Abstract
In vivo gold standard for the ante-mortem assessment of brain β-amyloid pathology is currently β-amyloid positron emission tomography or cerebrospinal fluid measures of β-amyloid42 or the β-amyloid42/β-amyloid40 ratio. The widespread acceptance of a biomarker classification scheme for the Alzheimer’s disease continuum has ignited interest in more affordable and accessible approaches to detect Alzheimer’s disease β-amyloid pathology, a process that often slows down the recruitment into, and adds to the cost of, clinical trials. Recently, there has been considerable excitement concerning the value of blood biomarkers. Leveraging multidisciplinary data from cognitively unimpaired participants and participants with mild cognitive impairment recruited by the multisite biomarker study of Alzheimer’s Disease Neuroimaging Initiative, here we assessed to what extent plasma β-amyloid42/β-amyloid40, neurofilament light and phosphorylated-tau at threonine-181 biomarkers detect the presence of β-amyloid pathology, and to what extent the addition of clinical information such as demographic data, APOE genotype, cognitive assessments and MRI can assist plasma biomarkers in detecting β-amyloid-positivity. Our results confirm plasma β-amyloid42/β-amyloid40 as a robust biomarker of brain β-amyloid-positivity (area under curve, 0.80–0.87). Plasma phosphorylated-tau at threonine-181 detected β-amyloid-positivity only in the cognitively impaired with a moderate area under curve of 0.67, whereas plasma neurofilament light did not detect β-amyloid-positivity in either group of participants. Clinical information as well as MRI-score independently detected positron emission tomography β-amyloid-positivity in both cognitively unimpaired and impaired (area under curve, 0.69–0.81). Clinical information, particularly APOE ε4 status, enhanced the performance of plasma biomarkers in the detection of positron emission tomography β-amyloid-positivity by 0.06–0.14 units of area under curve for cognitively unimpaired, and by 0.21–0.25 units for cognitively impaired; and further enhancement of these models with an MRI-score of β-amyloid-positivity yielded an additional improvement of 0.04–0.11 units of area under curve for cognitively unimpaired and 0.05–0.09 units for cognitively impaired. Taken together, these multi-disciplinary results suggest that when combined with clinical information, plasma phosphorylated-tau at threonine-181 and neurofilament light biomarkers, and an MRI-score could effectively identify β-amyloid+ cognitively unimpaired and impaired (area under curve, 0.80–0.90). Yet, when the MRI-score is considered in combination with clinical information, plasma phosphorylated-tau at threonine-181 and plasma neurofilament light have minimal added value for detecting β-amyloid-positivity. Our systematic comparison of β-amyloid-positivity detection models identified effective combinations of demographics, APOE, global cognition, MRI and plasma biomarkers. Promising minimally invasive and low-cost predictors such as plasma biomarkers of β-amyloid42/β-amyloid40 may be improved by age and APOE genotype.
Keywords: Alzheimer’s, β-amyloid, MRI, PET, plasma
Tosun et al. report a systematic comparison of β-amyloid positivity detection models, identifying effective combinations of demographics, APOE genotype, global cognitive measures, MRI and plasma biomarkers as promising minimally invasive and low-cost assessments to detect the β-amyloid positivity using florbetapir PET status as the ground-truth.
Graphical Abstract
Graphical Abstract.
Introduction
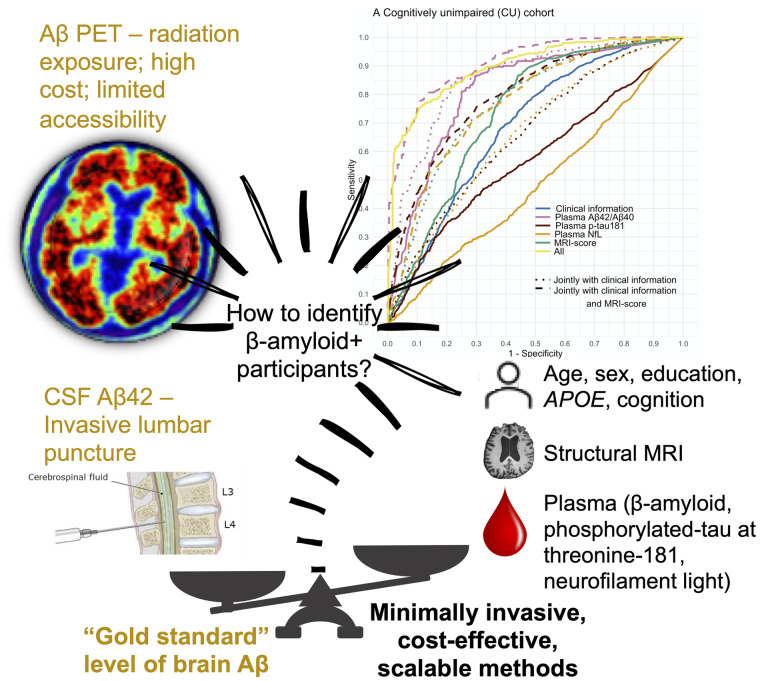
Alzheimer’s disease (AD), pathologically defined as the presence of plaques of β-amyloid (Aβ) protein, neurofibrillary tangles of tau protein and neurodegeneration (DeTure and Dickson, 2019), is the major cause of cognitive decline and dementia (2020). Currently, no treatment is approved that has been demonstrated to slow the progress of AD (Aisen, 2019). Historically, AD was diagnosed clinically through neurological and neuropsychological examinations to assess memory impairment and other thinking skills, judge functional abilities and identify behaviour changes, and exclude other causes than AD that could account for the dementia (McKhann et al., 2011). The ‘gold-standard’ method to confirm the presence of AD pathology is pathological examination of brains at autopsy (DeTure and Dickson, 2019). Since the turn of the century, the ability to diagnose AD pathology in living people has been made possible by the development of radioligands for Aβ positron emission tomographic (PET) scans (Klunk et al., 2004; Schilling et al., 2016) and tau PET scans (Marquie et al., 2015; Leuzy et al., 2019), magnetic resonance imaging (MRI) for neurodegeneration (Frisoni et al., 2010) and analysis of cerebrospinal fluid (CSF) for Aβ and tau species (Blennow, 2004; Holtzman, 2011). This has led to an in vivo biological framework of AD including Aβ, tau and neurodegeneration, based on the so-called A/T/N system (Jack et al., 2018). Indeed, the descriptive A/T/N system places Aβ+ individuals firmly on the AD continuum, whereas individuals with Aβ- profiles are considered either normal or possessing non-AD pathologic changes (Jack et al., 2018). Many trials, particularly the ones enrolling patients in earlier stages of disease, are therefore using either Aβ PET imaging or CSF Aβ42 levels as a critical step in clinical trial cohort enrichment (Sperling et al., 2014; Honig et al., 2018). Despite these advances, PET scans are quite expensive and not universally accessible. Although lumbar punctures are very safe (Peskind et al., 2009), there continues to be reluctance to CSF sample collection in the patient and professional population (Moulder et al., 2017). Therefore, there has been great interest in developing low cost, minimally invasive methods to detect AD Aβ pathology compared to PET scans and or CSF as the ‘gold standard’. Many publications (reviewed in Ashford et al.) have evaluated the role of demographics (Insel et al., 2016; Tosun et al., 2016; Jansen et al., 2018; Buckley et al., 2019; Ko et al., 2019; Maserejian et al., 2019), APOE ε4 (de Rojas et al., 2018; Jansen et al., 2018; Ten Kate et al., 2018; Ba et al., 2019; Buckley et al., 2019), cognition (Mielke et al., 2012; Burnham et al., 2014; Kandel et al., 2015; Burnham et al., 2016; Insel et al., 2016; Kim et al., 2018; Lee et al., 2018; Ba et al., 2019; Brunet et al., 2019; Maserejian et al., 2019; Ansart et al., 2020) and MRI measures (Tosun et al., 2013, 2014, 2016; Ten Kate et al., 2018; Petrone et al., 2019; Ansart et al., 2020; Ezzati et al., 2020) to detect AD Aβ pathology. More recently, there has been considerable excitement concerning the value of assays of plasma Aβ species and related proteins (Burnham et al., 2014, 2016; Kaneko et al., 2014; Fandos et al., 2017; Ovod et al., 2017; Park et al., 2017; de Rojas et al., 2018; Nakamura et al., 2018; Verberk et al., 2018; Westwood et al., 2018; Chatterjee et al., 2019; Chen et al., 2019; Goudey et al., 2019; Lin et al., 2019; Palmqvist et al., 2019a,b; Park et al., 2019; Perez-Grijalba et al., 2019; Vergallo et al., 2019), species of plasma tau, including phosphorylated tau (p-tau) forms (Mielke et al., 2018; Palmqvist et al., 2019b; Barthélemy et al., 2020; Janelidze et al., 2020a; Karikari et al., 2020; Palmqvist et al., 2020; Thijssen et al., 2020) and plasma neurofilament light (NfL) (Palmqvist et al., 2019b; Thijssen et al., 2020) to detect AD Aβ pathology. The first reports of reproducible high precision, high accuracy tests of plasma Aβ42/Aβ40 indicated high sensitivity and specificity for Aβ plaques as measured by mass spectrometry (Ovod et al., 2017; Nakamura et al., 2018). Subsequently, plasma measures of p-tau at residues 181 (Mielke et al., 2018) and 217 (Barthélemy et al., 2020; Palmqvist et al., 2020) indicated good performance relative to Aβ plaques and tau tangles. The performance of these tests is being evaluated and has been shown to detect PET Aβ-positivity conversion (Schindler et al., 2019), to be associated with cognitive decline and to correlate with AD pathology (Janelidze et al., 2020a). If proven useful, these alternative approaches to detect AD Aβ pathology may play an important role in drug discovery and in accelerating identification of risk factors for AD with greater precision.
