Abstract
The development of multidrug‐resistant bacteria has revealed the need for new antimicrobial compounds. Cannabis sativa preparations have a long history of medical applications, including the treatment of infectious diseases. This review collects the information about the activity of C. sativa extracts and its main components (cannabinoids and terpenes) against pathogenic bacteria and fungus, to assess its potential using as antimicrobial agents.
Keywords: antimicrobial activity, cannabinoids, Cannabis sativa, terpenes
Cannabis sativa preparations have a long history of medical applications, including the treatment of infectious diseases. This review collects the information about the activity of C. sativa extracts and its main components (cannabinoids and terpenes) against pathogenic bacteria and fungus. All the data presented in this work suggests that cannabinoids and related compounds demonstrated antibacterial activity against clinically important bacteria in the search of new tools as potential antimicrobial agents.
Abbreviations
- AEA
anandamide
- AraS
arachidonoyl serine
- BCE
before the common era
- CB1 and CB2
cannabinoid receptors type 1 and type 2
- CBC
cannabichromene
- CBCA
cannabichromene acid
- CBD
cannabidiol
- CBDA
cannabidiol acid
- CBDV
cannabidivarin
- CBE
cannabielsoin
- CBG
cannabigerol
- CBGA
cannabigerol acid
- CBL
cannabicyclol
- CBN
cannabinol
- CBND
cannabinodiol
- CBT
cannabitriol
- EOs
essential oils
- IL‐6
interleukin 6
- LPS
lipopolysaccharide
- MBC
minimum bactericidal concentration
- MIC
minimum inhibitory concentration
- MRSA
Methicillin‐Resistant Staphylococcus aureus
- MRSE
Methicillin‐Resistant Staphylococcus epidermidis
- MSSA
Methicillin‐Sensible Staphylococcus aureus
- OEA
oleoyl ethanolamide
- THCA
tetrahydrocannabinol acid
- TLR4
Toll‐like receptor 4
- TNF‐α
tumor necrosis factor α
- Δ8‐THC
Δ8‐tetrahydrocannabinol
- Δ9‐THC
Δ9‐tetrahydrocannabinol
- Δ9‐THCV
tetrahydrocannabivarin
1. INTRODUCTION
Bacterial resistance to antimicrobial therapy is a major concern all over the world. Infectious disease is the second leading cause of death resulting in 17 million people dying each year from bacterial infections. 1 The number of resistant organisms to one or more classes of antibiotics is unprecedented. There are more than 15 classes of antibiotics that target different structures of the bacterial cell and none has escaped a resistance mechanism. 2 The lack of alternatives to mitigate the antimicrobial resistance is based on the low number of new antibiotics developed and approved over the past decades. 3 In this scenario, alternatives strategies must be explored to mitigate resistant bacterial strains. 4 Historically, plants have been used as sources of natural products for human health, and many plant extracts and phytochemicals have shown antimicrobial properties against pathogens, including clinical resistant bacterial strains. 5 , 6 In some cases, the antibacterial activity of plant extracts was corroborated in animal models of infection. 7 , 8 Besides, synergistic effects could be achieved when plant extracts or their bioactive constituentsare combined with antibiotics to antagonize bacterial resistance mechanisms. 9
Cannabis sativais a herbaceous annual plant that has been used for centuries for textile, food, medical, recreational, and religious proposes. 10
The medicinal use of Cannabis has been well documented in many cultures. For example, the first evidence of medical use is attributed to the Chinese Emperor Shen Nung (2700 BCE) and the indications for Cannabis use included rheumatic pain, intestinal constipation, menstrual irregularities, and malaria among others. 11 In an Assyrian culture (1800 BCE) Cannabis was first used medicinally for grief, epilepsy, neuralgia, and pediculosis, 12 and in Ancient Egypt (1700 BCE) was used as a treatment for the eyes (possibly as an antiinflammatory agent). 13 In India, the earliest mention of Cannabis is in the Atharvaveda (1500 BCE) as a part of the mixture to release from anxiety. 14
Regarding the potential antimicrobial effects of Cannabis extracts, reports have been found from Egyptian papyruses, African natives, and European and South America folk medicine pointing the use as antiseptic to treat wounds and furuncles and to treat conditions such as erysipelas, anthrax, sepsis, dysentery, and malaria. 15
In the 19th century, the western medicine adopted the use of Cannabis with the works of Willian B. O’Shaughnessy and Jacques‐Joseph Moreau, describing the utility of Cannabis for rheumatism, convulsions, and muscular spasms of tetanus and rabies, and to treat mental illness respectively. 11 In 1851, Cannabis extract was listed in the United States Pharmacopeia and several medical articles were published in the United States and Europe. 16 However, despite the medical benefits of cannabis, its psychoactive effects and its use as a recreational intoxicant, made Cannabis a banned substance for therapeutic use from the British Pharmacopeia in 1932 10 and the US Pharmacopeia in 1941. 16
Since the structural elucidation and synthesis of Δ9‐tetrahydrocannabinol (Δ9‐THC) and cannabidiol (CBD) in the 1960s, the discovery of the endocannabinoid system (cannabinoids receptors, endogenous ligands, and enzymatic machinery) in the 1990s and the recognition of the role of the endocannabinoid system in health and disease, the scientific interest for Cannabis has increased significantly. 11 , 17 However, the utilization of Cannabis sativa and its active compounds against pathogenic bacteria had not taken the attention of the scientific community as compared to other medical applications such as antipsychotic, antiepileptic, anxiolytic, neuroprotective, anti‐inflammatory, analgesic, antiemetic, antidiabetic, and anticancer. 18 Nevertheless, recently some new developments related to the antimicrobial effects of cannabinoids have been published, repositioning Cannabis extracts and related compounds as potential antimicrobial agents.
This article aims to review the effect of Cannabis extracts and its outstanding active compounds (mainly cannabinoids and terpenes) against clinically important bacteria.
2. CANNABIS CHEMICAL COMPOSITION
C. sativa is a dioecious flowering plant with a complex chemical composition. 19 To the date, it has been reported a total number of 565 natural constituents, 120 of which correspond to the cannabinoid class. 20 The rest of Cannabis phytochemicals include primary metabolites such as amino acids, fatty acids, and steroids or secondary metabolites as terpenoids, flavonoids, stilbenoids, lignans, and alkaloids among others. 21
The term cannabinoid includes the compounds isolated from Cannabis plants (also named phytocannabinoids), the pharmacologically analogous synthetic cannabinoids, and the endogenous cannabinoids receptor ligands named endocannabinoids (Figure 1). 22
FIGURE 1.
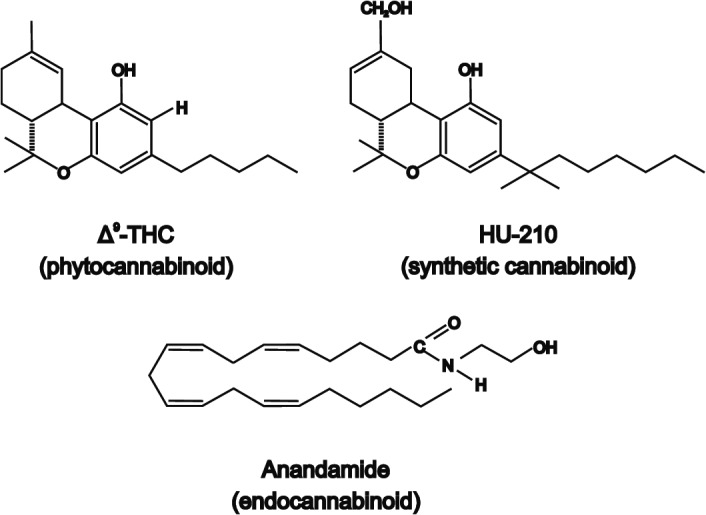
Schematical structure of the three different types of cannabinoids: endocannabinoids, phytocannabinoids, and synthetic cannabinoids. Adapted from [19]
Cannabinoids are a C21 terpene phenolic group of compounds, including their analogs and transformation products, predominantly produced by C. sativa. 23 Cannabinoids have been detected in different parts of the plant, however, they largely accumulate in the secretory cavity of the glandular trichomes of the female flowers. 24 In fresh Cannabis plants, cannabinoids are biosynthesized in their acid forms (carboxylated form) while during storage and smoking they are non enzymatically decarboxylated into their neutral forms. 25
Cannabinoids can be divided into 11 major structural types (Figure 2): Δ9‐tetrahydrocannabinol (Δ9‐THC), Δ8‐tetrahydrocannabinol (Δ8‐THC), cannabigerol (CBG), cannabichromene (CBC), cannabidiol (CBD), cannabinodiol (CBND), cannabielsoin (CBE), cannabicyclol (CBL), cannabinol (CBN), cannabitriol (CBT) and miscellaneous types. 20
FIGURE 2.
