Abstract
Aim
Neoadjuvant therapy and total mesorectal excision (TME) for rectal cancer are associated with bowel dysfunction symptoms known as low anterior resection syndrome (LARS). Our study compared the only two validated instruments—the LARS Questionnaire (LARS-Q) and the Memorial Sloan Kettering Bowel Function Instrument (MSK-BFI)—in rectal cancer patients undergoing sphincter-preserving TME.
Methods
190 patients undergoing sphincter-preserving TME for stage I–III rectal cancer completed the MSK-BFI and LARS-Q simultaneously at a median time of 12 (range 1–43) months after restoration of bowel continuity. Associations between the MSK-BFI total/subscale scores and the LARS-Q score were investigated using Spearman rank correlation (rs). Discriminant validity for the two questionnaires was assessed, and the questionnaires were compared with the European Quality of Life Instrument.
Results
Major LARS was identified in 62% of patients. The median MSK-BFI scores for no LARS, minor LARS, and major LARS were 76.5, 70, and 57, respectively. We found a strong association between MSK-BFI and LARS-Q (rs −0.79). The urgency/soilage subscale (rs −0.7) and the frequency subscale (rs −0.68) of MSK-BFI strongly correlated with LARS-Q. Low correlation was observed between the MSK-BFI diet subscale and LARS-Q (rs −0.39). On multivariate analysis, both questionnaires showed worse bowel function in patients with distal tumours. A low-to-moderate correlation with the European Quality of Life Instrument was observed for both questionnaires.
Conclusions
MSK-BFI and LARS-Q showed good correlation and similar discriminant validity. As the LARS-Q is easier to complete, it may be considered the preferred tool to screen for bowel dysfunction.
Introduction
Bowel dysfunction after sphincter-preserving total mesorectal excision (TME) encompasses a wide array of symptoms including faecal incontinence, increased bowel frequency or urgency, and difficulty with bowel evacuation. This complex of symptoms, referred to as low anterior resection syndrome (LARS) [1], may lead to a significant decline in quality of life (QoL) [2, 3].
Despite numerous publications measuring functional outcomes after TME in patients with rectal cancer, patient-reported outcomes for bowel function have been inconsistently measured using a wide variety of non-specific, non-validated tools [4]. In the past, commonly used bowel function questionnaires focused mainly on faecal incontinence [5], which does not encompass the full symptom spectrum of LARS. Two validated tools, the Memorial Sloan Kettering Bowel Function Instrument (MSK-BFI) [6] and the LARS Questionnaire (LARS-Q) [7], have been introduced as specific patient-reported outcome measures for bowel function after TME. Although both questionnaires were developed with the same purpose, they differ significantly in their clinical applicability and scope. While the LARS-Q is a quick and clinically easy-to-use tool, MSK-BFI is a more comprehensive instrument and may provide more in-depth evaluation of LARS. Controversy exists as to which of these 2 validated tools is better at assessing LARS. A recently published international expert consensus definition of LARS [4] raised the possibility of underestimation of bowel dysfunction as well as its impact on QoL using current validated instruments [8].
Our study aim was to evaluate the correlation between MSK-BFI and LARS-Q, their discriminant validity based on clinical variables, and their correlation with QoL after sphincter-preserving TME.
Methods
Patients
The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (MSK), and a waiver of informed consent was obtained. We retrospectively identified patients who underwent sphincter-preserving TME at MSK with reestablishment of gastrointestinal continuity between November 1, 2011, and August 31, 2017, for primary stage I, II, and III rectal adenocarcinoma located within 15 cm from the anal verge. Patients were excluded if they had a previous history of faecal incontinence or inflammatory bowel disease, had undergone extended resections, or had incomplete information for any question of either questionnaire. Standard demographic, clinical, surgical, and pathological data were collected.
Neoadjuvant treatment was based on patient-specific recommendations of the multidisciplinary disease management team and corresponded to 4 alternatives: (i) no neoadjuvant treatment (surgery alone); (ii) chemoradiation (CRT) alone, consisting of 50 or 50.4 Gy in 25 or 28 fractions, respectively, with concurrent infusional fluorouracil or oral capecitabine twice daily for 5 to 6 weeks; (iii) neoadjuvant chemotherapy alone, as either 8 cycles of mFOLFOX6 (leucovorin, fluorouracil, and oxaliplatin), 5 cycles of CAPOX (capecitabine and oxaliplatin) [9-11], or FLOX (weekly fluorouracil-leucovorin 7 and biweekly oxaliplatin) [12]; or (iv) a total neoadjuvant therapy, with neoadjuvant chemotherapy before or after CRT with a 2- to 3-week interval between therapies, as described in previous studies [13].
