Abstract
The salivary glands of insects play a key role in the replication cycle and vectoring of viral pathogens. Consequently, Musca domestica (L.) (Diptera: Muscidae) and the Salivary Gland Hypertrophy Virus (MdSGHV) serve as a model to study insect vectoring of viruses. A better understanding of the structural changes of the salivary glands by the virus will help obtain a better picture of the pathological impact the virus has on adult flies. The salivary glands are a primary route for viruses to enter a new host. As such, studying the viral effect on the salivary glands is particularly important and can provide insights for the development of strategies to control the transmission of vector-borne diseases, such as dengue, malaria, Zika, and chikungunya virus. Using scanning and transmission electron microscopic techniques, researchers have shown the effects of infection by MdSGHV on the salivary glands; however, the exact location where the infection was found is unclear. For this reason, this study did a close examination of the effects of the hypertrophy virus on the salivary glands to locate the specific sites of infection. Here, we report that hypertrophy is present mainly in the secretory region, while other regions appeared unaffected. Moreover, there is a disruption of the cuticular, chitinous lining that separates the secretory cells from the lumen of the internal duct, and the disturbance of this lining makes it possible for the virus to enter the lumen. Thus, we report that the chitinous lining acts as an exit barrier of the salivary gland.
Keywords: house fly, TEM, SEM, viral replication, Hytrosaviridae
Graphical Abstract
According to the World Health Organization, Vector-borne diseases (i.e., diseases transmitted through vectors, such as insects) account for more than 17% of all infectious diseases and cause more than 700,000 deaths annually (World Health Organization 2017). Some of the pathogens that use insects as vectors are chikungunya virus, Zika virus, yellow fever, dengue fever, malaria, etc. With 96 million cases per year, 80% of the world’s population is at risk of contracting a vector-borne disease (World Health Organization 2017). These dreadful statistics call out for the need for strategies to control the transmission of these diseases. The salivary glands of insects play a key role in the replication cycle and vectoring of viral pathogens. Importantly, these major human pathogens share features of their replication and transmission with the Salivary Gland Hypertrophy Virus (SGHVs) family suggesting that Musca domestica L. and the salivary gland hypertrophy virus (MdSGHV) may serve as a potential model, using a nonvertebrate pathogen to aid in the study of insect vectoring of viruses. More specifically, how they egress from the site of replication into the lumen of the gland facilitating their transmission.
Salivary gland hypertrophy viruses (SGHVs) have been found and studied in several species, including tsetse flies (Glossina pallidipes A. (Diptera: Glossinidae), Jaenson 1978, Guerra et al. 2013, Guerra et al. 2015, Glossina morsitans W. (Diptera: Glossinidae), Kokwaro et al. 1990). Studies using SGHVs in G. pallidipes proposed these viruses as part of a new virus family: Hytrosaviridae (Abd-Alla et al. 2009). The viruses are rod-shaped with large circular double-stranded DNA genomes (Coler et al. 1993, Lietze et al. 2011b). In M. domestica, the SGHVs reduce fertility in their hosts by inhibiting both mating receptivity and egg production in females, while the males remain fertile (Lietze et al. 2007, Prompiboon et al. 2010, Kariithi et al. 2017b). Furthermore, the virus is not transmitted sexually or vertically (Lietze et al. 2007).
While it has been well established that MdSGHV infected flies exhibit enlarged or hypertrophied salivary glands (Coler et al. 1993, Lietze et al. 2011c, Kariithi et al. 2017b, Schaler et al. 2018), the ultrastructural effects have not been shown. In fact, the ultrastructure and morphology, especially the SEM of the entire salivary glands of adult house flies, has not previously been done. Using electron microscopy, researchers have shown the hypertrophic effects of infection by MdSGHV in the salivary glands (Lietze et al. 2011c). However, the salivary glands extend throughout the entire adult body and are composed of different regions. Thus, it is difficult to pinpoint the exact location in this complex and multifunctional organ where these infections are taking place. To date, few studies have focused on elucidating changes in the morphology and physiology of the salivary glands resulting from infection of MdSGHV. Knowledge of the vectoring of viruses in the salivary glands is exceptionally important, as most viruses use the salivary glands of insects as an exit route to enter a new host.
