Abstract
Objective
This study aims to assess the magnitude and associated factors of poor medication adherence among diabetic and hypertensive patients visiting public health facilities in Addis Ababa, Ethiopia during the COVID-19 pandemic.
Methods
A multi-site cross-sectional design was conducted from 1st through 30th of August 2020 at public health facilities of the study area. Adult outpatients with T2DM and hypertension visiting hospitals and health centers were included in the study. A proportion to size allocation method was used to determine the required sample size per facility. Data was collected using the 8-item Morisky medication adherence scale. Descriptive statistics and binary logistic regression were used to analyze data. A 95% confidence interval and p≤0.05 statistical significance was considered to determine factors associated with poor medication adherence.
Results
A total of 409 patients were included in the present study. About 57% of the patients reported that the COVID-19 pandemic has posed negative impacts on either of their follow-up visits, availability of medications, or affordability of prices. And, 21% have reported that they have been affected in all aspects. The overall magnitude of poor medication adherence was 72%. Patients with extreme poverty were more likely to have good medication adherence (AOR: 0.59; 95%C.I: 0.36–0.97), whereas attendance to a health center (AOR: 1.71; 95%C.I: 1.02–2.85), presence of comorbidity (AOR: 2.05; 95%C.I: 1.13–3.71), and current substance use history (AOR: 11.57; 95%C.I: 1.52–88.05) predicted high odds of poor adherence.
Conclusion
Over a three-fourth of the patients, in the study setting, have poor adherence to their anti-diabetic and antihypertensive medications. Health facility type, income level, comorbidity, and current substance use history showed a statistically significant association with poor adherence to medication. Stakeholders should set alternative strategies as perceived impacts of the COVID-19 pandemic on medication adherence are high in the study area.
Introduction
Adherence to anti-diabetic and antihypertensive medications is an area of interest with marked implications to affect patient management outcomes. The World Health Organization (WHO) defined adherence in 2001 as the extent to which a patient follows medical instructions [1]. Whereas this concept accounts for a broader aspect in the medical practice, its correlation with the management and control of chronic illnesses as diabetes and hypertension is well documented. This is likely because, adherence in this group encompasses multiple dimensions, such as lifestyle changes, medications, patient attitudes, and provider-patient relationships among others [2, 3] with potential interaction in the longer or lifetime frames.
Measuring adherence provides information on patients’ behavior to medication and lifestyle practices [4]. Studies have documented that the level of medication adherence in developed nations remained, only, close to 50% [5–7]. This figure is even lower in the developing and middle-income countries owed to the paucity of healthcare inputs and limited access to the services. Reports of adherence to antihypertensive medications from China (65.1%), Gambia (27%), and Seychelles (26%) showed the magnitude and relevance of the problem [8–10].
Medication adherence studies on antidiabetic and antihypertensive medications have been conducted across regions of Ethiopia. Even though the measurement tool and design of the study are similar for most of the studies, there is a considerable variation in terms of the level of adherence by study settings and type of chronic illness considered [11–14]. Reports from the present study setting also revealed that adherence levels to anti-diabetic medications ranged from 51.3% to 76% [15–17].
Currently, when the focus of pharmaceutical companies is on battling the COVID-19 pandemic across the globe, low and middle-income countries (LMICs) like Ethiopia, might be worst affected due to the rather vulnerable and inadequate pharmaceutical manufacturing capacities in these countries to meet their general pharmaceutical needs particularly those for chronic diseases. With limited supply to meet the increased demand created, the market values of medicines for chronic diseases have escalated, making them unaffordable for several patients in LMICs who require them [13]. Besides, adequate therapeutic outcomes of chronic illnesses require a linear adherence to medications [18]. This, in turn, could be linked to accessibility, affordability that can, negatively, be impacted during the outbreak [19].
Also, even though more studies have been conducted on the topic [11–17], potential variation in findings is inevitable due to time, study design, perception, context, the population considered, sample size, and quality of care delivered to patients among others. The purpose of this study is to assess the level of medication adherence, patients’ perception of the impact of the COVID-19 pandemic, and factors associated with poor adherence to anti-diabetic and antihypertensive medications among patients visiting public health facilities in Addis Ababa.
