Abstract
Objectives
To determine the relationship between Growth-differentiation factor-15 (GDF-15) and clinical outcomes in ambulatory patients with heart failure and reduced ejection fraction (HFrEF).
Background
The prognostic utility of GDF-15, a member of the transforming growth factor-B cytokine family, among patients with HF is unclear.
Methods
We assessed GDF-15 levels (Elecsys, Roche Diagnostics) in 910 patients enrolled in the ‘Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training’ (HF-ACTION) trial, a randomized clinical trial of exercise training in patients with HFrEF. Median follow-up was 30 months. Cox proportional hazard models assessed the relationships between GDF-15 and clinical outcomes.
Results
The median GDF-15 level was 1596 pg/mL. Patients in the highest tertile of GDF-15 were older and had measures of more severe HF (higher NT-proBNP levels and lower peak oxygen uptake on cardiopulmonary exercise testing [CPX]). GDF-15 was a significant predictor of all cause death (unadjusted hazard ratio [HR] 2.03 per doubling of GDF-15, p<0.0001). This association persisted after adjustment for demographic, clinical, and biomarker including hs-TnT and NT-proBNP (HR 1.30 per doubling of GDF-15, p=0.029). GDF-15 did not improve discrimination (as measured by changes in c-statistics and the integrated discrimination improvement) on top of baseline variables including hs-TnT, NT-proBNP, or variables found in CPX testing,
Conclusions
In demographically diverse, well managed patients with HFrEF, GDF-15 provides independent prognostic information on top of established predictors of outcomes. These data support a possible role for GDF-15 in the risk stratification of patients with chronic HFrEF.
Condensed abstract
The prognostic utility of growth differentiating factor (GDF-15) among patients with chronic heart failure (HF) is unclear. We assessed GDF-15 levels in 910 patients enrolled in the ‘Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training’ (HF-ACTION) trial. GDF-15 was a significant predictor of all cause death (unadjusted hazard ratio [HR] 2.03 per doubling of GDF-15, p<0.0001). This association persisted after adjustment for demographic, clinical, and biomarker including hs-TnT and NT-proBNP (HR 1.30 per doubling of GDF-15, p=0.029). These data support a possible role for GDF-15 in the risk stratification of patients with chronic HF.
Trial registration
clinicaltrials.gov identifier NCT00047437.
Introduction
Chronic heart failure (HF) affects more than 5 million people in the United States and is one of the most common causes of rehospitalization1–3. The overall prognosis remains poor with 5-year mortality exceeding 50%, despite advances in therapy2,4,5. As the number of invasive and non-invasive HF therapies increase, risk stratification and prognostication become essential to identify patients that would most benefit from these treatments. Currently the US Food and Drug Administration has approved four biomarkers to aid in the prognostication of HF patients: B-type natriuretic peptide (BNP), N-terminal Pro-brain natriuretic peptide (NT-proBNP), galectin-3, and ST-2. Current HF guidelines indicate that measurement of other clinically available tests such as natriuretic peptides (NT-proBNP/BNP) and markers of myocardial injury (cardiac troponin T or I) may be considered for risk stratification5. Although a variety of other potentially prognostic biomarkers have been identified in heart failure, their clinical value remains uncertain.
GDF-15, a member of the transforming growth factor-β family, is secreted from a range of cells such as adipocytes and myocytes in response to cellular ischemia, mechanical strain, and oxidative stress6–9. Prior studies have suggested that GDF-15 provides prognostic utility across a spectrum of cardiovascular diseases on top of existing clinical risk factors and biomarkers10–15. However, robust data on GDF-15 in well treated patients with chronic HF are limited, especially in the context of other guideline recommended HF biomarkers such as natriuretic peptides and high-sensitivity troponin. A prior analysis from the Valsartan Heart Failure Trial (ValHeFT)16 identified that GDF-15 was independently associated with mortality; however, there was low use of evidence based medical therapy and a demographically homogenous patient population. An evaluation of the prognostic role of GDF-15 in a cohort of patients more reflective of current HF practice is needed. Furthermore, there is a paucity of data on the association between GDF-15 and other critical measures of HF status, such as exercise capacity. In the current study, we evaluated 1) the association between GDF-15 and other biologic covariates, 2) the association between GDF-15 functional status and exercise capacity, and 3) the relationship between GDF 15 and clinical outcomes in a well characterized cohort of ambulatory patients with HFrEF enrolled in the ‘Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training’ (HF-ACTION) trial.
Methods
The design, rationale, and primary results of the HF-ACTION trial (NCT00047437) have been previously reported17,18. Briefly, HF-ACTION was an NHLBI-funded randomized clinical trial evaluating the effects of exercise training in addition to usual care versus usual care alone on long-term morbidity and mortality in patients with symptomatic chronic HF and left ventricular systolic dysfunction (New York Heart Association [NYHA] class II–IV, left ventricular ejection fraction [LVEF] < 35%). Patients were on guidelines-based heart failure therapy prior to randomization. The study enrolled 2331 patients and had a primary composite end point of time to all-cause death or all-cause hospitalization. Patients had a median follow-up of 30 months. All deaths and first cardiovascular hospitalizations were adjudicated by a blinded independent clinical event committee. HF- ACTION was approved by local institutional review boards, and all enrolled patients provided written informed consent.
Biomarker assessment
A subset of patients enrolled in the HF-ACTION study agreed to participate in the biomarker substudy. Blood samples were obtained on the same day as baseline cardiopulmonary exercise (CPX) testing but before exercise. Samples were collected via peripheral vein into EDTA-containing tubes, centrifuged immediately, and stored at −70°C for subsequent analysis.
GDF-15 levels were measured in an core laboratory on baseline samples (n=910) using sensitive sandwich-immunoassay monoclonal antibodies (Elecsys GDF-15 Assay, Roche Diagnostics, Indianapolis, IN). Details on this assay have been previously described.19 The assay has a lower limit of detection of 400 pg/ml, an upper limit of detection of 20,000 pg/ml, an intra-assay coefficient of variation ≤ 3.0%, and an inter-assay coefficient of variation of ≤4.6 %. The core laboratory was blinded to all clinical data.
Clinical Outcomes
The primary clinical outcome of interest for the current analysis was the relationship between GDF-15 levels and all-cause mortality. The secondary outcomes of interest were (1) the composite of all-cause death or all-cause hospitalization (HF-ACTION primary endpoint); and (2) the composite of cardiovascular death or HF hospitalization. We also assessed the relationship between baseline GDF-15 and baseline assessments of functional capacity including NYHA class, 6 minute walk distance, and maximal oxygen consumption (peak VO2) by cardiopulmonary exercise testing (CPX).
Statistical Analysis
Baseline characteristics are presented as medians (25th, 75th percentiles) and number (percentages). The associations between GDF-15 tertile and baseline characteristics were compared using Kruskal-Wallis tests for continuous and ordinal variables and chi-square tests for categorical variables. Event rates by tertile of GDF-15 are shown with Kaplan-Meier curves. To assess the relationship between GDF-15 and baseline clinical and biomarker variables a model was developed using linear regression with backward elimination and alpha <0.1 for retention. Modeling for the time to event variables were done with complete case analysis. There were 716 patients with complete data for the all-cause death model, 646 for the all-cause death or hospitalization model, and 640 for the CV death or HF hospitalization model. GDF-15 was a continuous variable in all models but was log transformed for analysis because it was not normally distributed. For the clinical outcomes of interest, Cox proportional hazards models were utilized. Hazard ratios (HRs) were calculated for the log2such that the HR represents the risk per two fold greater value of GDF-15; this was utilized over or other log-transformed value to create a clinically more interpretable reference for the increase in GDF-15.