For optimal and generalizable operationalization of such imputation approaches for the presence of AD Aβ pathology, it is important to assess the independent and added value of each class of predictors (e.g. demographics, APOE ε4, cognition, plasma biomarkers, MRI, etc.) and the differences in their classification performances at different clinical stages. The Alzheimer’s Disease Neuroimaging Initiate (ADNI) is a large, multi-site, longitudinal study aimed at validating biomarkers for AD clinical trials (Weiner et al., 2017). ADNI participants have Aβ PET scans, lumbar punctures for CSF and blood drawn for plasma studies, therefore allowing for a head-to-head comparison. This study specifically aimed to assess (i) to what extent plasma Aβ42/Aβ40, NfL and p-tau181 biomarkers detect the presence of AD Aβ pathology (i.e. Aβ-positivity); (ii) to what extent the addition of demographic data, APOE genotype and cognitive assessments and (iii) MRI can assist plasma biomarkers in detecting Aβ-positivity and (iv) to what extent the stage of clinical diagnosis affects these relationships.
Materials and methods
Study design
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. Up-to-date information are available at www.adni-info.org.
Cohort
Subjects of this study were ADNI participants with known PET Aβ status and with plasma biomarker assessments for p-tau181, and NfL, clinical assessments and structural MRI within 6 months of Aβ PET imaging. A subset of the main study cohort also had plasma biomarker assessment for Aβ42/Aβ40. The primary focus of this study was to assess imputation of Aβ positivity from single time-point observations of clinical, neuroimaging and plasma biomarker data; therefore, a cross-sectional study design was used. Although longitudinal biomarkers, neuroimaging and clinical data are available for many ADNI participants, we considered only data from the first time-point with complete clinical, neuroimaging and biomarker assessments for each participant to avoid circular model training and assessment. Clinical assessment closest in time to Aβ PET imaging was used to define cognitively unimpaired (CU) and cognitively impaired (CI) diagnostic groups. The diagnostic criteria for ADNI participants were described previously (Petersen et al., 2010). Participant selection was made a priori from all ADNI subjects based on the availability of complete cross-sectional data as of 30 June 2020.
PET Aβ status
Mean tracer uptake in the cerebellar grey and white matter was computed and used as reference to generate whole-brain standardized uptake value ratio (SUVR) maps of florbetapir PET scans (Jagust et al., 2015). A composite region-of-interest consisting of middle frontal, anterior cingulate, posterior cingulate, inferior parietal, precuneus, supramarginal, middle temporal and superior temporal regions was used to compute a global SUVR for florbetapir. A threshold of SUVR ≥1.11 for florbetapir (Landau et al., 2013) was then used to determine the status of PET Aβ.
Demographics data
Age at florbetapir PET imaging, sex and years of education were included as demographic characteristics of each participant.
Apolipoprotein E genotyping
APOE genotyping was done by the ADNI Genetics Core using DNA from blood samples as detailed in Supplementary Material. APOE ε4 carrier status was considered as a predictor of Aβ-positivity in this study.
Global cognitive assessments
ADNI participants were assessed with a wide spectrum of clinical and cognitive tests (Weiner et al., 2017). In this study, we limited the global cognitive assessments to the Clinical Dementia Rating—Sum of Boxes (CDR–SB), the Alzheimer’s Disease Assessment Scale—Cognitive subscale 13-item (ADAS–Cog) and the Mini–Mental State Examination (MMSE) based on a 30-point questionnaire.
Plasma sample collection
Blood samples were obtained by venipuncture in EDTA tubes for plasma by following the ADNI protocol (Kang et al., 2015). Within 60 min, the samples were centrifuged at 3000 r.p.m. at room temperature, aliquoted and stored at −80°C. Samples underwent two freeze/thaws. Further details are provided in Supplementary Material.
Plasma Aβ42 and Aβ40
Plasma Aβ isoform concentrations were determined using immunoprecipitation combined with liquid chromatography tandem mass spectrometry (LC-MS/MS) as described previously (Ovod et al., 2017). Plasma aliquots were thawed at 21°C/800 RPM for 10 min and centrifuged at 21°C/10 000 RCF for 5 min prior to immunoprecipitation. Targeted Aβ42 and Aβ40 isoforms were immunoprecipitated with an anti-Aβ mid-domain antibody (HJ5.1) using a KingFisher (Thermo) automated immunoprecipitation platform. Immuno-enriched fractions were subsequently digested with Lys-N protease, generating Aβ28-42 and Aβ28-40 species, which were measured by LC-MS/MS (Ovod et al., 2017). Absolute Aβ isoform concentrations were determined with a 15 N-labelled internal standard for each isoform. The total levels of Aβ42 and Aβ40 were used to calculate the Aβ42/Aβ40 ratio.
Plasma p-tau181
Plasma p-tau181 was analysed by the Single-molecule array (Simoa) technique (Quanterix, Billerica, MA), using an assay developed in the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden (Karikari et al., 2020). The assay uses a combination of two monoclonal antibodies (Tau12 and AT270) and measures N-terminal to mid-domain forms of p-tau181 (Karikari et al., 2020). Calibrators were run as duplicates, whereas plasma samples were measured in singlicate. All the available samples were analysed in a single batch.
Plasma NfL
Plasma NfL was analysed by the Simoa technique (Quanterix, Billerica, MA). The assay uses a combination of monoclonal antibodies, and purified bovine NfL as a calibrator. Calibrators were run as triplicates, whereas plasma samples were measured in singlicate. All the available samples were analysed in a single batch.
MRI-score for Aβ-positivity
3T MRI data included a 3D MP-RAGE or IR-SPGR T1-weighted acquisition, as described online (http://adni.loni.usc.edu/methods/documents/mri-protocols). We employed a previously proposed methodology for assessing brain Aβ positivity status (Lang et al., 2019). Briefly, 972 ADNI patients with structural MRI scans and with known Aβ status based on either CSF or Aβ PET imaging were used to train a deep learning model. The deep learning model training cohort included individuals at different clinical stages (CU, subjective memory complaint, early/late MCI and dementia), but excluding the participants of this study with plasma biomarker data. The method yields a probabilistic score of Aβ-positivity between 0 and 1.
Statistical analysis
All analyses were performed on CU and CI data separately.
Demographic, clinical and biomarker characteristic differences between Aβ+ and Aβ− participants were described using two-sample t-test and the χ2 test for continuous and categorical variables, respectively.
Demographic characteristics (age, sex and years of education), APOE genotype, cognitive scores (MMSE, ADAS–Cog, and CDR–SB), plasma Aβ42/Aβ40, p-tau181 and NfL levels, and derived MRI-score were used as inputs to construct random forest (RF) classifiers to detect the Aβ-positivity using florbetapir PET status as the ground-truth. RF approach was pre-selected based on the classification performances that are previously reported (Fernández-Delgado et al., 2014) and flexibility of RF models to a mixture of numerical (age, years of education, cognitive scores, plasma levels and MRI-score) and categorical (sex and APOE genotype) features. A reference RF classifier was constructed from demographics and cognitive scores, referred as the clinical information here on. A second reference RF classifier was also constructed from MRI-score alone. To assess the added value of each class of variables (i.e. clinical, plasma and MRI classes), additional RF classifiers were constructed from (i) each plasma marker alone, (ii) each plasma marker jointly with clinical features, (iii) MRI-score jointly with clinical features and (iv) each plasma marker jointly with clinical features and MRI-score.
The RF model construction was repeated 10 times using different random seeds, and the average model performance was reported. Each data set (CU and CI data sets) was randomly divided into training and test data sets, using non-overlapping 80%/20% split. Each data set used the same partitioning for all classifiers for an unbiased comparison between classifiers (Vanschoren et al., 2012). The models were built on each training split, and the performance on the test data sets was evaluated, and this process was repeated 10 times. Performance was presented as mean and standard deviation over the model runs. We generated sensitivity–specificity curves based on model classifications on the test data. For each sensitivity–specificity curve, we also computed the area under curve (AUC) values. A confidence interval of 95% was chosen. AUC of two classifiers was compared with DeLong test (DeLong et al., 1988). Additionally, we computed accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) on each set of model classifications at classifier probability cut-off of 0.5.
Finally, for RF models with multiple variables, the mean decrease in accuracy caused by a variable was determined based on the out-of-bag error estimates. The more the accuracy of the RF decreases due to the exclusion of a single variable, the more important that variable was deemed for the classification of the data.
The main analyses reported below with PET Aβ-positivity as the gold-standard for Aβ-positivity were repeated with CSF Aβ-positivity and the results are shown in Supplementary Fig. 1. Results from another secondary analysis are also shown in Supplementary Fig. 2, in which each classifier model was considered in a sub-sample constraint by the plasma Aβ42/Aβ40 cohort where all relevant data was available, yielding a fixed sample size across all classifier models. Finally, the main analyses were repeated by restricting clinical information to age and APOE genotype (Supplementary Fig. 3).
All analyses were performed using the R language and environment for statistical computing version 4.0.1 (R Foundation for Statistical Computing).
Data availability
Data used in this study has been made publicly available by the ADNI in the Laboratory of Neuro Imaging (LONI) database.
