Abstract
Background.
Older patients are often considered high-risk surgical candidates for locally advanced esophageal cancer, and the benefit of surgery in this population is unclear. This national analysis examines the effect of age on esophagectomy outcomes and compares surgery versus chemoradiation in older patients.
Methods.
The National Cancer Database was used to identify patients with clinical stage II to III esophageal adenocarcinoma undergoing surgery or definitive chemoradiation between 2004 and 2015. Restricted cubic splines were used to examine the relationship between age and survival after esophagectomy, and maximally selected rank statistics were used to identify an age at which survival worsened. We used Cox proportional hazard models including an interaction term between age and treatment to compare overall survival, as well as survival of patients receiving esophagectomy versus definitive chemoradiation.
Results.
Of 17,495 patients, 11,680 underwent esophagectomy and 5815 received chemoradiation. Survival after esophagectomy worsened with increasing age and decreased considerably after age 73 (hazard ratio = 1.05, 95% confidence interval, 1.04-1.06, per increasing year after 73 versus hazard ratio = 1.01, 95% confidence interval, 1.00-1.01, per increasing year to 73; both P < .001). Chemoradiation was increasingly used over surgery as age increased. The interaction between age and treatment was significant, and a graph of this interaction demonstrated a survival benefit for surgery over chemoradiation at most ages, including octogenarians.
Conclusions.
Survival worsens with age after esophagectomy for locally advanced esophageal cancer. However, esophagectomy is associated with improved survival compared with definitive chemoradiation at most ages, including octogenarians. Esophagectomy may be considered over chemoradiation for patients who can tolerate surgery regardless of age.
Esophageal cancer is relatively rare, but it has one of the highest mortality rates of any cancer; more than 16,000 cancer-related deaths were predicted to occur in the United States in 2020.1 The mainstay of treatment for locally advanced esophageal cancer remains preoperative chemoradiation followed by esophagectomy, an operation that carries notable morbidity and mortality, with preoperative chemoradiation.2,3 As the world population is aging, and as the risk of esophageal cancer increases with age, more older patients will likely be diagnosed with esophageal cancer and considered for esophagectomy.4,5 Older patients, and particularly octogenarians, undergoing esophagectomy for cancer have been demonstrated to have increased perioperative morbidity and mortality.6-9 However, the role that age alone has, as opposed to the effect of concomitant comorbidities, is unclear.10-13 These patients may receive less aggressive treatment owing to advanced age, which may affect their overall survival.7,14,15 As the proportion of older patients increases, it is important to evaluate critically how best to care for these patients. We performed an analysis of the National Cancer Database (NCDB) to determine the impact of age on esophagectomy outcomes for patients with locally advanced esophageal cancer. We hypothesized that esophagectomy would be associated with a survival benefit compared with chemoradiation alone in older patients.
Patients and Methods
Data Source
We used the NCDB in this retrospective hospital-based analysis. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society.16 The data used in the study are derived from a deidentified NCDB file, representing clinical oncology data of more than 80% of newly diagnosed cancer cases nationwide from more than 1500 Commission on Cancer–accredited facilities.16 The data are collected by certified, independent tumor registrars.
Study Design
This retrospective analysis was deemed exempt by the institutional review board. All patients in the NCDB between 2004 and 2015 who were given a diagnosis of American Joint Commission on Cancer, Eighth Edition clinical stage II to III esophageal adenocarcinoma (cT2 3N0 2M0) were identified using International Classification of Diseases for Oncology, Third Edition histology codes. The cohort was limited to patients who underwent esophagectomy with or without neoadjuvant therapy or definitive concurrent chemoradiation (Figure 1). For chemoradiation cohorts, patients were only included if they received concurrent chemotherapy and radiation and a radiation dose of 40 Gy or more, in accordance with National Comprehensive Cancer Network recommendations. Patients were excluded if they received only adjuvant therapies, were missing survival data, or received chemoradiotherapy after being deemed unfit for surgery. The primary outcome was overall survival (OS).
Figure 1.