In this study, we investigated in detail the ultrastructural and morphological effects of MdSGHV on the salivary glands of a male adult house fly, M. domestica. At the same time, we provide SEM micrographs of the salivary pump, a structure usually ignored in adult dipterans. A better understanding of the structural effects on the salivary glands by the virus will help obtain a better picture of the pathological impact that the virus has on adult flies. Studying virus vectoring in M. domestica can provide important insights for the development of strategies to control the transmission of a range of vector-borne diseases.
Materials and Methods
Insects
Pupae of M. domestica were received from a laboratory colony at the U.S. Department of Agriculture (Agricultural Research Service, Gainesville, FL). The pupae were maintained at 24°C and 70% relative humidity under a photoperiod of 12:12h light–dark. Adult flies were given a diet of powdered milk and sugar at a 1:1 ratio.
Preparation of Crude MdSGHV Inoculum
MdSGHV was obtained from Dr. C. Geden, (U.S. Department of Agriculture – Agricultural Research Service in Gainesville, FL) and injected into the prothorax of healthy 1-day-old flies. To produce the MdSGHV inoculum, frozen (−20°C), 4-d-old virus-infected flies with the characteristic hypertrophied salivary glands were dissected. Following extraction of the infected salivary glands, a standard micro pestle was used to homogenize the glands in 500 μl phosphate-buffered saline (PBS). The homogenized solution was passed through a PBS-saturated 0.45 µm Acrodisc syringe filter, yielding 500 μl of virus-concentrated inoculum (containing two infectious gland pair equivalents (IGE), or 4 IGE/ml) for injections (Lietze et. al. 2007).
Production of Infected Flies and Dissections
Pupae of M. domestica were obtained from Dr. Geden, kept at 25°C and the adults were kept on a sugar-based diet. One-day-old male flies were injected following the method described by Lietze et al. (2007): MdSGHV inoculum (2.5 μl per fly) was injected into the prothorax of cold-immobilized (2 min, kept on ice) male flies (n = 10). A control group (n = 10) received 2.5 μl PBS via the same method of injection. Using a dissecting scope, salivary glands from five flies from both groups were dissected in PBS from 4 to 5 d old male flies that were cold-immobilized. Visual cues (e.g., light bluish coloration, enlargement of the glands) were used to confirm infection.
Scanning Electron Microscopy
Samples of normal and hypertrophied salivary glands were fixed in fixative (2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer containing 0.1% calcium chloride) overnight at 4°C. Subsequently, the samples were washed in 0.1 M cacodylate buffer three times for 10 min each and postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for 2 h at 4°C. Then, the glands were washed three times in the same buffer for 10 min. The samples were dehydrated through a series of steps in ethanol at progressively increasing concentrations (10, 30, 50, 70, 80, 90, and 100%) for 10 min. each at 4°C. The samples were dried using a critical point dryer (CPD020; Balzers Union AG, Balzars, Liechtenstein), and coated using a gold evaporator (MD010; Balzers Union AG). Once processed, the samples were observed under a JEOL JSM 5200 microscope (JEOL Ltd., Tokyo, Japan).
Transmission Electron Microscopy
Samples of normal salivary glands and hypertrophied salivary glands were fixed and dehydrated as described for SEM sample preparation (excluding the CPD), and then infiltrated in Epon resin (Electron Microscopy Sciences, Hatfield, PA), as described by Guerra et al. (2013). Thin sections were cut with Reichert Ultracut and LKB Nova ultramicrotomes (Reichert Technologies Life Sciences, Inc., Buffalo, NY). Semi-thin sections (1 μm thick) collected on slides were stained with toluidine blue and observed with a Zeiss Axiophot light microscope. Ultra-thin sections (60–80 nm) were collected on copper grids, stained with uranyl acetate and lead citrate, and observed at 120 kV using a JEOL JEM EX II TEM (Jeol Ltd.), equipped with a Veleta CCD camera (Olympus Corp., Tokyo, Japan). Pictures were processed using iTEM Version 5.0 (Olympus Soft Imaging Solutions Ltd., Tokyo, Japan).
Fluorescence Microscopy
Samples of normal and hypertrophied salivary glands were fixed in 4% paraformaldehyde in PBS at 25°C for 30 min, washed twice with PBS, and incubated with 0.5% Triton X-100 in PBS for 8 min. After three washes in PBS, the samples were treated with 2.5 μg/ml Alexa-conjugated Wheat Germ Agglutinin (WGA) (Thermo Fisher, Waltham, MA) at 25°C for 40 min. For double labeling, specimens were washed twice in PBS and incubated in 2.5 μg/ml DAPI (Thermo Fisher, Waltham, MA) at 25°C for 40 min. The samples were observed using a Nikon light microscope (Nikon TE2000) equipped with a color video camera (Roper Coolsnap HQ camera) and imaging software (NIS Elements AR imaging software).
