Abstract
People with HIV (PWH) taking antiretroviral therapy (ART) have persistent cognitive impairment. The prevalence of cognitive impairment is higher in women with HIV (WWH) compared to men with HIV (MWH), possibly due to sex differences in immune function. Here we report sex differences in cerebrospinal fluid (CSF) immune markers in relation to cognitive performance. A subset of 83 PWH on ART (52% WWH; mean age=37.6 years, SD=7.9) from the Rakai community cohort study Cohort and Rakai Health Sciences Program supported clinics in rural Uganda completed a neuropsychological (NP) assessment and a lumbar puncture. CSF was used to measure 16 cytokines/chemokines. Individual NP test z-scores were generated based on local normative data. A series of least absolute shrinkage and selection operator (lasso) regressions examined associations between CSF inflammatory markers and NP outcomes. Overall, there were no sex differences in CSF inflammatory marker levels. However, MWH displayed more associations between inflammatory markers and cognitive performance than WWH. Among MWH, inflammatory markers were associated with a number of cognitive domains, including attention, processing speed, fluency, executive function, learning and memory. MIP-1β, INF-γ, GM-CSF, IL-7 and IL-12p70 were associated with multiple domains. Among WWH, few inflammatory markers were associated cognition. Degree of associations between CSF inflammatory biomarkers and cognitive performance varied by sex in this young, ART-treated, Ugandan cohort. Further investigation into sex-specific inflammatory mechanisms of cognitive impairment among PWH is warranted to inform sex-specific management strategies.
Keywords: HIV, global health, cognition, sex, inflammation
1. Introduction
Globally, central nervous system (CNS) complications in HIV infection persist despite effective antiretroviral therapy (ART) and impact HIV care including ART adherence particularly in resource-limited settings. One of the most common CNS complications in the current ART era is cognitive impairment. In a recent comprehensive literature review, the median prevalence of cognitive impairment in people with HIV (PWH) was 41% (Rubin and Maki, 2019). Non-Western and Western cohorts often report similar rates of cognitive impairment (Saloner and Cysique, 2017). However, in low- and middle-income countries such as Uganda, higher rates of cognitive impairment are reported despite the negligible presence of HIV-associated non-AIDS comorbidities (e.g., cerebrovascular), illicit substance use, and non-ART drug use (Nakasujja et al., 2010).
One relatively under-studied determinant of cognitive impairment in PWH is biological sex which is particularly relevant as women with HIV (WWH) constitute more than half of all individuals with HIV globally and a staggering 59% of new HIV infections in sub-Saharan Africa are amongst women (amfAR, 2020). Similar to Western countries (Rubin et al., 2019a), WWH in sub-Saharan African countries report a higher prevalence of cognitive impairment compared to men with HIV (MWH) with the largest differences in the domains of learning and memory (Royal et al., 2016). Compared to MWH, WWH may be at greater risk for cognitive impairment because of social correlates of health, including poverty, low literacy levels and educational attainment, barriers to health care services, and environmental exposures leading to substance abuse/substance use disorders, poor mental health, and cognitive impairment (Maki and Martin-Thormeyer, 2009; Maki et al., 2015; Rubin et al., 2019a). Evidence also suggests that, when faced with challenges such as depression, WWH are more cognitively affected than MWH (Rubin et al., 2019c). In low- and middle-income countries, there appears to be a disproportionate burden of these cognitive heath risk factors in WWH (Kaharuza et al., 2006; Milanini et al., 2020; Saloner and Cysique, 2017; Vecchio et al., 2020).
Biological factors, such as sex hormones and hormonal milieus, and sex differences in immune factors may contribute to differences in cognition in PWH, but this is an understudied area of research. Sex differences in in immune function are well documented, and deserve increased attention in regions with a high prevalence of WWH such as Uganda. For example, women generally have a stronger innate and adaptive immune response compared to men (Klein and Flanagan, 2016; Markle and Fish, 2014). This robust and fast inflammatory response can result in efficient clearance of pathogens and establishment of vaccine immunity, but it can lead to pathological consequences of increased susceptibility to autoimmune disorders and severity of infectious disease manifestations (Klein and Flanagan, 2016). This was evident in the early HIV epidemic when men and women had similar disease progression despite women having a lower HIV viral load than men (Farzadegan et al., 1998; Gandhi et al., 2002; Sterling et al., 2001). Sex differences in the immune response to HIV infection (Gandhi et al., 2002; Griesbeck et al., 2015; Meier et al., 2009; Scully, 2018) may be significant contributors to these variations in pathogenesis. Since neuroinflammation and HIV disease progression are also implicated in cognitive impairment, immune sex differences may contribute to variations in cognitive manifestations. Delineating the specific patterns of associations between cerebrospinal fluid (CSF) markers of inflammation and domain-specific cognitive function in WWH and MWH separately may help to identify sex-specific therapeutic targets and disease pathways. In a unique rural Ugandan cohort of PWH, where half of the cohort is comprised of women, we aimed to examine the role of neuroinflammation in cognitive function. We hypothesized that there would be a different pattern of associations between neuroinflammatory markers and cognitive function in WWH and MWH in the context of antiretroviral therapy (ART) controlled HIV infection.
