Abstract
Splanchnic venous thrombosis is a rare type of venous thromboembolism, and its actual incidence is still unknown. Splenic infarction develops due to splenic vein (SV) thrombosis. Patients with COVID-19 may be exposed to a risk of thrombotic events, and the system affected at the highest level by coagulopathy is the respiratory system. The case presented here is splenic infarction that developed because of SV thrombosis, which is a rare form of venous thromboembolism.
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; IL-6, interleukin 6; INR, international normalized ratio; PV, portal vein; SARS-CoV-2, severe acute respiratory syndrome-related coronavirus; SMV, superior mesenteric vein; SV, splenic vein; SVT, splanchnic venous thrombosis; VS, vascular system
Keywords: 2019-nCoV disease, Splenic Infarction, Splanchnic venous thrombosis
Introduction
Coronavirus disease 2019 (COVID-19) was first reported in the city of Wuhan in China in December 2019. Here the newly identified human coronavirus was temporarily named as 2019-nCoV and then, with a common consensus, renamed as severe acute respiratory syndrome-related coronavirus “SARS-CoV-2” [1]. Chen et al. reported that most patients with COVID-19 had chronic diseases such as hypertension, diabetes mellitus, and cardiovascular diseases [2]. The SARS-CoV-2-ind-uced infection can be associated with coagulopathy, a finding consistent with infection-induced inflammatory changes as observed in patients with disseminated intravascular coagulopathy [3].
Case presentation
On May 22, 2020, a 46-year-old woman was admitted to the emergency department with abdominal pain that lasted for 3 days. She had diabetes mellitus type 2 and had been using metformin 1000 mg once a day for 10 years. She never smoked, had three births, and had no hereditary risk factors for thrombosis. The patient was diagnosed with COVID-19 with a SARS-CoV-2 real-time polymerase chain reaction test and thoracic computed tomography (CT) 25 days ago. In the first CT, ground-glass densities in a consolidated form with common peripheral localization were in line with moderate COVID-19, and the patient had no pathological findings in the abdominal sections (Fig. 1 ). She was hospitalized and received therapy of hydroxychloroquine sulfate, piperacillin-tazobactam, clarithromycin, and low-molecular-weight heparin (LMWH) for 5 days. At her discharge, LMWH treatment was continued with a dose of 6000 IU daily.
Fig. 1.
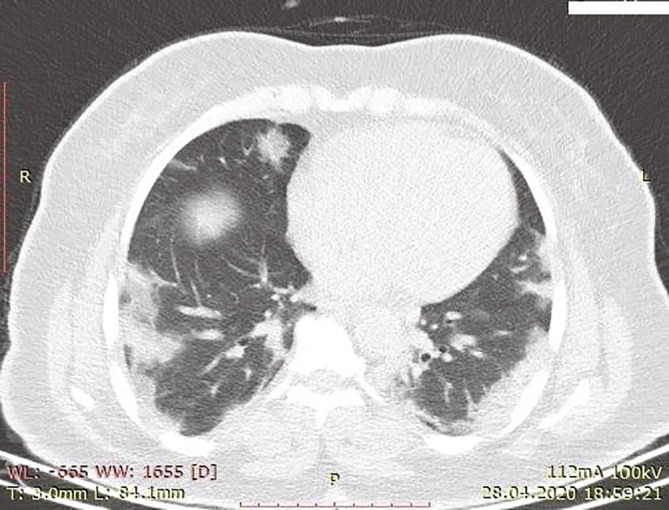
Consolidated ground-glass densities with common peripheral localization in both lungs.
Upon hospital admission, she also complained of fever. The temperature was 37 °C, respiratory count was 12/min, pulse rate was 88/min, and oxygen saturation was 97% at room air. Physical examination revealed general abdominal tenderness, which was more pronounced in the left hypochondrium. There were no pathological findings in the spleen in the upper abdominal sections (Fig. 2 ).
Fig. 2.