Results
The salivary glands of M. domestica consist of a common tubular and thin duct that then bifurcates at the thorax level and extends into the abdomen on both sides of the gut (Figs. 1A and 2A). The external morphology of healthy salivary glands was studied using light microscopy (Fig. 1). In healthy salivary glands, three sections are observed: secretory, absorptive, and proximal. The distal, secretory region extends from the abdomen into the thorax, and has a granular-like aspect, whereas the surface of the proximal region is devoid of this granular appearance. Moreover, light microscopy showed a defined internal duct that extends throughout the region uniformly but is more prominent in the secretory region (Fig. 1C). The healthy, normal salivary glands transition from the secretory region to the absorptive region in the thorax of the fly. The absorptive region was studied using light microscopy, which showed a change in color of the gland at this region, in comparison to the secretory region (Fig. 1A and B). Finally, there is a small salivary pump close to the mouth of the fly that is thought to control salivary secretion. Connected to the pump, there are two thick muscle fibers whose origin was not determined (Figs. 1B and 2A). After the pump, the common salivary duct reaches the mouth of the fly (Fig. 2A).
Fig. 1.
Light microscopy of the general organization of healthy salivary glands in Musca domestica. (A) The salivary glands are characterized by three regions: proximal, absorptive, and secretory. (B–C) Magnifications of these regions with the first two in B and secretory in C. Arrow is pointing at the salivary pump with a single pump muscle fiber. Scale bar = 115 μm. *, absorptive region.
Fig. 2.
Schematic of an adult fly depicting the salivary glands (black wavy lines) and differences in morphology whether normal (MdSGHV−) or infected (MdSGHV+). Scanning electron micrographs of three distinct sections of the glands are depicted: (A) the proximal region, composed of a salivary pump and duct that bifurcates and transitions into (B) the absorptive region. Bar = 25 μm. (C–E) The secretory region, found in the thorax and abdomen. Arrows indicate concave points versus smooth uniform exteriors in the middle of the glands. Bar = 10 μm; Bar = 12.5 μm. (F) Infected glands are smooth in this region. Bar = 37.5 μm. (G) The slightly swollen end of the gland is shown. Bar = 75 μm. (H) as well as its alterations by the virus. Scale bars = 37.5 μm. Image derived and modified from Fig. 3, Nayduch and Burrus (2017).
Scanning electron microscopy was used to further study the morphology of the salivary gland (Fig. 2). The secretory region makes up the majority of the glands, and its surface morphology is uniformly consistent. Moreover, scanning electron micrographs further showed the well-defined circular internal salivary duct (Fig. 2C). The granule-like surface may exhibit a concave region or dimple in the middle of the raised granular-like structure (Fig. 2D and E). Just before entering the head of the insect, the absorptive region loses the granular-like appearance altogether and transitions to the proximal region (Fig. 2B). This region is surrounded by a coat of epithelial cells. This last region continued in a duct joining a common salivary duct that extended into the head of the fly (Fig. 2A).
Infection with the SGH virus greatly enlarged the salivary glands and gave them a whitish and bluish appearance. The difference in diameter and color made infected glands easily distinguishable from noninfected glands. While the infection affected both individual glands uniformly, the secretory regions were primarily hypertrophic. Scanning electron micrographs revealed a swollen surface, with traces of where the granular-like structures used to be (Fig. 2F). However, the bulb-end, and the region before it, did not undergo major changes in its surface, in comparison to the rest of the secretory region (Fig. 2H). Meanwhile, infection with the SGH virus did not reveal major morphological changes in the absorptive or proximal regions.
TEM was used to further study the ultrastructure of the secretory region of the salivary glands. Similar to the light microscopy results, transmission electron micrographs revealed granule-like secretory cells, a central lumen, and a chitinous, cuticular matrix separating the cells from the lumen (Fig. 3A). TEM cross-sections of this region showed secretory cells containing several mitochondria and Golgi apparatus, and numerous electron-dense secretory granules (Fig. 3B–D). Moreover, adjacent cells were in contact along their plasma membrane by septate junctions (Fig. 3E). Further, TEM micrographs showed the presence of the internal duct and its cuticular structure, which extends through the entirety of this region (Fig. 3A, C, and D).
