductive development (Prasad et al., 2017; Ferguson et al., 2021), which shifts the dynamics between sources and sinks. Thus, a better understanding of these mechanisms is crucial to maintain crop productivity in a warmer world.
Alongside reduced photosynthesis, declines in leaf non-structural carbohydrate (NSC) contents have been reported in several crop species (including soybean, chickpea, castor bean, and maize) with short-term (≤7 d) exposure to heat stress (Kaushal et al., 2013; Ribeiro et al., 2014; Sun et al., 2016; Thomey et al., 2019). In tomato, maintained or higher levels of NSC in mature leaves were associated with heat tolerance under short-term heat stress (Zhou et al., 2017), which could help fuel increased respiration (Ferguson et al., 2021). However, under longer term heat stress, NSC accumulation in leaves and stems (tomato and rice, Zhang et al., 2012; Zhang et al., 2018) decreases root to shoot biomass ratio (castor bean, Ribeiro et al., 2014), and the reduced carbon export rate from leaves suggests a reduction in carbohydrate export towards sinks (maize, Suwa et al., 2010). Carbohydrate accumulation in mesophyll cells has been linked to down-regulation of photosynthetic capacity via negative feedback on Rubisco content and activity (Moore et al., 1999; Long et al., 2004). Yet any potential regulatory role for leaf carbohydrate accumulation observed during long-term heat stress remains unclear, due to the direct impact of temperature on Rubisco (see earlier).
Remobilization of NSCs stored in intermediate sinks, such as stems, contributes to grain allocation especially in cereal crops, and could help compensate for reduced A when heat stress occurs at certain development stages (Fig. 5) (Blum et al., 1994; Morita and Nakano, 2011; Zamani et al., 2014; Xu et al., 2020; Zhen et al., 2020; Ferguson et al., 2021). However, heat stress can also reduce stem NSC translocation efficiency decreasing yield further (Zamani et al., 2014; Zhen et al., 2020). Together, these studies suggest a negative impact of heat stress on carbohydrate translocation, especially towards the reproductive sinks, which highlights the importance of maintaining these functions to preserve yield in resilient crop cultivars.
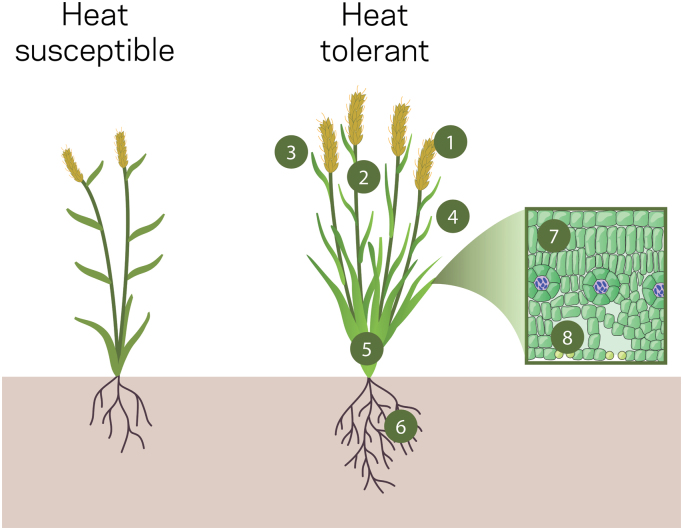
Fig. 5.
Structural and functional attributes that make a crop plant more susceptible (left) or tolerant (right) to heat stress. Numbers indicate the following: (1) higher invertase activity in spike/grain to maintain or increase carbohydrate import; (ii) remobilization of non-structural carbohydrates from the stems towards the spike/grain; (iii) short/erect flag leaf avoids direct light penetration and scorching, and has higher sucrose transporter expression to help maintain phloem loading and carbohydrate allocation to non-photosynthetic tissues; (iv) short/erect leaves avoid direct heat exposure, with angled leaves allowing light penetration lower into the canopy to help keep all leaves closer to temperature optimum; waxy leaves also help reduce water loss; (5) extra tillers and leaves to help maintain green leaf area and delay senescence; (6) more roots that reach deeper to access more soil moisture; (7) concentrated chlorophyll in the ‘sweet spot’ (i.e. not all in the top leaves) to improve leaf temperature optima; and (8) increased leaf stomatal density to improve CO2 entry into the leaves.
Various modifications in phloem structure and function, which may affect carbohydrate transport and allocation in response to elevated temperature and heat stress, have been described in several crop species (Fig. 5). At a biochemical level, intraspecific variation in rice shows that maintained or increased expression of sucrose transporters in leaves, stems, and grains is related to heat tolerance (Miyazaki et al., 2013; Phan et al., 2013; Zhang et al., 2018; Yaliang et al., 2020), particularly for transporters thought to be involved in phloem loading and apoplastic sucrose retrieval along the transport pathway (Scofield et al., 2007; Julius et al., 2017). These findings suggest that sucrose transporters are promising targets to develop heat-resilient crop cultivars. Invertases and sucrose synthases may also be interesting targets for crop improvement under heat stress (Julius et al., 2017; Xu et al., 2020). By catalysing sucrose degradation in sinks, they increase the amount of sucrose being unloaded from the phloem into these sinks. Increased or maintained expression and/or activity of invertases and sucrose synthases in reproductive sinks has been linked to heat tolerance in several crop species including rice, tomato, and chickpea (Pressman et al., 2006; Li et al., 2012; Kaushal et al., 2013; Phan et al., 2013; Li et al., 2015; Bahuguna et al., 2017; Rezaul et al., 2019; Yaliang et al., 2020). With photosynthetic improvements to heat stress, the enzymes involved in sucrose transport and metabolism may become increasingly important for ensuring increased photosynthates reach vegetative and reproductive sinks.
At a structural level, deposition of callose (a polysaccharide) and protein conformational change were observed in broad bean phloem following heat shock, resulting in blocked phloem transport (Furch et al., 2007). Heat-triggered callose deposition was also found in rice leaf and sheath plasmodesmata, especially in a heat-sensitive mutant with impaired carbohydrate translocation, potentially blocking phloem loading and/or unloading (Zhang et al., 2018). The underlying mechanisms of callose deposition in phloem under heat stress still need further investigation. Additionally, phloem anatomical features, such as the number and cross-sectional area of phloem cells, are correlated with photosynthetic capacity and environmental conditions (Cohu et al., 2014; Muller et al., 2014; Adams et al., 2016; Stewart et al., 2016). Elevated temperature decreased phloem cell number and area in an Arabidopsis ecotype from a cool climate, correlating with reduced photosynthetic capacity compared with growth at lower temperature (Adams et al., 2016; Stewart et al., 2016). This highlights the need for comparative studies in major food and fuel crops to inform acclimation potential to elevated temperatures, and identify anatomical features to select for future crop varieties.
Adding complexity: leaf interactions influence whole-plant responses to temperature
Scaling from enzymes functioning within a single leaf to a collective of leaves that make up a single plant adds a layer of complexity to the relationship between temperature and photosynthesis. The interaction of individual leaves within and among plants modifies the microclimate or phylloclimate (Chelle, 2005), causing variation in individual leaf temperatures within a crop plant. Leaf temperature depends on the leaf energy balance, including radiation, convection, and transpiration processes (Jones, 1993; Lambers et al., 1998). Shading of lower leaves by leaves higher in the canopy drives exponential declines in light availability in crop canopies (Monteith, 1965), while leaves and stems present physical barriers to wind, reducing wind speed with canopy depth (Jacobs et al., 1995). Air temperature, VPD, and [CO2] profiles influence gas exchange between the plant and the atmosphere. Thus, the interactions among all of these variables influence leaf temperature profiles with canopy depth.
Improving whole-plant photosynthesis has focused on the plant ‘ideotype’ that best intercepts light for optimal photon capture and utilization by light-harvesting complexes (Long et al., 2006b; Ort and Melis, 2011). While temperature effects are usually secondary to optimal photon capture, work to improve light distribution within plant canopies may alleviate some of the limitations posed by plant temperature gradients (Fig. 5). Modelling suggests that less light absorption by upper canopy leaves could result in cooler leaf temperatures at the top of the plant (Drewry et al., 2014), allowing those leaves to operate nearer Topt, which would be especially beneficial under heat stress conditions when gs is limited. Shifting a greater proportion of photosynthesis to the lower canopy where wind speeds are lower and humidity is higher could also increase water use efficiency (Drewry et al., 2014). However, the effects on leaf temperature remain uncertain.
How a crop plant develops under heat stress and what this means for photosynthesis and yield
While leaf temperatures higher than Topt directly affect whole-plant photosynthesis, they also have indirect impacts at plant and canopy scales across all stages of a plant’s life cycle. During the vegetative stage, deviation from a Topt alters plant development and subsequently limits A for biomass accumulation. Heat stress reduces germination, seedling vigour, and establishment in soybean and cowpea (Covell et al., 1986), and radicle elongation in rice (Han et al., 2009). In maize, extreme heat reduces, and can completely halt, coleoptile growth (Weaich et al., 1996). After plant establishment, heat stress can prevent leaf development (i.e. cassava, Burns et al., 2010), thereby preventing leaf area accumulation for photosynthetic gain to the plant canopy (Fig. 5). For example, daytime temperatures >33 °C and high night-time temperatures reduce leaf emergence and tillering in rice, thereby reducing plant biomass (Chaudhary and Ghildyal, 1970; Fahad et al., 2016a).
Heat damage to leaf photosynthetic pigments reduces photosynthetic efficiency during vegetative growth, which impacts biomass accumulation and development to reduce crop yield. For example, temperatures >35 °C negatively impact maize biomass accumulation due to degradation of chlorophyll, consequently reducing photosynthetic light absorption (Hatfield et al., 2011; Hussain et al., 2019). Premature loss of leaf chlorophyll due to heat stress accelerates mobilization of photosynthate to newer leaves and triggers early maturity of the whole plant (Nooden, 1986). This drives a shorter plant life cycle and reduces the grain-filling window—a critical yield determinant period for cereal plants. Heat-induced reductions in life cycle length have caused grain yield reduction in wheat (Camp et al., 1982; Nicolas et al., 1984; Reynolds et al., 1994; Benbella and Paulsen, 1998), rice (Fahad et al., 2016b), and maize (Ruiz-Vera et al., 2015).
