Abstract
Background
Strategies aimed at antiretroviral therapy (ART)–free remission will target individuals with a limited viral reservoir. We investigated factors associated with low reservoir measured as total human immunodeficiency virus type 1 (HIV-1) DNA in peripheral blood mononuclear cells (PBMCs) in perinatal infection (PaHIV).
Methods
Children from 7 European centers in the Early Treated Perinatally HIV Infected Individuals: Improving Children’s Actual Life (EPIICAL) consortium who commenced ART aged <2 years, and remained suppressed (viral load [VL] <50 copies/mL) for >5 years were included. Total HIV-1 DNA was measured by quantitative polymerase chain reaction per million PBMCs. Factors associated with total HIV-1 DNA were analyzed using generalized additive models. Age, VL at ART initiation, and baseline CD4% effects were tested including smoothing splines to test nonlinear association.
Results
Forty PaHIV, 27 (67.5%) female 21 (52.5%) Black/Black African, had total HIV-1 DNA measured; median 12 (IQR, 7.3–15.4) years after ART initiation. Eleven had total HIV-1 DNA <10 copies/106 PBMCs. HIV-1 DNA levels were positively associated with age and VL at ART initiation, baseline CD4%, and Western blot antibody score. Age at ART initiation presented a linear association (coefficient = 0.10 ± 0.001, P ≤ .001), the effect of VL (coefficient = 0.35 ± 0.1, P ≤ .001) noticeable >6 logs. The effect of CD4% (coefficient = 0.03 ± 0.01, P = .049) was not maintained >40%.
Conclusions
In this PaHIV cohort, reduced total HIV-1 DNA levels were associated with younger age and lower VL at ART initiation. The impact of early-infant treatment on reservoir size persists after a decade of suppressive therapy.
Keywords: adolescents, children, early treated, HIV-1, HIV reservoir, viral suppression
Initiation of antiretroviral therapy at a younger age and lower plasma viral load were associated with a lower HIV-1 viral reservoir in peripheral blood mononuclear cells after more than a decade of sustained virological suppression.
Despite the very significant gains made in the prevention of perinatal transmission of human immunodeficiency virus type 1 (HIV-1), globally >150 000 infected infants are born annually. Antiretroviral therapy (ART) has revolutionized survival; however, alone it is unable to confer a cure, a consequence of an inaccessible latent pool of HIV-1–infected reservoir cells. In both adults and infants, initiation of ART soon after infection minimizes the reservoir size in resting memory CD4+ T cells and limits the evolution of HIV-1 within reservoirs [1, 2]. In infants, early ART, typically defined as within 8–12 weeks of birth, is possible but challenging; those infected in utero can be identified within a few days of life, and those with intrapartum HIV-1 transmission by 2–6 weeks of age [3]. Rare case reports in perinatally infected individuals demonstrate that long-term viral control can be influenced by very early ART, although alone this is almost always insufficient for sustained viral control when subsequently stopped [4, 5]. Children differ immunologically from adults, with a more active thymus and greater capacity for immune regeneration, a reduced central memory T-cell compartment, and low immune activation state, a condition not conducive for HIV-1 reservoir seeding [6, 7]. Children starting ART within 3 months of birth remain frequently HIV-1 seronegative after losing maternal antibodies and lack HIV-specific cellular immune responses, making them potentially more susceptible to uncontrolled viral replication when ART is interrupted [8, 9]. Identifying factors and biomarkers, such as seronegativity, associated with limited viral reservoir size will potentially aid identification of participants for future proof-of-concept studies aimed at inducing ART-free viral remission [10].
The CARMA study (Child and Adolescent Reservoir Measurements on Early Suppressive ART) is part of the existing EPIICAL consortium (Early Treated Perinatally HIV Infected Individuals: Improving Children’s Actual Life; www.epiical.org), an international collaboration whose aim is to select promising HIV-1 therapeutic strategies and to identify candidates among perinatally HIV-1–infected children and adolescents and is described in detail previously [10].
The primary endpoint analyses of the CARMA cohort are presented, identifying factors that influence the establishment of a low total HIV DNA level in perinatally HIV-infected children and adolescents on suppressive ART.
