Abstract
Salmonella is a zoonotic pathogen that persists in poultry. Salmonella vaccines that can be delivered in-ovo can be cost-effective and can decrease Salmonella load in poultry. This study evaluates the efficacy of a Salmonella chitosan-nanoparticle (CNP) vaccine, administered in-ovo, in broilers. CNP vaccine was synthesized with Salmonella Enteritidis (SE) outer-membrane-proteins (OMPs) and flagellin proteins. At embryonic-d18, one-hundred-thirty-six eggs were injected with 200μl PBS or 1000μg CNP into the amniotic cavity. At d1-of-age, 132 chicks were allocated in 6 pens/treatment with 11 chicks/pen. At d7, birds were orally challenged with 1×109 CFU/bird SE. At d1, 8h-post-challenge, d14, and d21, serum anti-SE-OMPs IgY were analyzed. At d14 and d21, cloacal swabs and bile anti-SE-OMPs IgA, CD4+/CD8+-T-cell ratios, and ceca SE loads were analyzed. At d21, cecal tonsil IL-1β, IL-10, and iNOS mRNA were analyzed. Body-weight-gain (BWG) and feed-conversion-ratio (FCR) were recorded weekly. Data were analyzed by Student’s t-test at P<0.05. There were no significant differences in BWG or FCR between vaccinated birds compared to control. At d1, CNP-vaccinated birds had 5.62% greater levels (P<0.05) of anti-SE-OMPs IgY, compared to control. At 8h-post-challenge, CNP-vaccinated birds had 6.39% greater levels (P<0.05) of anti-SE-OMPs IgY, compared to control. At 2wk-post-challenge, CNP-vaccinated birds had 7.34% lower levels (P<0.05) of anti-SE-OMPs IgY, compared to control. At 1wk-post-challenge, CNP-vaccinated birds had 15.30% greater levels (P<0.05) of bile anti-SE-OMPs IgA, compared to control. At d14 and d21, CNP-vaccinated birds had 0.62 and 0.85 Log10 CFU/g, decreased SE ceca load (P<0.05), respectively, compared to control. There were no significant differences in CD4+/CD8+-T-cell ratios between vaccinated birds compared to control. There were no significant differences in IL-1β, IL-10, iNOS mRNA between vaccinated birds compared to control. Findings demonstrate that the in-ovo administration of CNP vaccine can induce an antigen-specific immune response against SE and can decrease SE cecal load in broilers.
Introduction
Salmonella is an enteric pathogen in poultry. Birds can have up to Log 5 of Salmonella CFU and remain asymptomatic [1]. In humans, more than 70% of salmonellosis cases in the United States have been attributed to the consumption of contaminated poultry products or eggs [2]. The Centers for Disease Control and Prevention estimates that Salmonella causes about 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States every year [3].
To prevent Salmonella infections and the economic burden it brings, the poultry industry employs the use of Salmonella vaccines, but currently, commercial vaccines have certain disadvantages. Live vaccines are not preferred due to the ability of the live strain to regain its virulence and spread through shedding, interfere with the salmonellosis monitoring program [4], and strict cold chain requirement to preserve their function. Killed vaccines are preferred, but the intramuscular route of administration is time-consuming, increases tissue damage, and requires additional vaccine administration costs. An oral-killed vaccine would circumvent the disadvantages of both live and killed vaccines while mimicking a natural infection and stimulating the mucosal and systemic immune responses [5]. However, there are currently no commercially available oral-killed Salmonella vaccines for poultry due to the acidic pH of the gastrointestinal tract (GIT) [6].
Nanoparticle vaccines are good carriers to deliver antigens through the oral route because the polymer coating that surrounds the vaccine antigens aids in preventing the degradation of the proteins of interest [7–9]. Chitosan nanoparticles intrinsically possess a positive surface charge that facilitates their adherence to the negatively charged mucus layers, ensuring delivery of the vaccine antigens [10, 11]. Also, nanoparticles can provide a slow release of the antigen within the GIT, which gives a constant stimulus to mucosal immune cells that can result in reducing the dosing frequency or the need for adjuvants [7]. Our polymeric nanoparticle vaccines, against Salmonella, have been previously characterized and used for antigen delivery in poultry [12–14]. Findings have shown that both, chitosan and polyanhydride, Salmonella-nanoparticle vaccines are safe with no adverse effects detected on bird performance or health and can significantly increase an antigen-specific humoral and mucosal immune response, as well as decrease the Salmonella load in the ceca.
It is important to have effective methods of mass vaccination because flock sizes of commercial poultry contain thousands of birds. In-ovo vaccines are a mass vaccination strategy that can reduce stressful post-hatching procedures [15]. In-ovo vaccines are currently approved for use in poultry flocks for different diseases, e.g., Marek’s disease. Typically, the in-ovo vaccine is injected into the amniotic sac and the amnion fluid containing the vaccine antigen gets swallowed by the embryo, giving way to “oral vaccination” at an early stage [16]. In-ovo vaccination induces early immunity with less interference from maternal antibodies [15]. Early immunity can also aid in significantly reducing the inflammatory response upon future encounters with the antigen [17]. Successful vaccination against Salmonella at an embryonic stage would be a useful prevention strategy for the poultry industry.
A Salmonella CNP vaccine was synthesized with crude-enriched outer membrane proteins (OMPs) and flagellin protein extracts from Salmonella Enteritidis (SE) and surface-tagged with flagellin proteins [12]. Previous findings demonstrate that the 1000μg CNP Salmonella vaccine can induce a specific immune response against Salmonella and has the potential to mitigate SE cecal colonization in broiler birds [13]. The objective of this study is to evaluate the efficacy of a novel chitosan nanoparticle oral vaccine (CNP) administered in-ovo for Salmonella control in broilers. We hypothesize that the in-ovo administration of the Salmonella CNP vaccine will increase anti-Salmonella antigen-specific IgY and IgA and decrease the SE loads in broilers.
Materials and methods
Isolation of outer membrane proteins
Outer membrane proteins from SE were isolated as described previously [12] with few modifications. Briefly, a bacterial culture, at its stationary phase, was washed with 10 mM Tris buffer (pH 7.5), the sediment was suspended in Tris-sucrose EDTA (pH 8), and incubated on ice for 90 min. The cell suspension was centrifuged at 16,000 ×g for 30 min and the supernatant was collected and centrifuged at 100,000 ×g for 60 min. The pellet containing OMPs enriched extract was freeze-dried with 5% sucrose as a cryoprotectant. The protein concentration was estimated using a micro-BCA protein assay kit (Thermo Scientific, MA) as per the manufacturer’s instructions.