Photosynthate availability and transport capacity from source tissues to reproductive tissues may also affect reproductive development (see above). For example, in some maize hybrids, kernel number and kernel weight correspond to source capacity during grain filling, suggesting that these yield components may be limited by photosynthate supply even under non-stressed conditions (Cerrudo et al., 2013). Therefore, detrimental effects of heat stress on leaf photosynthesis probably further impair grain development and yield where grain sink strength is high (Fig. 5). As discussed above, heat stress may also impair photosynthate transport between crop source and sink tissues (Suwa et al., 2010; Bagley et al., 2015). These studies emphasize the need for sufficient production of sugars through photosynthesis and maintenance of their transport, especially during heat stress. Although beyond the scope of this review, direct impacts of high temperature on reproductive structures also play a critical role in determining crop yields and will require engineering for greater tolerance to heat stress to ensure sufficient sink size for enhanced photosynthate production and transport (Barnabás et al., 2008; Ruiz-Vera et al., 2015; Ferguson et al., 2021).
Recent advances made at the plant level to improve understanding of temperature effects
Developing plant mechanisms to cope with heat stress is complicated by interacting climate factors and the geographical variability forecast for temperature (Long and Ort, 2010; Hoegh-Guldberg et al., 2018), with heat stress responses greatly influenced by region and environmental conditions. Further, a combination of traits and agronomic manipulations determine heat stress tolerance. The determination of heat-tolerant crop ‘ideotypes’ is a challenge for plant breeders, and has driven a push to locate quantitative trait loci (QTLs) and genetic markers for photosynthetic heat tolerance (Azam et al., 2014; Sharma et al., 2017). While progress has been made, searching for QTLs is a substantial task, given the combination of changing variables throughout a plant life cycle and the challenges in genotyping and phenotyping large germplasm sets at different growth stages.
Plant phenotyping may provide a quicker means of detecting plant heat stress responses given recent technological advances (Furbank et al., 2019; Furbank and Tester, 2011; Gao et al., 2020). For example, plant temperature stress causes stomatal responses detectable with thermal imaging (Stoll and Jones, 2007; Prashar and Jones, 2014) and visible scorching and damage detectable with red–green–blue imaging (Elazab et al., 2016). Photosynthetic responses are also detectable with chlorophyll fluorescence (Sharma et al., 2012; Jedmowski and Brüggemann, 2015) and hyperspectral analysis (Dobrowski et al., 2005). At the plant scale, recent advancements in field phenotyping have seen hyperspectral analysis used to predict photosynthetic capacity in field trials (Serbin et al., 2012; Yendrek et al., 2017; Silva-Perez et al., 2018; Fu et al., 2019, 2020; Furbank et al., 2019; Meacham-Hensold et al., 2019, 2020). Using these phenotyping tools to screen genetically targeted germplasm is required to target heat-tolerant traits for breeders.
Scaling from the leaf to the whole-plant level in translation of heat stress traits at a higher resolution remains an additional challenge. At the plant level, temperature responses are closely linked with irradiance profiles. Recent advances in functional and structural plant modelling (FSPM) (Vos et al., 2010; Evers et al., 2018) offer scope for deconstructing the relationship between irradiance gradients on whole-plant temperature profiles to pinpoint Topt for leaves at different plant canopy layers. The greater challenge in creating heat-resistant crops is pairing whole-plant FSPM, which considers leaf-level physiology to suggest heat-tolerant plant ideotypes, with tools to phenotype for genetic heat-tolerant markers across a range of species and environmental conditions.
Scaling from plants to ecosystem reinforces the complex relationship between temperature and photosynthesis
The effects of temperature on enzyme, leaf, and plant scales compound to impact crop photosynthesis and productivity at the ecosystem scale. This is due to the additive responses to the microclimate of all leaves and plants that make up a crop ecosystem (Bagley et al., 2015). The microclimate impacts crop productivity through the effects of atmospheric turbulence and wind changing the temperature, humidity, and light environment experienced by leaves at different heights within the canopy (Cleugh, 1998). While the speed at which a cropping system can respond to changes in light can reduce ecosystem photosynthesis (Kromdijk et al., 2016; Morales and Kaiser, 2020), increases in temperature are a crucial driver reducing photosynthesis and yields across the major cropping varieties (Lobell et al., 2014; Asseng et al., 2015; Liu et al., 2016; C. Zhao et al., 2017), and will be the focus of this section.
A key mechanism controlling the reduction in ecosystem photosynthesis at higher temperature is the link with atmospheric VPD (Bernacchi and VanLoocke, 2015). The amount of water vapour which air can hold at saturation (es) increases with temperature, while the actual water vapour of air at any given time (ea) remains relatively constant, resulting in increased atmospheric VPD—the difference between es and ea (Bernacchi and VanLoocke, 2015; Ficklin and Novick, 2017). Increasing atmospheric VPD has a feedback effect on plants, particularly on the stomata, whereby a drier atmosphere exerts a stronger pull on water from within leaves during photosynthesis (Lawson and Vialet-Chabrand, 2019). As discussed earlier, crops can close their stomata to conserve water, but this comes at the cost of photosynthesis, which reduces yield at the ecosystem scale if relied upon too often during the growing season.
Early lessons from FACE (Table 1) studies suggest that crop photosynthesis would be enhanced with higher [CO2], and water loss would decline with lower gs (Leakey et al., 2009). A recent update of the literature has confirmed that these conclusions hold for C3 and C4 crops (Ainsworth and Long, 2020). However, when FACE systems were coupled with increased temperature (T-FACE), canopy warming and periodic heat stress caused an acceleration in maize and soybean crop development and often decreased yield (Siebers et al., 2015; Ruiz-Vera et al., 2018), particularly when higher temperatures were coupled with water deficit (Gray et al., 2016). Even without supplemental heating through experimentation, hotter and drier growing seasons reduced wheat yield grown under FACE relative to FACE-grown plants under ‘typical’ growing seasons (Fitzgerald et al., 2016; Macabuhay et al., 2018). However, mixed results have been reported for rice grown at elevated temperature, probably due to latitudinal differences in average temperature maxima impacting rice grown in the tropics more than at higher latitudes (Lesk et al., 2016; Usui et al., 2016).
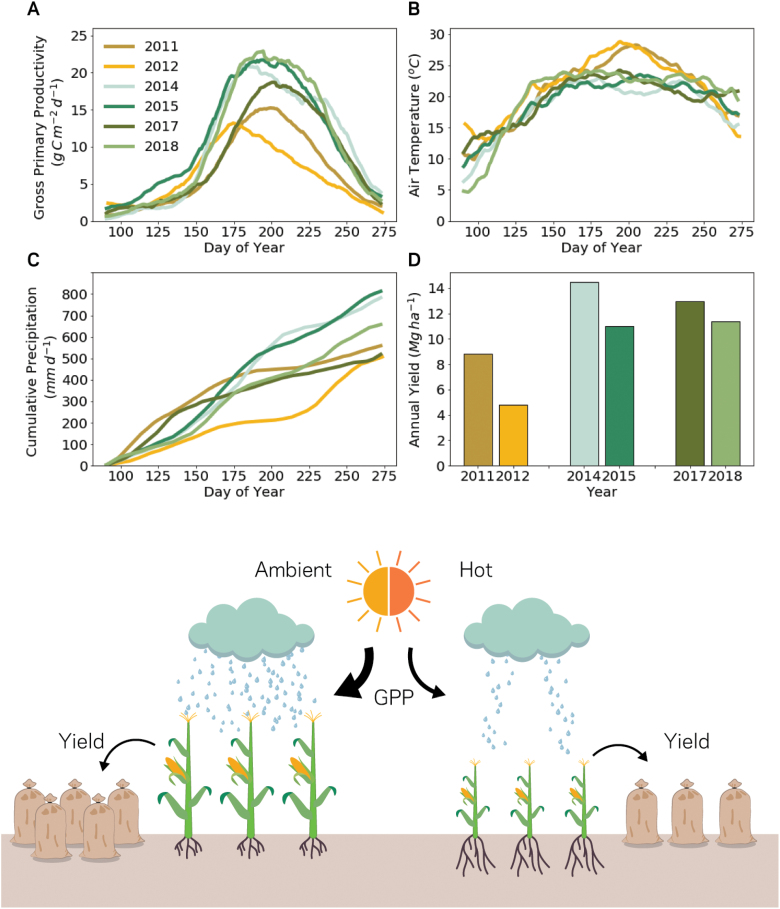
Crops grown under well-watered conditions can afford to maintain high A under elevated temperature for longer than crops grown under water stress (Fitzgerald et al., 2016). In regions of the world where increasing temperature is coupled with increasing rainfall, drought and heat stress impacts on crop photosynthesis and productivity may be minimized (Tesfaye et al., 2018). However, the timing and duration of rainfall events will be critical for determining the effectiveness of increased moisture as a buffer to hotter temperatures. For example, in the currently rain-fed and highly productive region of the Midwest United States, DeLucia et al. (2019) project that a water limit will be reached for maize productivity due to increased atmospheric VPD that will be driven by rising global temperature. Lobell et al. (2014) have shown that while maize yields have historically been increasing, the crop is very susceptible to drought and VPD stress. This impact on maize yield was evident in the 2012 drought experienced by the Midwest US during the growing season (Fig. 6). For cropping systems already reliant on irrigation, changes in mean annual rainfall associated with a warming world could be catastrophic for future yields if water resources become scarce. Shifting cropping systems that are primarily rain-fed to an irrigation-reliant system will place increased pressure on existing hydrological reserves to deliver water for agriculture in addition to metropolitan and natural systems (DeLucia et al., 2019).
Fig. 6.
The difference in gross primary productivity (GPP) and annual yield for maize across different climatic years, as indicated by air temperature and rainfall. (A–D) were produced using data from Ameriflux site Ui-C using processing protocols from Moore et al. (2020). The years 2013 and 2016 are omitted from (D) as these years were under a soybean rotation at the site.
Changes to the by-products of photosynthesis associated with rising temperature
Rising temperature at the ecosystem scale also affects carbon consumption processes that can impact short-term annual yield of cropping systems and their long-term ecological sustainability. For ecosystem-scale carbon cycle concepts, photosynthesis is referred to as gross primary productivity (GPP; Table 1) (Chapin et al., 2006). Changes to ecosystem autotrophic respiration (RA) and GPP as global temperature increases will be likely to mirror that of the processes described earlier, in that photosynthesis has a clear Topt and peak thermal response, and RA increases exponentially with rising temperature until acclimation occurs. However, what is less certain is the rate at which heterotrophic respiration (RH) will change as temperatures rise, particularly that of soil microbes (Bond-Lamberty and Thomson, 2010; von Haden et al., 2019). It is commonly accepted that ecosystem respiration (ER; combined RA and RH) increases with temperature (Lloyd and Taylor, 1994), and can acclimate under prolonged heat exposure (Way and Yamori, 2014). A recent synthesis has suggested that this has predictably responded to global warming, though there still remains large uncertainty surrounding the RH contribution in particular (Bond-Lamberty et al., 2018).
