Abstract
Introduction
E-cigarette or vaping product use associated lung injury (EVALI) has been an important health risk in both children and adults. The pathophysiology of EVALI is not well understood. However, it is speculated that certain substances such as Vitamin E Acetate (VEA), particularly in marijuana containing vape cartridges may result in lung injury and lead to respiratory dysfunction. EVALI is often seen in the absence of infections, but it has been found to be associated with both fungal and bacterial infections. Like EVALI, nontuberculous mycobacteria (NTM) pulmonary disease is also on the rise, but is primarily reported in immunocompromised individuals. Here, we present three immunocompetent individuals wherein pulmonary NTM infection co-occurred with vaping.
Methods
Medical information including patient history, laboratory, and radiograph reports were abstracted from electronic medical records from participating institutions located in the Bronx, NY, Philadelphia, PA, and Lexington, KY.
Results
All three cases were otherwise immunocompetent individuals with a significant history of vaping either nicotine and/or marijuana containing products. The pathogens isolated include Mycobacterium avium complex, M. xenopi, and M. gordonae. All three patients were treated for NTM.
Conclusion
There is little reported on the association between vaping and NTM. It is possible that vaping may have rendered these individuals to be more susceptible to NTM colonization and infection. The possible mechanisms of vaping lung injury and pulmonary NTM are discussed.
Introduction
The fit cases of e-cigarette or vaping product use associated lung injury (EVALI) were reported to the Centers for Disease Control and Prevention (CDC) in August 2019 [1]. Since then, the United States has faced an outbreak of EVALI, resulting in over 2400 hospitalizations across the country, afflicting mostly individuals under the age of 35 years old [2]. The exact toxicant causing EVALI is still unclear; however, a recent examination of bronchoalveolar lavage fluid (BALF) samples from patients with EVALI suggests that inhaled Vitamin E Acetate (VEA), a diluent in tetrahydrocannabinol (THC), may be responsible [2]. Other possible ingredients are other diluents such as propylene glycol, glycerin, chemical fl vors, and higher concentrations of nicotine, and fine and ultrafine particles [3]. Vaping may lead to lung injury by altering surfactant production and function, mucociliary function, and the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) channel, and by increasing the risk of bacterial infections in both animals [4] and humans [5].
Pulmonary nontuberculous mycobacteria (NTM) is uncommon and reported primarily in immunocompromised individuals [6]. For unclear reasons, the incidence of pulmonary NTM in immunocompetent individuals is increasing in the United States [7]. It is suspected that NTM infection in humans is acquired from bacteria-laden water, soil, or dust, although the source of infection cannot always be identified [8]. NTM includes all mycobacteria other than Mycobacterium tuberculosis (MTB), bovis, and leprae, and more than 150 species are known [7].
Little is known about the risk that vaping may pose on the development of NTM infection of the lungs. After reviewing the literature, we found one case of co-infection with Mycobacterium avium complex (MAC) in a case series on vaping in adolescents [9]. Here, we present three additional cases of co-infection with NTM in otherwise young healthy individuals with habitual use of e-cigarette or vaping and aim to establish the importance of this association (Table 1). We later discuss possible mechanisms leading to lung injury by this association and the medical implications of vaping and pulmonary NTM.
Table 1.