2. Methods
2.1. Participants
Four hundred ART-naïve PWH were enrolled into the Rakai Neurology Cohort Study (RNCS). Following enrollment, all participants were offered free ART initiation per Uganda national guidelines. For this analysis, we included 83 participants who were on ART and who consented to an optional lumbar puncture at the two-year study follow-up. This selection was due to our interest in examining the associations between inflammation and cognition in the context of ART. The full description of the RNCS can be found in previous publications (Saylor et al., 2019). In brief, the RNCS enrolled adult, ART-naïve PWH from communities in rural Rakai District, Uganda who were administered a sociodemographic and behavioral interview, depression screener (Center for Epidemiologic Studies Depression Scale [CES-D](Radloff, 1977)), functional assessments and laboratory measurements (i.e. HIV status, CD4 cell count, and plasma viral load) (Saylor et al., 2017; Saylor, 2017).
2.2. Standard protocol approvals, registrations, and patient consent
The study was approved by Western Institutional Review Board, the Uganda Virus Research Institute Research and Ethics Committee, and the Uganda National Council for Science and Technology. Participants provided written consent to participation with a separate written consent for LP.
2.3. CSF inflammatory markers
LPs were conducted by Medical Officers at the end of each study visit. CSF was aliquoted into centrifuge tubes (1ml in each tube) and stored at −80 degrees C. The CSF was used to measure 16 cytokines/chemokines via multiplex profiling with Luminex Platform (Human 17-Plex Panel, Bio-Rad, Hercules, CA) including: macrophage inflammatory protein-1 beta (MIP-1β), interleukin (IL)-6, interferon gamma (INF-γ), IL-5, granulocyte macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF)-α, IL-1β, IL-12, IL-4, monocyte chemoattractant protein-1 (MCP-1/CCL2), IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF), IL-7, IL-12p70, and IL-17. All assays were performed in accordance with the manufactures protocol. The assays have internal standard curves (n=8 points) which were run in duplicate. The percent coefficient of variation (CV) was less than 10% and standard curves had <10% CV as a quality control check. Percent undetectable for each CSF markers assessed was as follows: MIP-1β, GM-CSF, TNF-α, MCP-1, IL-10, and IL-7 (1%); IL-17 (4%); IL-6 (7%); IL-4 (16%); IL-12 (17%); IL-5 (19%); G-CSF (23%); IL-1β (24%); and IL-12p70 (69%). All samples assessed for INF-γ and IL-8 were detectable. Undetectable values were set to half the lower limit of detection. As the CSF markers were not normally distributed, we uniformly applied a log transformation to all markers. The log transformation adequately normalized all CSF marker distributions.
2.3. Neuropsychological (NP) Assessment
Participants completed a neuropsychological (NP) battery, described in earlier publications (Rubin et al., 2019b) that included the following domains with the associated tests in parentheses: (1) gait (Timed Gait) (2) gross motor (Finger Tapping [FTAP]), (3) fine motor (Grooved Pegboard [GPEG]), (4) processing speed (Color Trails-Trial 1, Symbol Digit Modalities Test [SDMT]), (5) semantic fluency (Animals), (6) attention (digit span [DSPAN] forward), (7) executive function (Color Trails-Trial 2), (8) working memory (DSPAN Backward), and (9 and 10) verbal learning and memory (WHO-UCLA Auditory Verbal Learning Test [AVLT]). The tests were scored with local normative data generated from 400 HIV-uninfected age- and sex-matched control participants to produce individual test Z-scores (Vecchio et al., 2020). In brief, each outcome was first regressed on age, education, and sex in the HIV-uninfected individuals. The unstandardized beta weights of each predictor, constant, and the standard error were used to calculate the predicted scores for each test. Lastly, to create Z-scores, with a mean of 0 and standard deviation of 1 in the controls, we estimated the difference between the predicted scores and observed (residual) scores (Z-score=[(observed score – predicted score)/standard error of the estimate of the regression]. These Z-scores served as outcome measures.
2.4. Statistical Analyses
Prior to analysis, all log transformed CSF markers and NP outcomes were standardized so that all variables were weighted equally in the statistical models. Spearman correlations were used to examine unadjusted associations between CSF markers and NP outcomes. A series of least absolute shrinkage and selection operator (lasso) regressions (Tibshirani, 1996) were used to examine adjusted associations between CSF inflammatory markers and NP outcomes in MWH and WWH separately. In brief, lasso regression is a type of linear regression often used in machine learning that uses regularization methods to identify optimal values of linear coefficients such that the important predictors receive non-zero values and irrelevant predictors are assigned coefficients of zero. Traditional linear regression is unable to build such clear boundary between important and unimportant predictors, yielding poorer interpretability compared to the lasso regression (Tibshirani, 1996). Each NP test outcome (e.g., Color Trails 1, SDMT) was used as the outcome in a separate lasso regression including all CSF biomarkers as potential predictors. A data reduction approach (i.e., principal components analysis-PCA), albeit possible, was not used on the 14 NP outcomes because the correlation structure (via Spearman) was not the same in WWH and MWH (Supplemental Figure 1) and would result in different NP outcomes contributing to different component scores. As a consequence, it would be difficult to discuss (even qualitatively) differences in the pattern of CSF biomarker and outcome associations by sex. For each lasso regression, the parameter controlling the number of non-zero coefficients was determined by cross-validation. Each lasso regression was then fitted 100 times, and CSF biomarkers significant on 90% of the regressions were considered significant in their association with that NP outcome. This procedure has been shown to yield satisfactory false discovery control (Romano et al., 2008). This model’s extensive fitting enhances the prediction accuracy and ability to interpret the relationship between individual inflammatory markers and NP performance by sex in the setting of multiple biomarkers. All models were adjusted for alcohol use and viral load as these were the only two variables that were associated with both CSF markers and NP outcomes and thus deemed confounders.
