Abstract
Background
Alaska Native (AN) infants are at risk for severe disease due to respiratory syncytial virus (RSV) and influenza. Maternal immunization protects young infants through transplacental antibody transfer. RSV- and influenza-specific transplacental antibody transfer in mother–infant pairs has not previously been evaluated in the AN population.
Methods
Serum samples collected during pregnancy and at birth from AN mother–infant pairs in the Yukon-Kuskokwim Delta region (YKD) of Alaska (2000–2011; n = 75) and predominantly white pairs in Seattle, Washington (2014–2016; n = 57), were tested for RSV and influenza antibody using a microneutralization and hemagglutination inhibition assay, respectively, and compared between sites.
Results
Mean RSV antibody concentrations in pregnant women in YKD and Seattle were similar (log2 RSV antibody 10.6 vs 10.7, P = .86), but cord blood RSV antibody concentrations were significantly lower in infants born to mothers in YKD compared with Seattle (log2 RSV antibody 11.0 vs 12.2, P < .001). Maternal and cord blood influenza antibody concentrations were lower for women and infants in YKD compared with Seattle for all 4 influenza antigens tested (all P < .05). The mean cord to maternal RSV antibody transfer ratio was 1.15 (standard deviation [SD], 0.13) in mother–infant pairs in Seattle compared with 1.04 (SD, 0.08) in YKD. Mean cord blood to maternal antibody transfer ratios for influenza antigens ranged from 1.22 to 1.42 in Seattle and from 1.05 to 1.59 in YKD.
Conclusions
Though the transplacental antibody transfer ratio was high (>1.0) for both groups, transfer ratios for RSV antibody were significantly lower in AN mother–infant pairs. Further studies are needed to elucidate the impact of lower transplacental antibody transfer on infant disease risk in rural Alaska.
Alaska Native and continental US mother-infant pairs have high transplacental antibody transfer ratios (>1.0) for influenza and respiratory syncytial virus, but anti-respiratory syncytial virus antibody levels are significantly lower in Alaska Native pairs than in those from the continental US.
Keywords: Alaska Native, influenza virus, maternal–child health, respiratory syncytial virus, transplacental antibody transfer
Similar RSV antibody levels in Alaska-native (AN) and non-AN women
AN infants’ cord blood RSV antibody levels lower
Maternal and cord blood influenza antibody levels lower in AN women and infants
AN mother–infant pairs’ RSV antibody transfer ratios significantly lower
Lower respiratory tract infection (LRTI) is the leading cause of childhood mortality worldwide. Alaska Native (AN) populations experience a disproportionate burden of respiratory syncytial virus (RSV) and influenza LRTI; one of the highest infant RSV-associated hospitalization rates worldwide is among AN in the Yukon-Kuskokwim Delta region (YKD) [1, 2]. Influenza-associated hospitalization rates are also higher among AN infants compared with the general US population [3]. In YKD, more than 60% of families live below the federal poverty line. In this population, household crowding, preterm birth, and lack of breastfeeding have been associated with increased risk for severe disease due to RSV and influenza [4].
Several lines of evidence support the specific role of maternal antibodies in protecting infants against viral LRTI. First, in prospective epidemiologic studies, higher cord blood RSV antibody concentrations are associated with decreased risk of severe infection in infants aged <6 months [5]. Second, palivizumab, a licensed monoclonal RSV-specific antibody, is known to prevent severe RSV-associated LRTI in high-risk infants [6]. Finally, maternal immunization, a strategy to protect the infant through transplacental antibody transfer [7], increases cord blood antibody titers and protects against influenza disease in infants [8–10]. Several maternal RSV vaccines are currently in clinical trials, with the goal of increasing infant RSV antibody concentrations to protect against severe LRTI [11, 12]. In the United States, Nepal, and Bangladesh, transplacental antibody transfer ratios of infant cord to maternal antibody have been shown to be efficient, with cord blood antibody concentrations higher than maternal antibody concentrations by the time of birth [13–15], and data from Seattle, Washington, prior to routine influenza vaccination show influenza antibody transfer ratios of 0.94–0.99 [9]. Given the increased risk of severe RSV and influenza in AN populations and the importance of maternal vaccine strategies as a public health intervention to protect infants by boosting their antibody levels, better understanding of the efficiency of transplacental antibody transfer in AN cohorts is needed to optimize maternal vaccination strategies.