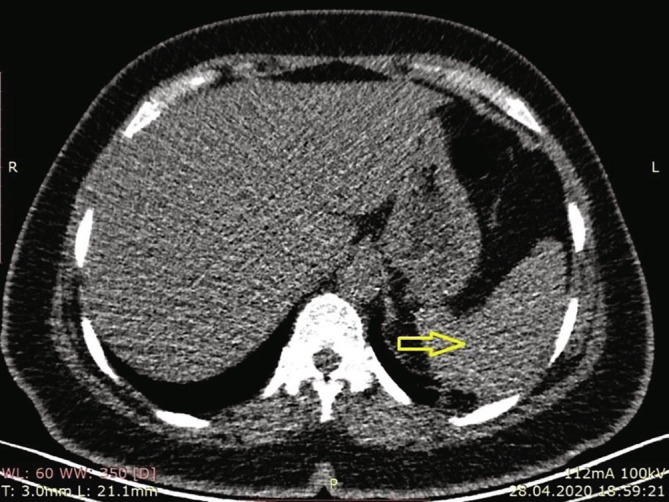
Significant pathological finding was not detected in the spleen in the sections above the upper abdomen in thoracic CT for COVID-19.
On laboratory examinations, following results were obtained: white blood cell, 26.490/mm3, neutrophil count, 76.30%; hemoglobin count, 10.1 g/dL; platelet count, 515.000; C-reactive protein level, 100.95 mg/L; D-dimer level, 11.73 mg/L; fibrinogen level, 714 mg/dL; prothrombin time, 11.9 s; activated partial thromboplastin time, 22.9 s; international normalized ratio (INR), 1.05; lactate dehydrogenase level, 610 U/L; aspartate aminotransferase level, 19 U/L; alanine aminotransferase level, 17 U/L; gamma-glutamyl transferase level, 150 U/L; alkaline phosphatase level, 117 U/L; interleukin-6 (IL-6) level, 37.91 pg/mL.
According to the results of the abdominal CT in our hospital, there were increases in the spleen sizes, and the spleen parenchyma densities decreased in cystic nature in Fig. 3 (Hounsfield unit: 8). The patient was admitted to the ward, and examination and treatment were initiated. In the CT angiography of the patient, vascular structures were natural in the arterial phase, and in the venous phase, filling defects were consistent with thrombus nearly completely filling the lumen in the portal vein (PV), superior mesenteric vein (SMV), and splenic vein (SV) (Fig. 4a, Fig. 4b, Fig. 4c ).
Fig. 3.
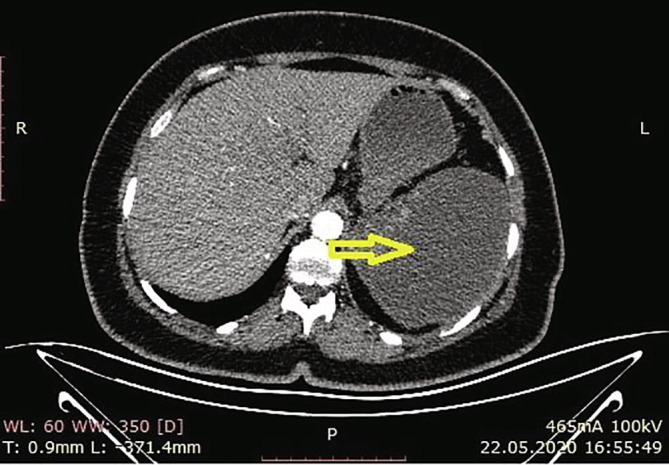
Loss in density in cystic nature in the spleen.
Fig. 4a.
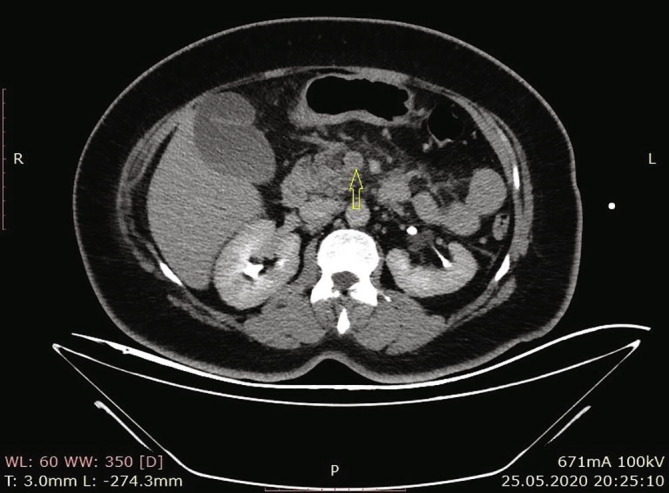
Filling effects in SMV consistent with thrombus that nearly completely fill the lumen.