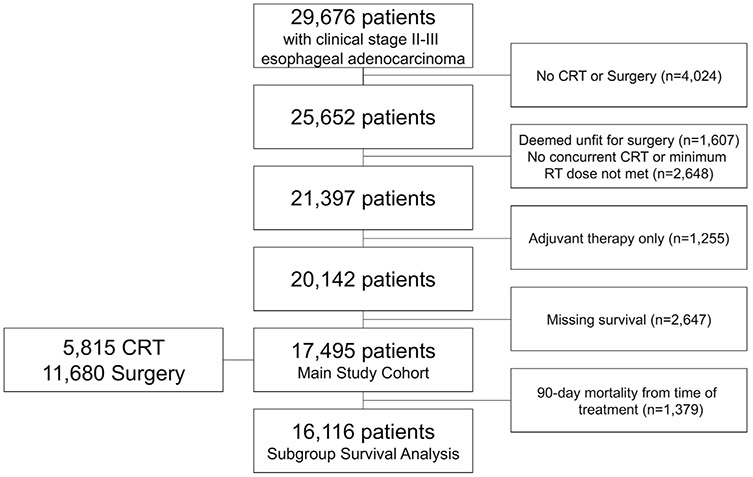
Patient selection scheme for study. (CRT, chemoradiation therapy; RT, radiation therapy.)
Statistical Analysis
COMPARISON OF ESOPHAGECTOMY OUTCOMES IN YOUNGER VERSUS OLDER PATIENTS.
In the first part of the study, we sought to identify the age at which survival after esophagectomy worsens for patients with locally advanced cancer and to compare outcomes of older and younger patients. A restricted cubic spline transformation using 5 prespecified knots of age compared with unadjusted OS demonstrated increasing hazard of mortality with age and a possible inflection point beyond about age 70 years (Figure 2).17,18 To identify this possible inflection exactly, we used maximally selected rank statistics. This approach identified a cut point in a continuous variable (age in this study), dividing a cohort into 2 groups based on an outcome measure of choice (OS in this study). A 2-sample linear rank statistic was computed for every value of age, and the value at which this standardized statistic is greatest was identified as the cut point age (Supplemental Figure 1). We compared baseline variables for patients, stratified by age based on this cut point, using Wilcoxon rank sum and Pearson’s chi-square tests for continuous and categorical measures, respectively. A multivariable Cox proportional hazards model was constructed to estimate survival using variables determined prospectively to be prognostically important based on prior studies and availability in the registry; these included gender, race, year of diagnosis, Charlson Deyo Comorbidity score, insurance status, facility location (metropolitan versus urban), facility type (academic versus nonacademic), distance to the treatment center, facility surgical volume, clinical stage (II versus III), tumor location (distal versus proximal thoracic/middle), and receipt of neoadjuvant chemotherapy or radiation. An intention-to-treat design was employed, so only variables known at the time of surgery were included in adjustment. Age was modeled as a continuous variable using piecewise linear functions and a knot at the proposed cut point of 73. We also performed a multivariable logistic regression using the same variables to examine factors associated with 90-day postoperative mortality.
Figure 2.
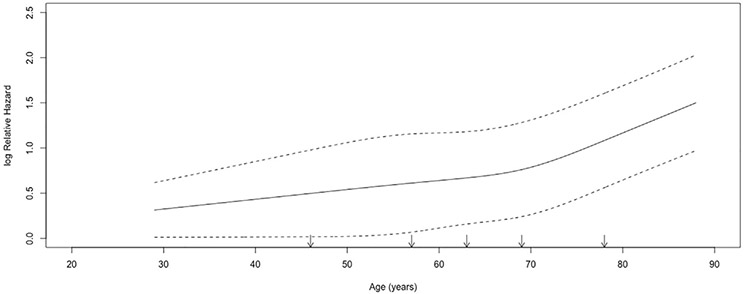
Restricted cubic spline transformation of unadjusted logarithmic hazard of mortality versus age in patients undergoing esophagectomy. Five prespecified knots were used in cubic spline transformation, denoted by arrows. Dotted lines represent bounds of 95% confidence interval.
COMPARISON OF ESOPHAGECTOMY VERSUS CHEMORADIATION.