Summary of NTM and vaping-related cases
| Case | Age and sex | Presenting symptoms | Vape content and duration | Pertinent Labs | NTM | Treatment |
|---|---|---|---|---|---|---|
| 1 | 18 YO female | 6 weeks of headache and 1 week of night sweats, fatigue, and cough | Cannabidiol oil twice weekly for 6 month | Positive serum Aspergillus antigen. Negative PPD, APB sputum smear × 2, and interferon-gamma release assay | M. xenopi | Rifabutin, Ethambutol, Moxifloxacin, inhaled Amikacin and Posaconazole |
| Normal CBC, IgA, G, and M, IgG subclasses, complement levels and rheumatologic diagnostics | ||||||
| BALF positive for Aspergillus fumigatus. but negative for AFB. | ||||||
| Lung tissue positive for NTM | ||||||
| 2 | 19 YO male | 1.5-year history of night sweats, productive cough, unintentional weight loss, and new-onset mild hemoptysis | Nicotine, marijuana (vaping, dabbing, and dripping) for 5 years | Negative coccidioidomycosis, cryptococcus, histoplasmosis, interferon-gamma release assay, and PPD | MAC | Tri-weekly Azithromycin, Rifampin, and Ethambutol |
| Normal CBC, IgM, IgG, vaccine titers, and CD3/CD4% pane. | ||||||
| Sweat chloride test normal | ||||||
| Sputum positive for Mycobacterium avium complex | ||||||
| BALF with neutrophilia and > 50% lipid-laden macrophages | ||||||
| 3 | 34 YO male | 3 months of productive cough, night sweats, unintentional weight loss, and new-onset mild hemoptysis | Flavored tobacco via Hookah for several years | Negative interferon-gamma release assay and HIV test | M. gordonae | Ethambutol, Rifampin, and Azithromycin |
| BALF cultures negative except for M.gordonas. Lymph node biopsy positive for M. gordonae by MALDI-TOF PCR. | ||||||
| Granulomatous inflammation without malignant cells |
NTM Nontuberculous mycobacterium, MAC Mycobacterium avium complex, PPD Shorthand for tuberculin skin test, AFB Acid fast bacteria, Ig Immunoglobulin, CBC Complete blood count BALF Bronchoalveolar lavage fluid
Methods
A retrospective chart review was conducted by three participating institutions on individuals who were otherwise healthy young adults with a history of vaping in the last 90 days who were found to have pulmonary NTM disease. Medical information including patient history, laboratory, and radiograph reports were abstracted from electronic medical records from either the Children’s Hospital at Montefiore in the Bronx, NY, the Children’s Hospital of Philadelphia in Philadelphia, PA, or the University of Kentucky College of Medicine in Lexington, KY. Each contributing institution received approval by their local IRB.
Results
Case 1
An 18-year-old female with asthma presented with 6 weeks of headache and 1 week of night sweats, fatigue, and cough. 6 months prior, she began vaping cannabinoid oil twice weekly. A chest CT showed a 5 × 9 cm cavity in the left upper lobe (LUL) and multiple bilateral calcifi nodules with bilateral mediastinal lymphadenopathy (Fig. 1a). Testing for MTB, other infectious causes, and workup for immunodeficiency and rheumatologic causes were negative. BALF and transbronchial biopsy cultures of the cavitary lesion were positive for Aspergillus fumigatus (AF) and negative for mycobacteria. This was followed by surgical resection