Abstract
Background:
Lung transplantation and related medications are associated with pathobiological changes that can induce frailty, a state of decreased physiologic reserve. Causes of persistent or emergent frailty after lung transplantation, and whether such transplant-related frailty is associated with key outcomes, is unknown.
Methods:
Frailty and HRQL were prospectively measured repeatedly for up to three-years after lung transplantation. Frailty, quantified by the Short Physical Performance Battery (SPPB), was tested as a time-dependent binary and continuous predictor. The association of transplant-related frailty with HRQL and mortality was evaluated using mixed effects and Cox regression models, respectively, adjusting for age, sex, ethnicity, diagnosis, and for body mass index and lung function as time-dependent covariates. We tested the association between measures of body composition, malnutrition, renal dysfunction, and immunosuppressants on the development of frailty using mixed effects models with time-dependent predictors and lagged frailty outcomes.
Results:
Among 259 adults (56% male; mean age 55.9 ± 12.3 years), transplant-related frailty was associated with lower HRQL. Frailty was also associated with a 2.5-fold higher mortality risk (hazard ratio [HR], 2.51; 95%CI: 1.21–5.23). Further, each 1-point worsening in SPPB was associated, on average, with a 13% higher mortality risk (HR, 1.13; 95%CI: 1.04–1.23). Secondarily, we found that sarcopenia, underweight and obesity, malnutrition, and renal dysfunction were associated with the development of frailty after transplant.
Conclusions:
Transplant-related frailty is associated with lower HRQL and higher mortality in lung recipients. Abnormal body composition, malnutrition, and renal dysfunction may contribute to the development of frailty after transplant. Confirming the role of these potential contributors and developing interventions to mitigate frailty may improve lung transplant success.
Introduction
Frailty reflects a state of risk in which physiologic reserves are either attenuated through pathologic aging or otherwise occupied by comorbid conditions1. In lung transplant candidates, pre-operative frailty is an independent risk factor for disability and waitlist mortality2–5. Further, pre-operative frailty is a risk factor for mortality after lung transplantation; in survivors however, it has been associated with a larger HRQL benefit from transplant2–5. While evidence has rapidly emerged, the clinical relevance of persistent or emergent frailty after transplantation remains unknown.
Many of the putative causes of frailty may be triggered or exacerbated by organ transplantation. Critical illness, debilitation, malnutrition, and cognitive impairments occur commonly during the perioperative period6–9. Later, immunosuppressive medications have off-target adverse effects including mitochondrial dysfunction and the development of adiposity and sarcopenia10–12. Immunosuppressants also cause numerous comorbidities including diabetes mellitus, hypertension, chronic kidney disease, and osteoporosis13–15. Further compounding these direct effects, disuse atrophy from low physical activity following lung transplantation is common16. The aggregate effects of these pathological changes could plausibly lead to persistent pre-operative frailty or new emergent frailty after transplant.
Pre-operative frailty persists in approximately 20% of adults undergoing lung transplantation and new frailty emerges after transplant in 5% of previously not frail candidates17. In both cases, frailty related to transplantation could potentially increase the risk for adverse outcomes in lung recipients. We investigated if frailty after lung transplantation was associated with HRQL and mortality. Additionally, we tested the association between potential transplant-related factors on the subsequent development of frailty.
Methods
Refer to the online Supplement for additional details on clinical immunosuppression protocols, care protocol, and outcome variables.
Study Design, Participants, and Study Aims
To assess the relationship of persistent or emergent frailty after transplant with HRQL and mortality, we analyzed data from the Breathe Again cohort. This single-center prospective cohort study followed adults undergoing first-time lung transplantation under the Lung Allocation Score (LAS) system between 2010 and 201718. Participants completed study visits that included measures of frailty and HRQL before and at 3-, 6-, 12-, 18-, 24-, 30-, and 36-months after transplant. For our primary analyses, we focused only on study visits performed after lung transplantation. Breathe Again was approved by our local Institutional Review Board; participants provided written informed consent.
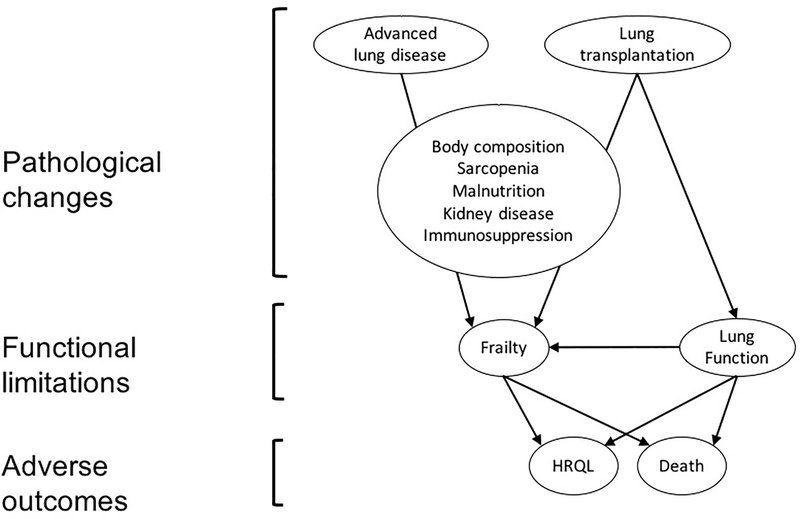
The conceptual model for this study is based on the disablement process19. Pathological changes lead to functional limitations (i.e., frailty) which, in turn, can result in disability, reduced HRQL, and death (Figure 1). Pathological changes that might induce frailty after transplant include the development of sarcopenia20, increased adiposity21, malnutrition22, and renal dysfunction23. These changes can be caused or exacerbated by chronic immunosuppression, in particular calcineurin inhibitors (CNI) and steroids.
Figure 1. Conceptual model of the relationship of frailty with key outcomes.
The pathological changes caused by both advanced lung disease and lung transplantation lead to functional limitations, which are impairments in physical or mental performance that are quantifiable in a laboratory-based setting (i.e., measures of frailty or lung function). Functional limitations, in turn, can result in disability, reduced health-related quality of life (HRQL), and death. Pathological changes that could induce frailty after transplant include changes in body composition with development of sarcopenia and increased adiposity, nutritional deficiency, and renal dysfunction. These changes can be caused or exacerbated by chronic immunosuppression, in particular calcineurin inhibitors and steroids.
We had two overarching study aims which involved two discrete sets of analyses. First, we aimed to evaluate the association between persistent or emergent (i.e., transplant-related) frailty on HRQL and mortality. Second, we aimed to identify potential causes of transplant-related frailty.