Recent advancements and prospects for monitoring crop canopies and improving management responses with rising temperature
There is an inherent need for the development of strategies to ensure crop productivity with global warming. Current agronomic practices rely on weather and climate forecasts to predict when cropping systems are likely to require irrigation or nutrient application. However, these meteorological services lack information on real-time carbon uptake and water loss from the cropping system of interest. Such information could advance understanding of crop responses to the environment and, where possible, lead to informed management decisions to minimize losses.
Eddy covariance flux towers monitor ecosystem photosynthesis, along with water use and a suite of common meteorological measurements including air temperature, solar radiation, wind, soil moisture/temperature, and humidity (Baldocchi et al., 2001). Yet, the data require large amounts of post-processing to generate complete time series for each measured variable (Isaac et al., 2017; Pastorello et al., 2020). Further, GPP is estimated (not measured) as the difference between the comparatively smaller net ecosystem exchange (NEE) of CO2 as the measured variable and ER estimated using nocturnal (Lloyd and Taylor, 1994) or diurnal (Lasslop et al., 2010) temperature response functions. While this approach is imperfect in many ways, it provides the most reliable and accurate means of quantifying, with high temporal precision, the rates of photosynthesis and respiration from cropping systems at the ecosystem scale.
With >900 sites registered as part of the FLUXNET community, there still remains a paucity of flux towers providing openly available long-term monitoring data (i.e. >5 years) from agricultural systems (Baldocchi et al., 2018; Cleverly et al., 2020; Pastorello et al., 2020). Increasing the number of flux towers operating in cropping systems in key climatic regions of the world, and making these data immediately and freely available through open-access licensing, will be an important step for improving current understanding of the wide-scale impact of rising temperature on crop ecosystem photosynthesis. The capacity to provide measurements of carbon and water fluxes in real-time is building (i.e. FluxSuite & SmartFlux from LICOR Biosciences, Lincoln, NE, USA or EasyFlux from Campbell Scientific, Logan, UT, USA), but delivering these data in real-time to land managers, as with weather forecasting, is lacking. While FLUXNET data require significant post-processing and data corrections, the end result is generally research related. Real-time output of fluxes with minimal processing may be suitable for land managers to make informed decisions. Given the link between ecosystem carbon and water fluxes, and crop photosynthetic efficiency and water stress, supplying these data in real-time would make a substantial contribution towards faster crop stress detection.
Flux tower networks also deliver important ground-truth data to validate satellite information that can be used to infer crop photosynthesis over landscape, regional, and global scales, which flux towers are incapable of completely capturing (i.e. measurement region of interest is usually between 200 m2 and 2000 m2). Satellite data products have typically relied on the calculation of vegetation indices from surface reflectance information, such as the normalized difference vegetation index (NDVI; Tucker, 1979), enhanced vegetation index (EVI; Huete et al., 2002), and photochemical reflectance index (PRI; Gamon et al., 1997) to provide indications of vegetation stress. However, these indices depend on changes in vegetation greenness to show variation in the index value, after which it can be too late to remedy vegetation stress. In addition, the indices typically measure top-of-canopy responses, so changes at lower canopy layers are missed.
Improvements in spectral sensing technology have led to the development of passive remote sensing of sun-induced chlorophyll fluorescence (SIF) as a proxy for real-time monitoring of photosynthesis (Meroni et al., 2009; Sun et al., 2017; Frankenberg and Berry, 2018). Chlorophyll fluorescence represents one of three fates of light energy absorbed by light-harvesting complexes within leaves; the other two being photochemistry and heat dissipation (Baker, 2008). Active measurement of chlorophyll fluorescence is a commonly used tool in plant physiology research, as these three light use pathways do not operate in isolation from each other. Chlorophyll fluorescence yield provides useful information on photosynthetic quantum efficiency and heat dissipation, which leads to its use in inferring A and in imaging to screen for genetic trait expression in plants (Murchie and Lawson, 2013). At scales from the ecosystem to globe, passive measurement of chlorophyll fluorescence as SIF relies on the spectral emission of SIF surrounding oxygen absorption bands (O2-A and O2-B) within a narrow spectral range (Meroni et al., 2009; Frankenberg and Berry, 2018).
Advancements in SIF monitoring in recent years have rapidly expanded, with studies demonstrating a strong correlation between crop GPP at the ecosystem (Miao et al., 2018; Wu et al., 2020), regional (Guan et al., 2016), and global scales (Guanter et al., 2014). The relationship between SIF and crop GPP has led to the use of SIF in detecting crop stress, as the two signals are inherently linked (Zarco-Tejada et al., 2012; Camino et al., 2019; Peng et al., 2020). Additional satellite sensing of land surface evapotranspiration (ET)—the ECOsystem Spaceborne Thermal Radiometer Experiment on Space Station (ECOSTRESS)—is also being used to assess ecosystem stress on daily time scales (Fisher et al., 2020). The combination of SIF and ECOSTRESS satellite products has the potential to greatly advance our understanding of ecosystem GPP in relation to ET, and how environmental stresses, such as increased temperature and heatwaves, are likely to impact crop productivity at regional to global scales. Granted, there still remain several unanswered questions surrounding the quantity of information provided by SIF, whether the signal is primarily affected by changes in canopy architecture or if it is a direct product of biochemistry (Magney et al., 2020). As these fundamental questions are answered, and with the addition of new satellite remote-sensing platforms to monitor SIF globally at high temporal resolution (i.e. TROPOMI, OCO-2, and GOME), SIF will certainly continue to advance as an important real-time tool for monitoring crop photosynthesis and productivity as global temperature rises.
Conclusion and future directions
This review provides a comprehensive evaluation of current understanding on how crop photosynthesis responds to temperature from the enzyme to ecosystem scale. The key conclusions for each scale are summarized as follows.
(i) Direct impacts of elevated temperature on photosynthetic enzymes involved in carbon assimilation are particularly damaging to C3 crops. Enzyme rates increase with temperature, but substrate specificity declines in the carbon-fixing enzyme Rubisco, which deactivates past optimal temperatures.
(ii) Stomata typically respond to temperature through the complex effects of heat on photosynthesis, VPD, transpiration, and plant water status. Stomatal conductance can change under temperature stress, and stomatal density and size can be altered if a plant develops under hotter conditions.
(iii) Photosynthate allocation from sources to sinks is impacted by heat stress through differential expression and activity of enzymes involved in sucrose transport and metabolism, as well as phloem structural changes.
(iv) At the whole-plant scale, leaf interactions create temperature gradients, and heat stress impairs plant development processes.
(v) The factors identified in (i)–(iv) act together to impact crop ecosystem photosynthesis and its response to temperature, the effects of which are typically seen as a cumulative response through the growing season and lead to reduced yield.
Ensuring our cropping systems remain resilient to rising temperatures will require integration of knowledge and information across scales. For each scale discussed, the areas of research needed to improve resiliency of cropping systems to rising temperature and heat stress are as follows.
(i) At the biochemical scale, most strategies for improving carbon fixation in a warmer climate involve enhancing Rubisco performance or minimizing the energy expended in photorespiration, but many remain to be tested in crop species or replicated field trials.
(ii) Altering stomatal anatomy and metabolism may help to reduce water loss from crops whilst maintaining photosynthetic rates to ensure high crop yields are maintained. However, the relationship between stomata and leaf hydraulic capacity should also be considered to maintain a balance between leaf water supply and demand.
(iii) At the transport system level, strategies need to be tested to help maintain photosynthate allocation from sources to sinks by increasing sucrose phloem loading in sources (e.g. increasing expression of leaf sucrose transporters) and sucrose phloem unloading in sinks (e.g. increasing invertase activity in reproductive sinks), as well as increasing remobilization of sugars stored in intermediate sinks.
(iv) Coupling whole-plant modelling of temperature gradients with phenotyping resources will allow identification and breeding of heat-resistant crop ideotypes.
(v) At the ecosystem scale, the implementation of faster crop stress detection systems will be critical for applying management strategies to combat temperature-related stress. These strategies may include combining ground-based measurements, such as those from flux towers, with satellite remote-sensing information, to provide closer to real-time monitoring of crop systems.
Acknowledgements
CEM and CJB acknowledge funding from the DOE Center for Advanced Bioenergy and Bioproducts Innovation (U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-SC0018420). APC, KM-H, PL, RAS, CB, TL, and CJB acknowledge funding from the research project Realizing Increased Photosynthetic Efficiency (RIPE) that is funded by the Bill & Melinda Gates Foundation, Foundation for Food and Agricultural Research (FFAR), and the UK Foreign Commonwealth & Development Office under grant no. OPP1172157. TL also acknowledges the BBSRC IWYP programme (grant no. BB/S005080/1). CJB also acknowledges support from the USDA to the Global Change and Photosynthesis Research Unit of the USDA Agricultural Research Service. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the U.S. Department of Agriculture or the U.S. Department of Energy.
Author contributions
CEM led manuscript preparation with contributions from all co-authors. Specifically, APC and CJB wrote the first section, TL and PL wrote the second section, KM-H and RAS wrote the third section, and CEM and CJB wrote the fourth section. CB led production of figures, with input from all other co-authors. Given the range of topics reviewed, CEM and APC share corresponding authorship.