Isolation of flagellin proteins
Flagellin proteins from SE were isolated as described previously [12]. Salmonella Enteritidis bacterial culture was grown on Trypticase soy agar plates and was inoculated into brain heart infusion broth and subsequently incubated for 48 h at 37°C, without shaking. The cells were washed with PBS (pH 7.4) and centrifuged at 7000 ×g for 30 min. The cell pellet was treated with 3M potassium thiocyanate (Sigma, MO) in PBS for 2 h at room temperature, under magnetic stirring. The cell suspension was centrifuged at 35,000 ×g for 30 min and the supernatant containing flagellin-protein-enriched extract was dialyzed once against PBS (pH 7.4), followed by Milli-Q water, and freeze-dried with 5% sucrose as a cryoprotectant. The protein concentration was estimated using micro-BCA protein assay kit as per the manufacturer’s instructions.
Preparation of nanoparticle vaccine
The CNP vaccine was synthesized at the Food Animal Health Research Program, The Ohio State University, USA. The OMPs and flagellin proteins were isolated from SE and the CNP vaccine was synthesized using the ionic gelation method, as described previously [12]. Briefly, a solution of 1.0% (w/v) low molecular weight chitosan (Sigma, MO) was prepared by slowly dissolving chitosan in an aqueous solution of 4.0% acetic acid. The solution was sonicated, adjusted to a 4.3 pH, and was filtered using a 0.44 μm syringe filter. Five milliliters of the 1.0% chitosan solution was added to 5 mL of dH2O and incubated with 2.5 mg OMPs and flagellar proteins. To form the nanoparticles, 2.5 mL of 1% (w/v) TPP in 2.5 mL deionized water was added to the above solution under magnetic stirring at room temperature. Afterward, 2.5 mg of flagellin protein in PBS was added to the nanoparticles to surface-conjugate the nanoparticles with flagellin proteins. The CNP vaccines were collected by centrifugation at 10,500 ×g for 10 minutes, lyophilized, and stored at -80°C until further use.
Experimental animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Georgia. Birds (Cobb-Vantress hatchery, Inc. Cleveland, GA, USA) had access to ad libitum feed and water. Birds were monitored at least once a day for dehydration, refusal to eat food, loss of body weight, diarrhea, bloody feces, and lethargy during the experimental period. All birds were euthanized by cervical dislocation. All birds were terminated at the end of the experimental period.
A total of one hundred thirty-six broilers eggs were vaccinated at embryonic day 18 of incubation. The eggs were randomly assigned to two treatment groups: control (sterile phosphate-buffered saline; PBS) and immunized (CNP). The eggshell was disinfected by spraying 1.5% hydrogen peroxide and was punctured using a sterile needle. Two hundred microliters of either PBS or 1000 μg vaccine in 200 μl PBS were injected into the amniotic cavity as described earlier [18]. The vaccinated eggs were incubated under standard hatchery settings. All the eggs were vaccinated and transferred from the incubator to the hatcher at embryonic day 18. At 21d of incubation, the percentage of hatchability was recorded. The hatchability was 97%.
At d1 of age, one hundred thirty-two chicks were randomly allocated to the two treatment groups in six pens (n = 6) with 11 chicks/pen. At d1, d7, d14, and d21 of age, body weight and feed consumption were recorded, and body weight gain (BWG) and feed consumption ratio (FCR) were calculated. Blood was collected at d1, 8h post-challenge, d14, and d21 of age, and serum was analyzed by ELISA for anti-OMPs specific IgY antibodies. At d7 of age, all the birds were orally inoculated with live SE (1 × 109 CFU/bird). Cloacal swabs and bile samples were collected at d14 and d21 of age for anti-OMPs specific IgA antibodies analysis. At d14 and d21 of age, spleen samples were analyzed for CD4+/CD8+ T cells by flow cytometry. At d14 and d21 of age, the birds’ ceca were collected for SE quantification by plating. At d21 of age, cecal tonsils were collected to analyze inflammatory cytokine (IL-1β), anti-inflammatory cytokine (IL-10), and induced nitric oxide synthase (iNOS) mRNA amounts by RT-PCR.
Anti-SE OMPs specific IgY and IgA antibodies in serum, cloacal swabs, and bile of chickens vaccinated in-ovo with CNP vaccine
Serum, cloacal swabs, and bile were collected from one bird/pen (n = 6) at d1, d7, d14, and d21 of age. The amounts of antigen-specific antibodies in serum, cloacal swab, and bile samples were determined by the enzyme-linked immunosorbent assay (ELISA), and was carried out as described previously [13]. For ELISA analysis, both treatment groups consisted of six samples, in duplicates, for each time point. Briefly, native SE OMPs were coated with either 2 μg/ml (IgY) or 7.5 μg/ml (IgA) on ELISA plates (Nunc MaxisorpTM, ThermoFisher Scientific, Waltham, MA). For analysis, 50 μl of serum and bile samples were diluted in 2.5% non-fat dry milk, and 50 μl of cloacal supernatants were added to the wells in duplicates. HRP-conjugated goat anti-chicken IgG (Southern Biotech, AL) (1: 10,000) or HRP-conjugated goat anti-chicken IgA (Gallus immunotech, NC) (1: 3000) in 2.5% skim milk powder in PBS- Tween 20 (PBST) were used as secondary antibodies. Optical density was measured as absorbance at 450 nm using a spectrophotometer (Biochek, Scarborough, ME) and values are reported as OD450.
CD4+ and CD8+ cell ratios in cecal tonsils of chickens vaccinated in-ovo with CNP vaccine
Cecal tonsil samples were collected from one bird/pen (n = 6) at d14 and d21 of age. For flow cytometry analysis, both treatment groups consisted of six samples, in duplicates, for each time point. Cecal tonsil samples were teased over a 0.4 μm cell strainer (Sigma, MO) with 2 mL RPMI-1640 media to obtain a single-cell suspension. Single-cell suspensions were concentrated for lymphocytes by density centrifugation over Histopaque (1.077 g/mL; Sigma, MO). For CD4+/CD8+ analysis, single-cell suspensions of the cecal tonsils (1 × 106 cells) were incubated with FITC-conjugated mouse anti-chicken CD4, PE-conjugated mouse anti-chicken CD8 (Southern Biotech, Birmingham, AL) at 1:200 dilution, and unlabeled mouse IgG at 1:200 dilution in a 96-well plate for 20 minutes. After incubation, cells were washed twice to remove unbound primary antibodies at 400 ×g for 5 minutes using wash buffer (1× PBS, 2 mM EDTA, 1.5% FBS). After washing, cells were analyzed using cytosoft software (Guava Easycyte, Millipore, Billerica, MA). The CD4+ and CD8+ and CD4+/CD8+ cell percentages were analyzed after gating cells based on forward-scatter and side-scatter plot for lymphocytes.