Results
The results of plasma Aβ42/Aβ40 for nine CU and nine CI participants failed quality control at measurement. No outliers (i.e. > 4 standard deviations of the mean) were detected in the plasma Aβ42/Aβ40 measurements. Samples from three CU and one CI participants were measured below the lower limit of quantification of 1.0 pg/ml for plasma p-tau181. We identified additional five CU and five CI participants with outlier values of plasma p-tau181 levels, who were discarded from subsequent analyses. Analytical sensitivity for plasma NfL was <1.0 pg/ml, and no sample contained NfL levels in plasma below the limit of detection, but 5 CUs and 11 CIs were excluded from our analyses due to outlier plasma NfL values. Participants with dementia were excluded for two main reasons. First, 91% of the AD participants (n = 235) with plasma NfL and plasma p-tau181 biomarker data were PET Aβ-positive. An unbiased classification performance analysis with a prevalence of 91% Aβ-positivity would have required a sample size of >500 (Hanczar et al., 2010). Second, cross-sectional plasma Aβ42/Aβ40 data was available only for non-demented participants. The final main study cohort was composed of 333 CU and 519 CI elderly individuals. Participant characteristics are reported in Table 1.
Table 1.
Sample demographics per clinical diagnostic group
| CU Aβ− | CU Aβ+ | P value | CI Aβ− | CI Aβ+ | P value | |
|---|---|---|---|---|---|---|
| Main cohort | ||||||
| N | 224 | 109 | 230 | 289 | ||
| Age (years) | 72.8 ± 6.2 | 74.6 ± 5.3 | 0.01 | 70.3 ± 7.9 | 73.3 ± 6.8 | <10−5 |
| Sex (Female %) | 52% | 36% | 0.005 | 56% | 56% | |
| Education (years) | 16.8 ± 2.5 | 15.9 ± 2.8 | 0.003 | 16.3 ± 2.5 | 15.9 ± 2.9 | 0.024 |
| APOE ε4 (carrier %) | 21% | 43% | <10−4 | 23% | 66% | <10−15 |
| MMSE | 29.1 ± 1.3 | 28.9 ± 1.1 | 28.4 ± 1.6 | 27.6 ± 1.8 | <10−6 | |
| CDR-SB | 0.06 ± 0.2 | 0.1 ±0.3 | 0.03 | 1.3 ± 0.8 | 1.6 ± 0.9 | <10−4 |
| ADAS-Cog | 5.5 ± 3.1 | 6.3 ± 3.0 | 0.02 | 7.8 ± 3.8 | 10.4 ± 4.6 | <10−10 |
| Plasma NfL (pg/ml) | 35.4 ± 15.8 | 39.4 ± 15.8 | 0.03 | 35.0 ± 18.7 | 43.3 ± 19.8 | <10−5 |
| Plasma p-tau181 (pg/ml) | 14.7 ± 10.6 | 16.9 ± 7.8 | 13.6 ± 8.6 | 21.6± 10.7 | <10−14 | |
| Plasma Aβ42/Aβ40 sub-cohort | ||||||
| N | 50 | 37 | 40 | 46 | ||
| Age (years) | 71.9 ± 6.1 | 75.3 ± 5.2 | 0.009 | 70.0 ± 7.9 | 73.1 ± 6.9 | |
| Sex (Female %) | 50% | 33% | 52% | 51% | ||
| Education (years) | 16.8 ± 2.6 | 16.1 ± 2.4 | 16.4 ± 2.5 | 16.0 ± 3.0 | ||
| APOE ε4 (carrier %) | 14% | 51% | 0.001 | 22% | 63% | 0.002 |
| MMSE | 29.2 ± 1.0 | 28.9 ± 1.0 | 28.5 ± 1.3 | 27.6 ± 2.0 | 0.04 | |
| CDR-SB | 0.04 ± 0.1 | 0.11 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 | ||
| ADAS-Cog | 5.5 ± 2.7 | 6.5 ± 3.1 | 7.0 ± 3.0 | 8.4 ± 3.4 | ||
| Plasma NfL (pg/ml) | 32.1 ± 15.8 | 36.1 ± 12.3 | 30.8 ± 11.3 | 37.7 ± 14.7 | 0.04 | |
| Plasma p-tau181 (pg/ml) | 13.5 ± 10.1 | 18.8 ± 7.7 | 0.01 | 14.5 ± 10.0 | 18.7 ± 7.6 | |
| Plasma Aβ42/Aβ40 | 0.12 ± 0.01 | 0.11 ± 0.01 | <10-6 | 0.13 ± 0.01 | 0.11 ± 0.009 | <10−10 |
CU, cognitively unimpaired elderly; CI, elderly individuals with mild cognitive impairment; APOE, apolipoprotein E; MMSE, Mini-Mental State Examination; CDR-SB, Clinical Dementia Rating—Sum of Boxes; ADAS-Cog, Alzheimer’s Disease Assessment Scale—Cognitive subscale 13-item; NfL, neurofilament light.
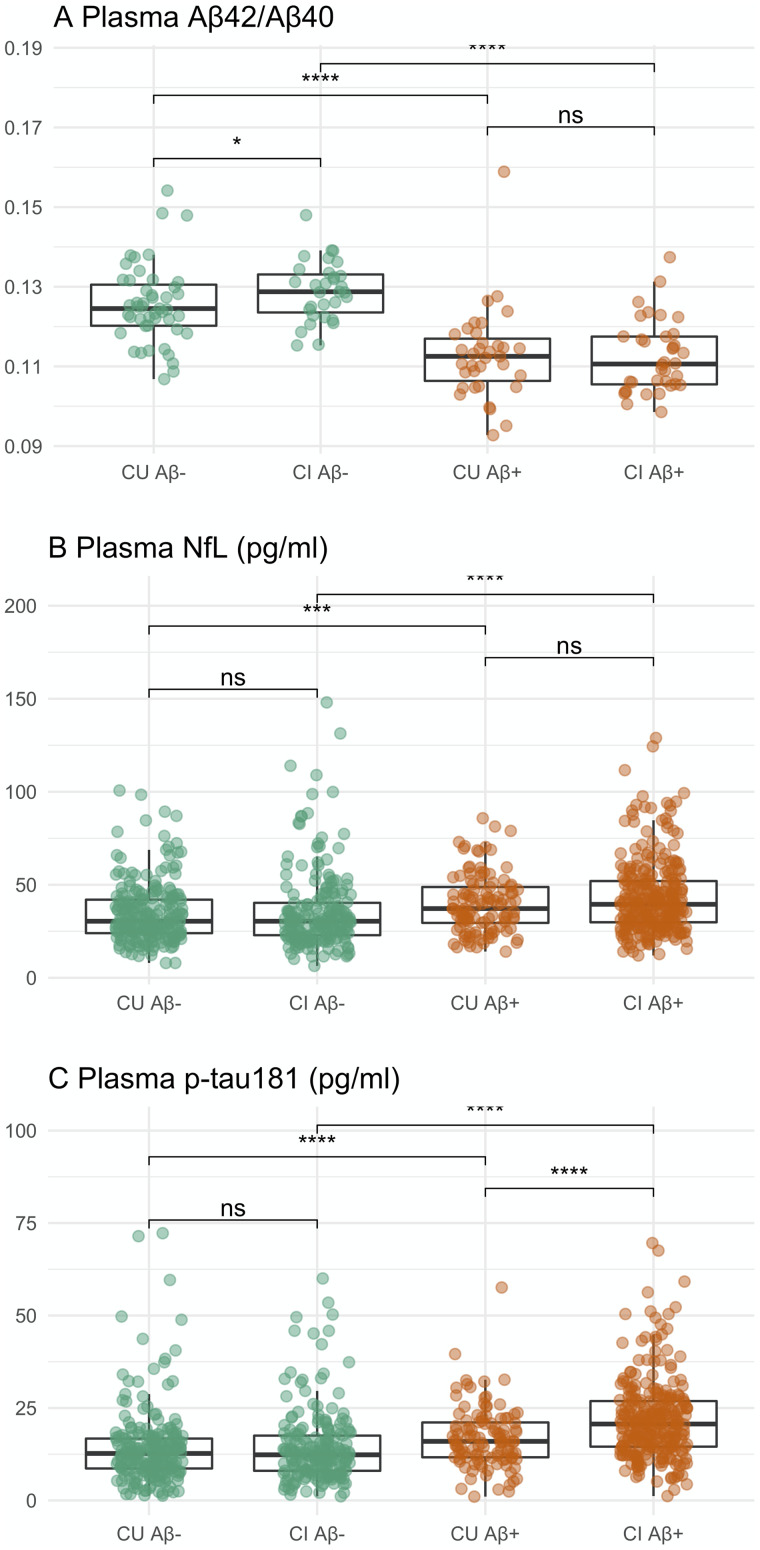
In brief, 33% of CU participants in the main study cohort were PET Aβ+. The frequency of APOE ε4 allele was higher among Aβ+ CUs compared to Aβ− CUs. Compared to Aβ− CUs, Aβ+ CUs were older with fewer females and had significantly fewer years of education, greater CDR-SB and ADAS-Cog scores, as well as greater plasma NfL levels (Fig. 1). Plasma p-tau181 levels were marginally higher in Aβ+ CUs compared to Aβ− CUs (P = 0.057). When controlled for age differences, Aβ− CUs and Aβ+ CUs did not differ in ADAS-Cog scores and plasma NfL levels. Demographic and clinical characteristics of CUs in the plasma Aβ42/Aβ40 sub-cohort did not differ from those of the main study CUs. Within the plasma Aβ42/Aβ40 sub-cohort, Aβ+ CUs had lower plasma Aβ42/Aβ40 compared to Aβ− CUs (Fig. 1; P < 10−6).