References
- Adams WW 3rd, Stewart JJ, Cohu CM, Muller O, Demmig-Adams B. 2016. Habitat temperature and precipitation of Arabidopsis thaliana ecotypes determine the response of foliar vasculature, photosynthesis, and transpiration to growth temperature. Frontiers in Plant Science 7, 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner H, Wilson RH, Bracher A, Calisse L, Bhat JY, Hartl FU, Hayer-Hartl M. 2017. Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science 358, 1272–1278. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. 2020. 30 years of free-air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Global Change Biology 27, 27–49. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Ort DR. 2010. How do we improve crop production in a warming world? Plant Physiology 154, 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258–270. [DOI] [PubMed] [Google Scholar]
- Akter N, Islam MR. 2017. Heat stress effects and management in wheat. A review. Agronomy for Sustainable Development 37, 1–17. [Google Scholar]
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. 2008. Heat stress: an overview of molecular responses in photosynthesis. Photosynthesis Research 98, 541–550. [DOI] [PubMed] [Google Scholar]
- Aphalo PJ, Jarvis PG. 1991. Do stomata respond to relative humidity? Plant, Cell & Environment 14, 127–132. [Google Scholar]
- Asseng S, Ewert F, Martre P, et al. 2015. Rising temperatures reduce global wheat production. Nature Climate Change 5, 143–147. [Google Scholar]
- Atkinson N, Mao Y, Chan KX, McCormick AJ. 2020. Condensation of Rubisco into a proto-pyrenoid in higher plant chloroplasts. Nature Communications 11, 6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam FI, Chang X, Jing R. 2014. Mapping QTL for chlorophyll fluorescence kinetics parameters at seedling stage as indicators of heat tolerance in wheat. Euphytica 202, 245–258. [Google Scholar]
- Bagley J, Rosenthal DM, Ruiz-Vera UM, Siebers MH, Kumar P, Ort DR, Bernacchi CJ. 2015. The influence of photosynthetic acclimation to rising CO2 and warmer temperatures on leaf and canopy photosynthesis models. Global Biogeochemical Cycles 29, 194–206. [Google Scholar]
- Bahuguna RN, Solis CA, Shi W, Jagadish KS. 2017. Post-flowering night respiration and altered sink activity account for high night temperature-induced grain yield and quality loss in rice (Oryza sativa L.). Physiologia Plantarum 159, 59–73. [DOI] [PubMed] [Google Scholar]
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology 59, 89–113. [DOI] [PubMed] [Google Scholar]
- Baldocchi D, Chu H, Reichstein M. 2018. Inter-annual variability of net and gross ecosystem carbon fluxes: a review. Agricultural and Forest Meteorology 249, 520–533. [Google Scholar]
- Baldocchi D, Falge E, Gu L, et al. 2001. FLUXNET: a new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bulletin of the American Meteorological Society 82, 2415–2434. [Google Scholar]
- Barnabás B, Jäger K, Fehér A. 2008. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell & Environment 31, 11–38. [DOI] [PubMed] [Google Scholar]
- Bathellier C, Yu LJ, Farquhar GD, Coote ML, Lorimer GH, Tcherkez G. 2020. Ribulose 1,5-bisphosphate carboxylase/oxygenase activates O2 by electron transfer. Proceedings of the National Academy of Sciences, USA 117, 24234–24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti DS, Naylor RL. 2009. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323, 240–244. [DOI] [PubMed] [Google Scholar]
- Bauer H, Ache P, Wohlfart F, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Hetherington AM, Hedrich R. 2013. How do stomata sense reductions in atmospheric relative humidity? Molecular Plant 6, 1703–1706. [DOI] [PubMed] [Google Scholar]
- Benbella M, Paulsen GM. 1998. Efficacy of treatments for delaying senescence of wheat leaves: II. Senescence and grain yield under field conditions. Agronomy Journal 90, 332–338. [Google Scholar]
- Bernacchi CJ, Pimentel C, Long SP. 2003. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell & Environment 26, 1419–1430. [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment 24, 253–259. [Google Scholar]
- Bernacchi CJ, VanLoocke A. 2015. Terrestrial ecosystems in a changing environment: a dominant role for water. Annual Review of Plant Biology 66, 599–622. [DOI] [PubMed] [Google Scholar]
- Betti M, Bauwe H, Busch FA, et al. 2016. Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. Journal of Experimental Botany 67, 2977–2988. [DOI] [PubMed] [Google Scholar]
- Blum A, Sinmena B, Mayer J, Golan G, Shpiler L. 1994. Stem reserve mobilisation supports wheat-grain filling under heat stress. Functional Plant Biology 21, 771. [Google Scholar]
- Bond-Lamberty B, Bailey VL, Chen M, Gough CM, Vargas R. 2018. Globally rising soil heterotrophic respiration over recent decades. Nature 560, 80–83. [DOI] [PubMed] [Google Scholar]
- Bond-Lamberty B, Thomson A. 2010. Temperature-associated increases in the global soil respiration record. Nature 464, 579–582. [DOI] [PubMed] [Google Scholar]
- Boyd RA, Gandin A, Cousins AB. 2015. Temperature responses of C4 photosynthesis: biochemical analysis of Rubisco, phosphoenolpyruvate carboxylase, and carbonic anhydrase in Setaria viridis. Plant Physiology 169, 1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher A, Whitney SM, Hartl FU, Hayer-Hartl M. 2017. Biogenesis and metabolic maintenance of Rubisco. Annual Review of Plant Biology 68, 29–60. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA. 2011. Passive origins of stomatal control in vascular plants. Science 331, 582–585. [DOI] [PubMed] [Google Scholar]
- Buckley TN, John GP, Scoffoni C, Sack L. 2017. The sites of evaporation within leaves. Plant Physiology 173, 1763–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A, Gleadow R, Cliff J, Zacarias A, Cavagnaro T. 2010. Cassava: the drought, war and famine crop in a changing world. Sustainability 2, 3572–3607. [Google Scholar]
- Caine RS, Yin X, Sloan J, et al. 2019. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytologist 221, 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino C, Gonzalez-Dugo V, Hernandez P, Zarco-Tejada PJ. 2019. Radiative transfer Vcmax estimation from hyperspectral imagery and SIF retrievals to assess photosynthetic performance in rainfed and irrigated plant phenotyping trials. Remote Sensing of Environment 231, 111186. [Google Scholar]
- Camp PJ, Huber SC, Burke JJ, Moreland DE. 1982. Biochemical changes that occur during senescence of wheat leaves: I. Basis for the reduction of photosynthesis. Plant Physiology 70, 1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva E, Scales JC, Madgwick PJ, Parry MA. 2015. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant, Cell & Environment 38, 1817–1832. [DOI] [PubMed] [Google Scholar]
- Cerrudo A, Di Matteo J, Fernandez E, Robles M, Pico LO, Andrade FH. 2013. Yield components of maize as affected by short shading periods and thinning. Crop and Pasture Science 64, 580. [Google Scholar]
- Chapin FS, Woodwell GM, Randerson JT, et al. 2006. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 9, 1041–1050. [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, Fleming A, Cuming AC, Gray JE, Beerling DJ. 2011. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology 21, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Chaudhary TN, Ghildyal BP. 1970. Influence of submerged soil temperature regimes on growth, yield, and nutrient composition of rice plant. Agronomy Journal 62, 281–285. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. 2003. Understanding plant responses to drought—from genes to the whole plant. Functional Plant Biology 30, 239–264. [DOI] [PubMed] [Google Scholar]
- Chelle M. 2005. Phylloclimate or the climate perceived by individual plant organs: what is it? How to model it? What for? New Phytologist 166, 781–790. [DOI] [PubMed] [Google Scholar]
- Chlingaryan A, Sukkarieh S, Whelan B. 2018. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: a review. Computers and Electronics in Agriculture 151, 61–69. [Google Scholar]
- Cleugh HA. 1998. Effects of windbreaks on airflow, microclimates and crop yields. Agroforestry Systems 41, 55–84. [Google Scholar]
- Cleverly J, Vote C, Isaac P, et al. 2020. Carbon, water and energy fluxes in agricultural systems of Australia and New Zealand. Agricultural and Forest Meteorology 287, 107934. [Google Scholar]
- Cochard H, Martin R, Gross P, Bogeat-Triboulot MB. 2000. Temperature effects on hydraulic conductance and water relations of Quercus robur L. Journal of Experimental Botany 51, 1255–1259. [PubMed] [Google Scholar]
- Cohu CM, Muller O, Adams WW 3rd, Demmig-Adams B. 2014. Leaf anatomical and photosynthetic acclimation to cool temperature and high light in two winter versus two summer annuals. Physiologia Plantarum 152, 164–173. [DOI] [PubMed] [Google Scholar]
- Covell S, Ellis RH, Roberts EH, Summerfield RJ. 1986. The influence of temperature on seed germination rate in grain legumes: I. A comparison of chickpea, lentil, soyabean and cowpea at constant temperatures. Journal of Experimental Botany 37, 705–715. [Google Scholar]
- Cowan IR, Farquhar GD. 1977. Stomatal function in relation to leaf metabolism and environment. Symposia of the Society for Experimental Biology 31, 471–505. [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2000. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proceedings of the National Academy of Sciences, USA 97, 13430–13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2002. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiology 129, 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Van De Loo FJ, Salvucci ME. 1997. The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiology 114, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidi D, Shamshoum M, Guo Z, et al. 2020. Highly active rubiscos discovered by systematic interrogation of natural sequence diversity. The EMBO Journal 39, e104081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen GE, Worrall D, Carmo-Silva E. 2020. An isoleucine residue acts as a thermal and regulatory switch in wheat Rubisco activase. The Plant Journal 103, 742–751. [DOI] [PubMed] [Google Scholar]
- DeLucia EH, Chen S, Guan K, et al. 2019. Are we approaching a water ceiling to maize yields in the United States? Ecosphere 10, e02773. [Google Scholar]
- Djanaguiraman M, Prasad PVV, Schapaugh WT. 2013. High day- or nighttime temperature alters leaf assimilation, reproductive success, and phosphatidic acid of pollen grain in soybean [Glycine max (L.) Merr.]. Crop Science 53, 1594–1604. [Google Scholar]
- Dobrowski SZ, Pushnik JC, Zarco-Tejada PJ, Ustin SL. 2005. Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sensing of Environment 97, 403–414. [Google Scholar]
- Drake JE, Tjoelker MG, Vårhammar A, et al. 2018. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biology 24, 2390–2402. [DOI] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ. 2013. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany 64, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry DT, Kumar P, Long SP. 2014. Simultaneous improvement in productivity, water use, and albedo through crop structural modification. Global Change Biology 20, 1955–1967. [DOI] [PubMed] [Google Scholar]
- Dusenge ME, Duarte AG, Way DA. 2019. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist 221, 32–49. [DOI] [PubMed] [Google Scholar]