Salmonella loads in the ceca of chickens vaccinated in-ovo with CNP vaccine
Ceca samples were collected from three birds from each pen (n = 6) at d14, and d21 of age, and samples were pulled and analyzed for SE loads by plating. Ceca samples were aseptically collected into stomacher bags, placed on ice, and transported to the laboratory. Ceca samples were macerated using a rubber mallet, and 3× (wt/vol) buffered peptone water (BPW) was added and stomached for 60 seconds and incubated for 12 h at 41°C for initial enrichment of the bacteria. A volume of 100 μL of ceca was serially diluted into 900 μL of BPW. From every dilution, a volume of 10 μL was plated in duplicates on Xylose Lactose Tergitol™ 4 (XLT4) agar plates. Plates were then incubated for 24 h at 42°C for confirmation of black colonies. Colonies were further confirmed using SYBR green qPCR with SE primers 5’-GCAGCGGTTACTATTGCAGC-3’ and 5’-CTGTGACAGGGACATTTAGCG-3’ [19]. Salmonella enumeration data were recorded as CFU/g of ceca and then transformed to Log 10 CFU/g of ceca for statistical analysis.
IL-1β, IL-10 and iNOS gene expression in the cecal tonsils of chickens vaccinated in-ovo with CNP vaccine
Cecal tonsil samples were collected from one bird/pen (n = 6) at d21 of age. For gene expression analysis, both treatment groups consisted of six samples, in duplicates, for each time point. Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. The isolated RNA was dissolved in Tris-EDTA (pH 7.5) buffer, and the concentration was determined by using NanoDrop™ 2000c Spectrophotometer (Thermo Fisher Scientific). The cDNA synthesis was performed with 2 μg of total RNA. The mRNA transcripts analyzed for the pro-inflammatory cytokine IL-1β, anti-inflammatory cytokine IL-10, and iNOS by RT-PCR (CFX96 Touch Real-Time System, BioRad) using iQ™ SYBR® Green Supermix (Bio-Rad, CA) after normalizing for housekeeping gene β-actin mRNA. Fold change from the reference was calculated, as explained previously [20]. The primers sequences used for RT-PCR analysis in this study are described in Table 1.
Table 1. Primers and PCR conditions for RT-PCR.
| Target Gene | Sequence | Annealing Temperature | Reference |
|---|---|---|---|
| IL-10-F | 5′-CATGCTGCTGGGCCTGAA-3′ | 57.5°C | [21] |
| IL-10-R | 3′ -CGTCTCCTTGATCTGCTTGATG-5′ | ||
| IL-1β -F | 5′-TCCTCCAGCCAGAAAGTGA-3′ | 57.0°C | [22] |
| IL-1β -R | 5′-CAGGCGGTAGAAGATGAAGC-3′ | ||
| β-actin-F | 5′-ACCGGACTGTTACCAACACC-3′ | 57.0°C | [23] |
| β-actin-R | 3′ -GACTGCTGCTGACACCTTCA-5′ |
Statistical analysis
For this experiment, the experimental unit was the pen, n = 6 pen/treatment, with 11 technical replicates as birds/pen. Serum, cloacal swabs, bile, and cecal tonsils samples were taken from 1 bird/pen at each time point, and samples were analyzed in duplicates. Ceca samples were taken from 3 bird/pen at each time point, and samples were pulled for each time point, and were analyzed in duplicates. All statistical differences between the two experimental treatments were determined using a parametric Student T-test. All statistical analyzes were performed using JMP Pro 14 (SAS Institute Inc., USA; 2018) with P-values <0.05 considered to be statistically significant.
Results
Hatchability and production performance of chickens vaccinated in-ovo with CNP vaccine
The hatchability of the eggs in the control group was 94%. There were no losses in the hatchability of the birds immunized with the in-ovo Salmonella CNP vaccine. Sixty-four out of the 68 eggs hatched in the group injected with PBS, while 68 out of the 68 eggs hatched in the group injected with the Salmonella CNP vaccine.
There were no significant differences (P>0.05) in the mean BWG and FCR of birds in any of the treatment groups at all-time points (Table 2).
Table 2. Production performance of chickens vaccinated with in-ovo CNP vaccine.
| Treatment | 0 to 21 | % mortality | |||||
|---|---|---|---|---|---|---|---|
| Feed intake (g) | SEM | BWG (g) | SEM | FCR | SEM | ||
| Control | 900.13 | 23.2 | 697 | 17.3 | 1.29 | 0.04 | 0 |
| CNP | 996.53 | 17.3 | 730 | 10.0 | 1.37 | 0.03 | 0 |
| P-value | 0.05 | 0.12 | 0.17 | ||||
The chitosan-based Salmonella CNP vaccine was synthesized with SE OMPs and flagellin proteins. At embryonic d18, broiler eggs were injected with 200μl of PBS or 1000μg CNP into the amniotic cavity. At d7, birds were orally challenged with 109 CFU/bird of SE. Production performance of the birds was monitored weekly and all birds were euthanized at 3 wk of age (d21). Data represents 6 pens/treatment (11 birds/pen). BWG: Body weight gain (g); FCR: Feed conversion ratio; Control: PBS-mock vaccination; CNP: chitosan nanoparticle vaccination. SEM: Standard error of mean. Data analyzed by parametric Student t-test.
Anti-SE OMPs specific IgY and IgA antibodies in serum, cloacal swabs, and bile of chickens vaccinated in-ovo with CNP vaccine
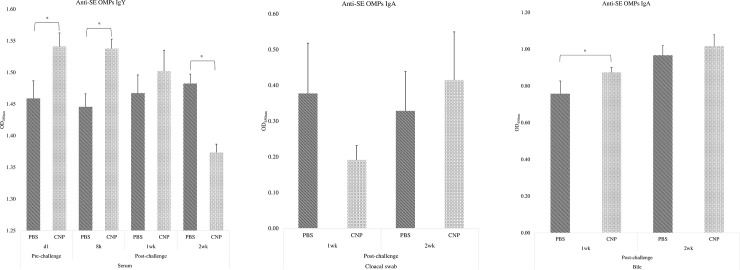
At d1, birds immunized with the in-ovo CNP had 5.62% higher levels (P<0.05) of serum anti-SE OMPs-specific IgY, compared to control (Fig 1A). At 8h post-challenge, birds immunized with the in-ovo CNP had 6.39% higher levels (P<0.05) of serum anti-SE OMPs-specific IgY, compared to control (Fig 1A). At 1wk post-challenge, birds in both treatment groups had no significant differences (P>0.05) in serum anti-SE OMPs-specific IgY (Fig 1A). At 2wk post-challenge, birds immunized with the in-ovo CNP had 7.34% lower (P<0.05) levels of serum anti-SE OMPs-specific IgY levels, compared to control (Fig 1A).