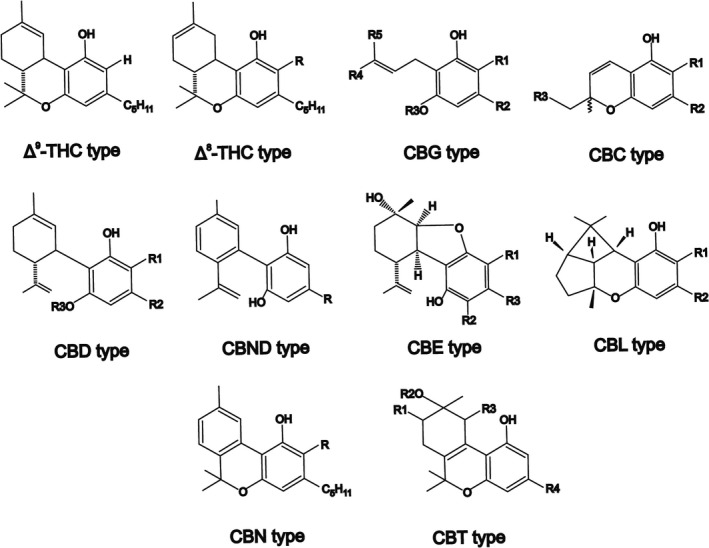
Major structural types of cannabinoids from C. sativa. Δ9‐tetrahydrocannabinol (Δ9‐THC), Δ8‐tetrahydrocannabinol (Δ8‐THC), cannabigerol (CBG), cannabichromene (CBC), cannabidiol (CBD), cannabinodiol (CBND), cannabielsoin (CBE), cannabicyclol (CBL), cannabinol (CBN), cannabitriol (CBT). Adapted from [19, 23]
Δ9‐THC, cannabinol (CBN), CBD, and cannabichromene (CBC) are the most abundant phytocannabinoids in Cannabis. 26 The acid form of cannabigerol (CBG), the cannabigerolic acid (CBGA), is the common precursor of tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA). 27 CBG appears as a relatively low concentration intermediate in the cannabis plant and among its suggested properties are anti‐proliferative, muscle relaxant, antidepressant, and analgesic effects. 28 Δ9‐THC is the main psychoactive constituent of Cannabis sativa and it was first identified, isolated, and synthesized by Gaoni and Mechoulam in 1964. 29 According to the phytocannabinoids profile, Cannabis sativa is divided into different chemical phenotypes where Δ9‐THC is the predominant cannabinoid in the drug‐type plants. 30 Δ9‐THC interacts with endogenous cannabinoid receptors types 1 and 2 (CB1 and CB2) and produces psychoactivity, analgesic, muscle relaxant, and antispasmodic effects. 31 CBN is the product of Δ9‐THC oxidation, therefore is a minor component of fresh Cannabis and exerts a weak CB1 and CB2 partial agonist. 18 CBC concentrations have been found higher in vegetative stage of cannabis and its appearance is related to the presence of Δ9‐THC. 32 CBC showed an antiinflamatory and antinociceptive activity and exerts a CB2 agonism 33 Meanwhile, CBD is the major non‐psychotropic constituent of the fiber‐type varieties of Cannabis sativa. 34 CBD has shown a variety of pharmacological effects and potential therapeutic applications that have taken the attention of many researchers in different areas. Within CBD applications are included inflammatory and neurodegenerative diseases, epilepsy, pain, anxiety, multiple sclerosis, and cancer among others 35 .
There are over 200 terpenes and terpenoids in Cannabis. 36 Terpenes are relatively volatile compounds with an isoprene structure while terpenoids are organic chemicals similar to terpenes but contain oxygen as an additional element in their composition. 37 Terpenes are divided into five classes namely monoterpenes (C10H16), sesquiterpenes (C15H24), diterpenes, triterpenes, and miscellaneous compounds of terpenoid origins. 38 Terpenes are the primary active components of essential oils (EOs) and exert their pharmacological activity through the interaction with different cell structures (i.e., cell membranes, ion channels, neurotransmitter receptors, second messenger systems, and enzymes). 28 Myrcene, alpha‐pinene, beta‐pinene, limonene, beta‐caryophyllene, alpha‐terpinolene, and alpha‐humulene are among the main components in Cannabis EOs (Figure 3). 39 , 40
FIGURE 3.
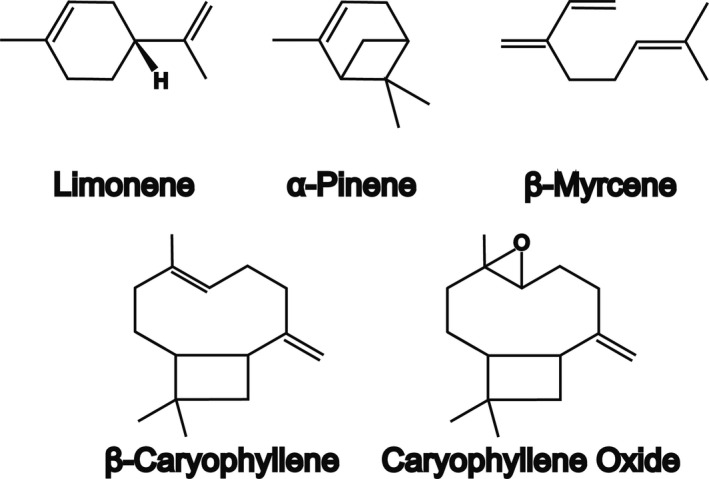
Chemical structure of some terpenes found in Cannabis sativa. Adapted from [28]
Monoterpenes, sesquiterpenes, and cannabinoids share a common precursor and most of them also concentrate in the glandular trichomes of Cannabis but generally, the content of terpenes present is smaller than cannabinoids one. 41 This content varies between different cannabis chemical phenotypes, but it can represent between 12.6% and 31.5% and 0.6%–3.3% of cannabis flower dry weight for cannabinoids and terpenes respectively. 42
Other chemical constituents identified in Cannabis include 50 hydrocarbons, 34 sugars, and related compounds, 27 nitrogenous compounds, 25 non‐cannabinoids phenols, 23 flavonoids, 18 amino acids, 13 simple ketones, 12 simple aldehydes, 12 simple esters, 11 steroids, 7 simple alcohols, 6 enzymes, 3 proteins, 2 pigments, 1 vitamin and 9 other elements. 23
As mentioned above, the complex mixture of chemical compounds within C. sativa makes it difficult to recognize which is the main component behind the antimicrobial effects. Thus, we will briefly describe the antimicrobial reports of different Cannabis extracts and, when is possible, we will focus on the activity of single components related to Cannabis such as cannabinoids and terpenoids.
3. IN VITRO ANTIMICROBIAL EFFECT OF C. Sativa DERIVATES
Whole Cannabis plant and leaves extracts, EOs, seed oils as well as isolated components such as cannabinoids have shown antimicrobial effects against pathogenic bacteria and fungus. 43 Alkaloids, flavonoids, peptides, tannins, and phenols are also known for their antimicrobial properties 37 and many of them are found in C. sativa. 23 Thus, many compounds present in Cannabis extracts could contribute to antimicrobial activity in addition to or synergistic manner.
The antimicrobial in vitro activity of different C. sativa derivatives and its related compounds are commented below and are summarized in Table 1.
TABLE 1.
Antimicrobial in vitro activity of C. sativa derivatives and related compounds
| Plant extract/isolate | Antimicrobial activity test | Outstanding antimicrobial activity | Mild or no antimicrobial activity | Cite | |
|---|---|---|---|---|---|
| Cannabis extracts | Different parts of Cannabis sativa var. indica extracts obtained with different solvents | Disc diffusion method | Antibacterial effect upon Gram‐positive bacteria: Staphylococcus aureus, Streptococcus alpha haemolyticus, Streptococcus beta haemolyticus, Enterococcus, Diplococcus pneumoniae, Bacillus subtilis, Bacillus anthracis, Corynebacterium diphtheriae, Corynebacterium cutis,Erysipelothrix rhusiopathiae Clostridium perfringens and Mycobacterium tuberculosis | Absence of activity against Escherichia coli, Salmonella typhi, Salmonella paratyphi B, Shigella Shigae, Pseudomona aeruginosa, Proteus vulgaris, fungi and yeast. | 15 |
| Aqueous, ethanolic and petroleum ether extracts of the leaves of Cannabis sativa L. and isolated acidic fraction from ethanolic extract and 2% Sodium Hydroxide extract |
Agar diffusion method Control: cephalexin 0,02 mg/ml and nystatin 0,1 mg/ml |
Ethanolic and petroleum ether extracts and the isolated acidic fractions showed marked activity against Gram‐positive bacteria (B. subtilis, Bacillus pumilus, S. aureus and Micrococcus flavus), Gram negative bacteria (P. vulgaris and Bordetella bronchiseptica) and fungi (Candida albicans and Aspergillus niger) at 1,5 and 10 mg/ml | Aqueous extract showed no activity against tested microorganism | 44 | |
| Cannabis sativa L. extracts from leaves and stems |
Disc diffusion method. Control: ticarcillin 75 µg and Chloranphenicol 30 µg |
Good antimicrobial activity against S. aureus ATCC 12600. | No activity against E. coli (ATCC 8677), P. aeruginosa (ATCC 9721) and C. albicans | 45 | |
| Oil of the seeds, petroleum ether and methanol extracts of the whole plant of Cannabis sativa |
Agar diffusion method. Control: Ampicillin, Benzyl penicillin, Cloxacillin and Gentamicin (5, 10, 20 and 40 µg/ml), Clotrimazole (5, 10 and 20 µg/ml) and nystatin (12,5, 25 and 50 µg/ml) MIC (agar plate dilution method) |
The oil of the seeds and the petroleum ether extract exerted pronounced antibacterial activity against B. subtilis and S. aureus. The oil of seeds exhibited high activity against P. aeruginosa and moderate activity against E. coli , while the petroleum ether extract exhibited high activity against E. coli. The methanol extract of the whole plant showed also pronounced antibacterial activity against B. subtilis and high activity against E. coli and P. aeruginosa. MIC of seeds methanol extract: 25 µg/ml for E. coli and S. aureus; 50 µg/ml for P. aeruginosa. MIC of whole plant methanol extract: 12,5 µg/ml for P. aeruginosa, 25 µg/ml E. coli and 50 µg/ml S. aureus. |
The oil of seed was inactive against A. niger and C. albicans. The petroleum ether extract of the whole plant was inactive against P. aeruginosa and both fungi. The methanol extract of the whole plant showed low activity against S. aureus and C. albicans and inactive against A. niger. |
46 | |
| Aquous and acetone extracts of leaves of Cannabis sativa |
Disc diffusion method. Control: not reported. |
Both extracts exherted a inhibition zone (from 3 to 12 mm depending on the concentration and the microorganism) against P. aeruginosa, Vibro cholerae, Cryptococcus neoformans and C. albicans | Not reported | 47 | |
| Acetone, methanol, ethanol and aqueous leaves extracts of Cannabis sativa |
Agar well diffusion method. Control: Ciprofloxacin and Anphotericin B MIC determination by modified agar well diffusion method |