The aim of the second part of the study was to understand whether definitive chemoradiation was associated with comparable survival to surgery for patients with locally advanced esophageal cancer, and especially how this relationship changes with age. Patients in the overall cohort were stratified by receipt of chemoradiation or surgery and baseline characteristics were compared using Wilcoxon rank sum and Pearson’s chi-square tests for continuous and categorical measures, respectively. In a Kaplan-Meier estimation of survival, the survival curves of both groups crossed around 3 months and the proportional hazards assumption was violated (Schoenfeld P < .001) (Supplemental Figure 2B). To resolve this, and because the aim of the study was to examine longer-term survival, a subgroup analysis was performed for multivariable analysis including only patients who survived at least 90 days from initiation of treatment. A multivariable Cox proportional hazards model was again constructed using identical variables as before, but including an interaction term between age and type of treatment. This interaction was significant, which suggested that age and type of treatment had a meaningful relationship with each other and survival. The interactions among age, treatment, and survival were graphed to better understand the relationships among these variables. Because the survival curves of the treatment groups appeared to start converging beyond about age 80, a subgroup survival analysis was also performed in octogenarians. Missing data were handled with complete case analysis given the high degree of completeness of the NCDB. All statistical analyses were performed with R software (version 3.5 for Mac, R Foundation for Statistical Computing, Vienna, Austria). Two-sided P values of .05 or less were considered statistically significant.
Results
Comparison of Esophagectomy Outcomes in Younger Versus Older Patients
A total of 11,680 patients underwent esophagectomy and met study criteria (Figure 1). A restricted cubic spline transformation showed that unadjusted survival worsened with increasing age, with a possible inflection point above about 70 years (Figure 2). Maximally selected rank statistics identified 73 years as the cutoff age above which survival worsened considerably for patients in this cohort (Supplemental Figure 1). Patients were then stratified by age based on this putative cutoff. Compared with younger patients, older patients were more likely to be female, have comorbidities, have government insurance, be treated at nonacademic centers, have stage II (rather than III) disease, and undergo surgery without neoadjuvant therapies (Table 1). Older patients were more likely to experience positive margins, 30-day postoperative mortality, and 90-day postoperative mortality as well. Unadjusted 5-year OS for younger and older patients was 38% (95% confidence interval [CI], 37-39) and 24% (95% CI, 22-27), respectively (Supplemental Figure 2A) from the time of treatment. In a multivariable Cox regression, older patients who underwent surgery had worse survival compared with younger patients (hazard ratio [HR] = 1.05, 95% CI, 1.04-1.06 per increasing year after age 73 versus HR = 1.01, 95% CI, 1.00-1.01 per increasing year to age 73; both P < .001) (Table 2). In a multivariable logistic regression, older patients were also more likely to experience 90-day postoperative mortality compared with younger patients (Supplemental Table 1).
Table 1.
Perioperative Characteristics of Younger (Age ≤73 Years) Versus Older (Age >73 Years) Patients Undergoing Esophagectomy Stratified Based on Age Cutoff Identified Through Maximally Selected Rank Statisticsa
| Characteristic | Younger (n = 10,122) |
Older (n = 1558) |
P |
|---|---|---|---|
| Age y | 62 (55-67) | 77 (75-79) | N/A |
| Sex (female) | 1107 (11) | 221 (14) | <.001 |
| Race | .07 | ||
| White | 9785 (98) | 1512 (98) | |
| Black | 144 (1) | 12 (1) | |
| Other | 109 (1) | 21 (1) | |
| Year of diagnosis | 2010 (2008-2013) | 2010 (2008-2013) | .28 |
| Charlson-Deyo score | <.001 | ||
| 0 | 7476 (74) | 1066 (68) | |
| 1 | 2125 (21) | 388 (25) | |
| ≥2 | 521 (5) | 104 (7) | |
| Insurance status | <.001 | ||
| Private | 5583 (56) | 144 (9) | |
| Government | 4123 (42) | 1384 (90) | |
| None | 203 (2) | 8 (1) | |
| Facility location | .61 | ||
| Metropolitan | 7721 (79) | 1188 (80) | |
| Urban | 1795 (18) | 260 (18) | |
| Rural | 232 (2) | 39 (2) | |
| Distance to treatment center, miles | 19 (8-49) | 17 (7-48) | .04 |
| Facility type | .006 | ||
| Academic/research | 5409 (54) | 788 (51) | |
| Annualized center surgical volume, cases/y | 7 (2-17) | 6 (2-14) | .11 |
| Clinical stage | <.001 | ||
| II | 5091 (50) | 926 (59) | |
| III | 5031 (50) | 632 (41) | |
| Distal primary tumor | 8743 (86) | 1339 (86) | .67 |
| Tumor size, mm | 40 (25-55) | 40 (25-53) | .39 |
| Minimally invasive approach | 1559 (15) | 265 (17) | .11 |
| Neoadjuvant therapies | |||
| Neoadjuvant chemotherapy | 8915 (89) | 1063 (69) | <.001 |
| Neoadjuvant radiation | 8587 (85) | 998 (64) | <.001 |
| Length of stay, d | 9 (7-14) | 10 (8-17) | <.001 |
| Unplanned 30-d readmission | 531 (5) | 98 (6) | .10 |
| Positive margins | 576 (6) | 125 (8) | <.001 |
| 30-d mortality | 265 (3) | 113 (7) | <.001 |
| 90-d mortality | 643 (6) | 228 (15) | <.001 |
All values are n (%) or median (interquartile range) unless otherwise stated.