Figure 1.
Plasma (A) Aβ42/Aβ40, (B) NfL concentrations and (C) p-tau181 concentrations categorized by clinical diagnosis and CSF Aβ-positivity. Plasma Aβ42/Aβ40 data was available for 173 individuals (Aβ− CU, n = 50; Aβ+ CU, n = 37; Aβ− CI, n = 40; Aβ+ CI, n = 46). Plasma p-tau181 and NfL data included 852 individuals (Aβ− CU, n = 224; Aβ+ CU, n = 109; Aβ− CI, n = 230; Aβ+ CI, n = 289). Unpaired two-samples t-test uncorrected significance levels at ****P < 0.00001; ***P < 0.0001; **P < 0.001; ns: P ≥ 0.5. CU, cognitively unimpaired elderly; CI, elderly individuals with mild cognitive impairment.
In total, 57% of CI participants in the main study cohort were PET Aβ+. Aβ+ CIs were older than Aβ− CIs with fewer years of education and a higher frequency of APOE ε4 allele. Compared to Aβ− CIs, Aβ+ CIs had greater clinical symptoms, with lower MMSE and higher CDR-SB and ADAS-Cog scores. Aβ+ CIs had significantly higher plasma p-tau181 and plasma NfL levels than Aβ− CIs (Fig. 1). Aβ− versus Aβ+ CI group differences in clinical scores and plasma levels were significant after controlling for age differences. Compared to the CIs in the main study cohort, CIs in the plasma Aβ42/Aβ40 sub-cohort had lower symptom severity (i.e. mean CDR-SB of 1.4 versus 0.7 with P < 10−15 and mean ADAS-Cog of 9.2 versus 7.8 with P = 0.002) and lower plasma NfL levels (39.5 versus 34.5 pg/ml with P = 0.01). Within the plasma Aβ42/Aβ40 sub-cohort, Aβ+ CIs had significantly lower plasma Aβ42/Aβ40 compared to Aβ− Cis (Fig. 1; P < 10−10).
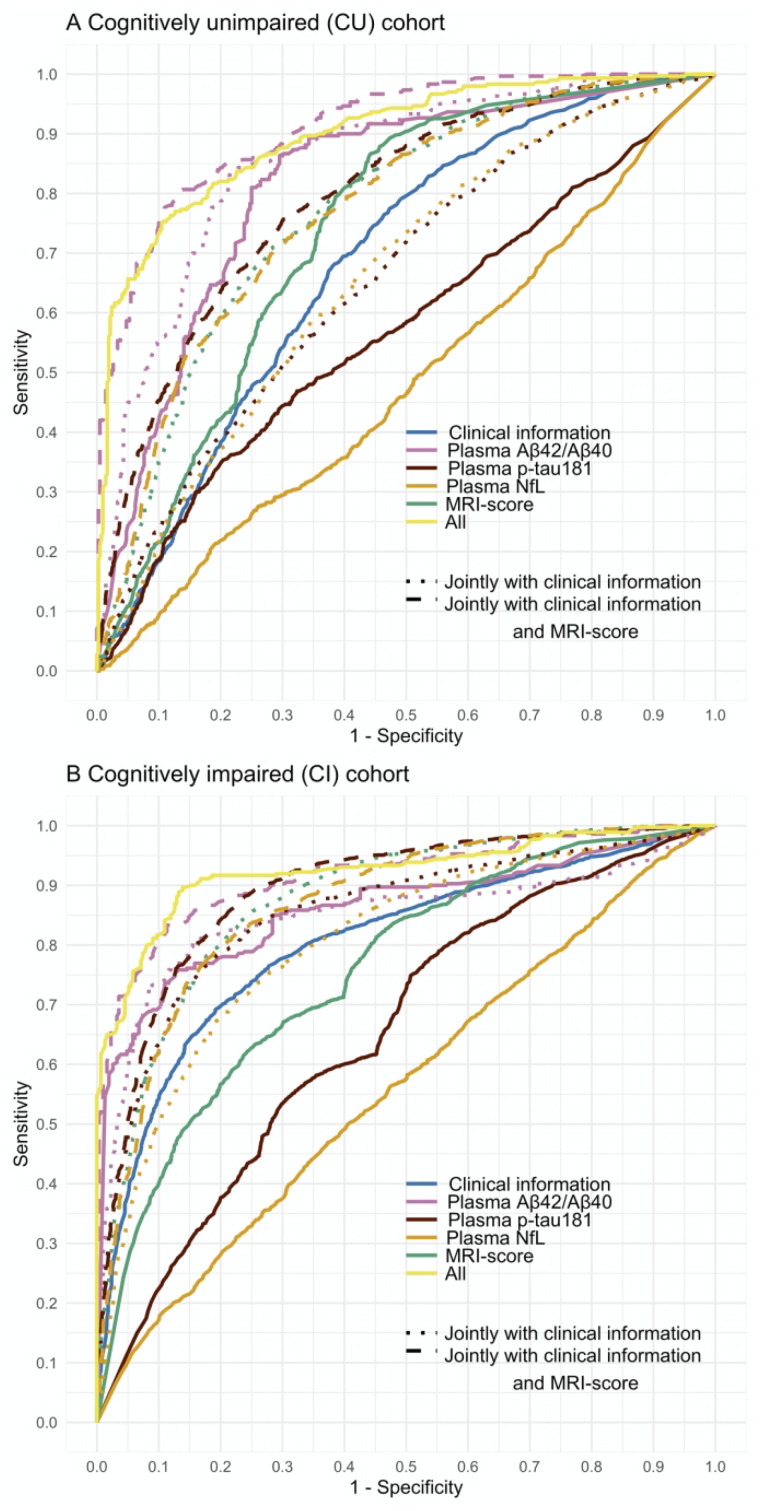
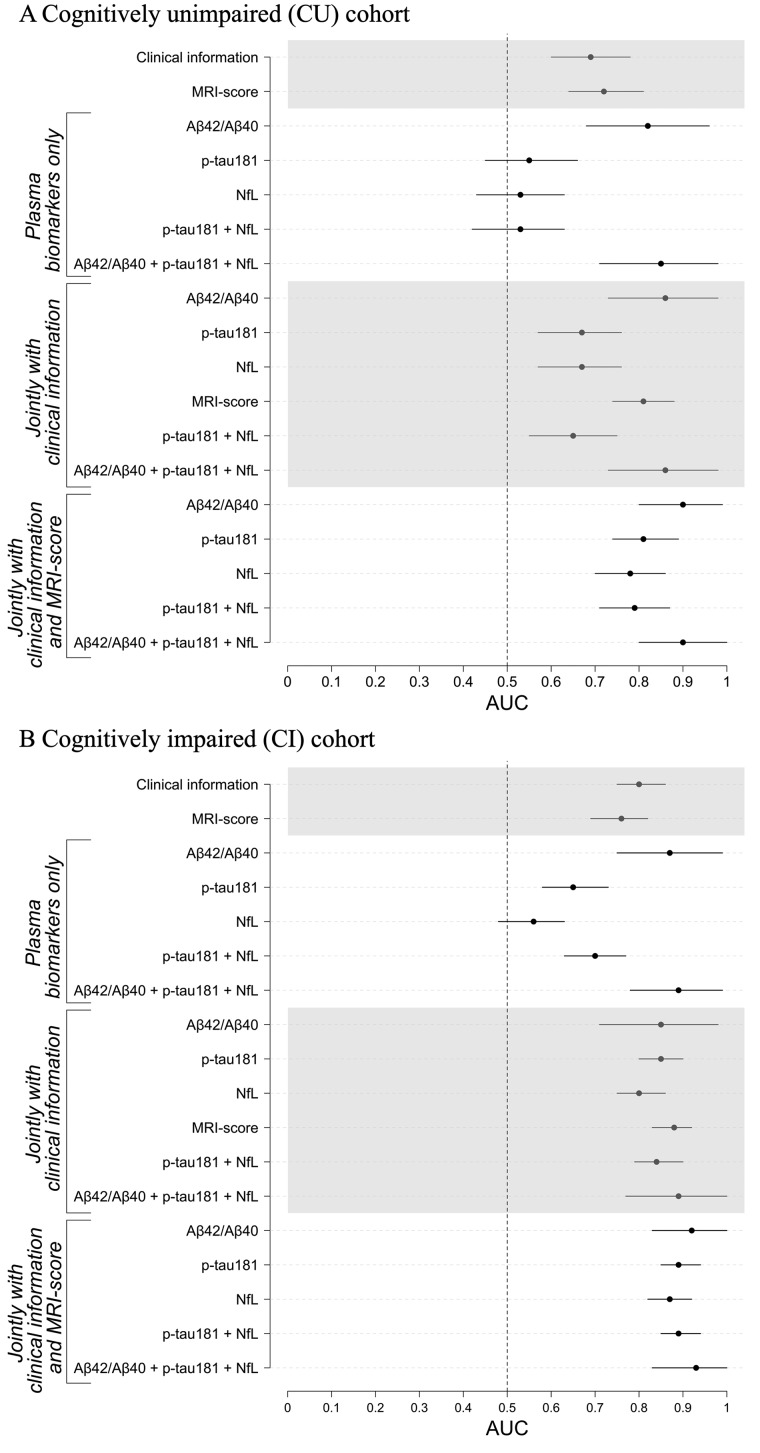
Performance of classifiers differentiating Aβ+ and Aβ− CU participants is shown and summarized in Figs. 2a and 3a and Table 2. A classifier constructed with only clinical information (i.e. demographics, APOE ε4 carrier status and global cognitive assessments) and a classifier constructed with only the MRI-score had similar performances (i.e. DeLong P = 0.06) with an accuracy of 67–68% in differentiating Aβ+ CUs and Aβ− CUs (Supplementary Fig. 4). Of these two classifiers, the MRI-score yielded better AUC (0.74 versus 0.69) reflected in higher NPV of MRI-score (76% versus 68%) and poor sensitivity of clinical information (3% versus 46%). When considered alone and together, plasma p-tau181 and plasma NfL did not differentiate Aβ+ and Aβ− CUs better than chance (Table 2; column (A)). In contrast, plasma Aβ42/Aβ40 alone differentiated Aβ+ CUs from Aβ− CUs with an accuracy of 72%, a PPV of 69% and an NPV of 76%, yielding an AUC of 0.80. The overall performance of plasma Aβ42/Aβ40 only classifier was similar to the performance of a classifier using MRI score and clinical information jointly (i.e. AUC of 0.80; DeLong P = 0.53), with plasma Aβ42/Aβ40 having slightly better PPV (69% versus 65%), whereas the multi-disciplinary MRI score and clinical information jointly having slightly better accuracy (i.e. 75% versus 72%) and NPV (i.e. 78% versus 76%). All three plasma biomarkers jointly differentiated Aβ+ CU and Aβ− CU at an improved accuracy of 77%, a PPV of 77% and an NPV of 80%, yielding an AUC of 0.83, but this was not significantly different than the performance of plasma Aβ42/Aβ40 alone classification (DeLong P = 0.09).