Methods
Study setting, design, and period
The study is conducted in Addis Ababa, the capital of Ethiopia. The 2021 estimated population of the city is over 4.8 Million [20]. There exist a total of 12 public hospitals [21] of which six are managed under the federal ministry of health (FMOH) whilst the rest five hospitals and 103 health centers are administered under the Addis Ababa health bureau (AAHB) [22]. The prevalence of diabetes mellitus and hypertension in public health facilities was reported to be 14.8% [23] and 32–34.7% [24, 25] respectively. A cross-sectional study design was conducted from 1st through 30th August 2020 at seven public health facilities to assess the adherence of patients to antidiabetic and antihypertensive medications during the COVID-19 pandemic. One of the facilities (Saint Paul’s hospital millennium medical college (SPHMMC)) was a teaching hospital under the ministry of health whereas, one general hospital (Ras Desta Damtew memorial hospital (RDDMH)), and five health centers namely; Arada, Lideta, Nifas Silk Lafto wereda 09, Akaki kality, and Bulbula are administered under the Addis Ababa regional health bureau.
Populations and inclusion criteria
The source population of this study was; all adult outpatients diagnosed with T2DM and hypertension in Addis Ababa, Ethiopia. All outpatients aged 18 years or above, those who have been on either anti-diabetic or antihypertensive medications for more than six months, visiting the selected public health facilities during the stated study period, and who provided informed consent were eligible in the study. Patients with known or suspected psychiatric problems and those attending to the emergency units were not included.
Sample size and sampling technique
The minimum sample size was estimated based on reports of adherence to antihypertensive [13] and anti-diabetic medications [15]. Of the two figures (66.7% and 51.3% respectively), the latter produced a higher required sample size. The single population proportion formula with a 95% confidence interval, and 5% tolerable error assumptions was employed in the calculation. Adding a 10% for non-response, a total of 422 participants was required for the study. Hospitals and health centers were selected purposively based on patient flow and socio-demographic diversity of catchment populations. A proportion to size allocation method was used to determine the required number of patients per health facility. Taking into account the COVID-19 risk, participants who consented to take part were recruited and included in the study using a consecutive sampling technique.
Data collection tool, procedures, and quality
Medication adherence was measured based on the 8-item Morisky medication adherence instrument [26]. Socio-demographic and clinical profiles were included with the questionnaire. After obtaining verbal informed consent, a face-to-face interview, using a pre-tested questionnaire, was done by trained data collectors. An Amharic (an official working language of Ethiopia) translated instrument was used during the data collection and back-translated to English for entry and analysis. Content validity of the tool was ensured by the study team. Supervision was undertaken throughout the data collection period.
Variables of the study
The dependent variables are adherence level and patients’ perception of the COVID-19 impact. On the other hand, the independent variables were socio-demographic variables, such as age, sex, health facility type, education, marital status, income level, current substance use history, presence of close people, number of close people, and Clinical variables, such as presence of comorbidity, disease duration, treatment duration, diagnosis type and presence of sleep disturbance.
Operational definitions
Good adherence
Refers to a patient’s overall score sum of 16 points from the 8-item Morisky’s medication adherence scale (MMAS). Items 1 through 7 were coded as 1 = yes, 2 = no except for item 5 in which no was rated as 1 and yes was rated as 2. For item 8, the Likert scaled scores of 1 to 5 were reverse coded as 2 = never and 1 = often to always.
Poor adherence
Refers to the MMAS-8 score summation of 8 through 15 where a patient missing at least one or more of the items of the scale was classified under poor adherence.
Current history of substance use
Patient reporting to have used either one or any combination of alcohol, Khat, or cigarettes in the past three months.
Close people
Refers to family members or relatives whom a patient can rely on or seek for any form of assistance during hard times.
Comorbidity
Was considered when a patient presents with one or more chronic conditions in addition to either diabetes or hypertension.
Ethical considerations
Ethical approval to conduct this study was obtained from Saint Paul’s Hospital Millennium Medical College (SPHMMC) institutional review board (IRB), and the Addis Ababa regional health bureau research ethics review committee. After ethical clearance was sought from the regional health bureau, a support letter was written to the respective health facilities before data collection. Verbal informed consent was obtained from each participant included in the study. Participation in the study was voluntary. Confidentiality of the data obtained was maintained throughout and after completion of the study. No personal identifiers were either included in the tool or were collected during the study.
Data analysis
After cleaning and coding manually, data was entered and checked for completeness and accuracy in statistical products for a social solution (SPSS) V.26.0. Both descriptive and inferential statistics were applied in the analysis. Tables and figures were used to present descriptive results. A Bi-variable analysis of all potential patient characteristics was done at p≤0.2 for potential association with poor adherence to anti-diabetic and antihypertensive medications. Variables that satisfied the first test were subsequently included in the multivariable logistic regression at a p≤0.05 level of significance. A 95% level of confidence was considered in both cases.
