Abstract
Objective:
This study aimed to examine the temporal activation of NF-κB and its relationship to the development of pain-related sensitivity and behavioral changes in a non-invasive murine knee loading model of PTOA.
Method:
Following knee injury NF-κB activity was assessed longitudinally via in vivo imaging in FVB.Cg-Tg(HIV-EGFP,luc)8Tsb/J mice. Measures of pain-related sensitivity and behavior were also assessed longitudinally for 16 weeks. Additionally, we antagonized NF-κB signaling via intra-articular delivery of an IκB kinase 2 antagonist to understand how local NF-κB inhibition might alter disease progression.
Results:
Following joint injury NF-κB signaling within the knee joint was transiently increased and peaked on day 3 with an estimated 1.35 p/s/cm2/sr (95% CI 0.913,1.792 p/s/cm2/sr) fold increase in signaling when compared to control joints. Injury also resulted in the long-term development of hindpaw allodynia. Hyperalgesia withdrawal thresholds were reduced at injured knee joints, with the largest reduction occurring 2 days following injury (estimate of between group difference 129.1 g with 95% CI 60.9,197.4 g), static weight bearing on injured limbs was also reduced. Local delivery of an NF-κB inhibitor following joint injury reduced chondrocyte death and also influenced the development of pain-related sensitivity but did not reduce long-term cartilage degeneration.
Conclusion:
These findings underscore the development of behavioral changes in this non-invasive loading model of PTOA and their relationships to NF-κB activation and pathology. They also highlight the potential chondroprotective effects of NF-κB inhibition shortly following joint injury despite limitations in preventing the long-term development of joint degeneration in this model of PTOA.
Keywords: post-traumatic osteoarthritis (PTOA), knee joint, drug delivery, inflammation, controlled release
INTRODUCTION
Osteoarthritis (OA) is one of the leading causes of disability in the United States. It is estimated that 1 in 3 individuals ages 18–64 have arthritis, and roughly 12% of all patients seeking treatment for symptomatic OA have had prior joint trauma1. Following traumatic joint injury roughly half of affected individuals will go on to develop post-traumatic osteoarthritis (PTOA) within 10–20 years2,3. Pathology following traumatic joint injury are varied but may include altered joint mechanics, inflammation, cell death, and alteration in matrix properties4. While the development of symptomatic PTOA generally occurs over several years, levels of pro-inflammatory cytokines have been reported to peak within days following joint injury in humans5,6. Furthermore, these pro-inflammatory mediators may remain elevated months after injury and are thought to cause permanent degenerative changes to the joint7,8. Monitoring of these acute changes and early intervention following joint injury may be helpful in slowing PTOA development.
Recently developed non-invasive tibial compression models of PTOA accurately capture some features of traumatic joint injury which lead to PTOA in humans8. Following injurious tibial compression, knee joints exhibit increased anterior-posterior joint laxity9. Synovial inflammation occurs within 3–5 days post-injury and is sustained for 8 weeks10,11. These models have recapitulated other hallmarks of PTOA such as an increased presence of matrix and bone remodeling markers and osteophyte formation following joint injury12. Furthermore, intra-cellular activation of NF-κB in cartilage and synovium has been reported 12 h post mechanical injury13. NF-κB activation is known to play a crucial role in the early development of PTOA and regulates a number of genes involved in inflammation and matrix remodeling14,15.
Increased NF-κB activity has been implicated in the development of pain following peripheral injury and recent data suggest NF-κB may be directly responsible for sensitization in some pain-related ion channels16–18. Our group has shown a correlation between NF-κB activation and pain-related sensitivity and behavioral change in a murine model of inflammatory arthritis19. Thus, early pharmacological inhibition of NF-κB can be a potential target for OA treatment. Various pre-clinical studies have shown protective effects of selective IκB kinase 2 (IKK-2) inhibitors, highlighting their potential as disease-modifying OA drugs20,21. Despite this, off-target effects upon systemic delivery have limited their translation beyond pre-clinical studies22,23. However, given the focal nature of diseases such as PTOA, local drug administration via intra-articular injection has the potential to enhance drug bioavailability in the injured joint which enables reduced total drug doses and limits the potential for off-target systemic effects.
In this study, we longitudinally tracked NF-κB activation in vivo and investigated multiple pain-related sensitivity and behavioral changes in a non-invasive mechanical loading model of PTOA. We further investigated the disease-modifying potential and biodistribution of intra-articularly injected PHA-408, a highly specific small molecule inhibitor of IKK-2 following a non-invasive mechanical joint injury. Our goal was to longitudinally examine the relationships between the development of pain-related sensitivity and changes in animal behavior, NF-κB activation in vivo, and the effect of a selective small molecule IKK-2 inhibitor delivered locally to the joint in a non-invasive model of PTOA.