Fig 1. Anti-SE OMPS IgY/IgA in serum, cloacal swab, and bile of chickens vaccinated with in-ovo CNP.
At embryonic d18, broiler eggs were vaccinated with either PBS (control) or 1000 μg CNP vaccine. At d7 of age, birds were challenged with live SE (1.0 × 109 CFU/bird). Blood, cloacal swabs, and bile samples were collected pre- and post-challenge and analyzed for anti-Salmonella antigen-specific IgY and IgA levels by ELISA. Results were reported as average optical density (OD) values. A–Serum OMPs IgY; B–Cloacal swab OMPs IgA; C–Bile OMPs IgA. Data analyzed by parametric Student t-test; Means+SEM.Bars. n = 6. "*" means P<0.05.
At 1wk and 2wk post-challenge, birds in both treatment groups had no significant differences (P>0.05) of cloacal swab anti-SE OMPs-specific IgA (Fig 1B).
At 1wk post-challenge, birds immunized with the in-ovo CNP had 15.30% higher levels (P<0.05) of bile anti-SE OMPs-specific IgA, compared to control (Fig 1C). At 2wk post-challenge, birds in both treatment groups had no significant differences (P>0.05) of cloacal swab anti-SE OMPs-specific IgA (Fig 1C).
CD4+ and CD8+ cell ratios in cecal tonsils of chickens vaccinated in-ovo with CNP vaccine
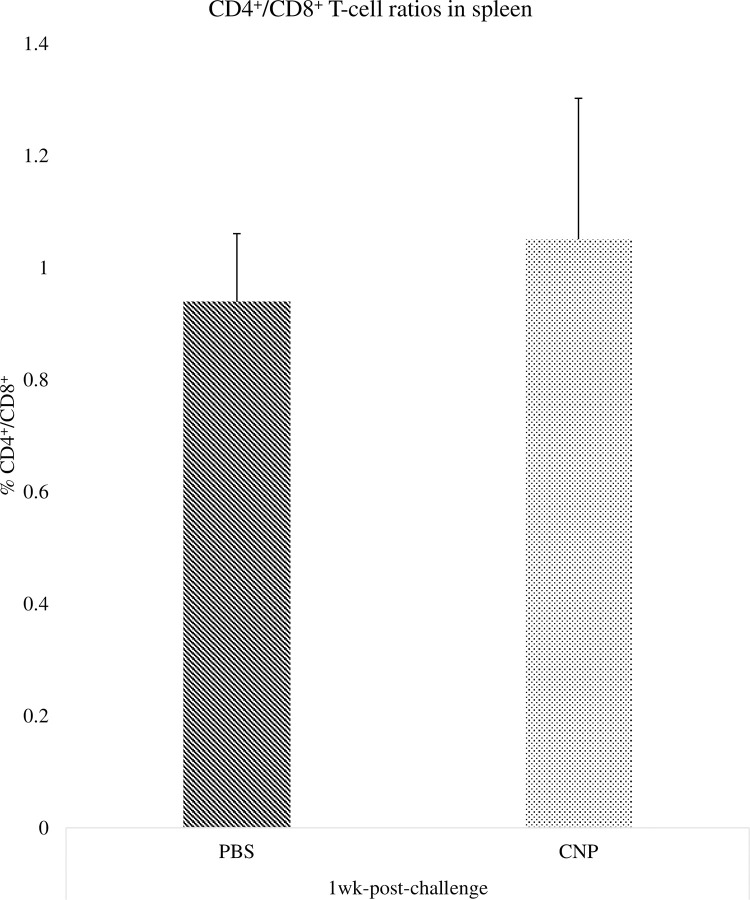
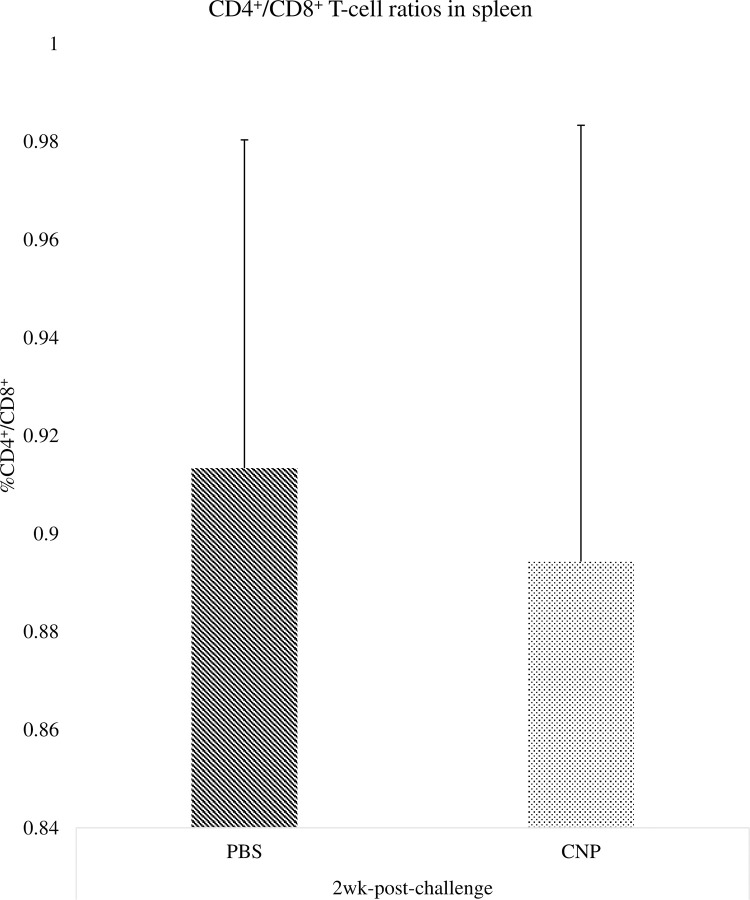
There were no significant differences (P>0.05) in the percentage of CD4+/CD8+ T-cells in cecal tonsils of birds at 1wk (Fig 2) and 2wk (Fig 3) post-challenge, compared to control.
Fig 2. CD4+/CD8+ cell percentages in cecal tonsils of chickens vaccinated with in-ovo CNP at 1wk-post-challenge.