- Elazab A, Ordóñez RA, Savin R, Slafer GA, Araus JL. 2016. Detecting interactive effects of N fertilization and heat stress on maize productivity by remote sensing techniques. European Journal of Agronomy 73, 11–24. [Google Scholar]
- Ermakova M, Arrivault S, Giuliani R, et al. 2020. Installation of C4 photosynthetic pathway enzymes in rice using a single construct. Plant Biotechnology Journal. doi: 10.1111/pbi.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JB, Letort V, Renton M, Kang M. 2018. Computational botany: advancing plant science through functional–structural plant modelling. Annals of Botany 121, 767–772. [Google Scholar]
- Fahad S, Hussain S, Saud S, et al. 2016. a. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiology and Biochemistry 103, 191–198. [DOI] [PubMed] [Google Scholar]
- Fahad S, Hussain S, Saud S, Khan F, Hassan S, Amanullah, Nasim W, Arif M, Wang F, Huang J. 2016b. Exogenously applied plant growth regulators affect heat-stressed rice pollens. Journal of Agronomy and Crop Science 202, 139–150. [Google Scholar]
- Faralli M, Matthews J, Lawson T. 2019. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Current Opinion in Plant Biology 49, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, McAusland L, Smith KE, Price AH, Wilson ZA, Murchie EH. 2020. Rapid temperature responses of photosystem II efficiency forecast genotypic variation in rice vegetative heat tolerance. The Plant Journal 104, 839–855. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Tidy AC, Murchie EH, Wilson ZA. 2021. The potential of resilient carbon dynamics for stabilising crop reproductive development and productivity during heat stress. Plant, Cell & Environment doi: 10.1111/pce.14015. [DOI] [PubMed] [Google Scholar]
- Ficklin DL, Novick KA. 2017. Historic and projected changes in vapor pressure deficit suggest a continental-scale drying of the United States atmosphere. Journal of Geophysical Research: Atmospheres 122, 2061–2079. [Google Scholar]
- Fischer RA, Rebetzke GJ. 2018. Indirect selection for potential yield in early-generation, spaced plantings of wheat and other small-grain cereals: a review. Crop and Pasture Science 69, 439–459. [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL. 1998. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475. [Google Scholar]
- Fisher JB, Lee B, Purdy AJ, et al. 2020. ECOSTRESS: NASA’s next generation mission to measure evapotranspiration from the international space station. Water Resources Research 56. [Google Scholar]
- Fitzgerald GJ, Tausz M, O’Leary G, et al. 2016. Elevated atmospheric [CO2] can dramatically increase wheat yields in semi-arid environments and buffer against heat waves. Global Change Biology 22, 2269–2284. [DOI] [PubMed] [Google Scholar]
- Flamholz AI, Prywes N, Moran U, Davidi D, Bar-On YM, Oltrogge LM, Alves R, Savage D, Milo R. 2019. Revisiting trade-offs between Rubisco kinetic parameters. Biochemistry 58, 3365–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O, et al. 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193–194, 70–84. [DOI] [PubMed] [Google Scholar]
- Flexas J, Carriquí M, Coopman RE, Gago J, Galmés J, Martorell S, Morales F, Diaz-Espejo A. 2014. Stomatal and mesophyll conductances to CO2 in different plant groups: underrated factors for predicting leaf photosynthesis responses to climate change? Plant Science 226, 41–48. [DOI] [PubMed] [Google Scholar]
- Florian A, Nikoloski Z, Sulpice R, Timm S, Araújo WL, Tohge T, Bauwe H, Fernie AR. 2014. Analysis of short-term metabolic alterations in Arabidopsis following changes in the prevailing environmental conditions. Molecular Plant 7, 893–911. [DOI] [PubMed] [Google Scholar]
- Flügel F, Timm S, Arrivault S, Florian A, Stitt M, Fernie AR, Bauwe H. 2017. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in Arabidopsis. The Plant Cell 29, 2537–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg C, Berry J. 2018. Solar induced chlorophyll fluorescence: origins, relation to photosynthesis and retrieval. In: Reference module in earth systems and environmental sciences. Elsevier, 143–162. [Google Scholar]
- Franks PJ. 2013. Passive and active stomatal control: either or both? New Phytologist 198, 325–327. [DOI] [PubMed] [Google Scholar]
- Fu P, Meacham-Hensold K, Guan K, Bernacchi CJ. 2019. Hyperspectral leaf reflectance as proxy for photosynthetic capacities: an ensemble approach based on multiple machine learning algorithms. Frontiers in Plant Science 10, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Meacham-Hensold K, Guan K, Wu J, Bernacchi C. 2020. Estimating photosynthetic traits from reflectance spectra: a synthesis of spectral indices, numerical inversion, and partial least square regression. Plant, Cell & Environment 43, 1241–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Jimenez-Berni JA, George-Jaeggli B, Potgieter AB, Deery DM. 2019. Field crop phenomics: enabling breeding for radiation use efficiency and biomass in cereal crops. New Phytologist 223, 1714–1727. [DOI] [PubMed] [Google Scholar]
- Furbank RT, Sharwood R, Estavillo GM, Silva-Perez V, Condon AG. 2020. Photons to food: genetic improvement of cereal crop photosynthesis. Journal of Experimental Botany 71, 2226–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Tester M. 2011. Phenomics—technologies to relieve the phenotyping bottleneck. Trends in Plant Science 16, 635–644. [DOI] [PubMed] [Google Scholar]
- Furch AC, Hafke JB, Schulz A, van Bel AJ. 2007. Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. Journal of Experimental Botany 58, 2827–2838. [DOI] [PubMed] [Google Scholar]
- Galmés J, Hermida-Carrera C, Laanisto L, Niinemets Ü. 2016. A compendium of temperature responses of Rubisco kinetic traits: variability among and within photosynthetic groups and impacts on photosynthesis modeling. Journal of Experimental Botany 67, 5067–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Copolovici LO, Hermida-Carrera C, Niinemets Ü. 2015. Temperature responses of the Rubisco maximum carboxylase activity across domains of life: phylogenetic signals, trade-offs, and importance for carbon gain. Photosynthesis Research 123, 183–201. [DOI] [PubMed] [Google Scholar]
- Gamon JA, Serrano L, Surfus JS. 1997. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 112, 492–501. [DOI] [PubMed] [Google Scholar]
- Gao G, Tester MA, Julkowska MM. 2020. The use of high throughout phenotyping for assessment of heat stress-induced changes in Arabidopsis. Plant Phenomics 2020, 3723916. doi: 10.34133/2020/3723916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SB, Dermody O, Klein SP, et al. 2016. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nature Plants 2, 16132. [DOI] [PubMed] [Google Scholar]
- Grossiord C, Buckley TN, Cernusak LA, Novick KA, Poulter B, Siegwolf RTW, Sperry JS, McDowell NG. 2020. Plant responses to rising vapor pressure deficit. New Phytologist 226, 1550–1566. [DOI] [PubMed] [Google Scholar]
- Guan K, Berry JA, Zhang Y, Joiner J, Guanter L, Badgley G, Lobell DB. 2016. Improving the monitoring of crop productivity using spaceborne solar-induced fluorescence. Global Change Biology 22, 716–726. [DOI] [PubMed] [Google Scholar]
- Guanter L, Zhang Y, Jung M, et al. 2014. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proceedings of the National Academy of Sciences, USA 111, E1327–E1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YP, Zhou HF, Zhang LC. 2006. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Scientia Horticulturae 108, 260–267. [Google Scholar]
- Han F, Chen H, Li XJ, Yang MF, Liu GS, Shen SH. 2009. A comparative proteomic analysis of rice seedlings under various high-temperature stresses. Biochimica et Biophysica Acta 1794, 1625–1634. [DOI] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. 2004. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant & Cell Physiology 45, 521–529. [DOI] [PubMed] [Google Scholar]
- Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Thomson AM, Wolfe D. 2011. Climate impacts on agriculture: implications for crop production. Agronomy Journal 103, 351–370. [Google Scholar]
- Hatfield JL, Prueger JH. 2015. Temperature extremes: effect on plant growth and development. Weather and Climate Extremes 10, 4–10. [Google Scholar]
- Hennacy JH, Jonikas MC. 2020. Prospects for engineering biophysical CO2 concentrating mechanisms into land plants to enhance yields. Annual Review of Plant Biology 71, 461–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Baldwin IT, Wu J. 2012. Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiology 158, 759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y. 2006. Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. Journal of Experimental Botany 57, 291–302. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Jacob D, Taylor M, et al. 2018. Impacts of 1.5 °C global warming on natural and human systems. In: Masson-Delmotte V, Zhai P, Pörtner HO, et al. , eds. Global warming of 1.5 °C. Geneva, Switzerland: IPCC, 177–311. [Google Scholar]
- Hu J, Yang QY, Huang W, Zhang SB, Hu H. 2014. Effects of temperature on leaf hydraulic architecture of tobacco plants. Planta 240, 489–496. [DOI] [PubMed] [Google Scholar]
- Huete A, Didan K, Miura T, Rodriguez EP, Gao X, Ferreira LG. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sensing of Environment 83, 195–213. [Google Scholar]
- Hughes J, Hepworth C, Dutton C, Dunn JA, Hunt L, Stephens J, Waugh R, Cameron DD, Gray JE. 2017. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiology 174, 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain HA, Men S, Hussain S, et al. 2019. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Scientific Reports 9, 3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Pachauri RK, Meyer LA, eds. Geneva, Switzerland: IPCC. [Google Scholar]
- IPCC . 2018. Summary for policymakers. In: Masson-Delmotte V, Zhai P, Pörtner HO, et al. , eds. Global warming of 1.5 °C. Geneva, Switzerland: IPCC. [Google Scholar]
- Isaac P, Cleverly J, McHugh I, van Gorsel E, Ewenz C, Beringer J. 2017. OzFlux data: network integration from collection to curation. Biogeosciences 14, 1–41. [Google Scholar]
- Jacobs AFG, van Boxel JH, El-Kilani RMM. 1995. Vertical and horizontal distribution of wind speed and air temperature in a dense vegetation canopy. Journal of Hydrology 166, 313–326. [Google Scholar]
- Jedmowski C, Brüggemann W. 2015. Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. Journal of Photochemistry and Photobiology. B, Biology 151, 153–160. [DOI] [PubMed] [Google Scholar]
- Jones MB. 1993. Plant microclimate. In: Hall DO, Scurlock JMO, Bolhar-Nordenkampf HR, Leegood RC, Long SP, eds. Photosynthesis and production in a changing environment. Dordrecht: Springer, 47–64. [Google Scholar]
- Julius BT, Leach KA, Tran TM, Mertz RA, Braun DM. 2017. Sugar transporters in plants: new insights and discoveries. Plant & Cell Physiology 58, 1442–1460. [DOI] [PubMed] [Google Scholar]
- Jumrani K, Bhatia VS, Pandey GP. 2017. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynthesis Research 131, 333–350. [DOI] [PubMed] [Google Scholar]
- Kapadiya KB, Singh C, Bhalara RL, Kandoliya UK, Dabhi KH. 2017. Effect of higher temperature on leaf anatomy of heat tolerance and heat susceptible wheat genotypes (Triticum aestivum L.) by scanning electron microscopy. Journal of Pharmacognosy and Phytochemistry 6, 2270–2277. [Google Scholar]