Fig. 3.
Transmission electron micrographs of the secretory region in the normal, uninfected salivary gland. (A) Micrographs show a sheath of glandular cells and a central lumen surrounded by a thick cuticular lining. Bar = 20 μm. (B–C) A central nucleus, large mitochondria, Golgi complexes, and numerous secretory granules are observed. The lumen is separated from the secretory cells by a cuticle. Bar = 2 μm; Bar = 1 μm. (D) The basal lamina is observed. Arrows point at junctions between adjacent cells. Bar = 2 μm. (E) A septate junction joins secretory cells together. Bar = 250 nm. Cu, cuticle; G, Golgi complex; Sj, septate junction; Lu, lumen; m, mitochondria; N, nucleus; Sg, *, secretory granules.
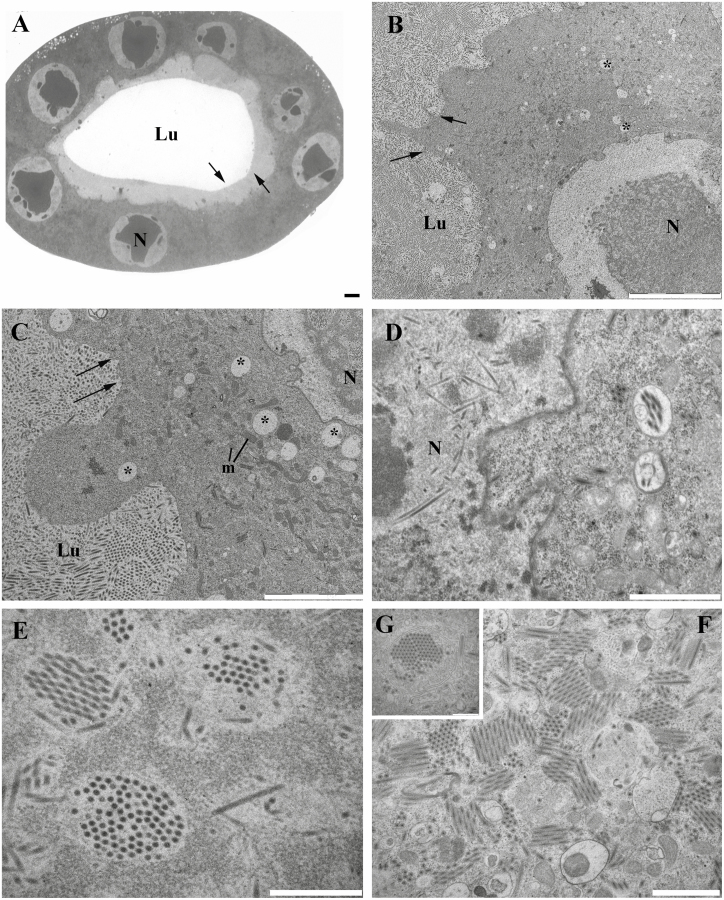
In the secretory region of MdSGHV-infected glands, the secretory cells underwent extensive ultrastructural changes. Light microscopy of a cross section revealed a collapsed lumen and enlarged nuclei (Fig. 4A). Transmission electron micrographs showed highly vacuolated cells and a significant enlargement of both the nucleus and cell (Fig. 4B and C). Numerous viral particles were observed in the nuclei and cytoplasm (Fig. 4D–G). In the nucleus of the cell, the virus replicated and assembled its nucleocapsid, and then the particles transition to the cytoplasm of the cells to further develop (Fig. 4D and E). Furthermore, the boundary between the cells and the lumen became unclear; i.e., the cuticle separating the two structures was disrupted or missing (Fig. 4A–C). Even further, viral particles were abundant in the lumen (Fig. 4B and C). These effects by SGHV infection were observed throughout the secretory region and extended into the distal bulb end.
Fig. 4.
Light and transmission electron micrographs of the secretory region of the hypertrophied salivary glands, infected by the SGH virus. (A) Light microscopy of the enlarged salivary gland. Nuclei are significantly enlarged. Lumen is collapsed. Arrows point at the lack of a visible cuticular lining. Bar = 10 um. (B–C) Transmission microscopy showing the enlarged nuclei and vacuolization of the secretory cells. Arrows point to the lack of a visible cuticular lining separating the lumen from the cells. Bar = 10 um; Bar = 5 um. (D) Virus particles replicate and form their nucleocapsids in the nucleus of the secretory cells. Bar = 1.5 um. (E–G) High magnification of the virus particles in the nucleus and cytoplasm, respectively. Bar = 500 nm; Bar = 1 um; Bar = 500 um. m, mitochondria; N, nuclei; Lu, lumen.