Methanolic extract showed marked antibacterial activities against B. subtilis and S. aureus and moderate against E. coli. The rest of the extracts showed moderate antibacterial activity specially agains Gram‐positive bacteria. Methanol extract was the most active showing MIC of 1,56 mg/ml against S. aureus and B. subtillis. |
The extracts showed no activity against P. aeruginosa, Saccharomyces cerevisiae and C. albicans. E. coli was the least sensitive with MIC of 50 mg/ml |
48 | |
| Cannabis indica leaves, stems and seeds extracts |
Agar well diffusion method Control: levofloxacin and clotrimazole (5 µg/mcL) |
The methanol extract of leaves was the most active against S. aureus, B. cereus, Klebsiella pneumoniae and Proteus mirabilis. P. aeruginosa was generally more sensible to the leaves extracts. The extracts of plant leaves, seeds and stems showed antifungal activities against A. niger, Aspergillus parasiticus, and Aspergillus oryzae |
E. coli, P. mirabilis and C. Albicans showed less sensitivity to the extracts. All strains showed no inhibition zone to at least one plant extract. |
49 | |
| Leaf extracts of Cannabis sativa |
Disc diffusion method. Control: penicillin, methicillin, cefoxitin and vancomycin |
Zones of inhibition against clinical and non‐clinical isolates of Methicillin Resistant S. aureus (MRSA). Synergism with other plants. | Not reported. | 50 | |
| Cannabis sativa L. seed extracts |
MIC well dilution method. Control: gentamycin and vancomycin |
Selective antimicrobial activity against pathogenic strains (Enterobacter aerogenes ATCC, Salmonella enterica ser. Typhimurium ATCC 14028, E. coli ATCC 25922, S. aureus ATCC 25923 and Enterococcus faecalis ATCC 29212) and no inhibitory effects on the growth of probiotic strains (Lactobacillus paracasei MB395, Lactobacillus reuteri DSM 20016, Lactobacillus brevis ATCC 14869, Lactobacillus plantarum MB91, Bifidobacterium bifidum B2009, Bifidobacterium longum Re11 and Bifidobacterium breve B632). MIC values of 1 mg/ml for E. coli ATCC 25922, S. typhimurium ATCC 14028, S. aureus ATCC 25923 and E. faecalis ATCC 29212 |
E. aerogenes ATCC 13048 MIC 2.5 mg/ml. | 51 | |
| S. aureus viability by a double‐staining fluorescence assay. Biofilm production and inhibition (crystal violet assay) | Reduction of cells viability and biofilm formation of S. aureus | Concentration lower than 0.5 mg/ml not completely inhibited the biofilm formation. | |||
| Cannabis EOs | EOs of five different cultivars of Cannabis sativa | Agar well diffusion method | Modest antimicrobial activity against some of the strains mentioned next: Acinetobacter calcoaceticus, Aeromonas hydrophyla, B. subtilis, Beneckea natriegens, Brevibacterium linens, Brochothrix thermosphacta, E. coli , Flavobacterium suaveolens, Yersinia enterocolitica, Micrococcus luteus and S. aureus | No activity against Alcaligenes faecalis, Citrobacter freundii, Enterobacter aerogenes, Erwinia carotovora, Klebsiella pneumoniae, Moraxella spp., Proteus vulgaris, Salmonella pullorum, Serratia marcescens and Streptococcus faecalis | 39 |
| EOs from three hemp‐type varieties of Cannabis sativa (Carmagnola, Fibranova and Futura) | MIC and MBC by broth dilution method |
Activity against Enterococcus hirae, Enterococcus faecium and Streptococcus salivarius subsp. thermophilus. The variety Futura also exhibited good activity against Clostridium spp., Gram‐negative bacteria and partially inhibited yeast growth. MBC of Futura EO was twice higher its MIC. |
Carmagnola and Fibranova EO MIC were above the detection limit for many Clostridia strains. The essential oils were unable to inhibit S. cerevisiae, and Torulospora delbrueckii and Zygosaccharomyces bailii (except Futura). |
40 | |
| EO from Cannabis Sativa variety Futura 75 |
Kirby–Bauer disc diffusion Method. Control: erythromycin 15 µg, tetracycline 30 µg, netilimicin 30 µg, levofloxacin 5 µg, cefoxitin 30 µg, linezolid 10 µg, rifampicin 30 µg, and gentamicin 10 µg MIC and MBC, Planktonic Susceptibility Assay Minimum Biofilm Eradication Concentration and Biofilm Eradication Assay and others. |
Antimicrobial and antibiofilm effects against five S. aureus strains (ATCC 29213 and clinical strains) and antibacterial effect against Helicobacter pylori. For S. aureus MIC values of 8 µg/ml and MBC values of 16 µg/ml. Minimum biofilm eradication concentration (MBEC) between 16 and 24 µg/ml. For H. pylori MIC 32 µg/ml |
Against Candida spp. and Malassezia spp. MIC value above 12,460 µg/ml | 66 | |
| EO from Cannabis Sativa variety Futura 75 | MIC and MBC by microdilution method, motility assay, flagela stain, electron microscopy examination, biofilm formation and others. |
Antibacterial activity against Listeria monocytogenes. MBC >2048 μg/ml Reduced flagelar motility, reduction in biofilm production among others |
Not reported | 67 | |
| Seventeen EOs from different fibre‐type varieties of Cannabis sativa |
Agar Well Disk Diffusion Assay. Control: ampicillin and ciprofloxacin MIC by microwell dilution method |
Good antibacterial activity of six EOs against the Gram‐positive bacteria (Staphylococcus spp., Enterococcus spp., L. monocytogenes and Bacillus spp.) |
Antimicrobial activity and MIC variable between strains and EOs. The majority of hemp EOs did not exploit any antibacterial activity on Staphylococcus bacterial strains. One EO showed no activity against Listeria strains |
53 | |
| Isolated cannabinoids | THC and CBD |
MIC by dilution method. Bacteriostatic and bactericide action against S. aureus by determination of number of living cells by standard pour‐plate method |
Antibacterial activity against S. aureus and Streptococcusspp. MIC values in the range of 1–5 µg/ml. THC and CBD resulted both bacteriostatic and bactericidal against S. aureus. |
Less activity in presence of horse blood in the agar. E. coli , S. typhi and P. vulgaris MIC >100 µg/ml. |
68 |
| CBC, homologs and isomers |
Agar well diffusion assay MIC by two‐fold serial dilution method. Control: streptomycin and anphotericin B. |
Strong antibacterial activity against B. subtillis, S. aureus and Mycobacterium smegmatis and mild to moderat activity against Trichophyton mentagrophytes | Poor to none activity agaist E. coli, P. aeruginosa, C. albicans, S. cerevisiae and A. niger | 26 | |
| CBC, analogs, homologs and isomers, CBG, homologs and isomers, Δ9‐THC, Δ8‐THC, CBD, CBN and CBL |
Agar well diffusion assay MIC by two‐fold serial dilution method Control: streptomycin and anphotericin B. |
CBC and CBG and theirs isomeres and homologs were the most active compounds against Gram‐positive bacteria and T. mentagrophytes | Mild inhibitory activity against Gram‐negative bacteria (E.coli and P. aeruginosa) and fungi | 69 | |
| CBD, CBC, CBG, THC and CBN, and related compunds |
MIC by plates dilution method. Control: norfloxacin, erythromycin, tetracycline and oxacillin. |
All major cannabinoids showed potent antibacterial activity against MRSA, with MIC values in the 0.5–2 μg/ml range. | Some compounds exhibited MIC values above 128 µg/ml and 256 µg/ml. | 72 | |
| (±)−4‐acetoxycannabichromene; (±)−3″‐hydroxy‐Δ(4″,5″)‐cannabichromene; (−)−7‐hydroxycannabichromene; (−)−7R‐cannabicoumarononic acid A;5‐acetyl−4‐hydroxycannabigerol; 4‐acetoxy−2‐geranyl−5‐hydroxy−3‐n‐pentylphenol; 8‐hydroxycannabinol; 8‐hydroxycannabinolic acid A and 2‐geranyl−5‐hydroxy−3‐n‐pentyl−1,4‐benzoquinone |
IC50determination. Control: ciprofloxacin and anphotericin B. |
4‐acetoxy‐2‐geranyl‐5‐hydroxy‐3‐n‐pentylphenol showed antibacterial activity againts S. aureus and MRSA, 8‐hydroxycannabinol showed antifungal activity against C. albicans) and 5‐acetyl‐4‐hydroxycannabigerol showed antileishmanial activity (Leishmania donovani) | The rest of the compounds tested showed weak antimicrobial activity. | 70 | |
| CBD |
Transmission electron miscrocopy, Western Blotting, proteomics, Disc diffusion test and others. Control: colistin (10 μg/ml), rifampicin (15 μg/ml), erythromycin (50 μg/ml), kanamycin (1,000 μg/ml) and vancomycin (5 μg/ml). |
Inhibition of membrane vesicule release from E. coli VCS257 CBD alone at 5 µM disminuyed E. coli cell viability CBD enhanced antibacterial effect of erythromycin and rifampicin against E. coli VCS257and increased the antibacterial effect of kanamycin against S. aureus subsp. aureus Rosenbach. |
Negligible inhibition of membrane vesicule release from S. aureus. CBD had no effect on S. aureus cell viability. CBD did not enhance bactericidal activity in the other combinations tested and reduced antibacterial effects of erythromycin and rifampicin against S. aureus. |
77 | |
| CBD, CBC. CBN, CBG and CBGA |
Colony count reduction. Control: comercial oral care products |
Reduced the bacterial colony count in dental plaque. | Not reported | 71 | |