Clinical Care Protocol
Our center recommends regular exercise to patients undergoing evaluation and while listed. If patients exhibit debilitation or limited exercise tolerance, we recommend pulmonary rehabilitation. If patients cannot access pulmonary rehabilitation programs, we recommend engaging in 30–40 minutes of a combination of aerobic and strength training exercises per day. The only exception to this practice is if candidates undergo an inpatient evaluation, however, in this circumstance the inpatient care includes aggressive physical therapy. While we recognize that pre-operative frailty is associated with poor outcomes after transplantation, we do not use frailty measures in making listing decisions or when making clinical care decisions.
Predictor variables
We defined transplant-related frailty as pre-operative frailty that persisted after transplant or that newly emerged after transplant. We quantified frailty using the Short Physical Performance Battery (SPPB), an aggregate score ranging from 0–12 based on tests of chair stands, balance, and gait speed24. Lower scores reflect worsening frailty. We used SPPB as both a binary (score ≤7) and a continuous predictor (range 0–12, minimal important difference [MID]=13,25. Because SPPB ≤9 is another frequently utilized cut-point2,26, we conducted a priori exploratory analyses using this alternative dichotomous cut-point, which are included in the supplement.
To identify potential causes of transplant-related frailty, we considered that abnormal body composition, malnutrition, renal dysfunction, and immunosuppression might lead to pathobiological changes resulting in frailty. Sarcopenia is defined as pathologically low muscle mass and strength or function27. Although we did not prospectively collect measures of muscle mass, we did measure grip strength using a handheld dynamometer at each study visit, as previously described2. We used a consensus definition of weak grip strength27 as a proxy of sarcopenia, in addition to grip strength as a continuous variable. Underweight and obesity status by body mass index (BMI) are also surrogates for low muscle mass and adiposity that have been associated with frailty28. We abstracted all BMI measurements from transplant up to 36 months after transplant. We defined underweight as a BMI <18.5 kg/m2 and obesity as a BMI ≥30 kg/m2.
For measures of malnutrition and renal dysfunction, we extracted all clinical laboratory measures of serum albumin and creatinine from our electronic medical record from transplant up to 36-months after transplant. We defined malnutrition as a serum albumin <3.5 mg/dl. For each creatinine value we calculated the estimated glomerular filtration rate (eGFR)29 and Chronic Kidney Disease (CKD) Stage30, which we further categorized as an ordinal variable including stage 1 or 2, stage 3, and stage 4 or 5.
For tacrolimus trough measurements, we extracted all clinical laboratory measures of tacrolimus trough levels from our electronic medical record from transplant up to 36-months after transplant. Finally, for a randomly selected sample of 50% of the cohort, we abstracted whether participants had received any prednisone “pulses” the first 12 months after transplant for treatment of acute rejection or infection with community-acquired respiratory viruses.
Outcome variables
We utilized several instruments to provide a comprehensive assessment of HRQL31. We assessed generic HRQL using the Medical Outcomes Survey Short Form-12 (SF12 v2) Physical and Mental Component Summary scores (SF12-PCS and SF12-MCS, respectively), respiratory-specific HRQL using the Airways Questionnaire 20-Revised (AQ20-R), and health-utility by the EuroQOL five-dimension three-level scale (EQ5D)32–36. Higher scores indicate better HRQL.
Survival was calculated in days from the date of transplant until death or re-transplantation. Since time-dependent predictors were collected up to 36-months after transplant, we applied administrative censoring at 48-months after lung transplant. Dates of death were verified in UCSF site-specific United Network for Organ Sharing reports.
Confounding and Precision Variables
Age at transplant, sex, race/ethnicity, pulmonary diagnosis37, and serial measures of BMI and forced expiratory volume in one second (FEV1) at the time of study visits were abstracted from medical records. We selected these variables based on their known or putative association with frailty and our outcomes of interest1,3,17,38–40.
Analysis Approach
To determine the proportion of frailty after transplant that was persistent or emergent, we compared the SPPB of each participant at all two continuous study visits where it was assessed, from the pre-operative visit through the 36-month post-transplant visit. As a binary measure, we defined frailty as persistent if the SPPB score remained ≤ 7, and emergent if the SPPB score declined from > 7 to ≤ 7 when comparing each visit with the preceding one. As a continuous measure, we defined emergent frailty as at least one-point worsening in SPPB from one visit to the next one.
The association of transplant-related frailty with HRQL and mortality
We tested whether frailty was associated with HRQL using linear mixed effects models with a subject-specific random effect to account for correlation among serial HRQL measures of the same individual. We considered frailty at each post-transplant study visit, as a time-dependent binary (SPPB ≤7) or continuous predictor (SPPB 0–12). We adjusted the models for age, sex, race/ethnicity, diagnosis37, and for BMI and FEV1 at each study visit.
To test the association between transplant-related frailty and mortality, we fitted Cox proportional hazards models with frailty as a time-dependent binary or continuous predictor. We adjusted for age, sex, race/ethnicity, diagnosis37, and for BMI and FEV1 at each study visit.
The models included all SPPB assessments participants had from 3- to 36-months after transplant. Participants generally experienced improvement of pre-operative frailty soon after transplant which stabilized by six-months after transplant17. In Cox regression, the last observation carried forward (LOCF) approach is commonly applied to occasional missing values for time-dependent predictors. To test the robustness of our estimates and the validity of our use of LOCF, we performed three sensitivity analyses. First, we performed two landmark analyses by restricting the model to study visits beginning at 6- and 12-months after transplant, respectively (e.g., dropping prior study visits). Resetting the “baseline” to 6- and 12-months removed the influence of previous frailty measures during the time that they were most dynamic. In the third analysis, we included SPPB assessments performed before transplant. This last analysis allowed us to evaluate the influence of frailty on survival across the pre- and post-transplant timespan.
We plotted Kaplan Meier curves stratified by three different groups of changes in frailty from 6- to 36-months or the participant’s last study visit. We defined the three groups as “unchanged” (participants who had no change in SPPB frailty); “improved” (SPPB score improved by ≥1 point); and “worsened” (SPPB score worsened by ≥1 point).
Predictors of transplant-related frailty
To test the association of transplant-related factors with the development of frailty, we performed analyses using mixed effects models with time-dependent predictors and lagged outcomes. For these analyses, frailty—both continuous (SPPB 0–12) and binary (SPPB ≤7)—was our outcome.
We tested the association between predictors collected in the 3–6-month time period preceding each study visit and the “lagged” outcome of frailty. This analysis approach allowed us to analyze the temporal relationship between predictors and subsequent measures of frailty.
To test the association between sarcopenia and frailty development, we utilized measures of grip strength from the study visit preceding the frailty visit of interest (e.g., grip strength at 3-months was used to predict frailty at 6-months).