MATERIALS AND METHODS
Design
This was a multicenter, cross-sectional study enrolling subjects from 7 European pediatric HIV clinical research centers contributing to EPIICAL, in England (3), Spain (2), and Italy (2).
Participants
Forty perinatally HIV-1–infected children who commenced ART within the first 24 months of life were included. Viral suppression (viral load [VL] <400 copies/mL) had to be achieved within 12 months of ART initiation, maintained for a minimum of 5 years with a VL of <50 copies/mL confirmed at enrollment. Children with current viral hepatitis or tuberculosis coinfection, malignancy, pregnancy, or concomitant immunosuppressive therapy were excluded. For each participant, data collection included demographics; age at HIV diagnosis, at ART start, and at time of analysis; and ART drug regimens ever received. Immunology (CD4 total, CD8 total, CD4% and CD8%, and CD4:CD8 ratio) and VL were recorded from diagnosis, ART initiation, throughout treatment, and at time of reservoir analysis. Viral load “blips” (defined as a rise in plasma VL from 50 to 399 copies/mL returning to <50 copies/mL on repeat sampling) and single annual viral “spikes” (defined as VL 400–999 copies/mL returning to <50 copies/mL on repeat sampling) were permitted.
Laboratory Analyses
Total HIV-1 DNA
Total HIV-1 DNA was measured in purified peripheral blood mononuclear cells (PBMCs) by real-time quantitative polymerase chain reaction (qPCR). Total nucleic acids were extracted from PBMCs on the QIAsymphony platform using the DSP Virus/Pathogen Mini Kit (Qiagen) according to the manufacturer’s protocol. Primers were designed for HIV-1 long terminal repeat and the genomic reference gene pyruvate dehydrogenase as previously described [11]. Quantification of cell-associated HIV-1 DNA by qPCR was determined using 20 μL of extract and a standard curve with known copy numbers in 10-fold dilutions. The limit of quantitation is 10 copies/106 PBMCs and the limit of detection is 1 copy/106 PBMCs. Assays were performed in triplicate.
Fourth-Generation Antibody/Antigen
Serological status was assessed by plasma venous sampling using the fourth-generation Abbott ARCHITECT HIV Ag/Ab Combo assay.
Western Blot: HIV-Specific Antibody Characterization
Plasma samples were tested as previously described [12]. Human immunodeficiency virus Western blot (WB) kit 2.2 (Medical Systems, Genova, Italy) was used, following the manufacturer’s instructions, to detect specific antibody (Ab) responses, in 20 μL of plasma, against 10 different HIV-1 viral proteins (gp160, gp120, p66, p55, p51, gp41, p39, p31, p24, and p17) as previously described [13]. Nitrocellulose strips containing HIV proteins incorporated through electrophoretic blotting were tested with plasma samples and with controls. The presence or absence of Abs to HIV was determined by comparing each nitrocellulose strip to the assay control strips tested with the nonreactive, strong reactive, and weak reactive controls. All of the WB strips were analyzed using the ImageJ software (ImageJ 1.52a; National Institutes of Health). The band intensity was calculated for each viral antigen in each sample, and compared with the intensity calculated in the strong reactive control. A score of 1 was assigned when the intensity of the band was ≥50% of those calculated in the strong positive control for the same antigen, whereas 0.5 was assigned when the band intensity ranged from 10% to 49% of those found in the positive control. Finally, negative results (WB score = 0) were assigned for band intensity ranging from 0% to 9% of those found in the positive control. A WB score was assigned to each patient by adding up the number of positive and weak responses (from 0 to 10).
Statistical Methods
Data were displayed as a whole and summarized by HIV-1 DNA reservoir (groups ≤10 copies vs >10 copies/106 PBMCs). HIV-1 DNA reservoir was dichotomized: low (≤10) vs high (>10) as per previous studies and reflecting the limit of assay quantitation. In multivariable models, HIV-1 DNA was used as a continuous variable. In case of continuous variables, median and interquartile range (IQR) were assessed for nonparametric variables and mean and standard deviation for parametric variables. In summary tables of categorical variables, counts and percentages were reported and comparisons were assessed using Fisher exact test. For normally distributed continuous variables, Student t test was performed, and Mann-Whitney U test when nonparametric.