3. Results
3.1. Participants
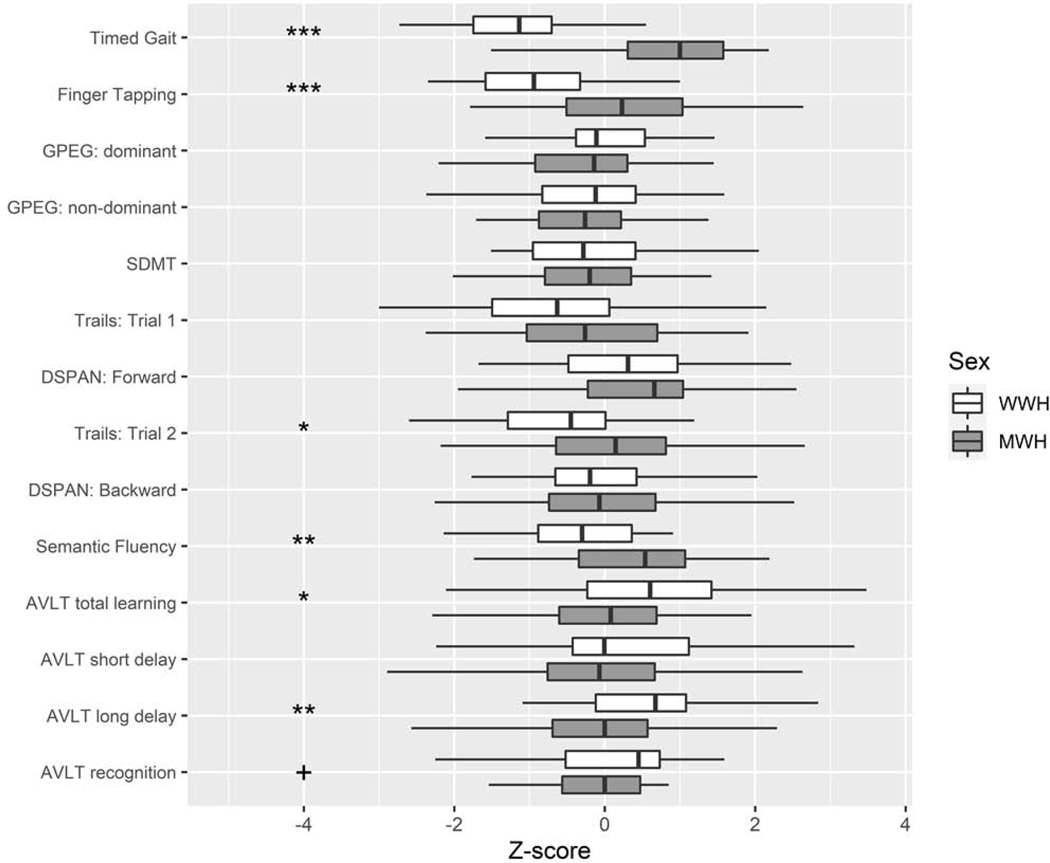
Eighty-three PWH on ART (52% MWH) had CSF and NP data available from the follow-up visit (Table 1). The mean age of this sample at follow-up was 37.6 years (SD=7.9). WWH and MWH were similar on most sociodemographic, clinical, and behavioral factors with the exceptions of higher alcohol and tobacco use among men in the last month compared to women, and women having a higher BMI than men (P’s<0.05). There were no differences in any of the CSF inflammatory marker levels by sex (Figure 1; Supplemental Table 1). The pattern of correlations between CSF markers were similar in WWH and MWH (Supplemental Table 2). Cognitively, MWH performed worse on timed gait, gross motor, executive function, and semantic fluency compared to WWH (P’s<0.05), whereas WWH performed worse on tests of learning and memory compared to MWH (P’s≤0.05) (Figure 2).
Table 1.
Sociodemographic, behavioral, and clinical characteristics in the overall sample and by sex.
| Overall (N=83) N (%) | Men (n=43) n (%) | Women (n=40) n (%) | P-value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 37.6 (7.9) | 39.0 (7.2) | 36.2 (8.4) | 0.10 |
| Education, mean (SD) | 5.4 (3.2) | 5.4 (3.0) | 5.3 (3.2) | 0.69 |
| BMI | 22.1 (3.0) | 21.4 (2.9) | 22.9 (3.0) | 0.02 |
| Tobacco use | 8 (10) | 8 (19) | 0 (0) | 0.004 |
| Alcohol use in the last month | 41 (49) | 30 (70) | 11 (27) | <0.001 |
| Narcotic use | 0 (0) | 0 (0) | 0 (0) | - |
| CES-D 16 cutoff | 3.8 (5.9) | 3.9 (7.2) | 3.7 (4.3) | 0.82 |
| Hypertension | 0 (0) | 0 (0) | 0 (0) | - |
| High cholesterol | 3 (4) | 2 (5) | 1 (2) | 0.60 |
| CD4 count, median (IQR) | 479 (271) | 487 (315) | 478 (201) | 0.45 |
| Undetectable HIV RNA (<40cp/ml) | 74 (89) | 38 (88) | 36 (90) | 0.81 |
| On ART | 83 (100) | 43 (100) | 40 (100) | - |
Note. ART=antiretroviral. BMI=body mass index; CES-D=Center for Epidemiologic Depression Scale; IQR=interquartile range. Sex differences were examined with independent T-tests for normally distributed continuous variables, Mann-Whitney U test for non-normally distributed variables (median, IQR are presented for these variables), and chi-square analysis for categorical variables.