Results
Profile of patients
A total of 409 patients were included in the present study. Even though few patients declined to take part during the subsequent recruitment (n = 13), the number of respondents was beyond the minimum requirement. The age of patients ranged from 19 to 95 with a mean of 56.5 and a standard deviation (SD) of 13.4 Years. The majority were in the age group of 45 or above (321,78.5%), females (229,6%), visiting hospitals (224,54.8%), not attended formal education (174, 42.5%), married (259, 63.3%), live with moderate poverty or better (222, 54.3%), have comorbidity (250, 61.0%), with 7 years or less mean duration of disease (277, 67.7%) and 7 years or less mean duration of treatment (257, 62.8%). Most of the participants had no current history of any substance use (92.2%), diagnosed with hypertension only (35.0%), had no sleep disturbance (68.7%), have close people around (88.5%), and live with 3–5 family members (59.7%) (Table 1). Among the comorbidities reported, hypertension (204, 81.6%), diabetes mellitus (179, 71.6%), heart disease (66, 26.4%), hypercholesterolemia (36, 14.4%), chronic asthma (23, 9.2%), and stroke (12, 4.8%) accounted for the top frequencies. A Cronbach’s alpha test of the reliability of the scale among the samples also showed an acceptable range for both hospitals (α = 0.88) and health centers (α = 0.63).
Table 1. Profile of T2DM and hypertensive patients visiting chronic care units of public health facilities in Addis Ababa, August 2020.
| Characteristic | Label | Frequency | % |
|---|---|---|---|
| Age (yrs.) | |||
| ≤45 | 88 | 21.5 | |
| >45 | 321 | 78.5 | |
| Sex | |||
| Male | 180 | 44 | |
| Female | 229 | 56 | |
| Visiting site | |||
| Health center | 185 | 45.2 | |
| Hospital | 224 | 54.8 | |
| Education | |||
| Not attended formal education | 174 | 42.5 | |
| Grades 1 to 12 | 165 | 40.4 | |
| Diploma or above | 70 | 17.1 | |
| Marital status | |||
| Unmarried | 53 | 13.0 | |
| Married | 259 | 63.3 | |
| Divorced/separated/widowed | 97 | 23.7 | |
| Income level (ETB) * | |||
| Extreme poverty | 187 | 45.7 | |
| Moderate poverty or better | 222 | 54.3 | |
| Presence of comorbidity | |||
| No | 159 | 38.9 | |
| Yes | 250 | 61.1 | |
| Disease duration** | |||
| ≤7 years | 277 | 67.7 | |
| >7 years | 132 | 32.3 | |
| Treatment duration** | |||
| ≤7 years | 257 | 62.8 | |
| >7 years | 152 | 37.2 | |
| Current substance use history | |||
| Yes | 32 | 7.8 | |
| No | 377 | 92.2 | |
| Diagnosis type | |||
| Type 2 diabetes mellitus | 132 | 32.2 | |
| Hypertension | 143 | 35.0 | |
| Both T2DM and hypertension | 134 | 32.8 | |
| Presence of sleep disturbance | |||
| Yes | 128 | 31.3 | |
| No | 281 | 68.7 | |
| Presence of close people | |||
| No | 47 | 11.5 | |
| Yes | 362 | 88.5 | |
| Number of family members | |||
| ≤2 | 79 | 19.3 | |
| 3–5 | 244 | 59.7 | |
| ≥6 | 86 | 21.0 |
*classified considered based on the World Bank’s definition of poverty [27].
** considered based on the mean duration (Yrs.) of diagnosis or initiation of treatment.
Patients’ perception of the impact of COVID-19 pandemic
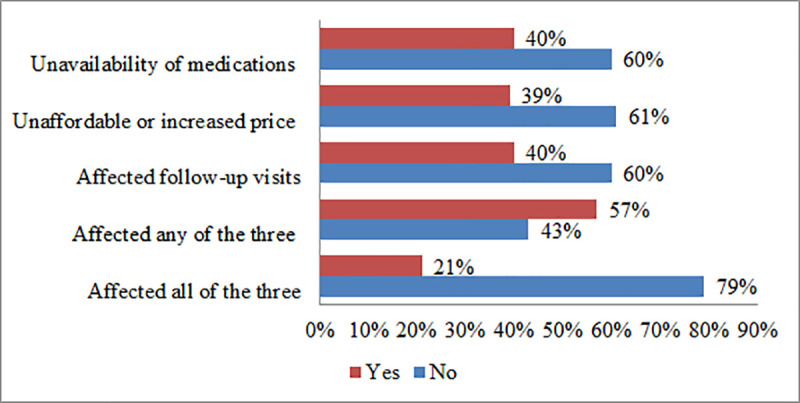
About 163(40%) of the patients reported that the COVID-19 pandemic has posed negative impacts on the availability of medications and their follow-up visits, whereas 160(39%) believed that it caused an unaffordable or increased price of medications. Two hundred thirty-four (57%) reported that they have faced one or more of the problems whilst 87(21%) stated that they come across all the three (Fig 1).