To describe the association between the HIV-1 reservoir and the different effect of each clinical, virological, and immunological feature, a generalized additive mixed model was applied. Backward stepwise elimination was applied to reach the final multivariable model, and Akaike information criteria (AIC) was used to identify the best-fitting model.
To analyze possible nonlinear associations, each variable was included in the model with smoothing splines. Smoothing parameters defining degrees of freedom (df) were selected according to nested AIC comparisons. ART combination at initiation was included as random intercept to correct for random variance across regimens. R software version 3.5.2 software was used for all analyses (R Development Core Team, 2008).
Ethical Considerations
The CARMA study was approved by ethics committees within each country and informed assent/consent obtained from adolescents and/or parent(s)/legal guardian in accordance with country-specific law. Each participant received a unique study number, under which data were pseudo-anonymized.
RESULTS
Forty perinatally HIV-1–infected children, 27 (67.5%) female, 21 (52.5%) Black/Black African, and 13 (32.5%) white, commenced ART at a median age of 4.08 (IQR, 0.25–6.23) months, CD4 count 1310 (IQR, 478–2376) cells/µL, CD4% 30.5 (IQR, 19.2–42.5), and log10 VL 5.28 (IQR, 4.07–5.70) copies/mL (Table 1). Median time to viral suppression was 4.69 (IQR, 2.52–6.26) months. In analysis at a median of 12 (IQR, 7.3–15.4) years after ART initiation, median CD4 count was 872 (IQR, 646–1053) cells/µL, CD4% 41.0 (IQR, 33.8–46.2), and total HIV-1 DNA <10 copies/106 PBMCs in 11 (27.5%) children. Of these 11 children, 5 were below the lower limit of detection (<1 copy/106 PBMCs), the remaining 6 children having low levels of detectable total HIV-1 DNA (1–9 copies/106 PBMCs). In the 35 children with a quantifiable total HIV-1 DNA level (≥1 copy/106 PBMCs), the median was 50.9 (IQR, 25.3–117.3) copies/106 PBMCs. Serological analyses were available for 39/40 children. Ten children were seronegative on fourth-generation HIV Ab/antigen (Ag) testing at time of analysis. Five of 10 (50%) children with total HIV-1 DNA <10 copies/106 PBMCs and 5 of 29 (17.2%) children with a reservoir >10 copies/106 PBMCs were seronegative (P = .087) (Table 1). Only 1 child with a reservoir below the limit of detection (<1 copy/106 PBMCs) had positive HIV serology. There was a suggestive association of children with a reservoir of <10 copies/106 PBMCs being Ab/Ag nonreactive compared with those with higher reservoir size (P = .087; Table 1).
Table 1.
Immunological and Virological Parameters Compared by Reservoir Size
| Parameter | All (N = 40) | >10 Copies/106 PBMCs (n = 29) | <10 Copies/106 PBMCs (n = 11) | P Value |
|---|---|---|---|---|
| Region, No. (%) | .062 | |||
| Italy | 16 (40.0) | 9 (31.0) | 7 (63.6) | |
| Spain | 5 (12.5) | 3 (10.3) | 2 (18.2) | |
| United Kingdom | 19 (47.5) | 17 (58.6) | 2 (18.2) | |
| Sex, female, No. (%) | 27 (67.5) | 17 (58.6) | 10 (90.9) | .068 |
| Race/ethnicity, No. (%) | .663 | |||
| White | 13 (32.5) | 8 (27.6) | 5 (45.5) | |