METHODS
Animal Model
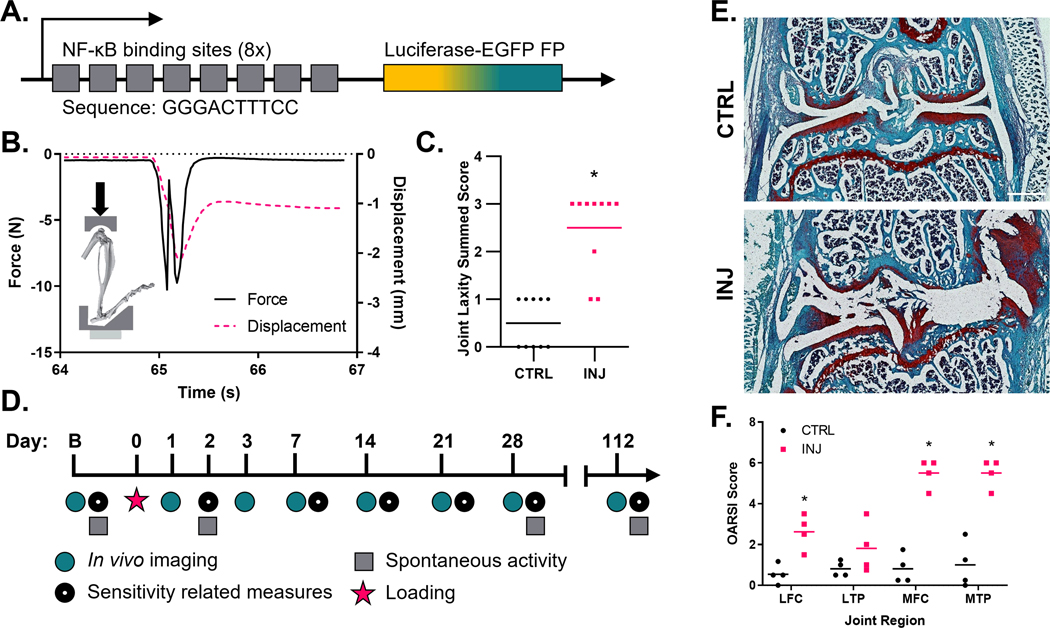
All procedures were performed with approval of the Washington University Institutional Animal Care and Use Committee. Transgenic FVB.Cg-Tg(HIV-EGFP,luc)8Tsb/J mice (Fig. 1A) (The Jackson Laboratory, Bar Harbor, ME) were acquired (male, 9–12 weeks of age) and acclimated to testing equipment for 1 week. Mice were maintained on a 12-h light–dark cycle with access to food and water ad libitum. All behavioral assessments were performed by the same blinded investigators and at the same time on each behavioral testing day. Mice were randomly assigned to one of three cohorts. Animals in cohort 1 were randomly assigned to sham control (CTRL, n=5), joint injury (INJ, n=6), or joint injury plus treatment (INJ/Tx, n=5) groups. Group sizes within cohort 1 were chosen following a power analysis; previous variance data for NF-κB driven luminescence were used to determine that n=6 animals/group would have at least 80% power to detect mean differences of (x̅2−x̅1)/σpooled=2 (mean difference of 2 in the size of pooled standard deviation) based on a two-sided independent t-test at a significance level of 0.0519. This power analysis assumed an average effect of the outcomes over time. Therefore, the actual power to detect mean difference between groups will be greater using the longitudinal measures analyzed by the linear mixed effects model. To induce joint injury, right knees were loaded as previously described by Rai and colleagues10. Knee joints were subjected to 60 cycles of 12 N axial compressive load. This protocol reproducibly induced a sudden drop in the axial force recorded on the time-force curve and increased anterior-posterior joint laxity via a joint laxity test (Supplemental Methods) in a separate cadaveric test cohort (Fig. 1B–C)10,11,24. CTRL animals were placed within the test frame and their knees were subjected to 10 min of static axial compressive load at 0.5 N. Animals in the INJ/Tx group received two intra-articular injections (30 G) of PHA-408 (3 μg/5 μl, sterile saline + 0.1% DMSO, Alchem Pharmtech Inc, Monmouth Junction, NJ), one immediately following injury and another 48 h post-injury. Animals were returned to their home cage (≤5 mice/cage) and allowed to freely ambulate upon recovery. Animals within cohort 1 were sacrificed 16 weeks following injury.
Figure 1. Study design.
(A) Representative NF-κB response element of FVB.Cg-Tg(HIV-EGFP, luc)8Tsb/J mice. (B) Custom-made mount for in vivo loading to induce non-invasive joint injury and corresponding time versus force and displacement curves showing one cycle of compressive loading with characteristic one-time mid-cycle drop in the time versus force curve coinciding with ligament injury. (C) Injured joints have increased anterior-posterior joint laxity as measured by a joint laxity score (n=10/group). (D) Study timeline showing repeated imaging and behavioral assessments. (E and F) Representative histology shows full thickness articular defects in injured knee-joints 112 days following injury and significant osteoarthritic progression in the lateral and medial femoral condyle (LFC/MFC) as well as the medial tibial plateau (MTP), n=4/group, scale bar = 500 μm, *=p<0.05.
Two additional cohorts of mice underwent joint injury as described above. Mice in cohort 2 received a single intra-articular injection of PHA-408 (3 μg) or vehicle (sterile saline + 0.1% DMSO) and were sacrificed 48 h post joint loading to study the immediate cellular effects of joint injury and PHA-408 on articular cartilage (n=4–6/group). Mice in cohort 3 received two intra-articular injections (t=0 and 48 h) of PHA-408, for the study of PHA-408 biodistribution (n=2–5/timepoint, Supplemental Methods).
In Vivo Luminescence Imaging of NF-κB Activity
To assess local NF-κB activation following joint injury, in vivo bioluminescent imaging was performed. At each imaging timepoint (Fig. 1D) mice received an injection of D-luciferin (150 mg/kg i.p., Sigma-Aldrich, St Louis, MO) and whole-body luminescent images were acquired with an in vivo imaging system (10 sec exposure, IVIS 50, PerkinElmer, Waltham, MA). NF-κB-driven luminescence was quantified in both ipsilateral and contralateral knees within an 8×10 mm elliptical region of interest (ROI) that was placed around the knee joint.
Pain-related Hindpaw Sensitivity
Mechanical allodynia was evaluated in both the ipsilateral and contralateral hindpaws using von Frey filaments. Filaments ranging from 0.02–2.0 g (starting filament, 0.4 g) were applied to the plantar region of a stationary, non-rearing, animal using the Chaplan “up-down” method25.
Pain-related Hyperalgesia
The threshold for contact hyperalgesia was tested at the knee joint using a small animal algometer (SMALGO, Bioseb, Vitrolles, FRA)26. Mice were scruffed and the hindlimb was held with the knee at a ~30° flexion angle. The tip of the SMALGO force transducer was applied to the lateral aspect of the knee at ~30 g/sec until the animal showed an escape response (flinch, head jerk, and/or squeak, to a maximum of 400 g). Three independent measurements were obtained and averaged for each hindlimb on each mouse on all behavioral testing days.
Static Weight Bearing
Mouse hind limb static weight bearing was measured using an incapacitance meter (BIO-SWB-TOUCH-M, Bioseb)19. Independent force measurements from both hindpaws were collected over a 3 sec interval; three independent measurements were obtained and averaged for each mouse on all behavioral testing days.