TME was performed using a standard open or minimally invasive approach (depending on the surgeon’s preference), usually 8 to 12 weeks after completion of neoadjuvant treatment in the CRT and total neoadjuvant therapy groups or 2–4 weeks after completion of neoadjuvant treatment in the neoadjuvant chemotherapy group. A colorectal or coloanal anastomosis was performed using either a hand-sewn or double-stapled technique depending on tumour location. A temporary diverting loop ileostomy was created depending on tumour location, patient characteristics, and surgeon’s judgment.
Postoperative bowel function measurement
Postoperative bowel function was assessed as part of routine clinical care using the MSK-BFI and LARS-Q follow-up questionnaires. Patients included in this study completed both questionnaires at the same time point and at a median time of 12 (range: 1–43) months after restoration of bowel continuity, using a previously validated electronic platform [14]. Three of the 190 questionnaires included in the present study were completed at 1 month; all other questionnaires were completed at least 3 months after restoration of bowel continuity. All questionnaires available were included in order to evaluate the relationship of both questionnaires at different time points and in different settings. Similarities and differences between the 2 instruments are summarized in Table 1.
Table 1.
Similarities and differences between MSK-BFI and LARS score
| Attribute | MSK-BFI | LARS-Q |
|---|---|---|
| Target population | Rectal Cancer Patients with Sphincter Preserving Surgery | |
| Purpose | Bowel Function Assessment | |
| Mode of administration | Self-Completed | Self-Completed |
| Number of questions | 18 | 5 |
| Dimensions of bowel function | Adopts a 4-week recall period | No specific recall period |
| Frequency | 6 items (Q1, Q5, Q8, Q9, Q10, Q11) | 1 item (Q3) |
| Urgency/soilage | 4 items (Q15, Q16, Q17, Q18) | 2 items (Q2, Q5) |
| Diet | 4 items (Q2, Q3, Q13, Q14) | No items |
| Other | ||
| Incomplete evacuation | 1 item (Q4) | No items |
| Continence of gas | 1 item (Q12) | 1 item (Q1) |
| Discrimination of gas vs stool | 1 item (Q7) | No items |
| Empty bowels within last 15 m | 1 item (Q6) | 1 item (Q4) |
| Summary scores(s) | Total score: sum of global score + independent items Global score: sum of the 3 sub-scales 3 sub-scales |
Total score |
| Item response scales | Integers from 1 to 5. Five categories for each question (never, rarely, sometimes, most of the time, always). |
Integers from 0 to 16. Different scoring for each question. Between 3 to 4 categories for item. |
| Scoring | Unweighted sum for each item, by sub-scale, global scale, and total scale. Range: 17–90; higher score = better bowel function. Corresponds to a continuous score, so far no clinical categories have been used. |
Weighted scoring. Final score is the sum for each individual item. Range: 0–42; lower score = better bowel function. Results discriminate 3 categories: 0–20 = No LARS, 21–29 = Minor LARS, 30–42 = Major LARS |
Abbreviations: MSK-BFI, Memorial Sloan Kettering Bowel Function Instrument; LARS, low anterior resection syndrome.
MSK-BFI scoring
The MSK-BFI 18-item questionnaire was specifically designed to assess bowel function after sphincter-preserving surgery for rectal cancer [6] and is the most comprehensive of the questionnaires currently in use [5]. A version of MSK-BFI and scoring instructions are in Appendix 1. MSK-BFI was meticulously formulated according to literature review, expert and patient input, factor analysis, and clinical relevance [6].
The main strength of the MSK-BFI is its detailed and thorough evaluation of LARS. Its diet, urgency, and frequency subscales potentially allow for a more personalized interpretation of the different dimensions of LARS. MSK-BFI uses a 4-week time period recall and an equal-weighting scoring system, with higher scores meaning better bowel function.
LARS-Q scoring
LARS-Q is a 5-item validated questionnaire assessing bowel function after sphincter-preserving surgery with or without radiotherapy for rectal cancer [7]. The items and scoring algorithm of LARS-Q are in Appendix 2.
The LARS-Q was initially developed and validated in a large cohort of Danish patients, who underwent curative low anterior resection, with or without radiotherapy for non-metastatic rectal cancer [7]. It has gained popularity in clinical practice due to its ease of use. Severity categories (score 0–20 = no LARS, score 21–29 = minor LARS, and score 30–42 = major LARS) facilitate early detection of patients who need further evaluation and attention (major LARS), since they report significantly worse QoL compared with those with no/minor LARS [2, 15]. The LARS-Q does not use a specific recall period or equal-weighting scoring, with higher scores meaning worse bowel function.