N/A, not applicable.
Table 2.
Cox Multivariable Regression of Factors Independently Associated With Survival in Patients Who Underwent Esophagectomya
| Predictor | Hazard Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Female sex (reference: male) | 0.85 | 0.79-0.93 | <.001 |
| Race (reference: white) | |||
| Black | 1.14 | 0.93-1.41 | .21 |
| Other | 0.95 | 0.73-1.24 | .71 |
| Year of diagnosis (per year) | 0.98 | 0.97-0.99 | <.001 |
| Charlson-Deyo score (reference: 0) | |||
| 1 | 1.11 | 1.04-1.18 | <.001 |
| ≥2 | 1.29 | 1.16-1.44 | <.001 |
| Insurance status (reference: private) | |||
| Government | 1.19 | 1.12-1.27 | <.001 |
| None | 1.14 | 0.94-1.38 | .20 |
| Facility location (reference: metropolitan) | |||
| Urban | 1.10 | 1.05-1.16 | <.001 |
| Distance to treatment center (per 10 miles) | 1.00 | 1.00-1.00 | .50 |
| Facility type (reference: nonacademic) | |||
| Academic/research | 0.97 | 0.91-1.03 | .26 |
| Annualized center surgical volume (per 10 cases/y) | 0.95 | 0.93-0.97 | <.001 |
| Clinical stage (reference: II) | |||
| III | 1.41 | 1.34-1.48 | <.001 |
| Distal tumor (reference: proximal thoracic/middle) | 0.91 | 0.85-0.97 | .007 |
| Neoadjuvant chemotherapy (reference: none) | 0.86 | 0.77-0.96 | .009 |
| Neoadjuvant radiation (reference: none) | 1.15 | 1.04-1.27 | .007 |
| Age (per increasing year) | |||
| Younger (<73) | 1.01 | 1.00-1.01 | <.001 |
| Older (>73) | 1.05 | 1.04-1.06 | <.001 |
N = 10,621 complete cases and 6230 events analyzed. Based on the inflection point identified by maximally selected rank statistics, age was treated as a continuous variable with piecewise linear functions and a knot at 73 years.