Spontaneous Activity
Freely selected activity was assessed by tracking mouse movement within a custom arena (40 cm x 40 cm x 40 cm)27–29. Movement within a 30 min recording period was analyzed (EthoVision, Noldus, Wageningen, NLD) to determine the change in distance traveled from data acquired at baseline.
In Situ Live-Dead Cellular Imaging
To determine the immediate effects of PHA-408 on chondrocyte cell viability, intact femurs from animals in cohort 2 were harvested 48 h following joint injury. Naive (n=4), INJ/Veh (n=6), and INJ/Tx (n=5) femurs were stained with Hoechst 33342 (10 μM, ThermoFisher, Waltham, MA), Calcein-AM (5 μM, Invitrogen, Carlsbad, CA), and Propidium Iodide (5 μM, ThermoFisher) for 1 h30. Following staining, intact femurs were imaged (FV1200, Olympus, Tokyo, JPN). Z-stacks (100 μM thickness) were acquired of each femoral condyle. Total cell number (Hoechst+) and membrane permeable cell number (PI+) were determined via an automated thresholding-based ImageJ script.
Histology
Mice were euthanized at the terminal timepoint (113 days) and ipsilateral knees collected for histology. All knees were fixed, decalcified, and cryoembedded. Serial coronal sections (8 μm) of ipsilateral knees from all groups were acquired, and two sections representing the most severe evidence of arthritic changes were chosen for staining with H&E, Safranin O, and fast green (n=4 knees/group were randomly selected). Stained sections were graded to consensus by two blinded graders using the Osteoarthritis Histopathology Assessment System (OARSI)31. Scores were obtained for the medial and lateral femoral condyle (MFC/LFC), and medial and lateral tibial plateau (MTP/LTP). Due to sectioning artifacts the LTP within a single INJ/Tx animal could not be scored.
Statistical Analysis
For longitudinal measures, a linear mixed model was used to test for differences of measured outcomes between CTRL and INJ as well as INJ and INJ/Tx groups over time considering group by time interaction. In vivo imaging data were log transformed (base 10) for statistical analysis to better meet the normality assumption. The linear mixed model was also performed to test for differences of cartilage OARSI scores between CTRL and INJ as well as INJ and INJ/Tx groups across different joint regions considering group by joint region interaction. Contrasts were used to test for mean difference between CTRL and INJ as well as INJ and INJ/Tx groups at different times and joint regions in longitudinal measures and OARSI scores, respectively. Relationships between NF-κB luminescence and allodynia in these cohorts were tested via Pearson correlation (Supplemental Methods). A repeated measures two-way ANOVA with Bonferroni adjustment for post-hoc comparisons was used to compare cell viability of mice from cohort 2. All statistical analyses were two-sided and significance was set at p < 0.05.
RESULTS
Joint Injury Leads to Increased NF-κB-Driven Luminescence
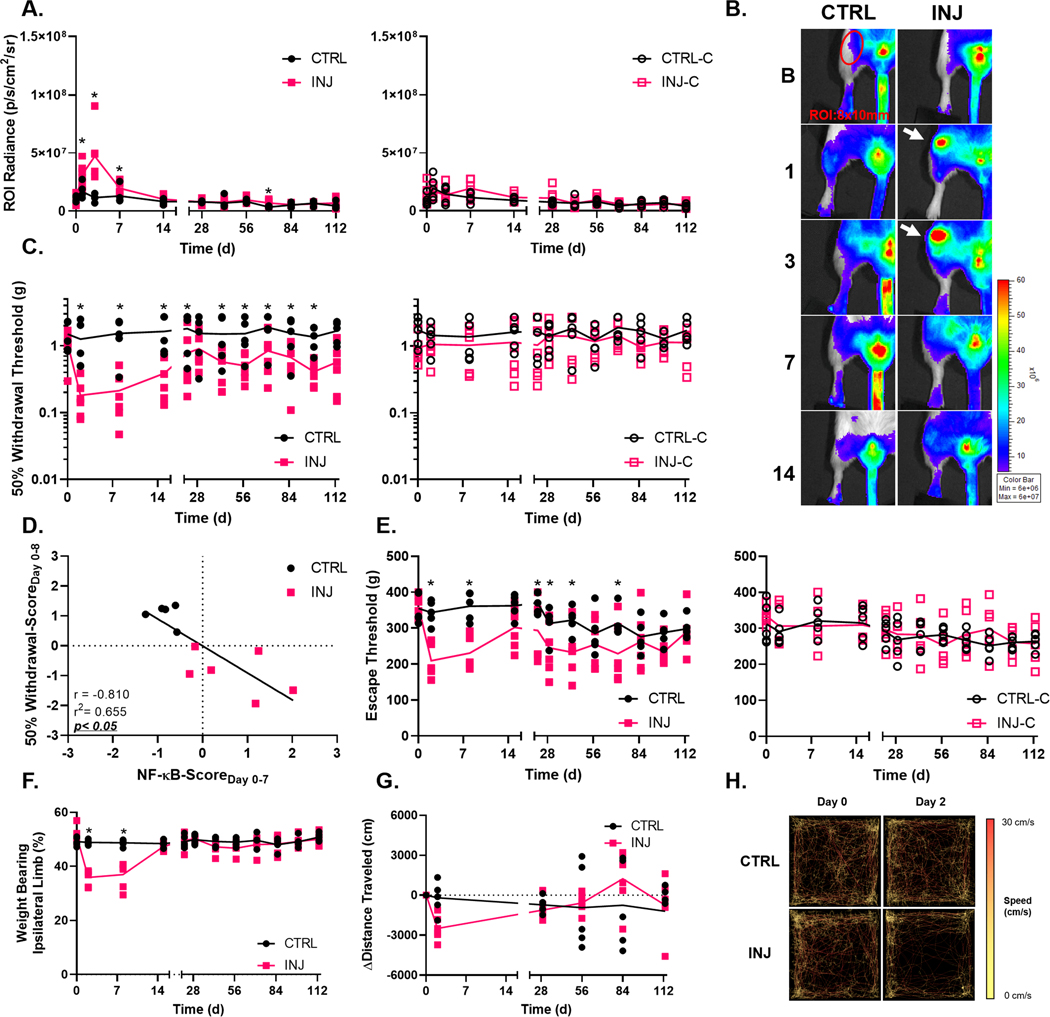
A transient increase in NF-κB-driven luminescence was observed in INJ ipsilateral limbs when compared to CTRL mice on days 1 (estimate of between group, log base 10 transformed, difference −0.639 p/s/cm2/sr with 95% CI −1.078,−0.200 p/s/cm2/sr), 3 (estimate of between group difference −1.353 p/s/cm2/sr with 95% CI −1.792,−0.914 p/s/cm2/sr), and 7 (estimate of between group difference −0.477 p/s/cm2/sr with 95% CI −0.916,−0.038 p/s/cm2/sr) following joint injury (Fig. 2A–B). Within injured ipsilateral limbs, the luminescence intensity peaked within the knee ROI on day 3 and decreased thereafter. NF-κB-driven luminescence was also slightly elevated in INJ ipsilateral knees on day 70 (estimate of between group difference −0.672 p/s/cm2/sr with 95% CI −1.111,−0.233 p/s/cm2/sr).