Infection with SGH virus did not show major ultrastructural changes of the surface of the absorptive and proximal regions, in comparison to the secretory region. In the infected proximal region, the coat of the epithelial cells was observed intact, as well as the overall structure of the pump. Likewise, the absorptive region of infected glands showed little disruption of the coat of cells surrounding the salivary duct.
The cuticular, chitinous lining was examined more closely using transmission electron and light microscopy (Fig 5A and C). To further investigate the molecular basis of the cuticle, and its disruption and destruction by the SGH virus, the salivary glands were stained with DAPI and WGA to identify the composition of the cuticle. Staining with DAPI revealed that each granular-like structure corresponds to an individual cell with a single central nucleus (Fig. 5E) that becomes enlarged postinfection (Fig. 5F). In healthy glands, the WGA stain showed the cuticular, chitinous lining separating the glandular cells from the lumen (Fig. 5E). The cuticle extended from the secretory to the absorptive region. Consistent with the electron and light microscopy observations (Fig. 5B and D), the infected glands were devoid of the cuticular lining (Fig. 5F).
Fig. 5.
Disruption of the chitinous, cuticular lining of the salivary duct by the SGH virus. (A) Transmission electron micrographs show the presence of a lining separating the secretory cells from the lumen in the healthy normal glands. Bar = 1 μm. (B) However, in the hypertrophied glands infected by the SGH virus, the lining is not observed. Bar = 1 μm. (C) Light microscopy of the healthy salivary glands shows the presence of a clear duct. Bar = 50 μm. (D) while infected glands do not. Bar = 15 μm. (E) Staining with WGA highlighted the cuticle. Bar = 50 μm. (F) And lack of it in the infected glands (arrows). Bar = 10 μm. Arrows point to the cuticle lining, and its disruption in the infected glands. Lu, lumen.
Discussion
In this study, a detailed description of the morphology and ultrastructure of the salivary glands of male M. domestica was obtained by electron and light microscopy techniques. Only males were used in this study due to the compounding difficulty of egg development and salivary gland removal in female flies. However, the female salivary glands are now being studied since both sexes show the pathology and both can transmit the virus. While viral infection among flies in nature likely occurs during feeding, attempts to feed the virus to adult flies are problematic in getting individual flies to ingest a virus solution, as well as in determining exact amounts and therefore does each fly receive. Furthermore, it is thought that adult flies acquire the virus by ingestion (Geden et al. 2008), but it is not known how the virus moves from the gut to the salivary gland of infected flies. Injecting the virus allows for certainty of infection of the glands using the same dosage. For this reason, one-day-old male flies were injected with either MdSGHV inoculum or saline, and the salivary glands of both groups were dissected for their study. Three main regions of the salivary glands were identified based on their location in the adult body, morphology, and ultrastructure: secretory, absorptive, and proximal. A similar organization of the salivary glands was observed in other species, such as G. pallidipes, which exhibited a distal secretory region and a proximal absorptive duct (Guerra et al. 2015).
The secretory region was characterized by a single layer of granular-like secretory cells surrounding an internal salivary duct. Many of these cells showed small concave depressions. This difference in morphology could be due to the level of secretory activity in the glands at the moment of dissection, which could affect the volume of the cell. Moreover, the secretory cells in this region contained a high number of mitochondria, Golgi apparatus, rough endoplasmic reticulum, and electron-dense secretory granules—all characteristic of secretory cells. In contrast, the absorptive region was devoid of the granular- or dome-like sheath covering the cells. Instead, a single layer of epithelial cells surrounded the internal duct. Similarly, the proximal region was surrounded by a single layer of epithelial cells and joined the common duct. Finally, a small, salivary pump was found in close proximity to the mouth. This salivary pump was previously described (Hewitt 1914) and sketched (in West 1951; drawing from Matheson 1944), but not imaged until now. The pump was connected to at least two muscle fibers and is thought to aid the release of saliva secretion. The report by Schneeberg and Beutel (2015) mentions that in Axymyiidae S. (Diptera: Axymyiidae), Deuterophlebiidae E. (Diptera: Deuterophlebiidae), and Limoniidae (Diptera: Tipulidae), the pump muscle is reduced while in Deuterophlebiids, which do not feed as adults, the salivary pump is superfluous. They also mention that the dipteran ground plan shows only one muscle going to the pump while the report by Liu and Hua (2010) is a more comprehensive review on salivary pumps. They report the panorpoid species they studied had two muscles going to the pump. Our study on a higher dipteran, however, shows two muscles and may represent a more evolutionary development. Finally, extending all throughout the gland, there is a chitinous, cuticular lining that separates the glandular cells from the lumen of the salivary duct.