Comparison of Esophagectomy Versus Chemoradiation
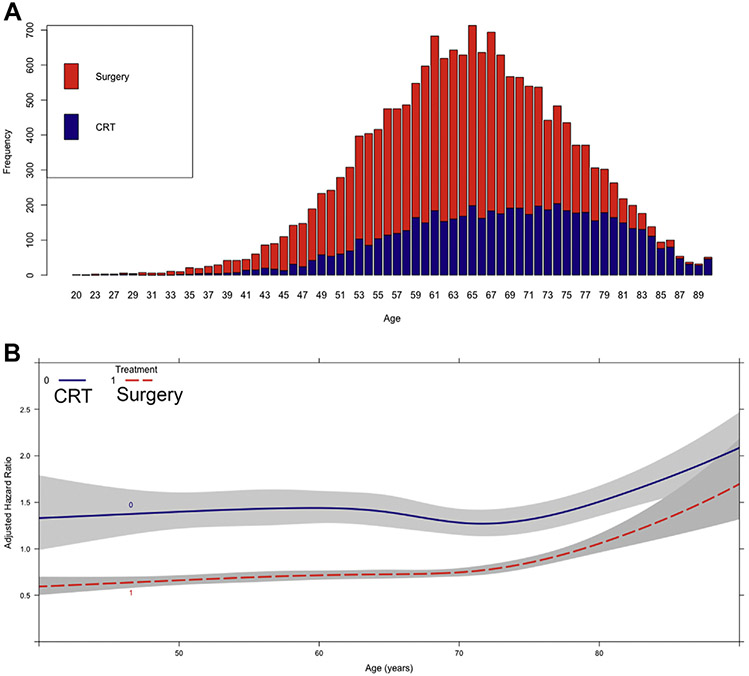
In the second part of the study, 17,495 patients met criteria, including 5815 who received chemoradiation (33%) and 11,680 who had surgery (67%). Compared with chemoradiation patients, surgery patients were more likely to be younger and male, have comorbidities, have private insurance, travel farther for treatment, be treated at an academic center, be treated at a higher volume surgery center, have stage II (rather than III) disease, and have a distal primary tumor (Table 3). Chemoradiation was increasingly performed over surgery as age increased (Figure 3A). Unadjusted 5-year OS was 19% (95% CI, 18-20) and 36% (95% CI, 35-37) for chemoradiation and surgery patients, respectively (Supplemental Figure 2B). Because the proportional hazards assumption was violated by the crossing survival curves at around 3 months, a subgroup of patients who survived at least 3 months from the initiation of treatment (chemoradiation or surgery) were then identified (Figure 1, Supplemental Figure 2C). In a multivariable Cox regression, esophagectomy was associated with improved survival over chemoradiation (HR = 0.34, 95% CI, 0.24-0.46; P < .001) (Table 4). The interaction between age and treatment was significant (Table 4). A graph of this interaction demonstrated that the survival curves of chemoradiation and surgery patients began to converge beyond age 80 years, although surgery was associated with improved survival at most ages (Figure 3B). In a subgroup analysis of 1099 patients older than age 80, the receipt of surgery was associated with improved survival compared with chemoradiation in multivariable regression (HR = 0.75, 95% CI, 0.59-0.95; P = .02).
Table 3.
Pretreatment Characteristics of Patients, Stratified by Receipt of Definitive Chemoradiation or Esophagectomya
| Characteristic | Chemoradiation (n = 5815) |
Esophagectomy (n = 11,680) |
P |
|---|---|---|---|
| Age y | 69 (61-77) | 63 (56-69) | <.001 |
| Female sex | 835 (14) | 1328 (11) | <.001 |
| Race | .005 | ||
| White | 5590 (97) | 11297 (98) | |
| Black | 115 (2) | 156 (1) | |
| Other | 70 (1) | 130 (1) | |
| Year of diagnosis | 2011 (2008-2013) | 2010 (2008-2013) | .001 |
| Charlson-Deyo score | <.001 | ||
| 0 | 4362 (75) | 8542 (73) | |
| 1 | 1064 (18) | 2513 (22) | |
| ≥2 | 389 (7) | 625 (5) | |
| Insurance status | <.001 | ||
| Private | 1521 (28) | 5727 (50) | |
| Government | 3715 (69) | 5507 (48) | |
| None | 121 (2) | 211 (2) | |
| Facility location | .94 | ||
| Metropolitan | 4419 (79) | 8909 (79) | |
| Urban | 1033 (19) | 2055 (18) | |
| Rural | 136 (2) | 271 (2) | |
| Distance to treatment center, miles | 11 (5-28) | 18 (7-50) | <.001 |
| Facility type | <.001 | ||
| Academic/research | 2107 (36) | 6197 (54) | |
| Annualized center surgical volume, cases/y | 2 (1-8) | 6 (2-16) | <.001 |
| Clinical stage | <.001 | ||
| II | 2756 (47) | 6017 (51) | |
| III | 3059 (53) | 5663 (49) | |
| Distal primary tumor | 4746 (82) | 10,082 (86) | <.001 |
| Follow-up (time to death or last follow-up from exposure), mo | 15 (7-30) | 24 (11-46) | <.001 |
All values are n (%) or median (interquartile range) unless otherwise stated.