Figure 2. Non-invasive joint injury alters NF-κB signaling, sensitivity to evoked mechanical stimuli, and static weight bearing.
(A and B) Increased luminescent signal was apparent in ipsilateral knees of the INJ group on days 1, 3, 7 and 70 following joint injury, with no changes in contralateral limbs. (C) Mice in the INJ group had persistent increased sensitivity in the hindpaw following injury. (D) Increased NF-κB signaling in joints was associated with reduced withdrawal thresholds in the first week of the study. (E) Mice in the INJ group also had a reduced threshold to noxious mechanical stimuli following joint injury. (F) Static weight bearing was altered up to one week following joint injury. (G) Mice in the INJ group exhibited a trend towards reduced activity, illustrated by animal matched representative traces (H), 2 days post joint injury. CTRL n=5, INJ n=6, *=p<0.05.
Joint Injury Alters Sensitivity to Mechanical Stimuli and Static Weight Bearing
Non-invasive joint injury induced changes to pain-related sensitivity. Increased hindpaw sensitivity to mechanical stimuli (allodynia) was observed on day 2 (estimate of between group difference 1.077 g with 95% CI 0.276,1.947 g), 8 (estimate of between group difference 1.315 g with 95% CI 0.446,2.185 g), 15 (estimate of between group difference 1.249 g with 95% CI 0.380,2.119 g), 22 (estimate of between group difference 1.033 g with 95% CI 0.163,1.902 g), 43 (estimate of between group difference 0.925 g with 95% CI 0.055,1.794 g), 57 (estimate of between group difference 1.010 g with 95% CI 0.141,1.879 g), 71 (estimate of between group difference 1.044 with 95% CI 0.174,1.913 g), 85 (estimate of between group difference 0.874 with 95% CI 0.004,1.743 g), and 99 (estimate of between group difference 0.943 g with 95% CI 0.073,1.812 g) following joint injury (Fig. 2C). Furthermore, increased NF-κB signaling in joints was associated with reduced withdrawal thresholds in the first week of the study (Fig. 2D).
Joint injury also led to hyperalgesia in the ipsilateral limbs of INJ group as measured by reduced hyperalgesia thresholds on days 2 (estimate of between group difference 129.1 g with 95% CI 60.89,197.4 g), 8 (estimate of between group difference 124.9 g with 95% CI 56.69,193.2 g), 22 (estimate of between group difference 76.81 g with 95% CI 10.86,142.8 g), 29 (estimate of between group difference 67.82 g with 95% CI 1.869,133.8 g), 43 (estimate of between group difference 89.51 g with 95% CI 23.56,155.5 g), and 71 (estimate of between group difference 84.29 g with 95% CI 18.34,150.24 g) following joint injury as compared to animals in the CTRL group (Fig. 2E). There were no observed differences in measured hyperalgesia thresholds for contralateral limbs between mice in the CTRL and INJ groups.
Following joint injury, animals in the INJ group exhibited reduced weight bearing on their ipsilateral limbs on days 2 (estimate of between group difference 12.96 % with 95% CI 10.10,15.81 %) and 8 (estimate of between group difference 11.76 % with 95% CI 8.899,14.61 %) (Fig. 2F).
Despite these differences in pain-related sensitivity, no significant alterations were observed in freely selected activity following joint injury (Fig. 2G,H).
Intra-articular Injection of PHA-408 Exhibits Chondroprotective Effects on Articular Cartilage and Shows Some Disease Modifying Potential to Longitudinal Measures Following Joint Injury
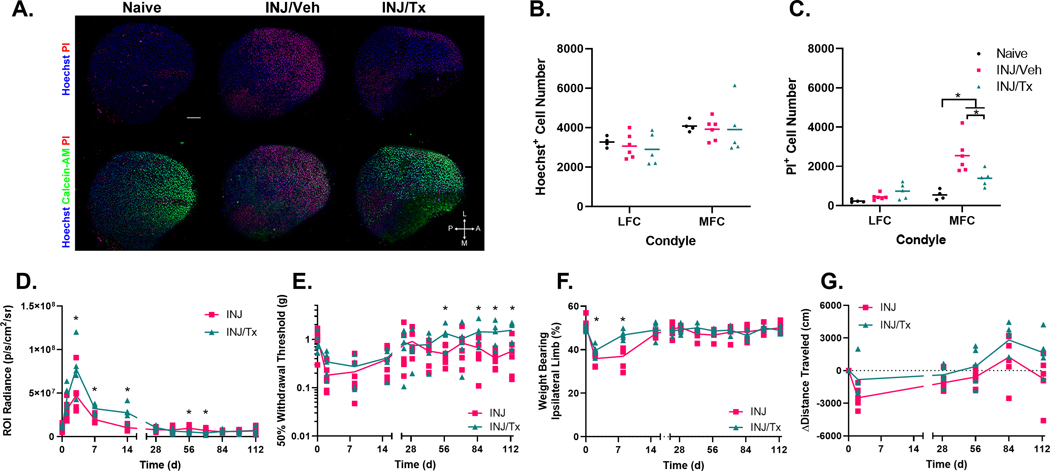
