Abstract
Background
Ischaemia–reperfusion (I/R) damage is a relevant cause of delayed graft function (DGF). Complement activation is involved in experimental I/R injury, but few data are available from kidney transplant (KT) patients. We studied the dynamics of membrane attack complex (C5b-9) as a soluble fraction (SC5b-9) and the histological deposit pattern of C3b, complement Factor H (FH) and C5b-9 in DGF patients.
Methods
We evaluated SC5b-9 levels in 59 recipients: 38 with immediate graft function and 21 with DGF. The SC5b-9 was measured at admission for KT and 7 days after KT. DGF-kidney biopsies (n = 12) and a control group of 1-year protocol biopsies without tissue damage (n = 4) were stained for C5b-9, C3b and FH.
Results
SC5b-9 increased significantly in DGF patients (Day 0: 6621 ± 2202 mAU/L versus Day 7: 9626 ± 4142 mAU/L; P = 0.006), while it remained stable in non-DGF patients. Days 0–7 increase >5% was the better cut-off associated with DGF versus non-DGF patient discrimination (sensitivity = 81%). In addition, SC5b-9 increase was related to DGF duration and worse graft function, and independently associated with DGF occurrence. SC5b-9, C3b and FH stains were observed in tubular epithelial cells basal membrane. DGF-kidney biopsies showed a more frequently high-intensity stain, a higher number of tubules with positive stain and larger perimeter of tubules with positive stains for SC5b-9, C3b and FH than control patients.
Conclusions
Both SC5b-9 levels and SC5b-9, C3b and FH deposits in tubular epithelial cells basal membrane are highly expressed in patients experiencing DGF. SC5b-9 levels increase could be useful as a marker of DGF severity.
Keywords: biomarkers, complement; delayed graft function; kidney biopsy; kidney transplantation
INTRODUCTION
Ischaemia–reperfusion (I/R) damage is one of the most important causes of delayed graft function (DGF) after kidney transplantation (KT) [1], a form of acute kidney injury (AKI) with a negative impact in graft outcomes [2–4].
Complement activation is implicated in the pathophysiology of I/R injury. It may occur through three different pathways (classic, alternative or lectin), due to the interaction with antibodies, pro-inflammatory proteins or mannose-binding lectin fragments [5–7]. Data extracted mostly from experimental studies have described a principal role of the complement classical pathway route activation in I/R injury in hearts, livers and intestines. However, in experimental models of I/R injury in kidneys, the alternative and lectin pathways are showed to be more relevant [5, 8], with some contradictory findings between animal models and clinical data [9].
Regardless of the mechanism, the three complement pathways converge in the assembly of C3 convertases that have the same function: cleavage of C3 into C3a and C3b fragments, with the latter union of C3b fragment to C3 convertase, producing C5 convertase. Finally, C5 convertases promote the complement cascade by cleaving C5 into C5a and C5b fragments. C5a initiates the building of the membrane attack complex (C5b-9) consisting of C5b, C6, C7, C8 and multiple C9 molecules [10]. C5b-9 has a critical role for defence against pathogens and has been implicated in the mechanisms of tissue injury during inflammation [5]. C3 and C5 components and the generation of anaphylatoxins (C3a and C5a) amplify the inflammatory response and provoke the release of opsonins such as C3b, which are important mediators in the process of antigen presentation and complement cascade amplification [11, 12].
Given the multiple effects that complement can exert, there are mechanisms to limit complement activation, where and when it occurs. One of the most relevant complement regulator is Factor H (FH), which accelerates the decay of the alternative pathway C3 convertase and is a co-factor of factor I-mediated cleavage and inactivation of C3b [13].