Figure 1.
Log CSF inflammatory levels in the overall sample and by sex. MWH=men with HIV; WWH=women with HIV.
Figure 2.
Neuropsychological performance in the overall sample and by sex. MWH=men with HIV; WWH=women with HIV. ***P<0.001; **P<0.01; *P<0.05; + P=0.05
3.2. Associations between CSF markers and NP performance in the overall sample
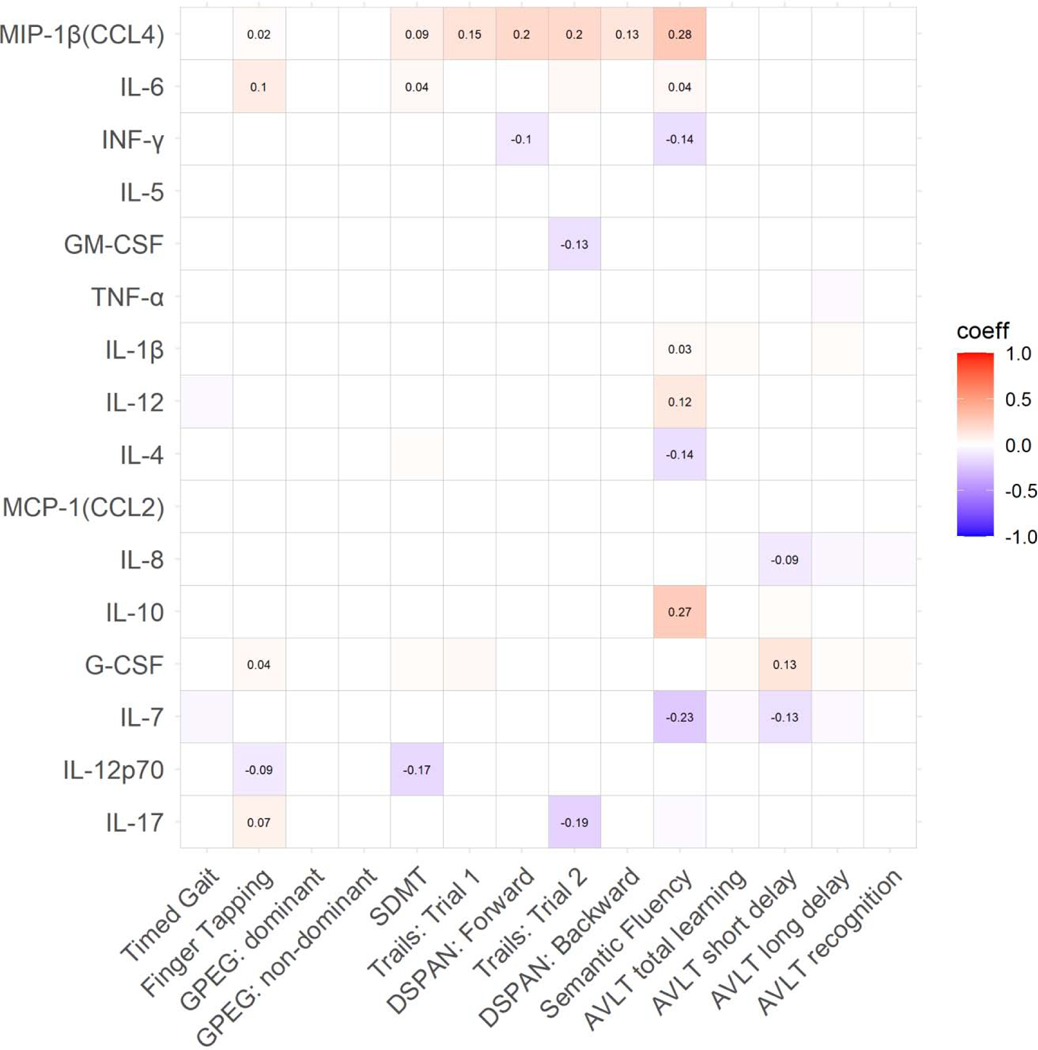
Correlations between the 16 CSF markers and cognitive outcomes are depicted in Supplemental Figure 3. In adjusted analyses, MIP-1β was associated with the largest number of outcomes (Figure 3; Supplemental Figure 5A). Lower MIP-1β levels related to poorer performance on half of the NP outcomes, including most cognitive domains, except fine motor and verbal learning and memory. Lower IL-6, IL-1β, IL-12, and IL-10, as well as higher INF-γ, IL-4, and IL-7 related to poorer semantic fluency. Lower G-CSF and higher IL-8 and IL-7 associated with poorer verbal memory (AVLT short delay), while lower IL-6 and G-CSF and higher IL-12p70 and IL-17 related to poorer gross motor function. Additionally, lower IL-6 and G-CSF related to poorer processing speed (SDMT). Finally, higher INF-γ related to poorer attention and higher GM-CSF and IL-17 to poorer executive function. None of the CSF markers related to fine motor and gait (Supplemental Table 4).