Local delivery of PHA-408 showed a short-term chondroprotective effect in the articular cartilage of the MFC following knee joint injury (Fig. 3A–C). The MFC of INJ/Tx joints had reduced cell death when compared to the MFC of INJ/Veh animals (estimate of between group difference −1155 PI+ cell number with 95% CI −1921,−389.4 PI+ cell number). INJ/Tx joints showed elevated NF-κB-driven luminescence following joint injury when compared to those of the untreated INJ group on days 3 (estimate of between group, log base 10 transformed, difference −0.536 p/s/cm2/sr with 95% CI −0.992,−0.080 p/s/cm2/sr), 7 (estimate of between group difference −0.501 p/s/cm2/sr with 95% CI −0.957,−0.045 p/s/cm2/sr), and 14 (estimate of between group difference −0.961 p/s/cm2/sr with 95% CI −1.417,−0.505 p/s/cm2/sr), however, at later timepoints NF-κB-driven luminescence was reduced within INJ/Tx joints when compared to INJ alone (day 56, estimate of between group difference 0.569 p/s/cm2/sr with 95% CI 0.113,1.025 p/s/cm2/sr; day 70, estimate of between group difference 0.559 p/s/cm2/sr with 95% CI 0.103,1.015 p/s/cm2/sr) (Fig. 3D). Animals in the INJ and INJ/Tx groups exhibited similar acute trends in the development of allodynia, however, animals in the INJ/Tx group had increased withdrawal threshold on days 57 (estimate of between group difference −0.965 g with 95% CI −1.828,−0.102 g), 85 (estimate of between group difference −0.893 g with 95% CI −1.756,−0.029 g), 99 (estimate of between group difference −1.165 g with 95% CI −2.028,−0.302 g), and 113 (estimate of between group difference −1.154 g with 95% CI −2.034,−0.275 g) following loading (Fig. 3E). Animals in the INJ/Tx group exhibited a more rapid recovery of uniform hindlimb static weight bearing following joint injury as evidenced by increased weight bearing on their ipsilateral hindpaw on days 2 (estimate of between group difference −3.725 % with 95% CI −6.698,−0.753 %) and 8 (estimate of between group difference −9.817 % with 95% CI −12.790,−6.845 %) when compared to INJ untreated animals (Fig. 3F). No differences were detected between INJ and INJ/Tx groups in distance traveled during open field assessments (Fig. 3G).
Figure 3. Intra-articular injection of PHA-408 exhibits short-term chondroprotective effects and shows some disease modifying potential to longitudinal measures following joint injury.
(A) Representative maximum intensity projected images of the mid-contact region of medial femoral condyles. (B) Joint injury did not lead to alterations in Hoechst+ cell number 48 h post joint injury. (C) In contrast, knee joint injury led to increased levels of PI+ cells, within the INJ/Veh (n=6) MFC when compared to naive joints (n=4), a single intra-articular injection of PHA-408 (3 μg, t=0 h) had potential to elicit a chondroprotective effect in the MFC of INJ/Tx (n=5) joints when compared to INJ/Veh treated joints (D) Mice in the INJ/Tx (n=5) group had increased NF-κB-driven luminescence shortly following joint injury, however, this was suppressed at later study timepoints when compared to INJ (n=6). (E) INJ/Tx mice showed a similar acute trend of a decreased withdrawal threshold following joint injury, however, later exhibited increased withdrawal thresholds when compared to INJ mice. (F) Drug treated mice showed a more rapid recovery of uniform static weight bearing between hindpaws following joint injury. (G) There were no differences between spontaneous activity in INJ and INJ/Tx groups. Scale bar = 100 μm, compass rose: L=lateral, A=anterior, M=medial, P=posterior. INJ data are replotted from Figure 2, *=p<0.05.
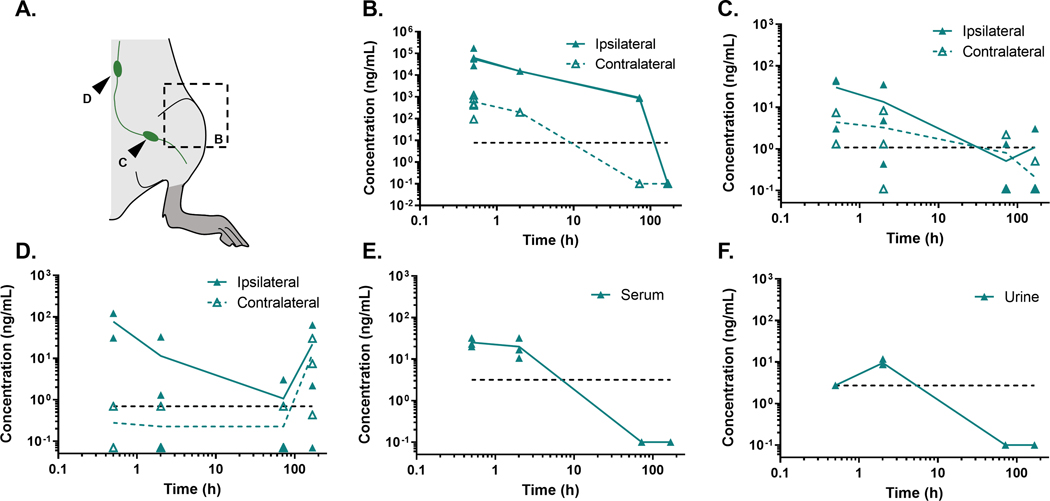
Following local delivery of PHA-408 to the knee joint (Fig. 4A), high levels of drug were detected within the injected knee joint for the first 72 h following intra-articular injection (Fig. 4B). Drug was not detected in knee joints one-week post injection. Drug levels above the lower detection limit persisted in contralateral uninjected limbs for 2 h following injection. Drug levels in major draining lymph nodes (Fig. 4C,D), serum (Fig. 4E), and urine (Fig. 4F) all exhibited a rapid reduction in drug concentration with time following intra-articular injection.
Figure 4. PHA-408 clearance from the joint.
(A) Schematic of tissues isolated for analysis. Following intra-articular injection of PHA-408, drug was rapidly cleared from knee joint tissues (B) as well as inguinal (C) and lumbar (D) lymph nodes, serum (E), and urine (F) following repeated intra-articular injections at t=0 h and t=48 h, dashed lines represent the lower detection limit of PHA-408 (n=2–5/timepoint).