The body composition predictor included the categories of underweight (BMI <18.5 kg/m2) and obesity (BMI ≥30 kg/m2), determined by the most extreme BMI recorded during the preceding time period. For malnutrition we used both continuous and binary predictors, a 1.0 g/dl-decrease in the worst albumin concentration and any occurrence of an albumin ≤3.5 in the preceding time period, respectively. For renal dysfunction, we used the worst eGFR as a continuous predictor and the worst CKD stage in the preceding time period as an ordinal predictor-- stage 1 or 2, stage 3, and stage 4 or 5.
To test the association between immunosuppression and transplant-related frailty, we defined variables to reflect average daily tacrolimus trough level (ng/ml) and prednisone pulse exposure (yes/no). In order to calculate the average daily tacrolimus trough level between study visits, we used a last observation carry forward method. For example, the trough level on a given day was carried forward until a new trough was obtained. This was continued such that each day between study visits had an assigned trough level. Then, we summed the trough level for each day and divided that by the number of days between study visits to estimate the average daily trough level (ng/ml) preceding each study visit.
To test the associations between the above predictors and subsequent frailty, we utilized linear mixed effects models for frailty as a continuous outcome and generalized linear mixed effects models for frailty as a binary outcome. Except for models examining renal dysfunction, all other models were adjusted for study visit, age, sex, and race. Since calculation of eGFR uses age, sex, and race, we adjusted the models examining renal dysfunction only for study visit.
Finally, we examined whether select pre-operative characteristics could predict frailty 6-months after transplant using univariate and multivariate logistic regression. Based on existing literature or biologic plausibility we examined age, gender, diagnosis, and frailty.
Analyses were performed using R Version 3.6 (R Foundation, Vienna, Austria) and STATA Version 14.2 (StataCorp, Texas, USA).
Results
Over the study period, 259 adults (56% male; mean age 55.9 ± 12.3 years), underwent lung transplantation and formed our study cohort (Table 1, Supplemental Figure 1). The median duration of uncensored follow-up was 43.4 months post-transplant (IQR 29.4– 64.5). There was no loss to follow up. Of participants with frailty after transplant (n = 55), 45 developed emergent frailty (82%) whereas 10 (18%) had frailty that persisted. Further, of 259 participants, 129 (50%) had at least a one-point worsening in SPPB at some point within the first three-years after lung transplantation (Supplemental Table 1).
Table 1.
Baseline characteristics.
| No. of subjects | 259 |
| Male, No. (%) | 146 (56.37) |
| Age, mean ± SD | 55.94 ± 12.27 |
| Age, No. (%) | |
| < 35 years | 23 (8.88) |
| 35–49 years | 42 (16.22) |
| 50–64 years | 126 (48.65) |
| ≥65 years | 68 (26.25) |
| Race, No. (%) | |
| White | 186 (71.81) |
| Black | 20 (7.72) |
| Hispanic | 34 (13.13) |
| Other | 19 (7.34) |
| Diagnosis, No. (%) | |
| A (e.g. Obstructive lung disease) | 42 (16.22) |
| B (e.g. Pulmonary Hypertension) | 10 (3.86) |
| C (e.g. Suppurative lung disease) | 23 (8.88) |
| D (e.g. Pulmonary Fibrosis) | 184 (71.04) |
| BMI (kg/m2), mean ± SD | 25.63 ± 4.31 |
| FEV1 % predicted, mean ± SD | 45.61 ± 20.51 |
| FEV1 % predicted, median (IQR) | 46.0 (28.0, 61.0) |
| 6 MWD (m), mean ± SD | 254.06 ± 140.87 |
| 6 MWD (m), median (IQR) | 263.0 (146.3, 366.0) |
| LAS at transplant, mean ± SD | 57.85 ± 21.47 |
| LAS at transplant, median (IQR) | 50.0 (39.0, 79.0) |
| Transplant type, No. (%) | |
| Bilateral | 238 (91.89) |
| Single | 15 (5.79) |
| Heart/Lung | 6 (2.32) |
| Inpatient at transplant, No. (%) | 85 (32.82) |
| Ventilator at transplant, No. (%) | 26 (10.04) |
| ECMO at transplant, No. (%) | 20 (7.72) |
| Pre-transplant SPPB, mean ± SD | 9.05 ± 3.04 |
| Pre-transplant SPPB, median (IQR) | 10.0 (8.0, 11.0) |
| Pre-transplant HRQL Scores, mean ± SD | |
| SF12-PCS | 23.67 ± 8.41 |
| SF12-MCS | 48.93 ± 10.47 |
| AQ20-R | 6.50 ± 3.73 |
| EQ5D | 0.65 ± 0.21 |
SPPB= Short Physical Performance Battery. COPD=Chronic Obstructive Pulmonary Disease, BMI=Body Mass Index, FEV1=Forced Expiratory Volume in the first second, 6 MWD=Six Minute Walk Distance, LAS=Lung Allocation Score, ECMO=Extracorporeal Membrane Oxygenation. Health-related quality of life instruments: generic-physical instrument: SF12-PCS (Short Form 12–Physical Component Score), range 0 to 100; generic-mental instrument: SF12-MCS (Short Form 12–Mental Component Score), range 0 to 100; respiratory-specific instrument: AQ20-R (Airways Questionnaire 20–Revised), range 0 to 20, reverse-coded for analysis; health-utility instrument: EQ5D (EuroQoL 5D), range -1.11 to 1. Data are presented as number of patients (percentage) or mean ± standard deviation.
The association of transplant-related frailty with HRQL and mortality
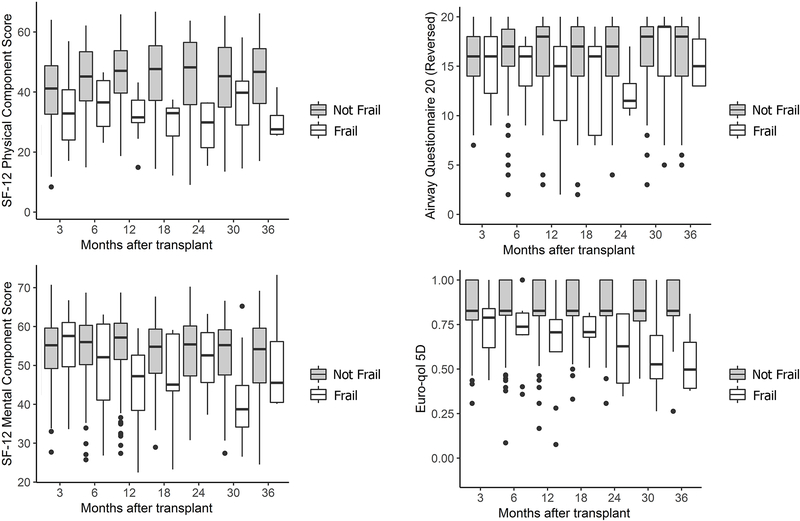
Across all study visits, transplant-related frailty was associated with generally poorer HRQL (Figure 2). In adjusted models, frailty (SPPB ≤7) was associated with lower SF12-PCS (difference [Δ], −5.76; 95%CI: −8.19, −3.30) and EQ5D measures (Δ, −0.11; 95%CI: −0.15, −0.08) (Table 2). Frailty was not associated with HRQL by SF12-MCS or by AQ20-R. Frailty defined as SPPB ≤9 was associated with lower HRQL by SF12-PCS, AQ20-R, and EQ5D (Supplemental Table 2).