Figure 3.
Significant associations (boxes with standardized beta coefficients) between CSF inflammatory markers and cognitive performance in the overall sample. Coeff=standardized beta coefficient. GPEG=Grooved Pegboard; SDMT=Symbol Digit Modalities Test; DSPAN=Digit Span; AVLT=Auditory Verbal Learning Test.
3.3. Associations between CSF markers and NP performance by sex
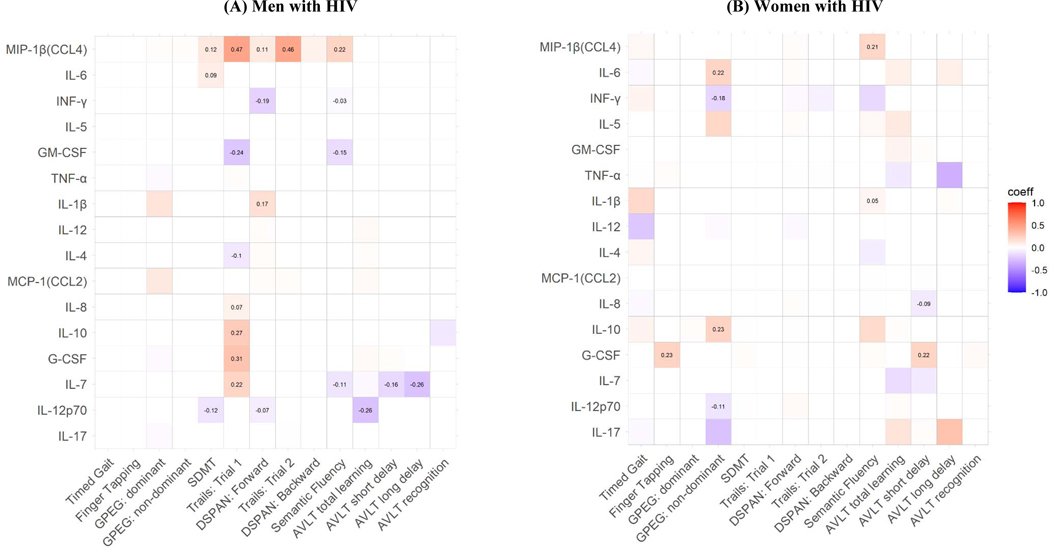
Correlations between the 16 CSF markers and cognitive outcomes by sex are shown in Supplemental Figure 4. While there were several CSF marker-NP outcome associations in the overall sample, a very different pattern of associations emerged when stratifying analyses by sex (Figure 4A; Supplemental Figure 5).
Figure 4.
Significant associations (boxes with standardized beta coefficients) between CSF inflammatory markers and cognitive performance by sex. Coeff=standardized beta coefficient. GPEG=Grooved Pegboard; SDMT=Symbol Digit Modalities Test; DSPAN=Digit Span; AVLT=Auditory Verbal Learning Test.
Consistent with the overall sample, MIP-1β was associated with the largest number of outcomes in MWH. In adjusted analyses, lower MIP-1β levels related to poorer performance most NP outcomes, including most cognitive domains, except motor (timed gain, gross and fine motor) and verbal learning and memory. In contrast to the overall sample, most CSF markers in MWH related to measures of processing speed rather than semantic fluency, including MIP-1β, IL-8, IL-10, G-CSF, IL-7, GM-CSF, IL-4, IL-6, and IL-12p70. Only MIP-1β, INF-γ, GM-CSF, and IL-7 related to semantic fluency. Lower IL-1β and higher INF-γ and IL-12p70 related to poorer attention. Finally, higher IL-7 and IL-12p70 related to poorer measures of learning and memory (all except AVLT recognition). None of the CSF markers were associated with timed gait, gross motor, fine motor, working memory or verbal recognition in MWH.
In contrast to MWH, there were few associations between CSF markers and NP outcomes among WWH (Figure 4B; Supplemental Figure 1C). IL-5, GM-CSF, TNF-α, IL-12, IL-4, MCP-1, IL-7, and IL-17 were unrelated to any NP outcome. Moreover, no one marker was associated with more than two NP outcomes. Lower IL-6 and IL-10, as well as higher INF-γ and IL-12p70 were associated with worse performance on GPEG non-dominant, lower MIP-1β and IL-1β related to poorer semantic fluency and lower G-CSF related to poorer performance on gross motor. Additionally, lower G-CSF and higher IL-8 were related to poorer verbal memory (AVLT short delay). None of the CSF markers were related to processing speed and executive function, attention, or working memory in WWH.