Fig. 4b.
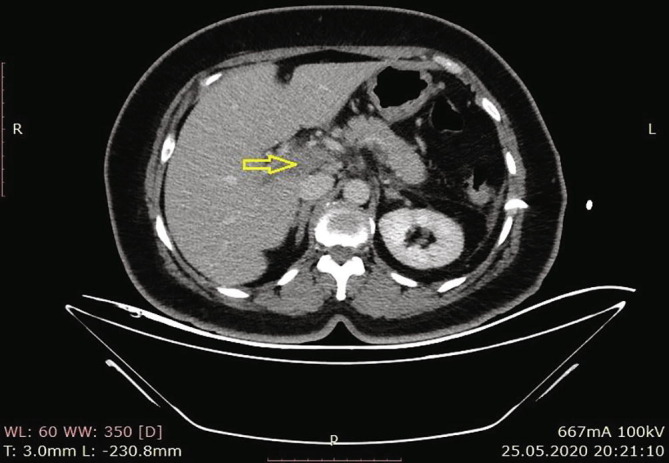
Filling effects in PV consistent with thrombus that nearly completely fill the lumen.
Fig. 4c.
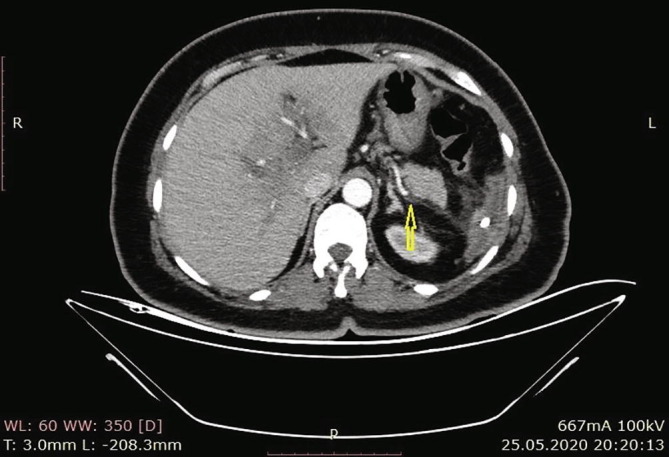
Filling effects in SV consistent with thrombus that nearly completely fill the lumen.
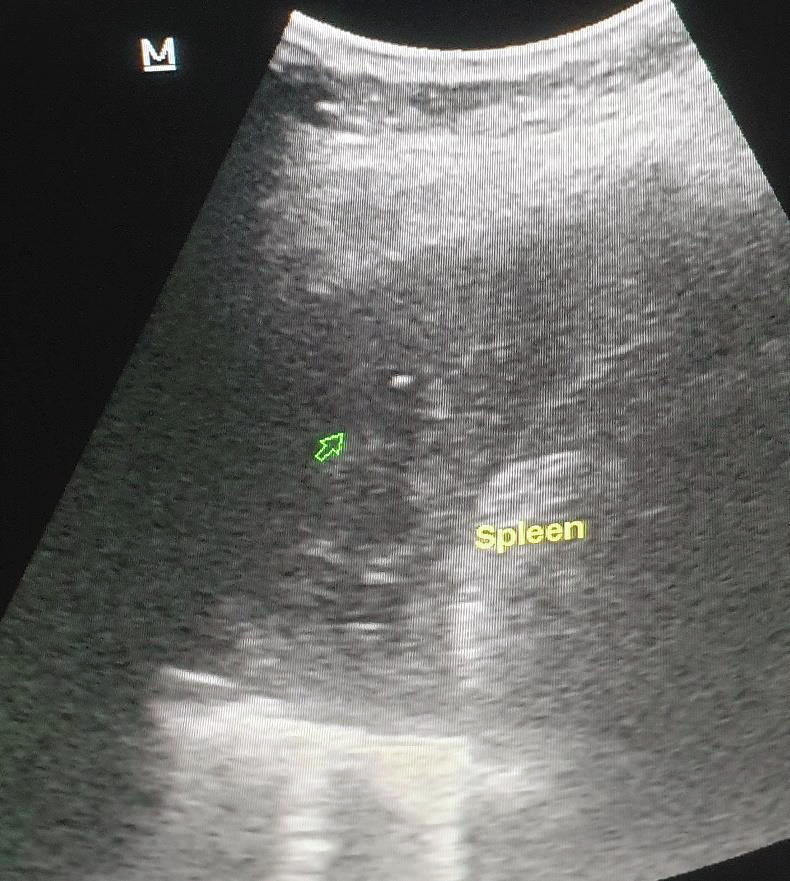
In Doppler ultrasonography, echogenicity that was consistent with thrombus was detected in PV, SMV, and SV, and no current was observed in the lumen (Fig. 5a, Fig. 5b ). Elevated infectious parameters have led to splenic abscesses secondary to splenic venous thrombosis. As the INR was normal, percutaneous drainage was planned by the interventional radiologist in operating room conditions. Percutaneous drainage was performed for severe fluid collection in the spleen (Fig. 6 ), and 1200 mL of infected hemorrhagic fluid was drained by the interventional radiologist. Then, antibiotic therapy (piperacillin + tazobactam) was started, and LMWH at 6000 IU 2 × 1 treatment dose was administered. The patient’s complaints regressed clinically and radiologically on the eighth day, and the patient was discharged with healing (Fig. 7 ).