Figure 2. Box plots of health-related quality of life (HRQL) by the presence of frailty after lung transplant.
Frailty was assessed with the Short Physical Performance Battery, which ranges from 0 to 12. Recipients with SPPB ≤ 7 were categorized as frail (white boxes), and those with SPPB >7 were categorized as not frail (gray boxes). HRQL was assessed by the SF12-Physical Component Score (generic-physical), the SF12-Mental Component Score (generic-mental), the Airway Questionnaire 20-Revised (respiratory specific), and the EuroQOL-5D (health utility).
Table 2.
Association of transplant-related frailty with health-related quality of life in lung recipients
| Predictor | Instrument | Instrument Type | Difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Frailty (SPPB ≤7) | SF12-PCS (MID=5) | Generic-Physical | −5.76 | (−8.19, −3.30) | <0.01 |
| SF12-MCS (MID=5) | Generic-Mental | −1.61 | (−3.76, 0.55) | 0.14 | |
| AQ20-R (MID=1.75) | Respiratory-Specific | −0.40 | (−1.15, 0.36) | 0.31 | |
| EQ5D (MID=0.06) | Health-Utility | −0.11 | (−0.15, −0.08) | <0.01 | |
| 1-point worsening in SPPB | SF12-PCS (MID=5) | Generic-Physical | −1.19 | (−1.50, −0.89) | <0.01 |
| SF12-MCS (MID=5) | Generic-Mental | −0.36 | (−0.63, −0.08) | 0.01 | |
| AQ20-R (MID=1.75) | Respiratory-Specific | −0.12 | (−0.21, −0.02) | 0.02 | |
| EQ5D (MID=0.06) | Health-Utility | −0.02 | (−0.02, −0.01) | <0.01 | |
Instruments: Short Physical Performance Battery (SPPB), range from 0 to 12 (Minimal Important Difference [MID] = 1); Short Form 12–Physical Component Score (SF12-PCS), range 0 to 100; Short Form 12–Mental Component Score (SF12-MCS), range 0 to 100; Airways Questionnaire 20–Revised (AQ20-R), range 0 to 20, which was reverse-coded for analysis; EuroQoL 5D (EQ5D), range -1.11 to 1, which measures health utility.
The association between frailty and HRQL was quantified by linear mixed-effects models considering frailty as a time-dependent predictor variable. All models were adjusted for pre-operative age, sex, race, diagnosis, and BMI and FEV1 at each study visit.
Data are presented as mean effect estimates with 95% confidence intervals (CI).
Frailty as a continuous predictor was associated with lower HRQL across all instruments. Each one-point worsening in SPPB was associated with lower SF12-PCS (Δ, −1.19; 95%CI: −1.50, −0.89), SF12-MCS (Δ, −0.36; 95%CI: −0.63, −0.08), AQ20-R (Δ, −0.12; 95%CI: −0.21, −0.02), and EQ5D (Δ, −0.02; 95%CI: −0.02, −0.01) (Table 2).
A total of 46 participants (18%) died over the study period. The majority died from chronic rejection (n=18), followed by cancer (n=4) and other infections (n=4) (Supplemental Table 3).
After controlling for covariates, including changes in allograft function and BMI, transplant-related frailty was associated, on average, with a 2.5-fold increased risk of death (HR, 2.51; 95%: CI 1.21, 5.23) (Table 3). The effect estimates and confidence intervals in our landmark sensitivity analyses were similar when setting the baseline SPPB at 6-months (HR 2.28; 95%CI: 1.07, 4.88), 12-months after transplant (HR 3.02; 95%CI: 1.37, 6.63), or when the pre-transplant SPPB was included (HR 3.20; 95%CI: 1.65, 6.21). Effect estimates were also largely unchanged when frailty was defined as SPPB ≤9 (Supplemental Table 4).
Table 3.
Association of transplant-related frailty with mortality in lung transplant recipients
| Baseline |
||||
|---|---|---|---|---|
| Model | Time-dependent predictor from baseline to 36 months post-transplant | 3-months Post-transplant | 6-months Post-transplant | 12-months Post-transplant |
| Unadjusted | Frailty (SPPB ≤7) | 4.06 (2.06, 7.98) | 3.83 (1.91, 7.68) | 4.62 (2.27, 9.39) |
| 1-point worsening in SPPB | 1.19 (1.11, 1.29) | 1.19 (1.10, 1.29) | 1.21 (1.12, 1.31) | |
| Adjusted | Frailty (SPPB ≤7) | 2.51 (1.21, 5.23) | 2.28 (1.07, 4.88) | 3.02 (1.37,6.63) |
| 1-point worsening in SPPB | 1.13 (1.04,1.23) | 1.13 (1.03, 1.23) | 1.15 (1.05, 1.25) | |
The Short Physical Performance Battery (SPPB) ranges from 0 to 12. Lower numbers reflect greater frailty. Mortality risk was estimated by Cox’s proportional hazards models with SPPB as a time-dependent predictor, including all SPPB measurements a participant had from baseline to 36 months post-transplant. Results when setting the baseline SPPB at different time points are shown side by side for comparison. All SPPB values before the specified baseline are excluded from the models. The number of participants alive at each of the baseline points is indicated. Models were adjusted for pre-operative age, sex, race, diagnosis, and BMI and FEV1 measured at each visit. Results represent hazard ratio for death with 95% confidence intervals noted in parenthesis.
After controlling for covariates, each 1-point worsening in frailty across the entire range of the SPPB was associated, on average, with a 13% increase in the risk of death (HR, 1.13; 95%CI: 1.04, 1.23). The effect estimates and confidence intervals in our landmark sensitivity analyses were similar when setting the baseline SPPB at 6-months (HR 1.13; 95%CI: 1.03, 1.23), 12-months after transplant (HR 1.15; 95%CI: 1.05, 1.25), or when the pre-transplant SPPB was included (HR 1.14; 95%CI: 1.06, 1.24).