| CBC, CBD, CBG, CBN, THC and precursors | Susceptibility test, MIC, biofilm formation with static abiotic solid‐surface assays, studies of mechanism and others. |
CBG, CBD, CBN, CBCA and THC MIC 2 µg/ml against MRSA. CBG MIC904 µg/mlfor MRSA isolates Antibacterial and antibiofilm activity of cannabinoids against MRSA. CBG targeted the cytoplasmic membrane of Gram‐positive bacteria and Gram‐negative bacteria (whose outer membranewas permeabilized). The combination of cannabinoids with polymyxin B was effective against multi‐drug resistant Gram‐negative pathogens. |
11‐nor−9‐carboxy‐Δ9‐THC, and 11‐hydroxy‐Δ9‐THC and cannabicylol were inactive against MRSA (MIC >32 µg/ml) MICs >128 μg/ml for E.coli |
73 | |
| CBD |
MIC determination Transmission electron microscopy, composition of peptidoglycan in HPLC chromatogram, qPCR and others. |
CBD MIC 4 µg/ml for MRSA, L. monocytogenes and MRSE and 8 µg/ml for E. faecalis. CBD potentiated the effect of bacitracinagainst Staphylococcus species, L. monocytogenes andE. faecalis. The combination CBD and bacitracin induced septa formations during cell division and membrane irregularities, and reduced the expression of the gene ezrAin S. aureus. |
MIC >128 µg/ml for P. aeruginosa, Salmonella typhimurium, K. pneumoniae, and E. coli. No synergims was found between CBD and bacitracin against Gram‐negative bacteria. CBD and bacitracin alone caused no morphological changes in S. aureus. In addition, CBD or combinated with bacitracin did not cause changes in the cell wall composition. |
78 | |
| CBCA and related synthetic analogues | MIC determination, time‐kill analysis, phase‐contrast and fluorescence microscopy | CBCA MIC 3,9 µM for MRSA, 7,8 µM for MSSA and 7,8 µM for vancomycin‐resistant E. faecalis. Potent and rapid bactericidal activity of CBCA against MRSA with low and high cell density. Bacterial degeneration and cell lysis of B.. subtilis due to membrane and nucleoid alteration. |
Synthetic CBCA analogues showed no antibacterial activity (with the exception of CBDVM against MRSA) In time‐kill analysis, the concentration of viable bacteria increased more than 5‐log after 24 h treatment with CBCA. |
76 | |
| CBD and CBDA | MIC determination by broth microdilution method, time‐kill analysis for S. aureus and synergy test with CBD and conventional antibiotics (clindamycin, ofloxacin, meropenem, tobramycin, teicoplanin, methicillin and vancomycin) on S aureus using checkboard method. | CBD MIC 1 µg/ml for S. aureus ATCC 25923 and MRSA (USA 300) and 2 µg/ml for S. epidermidis (CA71 and ATCC51625). CBDA MIC 2 µg/ml for S. aureus ATCC 25923 and 4 µg/ml for MRSA and S. epidermidis. CBD exherted rapid killing effect against S.aureus ATCC 25923 and MRSA. | Neither CBD or CBDA showed inhibitory activity against E. coli ATCC 25922 and P. aeruginosa PA01. In synergy test, CBD showed an indifferent effect with the antibiotics tested. | 75 | |
| Endocannabinoids and endocannabinoid‐like compounds | Anandamide (AEA) and arachidonoyl serine (AraS) |
MIC by standard broth microdilution method. Control: gentamycin. Biofilm formation by crystal violet staining, MBC, cell surface hydrophopicity test, cell agragation, membrane potential test and others |
Dose dependent antibiofilm activity against MRSA. Alterated biofilm associated properties (hydrophobicity and cell agregation) and modified bacterial membrane potential. |
AEA MIC >256 µg/ml AraS MIC variable between MRSA strains from 32 up to above 256 µg/ml |
74 |
| Anandamide (AEA), palmitoylethanolamide (PEA), oleoylethanolamide (OEA) and stearoylethanolamide (SEA) | Effect on planktonic cells and biofilm assay. | AEA and OEA exhibited a synergistic antibacterial effect with poly‐L‐lysine against Streptococcus mutans. AEA showed a partial antibiofilm formation effect in S. mutans. |
None of the agents showed MIC at all tested doses. PEA and SEA alone or in combination with poly‐L‐lisine had no effect on S. mutans growth or in biofilm formation. AEA alone and AEA mixed with poly‐L‐lysine at doses up to 12.5 μg/ml had no effect on bacterial growth. |
79 | |
| Synthetic cannabinoids | HU‐210 | MIC determination, bioluminescence assay, biofilm formation, biomass determination by crystal violet stain, qPCR, swimming motility assay and others | Interference of bacterial signal‐transduction systems in Vibrio harveyi by targeting the the AI−2 cascade. Reduction on swimming motility and biofilm formation. |
Growth of V. harveyi strains was unaffected at all tested concentrations(0.2–200 μg/ml) of HU−210. There was no uniform antibiofilm effect on all the strains tested. |
80 |
3.1. Cannabis crude extracts
Early reports on the antimicrobial activity of Cannabis come from Kabelík et al. in 1960. In this work, extracts from different parts of C. sativa var. indica were tested for antibacterial activity against Gram‐positive and Gram‐negative microorganisms. These extracts showed a bactericidal effect upon Gram‐positive bacteria and Mycobacterium tuberculosis but showed no effect on Gram‐negative bacteria as well as fungi and yeast assayed. 15
Crude aqueous, ethanolic, and petroleum ether extracts of the leaves of C. sativa along with isolated acidic fractions were studied for antimicrobial activity. Ethanolic and petroleum ether extracts, as well as the isolated acidic fraction, showed marked activity against Gram‐positive and Gram‐negative bacteria, and fungi (Candida albicans and Aspergillus niger), while the aqueous extract did not show any activity. 44
Leaves and stems extracts of C. sativa showed a wide inhibition zone diameter against Staphylococcus aureus assessed by a disc diffusion method. 45
Seed oil and organic solvent extract of the whole C. sativa plants were tested against Bacillus subtilis, S. aureus, Escherichia coli, Pseudomonas aeruginosa, A. niger, and C. albicans. Generally, as seen in previous reports a pronounced activity was found against Gram‐positive bacteria and moderate to none activity against Gram‐negative and fungi. 46
Antibacterial and antifungal activity of different extracts (aqueous and acetone extracts) of C. sativa leaves was tested by Lone and Lone (2012). In this work, both extracts showed inhibition zones against P. aeruginosa, Vibro cholerae, Cryptococcus neoformans, and C. albicans at 5 µg/ml and 10 µg/ml concentration. 47
Extracts of leaves of C. sativa and two other plants were obtained with four different solvents and tested for antimicrobial activity against six microbial cultures (two Gram‐positive, two Gram‐negative, and two yeast). C. sativa showed the best antibacterial activity and the methanolic extract of Cannabis was the most active with marked antibacterial activity against B. subtilis and S. aureus. 48
The antimicrobial activity of Cannabis (indica) leaves, stems, and seeds extracts was evaluated by Isahq et al. (2015). Six multidrug‐resistant bacterial strains (S. aureus, Bacillus cereus, E. coli, Klebsiella pneumoniae, P. aeruginosa, and Proteus mirabilis) and five fungal strains (Aspergillus and Candida genera) were used for susceptibility test. All extracts revealed a range of antimicrobial activity. 49
The antibacterial activity of dried leaves extracts of C. sativa and other Indian medicinal plants were determined using a disc diffusion method by Chakraborty et al. (2018). Clinical and non‐clinical isolates of Methicillin‐Resistant S. aureus (MRSA) were tested and the growth of all of them resulted inhibited by crude ethanol extracts of the assayed plants. Moreover, a synergism between Cannabis and the other extracts was found. 50
The effect of C. sativa seed extracts on pathogenic (Gram‐positive and Gram‐negative strains) and beneficial probiotic bacteria (Lactobacillus and Bifidobacterium) was evaluated. Hemp seed extract showed an inhibitory effect on pathogens while not marked antimicrobial activity against the tested probiotic strains was seen. Besides, Cannabis extracts also reduced cell viability and biofilm formation of S. aureus. 51
3.2. Cannabis EOs and terpenes
EOs are aromatic oily liquids obtained from plant material, chemically constituted mainly by a mixture of terpenoids compounds, which are responsible for the biological activity of EOs and particularly for the antimicrobial activity. 52 Additionally, low concentration of some cannabinoids such as CBD, CBC, and cannabidivarin (CBDV) can also be present in Cannabis EOs exerting synergistic interactions. 53
Antimicrobial activity of terpenes compounds such as beta‐caryophyllene, 54 , 55 , 56 , 57 , 58 caryophyllene oxide, 59 , 60 , 61 , myrcene, 62 limonene, 63 , 64 alpha‐pinene and beta‐pinene, 65 has already been reported. Regarding Cannabis, a moderate antimicrobial activity of different EOs from C. sativa was reported against potential pathogen microorganisms such as Acinetobacter calcoaceticus, B. subtilis, E. coli, Yersinia enterocolitica, Micrococcus luteus, and S. aureus. 39