Fig. 5a.
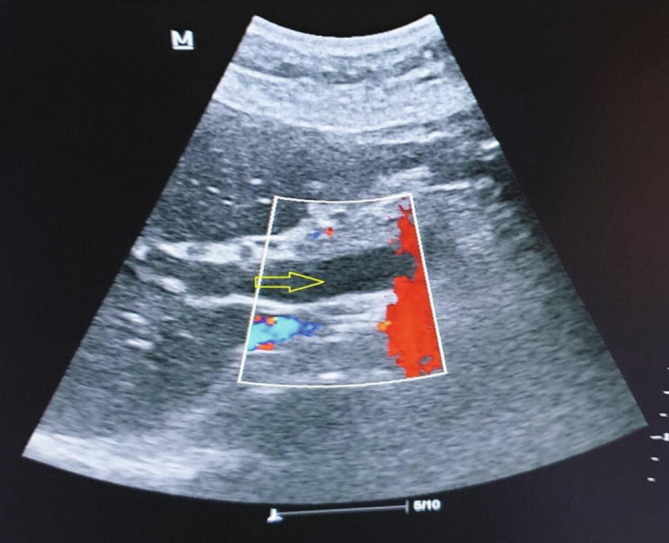
Hypoechogenicity consistent with thrombus with no current in the lumen in USI nearly completely filling the lumen in the portal vein.
Fig. 5b.
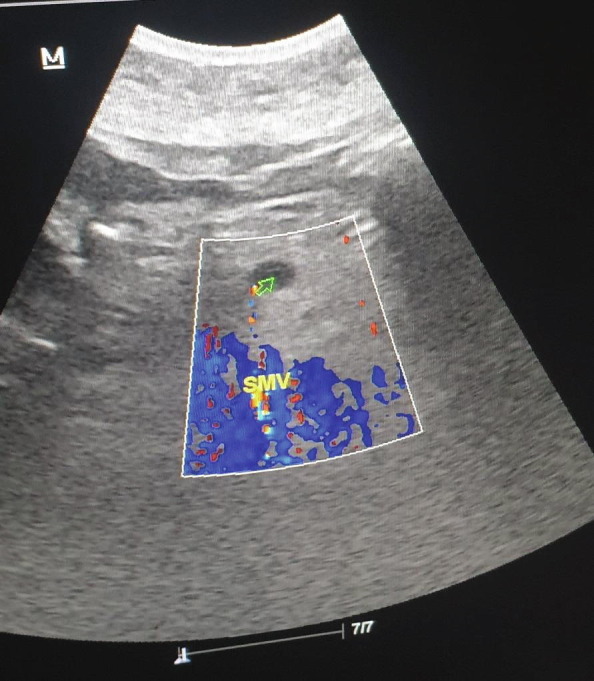
Hypoechogenicity consistent with thrombus with no current in the lumen in Doppler USI in the SMV nearly completely filling the lumen in the portal vein.
Fig. 6.