At embryonic d18, broiler eggs were vaccinated with either PBS (control) or 1000 μg CNP vaccine. At d7 of age, birds were challenged with live SE (1.0 × 109 CFU/bird). The CD4+/CD8+ T-cell percentage in cecal tonsils were analyzed by Flow Cytometry (Guava Eascyte; Millipore). The CD4+/CD8+ cell percentage was analyzed after gating cells based on forward-scatter and side-scatter plot for lymphocytes. Data analyzed by parametric Student t-test; Means+SEM.Bars. n = 6. "*" means P<0.05.
Fig 3. CD4+/CD8+ cell ratios in cecal tonsils of chickens vaccinated with in-ovo CNP at 2wk-post-challenge.
At embryonic d18, broiler eggs were vaccinated with either PBS (control) or 1000 μg CNP vaccine. At d7 of age, birds were challenged with live SE (1.0 × 109 CFU/bird). The CD4+/CD8+ T-cell percentage in cecal tonsils were analyzed by flow cytometry (Guava Eascyte; Millipore). The CD4+/CD8+ cell percentage was analyzed after gating cells based on forward-scatter and side-scatter plot for lymphocytes. Data analyzed by parametric Student t-test; Means+SEM.Bars. n = 6. "*" means P<0.05.
Salmonella loads in the ceca of chickens vaccinated in-ovo with CNP vaccine
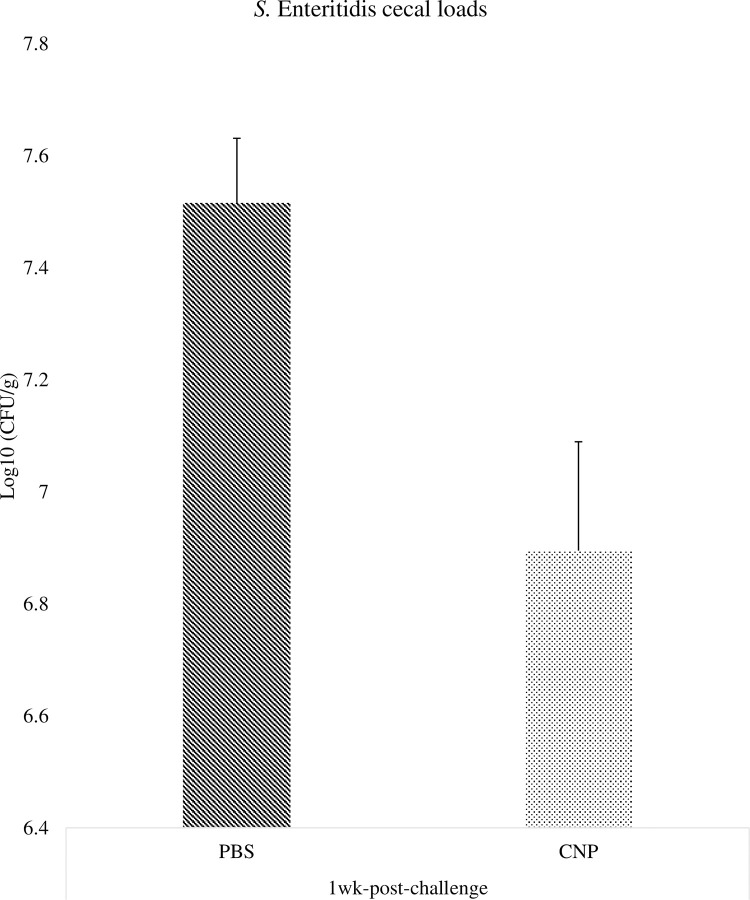
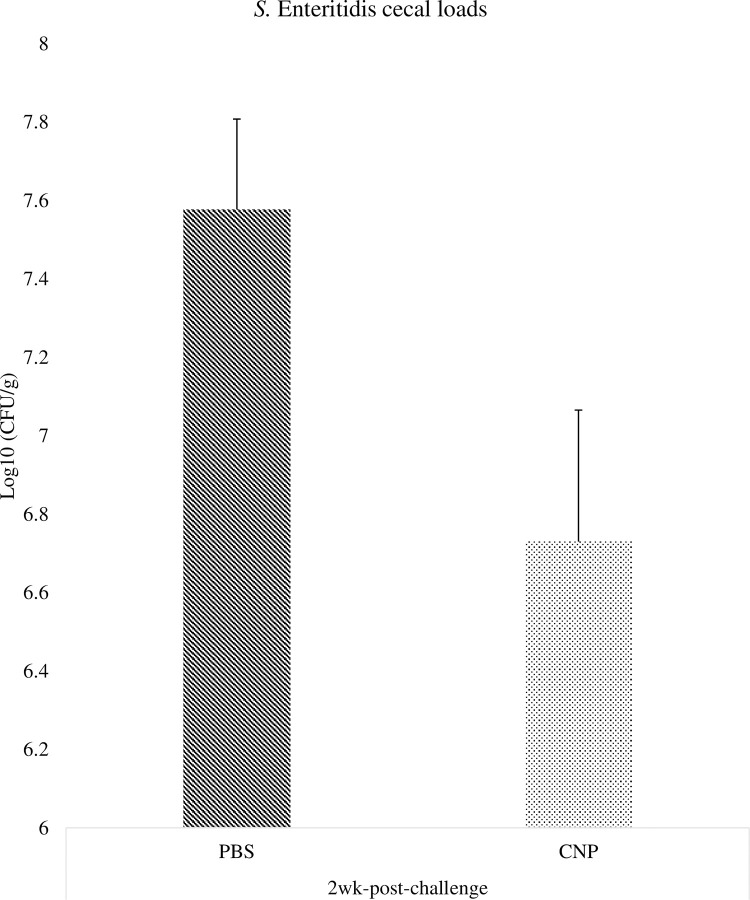
At 1wk post-challenge, birds immunized with the in-ovo CNP vaccine had a 0.62 Log 10 CFU/g reduction (P<0.05) of SE population in the ceca, compared to control (Fig 4). At 2wk post-challenge, birds immunized with the in-ovo CNP vaccine had a 0.85 Log 10 CFU/g reduction (P<0.05) of SE population in the ceca, compared to control (Fig 5).
Fig 4. Salmonella loads in the ceca of chickens vaccinated in-ovo with CNP at 1wk-post-challenge.
At embryonic d18, broilers egg were vaccinated with either PBS (control) or 1000 μg CNP vaccine. At d7 of age, birds were challenged with live SE (1.0 × 109 CFU/bird). Ceca samples were collected from 3 birds/pen, pulled, and stomached for enumeration by plating on XLT-4 agar. Salmonella enumeration data were recorded as CFU/g of ceca and then transformed to Log 10 CFU/g of ceca for statistical analysis. Data analyzed by parametric Student t-test; Means+SEM.Bars. n = 6. "*" means P<0.05.