The antimicrobial activity and terpene characterization of EOs extracted from the inflorescence of three different varieties of C. sativa were also assessed by Nissen et al., 2010. Besides, single terpenes standard antimicrobial activity was determined. All the EOs exhibited good antimicrobial effect, especially against Gram‐positive pathogens from Enterococcus and Streptococcus genera. One of the EOs (named Futura) gave satisfactory results against Clostridia, Gram‐negative pathogens, and some yeasts. Among terpenes standards, alpha‐pinene was the most effective against Gram‐positive and Gram‐negative bacteria. 40
A similar variety of C. sativa L. EO (Futura 75) showed antibacterial activity against clinically relevant (multidrug‐sensible and multidrug‐resistant) S. aureus and Helicobacter pylori strains. Besides, the EO demonstrated the capacity to eradicate biofilms developed by S. aureus. 66 Finally, C. sativa var. Futura 75 also showed moderated bactericidal activity against Listeria monocytogenes. It also affected L. monocytogenes virulence traits, reducing the motility, the invasion ability, and the biofilm formation capacity of the bacteria. 67
Recently, Iseppi et al. (2019) reported the chemical characterization of 17 EOs from Cannabis and its antibacterial activity. No antimicrobial activity was found against Gram‐negative bacteria, however, good antibiotic activity was observed against Gram‐positive microorganism. Some samples showed MIC values against Enterococcus, Listeria, Bacillus, and Staphylococcus similar toor lower than conventional antibiotics (ampicillin and ciprofloxacin). Among pure compounds, CBD, alpha‐pinene, beta‐pinene, and beta‐myrcene exhibited good antibiotic activity, especially toward Listeria and Enterococcus strains. 53
3.3. Cannabinoids
The first report addressed to quantified the antimicrobial activity of the purified cannabinoids of C. sativa (Δ9‐THC and CBD) was carried out by VanKlingeren & Ten Ham (1976). They established a bacteriostatic and bactericidal activity in the range of 1–5 µg/ml for S. aureus and Streptococcus spp. but found no activity against Gram‐negative bacteria. They also reported a decreased antibiotic activity in presence of horse serum. 68
Later on, Turner & ElSohly (1981) studied the biological activity of CBC, and related compounds, and found out anti‐inflammatory and antimicrobial effects of these compounds. They reported a strong antibacterial activity and a mild to the moderate antifungal activity of CBC and its isomers and homologs that, in some cases, showed larger inhibition zones than the positive control standards. 26 Besides, these researchers tested the antimicrobial activity of different cannabinoids against Gram‐positive bacteria, Gram‐negative bacteria, and fungi. Of those compounds, CBC and CBG, and their isomers and homologs were the most active compounds against bacteria and fungi assayed. 69
More recently, nine new cannabinoids were isolated and three of them namely 4‐acetoxy‐2‐geranyl‐5‐hydroxy‐3‐n‐pentylphenol, 8‐hydroxycannabinol and 5‐acetyl‐4‐hydroxycannabigerol showed antibacterial (S. aureus and MRSA), antifungal (C. albicans), and antileishmanial (Leishmania donovani) activity respectively. 70
Pure cannabinoids (CBD, CBC. CBN, CBG, CBGA) have also proven to be more effective in reducing the colony count of dental plaque‐associated bacterial strains as compared to commercial oral care products. 71
Concerning resistant bacteria, Appendino and co‐workers tested the five major cannabinoids (CBD, CBC, CBG, Δ9‐THC, and CBN) and related compounds against a variety of multidrug‐resistant S. aureus strains. All cannabinoids showed potent antibacterial activity, with MIC values in the 0.5–2 μg/ml range. Also, a relation between the chemical structure of cannabinoids and the antibiotic activity was found, suggesting a specific interaction to a bacterial target. 72 Following these results, Farha et al., 2020 proved that cannabinoids (CBC, CBD, CBG, CBN, Δ9‐THC, and precursors) have potent antibacterial activity against MRSA, inhibited MRSA biofilm formation, eradicated pre‐formed biofilms and stationary phase cells persistent to antibiotics. 73 Moreover, the endocannabinoid anandamide (AEA) and the endocannabinoid‐like, arachidonoyl serine (AraS) also were able to inhibit biofilm formation, the reduced metabolic activity of pre‐formed biofilms, and altered biofilm‐associated virulence factors such as hydrophobicity, cell aggregation, and spreading ability in MRSA. 74 Finally, recent reports demonstrated the antibacterial activity of CBD, CBDA 75 , and CBCA 76 against Gram‐positive bacteria, including MRSA.
Cannabinoids showed to improve the antimicrobial activity of conventional antibiotics against resistant bacteria and vice versa, some antibiotics have enhanced and extended the antimicrobial activity of cannabinoids. 73 , 77 , 78 CBD significantly enhanced the antibacterial effect of erythromycin and rifampicin against E. coli VCS257and increased the antibacterial effect of kanamycin against S. aureus subsp. aureus Rosenbach. 77 CBD also potentiates the effect of bacitracin against MRSA, Enterococcus faecalis, L. monocytogenes, and Methicillin‐Resistant Staphylococcus epidermidis (MRSE) and the combination reduced de MIC value of bacitracin by at least 64‐fold. In time‐kill assays, CBD and bacitracin showed synergistic and bactericidal effects. 78 The presence of a sublethal concentration of polymyxin B increased the activity of the five major cannabinoids against E. coli. Also, a synergy antimicrobial activity was determined between the combination of CBG and polymyxin B against multidrug‐resistant clinical isolates of Gram‐negative pathogens such as Acinetobacter baumannii, E. coli, K. pneumoniae, and P. aeruginosa. 73 Interactions between natural antimicrobial agents (i.e. poly‐L‐lysine) and endocannabinoids and endocannabinoids‐like compounds were also reported. AEA and oleoyl ethanol amide (OEA) showed synergistic antimicrobial effect with poly‐L‐lysine against Streptococcus mutans in terms of bacterial growth. The combination of AEA and poly‐L‐lysine also showed an antibiofilm effect. 79 However, positive interactions between pure cannabinods and conventional antibiotics are not always found. Recently, CBD showed indifference effect with the combination with different kind of antibiotics in synergy test against MRSA. 75
4. POTENTIAL ANTIMICROBIAL MODE OF ACTION OF COMPOUNDS FROM CANNABIS
Although the antibacterial mechanism of action of cannabinoids has remained elusive, recently some advances have been made.
One of the proposed modes of action of molecules found in Cannabis is related to the alteration of membrane permeability. The terpene Limonene destroyed the cell integrity and wall structure of L. monocytogenes leading to leakage of intracellular components. 64 β‐caryophyllene showed similar alterations in the B. cereus membrane. 58 CBG also has demonstrated to target the cytoplasmic membrane of Gram‐positive bacteria. Besides, the permeabilization of the outer membrane of Gram‐negative bacteria decreased the MIC of CBG for these bacteria (from >128 to 1 μg/ml) and allowed CBG to act in the inner membrane similarly as in Gram‐positive bacteria. 73 Microscopic evaluation of the effect of CBCA against B. subtilis also showed an alteration of the bacterial membrane and nucleoid, that lead to cell lysis. 76 CBD showed a membrane‐related activity causing a depolarization of the cytoplasmatic membrane and a disruption of the membrane potential in S. aureus. Furthermore, the combination CBD with bacitracin caused defects in cell division and cell envelope irregularities apparently due to a down‐regulation of a very important cell division gene called ezrA. 78
Another putative mode of action of cannabinoids is the alteration of cell communication via inhibition of membrane vesicles released by bacteria. CBD proved to be a strong inhibitor of membrane vesicle release from E. coli VCS257, although this action was insignificant in S. aureus subsp. aureus Rosenbach. 77 In this address, the synthetic cannabinoid HU‐210 also showed an effect on the bacterial communication system via inhibiting the quorum sensing (QS) system and QS‐mediated properties such as bioluminescence, biofilm formation, and swimming motility of Vibrio harveyi. 80
5. CANNABIS DERIVATES IN VIVO: INTERACTION WITH THE IMMUNE SYSTEM AND TOXICITY
Some concerns arise when thinking of Cannabis compounds as antimicrobials agents and they are related to in vivo efficacy, interactions with the immune system, pharmacokinetics, adverse effects, and toxicity.