Adjustment variables included demographics (age, sex, and race) and a comprehensive set of predictors that had previously been identified in the HF-ACTION cohort for each end point1,20 (Appendix Table 1a). Since CPX testing is not routinely available in some clinical settings, the adjusted models were examined with and without CPX variables. The baseline CPX variables used in the adjustment models included Peak VO2 by Weber class, ventricular conduction abnormality, VE/VCO2, and exercise duration. (Appendix Table 1b). Models were repeated with the inclusion of the GDF-15 x randomized treatment interaction term to assess for evidence of a differential treatment response to the study intervention based on GDF-15 levels. Model discrimination and risk prediction with and without GDF-15 were evaluated through the c-index, continuous net reclassification index (NRI), and integrated discrimination improvement (IDI). Individual biomarkers were also added to the clinical model separately and the measures of discrimination were compared. The proportional hazards assumption was checked for each endpoint in the full models (including demographic, clinical, CPX, and biomarker data) and no deviations were identified. For each model comparison, the summary measures were reported along with 95% bootstrap confidence intervals based on 999 replications.
Data were analyzed with SAS version 9.4. Statistical significance was based on a p value ≤ 0.05.
Results
Baseline Characteristics
Baseline characteristics for the HF-ACTION study population are shown in Table 1. The biomarker substudy cohort was broadly similar to the overall HF-ACTION population (data not shown). In general, the study population was similar to that from other chronic HFrEF clinical trials, with the exception of a high representation of non-white patients (34%) and women (29%) in the current study. Overall, the patients were medically well managed: 95% on beta blockers; 94% on angiotensin converting enzyme inhibitors (ACE-i) or angiotensin receptor blockers (ARB); and 45% on aldosterone antagonists.
Table 1:
Baseline patient characteristics by tertile of GDF-15
| Characteristic | Total population (HF-ACTION) (N=2331) | Lowest GDF-15 tertile <1173 pg/ml (N=303) | Intermediate GDF-15 tertile 1173 – 2252 pg/ml (N=304) | High GDF-15 tertile >2252 pg/ml (N=303) | P-value |
|---|---|---|---|---|---|
| Age, years | 59.3 (51.1, 68.0) | 50.5 (41.0, 57.7) | 60.9 (54.3, 68.4) | 66.5 (58.1, 75.6) | <0.0001 |
| Female | 661 (28.4) | 127 (41.9) | 75 (24.7) | 61 (20.1) | <0.0001 |
| White race | 1426 (62.1) | 150 (50.5) | 211 (69.9) | 215 (71.9) | <0.0001 |
| Ischemic etiology | 1197 (51.4) | 81 (26.7) | 175 (57.6) | 204 (67.3) | <0.0001 |
| HF hospitalization in prior 6 months | 610 (26.4) | 85 (28.3) | 68 (22.5) | 90 (29.9) | 0.098 |
| LVEF, % | 24.7 (20.0, 30.1) | 25.2 (20.0, 30.1) | 23.9 (18.8, 30.1) | 24.3 (19.8, 29.6) | 0.38 |
| Systolic blood pressure, mmHg | 111 (100, 126) | 116 (102, 126) | 112 (103, 127) | 110 (100, 127) | 0.30 |
| Heart rate, beats per min | 70 (63, 77) | 72 (64, 80) | 70 (64, 78) | 70 (64, 79) | 0.56 |
| Exercise duration, min (CPX test) | 9.6 (6.9, 12.0) | 11.0 (8.7, 13.7) | 10.0 (7.9, 11.6) | 7.5 (5.5, 10.0) | <0.0001 |
| Peak VO2, mL/kg/min (CPX test) | 14.4 (11.5, 17.7) | 16.5 (13.5, 19.6) | 14.9 (12.4, 17.5) | 12.3 (9.8, 14.7) | <0.0001 |
| VE / VCO2 slope | 32.6 (28.1, 38.5) | 29.6 (25.7, 33.9) | 32.1 (28.3, 37.0) | 36.7 (31.6, 44.0) | <0.0001 |
| 6-minute walk distance, m | 371 (299, 435) | 404 (331, 466) | 378 (314, 439) | 324 (258, 388) | <0.0001 |
| Paced | 536 (23.6) | 42 (14.0) | 83 (27.9) | 113 (38.4) | |
| Current NYHA heart failure class | <0.0001 | ||||
| II | 1477 (63.4) | 218 (71.9) | 204 (67.1) | 147 (48.5) | |
| III | 831 (35.6) | 83 (27.4) | 99 (32.6) | 147 (48.5) | |
| IV | 23 (1.0) | 2 (0.7) | 1 (0.3) | 9 (3.0) | |
| KCCQ symptom stability score | 0.8279 | ||||
| <50 | 186 (8.0) | 28 (9.3) | 20 (6.6) | 19 (6.3) | |
| 50 | 1704 (73.5) | 213 (70.8) | 223 (73.6) | 223 (74.3) | |
| >50 | 427 (18.4) | 60 (19.9) | 60 (19.8) | 58 (19.3) | |
| Diabetes | 748 (32.1) | 57 (18.8) | 104 (34.2) | 138 (45.5) | <0.0001 |
| Myocardial infarction | 979 (42.0) | 63 (20.8) | 145 (47.7) | 167 (55.1) | <0.0001 |
| Hypertension | 1388 (59.9) | 168 (55.8) | 199 (65.7) | 212 (70.2) | 0.0008 |
| Current smoker | 388 (16.7) | 47 (15.6) | 53 (17.5) | 48 (15.9) | 0.7856 |
| Body mass index, kg/m² | 29.9 (26.0, 35.1) | 32.1 (27.4, 37.7) | 29.4 (26.4, 35.1) | 29.8 (25.1, 34.0) | <0.0001 |
| Medication and devices | |||||
| ACE inhibitor or ARB | 2199 (94.3) | 293 (96.7) | 296 (97.4) | 277 (91.4) | 0.0009 |
| Beta-blocker | 2203 (94.5) | 295 (97.4) | 287 (94.4) | 280 (92.4) | 0.0002 |
| Beta-blocker dose, mg (N=2183) | 38 (25, 50) | 50 (25, 50) | 37.3 (19.0, 50.0) | 25 (13, 50) | 0.0053 |
| Aldosterone receptor antagonist | 1051 (45.1) | 147 (48.5) | 138 (45.4) | 121 (39.9) | 0.0989 |
| Digoxin | 1046 (44.9) | 132 (43.6) | 148 (48.7) | 155 (51.2) | 0.1620 |
| Loop diuretic | 1816 (77.9) | 216 (71.3) | 237 (78.0) | 265 (87.5) | <0.0001 |
| Loop diuretic dose, mg (N=1783) | 40 (40, 80) | 40 (30, 80) | 40 (40, 80) | 80 (40, 120) | <0.0001 |
| Implantable cardioverter-defibrillator | 938 (40.2) | 93 (30.7) | 142 (46.7) | 160 (52.8) | <0.0001 |
| Biventricular pacemaker | 419 (18.0) | 29 (9.6) | 66 (21.7) | 85 (28.1) | <0.0001 |
| Laboratory values | |||||
| hsTnT, mg/L | 14.8 (8.1, 24.9) | 8.0 (4.6, 13.6) | 14.5 (10.0, 22.6) | 24.9 (15.9, 39.0) | <0.0001 |
| GDF-15, pg/mL | 1596 (970, 2622) | 839 (645, 970) | 1596 (1364, 1836) | 3320 (2622, 4952) | <0.0001 |
| NT-proBNP, pg/mL | 815 (341, 1805) | 404 (176, 911) | 813 (378, 1526) | 2159 (900, 4577) | <0.0001 |
| Creatinine, mg/dL | 1.20 (1.00, 1.50) | 1.00 (0.90, 1.20) | 1.20 (1.00, 1.50) | 1.50 (1.20, 1.80) | <0.0001 |
| eGFR (MDRD) creatinine clearance, mL/min | 66.4 (50.6, 81.0) | 79.9 (68.7, 94.6) | 66.4 (49.6, 78.1) | 49.0 (38.9, 63.3) | <0.0001 |
| Blood urea nitrogen, mg/dL | 20 (15, 28) | 16 (13, 19) | 21 (15, 26) | 28 (21, 41) | <0.0001 |
| Characteristic | Total population (HF-ACTION) (N=2331) | Lowest GDF-15 tertile <1173 pg/ml (N=303) | Intermediate GDF-15 tertile 1173 – 2252 pg/ml (N=304) | High GDF-15 tertile >2252 pg/ml (N=303) | P-value |
| Hemoglobin, g/dL | 13.5 (12.3, 14.6) | 13.5 (12.5, 14.7) | 13.4 (12.3, 14.8) | 12.9 (11.7, 14.1) | <0.0001 |
Data are presented as medians (Q1–Q3) or percentages. BB , beta blocker; BMI, body mass index; BP, blood pressure; CPX, cardiopulmonary exercise; HF, heart failure; HR, hazard ratio; IVCD, intraventricular conduction delay; KCCQ, Kansas City Cardiomyopathy Questionnaire; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, amino-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; peak VO2, maximal oxygen consumption; and RBBB, right bundle branch block.