| Black/Black African | 21 (52.5) | 16 (55.15) | 5 (45.5) | |
| Other | 6 (15.0) | 5 (17.25) | 1 (9.09) | |
| Age at HIV diagnosis, mo | 4.17 (2.19–6.32) | 4.92 (3.08–6.49) | 3.61 (0.34–4.26) | .084 |
| AIDS diagnosis, No. (%) | .486 | |||
| No | 25 (62.5) | 17 (58.6) | 8 (72.7) | |
| Yes | 15 (37.5) | 12 (41.4) | 3 (27.3) | |
| Age at ART start, mo | 4.08 (0.25–6.23) | 4.59 (2.66–7.64) | 3.61 (0.10–4.49) | .071 |
| Follow-up, y | 13.0 (8.19–16.1) | 13.6 (8.29–16.4) | 10.7 (8.99–15.0) | .892 |
| Virology | ||||
| VL at diagnosis, log copies/mL | 5.60 (4.98–5.93) | 5.70 (5.25–6.00) | 4.66 (4.18–5.33) | .030 |
| VL at ART start, log copies/mL | 5.28 (4.07–5.70) | 5.48 (4.91–5.80) | 4.64 (4.00–5.18) | .119 |
| Time to viral suppression, mo | 4.69 (2.52–6.26) | 4.59 (3.21–6.46) | 4.79 (1.93–5.11) | .844 |
| VL blip. No. (%) | 11 (27.5) | 10 (34.5) | 1 (9.09) | .233 |
| Time to blip, y | 3.7 (2.35–7.26) | 3.31 (2.23–7.30) | 6.48 (6.48–6.48) | .723 |
| Viral spike, No. (%) | 5 (12.5) | 3 (10.3) | 2 (18.2) | .603 |
| Time to spike, y | 1.53 (0.86–3.53) | 3.52 (2.53–7.97) | 0.61 (0.48–0.73) | .200 |
| Immunology | ||||
| At diagnosis | ||||
| CD4 count | 1515 (637–2235) | 1510 (643–2120) | 1621 (827–2955) | .721 |
| CD4 % | 31.0 (18.0–38.0) | 34.0 (18.0–38.0) | 30.5 (25.2–39.0) | 1.000 |
| CD8 count | 1406 (720–2170) | 985 (639–1931) | 2170 (2115–3226) | .077 |
| CD8 % | 32.0 (25.0–40.0) | 29.0 (22.5–40.0) | 35.0 (33.0–47.0) | .157 |
| At ART start | ||||
| CD4 count | 1310 (478–2376) | 948 (478–2284) | 1506 (988–2965) | .590 |
| CD4 % | 30.5 (19.2–42.5) | 27.0 (19.0–41.0) | 33.0 (30.0–45.0) | .482 |
| CD8 count | 1068 (795–2129) | 960 (695–1688) | 2115 (1380–2170) | .166 |
| CD8 % | 31.0 (26.5–40.0) | 30.0 (25.0–40.0) | 34.0 (30.8–36.5) | .500 |
| CD4/CD8 | 0.84 (0.60–1.57) | 0.82 (0.59–1.98) | 0.89 (0.64–0.94) | .749 |
| At viral suppression | ||||
| CD4 count | 1710 (924–2263) | 1680 (891–2120) | 2028 (1535–2735) | .223 |
| CD4 % | 31.0 (25.4–44.0) | 30.0 (23.5–42.5) | 39.5 (30.0–46.2) | .161 |
| CD8 count | 1064 (826–1732) | 1064 (795–1716) | 1297 (878–1715) | .780 |
| CD8 % | 27.5 (18.5–32.2) | 28.0 (19.0–32.0) | 24.0 (18.0–32.0) | .784 |
| At reservoir analysis | ||||
| CD4 count | 872 (646–1053) | 877 (719–1043) | 630 (452–1080) | .440 |
| CD4 % | 41.0 (33.8–46.2) | 41.0 (36.0–46.0) | 39.0 (32.0–46.5) | .606 |
| CD8 count | 584 (402–708) | 636 (434–712) | 426 (292–642) | .142 |
| CD8 % | 26.0 (23.0–30.0) | 26.0 (23.0–30.0) | 25.0 (23.0–30.0) | .761 |
| CD4/CD8 | 1.54 (1.28–1.88) | 1.54 (1.31–1.88) | 1.54 (1.28–2.04) | .774 |
| Serology at reservoir analysis (n = 39) | n = 29 | n = 10 | ||
| Anti-CMV IgG, No. (%) | 1.000 | |||
| Negative | 10 (25.6) | 8 (27.6) | 2 (20.0) | |
| Positive | 29 (74.4) | 21 (72.4) | 8 (80.0) | |
| HIV Ag/Ab at reservoir analysis, No. (%) | .087 | |||
| Equivocal/positive | 29 (74.4) | 24 (82.8) | 5 (50.0) | |
| Nonreactive | 10 (25.6) | 5 (17.2) | 5 (50.0) | |
| Ag/Ab | 4.47 (0.74–26.8) | 6.02 (2.05–27.8) | 1.20 (0.10–3.21) | .087 |
| Western blot score | 1.0 (0.5–2.0) | 1.5 (0.5–2.0) | 0.75 (0.0–1.75) | .269 |
Data are presented as median (IQR) unless otherwise indicated.