To achieve a positive therapeutic outcome, drugs must reach an optimal concentration above the active threshold (the MIC for antimicrobial compounds) but below toxic levels. Furthermore, therapeutic success in antimicrobial therapies does not only depends on the MIC value, since the immunomodulation activity of the molecules also plays an important role. 81
5.1. Cannabinoids and immune system
The relation between the endocannabinoid system and the immune system and the impact of exogenous cannabinoids ligands on immune function especially during infections is not completely understood. 82 Many of the components of the endocannabinoid system have been involved in the immune system modulation through the regulation of the migration of hematopoietic stem and progenitor cells, the regulation of innate immune cell trafficking and functions, and the regulation of adaptive immunity (modulating T‐cells and B‐cells function). 83 However, there have been discrepancies between studies that demonstrate inhibitory effects on the immune system and others reporting a stimulatory action on immune cells, probably due to the complexity in the endocannabinoid network, the heterogeneity in types of cannabinoids and their effects, methods, and protocols utilized and to a biphasic response associated to the concentration of cannabinoids (stimulatory at nanomolar concentration and inhibitory at micromolar). 82
The challenge with lipopolysaccharide (LPS) in animal models can induce rapid organic responses characterized by an overwhelming innate immune reaction that resembles some initial characteristic of sepsis. 84 The deletion of the CB1 receptor in mice as well as the administration of a CB1 receptor antagonist has shown to prevent fever in a murine LPS model by inhibition of Toll‐like receptor 4 (TLR4)‐mediated cytokine production. Mice deficient for CB1 receptor had lower plasma interleukin 6 (IL‐6) and tumor necrosis factor α (TNF‐α) levels and did not exhibit hyperalgesia in response to LPS. 85 On the contrary, CB2 receptor activation is implicated in protective anti‐inflammatory responses in mice acute sepsis models, as the administration of a CB2 synthetic agonist probed to increase the survival rate and decreased the pro‐inflammatory cytokines release in splenocytes and macrophages in LPS treated animals, and the opposite effect was observed in know‐out CB2 mice. 86
In models of acute lung injury induced by LPS, Riveiro et al. reported that CBD has anti‐inflammatory effects and improved lung function, 87 , 88 while Karmaus et al., (2013) demonstrated that oral administration of CBD enhanced LPS‐induced pulmonary inflammation in mice. 89
Ambiguous results were also noticed between the antimicrobial in vitro results (mentioned before) and the in vivo models of infection tests, which in some cases have shown negative effects. 90 The combination of Δ9‐THC with purified LPS, heat‐killed E. coli, live E. coli or lipid A‐protein complexes enhanced mortality in mice. 91 The administration of Δ9‐THC, Δ8‐THC, CBD, and Cannabis extract also decreased the resistance of mice to L. monocytogenes infection in a dose‐dependent manner. 92 In agreement with these results, several works have shown that Δ9‐THC modulates the immune system and leads to a suppression of cellular function and cytokine production (specially interferon‐γ and interleukine 12) and increased mortality of mice to Legionella pneumophila infections. 93 , 94 , 95 However, the effect of cannabinoids on the levels of the proinflammatory cytokine has proven to have a beneficial effect on animal models of infection. In an animal model of meningitis, the administration of CBD (10 mg/kg) for nine days after the challenge with Streptococcus pneumoniae prevented the memory impairment in rats. 96 Besides, similar protective effects were seen in a sepsis animal model by cecal ligation and puncture, where CBD treatment improved impaired cognitive functions and decreased mortality of rats. 97 In a murine systemic model of infection of MRSA, Farha et al. reported that CBG showed a significant reduction in bacterial burden in the spleen at a dose of 100 mg/kg, comparable to vancomycin at similar doses. 73
5.2. Cannabinoids toxicity
Toxicity is probably the first limitation on cannabinoids uses. In animal models, high doses of THC produced hypothermia, hypo locomotion, catalepsy, and antinociception. 98 In rats, the median lethal dose (LD50) of Δ9‐THC administered orally was estimated between 800 and 1900 mg/kg, however in dogs and monkeys doses up to 3000 and 9000 mg/kg respectively were nonlethal. 99 CBD, CBG, CBC, Δ9‐Tetrahydrocannabivarin (Δ9‐THCV), CBDV and the acids forms of cannabinoids have shown weak or non‐psychoactivity thus they are more well‐tolerated. 18 Pharmacokinetic studies in mice and rats were performed with single doses of cannabinoids, given intraperitoneally and orally at doses of 120 mg/kg (CBD and CBG), 60 mg/kg (CBDV), and 30 mg/kg (Δ9‐THCV) with no signs of acute toxicity. 100 In dogs, escalated doses of CBD up to 62 mg/kg proved to be safe with only some mild adverse events (mainly gastrointestinal) comparable to the placebo group. 101 In humans, chronic use of CBD with doses up to 1500 mg/day were reported to be well tolerated. 102 A report from the WHO Expert Committee on Drug Dependence concluded that “CBD" is generally well tolerated and to have a good safety profile”, and that “CBD should not be scheduled within the International Drug Control Conventions”. 103
6. CONCLUSION
The bacterial resistance to conventional chemical antibiotics has led to the search for new alternative antimicrobial agents and strategies. Many plant extracts and their purified compounds have shown antimicrobial activity to a wide variety of pathogens.
Cannabinoids and related compounds from C. sativa exhibit numerous attractive pharmacological properties. Many of them have been extensively explored while others, such as the potential of Cannabis extracts and compounds as antibacterial agents, are still in a very early stage.
In vitro studies have shown that Cannabis extracts and EOs exert antimicrobial properties. There have been variable results in the spectrum of activity of Cannabis products probably due to variability in the extracts assayed and in the microbiological test utilized. It should having into account that most of the reports mentioned in this review, specially those using cannabis extracts, are a mixture of compounds not fully analytical assessed. In addition, on a microbiological point of view some conclusion were made based on diffusion technique methods that needs to be further complemented by dilution techniques. In general, Cannabis extracts and purified cannabinoids have proven to be more active against Gram‐positive bacteria, including multidrug‐resistant microorganisms. Cannabinoids have also exhibited antimicrobial effects against yeast and some Gram‐negative bacteria. Further, cannabinoids have also shown synergistic interaction enhancing the antibacterial effect of conventional antibiotics in vitro. This finding makes cannabinoids good candidates for the development of antimicrobial combined therapies to improve the outcome of the treatment of resistant bacteria.
The role of the endocannabinoid system in infectious diseases and the effects of the administration of cannabinoids compounds in pre‐clinic models of infections still needs to be elucidated. Considering the results previously mentioned, it seems that cannabinoids (specially Δ9‐THC) could compromise the immune system and make it ineffective against intracellular microorganisms but cannabinoids could be useful to protect from the attack of extracellular bacteria and the damage of excessive immune response in bacterial infections.
Although some advances have been made and several bacterial targets have been postulated, cannabinoids and terpenes specific antimicrobial mode of action still needs a better understanding.
Finally, the concerns on toxicity and adverse effects of the use of Cannabis extracts (in particular those which are highly concentrated in Δ9‐THC) could be left behind by using non‐psychotropic cannabinoids, that has proven a safety profile in animal models and potent in vitro antibacterial activity.
All the data presented in this work suggests that cannabinoids and other Cannabis components demonstrated some outstanding in vitro antibacterial features that should be further tested in vivo against clinically important bacteria in the search of new tools as potential antimicrobial agents.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 104 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 105
DISCLOSURE
None to declare.
DATA AVAILABILITY STATEMENT
None to declare.
REFERENCES
- 1. Martens E, Demain AL. The antibiotic resistance crisis, with a focus on the United States. J Antibiot. 2017;70(5):520‐526. 10.1038/ja.2017.30 [DOI] [PubMed] [Google Scholar]
- 2. Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12 Suppl):S122‐S129. 10.1038/nm1145 [DOI] [PubMed] [Google Scholar]
- 3. Andrei S, Valeanu L, Chirvasuta R, Stefan MG. New FDA approved antibacterial drugs: 2015–2017. Discoveries. 2018;6(1):e81. 10.15190/d.2018.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rios AC, Moutinho CG, Pinto FC, et al. Alternatives to overcoming bacterial resistances: state‐of‐the‐art. Microbiol Res. 2016;191:51‐80. 10.1016/j.micres.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Nascimento GGF, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic‐resistant bacteria. Braz J Microbiol. 2000;31(4):247–256. 10.1590/s1517-83822000000400003 [DOI] [Google Scholar]
- 6. Atef NM, Shanab SM, Negm SI, Abbas YA. Evaluation of the antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull Natl Res Cent. 2019;43(1). 10.1186/s42269-019-0184-9 [DOI] [Google Scholar]
- 7. Choi JG, Kang OH, Lee YS, et al. In vitro and in vivo antibacterial activity of Punica granatum peel ethanol extract against salmonella. Evid‐Based ComplAlt. 2011;1‐8. 10.1093/ecam/nep105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yunana BT, Bukar BB, Aguiyi JC. In vitro and in vivo evaluation of antibacterial activity of Bridelia ferruginea extracts on some clinical isolates. J Phytopharmacol. 2018;7(4):392‐398. [Google Scholar]