GDF-15 tertiles were identified as follows: lowest tertile < 1173 pg/ml; middle tertile 1173–2252 pg/ml; highest tertile >2252 pg/ml. Compared to patients in the lowest tertile of GDF-15 values, patients in the highest tertile of GDF-15 were older and had more markers of severe HF including higher prevalence of comorbidities, higher NYHA class, higher NT-proBNP, and higher hs-TnT. Use of evidence-based medical therapies tended to be lower in patients with higher GDF-15 levels and loop diuretic doses tended to be higher, again consistent with more severe HF in the patients with the highest GDF-15 values. In the overall HF-ACTION cohort, some differences in baseline characteristics were seen among patients who were included and excluded from multivariable modeling due to missing data; however, the missingness of data did not impact on clinical outcomes (data not shown).
Biologic Correlates of GDF-15
The independent variable most strongly associated with higher GDF-15 was higher hsTnT (p<0.0001; Table 2). In addition, older age, lower peak VO2, higher creatinine, higher NTproBNP were also found to be associated with higher GDF-15 levels after multivariable adjustment.
Table 2:
Association of GDF-15 with baseline clinical and biomarker variables
| Variable | Coefficient | 95% Confidence Limits | T | P-value |
|---|---|---|---|---|
| Log-2(hsTnT) | 0.18 | (0.12, 0.23) | 6.32 | <.0001 |
| Age, years | 0.01 | (0.01, 0.02) | 5.86 | <.0001 |