Figure 2.
Receiver-operating characteristic (ROC) analysis of Aβ positivity prediction in an ADNI cohort of (A) cognitively unimpaired (CU) and (B) cognitively impaired (CI) elderly individuals. Optimized ROC curves for classifiers constructed separately and jointly with demographic information (age, sex and years of education), APOE, clinical scores, plasma biomarkers (Aβ42/Aβ40, p-tau181 and NfL), and structural MRI-score when predicting Aβ-positivity using florbetapir PET as the ground truth in the ADNI study (n = 333 CUs and n = 519 CIs). To assess the added value of each class of variables (i.e. clinical, plasma and MRI classes), additional RF classifiers were constructed from (i) each plasma marker alone, (ii) each plasma marker jointly with clinical features, (iii) MRI-score jointly with clinical features and (iv) each plasma marker jointly with clinical features and MRI-score. Models including plasma Aβ42/Aβ40 were tested and validated in a cohort of n = 87 CUs and n = 86 CIs due to limited availability of plasma Aβ42/Aβ40 data. Error bars indicate union of 95% CIs from cross-validation iterations.
Figure 3.
Classifier performance metrics of Aβ positivity prediction in (A) cognitively unimpaired (CU) individuals and B) individuals with mild cognitive impairment (CI). Area under the curve (AUC) estimates with ±2 × standard variation error bars from cross-validation iterations are shown for classifiers constructed separately and jointly with demographic information (age, sex and years of education), APOE, clinical scores, plasma biomarkers (Aβ42/Aβ40, p-tau181 and NfL) and structural MRI-score when predicting Aβ-positivity using florbetapir PET as the ground truth in the ADNI study (n = 333 CUs and n = 519 CIs). To assess the added value of each class of variables (i.e. clinical, plasma and MRI classes), additional RF classifiers were constructed from (i) each plasma marker alone, (ii) each plasma marker jointly with clinical features, (iii) MRI-score jointly with clinical features and (iv) each plasma marker jointly with clinical features and MRI-score. Models including plasma Aβ42/Aβ40 were tested and validated in a cohort of n = 87 CUs and n = 86 CIs due to limited available of plasma Aβ42/Aβ40 data. Error bars indicate union of 95% CIs from cross-validation iterations.
Table 2.
Performance of classifier models in classifying Aβ+ CU individuals
| (A) Plasma biomarkers |
(B) Clinical information with and without plasma biomarkers |
(C) MRI—score with and without clinical information and plasma biomarkers |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUCa | Acc | PPV | NPV | Sens | Spec | AUCa | Acc | PPV | NPV | Sens | Spec | AUCa | Acc | PPV | NPV | Sens | Spec | |
| MRI score | 0.74 [0.66, 0.82] | 0.67 ± 0.04 | 0.48 ± 0.06 | 0.76 ± 0.03 | 0.46 ± 0.11 | 0.77 ± 0.04 | ||||||||||||
| Clinical informationb | 0.69 [0.60, 0.78] | 0.68 ± 0.01 | 0.45 ± 0.28 | 0.68 ± 0.01 | 0.03 ± 0.03 | 0.98 ± 0.01 | 0.80 [0.72, 0.87] | 0.75 ± 0.02 | 0.65 ± 0.06 | 0.78 ± 0.02 | 0.48 ± 0.09 | 0.88 ± 0.05 | ||||||
| Aβ42/Aβ40 | 0.80 [0.65, 0.94] | 0.72 ± 0.07 | 0.69 ± 0.12 | 0.76 ± 0.08 | 0.64 ± 0.18 | 0.77 ± 0.13 | 0.86 [0.73, 0.98] | 0.79 ± 0.05 | 0.77 ± 0.08 | 0.81 ± 0.06 | 0.71 ± 0.11 | 0.84 ± 0.07 | 0.90 [0.80, 1.00] | 0.83 ± 0.04 | 0.84 ± 0.08 | 0.83 ± 0.05 | 0.74 ± 0.10 | 0.89 ± 0.06 |
| p-tau181 | 0.55c [0.45, 0.66] | 0.62 ± 0.02 | 0.39 ± 0.04 | 0.71 ± 0.02 | 0.37 ± 0.07 | 0.73 ± 0.05 | 0.69 [0.60, 0.78] | 0.69 ± 0.01 | 0.58 ± 0.29 | 0.69 ± 0.01 | 0.07 ± 0.06 | 0.98 ± 0.03 | 0.80 [0.73, 0.88] | 0.76 ± 0.03 | 0.69 ± 0.07 | 0.78 ± 0.02 | 0.46 ± 0.06 | 0.90 ± 0.04 |
| NfL | 0.54c [0.44, 0.64] | 0.57 ± 0.03 | 0.31 ± 0.05 | 0.68 ± 0.02 | 0.31 ± 0.08 | 0.69 ± 0.04 | 0.68 [0.59, 0.77] | 0.68 ± 0.01 | 0.51 ± 0.33 | 0.68 ± 0.01 | 0.03 ± 0.02 | 0.98 ± 0.02 | 0.79 [0.71, 0.87] | 0.74 ± 0.03 | 0.64 ± 0.06 | 0.78 ± 0.02 | 0.45 ± 0.06 | 0.88 ± 0.04 |
| p-tau181 + NfL | 0.53c [0.42, 0.63] | 0.60 ± 0.04 | 0.34 ± 0.07 | 0.69 ± 0.02 | 0.26 ± 0.08 | 0.76 ± 0.05 | 0.65 [0.56, 0.75] | 0.69 ± 0.03 | 0.53 ± 0.10 | 0.72 ± 0.02 | 0.25 ± 0.08 | 0.90 ± 0.04 | 0.80 [0.72, 0.88] | 0.76 ± 0.03 | 0.66 ± 0.08 | 0.80 ± 0.02 | 0.54 ± 0.08 | 0.87 ± 0.05 |
| Aβ42/Aβ40 + p-tau181 + NfL | 0.83 [0.68, 0.97] | 0.77 ± 0.06 | 0.77 ± 0.13 | 0.80 ± 0.06 | 0.70 ± 0.12 | 0.83 ± 0.14 | 0.85 [0.72, 0.98] | 0.81 ± 0.04 | 0.82 ± 0.08 | 0.83 ± 0.06 | 0.73 ± 0.11 | 0.87 ± 0.08 | 0.91 [0.81, 0.99] | 0.82 ± 0.06 | 0.90 ± 0.08 | 0.79 ± 0.07 | 0.63 ± 0.14 | 0.95 ± 0.04 |
To assess the added value of each class of variables (i.e. clinical, plasma and MRI classes), additional RF classifiers were constructed from (i) each plasma marker alone, (ii) each plasma marker jointly with clinical features, (iii) MRI-score jointly with clinical features and (iv) each plasma marker jointly with clinical features and MRI-score.
95% Confidence intervals.
Demographics: Age, sex, years of education and APOE ε4 status; Global cognitive assessments: MMSE, ADAS-Cog and CDR–SB.
The confidence interval includes the axis y = x, suggesting that the classifier was not better than chance.