There is little information about the dynamics of complement components during I/R injury in KT recipients [14–16]. Some studies focused on soluble C5b-9 (SC5b-9) levels during the first minutes after unclamping with contradictory results [14, 15]. Regarding FH, most available data come from animal models and describe the ability to ameliorate partially I/R injury [17]. Moreover, most of these studies are focused on FH alteration during the liquid phase, and there are few data about the deposition of FH on cell surface in this setting. A previous study from our group reported higher intensity stain in kidney biopsies from patients with ischaemic AKI [16]. The C3d, a cleavage product from C3b, is considered a surrogate marker for complement activation, and has been described in kidney biopsies with the diagnosis of acute tubular necrosis [18], but there is less information regarding C3d stain in DGF patients.
Our principal aim was to evaluate the potential association between SC5b-9 levels and DGF in KT recipients. Additionally, we evaluated the histological pattern of three different complement components: C3d, FH and C5b-9.
MATERIALS AND METHODS
Study design and data collection
The study was undertaken in 115 KT recipients with available plasma samples. Their KT had been performed in our hospital between April 2016 and December 2017. We excluded KT from living donors (n = 25), KT from uncontrolled donation after cardio-circulatory death (n = 7), patients with acute rejection (n = 18) and patients with primary non-function (n = 6). Finally, we assessed SC5b-9 levels in 59 patients.
Clinical data were collected from our local transplant data base, which includes: baseline demographic characteristics from donors and recipients, transplant characteristics and clinical follow-up variables periodically registered, complications and patient/graft survival.
All participants gave written informed consent. This study adhered to the Principles of Helsinki Declaration and the Ethics Committee of the Hospital del Mar approved the protocol (CEIC 2010/3777/1).
Definitions
DGF was defined as the requirement for dialysis within the first week after transplantation.
Preemptive KT is defined as transplantation performed before initiation of maintenance dialysis.
Plasma samples and SC5b-9 measurements
Blood samples were prospectively collected at admission for KT day (Day 0) and at Day 7 after KT. They were collected in EDTA tubes to prevent further complement activation and stored in aliquots at −80°C until use. Sandwich ELISAs were used to measure SC5b-9 levels (#HK328, Hycult Biotechnology, Uden, The Netherlands). Briefly, samples and standards were incubated in microtiter wells coated with antibodies recognizing human C5b-9 (Clone 6G3) diluted samples to 1:45 concentration. Streptavidin–peroxidase conjugate was used to bind the biotinylated tracer antibody (dilution 1:12), and the enzyme reaction was stopped by the addition of oxalic acid. The absorbance was measured with a spectrophotometer at a wavelength of 450 nm (Tecan Infinite 200, TECAN Instruments, Männedorf, Zürich, Switzerland). A standard curve by plotting the absorbance versus the corresponding SC5b-9 concentrations was obtained. SC5b-9 were determined from the standard curve.
Immunohistochemistry in kidney tissue samples
C5b-9, F H and C3d deposition were assessed in kidney biopsies from 12 KT patients with DGF. Four patients with 1-year protocol graft biopsies with completely stable creatinine serum values since transplantation without proteinuria, anti-HLA antibodies or histological signs of renal dysfunction or rejection served as controls. The mean time between KT and kidney biopsy was 15 days [interquartile range (IQR) 10.7–29]. Biopsies from controls were performed at 12 months (IQR 12–16) after KT.
Frozen blocks on OCT resin were cut into 6-µm sections using the cryostat. Tissue sections onto Superfrost® glass slides were dried at room temperature. Samples were fixed in 10% buffered formalin and blocked for endogenous peroxidase activity by H2O2 3% in methanol. Goat serum 3% in TBS was used to block nonspecific sites. Primary antibodies against C5b-9 (#HM2167, anti-human terminal complex complement; Hycult Biotech, The Netherlands, Clone 6G3) [monoclonal antibody (mAb) 1:2000], FH (courtesy of Dr S. de Córdoba, CSIC, Clone 214) (mAb 1:1000) and C3d (courtesy of Dr S. de Córdoba, CSIC, Clone 12.3.3) (mAb 1:400) were incubated at 4°C overnight. Antigen–antibody reaction was detected by a goat anti-mouse immunoglobulins/HRP (#P0447, Dako). Sections were visualized with DAB Chromogen System (#K3467, Dako) and counterstained with haematoxylin.