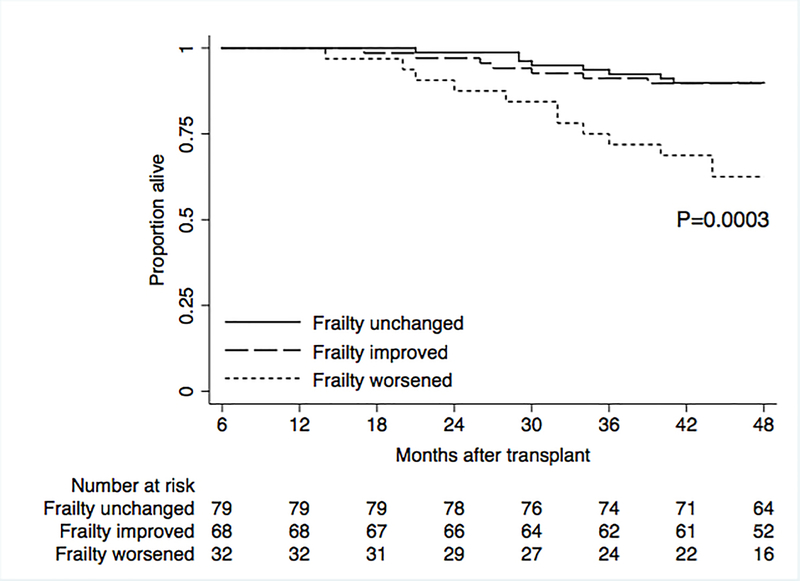
Kaplan Meier plots demonstrated lower survival among lung transplant recipients who had any decrease in SPPB compared to those whose SPPB remained stable or improved (p<0.001) (Figure 3).
Figure 3. Survival after lung transplant by frailty change.
Kaplan Meier plots stratified by three different groups of changes in frailty measured by the Short Physical Performance Battery (SPPB) from 6- to 36-months or the participant’s last study visit. We defined the three groups as “unchanged” (participants who had no change in SPPB frailty); “improved” (SPPB score improved by ≥1 point); and “worsened” (SPPB score worsened by ≥1 point).
Potential predictors of transplant-related frailty
In adjusted models, we found that sarcopenia, body composition, malnutrition, and renal dysfunction were all generally associated with subsequent frailty (Table 4). A weak grip at any point after transplant was associated, on average, with lower SPPB (Δ, −0.88; 95%CI: −1.20, −0.56) and increased odds of frailty (OR 7.14; 95%CI: 2.20, 23.18) at the next study visit. Underweight was associated, on average, with lower SPPB (Δ, −1.35; 95%CI: −2.08, −0.61) and a trend towards increased odds of frailty (OR 11.53; 95% CI: 0.90, 148.30) at the next study visit. Obesity was associated, on average, with four-fold increased odds of frailty at the next study visit (OR 4.33; 95%CI: 1.42, 13.18).
Table 4.
Predictors of subsequent frailty in lung transplant recipients
| Model | Time-dependent predictor* | Change in SPPB frailty (95% CI) |
Odds Ratio frail versus not frail (95% CI) |
|
|---|---|---|---|---|
| 1 | Sarcopenia Component | Per 1.0 kg decrease in grip strength | −0.08 (−0.10, −0.06) | 1.27 (1.12, 1.43) |
| 2 | Weak grip ¶ | −0.88 (−1.20, −0.56) | 7.14 (2.20, 23.18) | |
| 3 | Body Composition | Underweight (BMI <18.5 kg/m2) | −1.35 (−2.08, −0.61) | 11.53 (0.90, 148.30) |
| Obesity (BMI ≥ 30 kg/m2) | −0.17 (−0.56, 0.23) | 4.33 (1.42, 13.18) | ||
| 4 | Malnutrition | Per 1.0 g/dl decrease in albumin | −0.63 (−0.90, −0.36) | 1.92 (0.90, 4.09) |
| 5 | Albumin <3.5 g/dl ¶ | −0.70 (−1.04, −0.36) | 2.82 (0.99, 8.06) | |
| 6 | Renal dysfunction | Per 10-mL/min/1.73m2 decrease in eGFR | −0.18 (−0.27, −0.10) | 1.54 (1.18, 2.00) |
| 7 | CKD Stage 3 versus Stage 1 or 2 | −0.27 (−0.62, 0.08) | 3.80 (1.05, 13.72) | |
| CKD Stage 4 or 5 versus Stage 1 or 2 | −0.90 (−1.38, −0.42) | 6.20 (1.46, 26.32) | ||
Generalized linear mixed effects model with sarcopenia, body composition, malnutrition, and renal dysfunction as a time-dependent predictors and frailty as the “lagged” outcome.
Measures of frailty were obtained at study visits at 3-, 6-, 12-, 18-, 24-, 30-, and 36-months after lung transplantation. Measures of body composition, sarcopenia, nutrition, and renal function in the 3–6-month time period preceding each study visit were collected and used as time-dependent predictors of the subsequent, or “lagged” outcome of frailty.
Frailty was quantified using the Short Physical Performance Battery (SPPB), which ranges from 0–12 (minimal important difference = 1); lower scores denote worse frailty. Frailty was defined as a continuous measure and as a binary outcome (frail = SPPB ≤7).
Body composition was determined by the most extreme body mass index (BMI) in the interval prior to visit. Underweight and obesity were compared to a reference BMI of 18.5 to <30 kg/m2.
Grip strength was used as a proxy for sarcopenia. A weak grip was defined as proposed by the European Working Group on Sarcopenia in Older People27.
Malnutrition determined by the lowest serum albumin in the interval prior to visit, both as a continuous and a binary predictor.
Measures of renal function were calculated as the lowest estimated glomerular filtration rate (eGFR) and the worst chronic kidney disease (CKD) stage in the time period preceding each study visit. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.29
Models 1–5 were adjusted for study visit, age, sex, and race. Models 6 and 7 were adjusted for study visit.
Results represent the effect of sarcopenia, body composition, malnutrition, and renal dysfunction on SPPB score and odds ratio for frailty; 95% confidence intervals are noted in parenthesis.
Denotes that predictor is binary.
For malnutrition, each 1-g/dl decrease in albumin was associated, on average with lower SPPB (Δ, −0.63; 95%CI: −0.90, −0.36) and a trend towards increased odds of frailty (OR 1.92; 95% CI: 0.90, 4.09) at the next study visit.