4. Discussion
In this rural Ugandan cohort of PWH, we assessed associations between CSF neuroinflammation and cognitive function in a relatively young cohort on ART with few comorbidities. First, our findings indicate that inflammation, measured in CSF was associated with cognitive performance among the overall sample. All, but three, CSF markers were related to domain-specific cognitive function. Semantic fluency followed by gross motor and memory were among the strongest correlates of neuroinflammation in the overall sample. Second, despite similar levels of CSF markers between men and women, the quantity and patterns of CSF marker-cognition associations markedly varied by sex in this virally suppressed cohort, not confounded by the high rates of cardiovascular or metabolic complications and polypharmacy seen in US studies (Marcotte et al., 2013; Rubin et al., 2017; Schrier et al., 2015; Sundermann et al., 2018). Taken together, our findings underscores the significance of immune activation in the CNS as a mechanism for heterogeneous cognitive performance while on effective ART and the importance of considering sex differences in the cognitive correlates of neuroinflammation.
In the context of ART-mediated viral suppression, there is strong evidence that cognitive dysfunction is linked to persistent inflammation. Among the studied CSF markers to date, the most common ones linked to cognitive function include MCP-1, TNF-α, IL-6, IP-10/CXCL10, IL-8/CXCL8, INF-α, IL-16, ICAM5, microbial translocation markers, and growth factors (Bandera et al., 2019). Although we did not assess this entire panel of markers, of the markers that overlapped, we also found IL-6, IL-8, INF-α, and G-CSF to relate to cognition, albeit domain-specific rather than global cognitive function and the directionality of associations was not always consistent. For example, we found lower CSF G-CSF levels to relate to poorer gross motor and verbal memory whereas others demonstrate higher levels among cognitively impaired PWH compared to cognitively intact PWH (Yuan et al., 2015). Importantly, our participants were all on ART whereas less than half of participants in Yuan et al. (2015) were on ART. Thus, some of the inconsistencies in the direction of the associations may be driven by disease state and ART use. There may be a dynamic relationship between CSF inflammatory markers and cognition which depending on the disease state can lead to different outcomes (benefit vs. detrimental). It is important to note that the direction of some of the CSF marker-cognition associations were in the expected direction. For example, INF-γ, a cytokine involved in an array of immune modulation pathways, was associated with attention and motor function in MWH and WWH, respectively. This marker may be less dependent on disease state as prior to ART initiation, higher CSF levels of IFN-γ were associated with increased odds of symptomatic cognitive impairment (Abassi et al., 2017).
In the overall sample, there was only one CSF marker relating to the majority of cognitive domains. Unexpectedly, the marker was not MCP-1 or TNF-α but rather MIP-1β, a chemoattractant for monocytes and other immune cells with specificity for CCR5 receptors and coactivators of macrophages (Dorner et al., 2002). Specifically, we found that lower MIP-1β was related to poorer performance on half of the NP outcomes, suggesting a role in cognition more globally. While CSF MIP-1β has been associated with cognition, these associations have primarily been seen among ART-naïve PWH and in samples comprised of mostly MWH not on ART (Airoldi et al., 2012; Letendre et al., 1999). Additionally, among these subgroups, the pattern was such that higher levels were associated with cognitive impairment. In the context of ART-mediated viral suppression, there is less evidence linking lower MIP-1β and cognition. In our own work in the Women’s Interagency HIV Study, we did not find MIP-1β (mean or variability) to predict cognitive impairment (Rubin et al., 2018). Interestingly, consistent with our findings, higher CSF MIP-1β was found to relate to higher cognitive function cross-sectionally but predict cognitive decline over time in Alzheimer’s disease (Taipa et al., 2019). Again, the directionality may in part be dependent on disease state and the dynamic nature of inflammatory processes which could differentially impact cognitive function.
In the absence of sex-differences in overall levels of CSF inflammatory markers, the patterns of specific test performance associated with inflammatory makers diverged by sex. WWH had fewer associations between cognition and inflammatory markers than MWH. Eleven of the sixteen markers were associated with NP outcomes in MWH whereas only eight were significant among WWH. Among MWH, MIP-1β was correlated with most NP outcomes followed by IL-7 which plays a role in peripheral T-cell homeostasis (Fry and Mackall, 2005) and IL-12 p70, a proinflammatory cytokine released by T cells. In contrast, among WWH, each marker only correlated with one or two outcomes. One possibility is that these results support the idea that WWH are less sensitive to the effects of inflammation on cognition than MWH, which are often deleterious despite our mixed findings in the present study. Additional work is needed to better understand this pattern as it is in opposition to the predominant thought that women have more robust immune systems compared to men.
Not only did the number of associations differ by sex, but the cognitive correlates of neuroinflammation differed by sex. For MWH, measures of speed had the highest associations with nine CSF markers whereas attention and learning were associated with fewer immune markers. However, among WWH, motor and memory impairments related to IL-6, IL-8, IL-10, INF-γ, IL-12p70, G-CSF, collectively. Motor impairment has been implicated in WWH in previous studies (Maki et al., 2018; Rubin et al., 2019a). The association between immune markers and processing speed in MWH and memory impairment in WWH are also surprising, since those are typically female-dominant cognitive abilities (de Frias et al., 2006). Thus, the unique immune responses in virally suppressed PWH need further investigation in the context of HIV and looking at the direction of cognitive performance in relation to inflammation over time.