Histological Analysis Revealed Lack of PHA-408 Effect on OARSI Score
Knee joints from animals of the INJ group showed evidence of severe articular degeneration in the medial compartment of the joint compared to knee joints from the CTRL group (Fig. 1E); mean OARSI scores in MFC were 0.81 and 5.50 in CTRL and INJ joints respectively (estimate of between group difference −4.688 with 95% CI −5.915,−3.460), mean scores within the MTP were 1.00 in CTRL and 5.50 in INJ joints (estimate of between group difference −4.438 with 95% CI −5.665,−3.210) (Fig. 1F). In several cases, complete loss of cartilage on the MFC and MTP was observed at the terminal timepoint. The LFC and LTP of animals in the INJ group had mean OARSI grades of 2.63 and 1.81 respectively compared to mean scores of 0.54 and 0.81 in CTRL joints (estimate of between group difference in the LFC −2.125 with 95% CI −3.352,−0.8979). OARSI grades for articular cartilage from INJ and INJ/Tx groups showed similar levels of degeneration (Sup. Fig. 1).
DISCUSSION
In this work, we showed non-invasive joint injury led to unilateral disability that is consistent with changes in pain-related sensitivity and behavior seen in humans following acute traumatic joint injury4. Additionally, we investigated the therapeutic potential and residence time for a locally delivered IKK-2 inhibitor. While NF-κB was transiently elevated in the joint, giving evidence of inflammation post-injury, it rapidly returned to control levels. This transient elevation was associated with the onset of pain-related sensitivity, altered weight bearing, and cartilage degeneration. While intra-articular injection of PHA-408 exhibited a chondroprotective effect with increased cell viability following joint loading as well as some disease modifying effects to longitudinally assessed outcomes, it did not influence long-term degenerative changes to articular cartilage.
We and others have previously shown injection models of inflammatory arthritis result in transient changes to peripheral sensitivity following joint injury. Similarly, surgical destabilization models of PTOA consistently result in hindpaw allodynia when compared to sham operated animals; however, the onset, presentation, and duration of hindpaw allodynia is varied and different from that observed in this non-invasive PTOA model29,32,33. Recent work by Heegde and colleagues corroborates the findings of the current study and shows that mechanically-induced joint injury is associated with both acute onset and maintenance of hindpaw allodynia34. In this work, no alterations to thermal sensitivity at the hindpaw were observed following non-invasive knee joint injury (Sup. Fig 2); this is in contrast to the transient heat sensitivity observed by Temp and colleagues following medial meniscal transection surgery29. Additional studies are needed to shed light on the involvement of functional signaling changes in the innervating structures of the injured knee joint in order to interpret this observed sensitivity, as has been demonstrated to occur in the dorsal root ganglia following surgical destabilization of the meniscus and in the generation of inflammatory arthritis35,36.
Changes in non-evoked behaviors, such as weight bearing imbalance in stance, were observed shortly after joint injury in this study. However, only a modest reduction in freely selected ambulation was observed within the same acute post-injury period. Prior studies of mechanical and surgical joint injury models have reported similar trends in ambulatory behavior at early timepoints32,37,38. Our findings point to a consistent change in behavior under conditions that minimize animal fatigue and external stresses, such as is observed with prompted behaviors of gait or hyperalgesia. Mice are widely believed to compensate for experienced pain during periods of prompted stimulation such that activity changes associated with freely selected behaviors may be of particular value to understanding joint pathology. Future studies might benefit from assessments that allow for quantification of freely selected behaviors including animal rearing and place preference39. With this in mind, a careful understanding and pairing of complementary behavioral tests that assess different aspects of pain-related sensitivity in disease are important for accurate and consistent characterization of symptomatic profiles in animal models of PTOA.
The use of in vivo bioluminescent imaging allowed for the longitudinal tracking and quantification of activation of the NF-κB pathway in the knee joint. Our group has previously shown increased but transient, joint specific, NF-κB activation following intra-articular injection of mono-iodoacetate to induce inflammatory arthritis, while other models have led to prolonged elevation of NF-κB activation19. In particular, NF-κB-driven luminescence has been reported to remain elevated beyond 6 weeks following surgically induced destabilization of the medial meniscus in a similar reporter mouse line40. This suggests the systemic effects of surgery, with consequences for prolonged elevation of inflammatory mediators, may elicit sustained alterations in joint inflammation and associated pain-related sensitivity and behavioral changes.
A goal of the current study was to evaluate the effects of early and local administration of a small molecule inhibitor of IKK-2 on local NF-κB activation, pain-related sensitivity, behavior, and joint degeneration following joint injury. Based on NF-κB activation kinetics observed in the INJ group it was determined PHA-408 delivery in the first 72 h following injury, when NF-κB signaling in the joint peaked, may help to slow the progression of symptomatic PTOA. Intra-articular delivery of PHA-408 showed a transient chondroprotective effect in the articular cartilage of INJ/Tx knee joints 48 h following joint injury, as measured by increased cell viability compared to INJ/Veh control joints. Acute treatment with PHA-408 led to increased NF-κB-driven luminescence in INJ/Tx mice; although this result was somewhat surprising, similar pro-inflammatory effects have been previously reported following administration of small molecule NF-κB inhibitors such as bortezomib and BAY 11–708241. Prior studies have also shown chronic alterations to mechanical sensitivity as we observed here at later timepoints, and trends in improved joint function, following one-time dosing of the small molecule NF-κB inhibitors MG132 and SAR113945, respectively42,43. Future studies should seek to better understand how local delivery of these small molecule inflammatory antagonists may lead to these observed temporally distinct pleiotropic effects44.
Clearance and biodistribution data suggested clearance of freely administered PHA-408 from the knee joint, as well as major draining lymph nodes of the knee and systemic circulation, occurs shortly after local delivery; this may explain the limited efficacy of PHA-408 in reducing long-term degeneration to articular cartilage following injury. Others have reported a modest protective effect of local NF-κB inhibition on both the histological development of PTOA and hyperalgesia following a less severe, non-instability inducing, mechanical joint injury13,45. For these reasons, future work should seek to increase the duration of drug presence within the joint following injury either via increased dosing frequency or with sustained release formulations46–49. Taken together, these results suggest NF-κB inhibition may remain a viable strategy for targeting PTOA but that intra-articular small molecule delivery remains limiting due to the rapid clearance kinetics of low molecular weight compounds from the joint.