Figure 3.
(A) Proportion of patients, stratified by age, who underwent surgery versus chemoradiation therapy (CRT) for treatment of locally advanced esophageal cancer. (B) Interaction between age and type of treatment as a function of adjusted hazard ratio of mortality from a multivariable Cox proportional hazards model including an interaction term between age and treatment. X axis shows age in years whereas Y axis demonstrates the adjusted hazard ratio from the Cox model. Survival curves for patients who underwent CRT or surgery are depicted and modeled using restricted cubic splines with 5 prespecified knots. Gray areas represent bounds of 95% confidence interval.
Table 4.
Cox Multivariable Regression of Factors Independently Associated With Survival in Patients Treated With Definitive Chemoradiation or Surgerya
| Predictor | Hazard Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Female sex (reference: male) | 0.88 | 0.82-0.94 | <.001 |
| Race (reference: white) | |||
| Black | 1.03 | 0.87-1.22 | .75 |
| Other | 0.86 | 0.69-1.07 | .19 |
| Year of diagnosis (per year) | 0.98 | 0.98-0.99 | <.001 |
| Charlson-Deyo score (reference: 0) | |||
| 1 | 1.05 | 0.99-1.10 | .09 |
| ≥2 | 1.17 | 1.07-1.28 | <.001 |
| Insurance status (reference: private) | |||
| Government | 1.09 | 1.03-1.15 | .002 |
| None | 1.27 | 1.09-1.47 | .002 |
| Facility location (reference: metropolitan) | |||
| Urban | 1.08 | 1.04-1.13 | .001 |
| Distance to treatment center (per 10 miles) | 1.00 | 1.00-1.00 | .22 |
| Facility type (reference: nonacademic) | |||
| Academic/research | 1.01 | 0.96-1.06 | .78 |
| Annualized center surgical volume (per 10 cases/y) | |||
| Clinical stage (reference: II) | |||
| III | 1.38 | 1.32-1.44 | <.001 |
| Distal tumor (reference: proximal thoracic/middle) | 0.94 | 0.89-0.99 | .03 |
| Neoadjuvant chemotherapy (reference: none) | 0.88 | 0.78-0.99 | .03 |
| Neoadjuvant radiation (reference: none) | |||
| Treatment (reference: chemoradiation) | 1.14 | 1.02-1.26 | .02 |
| Esophagectomy | 0.34 | 0.24-0.46 | <.001 |
| Age (per y) | 1.00 | 1.00-1.01 | .02 |
| Interaction of age: treatment | <.001 |
A total of 13,966 complete cases and 8615 events were analyzed.
Comment
Although esophageal cancer is predominantly being a disease of the elderly population, with a peak incidence in the seventh decade, debate remains about the ideal management of locally advanced esophageal cancer in older patients.11-13 National Comprehensive Cancer Network guidelines recommend considering age when offering surgical resection versus definitive chemoradiation, and there is evidence that increased age, or at least age-associated concomitant comorbidities, lead to poorer surgical outcomes.2,6,7 This large, hospital-based analysis confirmed that survival after esophagectomy worsens with increasing age for patients with clinical stage II and III esophageal cancer, and further identified a precise cutoff in age after which the worsening survival significantly increases. However, although mortality after esophagectomy notably worsened beyond age 73 years, we found that surgery continued to be associated with a survival benefit, although an increasingly narrow one, over chemoradiation even for octogenarians.
The effect of age on perioperative and oncologic outcomes in locally advanced esophageal cancer has been studied in a number of single institutional studies, as well as a handful of database studies.11-15,19 The specific role that age has, as opposed to concomitant comorbidities, in worsening outcomes of esophagectomy in older patients has been difficult to delineate, but the data largely support not withholding surgery based solely on age.10,13,20-22 Many of these analyses evaluated older versus younger patients based on arbitrary cutoffs (ie, >65, >70, or >75 years) to define older and younger cohorts.11,21 Wright and colleagues6 used The Society of Thoracic Surgeons General Thoracic Database and determined that in patients undergoing esophagectomy for esophageal cancer, age 75 versus 55 was an important predictor of major morbidity, although age 65 versus 55 was not. We used rigorous statistical methods to determine an exact cutoff in age after which outcomes especially worsen (Figure 2, Supplemental Figure 1) and to use this cutoff to create cohorts of younger versus older patients. Our findings support other studies that showed worse outcomes for older patients, specifically that outcomes after esophagectomy worsen substantially above age 73, but they support that age alone should not preclude surgery because it is still associated with superior survival outcomes compared with definitive chemoradiation.