In this study, we compared RSV and influenza antibody concentrations and transplacental antibody transfer ratios in AN and Seattle cohorts.
METHODS
Seattle Cohort
An observational cohort of pregnant women was recruited from a midwife clinic in Seattle from 2014 through 2016 [16]. Women who had a healthy uncomplicated pregnancy, were ≥20 weeks’ gestation, and aged ≥18 years were eligible. Clinical and sociodemographic information was collected at enrollment and at birth. Maternal blood was collected during the second or third trimester, in the 2 weeks prior to birth, and at birth. Infant cord blood was collected at delivery.
YKD Cohort
Previously collected maternal serum and umbilical cord blood samples from AN mother–infant pairs enrolled at the Yukon-Kuskokwim Delta Regional Hospital in Bethel, Alaska, from 2000 through 2011 and banked at the Alaska Area Specimen Bank were analyzed. Serum samples were collected from AN mothers during a prenatal visit approximately 4 months before delivery and from umbilical cord blood collected at the time of delivery in a study of exposures to environmental pollutants (Maternal Organics Monitoring Study). RSV seasonality in YKD shifted to a later onset during the study time period, but similar to in the continental United States, nearly all cases occurred between October and May [17].
Laboratory Testing
Maternal serum and umbilical cord blood were processed and frozen at –30ºC. RSV-neutralizing antibody titers were measured with a microneutralization assay using 2-fold dilutions of serum incubated against RSV A2 strain in Hep-2 cells in 96-well plates at 37°C for 72 hours [15]. Cells were then washed, fixed, and stained with RSV F protein-specific mouse monoclonal primary antibody and a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G secondary antibody. Neutralization titer was defined as the minimal dilution of serum resulting in 50% color reduction compared with a positive control. World Health Organization (WHO) standardized RSV reference sera were included with every run [18]. Hemagglutination inhibition (HAI) assay antibody titers against H3N2, H1N1, B (Victoria), and B (Yamagata) influenza antigens were measured using a standard HAI assay [19]. Positive and negative controls and reference anti-sera were included with all runs. The following influenza antigens that corresponded to the appropriate year the sample was collected were used in the HAI assays: Seattle, 2015–2016 WHO antigens: influenza A (H1N1) pdm09 (A/California/07/2009 NYMC X-179A); influenza A (H3N2) (A/Switzerland/9715293/2013); influenza B Yamagata lineage (B/Phuket/3073/2013), and Victoria Lineage (B/Brisbane/60/2008); Bethel, 2011–2013 (H1N1)pdm09 (A/California/07/2009 NYMC X-179A); 2010–2012 (H3N2) control antigen, BPL-inactivated; 2010–2012, Yamagata lineage, ether-extracted; and 2010–2012 Victoria Lineage (B/Brisbane/60/2008). Influenza antigens from 2000–2002 were not available; therefore, serum samples from these years were not tested by HAI assay (n = 22).
Statistical Analyses
Gestational age was determined using the last menstrual period. Preterm birth was defined as birth <37 weeks’ gestation. Low birth weight (LBW) was defined as birth weight <2500 g. RSV and influenza HAI antibody titers were log2 transformed. Antibody titers were nonnormally distributed both before and after log transformation as measured by visual inspection and the Shapiro-Wilk test. Differences in demographic, antibody titer, and other variables between groups were assessed using the Wilcoxon-Mann-Whitney test, χ2 test, and Fisher exact test, as appropriate. Correlation was measured using Spearman rank correlation. For both influenza and RSV, multivariable linear regression models were used to evaluate the association between prenatal maternal antibody titers and infant cord blood titers, adjusting for preterm birth and/or LBW and season of birth (December–April vs May–November). For Seattle women, if a maternal sample was not available from the prenatal visit, a maternal sample from the time of delivery was used for analyses. To coincide with the timing of a theoretical maternal RSV vaccine administration, we additionally conducted an exploratory analysis of the subset of pregnant women with enrollment samples collected more than 2 weeks before delivery. All analyses were performed using R version 3.5.0 (R Foundation for Statistical Computing) in RStudio version 1.1.453 (RStudio, Inc.).