of the cavitary lesion. Tissue cultures isolated AF and she was started on voriconazole, which was later replaced with posaconazole. Tissue also grew acid fast bacteria (AFB) and mycobacterial polymerase chain reaction (PCR) identified M. xenopi, leading to initiation of ethambutol, azithromycin, and moxifloxacin. Intravenous amikacin was added, but due to hearing deficits, it was discontinued. The final NTM treatment regimen was rifabutin, ethambutol, moxifloxacin, and inhaled amikacin. Most of her clinical symptoms resolved in about one month. A chest CT 7 months later showed improvement in LUL findings, but several new nodules in the right lower lobe.
Fig. 1.
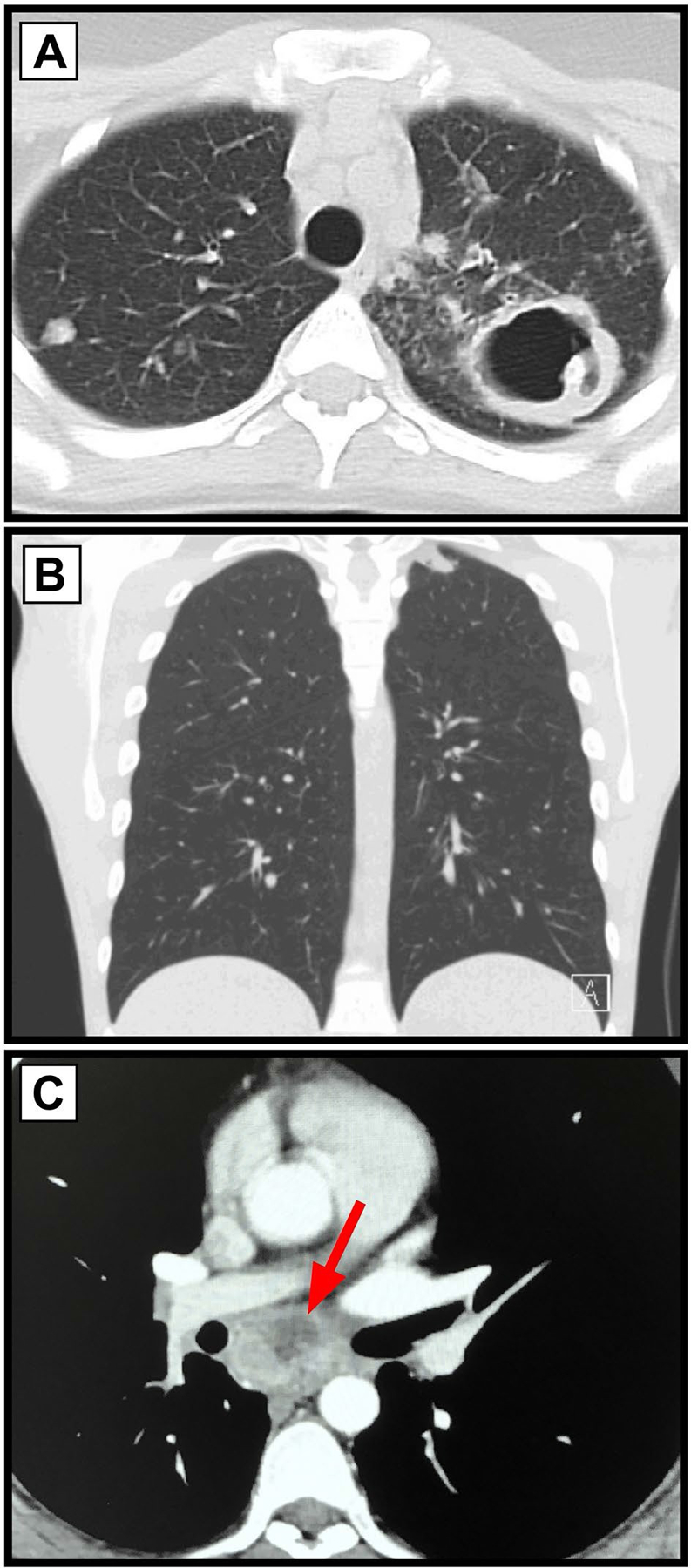
Chest CT imaging from the three separate cases. a Case 1 shows multiple bilateral cavitary and calcified pulmonary nodules, with a large 5 cm cavitary lesion in the left upper lobe, and bilateral lymphadenopathy. b Case 2 shows a solitary apical pulmonary nodules (1.4 × 1.0 cm). c Case 3 shows an enlarged (3.5 cm) central mediastinal lymph node with some cavitation (see arrow). Another necrotic mediastinal lymph node (2 cm) was present and not visualized here gordonae. He was treated with 6 months of ethambutol, rifampin, and azithromycin, with improvement of symptoms, and resolution of lymphadenopathy and no nodules or masses seen on follow-up chest CT.
Case 2
A 19-year-old male with a history of asthma, recent travel to southwest United States and history of incarceration, presented with a 1.5-year history of 40 lb unintentional weight loss, night sweats, productive cough, and new-onset hemoptysis. He had a 5-pack year history of cigarette smoking, vaping nicotine, and vaping, dabbing, and dripping tetrahydrocannabinol (THC) products. A chest CT (Fig. 1b) showed a solitary apical pulmonary nodule (1.4 × 1.0 cm). Testing for MTB, other infectious causes, workup for an immune dysfunction, and Cystic Fibrosis were negative. Three consecutive sputum samples and the BALF culture isolated MAC. BALF analysis showed neutrophilia and macrophages with positive Oil Red O staining. He was started on tri-weekly oral azithromycin, rifampin, and ethambutol. At the 2-month follow-up visit, his symptoms had improved with weight gain. BALF culture 5 months after therapy did not grow MAC with lipid-laden macrophages. He quit dabbing, vaping, and dripping THC oil products including nicotine, but continued to smoke cigarettes and marijuana.
Case 3
A 34-year-old male, otherwise healthy, presented with 3 months of cough productive of white sputum, night sweats, small volume hemoptysis, and 15 lb unintentional weight loss. He had a history of hookah smoking different brands of flavored tobacco for several years. A chest CT (Fig. 1c) showed two enlarged, necrotic mediastinal lymph nodes (3.5 × 2 cm). Testing for MTB and basic workup for an immunodeficiency was negative. Bronchoscopy for BALF and endobronchial ultrasound-guided lymph node biopsy was performed. Lymph node biopsy identifi M. gordonae by matrix-assisted laser desorption ionization-time of fl mass spectrometry (MALDI-TOF) assay with realtime PCR. Biopsy of the endobronchial mass found granulomatous inflammation, and the BALF culture isolated M.