Fig 1. Perception of T2DM and hypertensive patients on the impact of the COVID-19 pandemic visiting chronic care units of public facilities in Addis Ababa, August 2020.
Level of adherence to antidiabetic and antihypertensive medications
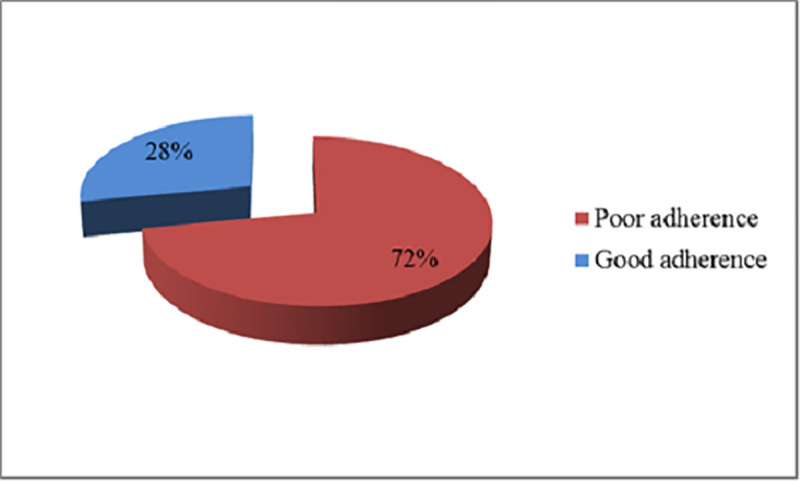
The level of adherence to antidiabetic and antihypertensive medications was measured using the 8-item Morisky medication adherence scale. Accordingly, the overall level of adherence was found to be 28% whilst 72% were poorly adherent missing at least one element from the scale (Fig 2).
Fig 2. Adherence level to antidiabetic and antihypertensive medications among patients visiting public health facilities during the COVID-19 pandemic in Addis Ababa, Ethiopia, August 2020.
Factors associated with poor medication adherence
Lastly, multiple characteristics of patients were tested against the presence of any potential association with poor medication adherence. Patients under extreme poverty were more likely to report a good adherence as compared to patients with moderate poverty or better average monthly income (AOR: 0.59; 95%C.I: 0.36–0.97). On the other hand, patients attending health centers (AOR: 1.71; 95%C.I: 1.02–2.85), having any comorbidity (AOR: 2.05; 95%C.I: 1.13–3.71), and current history of any substance use (AOR: 11.57; 95%C.I: 1.52–88.05) have shown a statistically significant positive association with poor medication adherence (Table 2).
Table 2. Factors associated with poor adherence to antidiabetic and antihypertensive medications among patients visiting public health facilities during the COVID-19 pandemic in Addis Ababa, Ethiopia, August 2020.
| Variable | Label | Level of adherence | COR (95%CI) | AOR (95%CI) | |
|---|---|---|---|---|---|
| Good (n) | Poor (n) | ||||
| Facility level | |||||
| Health centers | 38 | 147 | 1.98(1.27–3.12) | 1.71(1.02–2.85)* | |
| Hospitals | 76 | 148 | 1 | 1 | |
| Education | |||||
| Not attended formal education | 35 | 139 | 1.94(1.04–3.62) | 1.85(0.94–3.63) | |
| Primary/secondary education | 56 | 109 | 0.95(0.53–1.73) | 0.96(0.50–1.84) | |
| College/University education | 23 | 47 | 1 | 1 | |
| Average monthly income (ETB) d | |||||
| Extreme poverty | 58 | 129 | 0.75(0.49–1.16) | 0.50(0.27–0.89)* | |
| Moderate poverty or better | 56 | 166 | 1 | 1 | |
| Comorbid condition | |||||
| No | 56 | 103 | 1 | 1 | |
| Yes | 58 | 192 | 0.56(0.36–0.86) | 2.05(1.13–3.71)* | |
| Current history of substance use | |||||
| Yes | 1 | 31 | 13.27(1.79–98.39) | 11.44(1.50–87.11)* | |
| No | 113 | 264 | 1 | 1 | |
| Diagnosis type | |||||
| T2DM | 42 | 90 | 0.59(0.34–1.03) | 1.06(0.52–2.16) | |
| Hypertension | 43 | 100 | 0.64(0.37–1.11) | 0.92(0.48–1.77) | |
| Both T2DM and hypertension | 29 | 105 | 1 | 1 | |
| Presence of sleep disturbance | |||||
| Yes | 28 | 100 | 1.58(0.97–2.57) | 1.44(0.86–2.42) | |
| No | 86 | 195 | 1 | 1 | |
| Presence of close people around | |||||
| No | 7 | 40 | 2.40(1.04–5.52) | 2.03(0.84–4.90) | |
| Yes | 107 | 255 | 1 | 1 | |
*indicates statistical significance at P≤0.05. COR: crude odds ratio; AOR: adjusted odds ratio.