RESULTS
Overall, 57 mother–infant pairs in Seattle were enrolled from December 2014 through February 2016 and 75 mother–infant pairs in YKD from 2001 through 2011. The median age in the Seattle cohort was 33 years (range, 24–40), the majority were white (n = 50; 88%), and the majority received influenza vaccination during pregnancy (n = 41, 72%). All Seattle infants were born full-term, with median gestational age of 40 weeks (interquartile range [IQR], 39.0–41.0). The YKD cohort’s median age was 25 years (range, 16–41) and all women were AN. Vaccination data were not available, but samples were collected prior to a recommendation for routine influenza vaccination during pregnancy. Women in the YKD cohort were more likely to deliver preterm (6, 8% vs 0, 0%); P = .045; Table 1).
Table 1.
Comparison of Baseline Variables in Seattle, Washington, and Yukon-Kuskokwim Delta, Alaska, Mother–Infant Pairs
| Variable | Seattle, Washington, N = 57 | Yukon-Kuskokwim Delta, Alaska, N = 75 | P Value |
|---|---|---|---|
| Birth weight, g | .15 | ||
| >2500 | 48 (100%) | 70 (96%) | |
| <2500 | 0 (0%) | 3 (4%) | |
| Gestational age, weeks | .05 | ||
| < 37 | 0 (0%) | 6 (8%) | |
| ≥37 | 48 (100%) | 69 (92%) | |
| Child low birth weight and/or premature | .02 | ||
| No | 48 (100%) | 67 (89%) | |
| Yes | 0 (0%) | 8 (11%) | |
| Infant gender | .59 | ||
| Female | 28 (58%) | 40 (53%) | |
| Male | 20 (42%) | 35 (47%) |
In the Seattle cohort, maternal blood samples were collected a mean of 16 days (standard deviation [SD] = 17) before delivery. A total of 32 mothers had blood collected more than 2 weeks before delivery, and 15 were within 2 weeks of delivery. Nearly all (n = 56/57; 98%) had a prenatal sample. A total of 45 women had blood collected at the time of delivery, along with 46 infant cord blood samples. In the 75 women of the YKD cohort, prenatal blood samples were collected a mean of 137 days (SD = 64) before delivery. Cord blood samples from all infants were collected at the time of delivery.
RSV Antibody
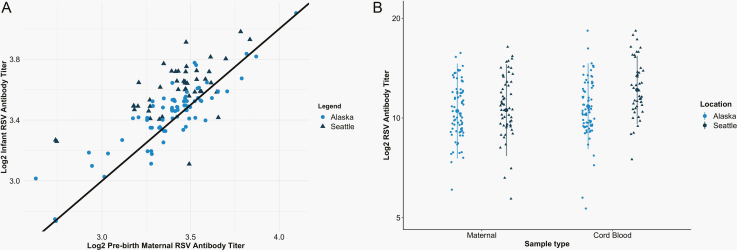
Mean RSV antibody concentrations in pregnant women in YKD and Seattle were similar before birth (log2 RSV ab 10.63 [SD = 1.70] vs 10.68 [SD = 1.58]; P = .68; Table 2, Figure 1). However, mean cord blood RSV antibody levels were significantly lower in cord blood of infants born to AN mothers compared with Seattle mothers (log2 RSV ab 10.97 [1.59] vs 12.24 [1.42]; P < .001) in multivariate regression adjusting for LBW, preterm birth, and season of birth. The median cord to maternal antibody ratio was 1.13 (IQR, 1.07–1.22) in mother–infant pairs in Seattle (n = 57) compared with 1.04 (IQR, 1.00–1.09) in YKD (n = 75). Pairs with ratios <1.00 were more likely to be LBW or preterm (75% of LBW preterm pairs had ratios <1.00 vs 27% of non-preterm or LBW pairs) or from YKD (45% of YKD pairs had ratios <1.00 vs 5% of Seattle pairs). Maternal and infant cord blood log2 RSV antibody titers were highly correlated in both Seattle (ρ = 0.65) and YKD (ρ = 0.88).