Samples were evaluated by two independent observers (C.E.A.-C. and J.G.) in a blinded manner using a semi-quantitative method, based on the percentage of stained tubules and the percentage of stained perimeter of the average stained tubule. The intensity of staining was graded as 0 (no staining), +1 (stain visible at 40× magnification), +2 (at 20×), +3 (at 10×) and +4 (at 2–4×). We considered ‘high-intensity’ as stain visible at or <10× magnification (+3 and +4), ‘diffuse stain’ as positive stain in >50% of all tubules from slide and ‘perimetral stain’ as 50% positive staining in >50% of tubular perimeter.
Statistical analysis
Quantitative variables with a normal distribution are expressed as mean [standard deviation (SD)] and the remaining as median and IQR. Means were compared with t-test for normally distributed variables and Mann–Whitney–Wilcoxon test or abnormally distributed ones. Comparisons of SC5b-9 levels between Days 0 and 7 within DGF and non-DGF patients were performed with Mann–Whitney–Wilcoxon test due to abnormal distribution. Categorical data were analysed by χ2 test. Risk factors for DGF were analysed by logistical regression analysis with a stepwise selection method. The factors included in the univariate analysis were: donor age, time on dialysis prior to transplant (months), recipient gender, previous transplants, diabetes mellitus (DM), peak of panel reactive antibody, cold ischaemia time (CIT), donor after cardiac death (DCD), donor terminal creatinine, donor weight, number of HLA mismatches and induction immunosuppression therapy, as well as Days 0–7 SC5b-9% increase. These were included in the analysis in basis of literature review [19] and clinical experience.
In the multivariate analysis, only those variables with a P < 0.05 were included.
Receiver operating characteristic (ROC) curves were constructed for assessing the discriminative ability of SC5b-9 levels increase Days 0–7 for estimating DGF, and the area under curve (AUC) was calculated. The value of marker defined as cut-off was determined by the maximum of Youden index (J = sensitivity + specificity − 1).
A P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and the STATA package version 15 (STATA Corp., College Station, TX, USA).
RESULTS
SC5b-9 levels
We analysed SC5b-9 levels at Days 0 and 7 post in 59 patients, 21 of them (35.6%) with DGF and 38 (64.4%) without DGF. Their clinical characteristics are summarized in Table 1. Patients in the DGF group were older, more frequently diabetics and received kidneys from older donors and more frequently controlled cardio-circulatory death type. All patients with DGF performed conventional haemodialysis, none required continuous techniques. Patients with DGF showed renal similar kidney function at 3 and 6 months, but worse renal function 1 year after KT (serum creatinine 1.78 ± 0.61 versus 1.35 ± 0.30 mg/dL in non-DGF patients) (Table 2).
Table 1.