In conclusion, the non-invasive model of PTOA described in this study may be valuable for understanding the early changes following acute traumatic joint injury and how they relate to pain-related sensitivity and behavioral changes without the confounding nature of a surgical incision. Further work to better dissect the temporal development and presentation of pain and sensitivity alterations in PTOA should be performed to help direct future treatment strategies that may prevent both the histological and painful progression of disease. Additionally, better understanding the spatiotemporal biodistribution and clearance behaviors of drugs following intra-articular injection may prove valuable in guiding future engineered intra-articular delivery approaches.
Supplementary Material
OARSI scoring of articular cartilage 16 weeks following joint injury showed similar levels of histological development of PTOA in INJ and INJ/Tx ipsilateral knee joints (n=4/group). Due to sectioning artifacts the LTP within a single INJ/Tx animal could not be scored.
Knee joint injury did not influence latency to withdrawal from hot (A) or cold (B) thermal stimuli presented at the ipsilateral hindpaw.
ACKNOWLEDGMENTS
The authors acknowledge Michael Brodt for assistance with tibial loading.
Funding sources: This study was supported by the NSF GRFP and NIH grants R01AR070975, P30AR057235, P30AR074992, R01AR068972, R01AR076758, P41EB002520, AG015768 and AG046927. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Competing interests: GM reports personal fees from Aclaris Therapeutics, Inc., outside the submitted work. All other authors have no competing interests with the content of this manuscript.
REFERENCES
- 1.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582–1587. doi: 10.1002/art.39692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178–2187. doi: 10.1002/art.11088 [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396 [DOI] [PubMed] [Google Scholar]
- 4.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. doi: 10.1002/jor.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31(2):315–321. doi: 10.1002/jor.22208 [DOI] [PubMed] [Google Scholar]
- 6.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10(1):93–96. doi: 10.1016/S0968-0160(02)00083-2 [DOI] [PubMed] [Google Scholar]
- 7.Swärd P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis) - a cross-sectional analysis. Osteoarthr Cartil. 2012;20(11):1302–1308. doi: 10.1016/j.joca.2012.07.021 [DOI] [PubMed] [Google Scholar]
- 8.Christiansen BA, Guilak F, Lockwood KA, Olson SA, Pitsillides AA, Sandell LJ, et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23(10):1627–1638. doi: 10.1016/j.joca.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsia AW, Anderson MJ, Heffner MA, Lagmay EP, Zavodovskaya R, Christiansen BA. Osteophyte formation after ACL rupture in mice is associated with joint restabilization and loss of range of motion. J Orthop Res. 2017;35(3):466–473. doi: 10.1002/jor.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rai MF, Duan X, Quirk JD, Holguin N, Schmidt EJ, Chinzei N, et al. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci Rep. 2017;7(45223):1–13. doi: 10.1038/srep45223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2012;20(7):773–782. doi: 10.1016/j.joca.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 12.Adebayo OO, Ko FC, Wan PT, Goldring SR, Goldring MB, Wright TM, et al. Role of subchondral bone properties and changes in development of load-induced osteoarthritis in mice. Osteoarthr Cartil. 2017;25(12):2108–2118. doi: 10.1016/j.joca.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, et al. Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci. 2016;113(41):E6199–E6208. doi: 10.1073/pnas.1608245113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.B. Marcu K, Otero M, Olivotto E, Maria Borzi R, B. Goldring M. NF-κB Signaling: Multiple Angles to Target OA. Curr Drug Targets. 2010;11(5):599–613. doi: 10.2174/138945010791011938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Chang SH, Mori D, Itoh S, Hirata M, Hosaka Y, et al. Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat Commun. 2016;7:1–12. doi: 10.1038/ncomms13336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, et al. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148(3):509–518. doi: 10.1016/j.pain.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, et al. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19(12):3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x [DOI] [PubMed] [Google Scholar]
- 18.Xie MX, Zhang XL, Xu J, Zeng WA, Li D, Xu T, et al. Nuclear Factor-kappaB Gates Nav1.7 Channels in DRG Neurons via Protein-Protein Interaction. iScience. 2019;19:623–633. doi: 10.1016/j.isci.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowles RD, Mata BA, Bell RD, Mwangi TK, Huebner JL, Kraus VB, et al. In vivo luminescence imaging of NF-κB activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol (Hoboken, NJ). 2014;66(3):637–646. doi: 10.1002/art.38279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbalaviele G, Sommers CD, Bonar SL, Mathialagan S, Schindler JF, Guzova JA, et al. A Novel, Highly Selective, Tight Binding IκB Kinase-2 ( IKK-2 ) Inhibitor : A Tool to Correlate IKK-2 Activity to the Fate and Functions of the Components of the Nuclear Factor- κB Pathway in Arthritis-Relevant Cells and Animal Models. J Pharmacol Exp Ther. 2009;329(1):14–25. doi: 10.1124/jpet.108.143800. [DOI] [PubMed] [Google Scholar]
- 21.Murahashi Y, Yano F, Kobayashi H, Makii Y, Iba K, Yamashita T, et al. Intra-articular administration of IκBα kinase inhibitor suppresses mouse knee osteoarthritis via downregulation of the NF-κB/HIF-2α axis. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-34830-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett J, Capece D, Begalli F, Verzella D, D’Andrea D, Tornatore L, et al. NF-κB in the crosshairs: Rethinking an old riddle. Int J Biochem Cell Biol. 2018;95(November 2017):108–112. doi: 10.1016/j.biocel.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168(1–2):37–57. doi: 10.1016/j.cell.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early Response of Mouse Joint Tissue to Noninvasive Knee Injury Suggests Treatment Targets. Arthritis Rheumatol. 2014;66(5):1256–1265. doi: 10.1002/art.38375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaplan S, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia evoked by unilateral ligation of the fifth and sixth lumbar nerves in the rat. J Neurosci. 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 26.Miller RE, Ishihara S, Tran PB, Golub SB, Last K, Miller RJ, et al. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI insight. 2018;3(6). doi: 10.1172/jci.insight.95704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. Mono-iodoacetate-induced experimental osteoarthritis. A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40(9):1670–1679. doi: 10.1002/art.1780400917 [DOI] [PubMed] [Google Scholar]