- 9. Wagner H. Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia. 2011;82(1):34‐37. 10.1016/j.fitote.2010.11.016 [DOI] [PubMed] [Google Scholar]
- 10. Bonini SA, Premoli M, Tambaro S, et al. Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol. 2018;227:300‐315. 10.1016/j.jep.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 11. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28(2):153‐157. 10.1590/s1516-44462006000200015 [DOI] [PubMed] [Google Scholar]
- 12. Russo EB. The pharmacological history of Cannabis. In: Pertwee R, ed. Handbook of cannabinoids. Oxford, UK: Oxford Univ. Press; 2014. [Google Scholar]
- 13. Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers. 2007;4(8):1614‐1648. 10.1002/cbdv.200790144 [DOI] [PubMed] [Google Scholar]
- 14. Russo EB. Cannabis in India: ancient lore and modern medicine. In: Mechoulam R, ed. Cannabinoids as therapeutics. Basel: Birkhäuser Verlag; 2005. [Google Scholar]
- 15. Kabelík J, Krejcí Z, Santavy F. Cannabis as a medicament. Bull Narc. 1960;12:5‐23. [Google Scholar]
- 16. Takakuwa KM, Schears RM. A history of the US medical cannabis movement and its importance to pediatricians: science versus politics in medicine’s greatest catch‐22. Clin Pediatr. 2019;58(14):1473‐1477. 10.1177/0009922819875550 [DOI] [PubMed] [Google Scholar]
- 17. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(S1):S163‐S171. 10.1038/sj.bjp.0706406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non‐psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515‐527. [DOI] [PubMed] [Google Scholar]
- 19. Hanus LO. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med Res Rev. 2009;29(2):213‐271. 10.1002/med.20135 [DOI] [PubMed] [Google Scholar]
- 20. ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. phytochemistry of Cannabis sativa L. Prog Chem Org Nat Prod. 2017;103:1‐36. 10.1007/978-3-319-45541-9_1 [DOI] [PubMed] [Google Scholar]
- 21. Flores‐Sanchez IJ, Verpoorte R. Secondary metabolism in cannabis. Phytochem Rev. 2008;7:615‐639. 10.1007/s11101-008-9094-4 [DOI] [Google Scholar]
- 22. Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96(4):1593‐1659. 10.1152/physrev.00002.2016 [DOI] [PubMed] [Google Scholar]
- 23. Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539‐548. 10.1016/j.lfs.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 24. Andre CM, Hausman JF, Guerriero G. Cannabis sativa: the plant of the thousand and one molecule. Front Plant Sci. 2016;7:19. 10.3389/fpls.2016.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taura F, Sirikantaramas S, Shoyama Y, Shoyama Y, Morimoto S. Phytocannabinoids in Cannabis sativa: recent studies on biosynthetic enzymes. Chem Biodivers. 2007;4(8):1649‐1663. 10.1002/cbdv.200790145 [DOI] [PubMed] [Google Scholar]
- 26. Turner CE, Elsohly MA. Biological activity of cannabichromene, its homologs and isomers. J Clin Pharmacol. 1981;21(S1):283S‐291S. 10.1002/j.1552-4604.1981.tb02606.x [DOI] [PubMed] [Google Scholar]
- 27. Sirikantaramas S, Taura F. Cannabinoids: biosynthesis and biotechnological applications. In: Chandra S, Lata H, ElSohly M, eds. Cannabis sativa L. ‐ Botany and Biotechnology. Cham: Springer; 2017. 10.1007/978-3-319-54564-6_8 [DOI] [Google Scholar]
- 28. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344‐1364. 10.1111/j.1476-5381.2011.01238.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J Am Chem Soc. 1964;86(8):1646‐1647. 10.1021/ja01062a046 [DOI] [Google Scholar]
- 30. Hartsel JA, Eades J, Hickory B, Makriyannis A. Chapter 53—Cannabis sativa and hemp. In: Gupta RC, ed. Nutraceuticals. Academic Press; 2016:735‐754. 10.1016/B978-0-12-802147-7.00053-X [DOI] [Google Scholar]
- 31. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9‐tetrahydrocannabinol, cannabidiol and delta9‐tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199‐215. 10.1038/sj.bjp.0707442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanuš LO, Meyer SM, Muñoz E, Taglialatela‐Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357‐1392. 10.1039/c6np00074f [DOI] [PubMed] [Google Scholar]
- 33. Udoh M, Santiago M, Devenish S, McGregor IS, Connor M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br J Pharmacol. 2019;176(23):4537‐4547. 10.1111/bph.14815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Appendino G, Chianese G, Taglialatela‐Scafati O. Cannabinoids: occurrence and medicinal chemistry. Curr Med Chem. 2011;18(7):1085‐1099. 10.2174/092986711794940888 [DOI] [PubMed] [Google Scholar]
- 35. Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133‐150. 10.1016/j.pharmthera.2017.02.041 [DOI] [PubMed] [Google Scholar]
- 36. Shapira A, Berman P, Futoran K, Guberman O, Meiri D. Tandem mass spectrometric quantification of 93 terpenoids in Cannabis using static headspace injections. Anal Chem. 2019;91(17):11425‐11432. 10.1021/acs.analchem.9b02844 [DOI] [PubMed] [Google Scholar]
- 37. Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant‐based antimicrobials‐ a review. Plants. 2017;6(2):16. 10.3390/plants6020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43(2):169‐234. 10.1021/np50008a001 [DOI] [PubMed] [Google Scholar]
- 39. Novak J, Zitterl‐Eglseer K, Deans SG, Franz CM. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001;16(4):259‐262. 10.1002/ffj.993 [DOI] [Google Scholar]
- 40. Nissen L, Zatta A, Stefanini I, et al. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia. 2010;81(5):413‐419. 10.1016/j.fitote.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 41. Potter DJ. The propagation, characterization and optimization of Cannabis sativa L. as a phytopharmaceutical. Ph.D., London: King's College; 2009. [Google Scholar]
- 42. Richins RD, Rodriguez‐Uribe L, Lowe K, Ferral R, O'Connell MA. Accumulation of bioactive metabolites in cultivated medical Cannabis. PLoS One. 2018;13(7):e0201119. 10.1371/journal.pone.0201119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan BA, Warner P, Wang H. Antibacterial properties of hemp and other natural fibre plants: a review. Bio Res. 2014;9(2):3642–3659. 10.15376/biores.9.2.3642-3659 [DOI] [Google Scholar]
- 44. Wasim K, Haq I, Ashraf M. Antimicrobial studies of the leaf of Cannabis sativa L. Pak J Pharm Sci. 1995;8(1):29‐38. [PubMed] [Google Scholar]
- 45. Borchardt JR, Wyse DL, Sheaffer CC, et al. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J Med Plant Res. 2008;2(5):98‐110. [Google Scholar]
- 46. Ali E, Almagboul A, Khogali S, Gergeir U. Antimicrobial activity of Cannabis sativa L. Chin Med. 2012;3(1):61‐64. 10.4236/cm.2012.31010 [DOI] [Google Scholar]
- 47. Lone TA, Lone RA. Extraction of cannabinoids from Cannabis sativa L plant and its potential antimicrobial activity. Univ J Med Dent. 2012;1(4):51‐55. [Google Scholar]
- 48. Kaur S, Sharma C, Chaudhry S, Aman R. Antimicrobial potential of three common weeds of kurukshetra: an in vitro study. Res J Microbiol. 2015;10:280‐287. 10.3923/jm.2015.280.287 [DOI] [Google Scholar]
- 49. Isahq MS, Afridi MS, Ali J, Hussain MM, Ahmad S, Kanwal F. Proximate composition, phytochemical screening, GC‐MS studies of biologically active cannabinoids and antimicrobial activities of Cannabis indica . Asian Pac J Trop Dis. 2015;5(11):897‐902. 10.1016/s2222-1808(15)60953-7 [DOI] [Google Scholar]
- 50. Chakraborty S, Afaq N, Singh N, Majumdar S. Antimicrobial activity of Cannabis sativa, Thuja orientalis and Psidium guajava leaf extracts against methicillin‐resistant Staphylococcus aureus . J Integr Med. 2018;16(5):350‐357. 10.1016/j.joim.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 51. Frassinetti S, Gabriele M, Moccia E, Longo V, Di Gioia D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT. 2020;124:109149. 10.1016/j.lwt.2020.109149 [DOI] [Google Scholar]
- 52. Gallucci MN, Oliva M, Casero C, et al. Antimicrobial combined action of terpenes against the food‐borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus . Flavour Fragr J. 2009;24(6):348‐354. 10.1002/ffj.1948 [DOI] [Google Scholar]
- 53. Iseppi R, Brighenti V, Licata M, et al. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre‐type Cannabis sativa L. (Hemp). Molecules. 2019;24(12):2302. 10.3390/molecules24122302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dahham SS, Tabana YM, Iqbal MA, et al. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β‐caryophyllene from the essential oil of Aquilaria crassna . Molecules. 2015;20(7):11808‐11829. 10.3390/molecules200711808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Su YC, Ho CL. Composition of the leaf essential oil of Phoebe formosana from Taiwan and its in vitro cytotoxic, antibacterial, and antifungal activities. Nat Prod Commun. 2016;11(6):845‐848. [PubMed] [Google Scholar]
- 56. Pieri FA, Souza MC, Vermelho LL, et al. Use of β‐caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet Res. 2016;12(1):216. 10.1186/s12917-016-0842-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Selestino Neta MC, Vittorazzi C, Guimarães AC, et al. Effects of β‐caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24‐h time‐kill curve studies. Pharm Biol. 2017;55(1):190‐197. 10.1080/13880209.2016.1254251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moo CL, Yang SK, Osman MA, et al. Antibacterial activity and mode of action of β‐caryophyllene on Bacillus cereus . Pol J Microbiol. 2020;69(1):1‐6. 10.33073/pjm-2020-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang D, Michel L, Chaumont JP, Millet‐Clerc J. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia. 1999;148(2):79‐82. 10.1023/a:1007178924408 [DOI] [PubMed] [Google Scholar]
- 60. Magiatis P, Skaltsounis AL, Chinou I, Haroutounian SA. Chemical composition and in‐vitro antimicrobial activity of the essential oils of three Greek Achillea species. Z Naturforsch C J Biosci. 2002;57(3‐4):287‐290. 10.1515/znc-2002-3-415 [DOI] [PubMed] [Google Scholar]
- 61. Bougatsos C, Ngassapa O, Runyoro DK, Chinou IB. Chemical composition and in vitro antimicrobial activity of the essential oils of two Helichrysum species from Tanzania. Z Naturforsch C J Biosci. 2004;59(5‐6):368‐372. 10.1515/znc-2004-5-614 [DOI] [PubMed] [Google Scholar]