Comparison between DGF and non-DGF patients
| Variables | DGF (n = 21) | Non-DGF (n = 38) | P-value |
|---|---|---|---|
| Recipient age, mean ± SD, years | 63 ± 11 | 56 ± 12 | 0.030 |
| Donor age, mean ± SD, years | 66 ± 14 | 57 ± 36 | 0.029 |
| Recipient sex (male, %) | 16 (76.2) | 20 (52.6) | 0.076 |
| Recipients with hypertension, % | 19 (90.4) | 34 (89.4) | 0.968 |
| Recipients with DM, % | 10 (47.6) | 7 (18.4) | 0.033 |
| Pre-KT SC5b-9 levels, mean ± SD, mAU/mL | 6621 ± 2201 | 5901 ± 3049 | 0.303 |
| Donor sex (male, %) | 14 (66.6) | 22 (61.1) | 0.508 |
| KDPI, mean ± SD | 84.8 ± 21.7 | 74.5 ± 27.4 | 0.153 |
| DCD, % | 13 (61.9) | 11 (28.9) | 0.014 |
| Time on dialysis prior to KT, median (IQR), months | 19 (14–37) | 20 (11–28) | 0.454 |
| CIT, median (IQR), h | 11 (7–17) | 12 (7–17) | 0.951 |
| Creatinine drop, median (IQR), days | 11 (10–15) | 2 (1–3) | <0.001 |
| Previous KT, % | 2 (9.5) | 5 (13.1) | 0.109 |
| Immunosuppression induction, % | |||
| Thymoglobulin | 0 | 1 (2.7) | 0.954 |
| Othersa | 21 (100) | 37 (97.3) | |
| Renal replacement therapy, % | |||
| Haemodialysis | 17 (80.9) | 23 (60.5) | 0.148 |
| Peritoneal dialysis | 4 (19.1) | 12 (31.6) | 0.370 |
| Preemptive KT | 0 | 3 (7.9) | 0.545 |
| 12-month creatinine, mean ± SD | 1.78 ± 0.61 | 1.35 ± 0.30 | 0.001 |
| Follow-up, median (IQR), months | 11 (4.5–12.1) | 12.1 (8.9–12.2) | 0.064 |
KDPI, Kidney Donor Profile Index.
aBasiliximab
Table 2.
Comparative in renal function among DGF patients with ΔC5b-9 >5% compared with DGF and ΔC5b-9 <5%
| Δ0–7 C5b-9 <5% (n = 5) | Δ0–7 C5b-9 >5% (n = 16) | P-value | |
|---|---|---|---|
| 3-month creatinine | 2.13 ± 1.07 | 2.34 ± 0.58 | 0.215 |
| 6-month creatinine | 1.91 ± 0.96 | 2.33 ± 0.58 | 0.081 |
| 12-month creatinine | 2.11 ± 1.85 | 2.37 ± 0.74 | 0.045 |
| 24-month creatinine | 1.50 ± 0.58 | 3.20 ± 1.96 | 0.020 |
No significant differences were found in Day 0 SC5b-9 levels between DGF and non-DGF patients (Table 1). These levels were similar between patients undergoing renal replacement therapy and preemptive KT patients (6273 ± 3048 mAU/L versus 6102 ± 2681 mAU/L; P = 0.835).
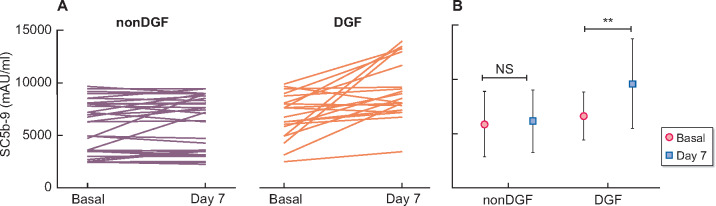
DGF patients presented similar levels at Day 0 and 7 (5902 ± 3049 mAU/L versus 6178 ± 2882 mAU/L; P = 0.686). While those patients with DGF showed a significant increase of SC5b-9 levels between Days 0 and 7 (6621 ± 2202 mAU/L versus 9625 ± 4142 mAU/L; P = 0.006, Figure 1). The post KT SC5b-9 levels were independent of creatinine serum value at Day 7 post-KT in non-DGF patients (data not show).
FIGURE 1:
Individual plasmatic SC5b-9 levels at Days 0 and 7 after KT among DGF and non-DGF patients (A), and plasmatic SC5b-9 levels expressed as mean ± SD (B). Data from 38 patients in the non-DGF group, 21 patients in the DGF group. NS, not significant. **P = 0.006.
Multivariate analysis showed an independent association between SC5b-9 levels increase between basal and Day 7 after KT and DGF (Table 2). Percentage SC5b-9 levels increase (Δ0–7 SC5b-9%) discriminative assessment analysed by ROC curve showed a good discriminative value for DGF with an AUC of 0.78, P < 0.001 (sensitivity 81%, specificity 66%), by cut-off point of 5%. This cut-off point was related with longer DGF duration (83% of patients with DGF longer than 10 days had Δ0–7 SC5b-9 >5%) and worse renal function 1 and 2 years after KT (Table 3).