Abbreviations: Ag/Ab, antigen/antibody; ART, antiretroviral therapy; CMV, cytomegalovirus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; PBMCs, peripheral blood mononuclear cells; VL, viral load.
Viral load at diagnosis was significantly lower in those with total HIV-1 DNA <10 copies/106 PBMCs (log10 VL, 5.70 [IQR, 5.25–6.00] copies/mL vs 4.66 [IQR, 4.18–5.33] copies/mL; P = .030; Table 1). Children with a reservoir of <10 copies/106 PBMCs were likely to be younger at HIV diagnosis (3.61 [median (IQR), 0.34–4.26] months vs 4.92 [IQR, 3.08–6.49] months) and at ART start (3.6 [median (IQR), 0.10–4.49] months vs 4.6 [IQR, 2.66–7.64] months), although this did not reach statistical significance. No differences were found in patients with low vs high reservoir related to clinical stage (AIDS vs non-AIDS) or time to viral suppression. The median age at ART initiation was 4.6 (IQR, 3.11–7.04) months in those with positive/equivocal serology and 3.1 (IQR, 0.21–3.80) months in those who were seronegative (Table 1). There was no association of age at ART with log10 VL at ART start and serological status at analysis 12 years later (log10 VL, 5.3 [IQR, 4.04–5.8] copies/mL in those with positive/equivocal serology and 5.02 [IQR, 4.22–5.4] copies/mL in those who were seronegative).
The number of patients ever experiencing a blip and/or spike and time from viral suppression to first blip and/or spike were comparable between groups (Table 1). Immunological parameters (total/% CD4, CD8, and CD4:CD8) did not differ between groups at diagnosis, ART initiation, viral suppression, or 12 years later at time of reservoir analysis.
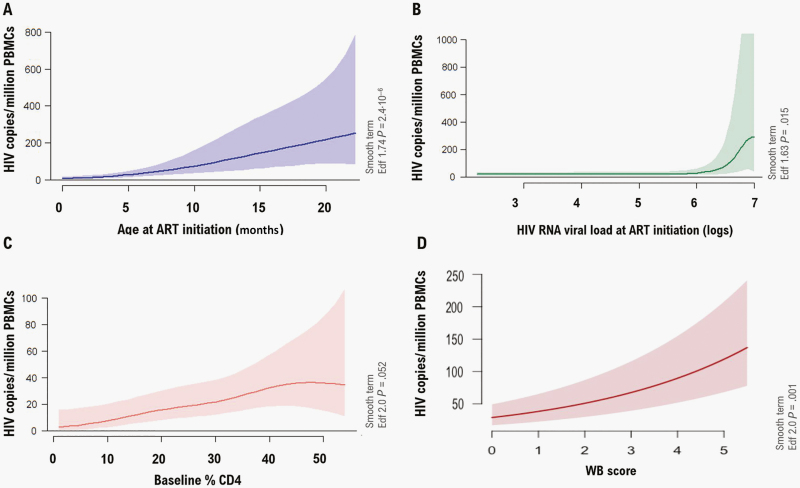
Only age and VL at ART initiation and baseline CD4% were selected to be included in the multivariable model to predict reservoir size. Total HIV-1 DNA levels were positively associated with age and VL at ART initiation and baseline CD4% (Figure 1). While age at ART initiation presented a linear association (coefficient = 0.10 ± 0.001, P ≤ .001, df = 2), the effect of VL (coefficient = 0.35 ± 0.10, P ≤ .001, df = 3) only was noticeable for individuals with VLs at ART initiation above 6 logs. The effect of baseline CD4% (coefficient = 0.03 ± 0.01, P = .049) was not maintained above 40%. Western blot score was significantly positively associated with HIV DNA reservoir adjusted for age and considering drug combinations as random intercepts (P < .001; Figure 1).