Studies have shown that chitin is a major component of insect cuticles and peritrophic matrices (Merzendorfer and Zimoch 2003). For this reason, we speculated that the cuticular lining delineating the internal salivary duct in M. domestica to be a chitinous structure as well. WGA is a lectin that binds internal sugar residues of glycoproteins and selectively recognizes and N-acetylglucosamine residues—the building block of chitin (Peters and Latka 1986). WGA has a stronger affinity to chitin labeling and thus has previously been used to localize this polymer (Peters and Latka 1986, Tonning et al. 2005, Pesch et al. 2015, Wells et al. 2017). Indeed, WGA labeling highlighted the cuticle, in accordance with this lining being a chitinous structure.
The salivary glands of M. domestica underwent profound morphological and ultrastructural changes caused by MdSGHV. The viral infection produced a significant hypertrophy in the secretory region while leaving the absorptive and proximal region nearly intact. SEM and TEM showed severe alterations of the granular-like secretory cells. Interestingly, the granular-like surface was lost and replaced by a smooth surface in infected glands due to enlargement of the cells. In some cases, SGH viruses induce hyperplasia, as in tsetse flies (Kariithi et al. 2017a). In fact, MdSGHV infected salivary glands were previously referred to as hyperplasia (Coler et al. 1993). However, TEM and DAPI staining confirmed that this change in the morphology of the surface was due to hypertrophy (Kariithi et al. 2017a).
Importantly, the secretory region was devoid of its chitinous, cuticular lining in infected flies. In TEM micrographs, it was difficult to distinguish the limits of the glandular cells and their respective lumen. Moreover, the lumen was characterized by and filled with a large number of viral particles. Staining with WGA further showed a lesser fluorescence, suggesting loss of the cuticle—a chitinous structure. This supported the hypothesis that cuticle disruption involved chitin degradation in virally infected flies. In fact, studies have reported upregulation of chitin-binding proteins including metalloproteases, as well as genes related to the ubiquitin-protease system, in flies infected by MdSGHV (Garcia-Maruniak et al. 2008, Kariithi et al. 2017b).
The infection did not show major alterations in the surface of the absorptive or proximal regions. SEM micrographs showed both regions unaffected by the virus, for the most part. Furthermore, staining with WGA showed the presence of the cuticle in these regions in both infected and noninfected flies.
The hypertrophic effects of MdSGHV, as well as its morphometrics, have been studied before (Lietze et al. 2011a). However, the exact location where the infection was found was unclear. In fact, we described using SEM the morphology of the entire healthy salivary gland, including the salivary pump, which had not been done before. Based on the results obtained in this study, we can conclude that the virus causes important ultrastructural and morphological alterations of the secretory cells, similar to those previously described by Lietze et al. (2011c). However, these alterations extend to a much lesser degree to the absorptive and proximal regions. The observations presented here highlight the ultrastructural and morphological changes induced by infection with MdSGHV, and thus could correlate to an alteration of gland function and/or be applied to other pathogen systems.
Interestingly, we observed a disruption of the cuticular, chitinous lining of the lumen in the secretory region. Based on this observation, we can hypothesize that the exhibited pathology could be a mechanism for the virus to infiltrate the glands and gain access to the saliva. In this manner, viral particles are able to reach the saliva, and travel from the infected fly to a new host. Thus, the cuticular chitinous lining may be serving as an infection escape barrier. In fact, similar salivary gland infection escape barriers have been described in mosquitoes (Romoser et al. 2005). A study further showed that a proteoglycan-dependent virus produced gross pathology, while its proteoglycan-independent variant resulted in minimal pathology (Ciano et al. 2014). The results presented here, in combination with previous studies, have revealed the importance of the cuticular lining for virus transmission in insects. In fact, this is the first study elucidating the potential of the cuticular lining to be an exit barrier in the salivary glands. Nonetheless, further studies are necessary to understand the mechanism of cuticle degradation, e.g., whether the viral encoded proteins disrupt the existing membrane or if virus replication reprograms the salivary cells to stop synthesis or induces autocatalysis of the lumen layer. Information on this mode of infection by the SGH virus can give us a better picture of viral pathology and vectoring in insects.