Quality of life
The European Quality of Life Instrument (EQ-5D-5L)[16] was used to evaluate overall QoL. It is divided into 2 sections: the EQ-5D-5L index and a visual analog scale (EQ-5D VAS). The EQ-5D-5L assesses health across 5 domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D VAS is a vertical measurement instrument with a range of 0 to 100, where 0 is the worst and 100 is the best imaginable health. The EQ-5D-5L has been widely used worldwide for more than 25 years in research and clinical settings, with the 5L version having an increased sensitivity due the improvement of different levels of evaluation and simpler task instructions of VAS scale.
Statistical analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Frequencies and percentages were calculated for categorical variables, and medians and ranges were calculated for continuous variables. A Spearman rank correlation between MSK-BFI total score (and its subscales) and LARS-Q was performed. A correlation was defined as strong if the absolute values of rs ≥ 0.7, moderate if rs was between 0.4 and 0.6, and low if rs was <0.4 [17].
To evaluate the discriminant validity of both MSK-BFI and LARS-Q, differences were explored using clinically relevant variables. LARS-Q was examined as a continuous variable and as a three-level categorical variable indicating severity (defined above), using analysis of variance to compare groups. Univariable and multivariable regression models with demographic and clinical variables as the explanatory variables and MSK-BFI or LARS-Q as the dependent variables were fit to the data. The Wald Chi-Square test was used to evaluate the significance of each variable. The multivariable model included known clinically relevant variables as well as variables found to be significantly associated with MSK-BFI and LARS-Q scores in the univariate analysis. If two variables were highly correlated, only one was include in the model. Finally, MSK-BFI and LARS-Q were correlated with each of the 5 EQ-5D items and with the total visual analog score. P values < 0.05 were considered significant.
This manuscript was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [18].
Results
Patient characteristics
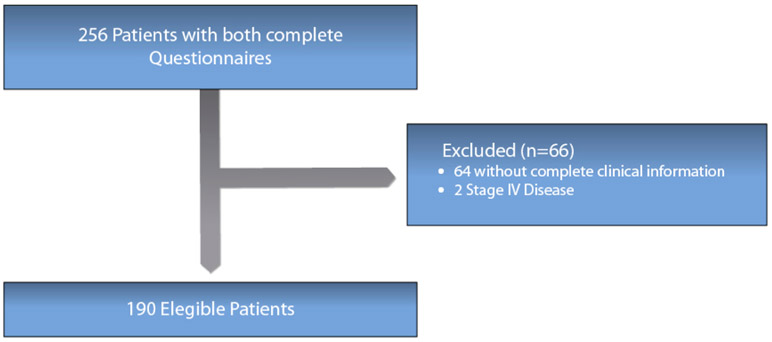
A total of 190 patients who completed both the BFI and the LARS-Q after stoma reversal were included in the analysis (Figure 1). Patient characteristics are described in Table 2. The median age was 53 years (range: 26–83 years). Median tumour distance from the anal verge was 9 cm (range: 1 –15 cm). Nearly 77% of patients received neoadjuvant therapy, with 17% of the total number of patients achieving a pathological complete response. A diverting loop ileostomy was performed in 76% of cases, with a median time of 12 (range 0.2–42) months between restoration of bowel continuity and questionnaire application. No patient required a new stoma during follow-up.
Figure 1.
Patients Flow Chart
Table 2.
Patient characteristics
| Characteristics | Cohort of patients undergoing LAR, n = 190 |
|---|---|
| Age, median (range) | 53 (26–83) |
| Female, n (%) | 91 (48) |
| BMI, median kg/m2 (range) | 27.1 (17.4–44.2) |
| Tumour distance from anal verge, median cm (range) | 9 (1–15) |
| Clinical AJCC stage, n (%) | |
| I | 32 (17) |
| II | 33 (17) |
| III | 125 (66) |
| Neoadjuvant therapy, n (%) | |
| No | 44 (23) |
| Chemoradiation alone | 22 (12) |
| Induction chemotherapy alone | 21 (11) |
| Total neoadjuvant therapy | 103 (54) |
| Surgical approach, n (%) | |
| Robotic | 139 (73) |
| Open | 36 (19) |
| Laparoscopic | 15 (8) |
| Hand-sewn anastomosis, n (%) | 26 (14) |
| Diverting loop ileostomy, n (%) | 144 (76) |
| Pathological AJCC stage, n (%) | |
| 0/Tis | 38 (20) |
| I | 73 (38) |
| II | 32 (17) |
| III | 47 (25) |
| Postoperative complications, n (%) | 43 (23) |
| Anastomotic leak | 11 (6) |
| Months between surgery and survey, median (range) | 12.0 (0.23-42) |
Abbreviations: LAR, low anterior resection; AJCC, American Joint Committee on Cancer.