Fig 5. Salmonella loads in the ceca of chickens vaccinated in-ovo with CNP at 2wk-post-challenge.
At embryonic d18, broiler eggs were vaccinated with either PBS (control) or 1000 μg CNP vaccine. At d7 of age, birds were challenged with live SE (1.0 × 109 CFU/bird). Ceca samples were collected from 3 birds/pen, pulled, and stomached for enumeration by plating on XLT-4 agar. Salmonella enumeration data were recorded as CFU/g of ceca and then transformed to Log 10 CFU/g of ceca for statistical analysis. Data analyzed by parametric Student t-test; Means+SEM.Bars. n = 6. "*" means P<0.05.
Cytokine gene expression in the cecal tonsils of chickens vaccinated in-ovo with CNP vaccine
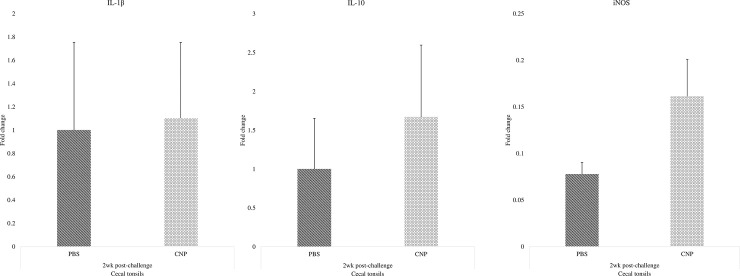
There were no significant differences (P>0.05) in the IL-1β (Fig 6A), IL-10 (Fig 6B), or iNOS (Fig 6C) mRNA amounts at 2wk post-challenge when compared to control.
Fig 6. Cytokine gene expression in the cecal tonsils of chickens vaccinated in-ovo with CNP.
At embryonic d18, broiler eggs were vaccinated with either PBS (control) or 1000 μg CNP vaccine. At d7 of age, birds were challenged with live SE (1.0 × 109 CFU/bird). Cecal tonsil samples were collected at 2wk post-challenge and analyzed for cytokine mRNA amounts by RT-PCR. Data represented fold change compared to control. A–IL-1β mRNA; B–IL-10 mRNA; C–iNOS mRNA. Data analyzed by parametric Student t-test; Means+SEM.Bars. n = 6. "*" means P<0.05.
Discussion
The physicochemical properties of the CNPs were determined in a previous study [12]. The average size of the nanoparticles was 168 nm, the entrapment efficiency of the nanoparticles was 70%, and the surface labeling efficiency of flagella on chitosan nanoparticles was 25%. The chitosan nanoparticles were found to be stable in both acidic and alkaline pH conditions with less than 10% and 0% protein release. The biocompatibility of chitosan nanoparticles using a hemolysis assay resulted in 0% hemolysis, demonstrating that the nanoparticles were biocompatible in chickens. Moreover, a previous study with empty CNPs alone showed they did not improve antibody response in the absence of a Salmonella antigen [12].
The findings from this study suggest that the novel CNP oral vaccine administered in-ovo for Salmonella control in broilers is a promising candidate vaccine against Salmonella. The mass administration of the Salmonella CNP vaccine ultimately induced an anti-Salmonella antigen-specific systemic and mucosal immune response and decreased SE cecal colonization in broilers.
A successful in-ovo vaccination should not decrease hatchability [24]. The limitations of the present study was the in-ovo vaccine was injected manually inside the embryo. Even with a manual in-ovo injection, a 97% hatchability was observed. The efficacy of both manual and automatic injections has been demonstrated [24]. Nevertheless, commercial application mandates automation, which can vaccinate a larger number of eggs efficiently, and reduce labor costs and the chances of human error. In-ovo vaccination is currently the standard procedure for Marek ’s disease and infectious bursal disease vaccines applied in hatcheries in the USA. Future studies should incorporate automation for the in-ovo vaccination of the Salmonella CNP vaccine as the vaccine has shown to have protective effects against SE and Heidelberg serovars [13].
There was no significant effect of the Salmonella CNP vaccine on the bird’s production performance. The results demonstrate that the embryonic vaccination had no negative effects on the production performance of broiler birds. The absence of difference in the performance can be seen as a positive result as the vaccine is protective against SE and does not significantly compromise the bird’s health. Previous studies have shown that chitosan nanoparticles are safe in chicken [12, 25, 26]. Other studies have shown that gavaging chickens with chitosan nanoparticles had no effect on mortality [25, 27]. Similarly, no mortality was observed during the 21-day experimental period. Our results demonstrated that the in-ovo administration of the Salmonella CNP candidate vaccine was safe with no adverse effects on bird performance.
The OMPs-specific IgY and IgA antibody responses were analyzed in serum, cloacal swabs, and bile samples. The in-ovo administration of the CNP vaccine induced antigen-specific antibody protection against SE at day-of-hatch and can potentially protect against SE infection as early as 8h post-challenge. Significant higher IgY antibody levels were also observed at 1wk post-challenge while at 2wk post-challenge the control animals displayed a significantly higher concentration of antibodies against SE in the serum. The increasing antibody levels can be attributed to a persistent infection in a carrier animal [28]. This may be because the non-vaccinated group had 0.62 Log 10 CFU/g more Salmonella load at 1wk post-challenge and hence can be expected to be in the process of eliminating the pathogen while the CNP vaccinated group had already cleared the infection by 0.85 Log 10 CFU/g at 2wk post-challenge, which is consistent with the CD4+/CD8+ cells percentage results obtained in this study.
Secretory IgA is the most abundant antibody class in intestinal secretions [29], and it serves as the first line of defense against enteric pathogens, such as Salmonella. Significantly higher bile anti-SE OMPs IgA levels were observed at 1wk post-challenge in birds vaccinated with CNP compared to that in the control group. The vaccine also increased antigen-specific IgA at 2wk post-challenge in bile and cloacal swabs. Our findings demonstrate that the in-ovo administration of the candidate vaccine can elicit a Salmonella-specific mucosal IgA response. Results are in agreement with previous findings, as the vaccine under study has shown to induce an antigen-specific mucosal IgA response in layers and broilers challenged with Salmonella [12, 13].
Our ELISA results demonstrated that the in-ovo administration of the Salmonella CNP vaccine can induce both antigen-specific mucosal and systemic immune responses against SE.