| Peak VO2, mL/kg/min | −0.04 | (−0.06, −0.03) | −5.69 | <.0001 |
| Log-2(creatinine) | 0.35 | (0.22, 0.48) | 5.25 | <.0001 |
| Log-2(NT-proBNP) | 0.08 | (0.05, 0.12) | 4.39 | <.0001 |
| LVEF, % | 0.01 | (0.007, 0.02) | 3.75 | 0.0002 |
| SBP, mmHg for values up to 125 | −0.008 | (−0.01, −0.003) | −3.49 | 0.0005 |
| SBP, mmHg for values above 125 | 0.008 | (0.002, 0.01) | 2.47 | 0.0136 |
| White race | 0.19 | (0.08,0.31) | 3.29 | 0.0011 |
| BMI, for values up to 35 | −0.02 | (−0.03,−0.008) | −3.20 | 0.0015 |
| Blood urea nitrogen (mg/dL) | 0.007 | (0.002, 0.01) | 2.98 | 0.0030 |
| Diabetes | 0.17 | (0.06, 0.28) | 2.96 | 0.0032 |
| Heart rate, beats per min | 0.006 | (0.001, 0.01) | 2.64 | 0.0086 |
| NYHA class III/IV | 0.12 | (0.01, 0.23) | 2.19 | 0.0292 |
GDF-15 and Symptom Status, Functional Capacity, and Exercise Performance
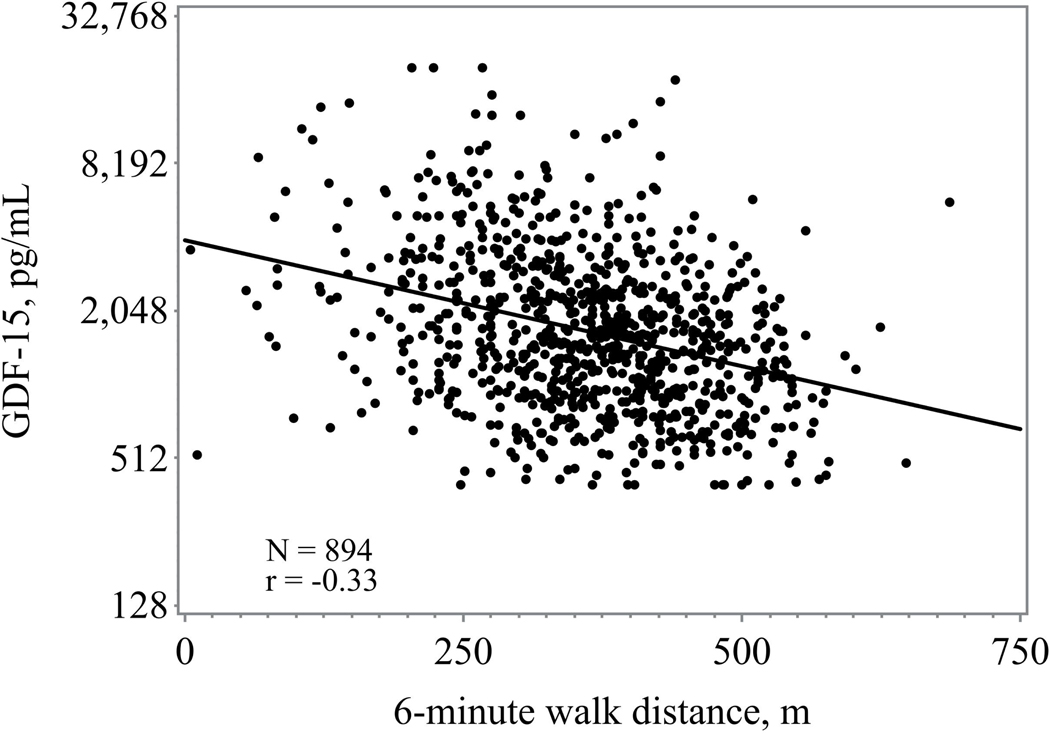
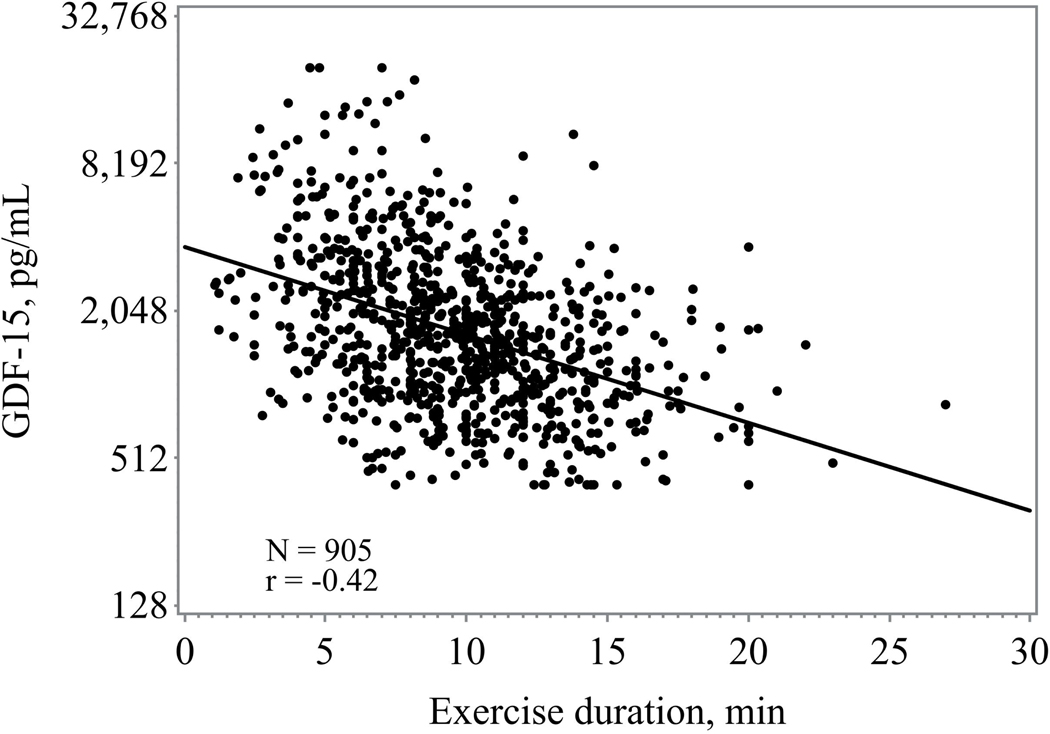
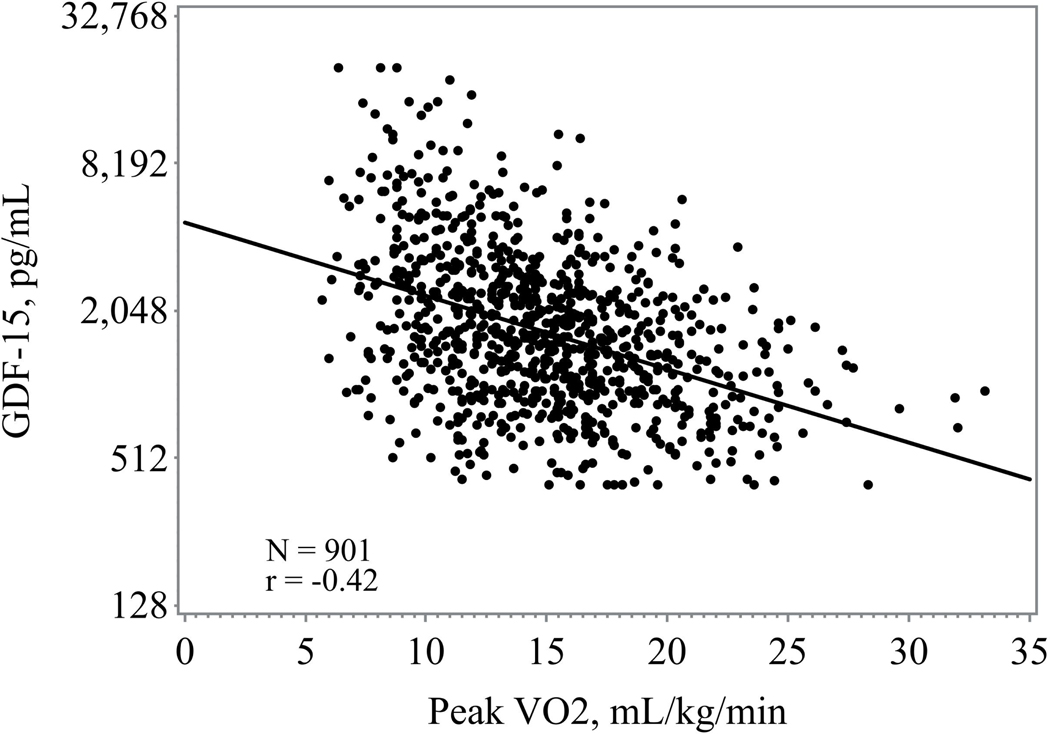
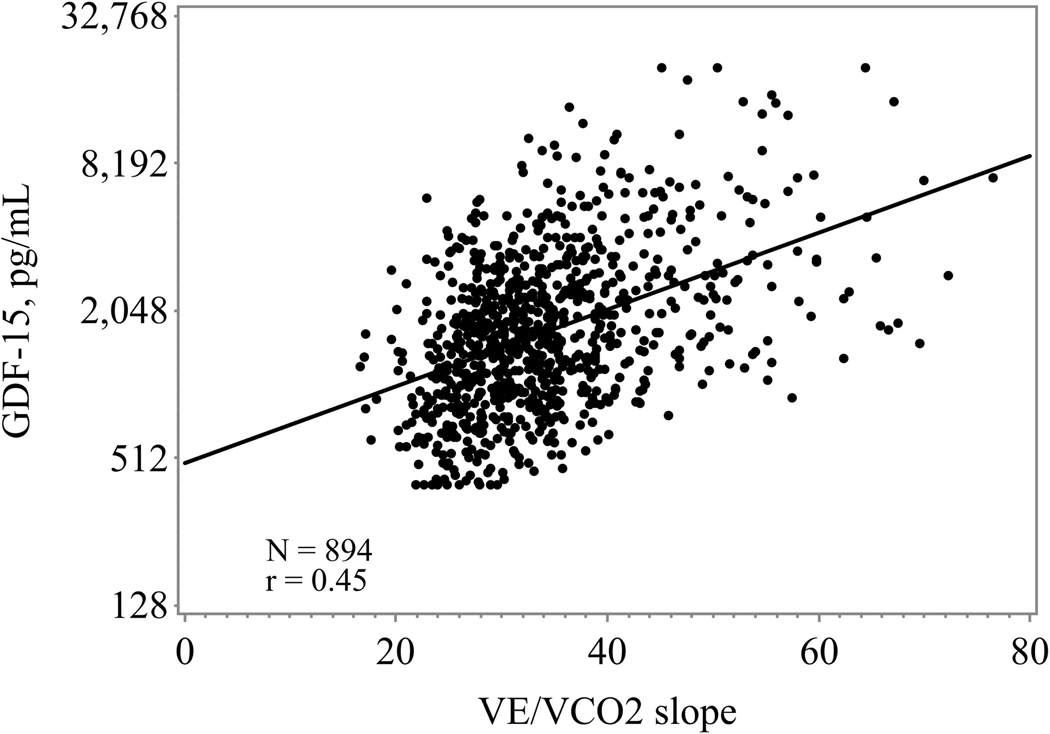
Higher GDF-15 levels were consistently associated with worsened 6-minute walk distance, exercise duration, peak VO2, and higher VE/VO2 slope (figure 1) Those in the highest tertile of GDF-15 had the lowest exercise duration and lowest peak VO2 (Table 1). When assessed as a continuous variable higher GDF-15 levels were associated with a lower baseline peak VO2 (r= −0.40; p <0.0001) and shorter 6-minute walk distance (r= −0.32, p <0.0001).