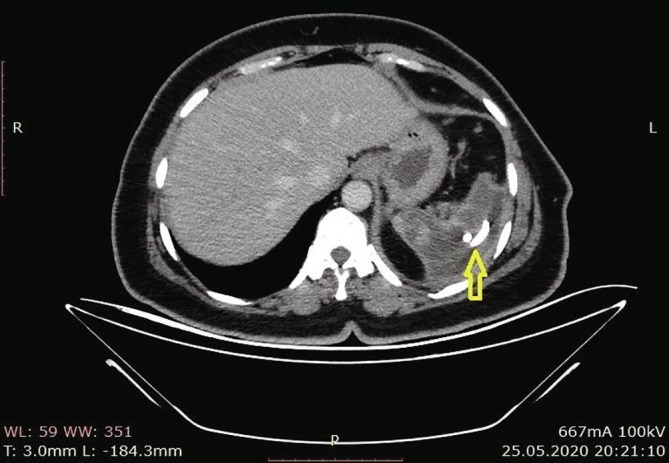
Image of drainage catheter in the spleen.
Fig. 7.
No collection was detected in the spleen after the drainage, and the echogenicity of the spleen is heterogeneous.
Discussion
Many studies had proved that patients with COVID-19 had a risk of thrombotic events. In these patients, hypercoagulability is associated with high D-dimer, fibrinogen, and IL-6 levels, which are also known as poor prognostic factors [4], [5]. In patients with COVID-19, the system affected at the highest level by coagulopathy is the respiratory system, and several studies reported that thrombus occurs in the pulmonary veins [6], [7]. The case presented here is a splenic infarction that developed because of SV thrombosis, which is a rarer form of venous thromboembolism.
Splanchnic venous thrombosis (SVT) refers to thrombosis occurring in the venous circulation that drains the digestive system from the lower end of the esophagus to the upper two-thirds of the rectum [8]. SVT is a rare type of venous thromboembolism, and although its actual incidence is still unknown, it is considered that it develops at a lower rate of at least 25-fold on an annual basis compared to lower extremity deep vein thrombosis and pulmonary embolism [9].
In CT angiography and Doppler ultrasonography of the patient, the presence of portal, superior mesenteric, and SV thrombus was shown. Splenic infarction develops due to SV thrombosis. In this patient, it was considered that two mechanisms might have played roles in the formation of the infarction. The first mechanism is that the infarction developed more likely secondary to the thrombus, which caused obliteration of the SV. The second mechanism is that the spleen could be attacked directly by the SARS-CoV-2 virus, even if there is insufficient evidence. Autopsies conducted on a limited number of patients who died because of COVID-19 showed that the spleen could be attacked directly by the virus, and degeneration and necrosis might develop in cells that can result in changes such as decreases in the T and B lymphocyte count and atrophy in lymphoid follicles [10].
It is considered that the treatment process was accelerated by the aspiration of a total of 1200 mL hemorrhagic fluid from the patient with percutaneous drainage. No complications developed during the intervention. It is already known that the venous circulation is provided by short gastric and left gastroepiploic veins when the SV is fully obliterated. Moreover, the highly recommended anticoagulant treatment for symptomatic patients with SVT in the current guidelines contributed to the treatment of the patient without splenectomy [11].
Conclusion
As a result, it is important to examine patients who have COVID-19 or are suspected to have it due to nonspecific complaints such as abdominal pain and nausea, considering that thromboembolic events may develop in abdominal vessels.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Coronaviridae Study Group oti The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;1 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020 doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han H, Yang L, Liu R, Liu F, Wu K-l, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med (CCLM). 2020;1(ahead-of-print). [DOI] [PubMed]
- 7.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., Qanadli S.D. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers K., Hannah P. CRC Press; 2017. Manual of venous and lymphatic diseases. [Google Scholar]
- 9.Wendelboe A.M., Raskob G.E. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 10.Xu X., Chang X., Pan H., Su H., Huang B., Yang M., et al. Pathological changes of the spleen in ten patients with new coronavirus infection by minimally invasive autopsies. Zhonghua Bing li xue za zhi = Chin J Pathol. 2020;49 doi: 10.3760/cma.j.cn112151-20200401-00278. E014-E. [DOI] [PubMed] [Google Scholar]
- 11.Kearon C., Akl E.A., Comerota A.J., Prandoni P., Bounameaux H., Goldhaber S.Z., et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2):e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]