The small sample size by sex and lack of HIV-uninfected control group were limitations of this study which prohibited additional analyses such as interactions between CSF markers and sex on NP outcomes. The small sample size also limited our ability to consider using multivariate multiple regression to model all NP test outcomes together instead of running separate regression models for each NP outcome which was the approach we selected for our proposed analyses. While a multivariate multiple regression model is possible (would require estimation of a 14 × 14 covariance matrix for the error term in the regression model), applying this model to a small sample dataset would result in estimates with a large degree of uncertainty. These statistical limitations due to our sample size lends the present findings to be considered preliminary and in part exploratory as we did not specify in our proposed hypothesis which sex would have stronger CSF marker-cognition associations, the expected direction of these associations, or the specific CSF markers or cognitive domains involved. Additionally, the individuals consenting to an LP were comprised of more women versus men who reported alcohol use and smoking; however, this pattern generalizes to the larger Rakai cohort of 312 PWH. We also did not have information on HIV disease duration as most participants were sourced from different facilities outside of the Rakai Health Sciences Program. However, we do know that the guidelines for ART initiation changed (initiate ART when CD4 <500) when this study began enrolling participants. Consequently, the majority of PWH were likely enrolled at the time of diagnosis. Also, this cross-sectional approach precludes causal assessment and the effects of transition from ART-naïve to treatment. Longitudinal studies in larger cohorts are warranted.
WWH have previously been shown to be more vulnerable to immune dysfunction and impaired cognition compared to men, including exhibiting higher CSF HIV viral loads (Hestad et al., 2012; Krebs et al., 2016; Royal et al., 2016). Here we find that MWH have a larger number of associations between CSF biomarkers and cognitive outcomes than WWH in the context of similar levels of CSF biomarkers of inflammation that were similar by sex overall. Some of these patterns indicate a positive relationship between immune regulation and cognitive performance, particularly in MWH. Our findings provide initial preliminary evidence that neuroinflammation may contribute to sex differences in cognition in PWH. Further investigation in larger cohorts and longitudinal studies may lead to delineation of sex-specific mechanisms of cognitive dysfunction in HIV and, possibly impact sex-specific screenings and management to limit the neurological complications of HIV in the ART era.
Supplementary Material
Highlights:
In the overall cohort of people living with HIV on antiretroviral therapy (ART), there were no sex differences in cerebrospinal fluid (CSF) inflammatory marker levels.
There were sex differences in cognitive test performance. For example, men with HIV (MWH) performed worse on cognitive tests of motor, executive function, and fluency compared to women with HIV (WWH).
MWH displayed more associations between inflammatory markers and cognitive performance than WWH
Among MWH, inflammatory markers (e.g. MIP-1β, INF-γ, GM-CSF, IL-7 and IL-12p70) were associated with 56% of the cognitive domains, including attention, processing speed, fluency, executive function, learning and memory.
Among WWH, inflammatory markers were associated with 33% of the domains (i.e. motor, fluency, and learning).
Acknowledgments
Funding
This study was sponsored by the NIH (MH120693, MH099733, MH075673, MH080661-08, L30NS088658, NS065729-05S2) with additional funding from the Johns Hopkins Center for Global Health. This study was also supported in part by the Division of Intramural Research, NIAID, NIH. We would also like to thank the participants and staff of the Rakai Health Sciences Program for their time and effort in the successful conduction of this study.
Footnotes
Conflicts of interest
All coauthors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abassi M, Morawski BM, Nakigozi G, Nakasujja N, Kong X, Meya DB, Robertson K, Gray R, Wawer MJ, Sacktor N, Boulware DR, 2017. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J Neurovirol 23, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi M, Bandera A, Trabattoni D, Tagliabue B, Arosio B, Soria A, Rainone V, Lapadula G, Annoni G, Clerici M, Gori A, 2012. Neurocognitive impairment in HIV-infected naive patients with advanced disease: the role of virus and intrathecal immune activation. Clinical & developmental immunology 2012, 467154. [DOI] [PMC free article] [PubMed] [Google Scholar]; amfAR, 2020. Statistics: Women and HIV/AIDS. amfAR. [Google Scholar]
- Bandera A, Taramasso L, Bozzi G, Muscatello A, Robinson JA, Burdo TH, Gori A, 2019. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front Aging Neurosci 11, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Nilsson LG, Herlitz A, 2006. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychology, development, and cognition. Section B, Aging, neuropsychology and cognition 13, 574–587. [DOI] [PubMed] [Google Scholar]
- Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, Radbruch A, Flesch IE, Kroczek RA, 2002. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci U S A 99, 6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D, 1998. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 352, 1510–1514. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Mackall CL, 2005. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol 174, 6571–6576. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM, 2002. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 35, 313–322. [DOI] [PubMed] [Google Scholar]
- Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, Palmer CD, Sirignano M, Beisel C, Hildebrandt H, Cenac C, Villani AC, Diefenbach TJ, Le Gall S, Schwartz O, Herbeuval JP, Autran B, Guery JC, Chang JJ, Altfeld M, 2015. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-alpha Production in Women. J Immunol 195, 5327–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR Jr., Imasiku ML, Kalima K, Heaton RK, 2012. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. J Nerv Ment Dis 200, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaharuza FM, Bunnell R, Moss S, Purcell DW, Bikaako-Kajura W, Wamai N, Downing R, Solberg P, Coutinho A, Mermin J, 2006. Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS Behav 10, S105–111. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nature reviews. Immunology 16, 626–638. [DOI] [PubMed] [Google Scholar]