Renal dysfunction was strongly associated with subsequent frailty. Every 10-mL/min/1.73m2 decrease in eGFR was associated, on average, with lower SPPB (Δ, −0.18; 95%CI: −0.27, −0.10) and increased odds of frailty (OR 1.54; 95%CI: 1.18, 2.00). CKD stage 4 or 5 versus CKD stage 1 or 2 was associated, on average, with lower SPPB (Δ, −0.90; 95% CI: −1.38, −0.42) and six-fold higher odds of frailty (OR 6.20; 95%CI: 1.46, 26.32). Defining frailty as SPPB ≤9 did not appreciably change our findings (Supplemental Table 5).
We found no association between tacrolimus trough level or receiving prednisone “pulses” with subsequent frailty (Supplemental Table 6).
Finally, in examining pre-operative characteristics, female sex and being frail before transplant were associated with frailty at 6-months after transplant (Supplemental Table 7). Age had no association with frailty in unadjusted or adjusted analyses (p=0.95) (Supplemental Table 7 and 8). In a multivariate analysis including sex, diagnosis, and pre-operative frailty, female sex and pre-operative frailty remained either statistically associated or of borderline association with persistent or emergent frailty six months after transplant.
Discussion
Frailty is the outcome of a process of physiological dysregulation that unfolds over time. Investigators in aging research have identified longitudinal examination of frailty trajectories in well-designed cohort studies as a priority to identify risk factors and design interventions to prevent and resolve frailty41. In this study, we found consistent associations between persistent or emergent frailty after lung transplantation and reduced HRQL and increased risk of death. Specifically, transplant-related frailty was associated with clinically meaningful reductions in physical HRQL and health-utility and with a 2.5-fold increased risk of death after accounting for covariates, including allograft function. Further, each one-point worsening in SPPB after transplant across the entire spectrum of the SPPB scale was associated, on average, with lower HRQL across all four instruments tested and with a 13% increased risk of death. We also found that sarcopenia, underweight and obesity, malnutrition, and renal dysfunction were associated with the development transplant-related frailty.
This study adds to emerging work investigating frailty in solid organ transplantation. Previous work has shown the association of pre-operative frailty with post-transplant mortality3,4. In this study, we now demonstrate that persistent or emergent frailty after lung transplantation is also associated with important clinical outcomes. Perhaps equally important, small differences in frailty scores—including those above our pre-specified frailty thresholds—have a strong association with HRQL and mortality, underscoring the semi-arbitrary nature of defining frail as an “all-or-nothing” state.
The relationship between frailty and HRQL is complex. The magnitude of the association between frailty and HRQL varied by HRQL instrument used and whether frailty was analyzed as a binary or continuous variable. Frailty as a binary predictor was associated with clinically meaningful impairments in generic physical HRQL and health-utility. It was not, however, associated with clinically significant impairments in generic mental or respiratory-specific HRQL. Though, each one-point worsening in SPPB was associated with poorer HRQL across all four instruments.
While the precise patho-biological mechanisms of frailty are undefined, it is possible that lung transplantation—and, perhaps, all of solid organ transplantation—may induce frailty in some individuals. Sarcopenia, disuse atrophy, malnutrition, and increased adiposity, among others are putative causes of frailty1,28,42–44. In the peri-operative period, critical illness, decreased mobility, and poor nutrition often occur7–9,45,46. Later, changes in peripheral muscle structure and function occur and sarcopenia emerges20,47. Further, renal dysfunction is strongly associated with frailty in non-transplant populations23. Conducting lagged analyses in our prospective cohort with repeated study visits over years allowed testing of the association of key potential predictors with the later occurrence of frailty.
Sarcopenia, thought of as a cardinal component of frailty48, may develop through multiple mechanisms. One potential mechanism of transplant-related frailty is immunosuppression, as CNI and mTOR inhibitors impair mitochondrial function, inhibit the expression of muscle hypertrophy and remodeling genes, and alter energy metabolism11,49–52. Corticosteroids are known to induce muscle atrophy through impairments in protein synthesis and activation of proteolysis53. Because we lacked a comparator group of transplant recipients who did not receive CNI and corticosteroids, we could not quantify their contribution to the development of frailty. Studies of human transplant recipients that include comparator groups may be hard to design but could help determine whether immunosuppression causes sarcopenia and frailty.
We found that being underweight or obese after transplant is associated with subsequent frailty. This observation supports the recently identified non-linear association between visceral adiposity and frailty21. Both very low and very high amounts of adiposity measured by bioelectrical impedance were associated with increased odds of frailty21. Despite the limitations of using BMI as a measure of body composition, our findings advance this association by establishing a temporal relationship. Whether the development of true sarcopenia (low muscle mass and function), adiposity, or both in combination (sarcopenic obesity) causes frailty after transplant still needs to be demonstrated, yet our findings support this hypothesis.
Similar to studies of community dwelling older individuals, we found that malnutrition (defined as an albumin ≤3.5g/dl) was associated with frailty after transplant22,54. A recent systematic review identified that low micronutrient intake was associated with increased risk of frailty, whereas higher protein intake, higher quality of diet, and diets higher in antioxidants were associated with lower risk frailty in older adults55. Taken together, these data suggest that evaluation of nutritional status and interventions to optimize it may prove effective in preventing or resolving transplant-related frailty.
The association between renal dysfunction and frailty after lung transplantation parallels findings in the general population, where decreases in eGFR and advanced CKD stages are associated with increased risk of frailty23. CKD is common after transplant and our findings highlight one potential novel pathway by which development of CKD after lung transplantation is associated with mortality56. While the mechanisms by which CKD leads to frailty remain to be elucidated, preventing kidney injury after transplant by considering alternative immunosuppression regimens and heightened attention to management of comorbidities may improve long term outcomes57.
The lack of association between age and frailty may be driven by a high frailty prevalence in younger patients with advanced lung disease and careful screening and selection of older patients deemed healthy enough to undergo lung transplant. This finding may not be generalizable to all patients referred for transplant evaluation. More broadly, our inability to predict frailty after transplant based on routinely collected clinical pre-operative variables underscores the need to develop more refined prognostic tools including advanced measures of body composition and biomarkers.
Clinically, our findings support a strategy of frailty surveillance which may identify those at risk for poor HRQL or death, independent of allograft function. Small decrements in measures of frailty—even above traditional frailty thresholds—could identify at-risk patients who might benefit from heightened attention and, possibly, nutritional or exercise-based interventions55,58–60.
Further research is needed to confirm our findings and identify the most robust clinical determinants and biochemical markers of transplant-related frailty. Interventions to prevent and resolve frailty throughout the life span of lung recipients, such as optimization of nutrition, physical conditioning, and minimizing exposure to nephrotoxic agents, are needed41,58–60. Further, heightened attention through rigorous control of co-morbidities associated with renal dysfunction such as diabetes, hypertension, and hypercholesterolemia may be of benefit.