Bowel function evaluation
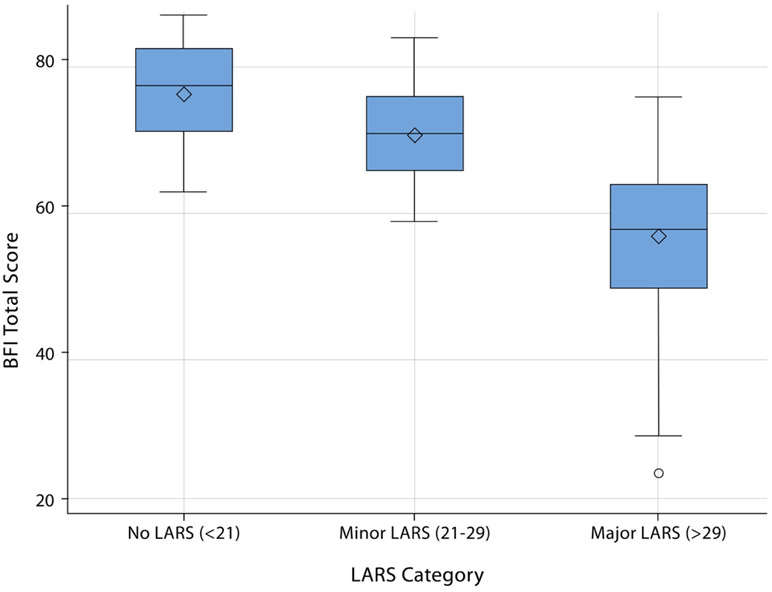
No, minor, and major LARS were present in 12.6%, 25.3%, and 62.1% of patients, respectively. The median MSK-BFI total score of the cohort was 62 (range 24–86). A statistically significant difference in median MSK-BFI total score was observed for each of the LARS score categories, with a median MSK-BFI total score of 76 for no LARS, 70 for minor LARS, and 56 for major LARS (Figure 2; analysis of variance F-test p < 0.001).
Figure 2.
Box-and-whiskers plot of the MSK-BFI total score when patients were divided into 3 groups, defined as no LARS (12.6%,), minor LARS (23.3%), and major LARS (62%).
Correlation between MSK-BFI and LARS-Q
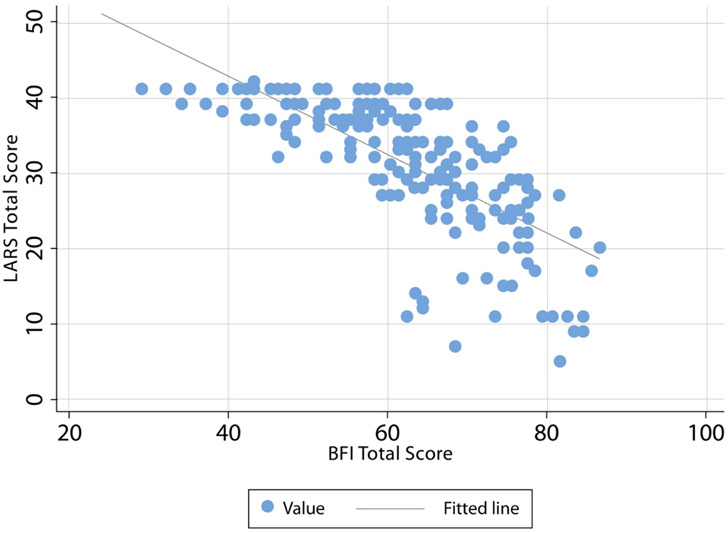
A strong negative correlation was observed between the MSK-BFI and LARS-Q (rs −0.79, Figure 3). When analyzing MSK-BFI subscales, the urgency/soilage subscale (rs 0.7) was strongly correlated with LARS-Q. The frequency subscale showed a moderate-strong correlation with LARS-Q (rs −0.68). Conversely, a low correlation was observed between the MSK-BFI diet subscale and LARS-Q (rs −0.39).
Figure 3.
MSK-BFI and LARS-Q score scatter plot. A strong negative correlation was observed between the two questionnaires (rs −0.79).
Discriminant validity of MSK-BFI and LARS-Q based on clinical variables
On univariate analysis, MSK-BFI total score was statistically different for proximal vs distal tumours (p < 0.001), handsewn vs stapled anastomosis (p < 0.001), presence vs absence of a diverting loop ileostomy (p = 0.003), presence of any postoperative complication (p = 0.042) and pathological stage II vs III tumours (p = 0.013) (Table 3). When analyzing MSK-BFI subscales, a similar discriminant validity of MSK-BFI total score was observed for the urgency/soilage and frequency subscales. No statistically significant differences were detected by the diet subscale. On univariate analysis, LARS-Q was statistically different for proximal vs distal tumours (p = 0.007) and handsewn vs stapled anastomosis (p = 0.049). The two questionnaires showed no difference in age, gender, clinical American Joint Committee on Cancer (AJCC) stage or type of surgical approach (open, laparoscopic, or robotic).