Table 2.
Summary Statistics of Respiratory Syncytial Virus and Influenza Antibody Titers for Seattle, Washington, and Yukon-Kuskokwim Delta, Alaska, Mother–Infant Pairs.
| Factor | Seattle, Washington | Yukon-Kuskokwim Delta, Alaska | P Value |
|---|---|---|---|
| Log2 respiratory syncytial virus neutralizing antibody | N = 57 | N = 75 | … |
| Maternal titer, median (IQR) | 10.9 (9.6–11.6) | 10.6 (9.6–11.1) | .68 |
| Cord blood titer, median (IQR) | 12.1 (11.1–13.1) | 11.1 (10.1–11.6) | <.001 |
| Log2 influenza hemagglutination inhibition assay antibody | N = 57 | N = 53 | |
| H3N2 | |||
| Maternal titer, median (IQR) | 5.3 (4.3–6.3) | 4.3 (2.3–5.3) | .001 |
| Cord blood titer, median (IQR) | 5.3 (4.3–6.3) | 3.3 (2.3–5.3) | <.001 |
| H1N1 | |||
| Maternal titer, median (IQR) | 5.3 (4.3–7.3) | 4.3 (3.3–5.6) | .03 |
| Cord blood titer, median (IQR) | 6.3 (4.3–7.3) | 5.3 (3.3–6.3) | .005 |
| B-Yamagata | |||
| Maternal titer, median (IQR) | 6.3 (5.3–7.3) | 5.3 (4.1–6.3) | <.001 |
| Cord blood titer, median (IQR) | 6.3 (5.3–7.3) | 5.3 (4.3–6.3) | .002 |
| B-Victoria | |||
| Maternal titer, median (IQR) | 5.3 (4.3–6.3) | 3.3 (3.3–4.3) | <.001 |
| Cord blood titer, median (IQR) | 5.3 (3.3–6.3) | 4.3 (3.3–5.3) | .003 |
When multiple samples were available, maternal titer was from the time point closest to birth.
Abbreviation: IQR = interquartile range.
Figure 1.
A, Comparison of RSV antibody in maternal (x-axis) and cord blood (y-axis) at time of delivery in Seattle, Washington, and Yukon- Kuskokwim Delta (YKD), Alaska, mother–infant pairs. When multiple samples were available, maternal titer was from the time point closest to birth. B, RSV antibody concentrations in mothers measured at 2 prenatal visits (visits 1 and 2) and in infants at delivery (cord blood) in Seattle and YKD mother–infant pairs. Abbreviation: RSV, respiratory syncytial virus.
RSV antibody concentrations at enrollment and birth in pregnant women from both sites were highly correlated (overall ρ = 0.87). Enrollment RSV antibody titers were also correlated to cord blood titers (ρ = 0.77). In the subset of women with enrollment samples collected more than 2 weeks before delivery (n = 32), the maternal RSV antibody titers at enrollment and birth remained highly correlated (ρ = 0.80), as did the maternal enrollment and infant cord blood antibody concentrations (ρ = 0.58).