When combined with clinical information (Table 2; column (B)), the predictive performance of the plasma p-tau181 and plasma NfL improved but not beyond the performance of the classifier constructed from clinical information alone (i.e. DeLong P = 0.18 and P = 0.08, respectively). Adding clinical information to the plasma Aβ42/Aβ40 classifier yielded better differentiation of Aβ+ CU and Aβ− CU cases with an accuracy of 79%, PPV of 77%, NPV of 81% and an AUC 0.86, with the greatest improvement in the PPV compared to plasma Aβ42/Aβ40 only and clinical information only classifiers. Further enhancing plasma NfL and plasma p-tau181 with the MRI score in addition to the clinical information improved classification accuracy by 5–8%, PPV by 13–22%, NPV by 8–11% and AUC by 0.10–0.14 (DeLong P < 10−14 and P < 10−21, respectively) but this was not better than the classifier constructed with the MRI-score and clinical information (i.e. DeLong P = 0.08 and P = 0.46, respectively) or the classifier based on plasma Aβ42/Aβ40 only (i.e. DeLong P = 0.07 and P = 0.88, respectively) as reported in Table 2 (column (C)). Of the three plasma biomarkers, Aβ42/Aβ40 in combination with the MRI-score and clinical information performed the best with an accuracy of 83% and AUC of 0.90, with a well-balanced PPV of 84% and NPV of 83%, which was significantly better than the performance of Aβ42/Aβ40 alone (i.e. DeLong P < 10−4) or in combination with clinical information (i.e. DeLong P = 0.02).
The full classifier model including all three plasma biomarkers, the MRI-score and clinical information had an accuracy of 82%, with a PPV of 90% and NPV of 79%. However, this was not significantly different from the classifier model with plasma Aβ42/Aβ40, MRI-score and clinical information (DeLong P = 0.61), suggesting minimal added value of plasma NfL and plasma p-tau181. The most significant variables in a decreasing order of importance based on mean decrease in accuracy analysis were plasma Aβ42/Aβ40, MRI-score, APOE ε4 status, MMSE, years of education and sex.
Performance of classifiers differentiating Aβ+ and Aβ− CI participants is shown and summarized in Figs. 2b and 3b and Table 3. Both clinical information-based and MRI-score-based classifiers were performed moderately well in differentiating Aβ+ and Aβ− CIs with an AUC of 0.81 and 0.76, accuracy of 74% and 67%, PPV of 76% and 70% and NPV of 73% and 63% (Supplementary Fig. 4), respectively. The MRI-score together with clinical information achieved an AUC of 0.88, with an accuracy of 81%, PPV of 82% and NPV of 80%, performing significantly better than clinical information only (DeLong P < 10−15) or MRI-score only (DeLong P < 10−39) models. In contrast to CU data, both plasma Aβ42/Aβ40 and plasma p-tau181, but not plasma NfL, separately detected Aβ-positivity in CIs with an average accuracy of 77% and 58%, PPV of 79% and 63%, NPV of 76% and 52%, yielding AUCs of 0.87 and 0.64, respectively. Enhancement with clinical information improved performance metrics of plasma p-tau181 and NfL, but not plasma Aβ42/Aβ40, classifiers by 15–23% (Table 3; column (B)). Plasma p-tau181 enhanced with clinical information perform similarly to plasma Aβ42/Aβ40. When further enhanced with the MRI-score in addition to the clinical information, classifier performance metrics for both plasma p-tau181 and plasma NfL increased by an additional 3–8%, with plasma p-tau181 performing slightly better with an accuracy of 82%, PPV of 83% and NPV of 82% (Table 3; column (C)). Similarly, the MRI-score enhanced classification performance of plasma Aβ42/Aβ40 more than clinical information (DeLong P < 10−4), reaching an AUC of 0.94 with an accuracy of 86%, PPV of 86% and NPV of 88%. The full classifier model, including all three plasma biomarkers, MRI-score, and clinical information achieved an AUC of 0.92 and an accuracy of 86%, with a PPV of 88% and NPV of 86%. This was not significantly different than the classifier model with plasma Aβ42/Aβ40, MRI-score and clinical information (DeLong P = 0.31), suggesting minimal added value of plasma NfL and plasma p-tau181. The most significant variables in a decreasing order of importance based on mean decrease in accuracy analysis were plasma Aβ42/Aβ40, MRI-score, APOE ε4 allele, age and CDR-SB.
Table 3.
Performance of classifier models in differentiating Aβ+ individuals with mild CI
| (A) Plasma biomarkers |
(B) Clinical information with and without plasma biomarkers |
(C) MRI-score with and without clinical information and plasma biomarkers |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUCa | Acc | PPV | NPV | Sens | Spec | AUCa | Acc | PPV | NPV | Sens | Spec | AUCa | Acc | PPV | NPV | Sens | Spec | |
| MRI score | 0.76 [0.70, 0.82] | 0.67 ± 0.02 | 0.70 ± 0.02 | 0.63 ± 0.03 | 0.72 ± 0.03 | 0.61 ± 0.04 | ||||||||||||
| Demographics + Clinical b | 0.81 [0.75, 0.87] | 0.74 ± 0.02 | 0.76 ± 0.02 | 0.73 ± 0.03 | 0.81 ± 0.03 | 0.66 ± 0.05 | 0.88 [0.83, 0.92] | 0.81 ± 0.02 | 0.82 ± 0.03 | 0.80 ± 0.03 | 0.85 ± 0.03 | 0.76 ± 0.04 | ||||||
| Aβ42/Aβ40 | 0.87 [0.75, 0.99] | 0.77 ± 0.06 | 0.79 ± 0.07 | 0.76 ± 0.08 | 0.79 ± 0.08 | 0.75 ± 0.10 | 0.85 [0.71, 0.99] | 0.79 ± 0.05 | 0.81 ± 0.10 | 0.77 ± 0.06 | 0.80 ± 0.07 | 0.77 ± 0.14 | 0.94 [0.87, 1.00] | 0.86 ± 0.05 | 0.86 ± 0.07 | 0.88 ± 0.08 | 0.90 ± 0.07 | 0.82 ± 0.1 |
| p-tau181 | 0.64 [0.56, 0.71] | 0.58 ± 0.03 | 0.63 ± 0.02 | 0.52 ± 0.03 | 0.61 ± 0.05 | 0.55 ± 0.05 | 0.85 [0.80, 0.90] | 0.79 ± 0.02 | 0.80 ± 0.02 | 0.78 ± 0.04 | 0.83 ± 0.04 | 0.73 ± 0.03 | 0.90 [0.86, 0.94] | 0.82 ± 0.02 | 0.83 ± 0.01 | 0.82 ± 0.04 | 0.86 ± 0.03 | 0.78 ± 0.02 |
| NfL | 0.56c [0.49, 0.64] | 0.54 ± 0.03 | 0.60 ± 0.02 | 0.48 ± 0.03 | 0.57 ± 0.03 | 0.51 ± 0.04 | 0.81 [0.75, 0.86] | 0.73 ± 0.02 | 0.75 ± 0.03 | 0.71± 0.03 | 0.79 ± 0.04 | 0.66 ± 0.05 | 0.87 [0.83, 0.92] | 0.81 ± 0.02 | 0.82 ± 0.03 | 0.79 ± 0.02 | 0.85 ± 0.03 | 0.75 ± 0.06 |
| p-tau181 + NfL | 0.70 [0.63, 0.77] | 0.66 ± 0.02 | 0.69 ± 0.02 | 0.62 ± 0.02 | 0.72 ± 0.03 | 0.58 ± 0.05 | 0.84 [0.79, 0.89] | 0.77 ± 0.03 | 0.78 ± 0.03 | 0.76 ± 0.04 | 0.83 ± 0.04 | 0.70 ± 0.06 | 0.89 [0.85, 0.93] | 0.82 ± 0.02 | 0.83 ± 0.03 | 0.81 ± 0.03 | 0.86 ± 0.03 | 0.77 ± 0.05 |
| Aβ42/Aβ40 + p-tau181 + NfL | 0.88 [0.76, 0.99] | 0.80 ± 0.05 | 0.81 ± 0.07 | 0.81 ± 0.08 | 0.84 ± 0.09 | 0.76 ± 0.11 | 0.89 [0.78, 1.00] | 0.82 ± 0.06 | 0.85 ± 0.10 | 0.81 ± 0.07 | 0.83 ± 0.07 | 0.82 ± 0.13 | 0.92 [0.82, 1.00] | 0.86 ± 0.05 | 0.88 ± 0.07 | 0.86 ± 0.06 | 0.87 ± 0.05 | 0.86 ± 0.09 |
To assess the added value of each class of variables (i.e. clinical, plasma and MRI classes), additional RF classifiers were constructed from (i) each plasma marker alone, (ii) each plasma marker jointly with clinical features, (iii) MRI-score jointly with clinical features and (iv) each plasma marker jointly with clinical features and MRI-score.
95% Confidence intervals.
Demographics: age, sex, years of education and APOE ε4 status; Clinical assessments: MMSE, ADAS-Cog and CDR-SB.
The confidence interval includes the axis y = x, suggesting that the classifier was not better than chance.
Discussion
The major findings of this multi-centre biomarker study were (i) of the three plasma biomarkers, when considered separately, Aβ42/Aβ40 consistently differentiated PET Aβ-positivity status both in CU and in CI participants, with a slightly better performance in CIs, whereas plasma p-tau181 showed moderate value for differentiating PET Aβ-positivity status in CI participants, and plasma NfL lacked Aβ-positivity stratification value both in CU and in CI participants; (ii) clinical information, dominated by APOE ε4 status and education in CU participants, and by APOE ε4 status and age in CI participants, as well as MRI-score independently differentiated PET Aβ− and Aβ+ both in CU and in CI participants; (iii) clinical information enhanced the performance of plasma biomarkers in differentiating PET Aβ− and Aβ+ participants by 0.06–0.14 units of AUC for CUs, and by 0.21–0.25 units for CIs and (iv) further enhancement of these models with an MRI-score yielded an additional improvement of 0.04–0.11 units of AUC for CUs and 0.05–0.09 units for CIs. Taken together, the results recapitulate plasma Aβ42/Aβ40 as a robust biomarker of brain Aβ-positivity and suggest that when combined with clinical information, plasma p-tau181 and NfL biomarkers, and an MRI-score, could effectively identify Aβ+ individuals with expected greater accuracy in the symptomatic individuals. Interestingly, when the MRI-score is considered in combination with clinical information, plasma p-tau181 and plasma NfL have minimal added value for brain Aβ-positivity stratification in this multi-centre ADNI cohort of CU and CI participants.