Table 3.
Univariate regression analysis of clinical variables vs MSK-BFI and LARS-Q scores in the study cohort
| Characteristic | MSK-BFI | LARS Total | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | p value | Estimate | 95% CI | p value | |
| Age | −0.079 | −0.23 to 0.07 | 0.313 | −0.027 | −0.084 to 0.137 | 0.635 |
| Male vs female | 2.077 | −1.28 to 5.43 | 0.225 | −0.049 | −2.481 to 2.383 | 0.968 |
| Tumour distance from the anal verge | 1.110 | 0.56 to 1.66 | <0.0001 | −0.557 | −0.961 to −0.152 | 0.007 |
| Clinical AJCC stage | ||||||
| I vs III | 2.894 | −1,68 to 7.47 | 0.215 | −0.337 | −3.654 to 2.979 | 0.842 |
| II vs III | −0.664 | −5.18 to 3.85 | 0.773 | 0.490 | −2.787 to 3.766 | 0.770 |
| Chemoradiation (yes vs no) | −3.097 | −6.44 to 0.25 | 0.070 | 1.915 | −0.508 to 4.339 | 0.121 |
| Surgical approach | ||||||
| Robotic vs open | −1.012 | −5.35 to 3.32 | 0.647 | 0.298 | −2.820 to 3.416 | 0.851 |
| Laparoscopic vs open | −0.533 | −7.66 to 6.59 | 0.883 | 2.614 | −7.873 to 2.373 | 0.293 |
| Handsewn anastomosis (yes vs no) | −9-090 | −13.81 to −4.37 | <0.001 | 3.512 | 0.012 to 7.012 | 0.049 |
| Diverting loop ileostomy (yes vs no) | −5.803 | 9.64 to −1.96 | 0.003 | 2.406 | −0.410 to 5.222 | 0.094 |
| Pathological AJCC stage | ||||||
| 0/Tis vs III | −0.050 | −4.99 to 4.89 | 0.984 | −1.381 | −4.999 to 2.237 | 0.454 |
| I vs III | 4.026 | −0.21 to 8.26 | 0.062 | −1.929 | −5.030 to 1.173 | 0.223 |
| II vs III | 6.610 | 1.42 to 11.8 | 0.013 | −3.692 | −7.493 to 0.109 | 0.057 |
| Postoperative complications | −4.119 | −8,10 to −0,14 | 0.042 | 0.613 | −2.290 to 3.515 | 0.679 |
| Anastomotic leak | 0.412 | −6.79 to 7.62 | 0.911 | −3.370 | −8.551 to 1.81 | 0.202 |
Abbreviations: AJCC, American Joint Committee on Cancer.
A multivariate analysis was performed to evaluate the effects of tumour distance from the anal verge, neoadjuvant chemoradiation, diverting loop ileostomy and pathological AJCC stage on total MSK-BFI and LARS-Q (Table 4). Distance of the tumour from the anal verge and pathological stage II vs III were the only variables that showed statistically significant differences for the two instruments.
Table 4.
Multivariate regression analysis of clinical variables vs MSK-BFI and LARS-Q scores in the study cohort
| Characteristic | MSK-BFI | LARS Total | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | p value | Estimate | 95% CI | p value | |
| Tumour distance from the anal verge | 0.939 | 0.305 to 1.574 | 0.004 | −0.544 | −1.019 to 0.069 | 0.025 |
| Chemoradiation (yes vs no) | −1.390 | −4.705 to 1.926 | 0.411 | 1.343 | −1.14 to 3.826 | 0.289 |
| Diverting loop ileostomy (yes vs no) | −2.431 | −6.769 to 1.907 | 0.272 | 0.544 | −2.705 to 3.792 | 0.743 |
| Pathological AJCC stage | ||||||
| 0/Tis vs III | 3.095 | −1.817 to 8.007 | 0.217 | −3.108 | −6.786 to 0.571 | 0.098 |
| I vs III | 4.992 | 2.918 to 0.932 | 0.016 | −2.437 | −5.477 to 0.603 | 0.116 |
| II vs III | 7.897 | 2.918 to 12.876 | 0.002 | −4.4 | −8.129 to −0.672 | 0.021 |
Abbreviations: AJCC, American Joint Committee on Cancer.