Influenza Antibody
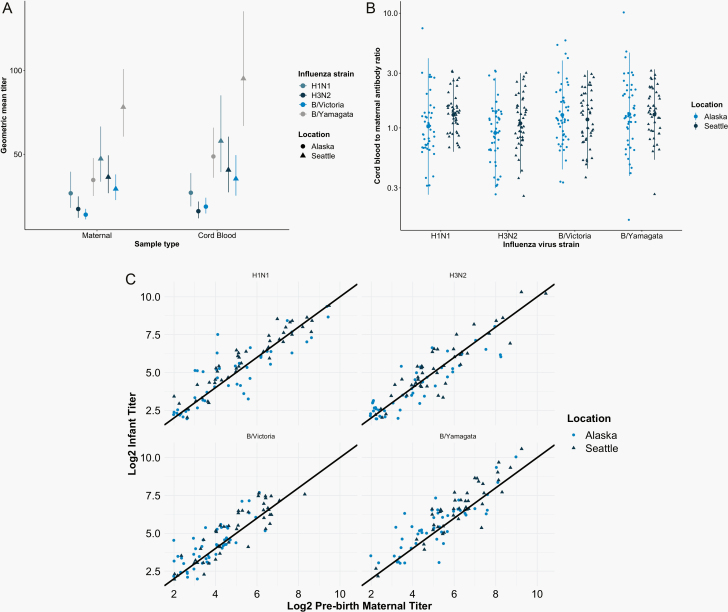
A total of 57 mother–infant pairs in Seattle and 54 mother–infant pairs in YKD were tested for influenza antibody. An additional 4 samples from Seattle and 4 samples from YKD had insufficient sera for testing. Median influenza antibody titers against influenza antigens were significantly greater in pregnant women in Seattle than in YKD (all P < .05; Table 2, Figure 2). Also, the median influenza antibody titers against all 4 influenza antigens were significantly higher in infants born to mothers in Seattle compared with AN mothers in YKD (all P < .01; Table 2, Figure 2). Importantly, the YKD cohort was enrolled in an era prior to recommended influenza vaccination during pregnancy, while the Seattle cohort was enrolled after this recommendation.
Figure 2.
A, Geometric mean titer (GMT) of hemagglutination inhibition antibodies in pregnant women and infants in Seattle, Washington, and Yukon-Kuskokwim Delta (YKD), Alaska. GMTs were measured at 2 prenatal visits (visits 1 and 2) and delivery (cord blood). Vertical bars indicate 95% CIs. B, Overall log10-transformed cord blood to maternal blood influenza antibody ratio in mother–infant pairs in Seattle and YKD for each of the 4 influenza antigens (H1N1, H3N2, B/Victoria, and B/Yamagata). Lines represent 2 standard deviations around the mean (large round dot). C, Comparison of log2 infant influenza antibody titer to prenatal maternal influenza antibody titer against H1N1 (R = 0.907), H3N2 (R = 0.904), B/Victoria (R = 0.878), and B/Yamagata (R = 0.867) antigens in mother–infant pairs in Seattle and YKD.
There was no statistically significant difference in the mean cord to maternal antibody ratios for influenza A or B between mother–infant pairs in Seattle or YKD. Both mean cord to maternal influenza A antibody ratios of mother–infant pairs in Seattle were not significantly greater than those of mother–infants pairs in the YKD cohort, with antibody ratios of 1.38 (SD = 0.51) and 1.35 (SD = 1.35) against H1N1 and 1.22 (0.53) and 1.05 (0.65) against H3N2 (all P > .1). Mean cord to maternal influenza B antibody ratios of mother–infant pairs in Seattle were not significantly less than those of mother–infant pairs in YKD, with ratios of 1.42 (SD = 0.54) and 1.59 (SD = 1.22) against B/Yamagata and 1.32 (SD = 0.58) and 1.51 (SD = 0.91) against B/Victoria (all P > .1; Table 3, Figure 2). There were no significant differences in the pairs with ratios <1.00 compared with those with ratios >1.00, and only 1 pair had ratios <1.00 for all 4 influenza strains tested (data not shown). Prenatal maternal and infant cord blood antibody titers at birth against H1N1, H3N2, B/Yamagata, and B/Victoria were strongly correlated (ρ = 0.907, 0.904, 0.878, and 0.867; Figure 2).
Table 3.