Discussion
The COVID-19 pandemic has severely affected health systems in general and follow-up service to chronic illnesses in particular [28–30]. Its impact in Sub-Saharan Africa is more pronounced as this region is often characterized by low health system infrastructure coupled with a growing burden of non-communicable diseases [31]. In Ethiopia too, most healthcare services have been disrupted due to the alarming spread of the pandemic and daily loaded terrifying news [32]. Augmented with less attention of the public to regularly practice preventive measures, more patients are still at risk of either its direct or indirect impacts militating their adherence to therapy. It is apparent from the present study that the majority of patients experienced negative impacts on either of their follow-visits, availability, or affordability of medications at least once during the outbreak. It was also noted that about a fifth of the patients in the present sample reported having faced all of the problems.
Evaluation of adherence to medication level could be regarded as a potential indicator for patients’ commitment to reverse an affected state of health [33]. Poor adherence to long-term medications is multifactorial often comprising socio-demographic factors, individual patient-related, therapy-related factors, and the health system among others [34]. As a result, achieving proper adherence remains to be a challenge both in the developed [5, 6] and developing [7–10] countries even though pharmacotherapy remains to be the mainstay of treatment especially among the older population [35].
The present study shows that level of poor adherence, where a patient failed to meet all the recommended criteria, was found to be significantly high (72%). This figure is higher as compared to the level of adherence to antihypertensive medications reported from Southwest Ethiopia (61.8%) by Asgedom and his colleagues [11], in Hawassa (67%) documented by Getnet et al. [12], and in Addis Ababa (66.7%) reported by Tibebu and his colleagues [13]. The highest level of adherence (75%) to antihypertensive medications was reported from Northwest Ethiopia as well [14]. More precisely, the earlier studies have indicated that at least half a proportion of the patients in Addis Ababa had a good level of adherence to their antihypertensive [13] and antidiabetic medications [15–17]. Whereas multiple contributors would result in a poor outcome, the impact of the COVID-19 pandemic six months before the study period was undeniably notable [32, 36]. Most patients have also implied in their report of perceived negative influence the outbreak had posed in terms of meeting with follow-up appointments, accessing medicines, and affording for prices. These factors are mentioned to account for a remarkable role in patients’ poor adherence to therapy [37, 38].
Studies documented that various factors have a link with poor adherence to medications among patients with chronic illness. These may include; lack of involvement of family and friends [39], economic difficulties [40], poor relationship between professional and patients [41], and side effects as well as the complexity of regimens [41, 42]. Furthermore, while patients with asymptomatic diseases have less incentive to adhere to medications, the presence of multiple comorbid conditions that are treated with more drugs can also impair attaining proper adherence [34, 43, 44]. The earlier studies done in Ethiopia have indicated that such contributors, as comorbidity [11–13], age group [12–14], level of knowledge about disease and medication [12–14, 16], and level of education [15, 16] were reported to be among the factors associated with medication adherence.
In paradox with the popular belief [40, 45], lower-income was found to be associated with higher odds of good adherence to anti-diabetic and antihypertensive medications in the current setting. Though the connection of income and health is well established in terms of either direct effect on material fulfillment or indirectly through ensuring social participation [46], the present association could be confounded with non-income factors. In the current Ethiopian context, all patients under the poverty line (45.7% of the respondents) are waived insurance fees that may improve their service utilization rate and access to medicines. Yet, adherence is a behavior of composite factors [34] that can be altered even apart from gaining access to service and medications. Taking into account non-compliance consequences, poor patients might tend to apply medical advice or maintain good relations with providers.