Discussion
We report pulmonary NTM in three otherwise healthy immunocompetent young individuals with a significant history of vaping. In all, NTM grew from either sputum or BALF. Of the three patients, two of them had a reported history of asthma. Although there is a suspected association with inhaled corticosteroid (ICS) therapy and pulmonary NTM [10], neither of the two patients in our cohort was being treated with ICS therapy. We cannot exclude that asthma was a contributing factor for the development of pulmonary NTM in either of our patients, but we believe clinicians should consider pulmonary NTM in asthmatics who are vaping and have pulmonary deterioration. In all three subjects, both vaping and pulmonary NTM infection contributed to their respiratory disease process [11]. Nevertheless, NTM infection or colonization is unusual in patients without an underlying immunodeficiency, significant lung disease, or pre-menopausal women [12, 13]. Thus, it would be important to establish the relationship between vaping, lung injury, and the immune system to better understand our findings.
Vaping involves inhaling and exhaling a vapor, which is produced by an e-cigarette or similar device. It should be emphasized that in addition to nicotine, the e-liquid in vaporizers contain diluents such as propylene glycol, vegetable glycerin-based liquid, and VEA, and flavoring products [2, 3]. In addition, many subjects who vape nicotine vape THC, marijuana, or other synthetic drugs [1]. Animal and human studies suggest that the vapor of such products may disrupt production and function of surfactant [14–16]. VEA in particular found in THC disrupts surfactant alignment with phospholipids, thereby resulting in EVALI, in humans [2].
Although vaping-related lung injury often occurs in the absence of infections, there have been cases of EVALI and co-infections reported in the literature [2, 9]. Furthermore, empiric antibiotics may be started to treat possible secondary infections until the diagnosis of EVALI is made, and antibiotics are recommended if a co-infection is identified or further suspected [17, 18]. Three recent case series on vaping mention the presence of possible or confirmed co-infections in adolescents and young adults [2, 9, 19]. A previous case series at one of the participating institutions from this case series described six patients with EVALI from ages 15 to 20 years who all required glucocorticoids, but also antibiotics to cover for possible infection [19]. In a case series of adults with probable EVALI without evidence of VEA in BALF, co-infection with methicillin-susceptible Staphylococcus aureus and coccidioidomycosis have been identified [2]. These individuals required both glucocorticoids and antibiotics and/or antifungals [2]. In another case series of eight adolescents ranging from ages 14 to 19 years old in the Northeast coast, three patients with vaping-related lung disease were found to either grow Mycoplasma, Mycobacterium avium and Staphylococcus Aureus, or Candida albicans [9]. It should be emphasized that the above series was the only report we found of NTM co-infection with EVALI. Similar to our findings, these three patients who vaped nicotine and THC were otherwise healthy with the exception of one patient with asthma. All were treated with antibiotics, except for one patient who was treated with both antibiotics and steroids [9]. A possible link between vaping and lung infection has also been seen in animal studies, where vaporized nicotine increased the risk of adhesion of pneumococci to respiratory cells and decreased the clearance of influenza A [4, 14].
Vaping increases the risk for lung infection by altering alveolar macrophage homeostasis and response to microbes may be altered in chronic vape users, due to upregulation of pulmonary phospholipid and fatty acyl triacylglycerol metabolism in the lungs [15]. NTM and other pathogenic mycobacteria are hydrophobic organisms that devote a large portion of their genome to lipid metabolism [14, 15]; therefore, the abundance of lipid-laden macrophages as seen in vaping cases provides an ideal environment for NTM growth. E-cigarette vapor exposure may also decrease neutrophil migration into infected spaces of the lungs, resulting in decreased reactive oxidative species production and increasing the burden of invasive bacterial infections [16].
It is possible that either the content within the vape cartridges, vape cartridges themselves, or hookah water reservoir used by the patients in this case series may have harbored NTM. NTM is able to survive in many unfavorable conditions, including environments with high temperatures and low oxygen content [20]. As vape cartridges or hookah devices contain a coil or coal source to heat the e-cigarette juice or hookah flavors, this may present as a favorable environment for NTM to grow in. “Hot tub lung,” a form of pulmonary NTM seen in immunocompetent individuals, further supports this speculation. In “hot tub lung,” environmental factors, such as the large number of organisms present in an indoor hot tub and aerosolized through tub jets, are thought to contribute to NTM pathogenicity [21].