Figure 1.
Generalized additive mixed multivariable model plots showing the association between the age at antiretroviral therapy (ART) (A), human immunodeficiency virus (HIV) RNA viral load (VL) at ART initiation (B), baseline % CD4 (C), and Western blot (WB) score (D) and the HIV reservoir size (HIV copies per million peripheral blood mononuclear cells [PBMCs]). ART combinations at initiation were included as random intercepts. The shaded areas indicate the 95% confidence intervals. Smooth terms are specified showing the effective degrees of freedom (Edf) for each of the variables included in the model. While age at ART initiation and WB score presented a linear association, the effect of VL was only noticeable for individuals with VLs at ART initiation above 6 logs. The effect of baseline CD4% was not maintained above 40%.
DISCUSSION
Of 40 HIV-1 perinatally infected children from 7 different sites across 3 European countries initiating ART in infancy (median age, 4 months), with viral suppression maintained for more than a decade, just over one-quarter had very low/undetectable total HIV-1 DNA levels (<10 copies/106 PBMCs). Patients with a lower reservoir size commenced ART earlier after birth and at a lower baseline VL. The association of earlier ART initiation following primary infection and a reduced reservoir size is well-established in adults and is increasingly recognized for children, further supported by this study. The impact of pretreatment VL on future reservoir size is less established. Baseline VL is associated with time to viral suppression, and subsequent risk of viral rebound in adult populations and with persistent residual low-level viremia on ultrasensitive assays [14, 15]. Perinatally HIV-1–infected infants have higher plasma VLs compared with older children and, despite higher CD4%, a longer time to viral suppression [16]. In a perinatal cohort from Europe and Thailand of >400 infants initiating therapy before 6 months of age, younger age, higher CD4%, and lower VL were independent predictors of viral suppression on multivariate analysis [17]. Starting ART even earlier, within the first 7 days of life, is associated with more rapid viral suppression [18]. The rate of viral suppression following ART initiation and length of time on suppressive ART has been shown to be associated with a reduced reservoir size, with a recent perinatal European cohort demonstrating a 10% reduction in total HIV-1 DNA for every year suppressed [11]. Reservoir size in children has been shown to continue to decline for more than a decade, but in adults appears to plateau after around 4 years of suppressive therapy [13, 19].
One-quarter of participants in our cohort are HIV-1 seronegative, 12 years after initiating suppressive ART, a rate comparable to other early-treated perinatal cohorts [9]. Previous studies have reported a correlation between earlier age at ART initiation and subsequent seronegativity (seropositivity odds ratio, 13.7 [95% confidence interval, 3.1–60.2]; P = .001) ART initiated 12–24 weeks vs <12 weeks) and with lower pretreatment VL [8, 9], although the latter association was not supported by our data, possibly due to an inadequate sample size and treatment initiation at a later stage (median age, 4 months) than in other cohorts [20, 21]. However higher Western blot score in multivariant analysis was strongly related to reservoir size, as reported in other cohorts [13, 21–23]. The close correlation found between the HIV-DNA and the magnitude of HIV-specific Ab response is consistent with the hypothesis that persistence of specific Abs is dependent on the HIV viral antigen exposure. Infancy represents a period of relative immunological immaturity characterized by the presence of maternal Abs in the infant’s blood that may hide the antigens. Suppression of viral replication through early ART in this period of life gives these children distinct HIV-specific humoral responses, which persists over time even after long-term viral suppression [24]. Quantitative HIV-1–specific antibodies have been shown to correlate and predict plasma HIV-1 RNA and cell-associated DNA levels [13, 23] and may potentially be an adjunctive tool to screen children, particularly in low-income settings, using small blood volumes for future intervention studies aimed at ART-free remission [23]. It remains unclear as to whether seronegativity will confer advantage in future treatment interruption studies in children, or potentially increase susceptibility to uncontrolled viral replication.