Table 3.
Multivariate lineal regression analysis to evaluate the association between SC5b-9 level increase and DGF
| OR (95% CI) | P-value | |
|---|---|---|
| ΔC5b-9 % | 1.030 (1.054–1.007) | 0.009 |
| KDPI | 1.014 (0.987–1.041) | 0.312 |
| Donor age (years) | 1.013 (0.965–1.064) | 0.336 |
| DM (recipient) | 1.504 (0.364–6.222) | 0.573 |
| DCD | 4.285 (1.066–17.23) | 0.040 |
Data from two independent models for evaluating the impact of increase C5b-9 increase (ΔC5b-9%) in DGF. One model was adjusted for KDPI score and the other one for donor age. Other variables included in the model were: recipient age, history of DM and DCD type. KDPI, Kidney Donor Profile Index; OR, odds ratio.
Immunohistochemistry in kidney tissue samples
Twelve kidney biopsies from patients with DGF were assessed. The median age of these patients was 61.0 ± 10.5 years, predominantly male (75% versus 16.7%), none of these patients had glomerulopathy related to complement alterations.
The mean (IQR) time between KT and kidney biopsy was 15 days (10.7–29). Biopsies from controls were performed at 12 months (12–16) after KT.
C5b-9-, C3b- and FH-positive staining were observed in tubular epithelial cells basal membrane. There was no staining in cytoplasmic, apical and/or lateral membrane in tubular cells. C5b-9 and C3b were diffusely positive in arterial endothelial cells and in endothelial glomerular cells in all cases, with variable intensity staining. FH was positive in some cases in arterial endothelial cells and in the mesangial areas of glomeruli, although intensity was not evaluated in this study.
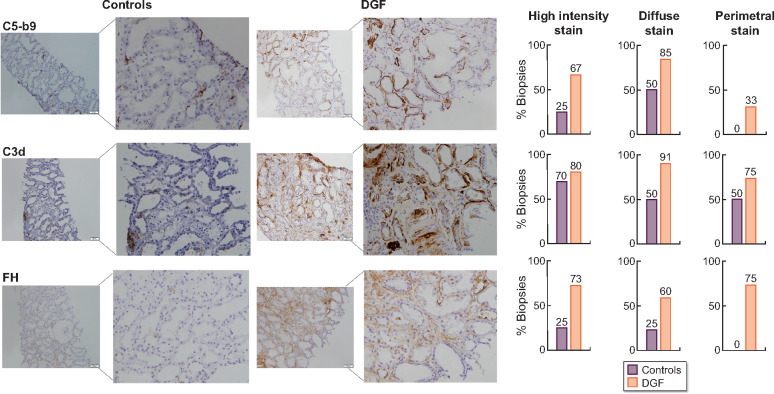
Regarding C5b-9 staining, biopsies from patients experiencing DGF showed more frequently high-intensity stain (67% versus 25%; P = 0.043) and diffuse stain (85% versus 50%; P = 0.052) compared with the control group. Perimetral stain was found only in biopsies from DGF patients (Figure 2).
FIGURE 2:
C5b-9, C3d and FH markers for complement activation in biopsies from KT patients with DGF and 1-year protocol biopsies without tissue damage as the control group. C5b-9, C3d and FH were evaluated in the tubular compartment using immunohistochemistry and semi-quantitative scoring. DGF biopsies showed more frequently high-intensity staining (stain visible at or less than ×10 magnification) for C5b-9 and FH than controls. C3d high intensity-stain was similar between DGF and controls. DGF biopsies showed more frequently diffuse staining (>50% of tubules) than controls. DGF biopsies also showed more frequently perimetral staining (positive staining in >50% of tubular perimeter) for C5b-9, C3d and FH than controls.