Although current guidelines for locally advanced esophageal cancer recommend trimodality therapy for management with induction chemoradiation followed by surgery, older patients have been shown to be less likely to be offered and to receive optimal therapies.14,22 Faiz and coworkers14 recently conducted a population-based study of 702 patients in the Netherlands with potentially curable esophageal cancer, evaluating the impact of age and comorbidities on the receipt of definitive chemoradiation versus trimodality therapy and subsequent outcomes. The authors found that survival for patients with esophageal adenocarcinoma was superior in the trimodality group compared with definitive chemoradiation irrespective of age or the number of comorbidities, but that older patients received different patterns of care: patients age 70 or greater were more likely to be treated with definitive chemoradiation in lieu of surgery, and 78% of patients aged greater than 75 years received definitive chemoradiation compared with only 33% of patients younger than age 60.14 Consistent with their findings, our study showed that most patients aged greater than 80 years underwent definitive chemoradiation instead of surgery (Figure 3A), and older patients who underwent esophagectomy were less likely to receive induction chemotherapy or radiation (Table 1). Given that older patients are less likely to receive optimal trimodality therapy and more likely to receive treatment with only a single modality, our study aimed to identify whether there was an age beyond which chemoradiation offered a survival benefit over surgery. We found that age and type of treatment had a significant interaction with each other and survival, and that surgery continued to have a long-term survival benefit over chemoradiation into the mid-eighties, although this relative survival benefit began to diminish past the mid-seventies (Figure 3B).
That fewer older patients are receiving surgical resection in favor of definitive chemoradiation may reflect nuanced clinical assessment of surgical candidacy, multidisciplinary decision-making, and patient preferences that we were unable to assess fully in our study. The appropriateness of surgery, with or without induction chemoradiation, must continue to be weighed against the less invasive and morbid option of definitive chemoradiation, particularly given the higher rates of early postoperative mortality seen in older patients.22 In our study, older patients had higher rates of both 30- and 90-day mortality compared with younger patients. In a multivariable logistic regression adjusting for patient, tumor, and treatment factors including comorbidity status, older patients were more likely to experience 90-day postoperative mortality compared with younger patients (Supplemental Table 1). This may reflect an increased level of concomitant comorbidities and frailty in the older population that is unable to be accounted for adequately in the Charlson Deyo Comorbidity score and our multivariable analysis, and the increased risk for postoperative mortality should be noted when considering surgical candidacy for older patients and discussed with patients considering treatment options. However, the study by Faiz and colleagues14 and our findings in the United States supporting improved long-term survival in patients receiving surgery over chemoradiation should also be taken into account when counseling patients on outcomes. These findings and the literature support not withholding surgery from patients based solely on age, and reinforce the need to consider other factors such as concomitant comorbidity, histology, patient preferences, and hospital volume when considering treatment options.14,22-25
Our study had a number of important limitations. As a retrospective database study, it was limited by selection bias, potential confounding, and a lack of granularity. Specifically, the NCDB does not contain data pertaining to clinical decision-making and the reasons for pursuing surgery or chemoradiation. However, patients deemed unfit for surgery were noted and excluded from the analysis to limit the effect of this bias. In addition, postoperative admission rates, although reported in the NCDB, are likely an underestimation of actual readmissions, because only readmissions to the same hospital where the surgery was performed are captured. Notable to our analysis, restaging information after neoadjuvant therapy is not provided, which likely affected clinical decisions for surgery versus chemoradiation. Furthermore, although the NCDB provides data on location and dose of radiation, it does not provide chemotherapy type or the number of cycles. Finally, although the NCDB contains limited information regarding complications and comorbidities, it does not contain quality of life metrics or patient preferences, which are important factors in patient outcomes and clinical decisions.