Our study has limitations. Our findings were derived from a single center with approaches to candidate selection and post-operative management that differ from other centers potentially limiting their generalizability. Additional studies are needed to determine if persistent and emergent frailty are similar in other centers and countries with different organ allocation systems. Our results might have differed had other measures of frailty been employed. Importantly, we reported summary scores for our measures of HRQL; frailty may have a differential impact on sub-domains of HRQL (e.g., physical functioning versus pain). We also did not assess some domains in HRQL such as cognitive function and depression that are important to lung recipients and potentially impacted by frailty. Our study was not originally designed to identify the spectrum of causes of frailty after lung transplant. The majority of transplant candidates were referred from outside institutions; thus, we lack accurate information about any immunosuppression received before transplant. Most notably, since all patients in our cohort received calcineurin inhibitors and corticosteroids, we were unable to optimally design an analysis approach that would allow us to determine the effect of specific immunosuppressive agents on transplant-related frailty. While it is possible that CNI and corticosteroids do not directly cause frailty, their threshold exposure sufficient to induce pathological changes that lead to frailty is unknown. Therefore, the lack of association between tacrolimus trough levels and prednisone administration with frailty in our study suggests the possibility that the doses administered after transplant exceed the minimum threshold needed to cause frailty in vulnerable individuals. Studies comparing different immunosuppressive regimens are needed to answer this unresolved question. Also, studies evaluating more accurate and precise measures of sarcopenia, body composition, nutrition, and other potential causes of frailty may improve our understanding of how it develops after transplant. For example, albumin was used as a surrogate for malnutrition. There are other factors in transplant recipients which can impact serum albumin, including critical illness, systemic inflammation, and hepatic and renal disease. Additionally, albumin is not a robust measure of malnutrition and so these analyses should be interpreted with caution. In our center, the practice of prescribing exercise in the immediate post-transplant period is based on clinical need—including discharge to acute rehabilitation facilities, hospital-based pulmonary rehabilitation, or home visits by physical therapists, and advising patients during clinic visits. Therefore, we were unable to evaluate the efficacy of exercise interventions to prevent or resolve frailty in this observational study.
Despite these limitations, our study has several strengths. We studied a relatively large and diverse cohort of lung recipients with repeated measures of frailty, lung function, and HRQL over several years. While we did have a modest amount of missing data, the majority of missing data were deemed missing at random and, as a result not likely to be a source of systematic bias. Further, our use of mixed effects models provide valid estimates for missing values 61. We leveraged the prospective collection of repeated measures by using mixed effects models and lagged analyses to identify novel findings. These early and, in many cases, substantial associations argue that a more rigorous and thorough investigation of how transplantation may induce frailty is needed. To our knowledge, this is the first study to assess the association between frailty after transplantation and key patient-centered outcomes and to identify potential transplant-related causes of frailty. Future studies with increased frequency of frailty and potential causal factor measurements over time would help answer some of the remaining questions—including causality--that our study could not address. In some ways, lung transplant practice with frequent follow-up visits, little to no loss to follow-up, and high event rates provides a unique “human model” to study the pathogenesis of frailty.
In sum, we found that persistent and emergent frailty after lung transplantation is associated with lower HRQL and mortality, independent of lung allograft function. Sarcopenia, underweight, obesity, malnutrition, and renal dysfunction are associated with the development of frailty after lung transplantation. Efforts to define frailty-specific interventions both before and after lung transplantation may help to maximize patient well-being and transplant success.
Supplementary Material
Key Messages:
What is the key question?
Is there a relationship between transplant-related frailty and health-related quality of life and mortality in lung recipients?
What is the bottom line?
Changes in body composition, nutritional status, and renal dysfunction are associated with the development of frailty after lung transplantation which, in turn, is associated with worse HRQL and increased risk of mortality.
Why read on?
Recognizing that lung transplantation, and potentially all solid-organ transplantation, can induce frailty in some individuals underscores the need to develop frailty-specific interventions to improve the overall success of transplantation.
Acknowledgements
We thank the patients who participated in this study.
Support statement
This work was supported by grants from the National Heart, Lung, and Blood Institute (K23 HL111115 and R01 HL134851) to JPS, and the Clinical Sciences Research & Development Service of the VA Office of Research and Development (career development award IK2CX001034 to JRG).
Competing Interests
Dr. John R. Greenland reports grants and personal fees from Thermo Fisher, Genentech, Atara Biotherapeutics, and BioMérieux, outside the submitted work. Dr. Paul J. Wolters reports grants from MedImmune, grants from Genentech, personal fees from Roche, personal fees from Blade Therapeutics, grants and personal fees from Boehringer Ingelheim, personal fees from Pliant, outside the submitted work.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. March 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 2.Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. American Journal of Respiratory and Critical Care Medicine. 2015;192:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: A prospective cohort study. Am J Transplant. April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant. February 2016;35(2):173–178. [DOI] [PubMed] [Google Scholar]
- 5.Rozenberg D, Mathur S, Wickerson L, Chowdhury NA, Singer LG. Frailty and clinical benefits with lung transplantation. J Heart Lung Transplant. October 2018;37(10):1245–1253. [DOI] [PubMed] [Google Scholar]
- 6.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. October 3 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummel NE, Balas MC, Morandi A, Ferrante LE, Gill TM, Ely EW. Understanding and reducing disability in older adults following critical illness. Crit Care Med. June 2015;43(6):1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional Trajectories Among Older Persons Before and After Critical Illness. JAMA Internal Medicine. 2015;175:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin MR, Arcasoy SM, Shah A, et al. Hypoalbuminemia and early mortality after lung transplantation: a cohort study. Am J Transplant. May 2012;12(5):1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito-Costa A, Pereira-da-Silva L, Papoila AL, et al. Factors Associated With Changes in Body Composition Shortly After Orthotopic Liver Transplantation: The Potential Influence of Immunosuppressive Agents. Transplantation. August 2016;100(8):1714–1722. [DOI] [PubMed] [Google Scholar]
- 11.Simon N, Morin C, Urien S, Tillement JP, Bruguerolle B. Tacrolimus and sirolimus decrease oxidative phosphorylation of isolated rat kidney mitochondria. Br J Pharmacol. January 2003;138(2):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary NS, Saigal S, Saraf N, et al. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant. March 2015;29(3):211–215. [DOI] [PubMed] [Google Scholar]