- 28.Leimer EM, Gayoso MG, Jing L, Tang SY, Gupta MC, Setton LA. Behavioral Compensations and Neuronal Remodeling in a Rodent Model of Chronic Intervertebral Disc Degeneration. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-39657-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temp J, Labuz D, Negrete R, Sunkara V, Machelska H. Pain and knee damage in male and female mice in the medial meniscal transection-induced osteoarthritis. Osteoarthr Cartil. 2020;28(4):475–485. doi: 10.1016/j.joca.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Mani SB, He Y, Hall AM, Xu L, Li Y, et al. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J Clin Invest. 2016;126(8):2893–2902. doi: 10.1172/JCI83676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18(SUPPL. 3):S17–S23. doi: 10.1016/j.joca.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 32.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthr Cartil. 2010;18(4):572–580. doi: 10.1016/j.joca.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 33.Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97(3):603–610. doi: 10.1016/j.pbb.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 34.Heegde F, Luiz AP, Magnusdottir R, Hopkinson M, Chang Y, Poulet B, et al. Osteoarthritis-related nociceptive behaviour following mechanical joint loading correlates with cartilage damage. Osteoarthr Cartil. Published online 2019. doi: 10.1016/j.joca.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 35.Miller RE, Kim YS, Tran PB, Ishihara S, Dong X, Miller RJ, et al. Visualization of Peripheral Neuron Sensitization in a Surgical Mouse Model of Osteoarthritis by In Vivo Calcium Imaging. Arthritis Rheumatol. 2018;70(1):88–97. doi: 10.1002/art.40342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brederson J-D, Chu KL, Xu J, Nikkel AL, Markosyan S, Jarvis MF, et al. Characterization and Comparison of Rat Monosodium Iodoacetate and Medial Meniscal Tear Models of Osteoarthritic Pain. J Orthop Res. Published online February 11, 2018:1–9. doi: 10.1002/jor.23869 [DOI] [PubMed] [Google Scholar]
- 37.Anderson MJ, Diko S, Baehr LM, Baar K, Bodine SC, Christiansen BA. Contribution of mechanical unloading to trabecular bone loss following non-invasive knee injury in mice. J Orthop Res. 2016;34(10):1680–1687. doi: 10.1002/jor.23178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruan MZC, Patel RM, Dawson BC, Jiang M-M, Lee BHL. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthr Cartil. 2013;21(9):1355–1364. doi: 10.1016/j.joca.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs BY, Allen KD. Factors affecting the reliability of behavioral assessments for rodent osteoarthritis models. Lab Anim. 2019;0(0):1–13. doi: 10.1177/0023677219867715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui Mingalone CK, Liu Z, Hollander JM, Garvey KD, Gibson AL, Banks RE, et al. Bioluminescence and second harmonic generation imaging reveal dynamic changes in the inflammatory and collagen landscape in early osteoarthritis. Lab Investig. Published online March 14, 2018:1–14. doi: 10.1038/s41374-018-0040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLoed AG, Sherrill TP, Cheng DS, Han W, Saxon JA, Gleaves LA, et al. Neutrophil-Derived IL-1β Impairs the Efficacy of NF-κB Inhibitors against Lung Cancer. Cell Rep. 2016;16(1):120–132. doi: 10.1016/j.celrep.2016.05.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartung JE, Eskew O, Wong T, Tchivileva IE, Oladosu FA, O’Buckley SC, et al. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav Immun. 2015;50:196–202. doi: 10.1016/j.bbi.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grothe K, Flechsenhar K, Paehler T, Ritzeler O, Beninga J, Saas J, et al. IκB kinase inhibition as a potential treatment of osteoarthritis – results of a clinical proof-of-concept study. Osteoarthr Cartil. 2017;25(1):46–52. doi: 10.1016/j.joca.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 44.Bailey KN, Furman BD, Zeitlin J, Kimmerling KA, Wu CL, Guilak F, et al. Intra-articular depletion of macrophages increases acute synovitis and alters macrophage polarity in the injured mouse knee. Osteoarthr Cartil. 2020;28(5):626–638. doi: 10.1016/j.joca.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan H, Duan X, Collins KH, Springer LE, Guilak F, Wickline SA, et al. Nanotherapy Targeting NF-kB Attenuates Acute Pain After Joint Injury. Precis Nanomedicine. 2019;2(1):245–248. doi: 10.33218/prnano2(1).181129.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burt HM, Tsallas A, Gilchrist S, Liang LS. Intra-articular drug delivery systems: Overcoming the shortcomings of joint disease therapy. Expert Opin Drug Deliv. 2009;6(1):17–26. doi: 10.1517/17425240802647259 [DOI] [PubMed] [Google Scholar]
- 47.Mwangi TK, Berke IM, Nieves EH, Bell RD, Adams SB, Setton LA. Intra-articular clearance of labeled dextrans from naive and arthritic rat knee joints. J Control Release. 2018;283(May):76–83. doi: 10.1016/j.jconrel.2018.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doan TN, Bernard FC, McKinney JM, Dixon JB, Willett NJ. Endothelin-1 inhibits size dependent lymphatic clearance of PEG-based conjugates after intra-articular injection into the rat knee. Acta Biomater. 2019;93:270–281. doi: 10.1016/j.actbio.2019.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holyoak DT, Wheeler TA, van der Meulen MCH, Singh A. Injectable mechanical pillows for attenuation of load-induced post-traumatic osteoarthritis. Regen Biomater. Published online 2019:1–9. doi: 10.1093/rb/rbz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OARSI scoring of articular cartilage 16 weeks following joint injury showed similar levels of histological development of PTOA in INJ and INJ/Tx ipsilateral knee joints (n=4/group). Due to sectioning artifacts the LTP within a single INJ/Tx animal could not be scored.
Knee joint injury did not influence latency to withdrawal from hot (A) or cold (B) thermal stimuli presented at the ipsilateral hindpaw.