On the other hand, attending a health center, having any comorbid condition and current history of any substance use have shown a statistically significant positive association with poor medication adherence. An increase in the odds of poor adherence among patients attending health centers would be related to patients’ health-seeking behavior, the severity of complications, patient loads, or perceived poor availability of COVID-19 preventive measures at these settings. That could be likely because, as primary healthcare units in the Ethiopian health system, health centers serve to catchment populations that do not need advanced diagnosis and treatment. This is in line with other studies that reported that having a comorbid condition [11] and substance use history [11, 47] were associated with high odds of poor medication adherence. This could also be attributed to the fact that patients may either not be able to comprehend the outcome of their disease and benefit of adherence to medications [48], experience side effects of polypharmacy [49] or fail to practice a healthy lifestyle.
This study has tried to present a city-wide comprehensive report on the medication adherence practice of patients visiting public health facilities in Addis Ababa, Ethiopia. Apart from being able to include various strata of facilities and patients, the findings can be easily compared to previous figures to evaluate the impact imposed by the COVID-19 pandemic on medication adherence. However, the observed association between dependent and independent variables may suffer from temporality. Inclusion of the only patients who were available during the study period could also pose a selection bias that might have affected the study results.
Conclusion
A significant proportion of patients with T2DM and hypertension in Addis Ababa have experienced negative impacts on either of their follow-visits, availability, or affordability of medications at least once during the COVID-19 outbreak. Over a three-fourth of the patients had poor adherence to their medications. Facility type, average monthly income, level of education, presence of comorbidity, and current histories of any substance use have shown a statistically significant association with poor adherence to antidiabetic and antihypertensive medications.
All concerned health authorities should take into account, and set multidisciplinary strategies to prevent impacts of the COVID-19 pandemic on medication adherence of patients with chronic illnesses.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank all patients who provided verbal consent to participate in this study. We also appreciate the kind assistance and facilitation gotten from all health facilities during the data collection process.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for the study was obtained from Saint Paul's Hospital Millennium Medical College. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sabate E, World Health Organization. Non-communicable Diseases and Mental Health Cluster. Adherence to long-term therapies: policy for action: meeting report, 4–5 June 2001. Geneva: World Health Organization; 2001. [Google Scholar]
- 2.Stanton AL. Determinants of adherence to medical regimens by hypertensive patients. Journal of Behavioral Medicine. 1987. 1987/08/01; 10(4):377–94. 10.1007/BF00846477 [DOI] [PubMed] [Google Scholar]
- 3.Rand CS. Measuring adherence with therapy for chronic diseases: implications for the treatment of heterozygous familial hypercholesterolemia. American Journal of Cardiology.1993, 72:68D–74D. 10.1016/0002-9149(93)90014-4 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). What is adherence. WHO.2003. pages 17–18. Available at: https://www.who.int/chp/knowledge/publications/adherence_Section1.pdf accessed on 24 October 2020. [Google Scholar]
- 5.Haynes RB, McDonald HP, Garg AX. Helping Patients Follow Prescribed Treatment: Clinical Applications. JAMA. 2002;288(22):2880–2883. 10.1001/jama.288.22.2880 [DOI] [PubMed] [Google Scholar]
- 6.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008; 336:1114–1117. 10.1136/bmj.39553.670231.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peacock E, Krousel-Wood M. Adherence to Antihypertensive Therapy. Med Clin North Am. 2017. January; 101(1):229–245. 10.1016/j.mcna.2016.08.005 ; PMCID: PMC5156530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bovet P, Burnier M, Madeleine G, Waeber B, Paccaud F. Monitoring one-year compliance to antihypertension medication in the Seychelles. Bull World Health Organ. 2002;80(1):33–9. ; PMCID: PMC2567628. [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Sande MA et al. Blood pressure patterns and cardiovascular risk factors in rural and urban Gambian communities. Journal of Human Hypertension. 2000, 14:489–496. 10.1038/sj.jhh.1001050 [DOI] [PubMed] [Google Scholar]