- 62. Inoue Y, Shiraishi A, Hada T, Hamashima H, Shimada J. The antibacterial effects of myrcene on Staphylococcus aureus and its role in the essential oil of the tea tree (Melaleuca alternifolia). Nat Med. 2004;58:10‐14. [Google Scholar]
- 63. Subramenium GA, Vijayakumar K, Pandian SK. Limonene inhibits streptococcal biofilm formation by targeting surface‐associated virulence factors. J Med Microbiol. 2015;64(8):879‐890. 10.1099/jmm.0.000105 [DOI] [PubMed] [Google Scholar]
- 64. Han Y, Sun Z, Chen W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes . Molecules. 2019;25(1):33. 10.3390/molecules25010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. da Silva Rivas AC, Lopes PM, de Azevedo Barros MM, Costa Machado DC, Alviano CS, Alviano DS. Biological activities of α‐pinene and β‐pinene enantiomers. Molecules. 2012;17(6):6305‐6316. 10.3390/molecules17066305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zengin G, Menghini L, Di Sotto A, et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: a multidisciplinary study. Molecules. 2018;23(12):3266. 10.3390/molecules23123266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marini E, Magi G, Ferretti G, et al. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. essential oil. Front Cell Infect Microbiol. 2018;8:293. 10.3389/fcimb.2018.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Klingeren B, Ten Ham M. Antibacterial activity of delta9‐tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek. 1976;42(1‐2):9‐12. 10.1007/BF00399444 [DOI] [PubMed] [Google Scholar]
- 69. Eisohly HN, Turner CE, Clark AM, Eisohly MA. Synthesis and antimicrobial activities of certain cannabichromene and cannabigerol related compounds. J Pharm Sci. 1982;71(12):1319‐1323. 10.1002/jps.2600711204 [DOI] [PubMed] [Google Scholar]
- 70. Radwan MM, Elsohly MA, Slade D, Ahmed SA, Khan IA, Ross SA. Biologically active cannabinoids from high‐potency Cannabis sativa . J Nat Prod. 2009;72(5):906‐911. 10.1021/np900067k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stahl V, Vasudevan K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: a preliminary observation. Cureus. 2020;12(1):e6809. 10.7759/cureus.6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure‐activity study. J Nat Prod. 2008;71(8):1427‐1430. 10.1021/np8002673 [DOI] [PubMed] [Google Scholar]
- 73. Farha MA, El‐Halfawy OM, Gale RT, et al. Uncovering the hidden antibiotic potential of Cannabis. ACS Infect Dis. 2020;6(3):338‐346. 10.1021/acsinfecdis.9b00419 [DOI] [PubMed] [Google Scholar]
- 74. Feldman M, Smoum R, Mechoulam R, Steinberg D. Potential combinations of endocannabinoid/endocannabinoid‐like compounds and antibiotics against methicillin‐resistant Staphylococcus aureus . PLoS One. 2020;15(4):e0231583. 10.1371/journal.pone.0231583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martinenghi LD, Jønsson R, Lund T, Jenssen H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules. 2020;10(6):900. 10.3390/biom10060900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Galletta M, Reekie TA, Nagalingam G, et al. Rapid antibacterial activity of cannabichromenic acid against methicillin‐resistant Staphylococcus aureus . Antibiotics (Basel). 2020;9(8):523. 10.3390/antibiotics9080523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kosgodage US, Matewele P, Awamaria B, et al. Cannabidiol is a novel modulator of bacterial membrane vesicles. Front Cell Infect Microbiol. 2019;9:324. 10.3389/fcimb.2019.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wassmann CS, Højrup P, Klitgaard JK. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram‐positive bacteria. Sci. Rep. 2020;10(1):4112. 10.1038/s41598-020-60952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Feldman M, Sionov R, SmoumR MR, Ginsburg I, Steinberg D. Comparative evaluation of combinatory interaction between endocannabinoid system compounds and poly‐L‐lysine against Streptococcus mutans growth and biofilm formation. Biomed Res Int. 2020;2020:1‐7. 10.1155/2020/7258380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Soni D, Smoum R, Breuer A, Mechoulam R, Steinberg D. Effect of the synthetic cannabinoid HU‐210 on quorum sensing and on the production of quorum sensing‐mediated virulence factors by Vibrio harveyi . BMC Microbiol. 2015;15:159. 10.1186/s12866-015-0499-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sadgrove NJ, Jones GL. From petri dish to patient: bioavailability estimation and mechanism of action for antimicrobial and immunomodulatory natural products. Front Microbiol. 2019;10:2470. 10.3389/fmicb.2019.02470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an ovComparative evaluation of combinatory interaction berview. Immunobiology. 2010;215(8):588‐597. 10.1016/j.imbio.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 83. Almogi‐Hazan O, Or R. Cannabis, the endocannabinoid system and immunity‐the journey from the bedside to the bench and back. Int J Mol Sci. 2020;21(12):4448. 10.3390/ijms21124448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Van der Poll T. Preclinical sepsis models. Surg Infect. 2012;13(5):287‐292. 10.1089/sur.2012.105 [DOI] [PubMed] [Google Scholar]
- 85. Duncan M, Galic MA, Wang A, et al. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R224‐R231. 10.1152/ajpregu.00104.2013 [DOI] [PubMed] [Google Scholar]
- 86. Gui H, Sun Y, Luo ZM, Su DF, Dai SM, Liu X. Cannabinoid receptor 2 protects against acute experimental sepsis in mice. Mediators Inflamm. 2013;2013:741303. 10.1155/2013/741303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ribeiro A, Ferraz‐de‐Paula V, Pinheiro ML, et al. Cannabidiol, a non‐psychotropic plant‐derived cannabinoid, decreases inflammation in a murine model of acute lung injury: a role for the adenosine A(2A) receptor. Eur J Pharmacol. 2012;678(1‐3):78‐85. 10.1016/j.ejphar.2011.12.043 [DOI] [PubMed] [Google Scholar]
- 88. Ribeiro A, Almeida VI, Costola‐de‐Souza C, et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS‐induced acute lung injury. Immunopharmacol Immunotoxicol. 2015;37(1):35‐41. 10.3109/08923973.2014.976794 [DOI] [PubMed] [Google Scholar]
- 89. Karmaus PW, Wagner JG, Harkema JR, Kaminski NE, Kaplan BL. Cannabidiol (CBD) enhances lipopolysaccharide (LPS)‐induced pulmonary inflammation in C57BL/6 mice. J Immunotoxicol. 2013;10(3):321‐328. 10.3109/1547691X.2012.741628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hernández‐Cervantes R, Méndez‐Díaz M, Prospéro‐García Ó, Morales‐Montor J. Immunoregulatory role of cannabinoids during infectious disease. NeuroImmunoModulation. 2017;24(4‐5):183‐199. 10.1159/000481824 [DOI] [PubMed] [Google Scholar]
- 91. Bradley SG, Munson AE, Dewey WL, Harris LS. Enhanced susceptibility of mice to combinations of delta 9‐tetrahydrocannabinol and live or killed gram‐negative bacteria. Infect Immun. 1977;17(2):325‐329. 10.1128/IAI.17.2.325-329.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Morahan PS, Klykken PC, Smith SH, Harris LS, Munson AE. Effects of cannabinoids on host resistance to Listeria monocytogenes and herpes simplex virus. Infect Immun. 1979;23(3):670‐674. 10.1128/IAI.23.3.670-674.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Klein TW, Newton CA, Nakachi N, Friedman H. Delta 9‐tetrahydrocannabinol treatment suppresses immunity and early IFN‐gamma, IL‐12, and IL‐12 receptor beta 2 responses to Legionella pneumophila infection. J Immunol. 2000;164(12):6461‐6466. 10.4049/jimmunol.164.12.6461 [DOI] [PubMed] [Google Scholar]
- 94. Klein TW, Cabral GA. Cannabinoid‐induced immune suppression and modulation of antigen‐presenting cells. J Neuroimmune Pharmacol. 2006;1(1):50‐64. 10.1007/s11481-005-9007-x [DOI] [PubMed] [Google Scholar]
- 95. Lu T, Newton C, Perkins I, Friedman H, Klein TW. Role of cannabinoid receptors in Delta‐9‐tetrahydrocannabinol suppression of IL‐12p40 in mouse bone marrow‐derived dendritic cells infected with Legionella pneumophila . Eur J Pharmacol. 2006;532(1–2):170‐177. 10.1016/j.ejphar.2005.12.040 [DOI] [PubMed] [Google Scholar]
- 96. Barichello T, Ceretta RA, Generoso JS, et al. Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur J Pharmacol. 2012;697(1‐3):158‐164. 10.1016/j.ejphar.2012.09.053 [DOI] [PubMed] [Google Scholar]
- 97. Cassol OJ Jr, Comim CM, Silva BR, et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010;1348:128‐138. 10.1016/j.brainres.2010.06.023 [DOI] [PubMed] [Google Scholar]
- 98. Beaulieu P. Toxic effects of cannabis and cannabinoids: animal data. Pain Res Manag. 2005;10(suppl A):23A‐26A. 10.1155/2005/763623 [DOI] [PubMed] [Google Scholar]
- 99. Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol. 1973;25(3):363‐372. 10.1016/0041-008x(73)90310-4 [DOI] [PubMed] [Google Scholar]
- 100. Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarin (CBDV), Δ⁹‐tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive‐compulsive behavior. Psychopharmacology. 2012;219(3):859‐873. 10.1007/s00213-011-2415-0 [DOI] [PubMed] [Google Scholar]
- 101. Vaughn D, Kulpa J, Paulionis L. Preliminary investigation of the safety of escalating cannabinoid doses in healthy dogs. Front Vet Sci. 2020;7:51. 10.3389/fvets.2020.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zuardi AW, Crippa JA, Hallak JE, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18(32):5131‐5140. 10.2174/138161212802884681 [DOI] [PubMed] [Google Scholar]
- 103. WHO Expert Committee on Drug Dependence, fortieth report. Geneva: World Health Organization; 2018 (WHO Technical Report Series, No. 1013). License: CC BY‐NC‐SA 3.0 IGO. Available online at https://apps.who.int/iris/bitstream/handle/10665/279948/9789241210225‐eng.pdf?ua=1. Accessed August 10, 2020.
- 104. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2019: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐D106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alexander SPH, Christopoulos A, Davenport AP, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176:S21‐S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None to declare.