Using a large, hospital-based database, we evaluated the effect of age on outcomes for patients with stage II to III esophageal cancer undergoing esophagectomy, and outcomes of patients with esophageal cancer receiving esophagectomy versus definitive chemoradiation. Among esophagectomy patients, survival outcomes are better in younger patients; however, a survival benefit remains for older patients undergoing esophagectomy with or without perioperative therapy compared with definitive chemoradiation, which persists even for octogenarians. Surgical candidacy should not be withheld from patients based solely on age, but clinical decision-making should consider age along with other factors such as patient preferences and fitness for surgery.
Supplementary Material
Acknowledgments
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the deidentified data used here; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Drs Farrow, Raman, and Voigt were supported by National Institutes of Health Surgical Oncology T-32 (5T32CA093245); Dr Jawitz was supported by Clinical Research T-32 (5T32HL069749).
Footnotes
The Supplemental Material can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2020.06.055] on http://www.annalsthoracicsurgery.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13:194–227. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright CD, Kucharczuk JC, O’Brien SM, Grab JD, Allen MS. Society of Thoracic Surgeons General Thoracic Surgery Database. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137:587–595 [discussion: 596]. [DOI] [PubMed] [Google Scholar]
- 7.van Gestel YR, Lemmens VE, de Hingh IH, et al. Influence of comorbidity and age on 1-, 2-, and 3-month postoperative mortality rates in gastrointestinal cancer patients. Ann Surg Oncol. 2013;20:371–380. [DOI] [PubMed] [Google Scholar]
- 8.Moskovitz AH, Rizk NP, Venkatraman E, et al. Mortality increases for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2006;82:2031–2036 [discussion: 2036]. [DOI] [PubMed] [Google Scholar]
- 9.Steyerberg EW, Neville BA, Koppert LB, et al. Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol. 2006;24:4277–4284. [DOI] [PubMed] [Google Scholar]
- 10.Sabel MS, Smith JL, Nava HR, Mollen K, Douglass HO, Gibbs JF. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol. 2002;9:210–214. [DOI] [PubMed] [Google Scholar]
- 11.Tapias LF, Muniappan A, Wright CD, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2013;95:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pultrum BB, Bosch DJ, Nijsten MW, et al. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133:1186–1192. [DOI] [PubMed] [Google Scholar]
- 14.Faiz Z, van Putten M, Verhoeven RHA, et al. Impact of age and comorbidity on choice and outcome of two different treatment options for patients with potentially curable esophageal cancer. Ann Surg Oncol. 2019;26:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppert LB, Lemmens VE, Coebergh JW, et al. Impact of age and co-morbidity on surgical resection rate and survival in patients with oesophageal and gastric cancer. Br J Surg. 2012;99:1693–1700. [DOI] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- 18.Hothorn T Package ‘maxstat’. Available at: https://cran.r-project.org/web/packages/maxstat/maxstat.pdf. Accessed April 24, 2020.
- 19.Poon RT, Law SY, Chu KM, Branicki FJ, Wong J. Esophagectomy for carcinoma of the esophagus in the elderly: results of current surgical management. Ann Surg. 1998;227:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Internullo E, Moons J, Nafteux P, et al. Outcome after esophagectomy for cancer of the esophagus and GEJ in patients aged over 75 years. Eur J Cardiothorac Surg. 2008;33:1096–1104. [DOI] [PubMed] [Google Scholar]
- 21.Jougon JB, Ballester M, Duffy J, et al. Esophagectomy for cancer in the patient aged 70 years and older. Ann Thorac Surg. 1997;63:1423–1427. [DOI] [PubMed] [Google Scholar]
- 22.Vlacich G, Samson PP, Perkins SM, et al. Treatment utilization and outcomes in elderly patients with locally advanced esophageal carcinoma: a review of the National Cancer Database. Cancer Med. 2017;6:2886–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derogar M, Orsini N, Sadr-Azodi O, Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 2012;30:1615–1619. [DOI] [PubMed] [Google Scholar]
- 24.Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg. 2003;75:337–341. [DOI] [PubMed] [Google Scholar]
- 25.Noordman BJ, de Bekker-Grob EW, Coene P, et al. Patients’ preferences for treatment after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg. 2018;105:1630–1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.