C3d stain showed similar intensity in both DGF biopsies and controls (80% versus 70%, P = 0.143); however, diffuse staining was more usually observed in biopsies from DGF patients (91% versus 50% controls; P = 0.049) and also perimetral (75% DGF versus 50% controls; P = 0.054) (Figure 2).
FH stain showed similar results. Biopsies from DGF patients presented more frequently higher intensity staining than controls (73% versus 25%; P = 0.036), more diffuse staining (60% versus 25% controls; P = 0.054) and only biopsies from DGF patients presented perimetral stain (Figure 2).
Among the 12 patients with DGF, three (25%) never recovered renal function, presenting all of them with Δ0-SC5b-9 >5% and intense, diffuse and positive staining in >50% of tubular perimeter for C5b-9, FH and C3b. Five DGF patients had 1-year protocol biopsy, and three of them showed more extensive interstitial fibrosis and tubular atrophy compared with biopsy performed during DGF period: all of them presented high-intensity and perimetral stain for C5b-9 and C3b staining. There were heterogeneous findings regarding FH staining.
DISCUSSION
This study shows that I/R injury activates complement system as reflected both by the increase of SC5b-9 levels and the local deposition of complement system fractions C5b-9, C3b and regulator FH in tubular epithelial cells basal membrane. We excluded patients who suffered from acute rejection since our aim was to evaluate the association between I/R injury and DGF caused mainly by complement system proteins fractions. To our knowledge, this is the first study that evaluates jointly the SC5b-9 levels increase and C5b-9, C3d and FH stain in KT patients.
Some studies have established complement’s critical role in post-transplant I/R injury [8, 20, 21]. Previous studies from our group reported a relationship between higher SC5b-9 levels and histologic C5b-9 deposits with the severity of AKI, including a small group of KT recipients with DGF [16, 22]. However, the independent impact of SC5b-9 with levels by multivariate analysis and pre-KT SC5b-9 levels were lacking in this study. Błogowski et al. [14] showed an increase in C5b-9 since the first minute after reperfusion in those patients that lately developed DGF, but they did not evaluate C5b-9 levels after the first 5 min. de Vries et al. [15] reported a transient increase in C5b-9 levels only the first seconds after reperfusion in brain-deceased and cardiac-death donor KT recipients, but not in living donor KT. The relationship between C5b-9 levels increase and DGF was not evaluated in this study.
Our data show that the complement cascade activation in the form of the increase of lytic C5b-9 fraction levels during the first week after KT was clearly increased in patients with DGF. Additionally, we found that those patients with DGF that experienced higher SC5b-9 increase presented more frequently worse graft function at 1 and 2 years and longer DGF duration. Similar to our results, in the study abovementioned from Błogowski et al. [14], authors also reported worse graft function in patients with higher C5b-9 increase after reperfusion, but the relationship between DGF duration and C5b-9 increase was not evaluated in this study.
To support the finding of complement activation in DGF cases, we also evaluated C5b-9, C3d and FH deposition in kidney biopsies from DGF patients. We found the presence of positive linear C5b-9, C3d and FH staining in tubular epithelial cell basal membrane. C5b-9 and FH stains were clearly more intense, more extensive and with higher perimeter staining in kidney biopsies from DGF patients compared with protocol biopsies from kidneys without signs of renal dysfunction or rejection. C3d stain showed similar intensity in both groups, but similar to C5b-9 and FH staining, biopsies from DGF patients showed more extensive and higher perimeter staining than controls. Previous studies from our group reported comparable findings in AKI biopsies that included a number of KT biopsies with DGF. Rodríguez et al. [16] reported a larger number of tubules with C5b-9 deposition and strong intensity for FH staining in AKI patients. This study stained the samples in fixed paraffin sections, and a different FH stain pattern with a tubular cytoplasmic distribution was noted. In our study, detection technique on frozen sections showed FH linear staining in the basal tubular membrane, with a similar pattern to C5b-9 and C3d staining, probably reflecting a more antibody specificity. As mentioned before, FH plays a fundamental role in complement regulation in soluble phase, helping to the decay and inactivation of C3b by Factor I and avoiding the additional deposition of C3b fragments and subsequent C5 convertase formation on cells surfaces [23]. However, this protective role seems insufficient to stop the complement damage in I/R injury [24]. The C3d is a cleavage product from C3b and its deposit reflects local activated complement. The fact that we found almost the same histological pattern of C3d, C5b-9 and FH in biopsies from the DGF group confirms a local complement activation in these patients. Otherwise, DGF patients with worse outcomes, such as never renal function recovery or more fibrosis in 1-year protocol biopsies, had more intense and diffuse staining. However, the small sample in this subgroup limits the interpretation and extrapolation of these results.