Figure 1.
a: Association between GDF-15 and 6-minute walk distance
b: Association between GDF-15 and exercise duration
c: Association between GDF-15 and peak V02
d: Association between GDF-15 and VE/V02 slope
GDF-15 and Clinical Endpoints
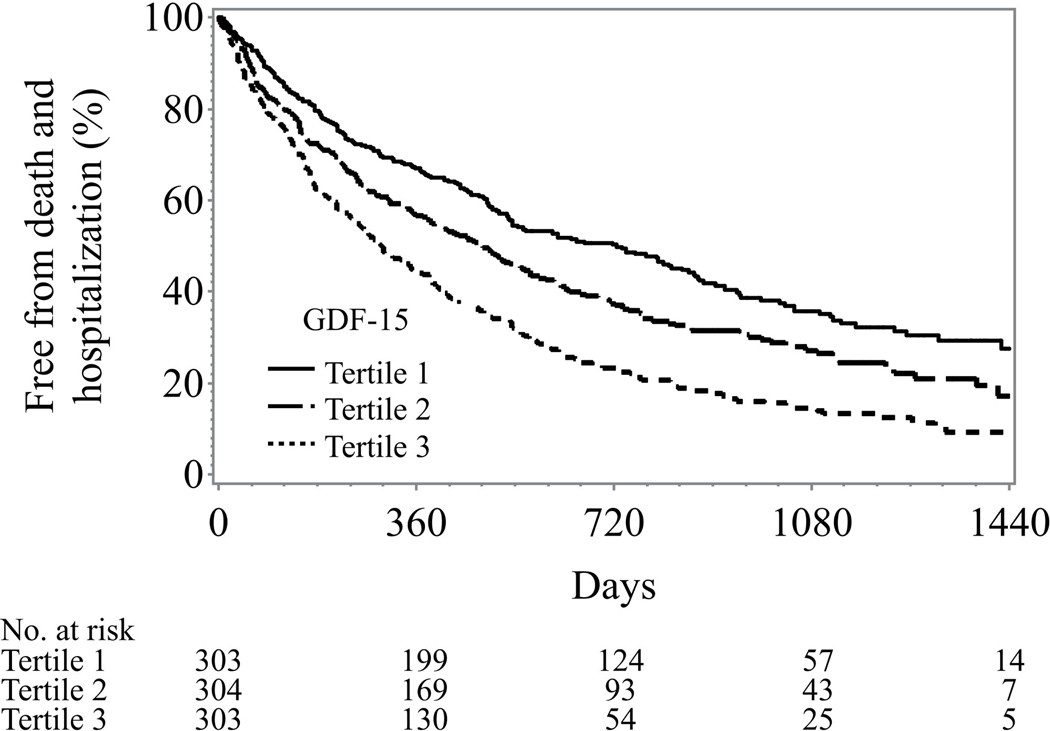
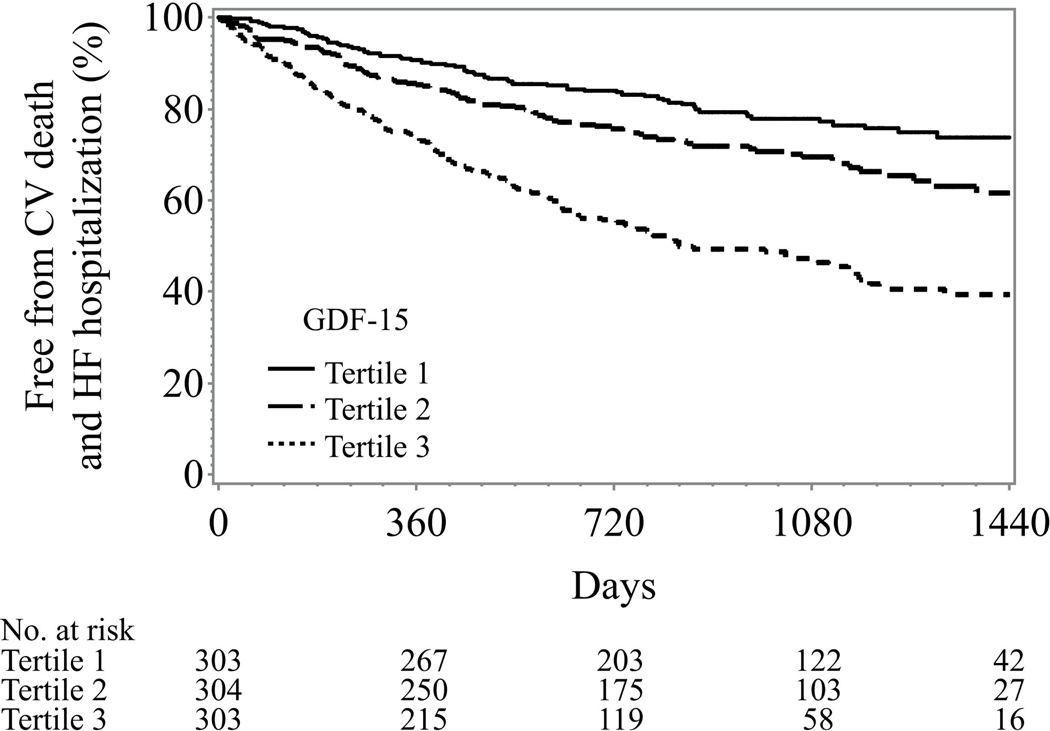
The median follow-up was 32 months and there were 171 deaths, 647 all-cause death or hospitalization events, and 301 cardiovascular death or HF hospitalization events in our study cohort. Generally, higher tertiles of GDF-15 were associated with worse clinical outcomes across all the clinical endpoints of interest (Figure 2). In univariable analyses, a two fold increased level of GDF15 was associated with a 203% increase in all cause mortality, a 32% increase in the composite of all cause death or all cause hospitalization, and a 61% increase in the composite of cardiovascular death or heart failure hospitalization (p < 0.0001) for all.
Figure 2: Unadjusted Kaplan-Meier curves of GDF-15 tertiles.