Acknowledgments
Thanks to Dr. Yasu Morita of the Microbiology Dept. at UMass Amherst for providing facilities and materials for fixation and embedding and to Shawheen Fagan and Marino Biagini for maintaining the house fly colony. Appreciation to Dr. Chris Geden for his weekly supply of fly pupae. This work was supported by the United States Department of Agriculture, National Institute of Food and Agriculture, Hatch MAS00527/S1076 to J.G.S. Thanks to Sarah Kiemle and Rebecca Reichel of the Microscopy Facilities at Mt. Holyoke College for providing facilities and materials for imaging.
Author Contributions
DMP: conducted the research and wrote the original draft; JGS: conceived the problem and reviewed subsequent drafts; AMF and GG: sectioned and imaged the TEM samples, as well as reviewed subsequent drafts. JB: reviewed and edited drafts and resource providing virus and virus handling input. The authors of this paper have not any involvement, financial or otherwise, that might potentially bias their work.
References Cited
- Abd-Alla, A. M., F. Cousserans, A. G. Parker, C. Jridi, M. Bergoin, and A. S. Robinson. . 2009. Quantitative PCR analysis of the salivary gland hypertrophy virus (GpSGHV) in a laboratory colony of Glossina pallidipes. Virus Res. 139: 48–53. [DOI] [PubMed] [Google Scholar]
- Ciano, K. A., J. J. Saredy, and D. F. Bowers. . 2014. Heparan sulfate proteoglycan: an arbovirus attachment factor integral to mosquito salivary gland ducts. Viruses. 6: 5182–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler, R. R., D. G. Boucias, J. H. Frank, J. E. Maruniak, A. Garcia-Canedo, and J. C. Pendland. . 1993. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 7: 275–282. [DOI] [PubMed] [Google Scholar]
- Garcia-Maruniak, A., J. E. Maruniak, W. Farmerie, and D. G. Boucias. . 2008. Sequence analysis of a non-classified, non-occluded DNA virus that causes salivary gland hypertrophy of Musca domestica, MdSGHV. Virology. 377: 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geden, C. J., V.-U. Lietze, and D. G. Boucias. . 2008. Seasonal prevalence and transmission of salivary gland hypertrophy virus of house flies (Diptera: Muscidae). J. Med. Entomol. 45: 10. [DOI] [PubMed] [Google Scholar]
- Guerra, L., J. G. Stoffolano, Jr, G. Gambellini, V. L. Masci, M. C. Belardinelli, and A. M. Fausto. . 2013. Ultrastructure of the salivary glands of non-infected and infected glands in Glossina pallidipes by the salivary glands hypertrophy virus. J. Invertebr. Pathol. 112 Suppl: S53–S61. [DOI] [PubMed] [Google Scholar]
- Guerra, L., J. G. Stoffolano, Jr, M. C. Belardinelli, G. Gambellini, A. R. Taddei, V. L. Masci, and A. M. Fausto. . 2015. Disruption of the salivary gland muscle in tsetse, Glossina pallidipes Austen, as a result of salivary gland hypertrophy virus infection. Med. Vet. Entomol. 29: 361–370. [DOI] [PubMed] [Google Scholar]
- Hewitt, C. G. 1914. The housefly: Musca domestica L.: its structure, habits, development, relation to disease and control. Cambridge University Press, New York, NY. [Google Scholar]
- Jaenson, T. G. 1978. Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes. Trans. R. Soc. Trop. Med. Hyg. 72: 234–238. [DOI] [PubMed] [Google Scholar]
- Kariithi, H. M., I. K. Meki, D. G. Boucias, and A. M. Abd-Alla. . 2017. Hytrosaviruses: current status and perspective. Curr. Opin. Insect Sci. 22: 71–78. [DOI] [PubMed] [Google Scholar]
- Kariithi, H. M., X. Yao, F. Yu, P. E. Teal, C. P. Verhoeven, and D. G. Boucias. . 2017. Responses of the housefly, Musca domestica, to the hytrosavirus replication: impacts on host's vitellogenesis and immunity. Front. Microbiol. 8: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokwaro, E. D., M. Nyindo, and M. Chimtawi. . 1990. Ultrastructural changes in salivary glands of tsetse, Glossina morsitans morsitans, infected with virus and rickettsia-like organisms. J. Invertebr. Pathol. 56: 337–346. [DOI] [PubMed] [Google Scholar]