Correlation of MSK-BFI and LARS-Q with EQ-5D
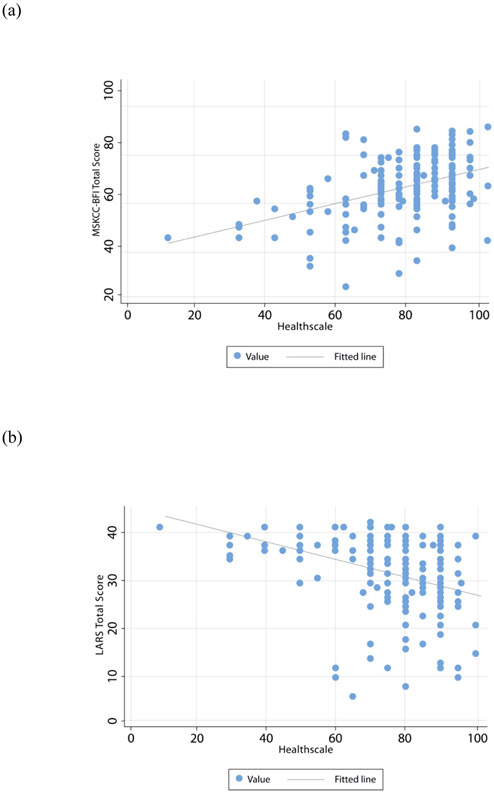
Overall, MSK-BFI total score and LARS-Q each had a low-to-moderate correlation with EQ-5D items, with higher correlation for questions related to alterations to usual activities and pain/discomfort. Both total scores show low-to-moderate strength correlations with the EQ-5D VAS (MSK-BFI rs 0.43; LARS-Q rs 0.39) (Figure 4).
Figure 4.
MSK-BFI (a) and LARS score (b) correlations with EQ-5D VAS (Healthscale). A low-to-moderate correlation was observed for both scores (rs 0.43 and −0.39, respectively).
Discussion
This study demonstrates a strong association between MSK-BFI and LARS-Q, with similar discriminant validity based on clinical variables (proximal vs distal tumors) and handsewn vs stapled anastomosis on univariate analysis. On multivariate analysis, we found that distal tumours and pathological AJCC stage III vs II were significantly associated with worse bowel function for both MSK-BFI and LARS-Q. Both the urgency/soilage subscale and the frequency subscale of MSK-BFI showed similar discriminant validity, while the diet subscale was not associated with any clinical variables. Finally, a low-to-moderate correlation with the EQ-5D VAS was observed for both scores.
The strong association between both questionnaires was not unexpected. Both instruments share a similar methodology and validation process, including correlation with relevant clinical variables and comparison with the same QoL instruments (EORTC QLQ-C30 [European Organization for Research and Treatment of Cancer quality of life questionnaire C30]) for their validation [6, 7]. On univariate analysis, MSK-BFI showed stronger correlation with previously established LARS-related variables such as type of anastomosis, presence of diverting loop ileostomy, postoperative complications, and pathological stage. This may suggest that MSK-BFI is better suited for a more comprehensive and in-depth evaluation of LARS. Alternatively, we may hypothesize that this distinction is related to the fact that our patient cohort was similar to the one used to validate MSK-BFI rather than the one used to validate LARS-Q. On multivariate analysis, both MSK-BFI and LARS-Q were only related to tumour distance from the anal verge and AJCC pathological stage III vs II. The absence of correlation with known variables such as neoadjuvant therapy may be explained by collinearity with pathological stage.
When comparing the 3 individual MSK-BFI subscales with LARS-Q, the frequency and urgency/soilage subscales were found to have the strongest correlation. This is expected, because LARS is considered to be mainly driven by urgency as one of the cardinal symptoms. As expected, the diet subscale was not correlated with LARS-Q, which does not include any question related to diet modifications. However, an important finding of our study is that the MSK-BFI diet subscale is not strongly correlated with relevant clinical variables and QoL questionnaires used so far in rectal cancer. Our findings raise questions about its clinical usefulness and its inclusion in the future development of questionnaires to measure LARS.
Our results corroborate the study by Liapi et al [19] that compared LARS-Q and MSK-BFI scores in a cohort of 112 patients. This study showed similar correlation between the two scores as observed in our study, as well as with the EORTC QoL instrument. Although we used a different QoL questionnaire (EQ-5D-5L), correlation between the two questionnaires was present. These findings may demonstrate the ability of both questionnaires to show changes in QoL regardless of the instrument used.
Our study was able to show statistically significant variations in the median MSK-BFI total score when evaluating each one of the LARS score categories. Although this finding may suggest potential cut-off points, the scope of the MSK-BFI score was never expected to define categories. In addition, there is considerable overlap between the MSK-BFI score and the LARS score categories, raising doubt about its clinical usefulness. In this context, it is still necessary to define a minimal clinically important difference for the MSK-BFI score to implement its use in clinical practice.