- Krebs SJ, Slike BM, Sithinamsuwan P, Allen IE, Chalermchai T, Tipsuk S, Phanuphak N, Jagodzinski L, Kim JH, Ananworanich J, Marovich MA, Valcour VG, 2016. Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV infected individuals. AIDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Lanier ER, McCutchan JA, 1999. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J Infect Dis 180, 310–319. [DOI] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E, 2009. HIV, cognition and women. Neuropsychol Rev 19, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, Valcour V, Young MA, Becker JT, Martin EM, Neuropsychology Working Groups of the Women’s Interagency, H.I.V.S., the Multicenter, A.C.S., 2018. Differences in Cognitive Function Between Women and Men With HIV. J Acquir Immune Defic Syndr 79, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K, 2015. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Deutsch R, Michael BD, Franklin D, Cookson DR, Bharti AR, Grant I, Letendre SL, Group C, 2013. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol 8, 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Fish EN, 2014. SeXX matters in immunity. Trends Immunol 35, 97–104. [DOI] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M, 2009. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanini B, Allen I, Paul R, Bahemana E, Kiweewa F, Nambuya A, Maswai J, Langat R, Owuoth J, Martin S, Possin K, Esber A, Polyak C, Ake JA, Valcour V, 2020. Frequency and Predictors of HIV-Related Cognitive Impairment in East Africa: The Africa Cohort Study (AFRICOS). J Acquir Immune Defic Syndr 83, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasujja N, Skolasky RL, Musisi S, Allebeck P, Robertson K, Ronald A, Katabira E, Clifford DB, Sacktor N, 2010. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC psychiatry 10, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Romano JP, Shaikh AM, Wol M, 2008. Control of the false discovery rate under dependence using the bootstrap and subsampling. Test 17, 417–442. [Google Scholar]
- Royal W 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA, 2016. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PLoS One 11, e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Benning L, Keating SM, Norris PJ, Burke-Miller J, Savarese A, Kumanan KN, Awadalla S, Springer G, Anastos K, Young M, Milam J, Valcour VG, Weber KM, Maki PM, 2018. Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J Neurovirol 24, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Maki PM, 2019. HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Curr HIV/AIDS Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, Villacres MC, Jiang X, Adimora AA, Waldrop-Valverde D, Vance DE, Bolivar H, Alden C, Martin EM, Valcour VG, Women’s Interagency HIVS, 2017. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 89, 1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Neigh GN, Sundermann EE, Xu Y, Scully EP, Maki PM, 2019a. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr Psychiatry Rep 21, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Saylor D, Nakigozi G, Nakasujja N, Robertson K, Kisakye A, Batte J, Mayanja R, Anok A, Lofgren SM, Boulware DR, Dastgheyb R, Reynolds SJ, Quinn TC, Gray RH, Wawer MJ, Sacktor N, 2019b. Heterogeneity in neurocognitive change trajectories among people with HIV starting antiretroviral therapy in Rakai, Uganda. J Neurovirol 25, 800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Springer G, Martin EM, Seaberg EC, Sacktor NC, Levine A, Valcour VG, Young MA, Becker JT, Maki PM, Neuropsychology Working Groups of the Women’s InterAgency, H.I.V.S., the Multicenter, A.C.S., 2019c. Elevated depressive symptoms are a stronger predictor of executive dysfunction in HIV-infected women than men. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, Cysique LA, 2017. HIV-Associated Neurocognitive Disorders: A Global Perspective. J Int Neuropsychol Soc 23, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Kumar A, Nakigozi G, Anok A, Batte J, Kisakye A, Mayanja R, Nakasujja N, Robertson KR, Gray RH, Wawer MJ, Pardo CA, Sacktor N, 2019. Interleukin-6 is associated with mortality and neuropsychiatric outcomes in antiretroviral-naive adults in Rakai, Uganda. J Neurovirol 25, 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Nakigozi G, Nakasujja N, Robertson K, Gray RH, Wawer MJ, Sacktor N, 2017. Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda. Neurology 89, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor DNG,; Nakasujja N; Robertson K; Gray R; Wawer M and Sacktor N, 2017. Peripheral Neuropathy in HIV-Infected and Uninfected Patients in Rakai, Uganda. Neurology 89, 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, Letendre S, Group H, 2015. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 10, e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully EP, 2018. Sex Differences in HIV Infection. Curr HIV/AIDS Rep 15, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC, 2001. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 344, 720–725. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Heaton RK, Pasipanodya E, Moore RC, Paolillo EW, Rubin LH, Ellis R, Moore DJ, Group H, 2018. Sex differences in HIV-associated cognitive impairment. AIDS 32, 2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipa R, das Neves SP, Sousa AL, Fernandes J, Pinto C, Correia AP, Santos E, Pinto PS, Carneiro P, Costa P, Santos D, Alonso I, Palha J, Marques F, Cavaco S, Sousa N, 2019. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol Aging 76, 125–132. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, 1996. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society: Series B (Methodological) 58, 267–288. [Google Scholar]
- Vecchio A, Robertson K, Saylor D, Nakigozi G, Nakasujja N, Kisakye A, Batte J, Mayanja R, Anok A, Reynolds SJ, Quinn TC, Gray R, Wawer MJ, Sacktor N, Rubin LH, 2020. Neurocognitive Effects of Antiretroviral Initiation Among People Living With HIV in Rural Uganda. J Acquir Immune Defic Syndr 84, 534–542. [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D, 2015. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. BioMed research international 2015, 506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.