- Lietze, V. U., C. J. Geden, P. Blackburn, and D. G. Boucias. . 2007. Effects of salivary gland hypertrophy virus on the reproductive behavior of the housefly, Musca domestica. Appl. Environ. Microbiol. 73: 6811–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietze, V. U., A. M. Abd-Alla, and D. G. Boucias. . 2011a. Two hytrosaviruses, MdSGHV and GpSGHV, induce distinct cytopathologies in their respective host insects. J. Invertebr. Pathol. 107: 161–163. [DOI] [PubMed] [Google Scholar]
- Lietze, V. U., A. M. Abd-Alla, M. J. Vreysen, C. J. Geden, and D. G. Boucias. . 2011b. Salivary gland hypertrophy viruses: a novel group of insect pathogenic viruses. Annu. Rev. Entomol. 56: 63–80. [DOI] [PubMed] [Google Scholar]
- Lietze, V. U., T. Z. Salem, P. Prompiboon, and D. G. Boucias. . 2011c. Tissue tropism of the Musca domestica salivary gland hypertrophy virus. Virus Res. 155: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., and B. Hua. . 2010. Histology and ultrastructure of the salivary glands and salivary pumps in the scorpionfly Panorpa obtusa (Mecoptera: Panorpidae). Acta Zool. 91: 457–465. [Google Scholar]
- Matheson, R. 1944. Entomology for introductory courses. Comstock Publishing Company, Ithaca, NY. [Google Scholar]
- Merzendorfer, H., and L. Zimoch. . 2003. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 206: 4393–4412. [DOI] [PubMed] [Google Scholar]
- Nayduch, D., and R. G. Burrus. . 2017. Flourishing in Filth: House Fly–Microbe Interactions Across Life History. Ann. Entomol. Soc. Am. 110: 6–18. [Google Scholar]
- Pesch, Y. Y., D. Riedel, and M. Behr. . 2015. Obstructor A organizes matrix assembly at the apical cell surface to promote enzymatic cuticle maturation in Drosophila. J. Biol. Chem. 290: 10071–10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, W., and I. Latka. . 1986. Electron microscopic localization of chitin using colloidal gold labelled with wheat germ agglutinin. Histochemistry. 84: 155–160. [DOI] [PubMed] [Google Scholar]
- Prompiboon, P., V. U. Lietze, J. S. Denton, C. J. Geden, T. Steenberg, and D. G. Boucias. . 2010. Musca domestica salivary gland hypertrophy virus, a globally distributed insect virus that infects and sterilizes female houseflies. Appl. Environ. Microbiol. 76: 994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romoser, W. S., M. J. Turell, K. Lerdthusnee, M. Neira, D. Dohm, G. Ludwig, and L. Wasieloski. . 2005. Pathogenesis of Rift Valley fever virus in mosquitoes--tracheal conduits & the basal lamina as an extra-cellular barrier. Arch. Virol. Suppl. 19: 89–100. [DOI] [PubMed] [Google Scholar]
- Schaler, J., J. Stoffolano, A. M. Fausto, G. Gambellini, and J. Burand. . 2018. Effect of diet on adult house fly (Diptera: Muscidae) injected with the salivary gland hypertrophy virus (MdSGHV). J. Insect Sci. 18: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberg, K., and R. G. Beutel. . 2014. The evolution of head structures in lower Diptera. Sci. Res. doi: 10.14293/S2199-1006.1.SOR-LIFE.ALTCE1.v2 [DOI] [Google Scholar]
- Tonning, A., J. Hemphälä, E. Tång, U. Nannmark, C. Samakovlis, and A. Uv. . 2005. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell. 9: 423–430. [DOI] [PubMed] [Google Scholar]
- Wells, M. B., J. Villamor, and D. J. Andrew. . 2017. Salivary gland maturation and duct formation in the African malaria mosquito Anopheles gambiae. Sci. Rep. 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, L. S. 1951. The housefly. Comstock Publishing Company, Ithaca. [Google Scholar]
- World Health Organization. 2017. Global vector control response 2017–2030. World Health Organization, Geneva. [Google Scholar]