For unclear reasons, the prevalence of pulmonary NTM disease has increased over the past 30 years [21]. In the United States, the incidence of pulmonary NTM disease is 3.2–9.8 per 100,000 people [22], and may differ based off of geographical location [21]. For example, MAC is commonly found in North America and East Asia, but M. xenopi is mostly seen in Europe [22]. M. gordonae is commonly found in the environment, yet due to its low virulence, it is typically seen as a contaminant with lower clinical significance to other more common NTM species [23]. Due to their ubiquitous presence, it may not be surprising that MAC and M. gordonae were isolated in our subjects. However, M. xenopi is a species of NTM that is not as commonly seen in North America. Furthermore, a distinct sequence type of M. xenopi has been found to cause pulmonary disease in immunocompetent individuals with no significant underlying pulmonary conditions [24]. It is also important to mention that NTM pulmonary disease has been linked to co-infection with other bacteria and fungi [21], especially AF with M. xenopi [25].
Our case series has certain limitations. Due to the nature of this report, we cannot establish a cause and eff relationship between vaping and pulmonary NTM. Although there were commonalities between these three cases, there were differences in smoking practices and pre-existing medical history, and at this time, the details from this case series should not be generalized. Of the three cases, two had asthma not being treated with ICS. The history recorded from these patients is also subject to recall bias. As there is only a sparse amount of case series associating vaping practices with pulmonary NTM, we hope that this will give rise to future research studies investigating this relationship.
Conclusion
The cases presented here highlight that pulmonary NTM should be considered in young adults with a history of vaping, especially in the setting of asthma with or without ICS therapy. Vaping has been linked to the disruption of surfactant, alveolar macrophage homeostasis, and innate immune function of the lungs [14, 16, 26]. Treatment of EVALI, especially long-term care, may be challenging when there is associated pulmonary NTM. NTM may also co-exist with fungal infections [25]. Notably, NTM and other microbes may be present in e-fluid, hookah water reservoirs, or even already present in the airways. Further research into how vaping may alter pulmonary surfactant function, macrophage, and neutrophil function to enable NTM growth is warranted.
References
- 1.Perrine CG, Pickens CM, Boehmer TK, King BA, Jones CM, DeSisto CL, Duca LM, Lekiachvili A, Kenemer B, Shamout M, Landen MG, Lynfi R, Ghinai I, Heinzerling A, Lewis N, Pray IW, Tanz LJ, Patel A, Briss PA (2019) Characteristics of a multistate outbreak of lung injury associated with E-cigarette use, or vaping—United States, 2019. MMWR Morb Mortal Wkly Rep 68:860–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D, Corstvet J, Cowan E, De Jesus VR, Espinosa P, Fernandez C, Holder C, Kuklenyik Z, Kusovschi JD, Newman C, Reis GB, Rees J, Reese C, Silva L, Seyler T, Song MA, Sosnoff C, Spitzer CR, Tevis D, Wang L, Watson C, Wewers MD, Xia B, Heitkemper DT, Ghinai I, Layden J, Briss P, King BA, Delaney LJ, Jones CM, Baldwin GT, Patel A, Meaney-Delman D, Rose D, Krishnasamy V, Barr JR, Thomas J, Pirkle JL, Lung injury response laboratory working G (2020) Vitamin E acetate in bronchoalveolar-lavage fl associated with EVALI. N Engl J Med 382:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths A, Rauzi A, Stadheim K, Wheeler W (2020) Lung injury associated with E-cigarette or vaping product use. Pediatr Ann 49:e93–e98 [DOI] [PubMed] [Google Scholar]
- 4.Miyashita L, Suri R, Dearing E, Mudway I, Dove RE, Neill DR, Van Zyl-Smit R, Kadioglu A, Grigg J (2018) E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur Respir J 51:1701592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozier J, Zakarya R, Chapman DG, Oliver BGG (2020) How harmless are E-cigarettes? effects in the pulmonary system. Curr Opin Pulm Med 26:97–102 [DOI] [PubMed] [Google Scholar]
- 6.Wagner D, Young LS (2004) Nontuberculous mycobacterial infections: a clinical review. Infection 32:257–270 [DOI] [PubMed] [Google Scholar]