Log2 Respiratory Syncytial Virus and Log2 Influenza Antibody Cord Blood to Maternal Blood Ratios for Seattle, Washington, and Yukon-Kuskokwim Delta, Alaska, Mother–Infant Pairs.
| Antibody Ratio | Seattle, Washington | Yukon-Kuskokwim Delta, Alaska | P Value |
|---|---|---|---|
| Respiratory syncytial virus neutralizing antibody | N = 57 | N = 75 | … |
| CB:MB ratio, mean (SD) | 1.15 (0.13) | 1.04 (0.08) | <.001 |
| Influenza hemagglutination inhibition assay antibody | N = 57 | N = 53 | … |
| H3N2 | |||
| CB:MB ratio, mean (SD) | 1.22 (0.53) | 1.05 (0.65) | .17 |
| H1N1 | |||
| CB:MB ratio, mean (SD) | 1.38 (0.51) | 1.35 (1.35) | .87 |
| B-Yamagata | |||
| CB:MB ratio, mean (SD) | 1.42 (0.54) | 1.59 (1.22) | .38 |
| B/Victoria | |||
| CB:MB ratio, mean (SD) | 1.32 (0.58) | 1.51 (0.91) | .24 |
Abbreviations: CB:MB = cord blood to maternal blood ratio; SD = standard deviation.
In the YKD cohort, 8 infants were preterm or LBW (11%; Table 1), and maternal RSV and influenza antibody titers did not differ in mothers of infants born premature or LBW compared with those born term and not LBW (Supplementary Table). No infants were born preterm or LBW in the Seattle cohort.
DISCUSSION
This study characterizes RSV and influenza transplacental antibody transfer in AN mother–infant pairs in comparison to a Seattle cohort. In these 2 populations, efficient transplacental RSV and influenza antibody transfer was observed (ie, transfer ratio >1.0). RSV antibody titers in pregnant women were similar between the 2 groups, though cord blood RSV antibody titers were significantly lower in the YKD group.
AN infants have high rates of hospitalization due to influenza, and the AN population is considered a CDC priority group for influenza vaccination. Influenza vaccination during pregnancy protects the mother and the infant from influenza illness through boosting of serum and breast milk antibody titers. Importantly, timing of vaccination in the second vs third trimester does not impact efficacy in infants [20–25]. Our results are consistent with those from several previous maternal influenza vaccine studies that demonstrated transplacental antibody transfer ratios >1.00 [10, 26]. In our study, influenza antibody titers were greater in Seattle-based pregnant women than in YKD-based women across all antigens tested. This is likely because the study was performed using samples collected from AN cohorts before routine influenza vaccination for pregnant women was standard, while the Seattle cohort had high rates of influenza vaccination consistent with contemporary CDC and WHO recommendations [27]. We did find that antibody titers against influenza B were higher than for influenza A. This may reflect circulating influenza strains in the area during the surveillance period.
Maternal vaccination against RSV may protect very young infants until several months of age, when they can generate a more effective immune response to vaccination [7]. Multiple maternal RSV vaccine candidates are in clinical trials, and as with influenza vaccine, studies support the role of serum and breast milk antibody in protection of infants from severe disease [5, 28–30]. Previous studies in the AN population have examined cord blood RSV antibody titers and did not find a relationship between higher cord blood antibody titers and RSV disease incidence; however, among hospitalized infants in the YKD, lower cord blood RSV antibody titers have been associated with more severe disease [2]. Motavizumab, a monoclonal antibody against RSV, was recently studied in AN infants and was found to have efficacy against RSV-associated hospitalization. This demonstrates the role of high-titered RSV antibody in prevention of severe RSV disease in AN infants. In our study, we found that RSV antibody transfer ratios were >1.00 on average in both cohorts, though lower in the YKD compared with the Seattle cohort, and pairs with ratios <1.00 were more likely to be LBW or preterm or from YKD. Unfortunately, data on the characteristics of YKD pairs were limited and did not include data on crowding or access to running water; this limited our ability to determine whether there were additional factors associated with low transfer ratios. The ratios observed in YKD were similar to those from studies conducted by our group in Nepal and Bangladesh—populations with shared risk factors for severe RSV disease, such as household crowding and lack of running water [14, 15]. The difference in the log2 RSV antibody concentrations between infants born in Seattle and YKD was 1.2, which likely was clinically meaningful, as Piedra et al showed that an increased log2 1.0 titer decreased the risk of hospitalization [29].