Legend: GDF-15 levels: lowest tertile < 1173 pg/ml; middle tertile 1173–2252 pg/ml; highest tertile >2252 pg/ml
A: All-cause death
B: All-cause death and hospitalization
C: Cardiovascular death and heart failure hospitalization
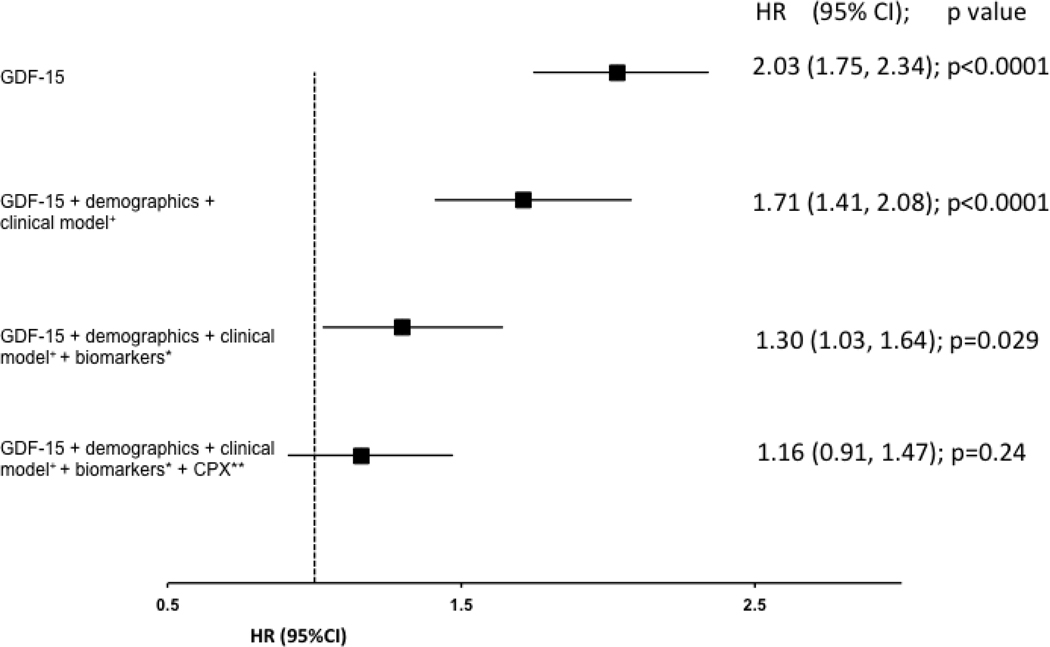
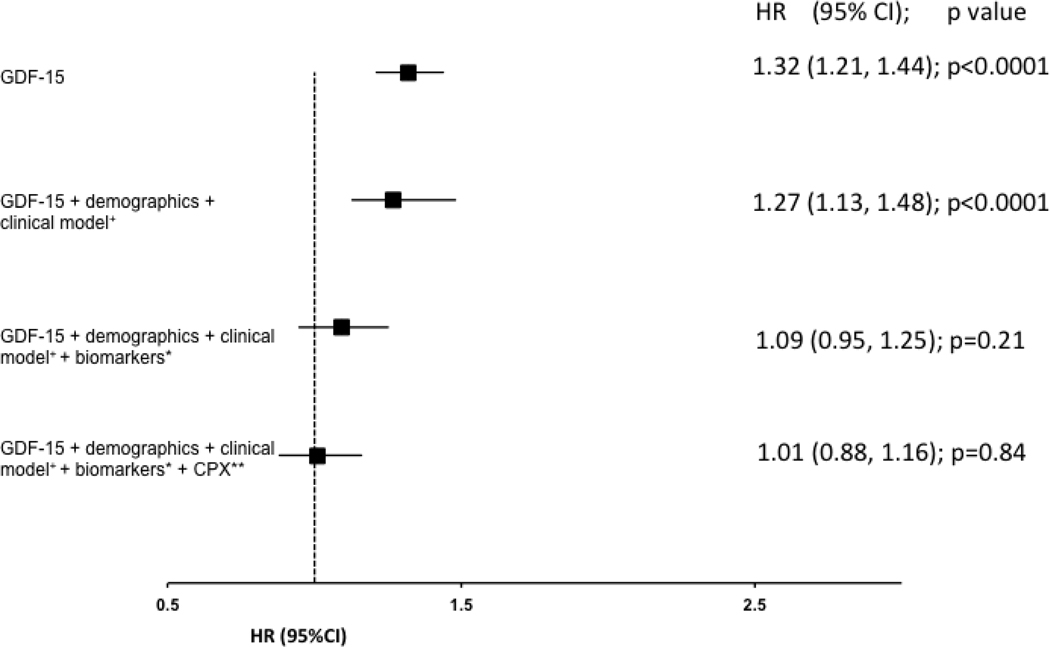
In order to assess the independent association of GDF15 in the context of other commonly clinically available data, we created a series of multivariable models that sequentially included clinical and demographic variables, other guideline recommended biomarkers (NT-proBNP and hs-Trop T), and variables obtained from CPX testing. After adjustment for demographic and clinical variables only, a two-fold increase in the concentration of GDF-15 was associated with a 71% increased risk of all-cause death (p <0.0001; Figure 3A), 27% increased risk in all-cause death or rehospitalization (p < 0.0001; Figure 3B), and a 34% increased risk of cardiovascular death or HF rehospitalization (p =0.0005; Figure 3C). After additionally adjusting for both hs-TnT and NT-proBNP on top of clinical and demographic variables, a doubling of the baseline concentration of GDF-15 was still associated with a 30% increased risk of all-cause death (p=0.03; figure 3A). After further adjusting for CPX variables in addition to the clinical model and biomarkers, GDF-15 was no longer significantly associated with any of the clinical endpoints of interest. There were no significant interactions between GDF-15 and randomized treatment assignment (exercise training v. control) for any of the clinical endpoints (all p>0.14), suggesting that GDF-15 did not identify patients more or less likely to respond to exercise training.
Figure 3.
a: Full model adjusted for demographic variables and serum creatinine (truncated at 2.3), BMI, loop diuretic dose (truncated at 100), CCS angina class (0, 1, ≥2), and LVEF; * Biomarkers refers to NT-proBNP and HsTnT; ** CPX refers to baseline cardiopulmonary exercise stress testing variables which includes exercise duration and ventricular conduction.
b: Clinical model adjusted for KCCQ symptom stability score (3 categories: <50, 50, >50), BUN, United States, LVEF, BB dose (truncated at 50), moderate or severe MR; *Biomarkers refers to NT-proBNP and HsTnT; ** CPX refers to baseline cardiopulmonary exercise stress testing variables which includes Peak VO2 characterized by Weber class, and ventricular conduction on the baseline CPX test
c: Clinical model adjusted for Loop diuretic dose (truncated at 100), LVEF, moderate or severe MR, KCCQ symptom stability score (3 categories: <50, 50, >50), BUN (truncated at 39);* Biomarkers refers to NT-proBNP and HsTnT; ** CPX refers to baseline cardiopulmonary exercise stress testing variables which includes Peak VO2 characterized by Weber class, VE/VCO2, ventricular conduction on the baseline CPX test.
Prognostic Utility of GDF-15
The prognostic utility of GDF-15 on top of the clinical models, biomarkers (NT-proBNP, and hs-TnT), and CPX variables are shown in Table 3. GDF-15 improves discrimination of all-cause death on top of the clinical model, particularly the discrimination of non-events. When comparing the addition of biomarkers to the clinical model, all biomarker improve discrimination of all-cause death individually. However, NTproBNP appears to have the greatest magnitude of improvement in discrimination, followed by GDF-15 (appendix table 2). While the addition of GDF-15 incrementally increased the C-index for all-cause death on top of clinical, biomarker, and CPX variables, the improvement was not significant overall. Similarly, GDF-15 did not significantly improve discrimination for the composite outcomes of all-cause death or hospitalization and cardiovascular death or HF hospitalization.