- 7.Johnson MM, Odell JA (2014) Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 6:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffi h DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, Subcommittee ATSMD, American Thoracic S, Infectious Disease Society of A (2007) An offi ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416 [DOI] [PubMed] [Google Scholar]
- 9.Kass AP, Overbeek DL, Chiel LE, Boyer EW, Casey AMH (2020) Case series: Adolescent victims of the vaping public health crisis with pulmonary complications. Pediatr Pulmonol 55:1224–1236 [DOI] [PubMed] [Google Scholar]
- 10.Brode SK, Campitelli MA, Kwong JC, Lu H, Marchand-Austin A, Gershon AS, Jamieson FB, Marras TK (2017) The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J 50:1700037. [DOI] [PubMed] [Google Scholar]
- 11.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J (2020) Pulmonary illness related to E-cigarette use in illinois and wisconsin—final report. N Engl J Med 382:903–916 [DOI] [PubMed] [Google Scholar]
- 12.Rivero-Lezcano OM, Gonzalez-Cortes C, Mirsaeidi M (2019) The unexplained increase of nontuberculous mycobacteriosis. Int J Mycobacteriol 8:1–6 [DOI] [PubMed] [Google Scholar]
- 13.Mirsaeidi M, Sadikot RT (2015) Gender susceptibility to mycobacterial infections in patients with non-CF bronchiectasis. Int J Mycobacteriol 4:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, You R, Hong MJ, Baghaei N, Song LZ, Porter P, Putluri N, Salas R, Gilbert BE, Levental I, Campen MJ, Corry DB, Kheradmand F (2019) Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 129:4290–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur G, Pinkston R, McLemore B, Dorsey WC, Batra S (2018) Immunological and toxicological risk assessment of e-cigarettes. Eur Respir Rev 27:170119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corriden R, Moshensky A, Bojanowski CM, Meier A, Chien J, Nelson RK, Crotty Alexander LE (2020) E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol 318:C205–C214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson K, Brancato A, Heetderks P, Mansour W, Matheis E, Nario M, Rajagopalan S, Underhill B, Wininger J, Fox D (2019) Outbreak of electronic-cigarette-associated acute lipoid pneumonia - North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep 68:784–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel DA, Jatlaoui TC, Koumans EH, Kiernan EA, Layer M, Cates JE, Kimball A, Weissman DN, Petersen EE, Reagan-Steiner S, Godfred-Cato S, Moulia D, Moritz E, Lehnert JD, Mitchko J, London J, Zaki SR, King BA, Jones CM, Patel A, Delman DM, Koppaka R, Lung Injury Response Clinical Working G, Lung Injury Response Epidemiology/Surveillance G (2019) Update: interim guidance for health care providers evaluating and caring for patients with suspected E-cigarette, or vaping, product use associated lung injury—United States, October 2019. MMWR Morb Mortal Wkly Rep 68:919–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina MD, Levin TL, Conrad LA, Bidiwala A (2020) Vaping associated lung injury: a potentially life-threatening epidemic in US youth. Pediatr Pulmonol 55:1705. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya J, Mohandas S, Goldman DL (2019) Nontuberculous mycobacterial infections in children. Pediatr Rev 40:179–190 [DOI] [PubMed] [Google Scholar]
- 21.Stout JE, Koh WJ, Yew WW (2016) Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 45:123–134 [DOI] [PubMed] [Google Scholar]
- 22.Prevots DR, Marras TK (2015) Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wassilew N, Hoffmann H, Andrejak C, Lange C (2016) Pulmonary disease caused by non-tuberculous mycobacteria. Respiration 91:386–402 [DOI] [PubMed] [Google Scholar]
- 24.Hirama T, Marchand-Austin A, Ma J, Alexander DC, Brode SK, Marras TK, Jamieson FB (2018) Mycobacterium xenopi genotype associated with clinical phenotype in lung disease. Lung 196:213–217 [DOI] [PubMed] [Google Scholar]
- 25.Andrejak C, Lescure FX, Pukenyte E, Douadi Y, Yazdanpanah Y, Laurans G, Schmit JL, Jounieaux V, Xenopi G (2009) Mycobacterium xenopi pulmonary infections: a multicentric retrospective study of 136 cases in north-east France. Thorax 64:291–296 [DOI] [PubMed] [Google Scholar]
- 26.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, Thickett DR (2018) Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73:1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]