- 13.Hackman KL, Bailey MJ, Snell GI, Bach LA. Diabetes is a major risk factor for mortality after lung transplantation. Am J Transplant. February 2014;14(2):438–445. [DOI] [PubMed] [Google Scholar]
- 14.Sithamparanathan S, Thirugnanasothy L, Clark S, et al. Observational study of lung transplant recipients surviving 20 years. Respir Med. August 2016;117:103–108. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari SL, Nicod LP, Hamacher J, et al. Osteoporosis in patients undergoing lung transplantation. Eur Respir J. November 1996;9(11):2378–2382. [DOI] [PubMed] [Google Scholar]
- 16.Langer D Rehabilitation in Patients before and after Lung Transplantation. Respiration. 2015;89:353–362. [DOI] [PubMed] [Google Scholar]
- 17.Venado A, McCulloch C, Greenland JR, et al. Frailty trajectories in adult lung transplantation: A cohort study. J Heart Lung Transplant. July 2019;38(7):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer JP, Katz PP, Soong A, et al. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am J Transplant. May 2017;17(5):1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jette AM. Disablement outcomes in geriatric rehabilitation. Med Care. June 1997;35(6 Suppl):JS28–37; discussion JS38–44. [DOI] [PubMed] [Google Scholar]
- 20.Rozenberg D, Wickerson L, Singer LG, Mathur S. Sarcopenia in lung transplantation: a systematic review. J Heart Lung Transplant. December 2014;33(12):1203–1212. [DOI] [PubMed] [Google Scholar]
- 21.Anderson MR, Kolaitis NA, Gao Y, et al. A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transplant. July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gingrich A, Volkert D, Kiesswetter E, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. April 27 2019;19(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. Jan-Feb 2017;68:135–142. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. March 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 25.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. May 2006;54(5):743–749. [DOI] [PubMed] [Google Scholar]
- 26.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. December 22 2016;14(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. July 2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Esquinas E, Jose Garcia-Garcia F, Leon-Munoz LM, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in Spain. Obesity (Silver Spring). April 2015;23(4):847–855. [DOI] [PubMed] [Google Scholar]
- 29.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. September 2010;56(3):486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. July 15 2003;139(2):137–147. [DOI] [PubMed] [Google Scholar]
- 31.Singer JP, Chen J, Katz PP, Blanc PD, Kagawa-Singer M, Stewart AL. Defining novel health-related quality of life domains in lung transplantation: a qualitative analysis. Qual Life Res. December 4 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey - Construction of scales and preliminary tests of reliability and validity. Medical Care. March 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 33.Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Current Opinion in Rheumatology. March 2002;14(2):109–114. [DOI] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life - The remarkable universality of half a standard deviation. Medical Care. May 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Eisner MD, Katz PP, Yelin EH, Blanc PD. Measuring disease-specific quality of life in obstructive airway disease - Validation of a modified version of the airways questionnaire 20. Chest. June 2006;129(6):1644–1652. [DOI] [PubMed] [Google Scholar]
- 36.Euroqol Williams A. - a New Facility for the Measurement of Health-Related Quality-of-Life. Health Policy. December 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 37.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. [DOI] [PubMed] [Google Scholar]
- 38.Espinoza SE, Jung I, Hazuda H. Lower frailty incidence in older Mexican Americans than in older European Americans: the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. November 2010;58(11):2142–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. November 01 2014;190(9):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant. October 2018;37(10):1169–1183. [DOI] [PubMed] [Google Scholar]
- 41.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. October 12 2019;394(10206):1365–1375. [DOI] [PubMed] [Google Scholar]
- 42.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. June 2006;54(6):991–1001. [DOI] [PubMed] [Google Scholar]
- 43.Stenholm S, Sallinen J, Koster A, et al. Association between obesity history and hand grip strength in older adults--exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci. March 2011;66(3):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stenholm S, Strandberg TE, Pitkala K, Sainio P, Heliovaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. January 2014;69(1):73–78. [DOI] [PubMed] [Google Scholar]
- 45.Gannon WD, Lederer DJ, Biscotti M, et al. Outcomes and Mortality Prediction Model of Critically Ill Adults With Acute Respiratory Failure and Interstitial Lung Disease. Chest. June 2018;153(6):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors Associated with Functional Recovery among Older Intensive Care Unit Survivors. Am J Respir Crit Care Med. August 1 2016;194(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaufelberger M, Eriksson BO, Lönn L, Rundqvist B, Sunnerhagen KS, Swedberg K. Skeletal muscle characteristics, muscle strength and thigh muscle area in patients before and after cardiac transplantation. Eur J Heart Fail. January 2001;3(1):59–67. [DOI] [PubMed] [Google Scholar]
- 48.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lombardi A, Trimarco B, Iaccarino G, Santulli G. Impaired mitochondrial calcium uptake caused by tacrolimus underlies beta-cell failure. Cell Commun Signal. November 2017;15(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercier JG, Hokanson JF, Brooks GA. Effects of cyclosporine A on skeletal muscle mitochondrial respiration and endurance time in rats. Am J Respir Crit Care Med. May 1995;151(5):1532–1536. [DOI] [PubMed] [Google Scholar]
- 51.Wang XN, Williams TJ, McKenna MJ, et al. Skeletal muscle oxidative capacity, fiber type, and metabolites after lung transplantation. Am J Respir Crit Care Med. July 1999;160(1):57–63. [DOI] [PubMed] [Google Scholar]
- 52.Illsinger S, Göken C, Brockmann M, et al. Effect of tacrolimus on energy metabolism in human umbilical endothelial cells. Ann Transplant. 2011. Apr-Jun 2011;16(2):68–75. [DOI] [PubMed] [Google Scholar]
- 53.Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. October 2013;45(10):2163–2172. [DOI] [PubMed] [Google Scholar]
- 54.Wei K, Nyunt MSZ, Gao Q, Wee SL, Ng TP. Frailty and Malnutrition: Related and Distinct Syndrome Prevalence and Association among Community-Dwelling Older Adults: Singapore Longitudinal Ageing Studies. J Am Med Dir Assoc. December 1 2017;18(12):1019–1028. [DOI] [PubMed] [Google Scholar]
- 55.Lorenzo-Lopez L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodriguez-Villamil JL, Millan-Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. May 15 2017;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. September 4 2003;349(10):931–940. [DOI] [PubMed] [Google Scholar]
- 57.Schena FP, Pascoe MD, Alberu J, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. January 27 2009;87(2):233–242. [DOI] [PubMed] [Google Scholar]
- 58.Fairhall N, Langron C, Sherrington C, et al. Treating frailty--a practical guide. BMC Med. 2011;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Latham NK, Harris BA, Bean JF, et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. February 19 2014;311(7):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singer JP, Soong A, Bruun A, et al. A mobile health technology enabled home-based intervention to treat frailty in adult lung transplant candidates: A pilot study. Clin Transplant. June 2018;32(6):e13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laird NM. Missing data in longitudinal studies. Stat Med. Jan-Feb 1988;7(1–2):305–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.