Plasma Aβ42/Aβ40 detects PET Aβ-positivity
The first major finding was that plasma Aβ42/Aβ40 was a robust biomarker of PET Aβ-positivity independent of clinical diagnosis, whereas plasma p-tau181 detected PET Aβ-positivity only in CIs with a moderate accuracy, and plasma NfL lacked value for stratification of PET Aβ+ and PET Aβ− cases both in CU and in CI cohorts. It should be noted that this finding was replicated when the modelling and testing of all classifiers were repeated on the plasma Aβ42/Aβ40 sub-cohort to mitigate the potential influence of sample size and sub-cohort characteristics in comparisons of classifiers (Supplementary Fig. 2). Of the three plasma biomarkers considered in this study, Aβ42/Aβ40 has been the most extensively studied in the literature. Recent studies, particularly the ones using highly sensitive mass spectrometry, have repeatedly reported a strong correlation between plasma Aβ42/Aβ40 and the gold-standard CSF and PET Aβ measures (Janelidze et al., 2016; Ovod et al., 2017; Nakamura et al., 2018; Schindler et al., 2019). Consistent with our findings, plasma Aβ42/Aβ40, especially when combined with age and APOE ε4 status, have been shown to accurately stratify Aβ+ individuals (e.g. AUC, 0.80–0.85) in the AD continuum (Palmqvist et al., 2019b; Schindler et al., 2019). The slightly superior performance of plasma Aβ42/Aβ40 in this study (cf. Supplementary Fig. 3) compared to the previous reports of 0.79–0.82 AUC for the detection of Aβ-positivity in CU participants (Fandos et al., 2017; de Rojas et al., 2018; Chatterjee et al., 2019) and 0.90 AUC for CIs (Lin et al., 2019) might be due to high molecular specificity and detection sensitivity of LC-MS/MS technique used to analyse the ADNI plasma samples. This observation is consistent with the notion that the different assays for plasma Aβ42/Aβ40 may have different precision and, in particular, mass spectrometry-based assays compared to immunoassays might be more accurate and robust in measuring levels of plasma Aβ species as biomarker of brain Aβ (Zetterberg, 2019). Another factor contributing to the high performance of the Aβ42/Aβ40 ratio, as compared with single biomarkers, is that between-individual differences in basal ‘total’ Aβ secretion are compensated for in the ratio, by dividing with Aβ40, whereas such differences in plasma NfL and p-tau181 levels, MRI measures or cognitive abilities might introduce variability in these measures.
Plasma p-tau181 presented a more complex picture as a candidate biomarker of brain Aβ-positivity
Assays for the quantification of plasma p-tau181 are very recently developed (Zetterberg and Blennow, 2020) and are still under extensive investigation to fully understand the role of different plasma tau species as peripheral markers of AD pathophysiology. Compared to the limited number of previously reported evaluations of plasma p-tau181 as a biomarker of brain Aβ-positivity in other research and clinical cohorts (Mielke et al., 2018; Palmqvist et al., 2019b; Barthélemy et al., 2020; Janelidze et al., 2020a; Karikari et al., 2020; Thijssen et al., 2020), ADNI plasma p-tau181 levels measured by the Simoa assay differentiated between PET Aβ+ and PET Aβ− ADNI CI participants with an inferior accuracy (AUC, 0.64). Furthermore, this biomarker had no stratification value for PET Aβ-positivity within the ADNI CU participants (AUC, 0.55). The addition of clinical information to this base model increased AUC for the classification of Aβ+ versus Aβ− by 0.14–0.69 in CUs and by 0.21–0.85 in CIs. The subsequent addition of an MRI-score to this model further increased AUC for the classification of Aβ+ versus Aβ− by 0.11–0.80 in CUs and by 0.05–0.90 in CIs, bringing its classification performance to a clinically acceptable level.
Potential sources of the discrepancy between our results and those of other groups may include differences in the plasma analysis assays, diagnostic composition and demographic characteristics of the study cohorts, methodology used to determine ground-truth brain Aβ-positivity status and data analytics. One of the earliest plasma p-tau181 studies on a Meso Scale Discovery (MSD) platform reported that plasma p-tau181 as a good biomarker of the elevated brain Aβ with an AUC of 0.7 in CU and 0.85 in MCI participants in their discovery cohort but this study lacked internal validation or replication in an external validation cohort (Mielke et al., 2018). Another study (Barthélemy et al., 2020) reported high specificity of plasma p-tau181, measured by a highly sensitive mass spectrometry assay, for Aβ plaque pathology in their discovery cohort (n = 34; including clinically diagnosed CU, MCI and AD individuals) and then replicated their findings with an AUC of 0.72 to differentiate Aβ− and Aβ+ individuals in an independent replication cohort of CUs, MCIs and ADs (n = 92) but the performance within CU only or MCI only sub-cohorts was not statistically significant. Similarly, a larger multi-cohort study which included individuals with various clinical diagnoses including CU, MCI and AD reported a stepwise increase in plasma p-tau181 levels, measured on the MSD platform, with both Aβ-positivity and cognitive impairment and achieved an AUC of 0.81 in differentiating Aβ− and Aβ+ individuals, which was increased to 0.84 with the addition of plasma Aβ42/Aβ40 (Janelidze et al., 2020a).
The age of cohort participants may also influence the ability of plasma p-tau181 to detect Aβ-positivity status. For instance, a multi-cohort study used the Simoa assay to measure plasma p-tau181 in four different cohorts (Karikari et al., 2020) and found that plasma p-tau181 biomarker discriminated Aβ+ CU older adults and individuals with CI from Aβ− CU older adults and young adults with an AUC of 0.76–0.88 across cohorts. However, the CU older adults in this study were on average 10 years younger than ADNI participants, raising the question about age-dependent sensitivity of plasma p-tau181 to AD-related Aβ pathology. Similarly, another small cohort study of CU and CI participants, who were on average 13 years younger than ADNI participants, reported an excellent AUC of 0.86 in CU and 0.94, although not internally validated or replicated in an external cohort, in differentiating PET Aβ+ and PET Aβ− CIs with plasma p-tau181 levels (Thijssen et al., 2020). It is highly likely that younger Aβ+ participants might have greater pathophysiological changes than the older ADNI participants in response to Aβ toxicity, which might be a driving factor for increased plasma p-tau181 levels. Indeed, it is well established that younger individuals who are Aβ+ have more brain tau deposition than older individuals who are Aβ+ (Schöll et al., 2017). Furthermore, previous studies found that the strong correlations between plasma p-tau181 and Aβ PET are often in the Aβ+ but not in Aβ− individuals (Janelidze et al., 2020a) and that increased plasma p-tau181 levels might be initiated by accumulation of Aβ beyond the positivity threshold, and continue to increase as Aβ further accumulates in the brain even during early stages of tau pathology as measured by Braak & Braak staging at autopsy or tau PET during life (Janelidze et al., 2020a; Karikari et al., 2020). Evidence from these recent studies together with the stronger association of plasma p-tau181 with brain Aβ burden in younger cohorts might suggest that plasma p-tau181 is unlikely to be a direct measure of Aβ pathology but instead a marker of tau pathology. Our finding that plasma p-tau181 has moderate stratification accuracy for PET Aβ-positivity only at the symptomatic disease stage suggests that p-tau181 detects Aβ-positivity only once a significant tau pathology, which is closely associated with symptoms, is detectable.
Plasma NfL was a poor biomarker of PET Aβ-positivity
The relatively poor performance of plasma NfL in differentiating Aβ+ and Aβ− ADNI individuals, either symptomatic or asymptomatic, is largely consistent with the previous literature. Previous studies found no evidence that plasma NfL was related to Aβ or tau pathology as measured by PET or even synaptic dysfunction as measured by fluorodeoxyglucose-PET imaging, repeatedly emphasizing that plasma NfL is more likely to be a marker of all cause neurodegeneration (Mattsson et al., 2019; Mielke et al., 2019; Janelidze et al., 2020a; Thijssen et al., 2020). Finally, our finding that plasma p-tau181 and plasma NfL did not improve Aβ-positivity stratification accuracy above and beyond the plasma Aβ42/Aβ40 was consistent with the previous studies on other AD research cohorts (Palmqvist et al., 2019b).