To our knowledge, this is the first study that correlates SC5b-9 levels increase during the first week after KT with DGF duration and more interestingly, medium-term renal function. According to this finding, ΔC5b-9 (0–7) may be considered as a promising marker to DGF severity. In addition, possible complement-dependent DGF cases might benefit from treatment with complement inhibitors.
A reasonable concern about our findings could be the influence of haemodialysis or glomerular filtration rate in SC5b-9 levels. Some studies reported that complement proteins (including C5b-9) might increase due to blood–membrane contact in the dialyser [25, 26]; however, this reaction is not described with the modern membranes used in our patients. Furthermore, we did not find differences between baseline levels in patients on haemodialysis before KT, peritoneal dialysis or preemptive KT patients, so it is unlikely that treatment with haemodialysis could have significantly influenced our results. Regarding influence by effective glomerular filtration rate, we did not find differences in SC5b-9 levels between non-DGF patients with different creatinine serum values at Day 7 after KT.
The limitations of our study are derived from its single-centre and small sample size, with a number of cases that needed to be excluded due to missing blood sample for C5b-9 determination. We believe that the exclusion of the patients with missing samples was not associated with any recipient or donor characteristics and thus should not bias our results. Other limitations were the exclusion of KT from living donors and uncontrolled cardio-circulatory death donors; the reason for excluding this patient was the under representation of these groups in the global sample. In addition, the small sample size even could reduce the statistical power of the multivariable linear regression analysis.
In conclusion, complement activation during the peritransplant period could be related to the severity of graft injury and the presence of DGF. Therefore, the determination of C5b-9 levels could be useful to identify patients with possible complement-dependent graft injury that might benefit from complement inhibitor therapies. Additional studies are needed to improve the diagnostic accuracy from this marker.
ACKNOWLEDGEMENTS
We thank the study coordinators Anna Faura, Sara Alvarez, Montserrat Folgueiras and María Vera for their continuous technical support. This work was carried out by C.E.A.-C. as part of his thesis project in the Department of Medicine, Universitat Autònoma of Barcelona.
FUNDING
C.E.A.-C. has support from a Rio Hortega contract CM17/00067, ISCIII. This study had funds from FIS-FEDER PI16/0617 and Redinren RD16/0009/0013.
AUTHORS’ CONTRIBUTIONS
C.E.A.-C., M.R., E.R. and J.P. designed the study, performed the analysis and validated the data. J.G. contributed to histological analyses. C.E.A.-C. drafted the initial report, while all authors contributed to the final manuscript and approved it.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format. The authors declare no conflicts of interest.