Interestingly, our findings indicate that the in-ovo CNP vaccinated group had a numerical increase in the frequency of CD4+ T-cells at 1wk post-challenge when compared to control. The immune response of Salmonella-specific CD4+ T-cells suggests a way for the host to quickly produce interferon-γ to activate macrophages and protect against Salmonella [29]. Nevertheless, is important to recall that despite the rapid development of a large number of CD4+ T-cells during primary Salmonella infection, there is actually very little evidence to suggest that, at this early stage of infection, they contribute to bacterial clearance [30]. Mostly because at an early phase of Salmonella infection, Salmonella can survive and replicate within macrophages [30]. At 2wk post-challenge, the PBS (control) group had higher CD4+ T-cells frequency, indicating that the non-vaccinated group could still be trying to eliminate the infection at 2 wk post-challenge, as supported by our serology findings, whilst the CNP vaccinated birds have cleared the SE infection.
Birds can remain asymptomatic with up to Log 5 of Salmonella CFU [1]. This resistance is thought due to the activation of T-regulatory cells and their suppressive immune response by IL-10 [31]. For this experiment, both treatment groups were kept under the same experimental conditions; thus, all birds were challenged with SE. The SE load in ceca of birds immunized with the in-ovo CNP vaccine was reduced compared to the control group. Reduction in SE intestinal loads is critical to prevent the transfer of Salmonella from poultry to humans. A successful Salmonella vaccine in the poultry industry should ideally reduce the total intestinal bacterial loads and shed in feces; however, this has yet not been feasible. As an alternative, an efficient vaccine that can reduce Salmonella loads is combined with other on-farm control strategies, to ultimately decrease foodborne illness risks for humans [32]. Findings demonstrate that the CNP vaccine is a promising candidate to mitigate Salmonella in broilers, as its in-ovo administration has shown to decrease SE cecal colonization.
In-ovo immunization with CNP Salmonella vaccine had no negative effects on anti-inflammatory IL-10 or pro-inflammatory IL-1β mRNA expression in cecal tonsils. However, there was an increase in both IL-10 and IL-1β mRNA expression in caecal tonsils of CNP vaccinated broilers, compared to control group. This is consistent with what has been found in previous research with chitosan nanoparticle vaccines [12, 13]. The oral gavage of the CNP Salmonella vaccine has shown to induce balanced levels of IL-1β and IL-10 cytokines in layers and broilers challenged with SE. The higher IL-1β levels are attributed to the intrinsic adjuvant composition of the vaccine that can induce a predominantly Th1-type inflammatory response [33]. The anti-inflammatory IL-10 cytokine may be increased to reduce the host tissue damage in response to the inflammation caused by Salmonella infections [34].
In-ovo immunization with CNP Salmonella vaccine had no negative effects on iNOS mRNA expression in cecal tonsils. However, there was a numerical increase in iNOS mRNA expression in caecal tonsils of CNP vaccinated broilers, compared to the control group. Results are in agreement with previous findings, as the oral gavage of the CNP Salmonella vaccine has been shown to induce higher levels of iNOS in broilers challenged with SE [13]. The iNOS is a key enzyme in the macrophage inflammatory response, as it is the source of nitric oxide (NO) that is potently induced in response to pro-inflammatory stimuli and will function to kill pathogens like Salmonella [35]. However, NO production can decrease the production performance of broilers [36], which was not the case in this study, and remains consistent with our previous findings [13]. Overall, the in-ovo administration of the CNP vaccine had no negative effects on the birds’ immune status.
Conclusion
The oral gavage of the CNP Salmonella vaccine has previously been shown to induce significantly higher antigen-specific IgA response in bile, serum, cloacal swab, and tracheal wash samples of layers challenged with Salmonella [12]. The oral gavage of 1000μg CNP Salmonella vaccine has previously been shown to induce significantly higher antigen-specific IgY and IgA antibodies in serum, cloacal swab, and bile samples of broilers challenged with Salmonella [13]. The results of the present study showed that the CNP can also be mass administered by in-ovo, making it a potential vaccine candidate to mitigate Salmonella in poultry. Findings demonstrate that the in-ovo vaccination of the CNP Salmonella vaccine can induce antigen-specific systemic and mucosal immune response without causing significant hatchability losses or altering the performance of broilers. The vaccine under study demonstrated promising results on significantly reducing SE cecal colonization in birds. Also, the in-ovo CNP vaccine had no negative effects on the bird’s production performance, CD4+/CD8+ T-cells frequency, pro- and anti-inflammatory cytokines levels, or iNOS levels of birds. Future research will further study the vaccine’s potential for mass vaccination methods.
Acknowledgments
The authors would like to thank Revathi Shanmugasundaram, Mohamad Mortada, Gabriel Akerele, Nour Ramadan, Ragini Reddyvari, Theros Ng, and Jarred Oxford, for assisting with sample collections. We would also like to thank the UGA PRC staff, for their assistance during our project.
Abbreviations
- CNP
Chitosan nanoparticle
- SE
Salmonella Enteritidis
- OMPs
Outer membrane proteins
- BWG
Body weight gain
- FCR
Feed conversion ratio
Data Availability
All relevant data are within the manuscript.
Funding Statement
This research was partially supported by Hatch grant and USDA-NIFA grant 2017-05035 and 58-6040-8-034, awarded to RKS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of authors in this manuscript is mentioned in authors contribution section.