Potential causes of different transfer ratios for influenza and RSV were not explained by LBW, preterm birth, or seasonality. Another potential cause could be differences in neonatal fc gamma receptor binding between the 2 pathogens [31]. To test this, our group is working to use systems serology profiling to understand the role of antibody glycosylation, subtype, and subclass in antibody transfer by pathogen.
Study limitations include differences between cohorts in the timing of sample collection during pregnancy and that the samples were collected in different years in the 2 cohorts. However, previous studies have shown that RSV neutralizing antibody levels are stable during pregnancy [15], and for Seattle women in our study with multiple levels checked, the mean change in titer was 0 (SD = 0.79). Additional limitations included lack of measurement of epitope-specific antibodies, such as RSV prefusion antibody, which may be a more specific correlate of protection than RSV neutralizing antibody [32]. Furthermore, for influenza, the HAI assay may not be the optimal correlate of protection against influenza in adults or infants. In other studies, neuraminidase or microneutralization assays have been shown to be independent correlates of protection against influenza [33]. Also, no data were collected on either group regarding chronic inflammation or other systemic conditions that may differentially impair antibody transfer by subclass. Last, some women had blood collected more than 2 weeks prior to the time of delivery, which may limit comparisons between groups.
Overall, we find that compared with a healthy population of mother–infant pairs in Washington state, AN infants have significantly lower cord blood RSV antibody concentrations. Since many factors are linked to severe disease in AN infants, these results warrant further studies to understand the specific contribution and impact of RSV and influenza transplacental antibody transfer. Furthermore, this helps identify a high-risk population who would benefit from prioritization for studies of RSV and influenza vaccination during pregnancy or other novel approaches for protection of newborns, such as long-acting passive antibody [34].
Supplementary Material
Notes
Human participant approval was obtained from the Seattle Children’s Institutional Review Board, the Alaska Native Tribal Health Consortium, the Centers for Disease Control and Prevention (CDC) Arctic Investigations Program, the Yukon-Kuskokwim Delta Regional Hospital, and the University of Washington.
Acknowledgments. We acknowledge and thank the Northwest Hospital Midwives Clinic, Northwest Hospital and Medical Center, for their participation in the Seattle, Washington, study.
Author contributions. Conceptualization (H. Y. C., J. A. E., R. S.); data curation (all authors); formal analysis (H. Y. C., K. L. N., S. C., R. S.); funding acquisition (H. Y. C.); investigation (all authors); methodology (H. Y. C., K. L. N., J. A. E., R. S.); project administration (H. Y. C., K. L. N., R. S.); resources (H. Y. C.); software (H. Y. C., K. L. N., S. C.); supervision (H. Y. C., J. A. E., R. S.); validation (H. Y. C., K. L. N.); visualization (H. Y. C., K. L. N., S. C.); roles/writing the original draft (H. Y. C., K. L. N., J. A. E., S. C., K. C., K. L., R. S.); and writing, review and editing (all authors).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH; K23-AI103105 to H. Y. C.) and the National Center for Advancing Translational Sciences of the NIH under UL1 TR002319.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karron RA, Singleton RJ, Bulkow L, et al. Severe respiratory syncytial virus disease in Alaska native children. RSV Alaska Study Group. J Infect Dis 1999; 180:41–9. [DOI] [PubMed] [Google Scholar]
- 3. Gounder PP, Callinan LS, Holman RC, et al. Influenza hospitalizations among American Indian/Alaska native people and in the United States general population. Open Forum Infect Dis 2014; 1:ofu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bulkow LR, Singleton RJ, Karron RA, Harrison LH; Alaska RSV Study Group . Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics 2002; 109:210–6. [DOI] [PubMed] [Google Scholar]
- 5. Glezen WP, Paredes A, Allison JE, et al. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 6. Groothuis JR, Nishida H. Prevention of respiratory syncytial virus infections in high-risk infants by monoclonal antibody (palivizumab). Pediatr Int 2002; 44:235–41. [DOI] [PubMed] [Google Scholar]