Patient-reported outcomes in rectal cancer have garnered growing interest, especially in the context of high rates of sphincter-preserving rectal resections and, more recently, secondary to organ preservation in complete clinical response after neoadjuvant treatment [20-22]. However, the heterogeneity of symptoms, as well as the variability in the definition of LARS, has made it difficult to correctly quantify the real burden of LARS [4, 23]. Which tool is best to measure LARS is still undetermined. Although both MSK-BFI and LARS-Q are validated and both attempt to evaluate bowel function in LARS patients, each has unique characteristics and limitations. Weighted scoring systems, such as LARS-Q, give certain variables more influence in the overall measure over other variables. In the LARS patients, for example, one could posit that urgency has more influence than other variables and should be reflected with a higher weight when considering an overall measure of a rectal cancer patient’s quality of life. This is in contrast to MSK-BFI, where no variables or submeasures are given a higher weight than others. In general, unweighted measures such as MSK-BFI have better psychometric properties over a wide range of study participants and better adapt to changes over time than weighted scoring methods [24]. In an attempt to standardize the definition of LARS, a recent international expert consensus has been published [3]. This LARS definition focuses on 8 symptoms and 8 consequences that capture essential aspects of the syndrome. This novel separation of symptoms and consequences may enhance sensitivity for detecting changes in LARS over time and with intervention. This new definition will raise new challenges, as our current data reveal that both scores may be unable to fully characterize LARS and do not truly assess relevant issues such as personal, return-to-work, and social sequelae that these patients experienced. Our study may shed light upon the strengths and limitations of each questionnaire and may prove useful for eventual development of novel tools to capture LARS.
The main strengths of our study are the large sample of patients and the simultaneous use of both questionnaires at the same time interval, with a median time of about a year since restoration of bowel continuity. The comparison of MSK-BFI and LARS-Q helped us determine potential cut-off points for MSK-BFI, as well as what designates a minimal clinically important difference in the MSK-BFI score, both not previously described. These potential cut-offs need to be tested and validated before their clinical application and utility in the development of more precise tools to assess LARS. Our data provide insight into the variables necessary to consider for the development of future LARS patient-reported outcome measures for detection, assessment of variation over time, and ability to capture effects of interventions on LARS symptomatology.
Limitations of our study include the use of tumour distance from the anal verge, rather than distance of anastomosis from the anal verge, with the latter described as a more precise predictor of LARS [25]. This choice was necessary to standardize the results, as some patients had missing data regarding exact height of anastomosis at time of surgery, though it is safe to assume that for each proctectomy a safe oncological margin was achieved. Our cohort consisted of younger patients (median age 53 years), consistent with the increased incidence of young-onset colorectal cancer; this may limit the applicability of our findings to older patients. Another limitation of our study was that questionnaires were not administered at the same time point after restoration of gastrointestinal continuity, including three questionnaires that were filled out 1 month after stoma reversal. Exclusion of these three patients did not alter the findings of our analysis; a decision was made to include them in our analysis in an effort to understand how the two questionnaires correlate at different time points. It is important to note that a similar degree of correlation between the two scores was reported by Liapi et al using an older cohort of patients [19]. In any study of bowel function after low anterior resection, the uncertainty of baseline bowel dysfunction in patients with rectal cancer may be an additional limitation. Normative LARS score data have shown prevalence of major LARS in approximately 19% of previously healthy women [26]. However, bowel function instruments are not used in comparison to a baseline examination, as baselfvine symptoms may be unreliable in rectal cancer patients due to the presence of the rectal tumour. Additionally, the lack of comparison between these 2 patient-reported outcome measures at multiple timepoints may be a limitation in assessing how MSK-BFI and LARS-Q correlate over time. However, so far there has been no report showing that the MSK-BFI score improves over time. An analysis of LARS-Q at different time points has not shown significant statistical variation over time [27, 28].
In conclusion, both MSK-BFI and LARS-Q showed good correlation and similar discriminant validity for bowel dysfunction after sphincter-preserving TME, with MSK-BFI being more suitable as a comprehensive, detailed instrument in comparison with a rapid-screening tool like the LARS-Q. The two scores appear to be equivalent in assessing bowel dysfunction and QoL. As the LARS-Q is easier to complete, it may be considered the preferred tool to screen for bowel dysfunction at present. Future tools may be able to better separate symptoms of bowel dysfunction and their individual impact on personal, social, professional and mental well-being, as well as the ability of individuals to adapt to change over time.
Supplementary Material
What does this paper add to the literature?
Controversy exists as to which is the best validated questionnaire to assess low anterior resection syndrome (LARS). This study demonstrated good correlation and similar discriminant validity between the only two validated questionnaires. The LARS Questionnaire may be the simplest and fastest tool for assessing LARS.
Acknowledgments
Funding: NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure: Dr. Garcia-Aguilar has received honoraria from Medtronic and Johnson & Johnson and holds equity in Intuitive Surgical, Inc.