- 10.Lee GK, Wang HH, Liu KQ, Cheung Y, Morisky DE, Wong MC. Determinants of medication adherence to antihypertensive medications among a Chinese population using Morisky Medication Adherence Scale. PLoS One. 2013. April 25;8(4):e62775. 10.1371/journal.pone.0062775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asgedom SW, Atey TM, Desse TA. Antihypertensive medication adherence and associated factors among adult hypertensive patients at Jimma University Specialized Hospital, southwest Ethiopia. BMC Res Notes. 2018; (11):27. 10.1186/s13104-018-3139-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Getenet A, Tesfa M, Ferede A, Molla Y. Determinants of adherence to antihypertensive medications among adult hypertensive patients on follow-up in Hawassa Referral Hospital: A case-control study. JRSM Cardiovascular Disease. 2019; 8:1–8. 10.1177/2048004019892758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibebu A, Mengistu D, Bulto LN. Adherence to prescribed antihypertensive medications and associated factors for hypertensive patients attending chronic follow-up units of selected public hospitals in Addis Ababa, Ethiopia. Int J Health Sci (Qassim). 2017; 11 (4). 47–52. [PMC free article] [PubMed] [Google Scholar]
- 14.Teshome DF, Bekele KB, Habitu YA, and Gelagay AA. Medication adherence and its associated factors among hypertensive patients attending the Debre Tabor General Hospital, northwest Ethiopia. Integr Blood Press Control.2017; 10:1–7. 10.2147/IBPC.S128914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesfin Y, Assegid S, Beshir M. Medication Adherence among Type 2 Diabetes Ambulatory Patients in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. Epidemiology (Sunnyvale).2017; 7: 322. [Google Scholar]
- 16.Ali M, Alemu T, Sada O. Medication adherence and its associated factors among diabetic patients at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. BMC Res Notes. 2017;10:676. 10.1186/s13104-017-3025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demoz GT, Berha AB, Alebachew Woldu M, Yifter H, Shibeshi W, Engidawork E. Drug therapy problems, medication adherence and treatment satisfaction among diabetic patients on follow-up care at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS ONE.2019; 14(10):e0222985. 10.1371/journal.pone.0222985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, et al. Interventions to Improve Adherence to Self-administered Medications for Chronic Diseases in the United States: A Systematic Review. Ann Intern Med. 2012; 157(11):785–795. 10.7326/0003-4819-157-11-201212040-00538 [DOI] [PubMed] [Google Scholar]
- 19.Kretchy IA, Asiedu-Danso M, Kretchy JP. Medication management and adherence during the COVID-19 pandemic: Perspectives and experiences from low-and middle-income countries. Res Social Adm Pharm. 2021. January;17(1):2023–2026. 10.1016/j.sapharm.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Bank Group. Addis Ababa, Ethiopia: Enhancing Urban Resilience. Washington DC. 2015. Pages 25–30. Available at http://documents1.worldbank.org/curated/en/559781468196153638/pdf/Addis-Ababa-Enhancing-Urban-Resilience-city-strength-resilient-cities-program.pdf. Accessed on 17 March 2021.
- 21.Misganaw A, Mariam DH, Araya T, Ayele K. Patterns of mortality in public and private hospitals of Addis Ababa, Ethiopia. BMC Public Health. 2012; 12, 1007. 10.1186/1471-2458-12-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asemahagn MA. Knowledge and experience sharing practices among health professionals in hospitals under the Addis Ababa health bureau, Ethiopia. BMC Health Serv Res.2014; 14, 431. 10.1186/1472-6963-14-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahile AT, Bekele GE. Prevalence of Diabetes Mellitus and Associated Factors in Addis Ababa Public Health Facilities, Addis Ababa, Ethiopia, 2016. Diabetes Metab Syndr Obes. 2020;13:501–508. 10.2147/DMSO.S237995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abebe S, Yallew WW. Prevalence of hypertension among adult outpatient clients in hospitals and its associated factors In Addis Ababa, Ethiopia: a hospital-based cross-sectional study. BMC Res Notes. 2019; 12, 87. 10.1186/s13104-019-4127-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekele GE, Tadesse T, Negaw R, Zewde T. Magnitude and associated factors of hypertension in Addis Ababa public health facilities, Ethiopia. MOJ Public Health. 2018;7(6):280–286. 10.15406/mojph.2018.07.00252 [Google Scholar]
- 26.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Castañeda A, Doan D, Newhouse D, Nguyen MC, Uematsu H, Azevedo JP. Who Are the Poor in the Developing World? Poverty and Shared Prosperity Report 2016: Taking on Inequality. Policy Research Working Paper 7844. World Bank Group. 2016. Available at: http://econ.worldbank.org. Accessed on 10 October 2020.
- 28.Danhieux K, Buffel V, Pairon A, Benkheil A, Remmen R, Wouters E, et al. The impact of COVID-19 on chronic care according to providers: a qualitative study among primary care practices in Belgium. BMC Fam Pract. 2020; 21, 255. 10.1186/s12875-020-01326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chudasama YV, Gillies CL, Zaccardi F, Coles B, Davies MJ, Seidu S, et al. Impact of COVID-19 on routine care for chronic diseases: A global survey of views from healthcare professionals, Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020; 14 (5):965–967. 10.1016/j.dsx.2020.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO). COVID-19 significantly impacts health services for non-communicable diseases. Geneva.2020. Available at: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases accessed on October 25, 2020.