- 7. Chu HY, Englund JA. Maternal immunization. Clin Infect Dis 2014; 59:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shakib JH, Korgenski K, Presson AP, et al. Influenza in infants born to women vaccinated during pregnancy. Pediatrics. 2016; 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Englund JA, Mbawuike IN, Hammill H, et al. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis 1993; 168:647–56. [DOI] [PubMed] [Google Scholar]
- 10. Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy–antibody responses in mothers and infants. N Engl J Med 2010; 362:1644–6. [DOI] [PubMed] [Google Scholar]
- 11. Englund JA, Chu HY. Vaccines against respiratory syncytial virus: the time has come. J Infect Dis 2017; 215:4–7. [DOI] [PubMed] [Google Scholar]
- 12. Glenn GM, Smith G, Fries L, et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine 2013; 31:524–32. [DOI] [PubMed] [Google Scholar]
- 13. Suara RO, Piedra PA, Glezen WP, et al. Prevalence of neutralizing antibody to respiratory syncytial virus in sera from mothers and newborns residing in the Gambia and in the United States. Clin Diagn Lab Immunol 1996; 3:477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu HY, Tielsch J, Katz J, et al. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol 2017; 95:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu HY, Steinhoff MC, Magaret A, et al. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother–infant pairs in Bangladesh. J Infect Dis 2014; 210:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray AF, Englund JA, Tielsch JM, et al. Measles and rubella seroprevalence in mother–infant pairs in rural Nepal and the United States: pre- and post-elimination populations. Am J Trop Med Hyg 2018; 99:1342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruden DJ, Singleton R, Hawk CS, et al. Eighteen years of respiratory syncytial virus surveillance: changes in seasonality and hospitalization rates in southwestern Alaska native children. Pediatr Infect Dis J 2015; 34:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDonald JU, Rigsby P, Dougall T, Engelhardt OG; Study Participants . Establishment of the first WHO international standard for antiserum to respiratory syncytial virus: report of an international collaborative study. Vaccine 2018; 36:7641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization Collaborating Center for Surveillance EaCoI. The 2017–2018 WHO Influenza Reagent Kit for Identification of Influenza Isolates. Atlanta, GA: Centers for Disease Control; 2018. [Google Scholar]
- 20. Steinhoff MC, Katz J, Englund JA, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017; 17:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tapia MD, Sow SO, Tamboura B, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016; 16:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madhi SA, Cutland CL, Kuwanda L, et al. ; Maternal Flu Trial Team . Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371:918–31. [DOI] [PubMed] [Google Scholar]
- 23. Nunes MC, Cutland CL, Jones S, et al. ; Maternal Flu Trial Team . Duration of infant protection against influenza illness conferred by maternal immunization: secondary analysis of a randomized clinical trial. JAMA Pediatr 2016; 170:840–7. [DOI] [PubMed] [Google Scholar]
- 24. Schlaudecker EP, Steinhoff MC, Omer SB, et al. IgA and neutralizing antibodies to influenza A virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One 2013; 8:e70867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–64. [DOI] [PubMed] [Google Scholar]
- 27. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eick A, Karron R, Shaw J, et al. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J 2008; 27:207–12. [DOI] [PubMed] [Google Scholar]
- 29. Piedra PA, Jewell AM, Cron SG, et al. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003; 21:3479–82. [DOI] [PubMed] [Google Scholar]
- 30. Mazur NI, Horsley NM, Englund JA, et al. Breast milk prefusion F immunoglobulin G as a correlate of protection against respiratory syncytial virus acute respiratory illness. J Infect Dis 2019; 219:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jennewein MF, Goldfarb I, Dolatshahi S, et al. Fc glycan-mediated regulation of placental antibody transfer. Cell 2019; 178:202–15.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ngwuta JO, Chen M, Modjarrad K, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7:309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monto AS, Petrie JG, Cross RT, et al. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis 2015; 212:1191–9. [DOI] [PubMed] [Google Scholar]
- 34. Domachowske JB, Khan AA, Esser MT, et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion f-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J 2018; 37:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.