References
- 1.Stern NJ, Cox NA, Bailey JS, Berrang ME, Musgrove MT. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult Sci. 2001. 10.1093/ps/80.2.156 [DOI] [PubMed] [Google Scholar]
- 2.Andino A, Hanning I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Vol. 2015, Scientific World Journal. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Salmonella [Internet]. 2020. Available from: https://www.cdc.gov/salmonella/index.html. [Google Scholar]
- 4.Guo R, Jiao Y, Li Z, Zhu S, Fei X, Geng S, et al. Safety, protective immunity, and DIVA capability of a rough mutant Salmonella Pullorum vaccine candidate in broilers. Front Microbiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revolledo L, Ferreira AJP. Current perspectives in avian salmonellosis: Vaccines and immune mechanisms of protection. J Appl Poult Res. 2012;21(2):418–31. [Google Scholar]
- 6.Kang SH, Hong SJ, Lee YK, Cho S. Oral vaccine delivery for intestinal immunity-biological basis, barriers, delivery system, and M cell targeting. Polymers. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. 2012;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014. 10.1007/s11095-014-1419-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, et al. Nanoparticle vaccines. Vol. 32, Vaccine. 2014. p. 327–37. 10.1016/j.vaccine.2013.11.069 [DOI] [PubMed] [Google Scholar]
- 10.Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017. 10.3390/pharmaceutics9040053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung RCF, Ng TB, Wong JH, Chan WY. Chitosan: An update on potential biomedical and pharmaceutical applications. Vol. 13, Marine Drugs. 2015. p. 5156–86. 10.3390/md13085156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renu S, Markazi AD, Dhakal S, Lakshmanappa YS, Shanmugasundaram R, Selvaraj RK, et al. Oral deliverable mucoadhesive Chitosan-Salmonella subunit nanovaccine for layer chickens. Int J Nanomedicine. 2020. 10.2147/IJN.S238445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acevedo-Villanueva KY, Lester B, Renu S, Han Y, Shanmugasundaram R, Gourapura R, et al. Efficacy of chitosan-based nanoparticle vaccine administered to broiler birds challenged with Salmonella. PLoS One. 2020. 10.1371/journal.pone.0231998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renu S, Markazi AD, Dhakal S, Lakshmanappa YS, Gourapura SR, Shanmugasundaram R, et al. Surface engineered polyanhydride-based oral Salmonella subunit nanovaccine for poultry. Int J Nanomedicine. 2018. 10.2147/IJN.S185588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes JIM, Prokoski K, Oliveira BC, Oro CS, Oro PJ, Fernandes NLM. Evaluation of incubation yield, vaccine response, and performance of broilers submitted to in-Ovo vaccination at different embryonic ages. Rev Bras Cienc Avic. 2016. [Google Scholar]
- 16.Godlewska R, Kuczkowski M, Wyszyńska A, Klim J, Derlatka K, Woźniak-Biel A, et al. Evaluation of a protective effect of in ovo delivered Campylobacter jejuni OMVs. Appl Microbiol Biotechnol. 2016. 10.1007/s00253-016-7699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez Carlos, Arbe Miren, Cruz Paola, Lozano F, Fabio de Souza, Joreci Federizzi EJ, et al. IN OVO VACCINATION Introducing a new approach to in ovo vaccination for modern hatcheries [Internet]. 2016. Available from: http://www.positiveaction.info/digital/Supplements/In_Ovo_Vaccination/pdf/In-Ovo_Supplement.pdf. [Google Scholar]
- 18.Sharma JM. Embryo Vaccination with Infectious Bursal Disease Virus Alone or in Combination with Marek’s Disease Vaccine. Avian Dis. 1985. [PubMed] [Google Scholar]
- 19.Woodward MJ, Kirwan SES. Detection of Salmonella enteritidis in eggs by the polymerase chain reaction. Vet Rec. 1996. 10.1136/vr.138.17.411 [DOI] [PubMed] [Google Scholar]
- 20.Shanmugasundaram R, Mortada M, Cosby DE, Singh M, Applegate TJ, Syed B, et al. Synbiotic supplementation to decrease Salmonella colonization in the intestine and carcass contamination in broiler birds. PLoS One. 2019. 10.1371/journal.pone.0223577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, et al. Cloning and Characterization of Chicken IL-10 and Its Role in the Immune Response to Eimeria maxima. J Immunol. 2004. 10.4049/jimmunol.173.4.2675 [DOI] [PubMed] [Google Scholar]
- 22.Morris A, Shanmugasundaram R, Lilburn MS, Selvaraj RK. 25-Hydroxycholecalciferol supplementation improves growth performance and decreases inflammation during an experimental lipopolysaccharide injection. Poult Sci. 2014. 10.3382/ps.2014-03939 [DOI] [PubMed] [Google Scholar]
- 23.Markazi A, Luoma A, Shanmugasundaram R, Mohnl M, Murugesan GR, Selvaraj R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult Sci. 2018. 10.3382/ps/pey234 [DOI] [PubMed] [Google Scholar]
- 24.Peebles ED. In ovo applications in poultry: A review. Poultry Science. 2018. 10.3382/ps/pey081 [DOI] [PubMed] [Google Scholar]
- 25.Zhao K, Chen G, Shi X ming, Gao T ting, Li W, Zhao Y, et al. Preparation and Efficacy of a Live Newcastle Disease Virus Vaccine Encapsulated in Chitosan Nanoparticles. PLoS One. 2012;7(12):1–11. 10.1371/journal.pone.0053314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akerele G, Ramadan N, Renu S, Renukaradhya GJ, Shanmugasundaram R, Selvaraj RK. In vitro characterization and immunogenicity of chitosan nanoparticles loaded with native and inactivated extracellular proteins from a field strain of Clostridium perfringens associated with necrotic enteritis. Vet Immunol Immunopathol [Internet]. 2020;224(December 2019):110059. Available from: 10.1016/j.vetimm.2020.110059 [DOI] [PubMed] [Google Scholar]
- 27.Renu Sankar, Markazi Ashley D., Dhakal Santosh, Yashavanth Shaan Lakshmanappa, Revathi Shanmugasundaramand GJR. Engineering of Targeted Mucoadhesive Chitosan Based Salmonella Nanovaccine for Oral Delivery in Poultry. J Immunol. 2018;200(1). [Google Scholar]
- 28.Hansen KR, Nielsen LR, Lind P. Use of IgG avidity ELISA to differentiate acute from persistent infection with Salmonella Dublin in cattle. J Appl Microbiol. 2006; 10.1111/j.1365-2672.2005.02758.x [DOI] [PubMed] [Google Scholar]
- 29.Abbas Abul K., Lichtman AH, Pillai S. Cellular and Molecular Immunology. Vol. 8a ed., Elsevier. 2015. 544 p. [Google Scholar]
- 30.Griffin AJ, McSorley SJ. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunology. 2011. 10.1038/mi.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanmugasundaram R, Kogut MH, Arsenault RJ, Swaggerty CL, Cole K, Reddish JM, et al. Effect of Salmonella infection on cecal tonsil regulatory T cell properties in chickens. Poult Sci. 2015;94(8):1828–35. 10.3382/ps/pev161 [DOI] [PubMed] [Google Scholar]
- 32.Cogan TA, Humphrey TJ. The rise and fall of Salmonella Enteritidis in the UK. In: Journal of Applied Microbiology Symposium Supplement. 2003. [DOI] [PubMed]
- 33.Renu Sankar, Gourapura Renukaradhya, Selvaraj Ramesh. Nanoparticle Patent No. 10336-342WO1. 2017. [Google Scholar]
- 34.Peñaloza HF, Noguera LP, Riedel CA, Bueno SM. Expanding the current knowledge about the role of interleukin-10 to major concerning bacteria. Front Microbiol. 2018;9(SEP):1–8. 10.3389/fmicb.2018.02047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeill E, Crabtree MJ, Sahgal N, Patel J, Chuaiphichai S, Iqbal AJ, et al. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic Biol Med. 2015; 10.1016/j.freeradbiomed.2014.10.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007. 10.1007/s10787-007-0013-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.