Our study has several limitations, being a cross-sectional analysis of reservoir dynamics in children and adolescents commencing ART a decade ago with limited numbers reducing analytical power. However, this reflects the clinical reality; very small numbers of children who start ART in infancy achieve and consistently maintain viral control for many years. For this reason, the study entry criteria were broad: ART initiation before the age of 2 years with viral suppression for at least 5 years. Yet of >400 children aged 7–18 years in pediatric care in 7 European centers, <1 in 10 fulfilled the criteria. The multicenter, multicountry enrollment, the length of sustained viral suppression, and pretreatment VL data frequently absent in perinatal cohorts adds weight to our data. Although quantification of total HIV-1 DNA overestimates the size of the reservoir, lack of universal standards make between-study comparisons complex, particularly as many data sets are single-cohort studies. Subsequent immunological and virological secondary endpoint analyses in the CARMA cohort are ongoing; cell-associated HIV-1 RNA and DNA, near full-length single-genome sequencing to infer on intact provirus, and the association of natural killer cell function with HIV-1 DNA and T-cell markers are to be presented at the Conference on Retroviruses and Opportunistic Infections 2020 (www.croiconference.org/abstracts/search-abstracts/). Following this initial cross-sectional analysis, the CARMA participants are being offered enrollment in a longitudinal follow-on study, looking at changing reservoir dynamics as they enter early adult life. Additional cohorts of early-treated children on sustained suppressive ART from South Africa and Thailand are being invited to join the CARMA cohort to explore potential differences in high-prevalence settings.
Despite variations between studies, time to ART start, pretreatment VL, and length of viral suppression remain consistent markers associated with the likelihood of a smaller reservoir size. Children and adolescents who started ART in the first months of life, with a lower VL and who have remained suppressed, may be preferred candidates for future intervention studies aiming for ART-free remission.
CONCLUSIONS
One-quarter of early-treated, perinatally infected children have low reservoir 12 years after enrollment. Initiation of ART at a younger age and lower plasma VL were associated with a lower HIV-1 viral reservoir in PBMCs a decade later in the context of sustained virological suppression. While the clinical benefits of early-infant ART are profound, whether a smaller latent reservoir can confer future ART-free advantage remains an elusive question for a population that currently faces a lifetime on ART.
Supplementary Material
Notes
Acknowledgments. The writing group would like to thank all of the young people who contributed their time and blood samples to the Child and Adolescent Reservoir Measurements on Early Suppressive ART (CARMA) study. The writing group would also like to thank Viviana Gianuzzi, Annalisa Landi, Francesca Rocchi, Maria Grazia Lain, Alessandra Nardone, and Silvia Faggion for their support in the trial regulatory aspects.
Financial support. CARMA was supported by the EPIICAL (Early-Treated Perinatally HIV-Infected Individuals: Improving Children’s Actual Life With Novel Immunotherapeutic Strategies) project, funded through an independent grant by ViiV Healthcare United Kingdom. This work is part of the EPIICAL project (http://www.epiical.org/), supported by the PENTA-ID foundation (http://penta-id.org/).