- Kattge J, Knorr W. 2007. Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant, Cell & Environment 30, 1176–1190. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Awasthi R, Gupta K, Gaur P, Siddique KHM, Nayyar H. 2013. Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Functional Plant Biology 40, 1334–1349. [DOI] [PubMed] [Google Scholar]
- Kim SY, Slattery RA, Ort DR. 2020. A role for differential Rubisco activase isoform expression in C4 bioenergy grasses at high temperature. GCB Bioenergy 13, 211– 223. [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Long SP. 2016. One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proceedingsof the Royal Society B: Biological Sciences 283, 20152578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis A, Bar-Even A. 2019. Synthetic biology approaches for improving photosynthesis. Journal of Experimental Botany 70, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Li C, Portis AR Jr. 2009. Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynthesis Research 100, 143–153. [DOI] [PubMed] [Google Scholar]
- Kumarathunge DP, Medlyn BE, Drake JE, et al. 2019. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytologist 222, 768–784. [DOI] [PubMed] [Google Scholar]
- Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu G. 2007. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. The Plant Cell 19, 3230–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. 1998. Plant physical ecology. New York: Springer. [Google Scholar]
- Lasslop G, Reichstein M, Papale D, Richardson AD, Arneth A, Barr A, Story P, Wohlfahrt G. 2010. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Global Change Biology 16, 187–208. [Google Scholar]
- Law RD, Crafts-Brandner SJ. 2001. High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Archives of Biochemistry and Biophysics 386, 261–267. [DOI] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ, Salvucci ME. 2001. Heat stress induces the synthesis of a new form of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in cotton leaves. Planta 214, 117–125. [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR. 2014. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164, 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Matthews J. 2020. Guard cell metabolism and stomatal function. Annual Review of Plant Biology 71, 273–302. [DOI] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D. 2014. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytologist 203, 1064–1081. [DOI] [PubMed] [Google Scholar]
- Lawson T, Vialet-Chabrand S. 2019. Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist 221, 93–98. [DOI] [PubMed] [Google Scholar]
- Lawson T, von Caemmerer S, Baroli I. 2010. Photosynthesis and stomatal behavior. In: Lüttge U, Beyschlag W, Büdel B, Francis D, eds. Progress in Botany, Vol. 72. Berlin, Heidelberg: Springer, 265–304. [Google Scholar]
- Leakey AD, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. 2009. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany 60, 2859–2876. [DOI] [PubMed] [Google Scholar]
- Le Quéré C, Jackson RB, Jones MW, et al. 2020. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nature Climate Change 10, 647–653. [Google Scholar]
- Lesk C, Rowhani P, Ramankutty N. 2016. Influence of extreme weather disasters on global crop production. Nature 529, 84–87. [DOI] [PubMed] [Google Scholar]
- Li X, Lawas LM, Malo R, et al. 2015. Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant, Cell & Environment 38, 2171–2192. [DOI] [PubMed] [Google Scholar]
- Li Z, Palmer WM, Martin AP, Wang R, Rainsford F, Jin Y, Patrick JW, Yang Y, Ruan YL. 2012. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. Journal of Experimental Botany 63, 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Stone WD, Chaudhari V, Hanson MR. 2020. Small subunits can determine enzyme kinetics of tobacco Rubisco expressed in Escherichia coli. Nature Plants 6, 1289–1299. [DOI] [PubMed] [Google Scholar]
- Liu B, Asseng S, Müller C, et al. 2016. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nature Climate Change 6, 1130–1136. [Google Scholar]
- Lloyd J, Taylor JA. 1994. On the temperature dependence of soil respiration. Functional Ecology 8, 315–323. [Google Scholar]
- Lobell DB, Gourdji SM. 2012. The influence of climate change on global crop productivity. Plant Physiology 160, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL. 2014. Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344, 516–519. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J. 2011. Climate trends and global crop production since 1980. Science 333, 616–620. [DOI] [PubMed] [Google Scholar]
- Long BM, Hee WY, Sharwood RE, et al. 2018. Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nature Communications 9, 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell & Environment 14, 729–739. [Google Scholar]
- Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR. 2006a. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. 2004. Rising atmospheric carbon dioxide: plants FACE the future. Annual Review of Plant Biology 55, 591–628. [DOI] [PubMed] [Google Scholar]
- Long SP, Ort DR. 2010. More than taking the heat: crops and global change. Current Opinion in Plant Biology 13, 241–248. [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006b. Can improvement in photosynthesis increase crop yields? Plant, Cell & Environment 29, 315–330. [DOI] [PubMed] [Google Scholar]
- López-Calcagno PE, Fisk S, Brown KL, Bull SE, South PF, Raines CA. 2019. Overexpressing the H-protein of the glycine cleavage system increases biomass yield in glasshouse and field-grown transgenic tobacco plants. Plant Biotechnology Journal 17, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Percy RG, Qualset CO, Zeiger E. 1998. Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. Journal of Experimental Botany 49, 453–460. [Google Scholar]
- Lu Z, Quiñones MA, Zeiger E. 2000. Temperature dependence of guard cell respiration and stomatal conductance co-segregate in an F2 population of Pima cotton. Australian Journal of Plant Physiology 27, 457–462. [Google Scholar]
- Lundgren MR. 2020. C2 photosynthesis: a promising route towards crop improvement? New Phytologist 228, 1734–1740. [DOI] [PubMed] [Google Scholar]
- Macabuhay A, Houshmandfar A, Nuttall J, Fitzgerald GJ, Tausz M, Tausz-Posch S. 2018. Can elevated CO2 buffer the effects of heat waves on wheat in a dryland cropping system? Environmental and Experimental Botany 155, 578–588. [Google Scholar]
- Magney TS, Barnes ML, Yang X. 2020. On the covariation of chlorophyll fluorescence and photosynthesis across scales. Geophysical Research Letters. [Google Scholar]
- Martin-Avila E, Lim YL, Birch R, Dirk LMA, Buck S, Rhodes T, Sharwood RE, Kapralov MV, Whitney SM. 2020. Modifying plant photosynthesis and growth via simultaneous chloroplast transformation of Rubisco large and small subunits. The Plant Cell 32, 2898–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. 2005. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436, 866–870. [DOI] [PubMed] [Google Scholar]
- Matthews JSA, Lawson T. 2019. Climate change and stomatal physiology. Annual Plant Reviews 2, 713–752. [Google Scholar]
- McAdam SA, Brodribb TJ. 2014. Separating active and passive influences on stomatal control of transpiration. Plant Physiology 164, 1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T. 2016. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytologist 211, 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham-Hensold K, Fu P, Wu J, et al. 2020. Plot-level rapid screening for photosynthetic parameters using proximal hyperspectral imaging. Journal of Experimental Botany 71, 2312–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham-Hensold K, Montes CM, Wu J, et al. 2019. High-throughput field phenotyping using hyperspectral reflectance and partial least squares regression (PLSR) reveals genetic modifications to photosynthetic capacity. Remote Sensing of Environment 231, 111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B, Kilk K, Kollist H. 2018. Stomatal VPD response: there is more to the story than ABA. Plant Physiology 176, 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni M, Rossini M, Guanter L, Alonso L, Rascher U, Colombo R, Moreno J. 2009. Remote sensing of solar-induced chlorophyll fluorescence: review of methods and applications. Remote Sensing of Environment 113, 2037–2051. [Google Scholar]
- Miao G, Guan K, Yang X, et al. 2018. Sun-induced chlorophyll fluorescence, photosynthesis, and light use efficiency of a soybean field from seasonally continuous measurements. Journal of Geophysical Research: Biogeosciences 123, 610–623. [Google Scholar]
- Miyazaki M, Araki M, Okamura K, Ishibashi Y, Yuasa T, Iwaya-Inoue M. 2013. Assimilate translocation and expression of sucrose transporter, OsSUT1, contribute to high-performance ripening under heat stress in the heat-tolerant rice cultivar Genkitsukushi. Journal of Plant Physiology 170, 1579–1584. [DOI] [PubMed] [Google Scholar]
- Monteith JL. 1965. Light distribution and photosynthesis in field crops. Annals of Botany 29, 17–37. [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR. 1999. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant, Cell & Environment 22, 567–582. [Google Scholar]
- Moore CE, Berardi DM, Blanc-Betes E, et al. 2020. The carbon and nitrogen cycle impacts of reverting perennial bioenergy switchgrass to an annual maize crop rotation. GCB Bioenergy 12, 941–954. [Google Scholar]
- Morales A, Kaiser E. 2020. Photosynthetic Acclimation to Fluctuating Irradiance in Plants. Frontiers in Plant Science 11, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Nakano H. 2011. Nonstructural carbohydrate content in the stem at full heading contributes to high performance of ripening in heat-tolerant rice cultivar Nikomaru. Crop Science 51, 818–828. [Google Scholar]
- Mott KA, Peak D. 2013. Testing a vapour-phase model of stomatal responses to humidity. Plant, Cell & Environment 36, 936–944. [DOI] [PubMed] [Google Scholar]
- Muller O, Stewart JJ, Cohu CM, Polutchko SK, Demmig-Adams B, Adams WW 3rd. 2014. Leaf architectural, vascular and photosynthetic acclimation to temperature in two biennials. Physiologia Plantarum 152, 763–772. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Lawson T. 2013. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany 64, 3983–3998. [DOI] [PubMed] [Google Scholar]
- Nicolas ME, Gleadow RM, Dalling MJ. 1984. Effects of drought and high temperature on grain growth in wheat. Australian Journal of Plant Physiology 11, 553–66. [Google Scholar]
- Niinemets U, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. 2009. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. Journal of Experimental Botany 60, 2249–2270. [DOI] [PubMed] [Google Scholar]
- Nooden LD. 1986. Whole plant senescence. In: Leshem YY, Halevy A, Frenkel C, eds. Processes and control of plant senescence. New York: Elsevier, 119–126. [Google Scholar]
- Novick KA, Ficklin DL, Stoy PC, et al. 2016. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nature Climate Change 1, 1–5. [Google Scholar]
- Ogren WL, Bowes G. 1971. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nature 230, 159–160. [DOI] [PubMed] [Google Scholar]