References
- 1.Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol 2012; 13: e403–8. [DOI] [PubMed] [Google Scholar]
- 2.Emmertsen KJ, Laurberg S, Rectal Cancer Function Study G. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg 2013; 100: 1377–87. [DOI] [PubMed] [Google Scholar]
- 3.Keane C, Fearnhead NS, Bordeianou LG, Christensen P, Basany EE, Laurberg S, et al. International consensus definition of low anterior resection syndrome. Dis Colon Rectum 2020; 63: 274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane C, Wells C, O'Grady G, Bissett IP. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis 2017; 19: 713–22. [DOI] [PubMed] [Google Scholar]
- 5.Chen TY, Emmertsen KJ, Laurberg S. What are the best questionnaires to capture anorectal function after surgery in rectal cancer? Curr Colorectal Cancer Rep 2015; 11: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temple LK, Bacik J, Savatta SG, Gottesman L, Paty PB, Weiser MR, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum 2005; 48: 1353–65. [DOI] [PubMed] [Google Scholar]
- 7.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012; 255: 922–8. [DOI] [PubMed] [Google Scholar]
- 8.Ribas Y, Aguilar F, Jovell-Fernández E, Cayetano L, Navarro-Luna A, Muñoz-Duyos A. Clinical application of the LARS score: results from a pilot study. Int J Colorectal Dis 2017; 32: 409–18. [DOI] [PubMed] [Google Scholar]
- 9.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011; 29: 1465–71. [DOI] [PubMed] [Google Scholar]
- 10.Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015; 33: 3733–40. [DOI] [PubMed] [Google Scholar]
- 11.Schmoll HJ, Twelves C, Sun W, O'Connell MJ, Cartwright T, McKenna E, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 2014; 15: 1481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yothers G, O'Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29: 3768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018; 4: e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett AV, Keenoy K, Shouery M, Basch E, Temple LK. Evaluation of mode equivalence of the MSKCC Bowel Function Instrument, LASA Quality of Life, and Subjective Significance Questionnaire items administered by web, interactive voice response system (IVRS), and paper. Qual Life Res 2016; 25: 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juul T, Ahlberg M, Biondo S, Espin E, Jimenez LM, Matzel KE, et al. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum 2014; 57: 585–91. [DOI] [PubMed] [Google Scholar]
- 16.Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013; 22: 1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg 2018; 126: 1763–8. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liapi A, Mavrantonis C, Lazaridis P, Kourkouni E, Zevlas A, Zografos G, et al. Validation and comparative assessment of low anterior resection syndrome questionnaires in Greek rectal cancer patients. Ann Gastroenterol 2019; 32: 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, et al. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard resection - a matched-controlled study. Dis Colon Rectum 2017; 60: 1032–40. [DOI] [PubMed] [Google Scholar]
- 21.Quezada-Diaz F, Jimenez-Rodriguez RM, Pappou EP, Joshua Smith J, Patil S, Wei I, et al. Effect of neoadjuvant systemic chemotherapy with or without chemoradiation on bowel function in rectal cancer patients treated with total mesorectal excision. J Gastrointest Surg 2019; 23: 800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quezada-Diaz FF, Smith JJ, Jimenez-Rodriguez RM, Wasserman I, Pappou EP, Patil S, et al. Patient-reported bowel function in patients with rectal cancer managed by a watch-and-wait strategy after neoadjuvant therapy: a case-control study. Dis Colon Rectum 2020; 63: 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman SJ, Bolton WS, Corrigan N, Young N, Jayne DG. A cross-sectional review of reporting variation in postoperative bowel dysfunction after rectal cancer surgery. Dis Colon Rectum 2017; 60: 240–7. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MA. Using multi-item psychometric scales for research and practice in human resource management. Human Resource Management 2018; 57: 739–50. [Google Scholar]
- 25.Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, et al. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut 2018; 67: 688–96. [DOI] [PubMed] [Google Scholar]
- 26.Juul T, Elfeki H, Christensen P, Laurberg S, Emmertsen KJ, Bager P. Normative data for the low anterior resection syndrome score (LARS score). Ann Surg 2019; 269: 1124–8. [DOI] [PubMed] [Google Scholar]
- 27.Pieniowski EHA, Palmer GJ, Juul T, Lagergren P, Johar A, Emmertsen KJ, et al. Low anterior resection syndrome and quality of life after sphincter-sparing rectal cancer surgery: a long-term longitudinal follow-up. Dis Colon Rectum 2019; 62: 14–20. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg S, Asplund D, Bisgaard T, Bock D, Gonzalez E, Karlsson L, et al. Low anterior resection syndrome in a Scandinavian population of patients with rectal cancer: a longitudinal follow-up within the QoLiRECT study. Colorectal Dis Published online April 28, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.