- 31.Amu H, Dowou R, Boateng LA, Tarkang EE. Implications of COVID-19 for the management of chronic non-communicable diseases in sub-Saharan Africa: application of the chronic care model. Pan African Medical Journal. 2020;35(2):94. 10.11604/pamj.supp.2020.35.24047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biadgilign S, Yigzaw M. COVID-19 in Ethiopia: current situation, missed opportunities, and the risk of health system disruptions. Pan African Medical Journal. 2020;35(2):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. 10.4065/mcp.2010.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35 (3):207. . [PubMed] [Google Scholar]
- 35.Midão L, Giardini A, Menditto E, Kardas P and Costa E. Adherence to Medication in Older Adults as a Way to Improve Health Outcomes and Reduce Healthcare System Spending. In: D’Onofrio G, editor. Gerontology. July 4th 2018. DOI: 10.5772/intechopen.72070. Available from: https://www.intechopen.com/books/gerontology/adherence-to-medication-in-older-adults-as-a-way-to-improve-health-outcomes-and-reduce-healthcare-sy accessed on 26 October 2020. [DOI]
- 36.Kassaw C, Pandey D. The Current Mental Health Crisis of COVID-19 Pandemic Among Communities Living in Gedeo Zone Dilla, SNNP, Ethiopia. J. Psychosoc. Rehabil. Ment. Health. 2020. 10.1007/s40737-020-00192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips J. Evaluating patient compliance: Effect of appointment reminder systems on attendance. Master of Public Health Program Student Publications. 2008. Available at: http://corescholar.libraries.wright.edu/cgi/viewcontent.cgi?article=1057&context=mph accessed on 27 October 2020. [Google Scholar]
- 38.Demoz GT, Wahdey S, Bahrey D, Kahsay H, Woldu G, Niriayo YL, et al. Predictors of poor adherence to antidiabetic therapy in patients with type 2 diabetes: a cross-sectional study insight from Ethiopia. Diabetol Metab Syndr. 2020; 12, 62. 10.1186/s13098-020-00567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller TA, Dimatteo MR. Importance of family/social support and impact on adherence to diabetic therapy. Diabetes Metab Syndr Obes: Targets and Therapy. 2013; 6:421–426. 10.2147/DMSO.S36368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kardas P. Ascertaining Barriers for Compliance: Policies for safe, effective and cost-effective use of medicines in Europe. ABC Project Final Report. Medical University of Lodz. 2012. http://abcproject.eu/img/abc%20 final.pdf [Google Scholar]
- 41.Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005. September;1(3):189–199. [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clinic Proceedings. 2011;86(4):304–314. 10.4065/mcp.2010.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osterberg L, Blaschke T. Adherence to medication. The New England Journal of Medicine. 2005;353:487–497. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 44.Stewart D, Mair A, Wilson M, Kardas P, Lewek P, Alonso A, et al. Guidance to manage inappropriate polypharmacy in older people: Systematic review and future developments. Expert Opin Drug Saf. 2017. November 25;16(2):203–213. 10.1080/14740338.2017.1265503 [DOI] [PubMed] [Google Scholar]
- 45.Rathish D, Hemachandra R, Premadasa T, Ramanayake S, Rasangika C, Roshiban R, et al. Comparison of medication adherence between type 2 diabetes mellitus patients who pay for their medications and those who receive it free: a rural Asian experience. J Health Popul Nutr. 2019; 38 (4). 10.1186/s41043-019-0161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marmot M. The Influence of Income on Health: Views of an Epidemiologist. Health Aff(Millwood). 2002; 21 (2). 10.1377/hlthaff.21.2.31 [DOI] [PubMed] [Google Scholar]
- 47.Eticha T, Teklu A, Ali D, Solomon G, Alemayehu A. Factors associated with medication adherence among patients with schizophrenia in Mekelle, Northern Ethiopia. PLoS One. 2015;10(3):e0120560. Published 2015 Mar 27. 10.1371/journal.pone.0120560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saqib A, Atif M, Ikram R, Riaz F, Abubakar M, Scahill S. Factors affecting patients’ knowledge about dispensed medicines: A Qualitative study of healthcare professionals and patients in Pakistan. PLoS ONE.2018; 13(6): e0197482. 10.1371/journal.pone.0197482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atinga RA, Yarney L, Gavu NM. Factors influencing long-term medication non-adherence among diabetes and hypertensive patients in Ghana: A qualitative investigation. PLoS ONE. 2018; 13(3): e0193995. 10.1371/journal.pone.0193995 [DOI] [PMC free article] [PubMed] [Google Scholar]