- Orr DJ, Alcântara A, Kapralov MV, Andralojc PJ, Carmo-Silva E, Parry MA. 2016. Surveying Rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiology 172, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Melis A. 2011. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiology 155, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, et al. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proceedings of the National Academy of Sciences, USA 112, 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorello G, Trotta C, Canfora E, et al. 2020. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Scientific Data 7, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Guan K, Zhou W, Jiang C, Frankenberg C, Sun Y, He L, Köhler P. 2020. Assessing the benefit of satellite-based solar-induced chlorophyll fluorescence in crop yield prediction. International Journal of Applied Earth Observation and Geoinformation 90, 102126. [Google Scholar]
- Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J. 2017. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Frontiers in Plant Science 8, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo JA, Carmo-Silva E, Hermida-Carrera C, Flexas J, Galmés J. 2016. Acclimation of biochemical and diffusive components of photosynthesis in rice, wheat, and maize to heat and water deficit: implications for modeling photosynthesis. Frontiers in Plant Science 7, 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SE, Alexander LV, Nairn JR. 2012. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophysical Research Letters 39, 2012GL053361. [Google Scholar]
- Phan TTT, Ishibashi Y, Miyazaki M, Tran HT, Okamura K, Tanaka S, Nakamura J, Yuasa T, Iwaya-Inoue M. 2013. High temperature-induced repression of the rice sucrose transporter (OsSUT1) and starch synthesis-related genes in sink and source organs at milky ripening stage causes chalky grains. Journal of Agronomy and Crop Science 199, 178–188. [Google Scholar]
- Pokharel M, Chiluwal A, Stamm M, Min D, Rhodes D, Jagadish SVK. 2020. High night-time temperature during flowering and pod filling affects flower opening, yield and seed fatty acid composition in canola. Journal of Agronomy and Crop Science 206, 579–596. [Google Scholar]
- Posch BC, Kariyawasam BC, Bramley H, Coast O, Richards RA, Reynolds MP, Trethowan R, Atkin OK. 2019. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. Journal of Experimental Botany 70, 5051–5069. [DOI] [PubMed] [Google Scholar]
- Prasad PVV, Bheemanahalli R, Jagadish SVK. 2017. Field crops and the fear of heat stress: opportunities, challenges and future directions. Field Crops Research 200, 114–121. [Google Scholar]
- Prasad PVV, Djanaguiraman M. 2011. High night temperature decreases leaf photosynthesis and pollen function in grain sorghum. Functional Plant Biology 38, 993–1003. [DOI] [PubMed] [Google Scholar]
- Prasad PVV, Pisipati SR, Ristic Z, Bukovnik U, Fritz AK. 2008. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Science 48, 2372–2380. [Google Scholar]
- Prashar A, Jones H. 2014. Infra-red thermography as a high-throughput tool for field phenotyping. Agronomy 4, 397–417. [Google Scholar]
- Pressman E, Harel D, Zamski E, Shaked R, Althan L, Rosenfeld K, Firon N. 2006. The effect of high temperatures on the expression and activity of sucrose-cleaving enzymes during tomato (Lycopersicon esculentum) anther development. Journal of Horticultural Science and Biotechnology 81, 341–348. [Google Scholar]
- Raven PH, Evert RF, Eichhorn SE. 2005. Biology of plants. New York: W.H. Freeman and Co. Ltd. [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA. 2013. Yield trends are insufficient to double global crop production by 2050. PLoS One 8, e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Balota M, Delgado M, Amani I, Fischer R. 1994. Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Functional Plant Biology 21, 717. [Google Scholar]
- Rezaul IM, Baohua F, Tingting C, Weimeng F, Caixia Z, Longxing T, Guanfu F. 2019. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiologia Plantarum 165, 644–663. [DOI] [PubMed] [Google Scholar]
- Ribeiro PR, Fernandez LG, de Castro RD, Ligterink W, Hilhorst HW. 2014. Physiological and biochemical responses of Ricinus communis seedlings to different temperatures: a metabolomics approach. BMC Plant Biology 14, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues WP, Martins MQ, Fortunato AS, et al. 2016. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Global Change Biology 22, 415–431. [DOI] [PubMed] [Google Scholar]
- Rogiers SY, Hardie WJ, Smith JP. 2011. Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Australian Journal of Grape and Wine Research 17, 147–152. [Google Scholar]
- Ross BL, Trudeau D, Bar-Even A, Tawfik D. 2020. Developing a carbon-conserving photorespiration bypass pathway through ancestral genome mining and engineering. Biophysical Journal 118, 45a. [Google Scholar]
- Ruiz-Vera UM, Siebers MH, Drag DW, Ort DR, Bernacchi CJ. 2015. Canopy warming caused photosynthetic acclimation and reduced seed yield in maize grown at ambient and elevated [CO2]. Global Change Biology 21, 4237–4249. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vera UM, Siebers MH, Jaiswal D, Ort DR, Bernacchi CJ. 2018. Canopy warming accelerates development in soybean and maize, offsetting the delay in soybean reproductive development by elevated CO2 concentrations. Plant, Cell & Environment 41, 2806–2820. [DOI] [PubMed] [Google Scholar]
- Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, Moshelion M. 2010. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiology 152, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30, 1086–1106. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD. 1987. The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiology 84, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda K, Yamamoto A, Ishikawa C, Taniguchi Y, Matsumura H, Fukayama H. 2020. Effects of introduction of sorghum RbcS with rice RbcS knockdown by RNAi on photosynthetic activity and dry weight in rice. Plant Production Science doi: 10.1080/1343943X.2020.1847669. [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ. 2004. Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiology 134, 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E. 2001. Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiology 127, 1053–1064. [PMC free article] [PubMed] [Google Scholar]
- Šantrůcek J, Sage RF. 1996. Acclimation of stomatal conductance to a CO2-enriched atmosphere and elevated temperature in Chenopodium album. Australian Journal of Plant Physiology 23, 467–478. [Google Scholar]
- Savir Y, Noor E, Milo R, Tlusty T. 2010. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proceedings of the National Academy of Sciences, USA 107, 3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, Atwell BJ, Muylaert S, Reusel BV, Ruiz GA, Rie JV, Gallé A. 2018. A thermotolerant variant of Rubisco activase from a wild relative improves growth and seed yield in rice under heat stress. Frontiers in Plant Science 9, 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, Bautsoens N, den Boer B, Van Rie J, Gallé A. 2019. A conserved sequence from heat-adapted species improves Rubisco activase thermostability in wheat. Plant Physiology 181, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro AP, Gallé A, Van Rie J, Carmo-Silva E, Salvucci ME, Atwell BJ. 2016. Heat tolerance in a wild Oryza species is attributed to maintenance of Rubisco activation by a thermally stable Rubisco activase ortholog. New Phytologist 211, 899–911. [DOI] [PubMed] [Google Scholar]
- Schrader SM, Kane HJ, Sharkey TD, von Caemmerer S. 2006. High temperature enhances inhibitor production but reduces fallover in tobacco Rubisco. Functional Plant Biology 33, 921–929. [DOI] [PubMed] [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD. 2004. Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant, Cell & Environment 27, 725–735. [Google Scholar]
- Schulze E, Lange OL, Kappen L, Evenari M, Buschbom U. 1975. The role of air humidity and leaf temperature in controlling stomatal resistance of Prunus armeniaca L. under desert conditions: II. The significance of leaf water status and internal carbon dioxide concentration. Oecologia 18, 219–233. [DOI] [PubMed] [Google Scholar]
- Scofield GN, Hirose T, Aoki N, Furbank RT. 2007. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. Journal of Experimental Botany 58, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Serbin SP, Dillaway DN, Kruger EL, Townsend PA. 2012. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. Journal of Experimental Botany 63, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DK, Andersen SB, Ottosen CO, Rosenqvist E. 2012. Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Functional Plant Biology 39, 936–947. [DOI] [PubMed] [Google Scholar]
- Sharma DK, Torp AM, Rosenqvist E, Ottosen CO, Andersen SB. 2017. QTLs and potential candidate genes for heat stress tolerance identified from the mapping populations specifically segregating for Fv/Fm in wheat. Frontiers in Plant Science 8, 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharwood RE. 2017. Engineering chloroplasts to improve Rubisco catalysis: prospects for translating improvements into food and fiber crops. New Phytologist 213, 494–510. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, Ghannoum O, Kapralov MV, Gunn LH, Whitney SM. 2016. Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis. Nature Plants 2, 16186. [DOI] [PubMed] [Google Scholar]
- Shen BR, Wang LM, Lin XL, et al. 2019. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Molecular Plant 12, 199–214. [DOI] [PubMed] [Google Scholar]
- Shivhare D, Mueller-Cajar O. 2017. In vitro characterization of thermostable CAM Rubisco activase reveals a Rubisco interacting surface loop. Plant Physiology 174, 1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebers MH, Slattery RA, Yendrek CR, Locke AM, Drag D, Ainsworth EA, Bernacchi CJ, Ort DR. 2017. Simulated heat waves during maize reproductive stages alter reproductive growth but have no lasting effect when applied during vegetative stages. Agriculture, Ecosystems and Environment 240, 162–170. [Google Scholar]
- Siebers MH, Yendrek CR, Drag D, Locke AM, Rios Acosta L, Leakey AD, Ainsworth EA, Bernacchi CJ, Ort DR. 2015. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Global Change Biology 21, 3114–3125. [DOI] [PubMed] [Google Scholar]
- Silva-Perez V, Molero G, Serbin SP, Condon AG, Reynolds MP, Furbank RT, Evans JR. 2018. Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. Journal of Experimental Botany 69, 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, Faralli M, Ramamoorthy S, Lawson T. 2020. Photosynthesis in non-foliar tissues: implications for yield. The Plant Journal 101, 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, López-Calcagno PE, Raines CA. 2019. Feeding the world: improving photosynthetic efficiency for sustainable crop production. Journal of Experimental Botany 70, 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery RA, Ort DR. 2019. Carbon assimilation in crops at high temperatures. Plant, Cell & Environment 42, 2750–2758. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2017. Short-term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Global Change Biology 23, 4840–4853. [DOI] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL. 1982. Genetic modification of photorespiration. Trends in Biochemical Sciences 7, 171–174. [Google Scholar]