Table 3:
Prognostic Utility of GDF-15
| Model | C without GDF-15 | C with GDF-15 | Overall NRI | NRI for events | NRI for non-events | IDI |
|---|---|---|---|---|---|---|
| All-cause death | ||||||
| Clinical model | 0.704 (0.659, 0.749) | 0.735 (0.690, 0.779) | 0.298 (0.081, 0.516) | 0.044 (−0.131, 0.227) | 0.254 (0.155, 0.342) | 0.043 (0.025, 0.062) |
| Clinical model + CPX | 0.751 (0.709, 0.794) | 0.759 (0.717, 0.801) | 0.150 (−0.071, 0.377) | −0.029 (−0.201, 0.150) | 0.179 (0.087, 0.272) | 0.009 (−0.002, 0.019) |
| Clinical model + biomarkers | 0.755 (0.713, 0.797) | 0.759 (0.717, 0.801) | 0.105 (−0.124, 0.339) | 0.058 (−0.116, 0.236) | 0.046 (−0.050, 0.140) | 0.004 (−0.005, 0.011) |
| Clinical model + biomarkers + CPX | 0.770 (0.730, 0.810) | 0.772 (0.732, 0.812) | −0.038 (−0.263, 0.192) | −0.044 (−0.217, 0.136) | 0.006 (−0.099, 0.106) | −0.001 (−0.005, 0.004) |
| All-cause death or hospitalization | ||||||
| Clinical model | 0.612 (0.584, 0.640) | 0.623 (0.595, 0.650) | 0.090 (−0.242, 0.378) | −0.030 (−0.122, 0.066) | 0.120 (−0.164, 0.369) | 0.008 (0.002, 0.015) |
| Clinical model + CPX | 0.637 (0.610, 0.664) | 0.640 (0.613, 0.668) | −0.181 (−0.500, 0.111) | −0.101 (−0.190, −0.002) | −0.080 (−0.379, 0.170) | 0.002 (−0.002, 0.006) |
| Clinical model + biomarkers | 0.636 (0.609, 0.663) | 0.639 (0.612, 0.666) | −0.272 (−0.632, 0.039) | −0.105 (−0.193, −0.009) | −0.168 (−0.490, 0.099) | −0.004 (−0.007, −0.002) |
| Clinical model + biomarkers + CPX | 0.649 (0.623, 0.676) | 0.650 (0.623, 0.676) | −0.404 (−0.737, −0.102) | −0.144 (−0.234, −0.046) | −0.260 (−0.569, −0.007) | 0.000 (−0.001, 0.000) |
| CV death or HF hospitalization | ||||||
| Clinical model | 0.715 (0.680, 0.749) | 0.725 (0.691, 0.759) | 0.111 (−0.086, 0.317) | 0.013 (−0.113, 0.155) | 0.098 (−0.015, 0.219) | 0.007 (0.000, 0.015) |
| Clinical model + CPX | 0.730 (0.696, 0.764) | 0.733 (0.700, 0.767) | 0.028 (−0.168, 0.238) | −0.028 (−0.159, 0.118) | 0.056 (−0.061, 0.177) | 0.000 (−0.005, 0.004) |
| Clinical model + biomarkers | 0.752 (0.720, 0.784) | 0.752 (0.719, 0.784) | −0.063 (−0.250, 0.144) | −0.070 (−0.201, 0.079) | 0.007 (−0.106, 0.123) | −0.001 (−0.003, 0.000) |
| Clinical model + biomarkers + CPX | 0.755 (0.723, 0.787) | 0.756 (0.723, 0.788) | −0.084 (−0.281, 0.123) | −0.091 (−0.230, 0.057) | 0.007 (−0.106, 0.123) | −0.001 (−0.001, 0.000) |
CV: cardiovascular; HF: heart failure; CPX: cardiopulmonary exercise stress testing; NRI net reclassification index; IDI integrated discriminatory improvement
Bolded text highlights the results where by GDF-15 significantly increases the C-statistic, overall NRI, and IDI.
Discussion
Assessing the clinical utility of new and existing biomarkers remains an important goal in heart failure. The present analyses from the HF-ACTION study adds a number of important findings regarding the role of between GDF-15 and HF: (1) a two fold greater concentration of GDF-15 is associated with a 30% greater risk of all-cause death despite comprehensive multivariable adjustment including hs-TnT and NT-proBNP; (2) greater GDF-15 levels were associated with worse symptom burden and functional capacity, including baseline NYHA class, 6-minute walk distance, and peak VO2; and (3) the strongest baseline predictor of higher GDF-15 levels was greater Hs-TnT. Since it was a large multicenter cohort of demographically diverse, ambulatory, chronic HFrEF patients, the HF-ACTION trial allowed for a comprehensive assessment of GDF-15’s association with cardiovascular outcomes,. The range of outcomes in HFACTION, including CPX data available through HF-ACTION provides a unique opportunity to evaluate the association between GDF-15, functional capacity, and exercise performance.
Association of GDF-15 with baseline clinical and biomarker variables
Cellular expression of GDF-15 increases in response to various stressors including reactive oxygen species and proinflammatory cytokines8,21. In murine models, GDF-15 inhibits apoptosis, hypertrophy, and adverse cardiac remodeling8,9,22. GDF-15 is not normally expressed in the heart; however, it can be induced in experimental models of myocardial ischemia, pressure/volume overload, and dilated cardiomyopathy8,9. Our study expands upon these findings; we identified that GDF-15 levels were closely associated with hsTnT and NT-proBNP (Table 2), suggesting that cardiac ischemia and cardiomyocyte stretch play a role in GDF-15 expression. Our also study aligns with prior literature suggesting that GDF-15 is closely associated with multiple biologic pathways including renal dysfunction and abnormal glucose regulation11,16,23,24.
Association between GDF-15 and Cardiovascular Outcomes
Current HF guidelines indicate that natriuretic peptides and biomarkers of myocardial injury (cardiac troponin T or I) may be considered for ambulatory risk stratification5. Our findings suggest that GDF-15 may also play an important role in ambulatory risk stratification. In the ValHeFT analysis, after adjustment for clinical and biomarker variables, a 100 pg/ml increase in GDF-15 was associated with an increased risk of all-cause death(HR 1.007 95% CI 1.001–1.014; p = 0.02)16. Compared to ValHeFT, our patient population was younger (median age 59 years versus 63), had increased non-white participants (36% versus 16%), higher proportion of patients with hypertension (64% versus 7%), had lower median GDF-15 levels (1596 pg/ml versus 2040 pg/ml), and greater use of evidence based medical therapy (beta-blocker use 95% versus 33%; spironolactone use 45% versus 2%). Despite these difference, our study still demonstrated that in a demographically diverse, well-medically managed group of chronic HF patients, GDF-15 was associated with an increased risk of all-cause mortality even after adjusting for commonly available demographic, clinical, and laboratory variables.
Compared to other biomarkers, GDF-15 appears to have a robust association with all-cause mortality. Prior studies from HF-ACTION have evaluated the fibrosis marker galectin-325 and the interleukin signaling ligand soluble ST226. Galectin-3 was not associated with an increased risk of all-cause death after adjustment for clinical variables and NT-proBNP (adjusted HR 1.06; p=0.30)25. A doubling of ST2 was associated with increased risk of all-cause death (adjusted HR 1.42; p=0.007)26;however, this study did not adjust for troponin, which is a powerful predictor of mortality in patients with HF26.
In our study, GDF-15 does not provide any additional prognostic information when CPX variables were added to demographic, clinical, laboratory, and biomarker data. Similar results have been seen with other biomarkers including ST2 and galectin-3.25,26 Our results suggest that the unique prognostic information provided by GDF-15 are also captured by variables derived from CPX testing. Abnormal CPX measures inherently indicate worse HF and may reduce the association between GDF-15 and cardiovascular outcomes. As CPX testing is not widely available or performed in the routine management of heart failure patients in clinical practice28, our results demonstrate the strength of GDF-15’s association with clinical outcomes of interest on top of routinely available clinical and biomarker variables.
GDF-15 significantly improved discrimination for all-cause mortality on top of clinical variables; however, when CPX variables or additional biomarkers were added to the clinical model for all-cause death, model discrimination did not improve after the addition of GDF-15. In the ValHeFT study, the addition of GDF-15 to clinical and biomarker variables (BNP, hs-CRP, and hs-TnT) did improve the c-index for mortality (c-index from 0.73 to 0.76; p=0.02) and the IDI for mortality was borderline significant (p=0.06)16. Among patients with chronic HF in a Singapore cohort, GDF-15 also improved discrimination on top of a clinical model with NT-proBNP and hs-TnT (c-index from 0.72–0.74; p=0.0019).29 In our study, the discriminatory ability of biomarkers (hs-TnT and NT-proBNP) and CPX variables on top of baseline clinical variables for the outcome of all-cause mortality was already very high as reflected by a C-index of 0.77 (table 3). While GDF-15 improved the C-index for all-cause mortality, on top of the baseline biomarkers and CPX variables, this increase was not significant. This finding likely reflects the challenge of improving discrimination on top of an already robust model. However, combined with the prior analyses of GDF-15 among patients with HF, the totality of evidence suggest that GDF-15 may improve discrimination above and beyond clinical variables and established biomarkers.