REFERENCES
- 1. Zhao H, Alam A, Soo AP. et al. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine 2018; 28: 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ojo AO, Wolfe RA, Held PJ. et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997; 63: 968–974 [DOI] [PubMed] [Google Scholar]
- 3. Yarlagadda SG, Coca SG, Formica RN. et al. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 2008; 24: 1039–1047 [DOI] [PubMed] [Google Scholar]
- 4. Pascual J, Pérez-Sáez MJ, Mir M. et al. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev 2012; 26: 280–290 [DOI] [PubMed] [Google Scholar]
- 5. Diepenhorst GMP, van Gulik TM, Hack CE.. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg 2009; 249: 889–899 [DOI] [PubMed] [Google Scholar]
- 6. Zipfel PF, Skerka C.. Complement regulators and inhibitory proteins. Nat Rev Immunol 2009; 9: 729–740 [DOI] [PubMed] [Google Scholar]
- 7. Damman J, Schuurs TA, Ploeg RJ. et al. Complement and renal transplantation: from donor to recipient. Transplantation 2008; 85: 923–927 [DOI] [PubMed] [Google Scholar]
- 8. Zhou W, Farrar CA, Abe K. et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 2000; 105: 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Pol P, Roos A, Berger SP. et al. Natural IgM antibodies are involved in the activation of complement by hypoxic human tubular cells. Am J Physiol Renal Physiol 2011; 300: F932–F940 [DOI] [PubMed] [Google Scholar]
- 10. Sarma JV, Ward PA.. The complement system. Cell Tissue Res 2011; 343: 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo RF, Ward PA.. Role of C5a in inflammatory responses. Annu Rev Immunol 2005; 23: 821–852 [DOI] [PubMed] [Google Scholar]
- 12. Zhou W. The new face of anaphylatoxins in immune regulation. Immunobiology 2012; 217: 225–234 [DOI] [PubMed] [Google Scholar]
- 13. Harrison RA, Lachmann PJ.. The physiological breakdown of the third component of human complement. Mol Immunol 1980; 17: 9–20 [DOI] [PubMed] [Google Scholar]
- 14. Błogowski W, Dołęgowska B, Sałata D. et al. Clinical analysis of perioperative complement activity during ischemia/reperfusion injury following renal transplantation. Clin J Am Soc Nephrol 2012; 7: 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Vries DK, van der Pol P, van Anken GE. et al. Acute but transient release of terminal complement complex after reperfusion in clinical kidney transplantation. Transplant J 2013; 95: 816–820 [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez E, Gimeno J, Arias-Cabrales C. et al. Membrane attack complex and factor H in humans with acute kidney injury. Kidney Blood Press Res 2018; 43: 1655–1665 [DOI] [PubMed] [Google Scholar]
- 17. Renner B, Ferreira VP, Cortes C. et al. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int 2011; 80: 165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thurman JM, Scott Lucia M, Ljubanovic D. et al. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int 2005; 67: 524–530 [DOI] [PubMed] [Google Scholar]
- 19. Irish WD, Ilsley JN, Schnitzler MA. et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant 2010; 10: 2279–2286 [DOI] [PubMed] [Google Scholar]
- 20. Chun NH, Horwitz JK, Heeger PS.. Role of complement activation in allograft inflammation. Curr Transpl Rep 2019; 6: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang M, Takahashi K, Alicot EM. et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol 2006; 177: 4727–4734 [DOI] [PubMed] [Google Scholar]
- 22. Rodríguez E, Riera M, Barrios C. et al. Value of plasmatic membrane attack complex as a marker of severity in acute kidney injury. BioMed Res Int 2014; 2014: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferreira VP, Pangburn MK, Cortés C.. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol 2010; 47: 2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goetz L, Laskowski J, Renner B. et al. Complement factor H protects mice from ischemic acute kidney injury but is not critical for controlling complement activation by glomerular IgM. Eur J Immunol 2018; 48: 791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheung AK, Parker CJ, Wilcox L. et al. Activation of the alternative pathway of complement by cellulosic hemodialysis membranes. Kidney Int 1989; 36: 257–265 [DOI] [PubMed] [Google Scholar]
- 26. Hauser AC, Derfler K, Stockenhuber F. et al. Generation of the membrane attack complex during haemodialysis: impact of classical and alternative pathway components. Clin Sci 1990; 79: 471–476 [DOI] [PubMed] [Google Scholar]