- Sonawane BV, Sharwood RE, von Caemmerer S, Whitney SM, Ghannoum O. 2017. Short-term thermal photosynthetic responses of C4 grasses are independent of the biochemical subtype. Journal of Experimental Botany 68, 5583–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South PF, Cavanagh AP, Liu HW, Ort DR. 2019. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363, eaat9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JJ, Demmig-Adams B, Cohu CM, Wenzl CA, Muller O, Adams WW 3rd. 2016. Growth temperature impact on leaf form and function in Arabidopsis thaliana ecotypes from northern and southern Europe. Plant, Cell & Environment 39, 1549–1558. [DOI] [PubMed] [Google Scholar]
- Stoll M, Jones HG. 2007. Thermal imaging as a viable tool for monitoring plant stress. Journal International des Sciences de la Vigne et du Vin 41, 77–84. [Google Scholar]
- Sun CX, Gao XX, Li MQ, Fu JQ, Zhang YL. 2016. Plastic responses in the metabolome and functional traits of maize plants to temperature variations. Plant Biology 18, 249–261. [DOI] [PubMed] [Google Scholar]
- Sun Y, Frankenberg C, Wood JD, et al. 2017. OCO-2 advances photosynthesis observation from space via solar-induced chlorophyll fluorescence. Science 358, eaam5747. [DOI] [PubMed] [Google Scholar]
- Suwa R, Hakata H, Hara H, El-Shemy HA, Adu-Gyamfi JJ, Nguyen NT, Kanai S, Lightfoot DA, Mohapatra PK, Fujita K. 2010. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiology and Biochemistry 48, 124–130. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sugano SS, Shimada T, Hara-Nishimura I. 2013. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist 198, 757–764. [DOI] [PubMed] [Google Scholar]
- Tcherkez G. 2016. The mechanism of Rubisco-catalysed oxygenation. Plant, Cell & Environment 39, 983–997. [DOI] [PubMed] [Google Scholar]
- Tcherkez GG, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye K, Kruseman G, Cairns JE, Zaman-Allah M, Wegary D, Zaidi PH, Boote KJ, Rahut D, Erenstein O. 2018. Potential benefits of drought and heat tolerance for adapting maize to climate change in tropical environments. Climate Risk Management 19, 106–119. [Google Scholar]
- Teskey R, Wertin T, Bauweraerts I, Ameye M, McGuire MA, Steppe K. 2015. Responses of tree species to heat waves and extreme heat events. Plant, Cell & Environment 38, 1699–1712. [DOI] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. 1999. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401, 914–917. [Google Scholar]
- Thomey ML, Slattery RA, Köhler IH, Bernacchi CJ, Ort DR. 2019. Yield response of field-grown soybean exposed to heat waves under current and elevated [CO2]. Global Change Biology 25, 4352–4368. [DOI] [PubMed] [Google Scholar]
- Timm S, Florian A, Arrivault S, Stitt M, Fernie AR, Bauwe H. 2012. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Letters 586, 3692–3697. [DOI] [PubMed] [Google Scholar]
- Timm S, Giese J, Engel N, Wittmiß M, Florian A, Fernie AR, Bauwe H. 2018. T-protein is present in large excess over the other proteins of the glycine cleavage system in leaves of Arabidopsis. Planta 247, 41–51. [DOI] [PubMed] [Google Scholar]
- Timm S, Wittmiß M, Gamlien S, Ewald R, Florian A, Frank M, Wirtz M, Hell R, Fernie AR, Bauwe H. 2015. Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. The Plant Cell 27, 1968–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm S, Woitschach F, Heise C, Hagemann M, Bauwe H. 2019. Faster removal of 2-phosphoglycolate through photorespiration improves abiotic stress tolerance of Arabidopsis. Plants 8, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricker PJ, ElHabti A, Schmidt J, Fleury D. 2018. The physiological and genetic basis of combined drought and heat tolerance in wheat. Journal of Experimental Botany 69, 3195–3210. [DOI] [PubMed] [Google Scholar]
- Trudeau DL, Edlich-Muth C, Zarzycki J, et al. 2018. Design and in vitro realization of carbon-conserving photorespiration. Proceedings of the National Academy of Sciences, USA 115, E11455–E11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker CJ. 1979. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sensing of Environment 8, 127–150. [Google Scholar]
- Urban J, Ingwers MW, McGuire MA, Teskey RO. 2017. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides×nigra. Journal of Experimental Botany 68, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui Y, Sakai H, Tokida T, Nakamura H, Nakagawa H, Hasegawa T. 2016. Rice grain yield and quality responses to free-air CO2 enrichment combined with soil and water warming. Global Change Biology 22, 1256–1270. [DOI] [PubMed] [Google Scholar]
- Vico G, Way DA, Hurry V, Manzoni S. 2019. Can leaf net photosynthesis acclimate to rising and more variable temperatures? Plant, Cell & Environment 42, 1913–1928. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2020. Rubisco carboxylase/oxygenase: from the enzyme to the globe: a gas exchange perspective. Journal of Plant Physiology 252, 153240. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell & Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
- von Haden AC, Marín-Spiotta E, Jackson RD, Kucharik CJ. 2019. Soil microclimates influence annual carbon loss via heterotrophic soil respiration in maize and switchgrass bioenergy cropping systems. Agricultural and Forest Meteorology 279, 107731. [Google Scholar]
- Vos J, Evers JB, Buck-Sorlin GH, Andrieu B, Chelle M, de Visser PH. 2010. Functional–structural plant modelling: a new versatile tool in crop science. Journal of Experimental Botany 61, 2101–2115. [DOI] [PubMed] [Google Scholar]
- Walker B, Ariza LS, Kaines S, Badger MR, Cousins AB. 2013. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant, Cell & Environment 36, 2108–2119. [DOI] [PubMed] [Google Scholar]
- Walker BJ, VanLoocke A, Bernacchi CJ, Ort DR. 2016. The costs of photorespiration to food production now and in the future. Annual Review of Plant Biology 67, 107–129. [DOI] [PubMed] [Google Scholar]
- Wang D, Heckathorn SA, Wang X, Philpott SM. 2012. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169, 1–13. [DOI] [PubMed] [Google Scholar]
- Wang D, Li XF, Zhou ZJ, Feng XP, Yang WJ, Jiang DA. 2010. Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiologia Plantarum 139, 55–67. [DOI] [PubMed] [Google Scholar]
- Way DA. 2011. The bigger they are, the harder they fall: CO2 concentration and tree size affect drought tolerance. Tree Physiology 31, 115–116. [DOI] [PubMed] [Google Scholar]
- Way DA, Yamori W. 2014. Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynthesis Research 119, 89–100. [DOI] [PubMed] [Google Scholar]
- Weaich K, Bristow KL, Cass A. 1996. Modeling preemergent maize shoot growth: I. physiological temperature conditions. Agronomy Journal 88, 391–397. [Google Scholar]
- Weyers JDB, Lawson T. 1997. Heterogeneity in stomatal characteristics. Advances in Botanical Research 26, 317–352. [Google Scholar]
- Weyers JDB, Lawson T, Peng ZY. 1997. Variation in stomatal characteristics at the whole-leaf level. In: van Gardingen P, Foody G, Curran P, eds. Scaling-up: from cell to landscape. Cambridge: Cambridge University Press, 129–149. [Google Scholar]
- Wolfenden R, Snider MJ. 2001. The depth of chemical time and the power of enzymes as catalysts. Accounts of Chemical Research 34, 938–945. [DOI] [PubMed] [Google Scholar]
- Wu A, Hammer GL, Doherty A, von Caemmerer S, Farquhar GD. 2019. Quantifying impacts of enhancing photosynthesis on crop yield. Nature Plants 5, 380–388. [DOI] [PubMed] [Google Scholar]
- Wu G, Guan K, Jiang C, et al. 2020. Radiance-based NIRv as a proxy for GPP of corn and soybean. Environmental Research Letters 15, 034009. [Google Scholar]
- Xie X, Wang Y, Williamson L, et al. 2006. The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Current Biology 16, 882–887. [DOI] [PubMed] [Google Scholar]
- Xu J, Henry A, Sreenivasulu N. 2020. Rice yield formation under high day and night temperatures—a prerequisite to ensure future food security. Plant, Cell & Environment 43, 1595–1608. [DOI] [PubMed] [Google Scholar]
- Yaliang W, Yikai Z, Qinghua S, et al. 2020. Decrement of sugar consumption in rice young panicle under high temperature aggravates spikelet number reduction. Rice Science 27, 44–55. [Google Scholar]
- Yamori W, Hikosaka K, Way DA. 2014. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research 119, 101–117. [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hanba YT, Terashima I. 2006. Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant & Cell Physiology 47, 1069–1080. [DOI] [PubMed] [Google Scholar]
- Yang D, Peng S, Wang F. 2020. Response of photosynthesis to high growth temperature was not related to leaf anatomy plasticity in rice (Oryza sativa L.). Frontiers in Plant Science 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendrek CR, Tomaz T, Montes CM, Cao Y, Morse AM, Brown PJ, McIntyre LM, Leakey AD, Ainsworth EA. 2017. High-throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiology 173, 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani MM, Nabipour M, Meskarbashee M. 2014. Stem water soluble carbohydrate remobilization in wheat under heat stress during the grain filling. International Journal of Agriculture and Biology 16, 401–405. [Google Scholar]
- Zarco-Tejada PJ, González-Dugo V, Berni JAJ. 2012. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sensing of Environment 117, 322–337. [Google Scholar]
- Zhang CX, Feng BH, Chen TT, Fu WM, Li HB, Li GY, Jin QY, Tao LX, Fu GF. 2018. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source–sink relationship and sugars allocation. Environmental and Experimental Botany 155, 718–733. [Google Scholar]
- Zhang J, Jiang X, Li T, Chang T. 2012. Effect of elevated temperature stress on the production and metabolism of photosynthate in tomato (Lycopersicon esculentum L.) leaves. Journal of Horticultural Science and Biotechnology 87, 293–298. [Google Scholar]
- Zhao C, Liu B, Piao S, et al. 2017. Temperature increase reduces global yields of major crops in four independent estimates. Proceedings of the National Academy of Sciences, USA 114, 9326–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W-L, Siddiq Z, Fu PL, Zhang JL, Cao KF. 2017. Stable stomatal number per minor vein length indicates the coordination between leaf water supply and demand in three leguminous species. Scientific Reports 7, 2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen F, Zhou J, Mahmood A, Wang W, Chang X, Liu B, Liu L, Cao W, Zhu Y, Tang L. 2020. Quantifying the effects of short-term heat stress at booting stage on nonstructural carbohydrates remobilization in rice. Crop Journal 8, 194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Xu M, Hou R, Shen R, Qiu S, Ouyang Z. 2013. Effects of experimental warming on stomatal traits in leaves of maize (Zea mays L.). Ecology and Evolution 3, 3095–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Kjaer KH, Rosenqvist E, Yu X, Wu Z, Ottosen C-O. 2017. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. Journal of Agronomy and Crop Science 203, 68–80. [Google Scholar]
- Zhou Y, Whitney S. 2019. Directed evolution of an improved Rubisco; in vitro analyses to decipher fact from fiction. International Journal of Molecular Sciences 20, 5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Jin Z, Zhuang Q, Ciais P, Bernacchi C, Wang X, Makowski D, Lobell D. 2018. The important but weakening maize yield benefit of grain filling prolongation in the US Midwest. Global Change Biology 24, 4718–4730. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Portis AR, Long SP. 2004. Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant, Cell & Environment 27, 155–165. [Google Scholar]