Strengths and Limitations
This was a post-hoc study and is subject to the limitation of these types of analyses. Patients enrolled in the biomarker substudy were predominantly from the US. As with most clinical trials, our patient population differed from the broader heart failure population as seen in HF registries30, although HF ACTION was notable for enrolling a higher proportion of non white patients and women than most other heart failure clinical trials. Furthermore, the patients in HF ACTION had a higher usage of evidence based therapies compared to other cohort evaluating the prognostic role of GDF-15 among patients with HF. Attempting to assess the association of GDF-15 and individual outcomes over an optimized set of variables may have contributed to the difficulties in showing a significant association with outcomes besides all-cause mortality. Patients enrolled in the HF ACTION had impaired ejection fraction (LVEF < 0.35); as such our results cannot be extrapolated to other HF populations such as patients with heart failure and preserved ejection fraction (HFpEF). Our study revealed that patients with higher GDF-15 had worsened peak VO2; however, all patients had to consent and be able to perform a CPX test which may not reflect the overall HF population.
Conclusion
In this analysis of a large clinical study with well-treated chronic HFrEF patients, increasing baseline GDF-15 was associated with long-term adverse cardiovascular outcomes. Even after comprehensive multivariable adjustment including demographics, clinical variables, and biomarkers (hs-TnT and NT-proBNP), double the concentration GDF-15 was significantly associated with all-cause mortality. Further adjustment for CPX derived variables attenuated the value of GDF-15, but CPX testing is not widely used in routine clinical practice. These data suggest a potential role for GDF-15 in risk stratification among patients with chronic HFrEF.
Supplementary Material
Perspective.
Competency in Medical Knowledge
GDF-15, a member of the transforming growth factor-B cytokine family, is an emerging biomarker used for risk stratification among patients with cardiovascular diseases. In our analysis of a demographically diverse, well managed patients with chronic HFrEF, GDF-15 provides independent prognostic information on top of established predictors of outcomes including hs-TnT and NT-proBNP.
Translational outlook
Our findings suggest a possible role for GDF-15 in the risk stratification of patients with chronic HFrEF. Future studies evaluating the mechanistic role of GDF-15 in patients with HF may lead to new therapeutic targets.
Acknowledgements
1. Abhinav Sharma: Received funding from the Alberta Innovates Health Solution Clinician Scientist fellowship, Roche Diagnostics, and the Bayer-Vascular Canadian Cardiovascular Society research grant.
2. Susanna R. Stevens: No COI
3. Joseph Lucas: No COI
4. Mona Fiuzat: Research funding from Roche
5. Kirkwood F. Adams: Research funding and consultation from Roche.
6. David J. Whellan: No COI
7. Mark P. Donahue: No COI
8. Dalane W. Kitzman: No COI
9. Ileana L. Piña: No COI
10. Faiez Zannad: personal fees from Roche Diagnostics, during the conduct of thestudy; personal fees from Janssen, personal fees from Bayer, personal fees from Pfizer, personal fees from Novartis, personal fees from Boston Scientific, personal fees from Resmed, personal fees from Amgen, personal fees from CVRx, personal fees from Quantum Genomics, personal fees from Takeda, personal fees from General Electric, personal fees from Boehringer, personal fees from Relypsa, personal fees from ZS Pharma, personal fees from AstraZeneca, personal fees from GSK, other from cardiorenal, other from CVCT, outside the submitted work; In addition, Dr. Zannad has a patent null issued.
11. William E. Kraus: No COI
12. Christopher M. O’Connor: Research funding from Roche
13. G. Michael Felker: Research funding from Roche
Footnotes
conflict of interest/disclosures:
All other authors report no conflicts of interest.
References:
- 1.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168(4):418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385(9970):812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Mair J, Mueller C, et al. Recommendations for the use of natriuretic peptides in acute cardiac care: A position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. European Heart Journal. 2012;33(16):2001–2006. doi: 10.1093/eurheartj/ehq509. [DOI] [PubMed] [Google Scholar]
- 7.Kempf T, Zarbock A, Widera C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17(5):581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 8.Kempf T, Eden M, Strelau J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98(3):351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Kimball TR, Lorenz JN, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98(3):342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 11.Wallentin L, Hijazi Z, Andersson U, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130(21):1847–1858. doi: 10.1161/CIRCULATIONAHA.114.011204. [DOI] [PubMed] [Google Scholar]
- 12.Wollert KC, Kempf T, Lagerqvist B, Lindahl B. Growth Differentiation Factor 15 for Risk Stratification and Selection of an Invasive Treatment Strategy in Non–ST-Elevation Acute Coronary Syndrome. Circulation. 2007;116(14):1540–1548. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 13.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-Differentiation Factor-15 Is a Robust, Independent Predictor of 11-Year Mortality Risk in Community-Dwelling Older Adults The Rancho Bernardo Study. Circulation. 2011;123(19):2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggers KM, Kempf T, Lagerqvist B, et al. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-ST-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3(1):88–96. doi: 10.1161/CIRCGENETICS.109.877456. [DOI] [PubMed] [Google Scholar]
- 15.Dallmeier D, Brenner H, Mons U, Rottbauer W, Koenig W, Rothenbacher D. Growth Differentiation Factor 15, Its 12-Month Relative Change, and Risk of Cardiovascular Events and Total Mortality in Patients with Stable Coronary Heart Disease: 10-Year Follow-up of the KAROLA Study. Clin Chem. 2016;62(7):982–992. doi: 10.1373/clinchem.2016.254755. [DOI] [PubMed] [Google Scholar]
- 16.Anand IS, Kempf T, Rector TS, et al. Serial Measurement of Growth-Differentiation Factor-15 in Heart Failure: Relation to Disease Severity and Prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122(14):1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 17.Whellan DJ, O’Connor CM, Lee KL, et al. Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION): Design and rationale. American Heart Journal. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wollert KC, Kempf T, Giannitsis E, et al. An Automated Assay for Growth Differentiation Factor 15. J App Lab Med 2017;1(5):510–521. doi: 10.1373/jalm.2016.022376 [DOI] [PubMed] [Google Scholar]
- 20.O’Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circulation: Heart Failure. 2012;5(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. AJC. 2005;95(8):948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Kim H-N, Januzzi JL. Natriuretic peptide testing in heart failure. Circulation. 2011;123(18):2015–2019. doi: 10.1161/CIRCULATIONAHA.110.979500. [DOI] [PubMed] [Google Scholar]
- 23.Kempf T, Horn-Wichmann R, Brabant G, et al. Circulating Concentrations of Growth-Differentiation Factor 15 in Apparently Healthy Elderly Individuals and Patients with Chronic Heart Failure as Assessed by a New Immunoradiometric Sandwich Assay. Clin Chem. 2006;53(2):284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 24.Kempf T, Haehling von S, Peter T, et al. Prognostic Utility of Growth Differentiation Factor-15 in Patients With Chronic Heart Failure. J Am Coll Cardiol. 2007;50(11):1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 25.Felker GM, Fiuzat M, Shaw LK, et al. Galectin-3 in Ambulatory Patients With Heart Failure: Results From the HF-ACTION Study. Circulation: Heart Failure. 2012;5(1):72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felker GM, Fiuzat M, Thompson V, et al. Soluble ST2 in Ambulatory Patients With Heart Failure: Association With Functional Capacity and Long-Term Outcomes. Circulation: Heart Failure. 2013;6(6):1172–1179. doi: 10.1161/CIRCHEARTFAILURE.113.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O’Connor CM, Felker GM. Troponin Elevation in Heart Failure. JACC. 2010;56(14):1071–1078. doi: 10.1016/j.jacc.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Balady GJ, Arena R, Sietsema K, et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults: A Scientific Statement From the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 29.Chan MMY, Santhanakrishnan R, Chong JPC, et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. European Journal of Heart Failure. 2015;18(1):81–88. doi: 10.1002/ejhf.431. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122(6):585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.