Recruiting site Partners are listed in Supplementary Appendix 1, Virology and Immunology Partners are listed in Supplementary Appendix 2 and the members of the EPIICAL Consortium are listed in Supplementary Appendix 3.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Klein N, Palma P, Luzuriaga K, et al. Early antiretroviral therapy in children perinatally infected with HIV: a unique opportunity to implement immunotherapeutic approaches to prolong viral remission. Lancet Infect Dis 2015; 15:1108–14. [DOI] [PubMed] [Google Scholar]
- 2. Foster C, Pace M, Kaye S, et al. CHERUB Investigators . Early antiretroviral therapy reduces HIV DNA following perinatal HIV infection. AIDS 2017; 31:1847–51. [DOI] [PubMed] [Google Scholar]
- 3. Tagarro A, Chan M, Zangari P, et al. EPIICAL Consortium . Early and highly suppressive ART are the main factors associated with low viral reservoir in European perinatally HIV infected children. JAIDS 2018; 79:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pasvol TJ, Foster C, Fidler S. Novel therapies/hopes for HIV cure in perinatally acquired HIV-positive adolescents. Curr Opin HIV AIDS 2018; 13:281–7. [DOI] [PubMed] [Google Scholar]
- 6. Muenchhoff M, Prendergast AJ, Goulder PJ. Immunity to HIV in early life. Front Immunol 2014; 5:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. HIV-NAT 194 Study Group . Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28:1015–20. [DOI] [PubMed] [Google Scholar]
- 8. Payne H, Mkhize N, Otwombe K, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis 2015; 15:803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fidler K, Foster C, Lim E, et al. Reactivity of routine HIV antibody tests in children with perinatally-acquired HIV-1 in England: cross sectional analysis. Pediatr Infect Dis J 2019; 38:146–8. [DOI] [PubMed] [Google Scholar]
- 10. Palma P, Foster C, Rojo P, et al. The EPIICAL project: an emerging global collaboration to investigate immunotherapeutic strategies in HIV-infected children. J Virus Erad 2015; 1:134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tagarro A, Chan M, Zangari P, et al. Early and highly suppressive antiretroviral therapy are main factors associated with low viral reservoir in European perinatally HIV-infected children. J Acquir Immune Defic Syndr 2018; 79:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rocca S, Zangari P, Cotugno N, et al. Human immunodeficiency virus (HIV) antibody repertoire estimates reservoir size and time of antiretroviral therapy initiation in virally suppressed perinatally HIV-infected children. J Pediatric Infect Dis Soc 2019; 8:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Connor J, Smith C, Lampe FC, et al. UK Collaborative HIV Cohort (CHIC) Study . Durability of viral suppression with first-line antiretroviral therapy in patients with HIV in the UK: an observational cohort study. Lancet HIV 2017; 4:e295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKinnon E, Castley A, Payne L, et al. Determinants of residual viraemia during combination HIV treatment: impacts of baseline HIV RNA levels and treatment choice. HIV Med 2016; 17:495–504. [DOI] [PubMed] [Google Scholar]
- 16. Ásbjörnsdóttir KH, Hughes JP, Wamalwa D, et al. Differences in virologic and immunologic response to antiretroviral therapy among HIV-1-infected infants and children. AIDS 2016; 30:2835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) and Early-Treated Perinatally HIV-Infected Individuals: Improving Childrenʼs Actual Life With Novel Immunotherapeutic Strategies (EPIICAL) Study Groups. Predictors of faster virological suppression in early treated infants with perinatal HIV from Europe and Thailand. AIDS 2019; 33:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tagarro A, Dominguez-Rodriguez S, Palma P, et al. EPIICAL Consortium . Neonatal ART <7 days versus 7–28 days reduced time to viral suppression. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 4–7 March 2019. Abstract 44.
- 19. Uprety P, Patel K, Karalius B, et al. Pediatric HIV/AIDS Cohort Study (PHACS) . Human immunodeficiency virus type 1 DNA decay dynamics with early, long-term virologic control of perinatal infection. Clin Infect Dis 2017; 64:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhn L, Schramm DB, Shiau S, et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS 2015; 29:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martínez-Bonet M, Puertas MC, Fortuny C, et al. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 2015; 61:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Persaud D, Patel K, Karalius B, et al. Pediatric HIV/AIDS Cohort Study . Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McManus M, Henderson J, Gautam A, et al. PACTG 356 Investigators . Quantitative human immunodeficiency virus (HIV)-1 antibodies correlate with plasma HIV-1 RNA and cell-associated DNA levels in children on antiretroviral therapy. Clin Infect Dis 2019; 68:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cotugno N, Morrocchi E, Rinaldi S, et al. EPIICAL Consortium . Early antiretroviral therapy-treated perinatally HIV-infected seronegative children demonstrate distinct long-term persistence of HIV-specific T-cell and B-cell memory. AIDS 2020; 34:669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.