Clinical information and MRI-score independently differentiated PET Aβ+ and Aβ− ADNI individuals
To date, the most common candidate predictors considered for Aβ-positivity were age, APOE genotype and measures of cognition, largely because they are easier to collect with widely available standardized protocols. Of these, age has been the most common predictor of elevated brain Aβ followed by the APOE genotype (reviewed in Ashford et al. (2021)), consistent with the notion that after advanced age, APOE ε4 genotype is a major risk factor for developing AD (Payami et al., 1997). Consistent with the prior knowledge, age and APOE genotype were important predictors of Aβ-positivity for ADNI CU and CI participants (cf. Supplementary Fig. 3). In the main analyses, we observed that the ability of clinical information to differentiate Aβ+ and Aβ− participants improved, especially in terms of sensitivity, with increasing severity of clinical diagnosis. Indeed, measures of global cognition, such as MMSE and CDR-SB, had greater influence in the classifier model for Aβ-positivity within the CI participants. Consistent with our findings, accumulating evidence suggests that elevated Aβ is associated with risk of cognitive worsening and may indicate a pre-symptomatic stage of disease (Roe et al., 2013; Donohue et al., 2017). As the relationships between cognition and AD biomarkers are expected to be subtle, the global measures of cognition may have insufficient sensitivity among CUs to reliable detect pre-symptomatic expression of Aβ pathology, as reflected in our results with extremely low sensitivity of clinical information in detecting Aβ-positivity in CUs.
MRI-score of brain Aβ alone stratified Aβ+ and Aβ− participants with an AUC of 0.74 in ADNI CUs and an AUC of 0.76 in ADNI CIs with a substantially increased sensitivity. When combined with clinical information, MRI-score performed as well as, or, in CIs, even better than, the best performing plasma biomarker, Aβ42/Aβ40. Although structural T1-weighted MRI is not a molecular imaging modality directly targeting quantification of protein accumulation in the brain, MRI has been a gold standard for neurodegeneration (Jack et al., 2004). The evidence for a relationship between Aβ deposition and neurodegeneration has been previously demonstrated in very early AD and even in asymptomatic individuals (Bourgeat et al., 2010; Chételat et al., 2010). In a similar manner to plasma p-tau181, the value of the MRI-score for Aβ-positivity might be a reflection of neurodegenerative processes due to Aβ toxicity, yet we observed that the MRI-score outperformed the plasma p-tau181. The brain Aβ deposition has a spatially distinct signature of cortical atrophy (Bourgeat et al., 2010; Chételat et al., 2010; Tosun et al., 2011) and MRI-based correlates of brain Aβ deposition compared to plasma analytes might have the advantage of capturing this spatial information. Furthermore, although structural T1-weighted imaging has been traditionally considered to reveal fat and water distribution and distinguish tissue types, cellular changes associated with neuropathology might also influence the MRI contrast as well as the MRI intensity quality, such as the grey value distribution, texture features and spatial heterogeneity (Sørensen et al., 2016; Feng et al., 2019; Ranjbar et al., 2019). Our results also suggest that deep learning, the computational approach used in this study to construct MRI-scores, might efficiently quantify Aβ toxicity from structural MRI because of its high automatic feature learning and visual pattern recognition abilities (LeCun et al., 2015).
Both clinical information and MRI-score enhanced performance of plasma biomarkers in identifying PET Aβ-positivity
One interesting observation was that although when combined with clinical information and MRI-score, plasma p-tau181 and NfL biomarkers could effectively identify Aβ+ symptomatic individuals, plasma p-tau181 and plasma NfL did not contribute to the detection of brain Aβ above and beyond the classification power of clinical information and MRI-score jointly, particularly in CUs. This is a particularly important criterion in the selection of candidate cost-effective and rapid screening tools for broad implementation in clinical and drug trial settings. Demographics and global cognitive measures are an integral part of the clinical assessment. MRI has long played a role in inclusion and exclusion criteria in patient recruitment and ruling out other causes of cognitive symptoms (Frisoni et al., 2010). Furthermore, MRI has been routinely acquired in clinical trials to identify and monitor adverse events (Cash et al., 2014). Plasma biomarkers, therefore, should have a classification ability as good as or better than clinical information and MRI separately and in combination in order to be a practical non-invasive screener.
Our results in this ADNI study, although limited to CU and CI participants, suggest that plasma Aβ42/Aβ40 but not plasma p-tau181 and plasma NfL might have added value in screening for brain Aβ-positivity. It is also important to emphasize that plasma assays target brain-derived proteins that are present at extremely low concentrations in the peripheral circulation and originate not only in the brain but almost all peripheral cells (Roher et al., 2009). What plasma Aβ measures mean biologically and to what extent the variances seen in plasma Aβ levels reflect brain pathology especially in the CU and CI clinical groups in which greater heterogeneity in comorbid conditions is expected are questions still warrant further investigations. These limitations may make the use of the plasma Aβ biomarkers to predict the AD pathology more difficult at the individual level. Despite the inferior performance of plasma p-tau181 in detecting AD Aβ-positivity observed in this ADNI cohort, this biomarker may have different utility. Plasma p-tau181 can be robustly measured in plasma and is highly specific for AD pathology (Mielke et al., 2018), making it an attractive screening tool for brain Aβ and tau pathologies jointly as required for A/T/N biomarker profiling (Jack et al., 2018) linked to differential trajectories of disease progression (Altomare et al., 2019; Jack et al., 2019; Ebenau et al., 2020). Further studies are warranted to better understand the behaviour of plasma p-tau181 as a biomarker of the burden of the disease at different disease stages (Lantero Rodriguez et al., 2020). Given that Aβ-positivity assessment using either CSF or PET is independent of clinical diagnosis, clinical stage-dependent classifier performance might be a concern if these plasma biomarkers are operationalized in clinical practice. In our analysis, a similar clinical diagnosis-dependent gradual increase in classification performance was observed in Aβ-positivity classifier constructed with clinical information and to a lesser extent with MRI-score.
Study design-specific considerations
There are multiple strengths to the study including the large sample size, well-characterized participants, and availability of plasma analytes, Aβ PET imaging and structural MRI, all assessed within a short period of time. A limitation of this in vivo study was the use of Aβ PET as the gold standard for brain Aβ-positivity rather than the true gold standard of neuropathology. A limitation of plasma analyte comparisons is that different techniques were used, namely Simoa for p-tau181 and NfL and LC-MS/MS for Aβ42/Aβ40. Despite the superior specificity, mass spectrometry has the disadvantage of being more expensive and requiring more expertise than immunoassays, which are easily analysed by laboratories that routinely run blood tests. Another limitation of the study is the potential pre-analytical variability since the blood samples were collected at multiple ADNI sites. Although the collection site as a categorical variable had no significant effect on ADNI plasma levels, multi-centre studies of plasma analytes still require further investigation for standardization of protocols to reduce measurement variability (Rozga et al., 2019). We should also note that this study was limited to plasma p-tau181. Other blood immunoassays targeting tau species, specifically the very recently reported plasma pTau-217, might be promising biomarkers for AD Aβ pathology (Janelidze et al., 2020b). Finally, we should further emphasize that this study is based on a convenience cohort where the degree of true population representation is not known. Most notable, about 47% of CU and 19% of CI ADNI participants who were CSF p-tau positive were PET Aβ−, suggesting non-AD aetiology of their tau pathology that might have particularly impacted the observed plasma p-tau181 levels (Benussi et al., 2020). Additionally, the PPV and NPV performance of the classifier models considered in this study was limited by the prevalence of the PET Aβ-positivity in the selected ADNI cohort and may not be directly comparable to other studies with different PET Aβ-positivity prevalence.
Conclusion
In summary, in vivo gold standard for brain Aβ burden assessment is currently Aβ PET or lumbar puncture for CSF Aβ42 (Tapiola et al., 2009; Palmqvist et al., 2016). The widespread acceptance of biomarker classification scheme for the AD continuum (Jack et al., 2018) has ignited interest in more affordable and accessible approaches to detect AD Aβ pathology, a process that often slows down the recruitment into, and adds to the cost of, clinical trials. To this end, our systematic comparison of Aβ-positivity stratification models that use minimally invasive and low-cost measures identified demographics, APOE genotype, global cognitive measures, MR imaging, plasma Aβ measures, plasma p-tau181 and plasma NfL biomarkers, some alone and some in combination, as promising Aβ-positivity classifiers. Advances in ultrasensitive assays for plasma analytes as well as in computational classifier techniques combining multidisciplinary information further promise reduce the difficulty and cost of screening participants with AD Aβ pathology.
Supplementary Material
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is co-ordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data is disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding
This work is funded by the National Institutes of Health Grant U01 AG024904.
Conflicts of interest
Dr Jack serves on an independent data monitoring board for Roche, has consulted for and served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic.
Dr Saykin reports grants from NIH Grants, non-financial support from Eli Lilly/Avid Radiopharmaceuticals, other from Bayer Oncology, grants and other from Arkley BioTek, personal fees and other from Springer Nature, outside the submitted work.
Dr Shaw reports grants from NIA, during the conduct of the study.
Dr Jagust reports personal fees from Genentech, personal fees from Biogen, personal fees from Novartis, personal fees from Bioclinica, personal fees from Grifols, personal fees from Curasen, outside the submitted work.
Dr Aisen reports grants from Janssen, grants from NIA, grants from FNIH, grants from Alzheimer’s Association, grants from Eisai, personal fees from Merck, personal fees from Biogen, personal fees from Roche, personal fees from Lundbeck, personal fees from Proclara, personal fees from Immunobrain Checkpoint, outside the submitted work.
Dr Zetterberg has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Dr Blennow has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Dr Bateman co-founded C2NDiagnostics. Washington University and RJB have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labelling kinetics and blood plasma assay) licenced by Washington University to C2N Diagnostics. Dr Bateman receives income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with Dr Bateman as coinventor, has submitted the US provisional patent application ‘Plasma Based Methods for Detecting CNS Amyloid Deposition’. Dr Bateman consults for Roche, Genentech, AbbVie, Pfizer, Boehringer-Ingelheim and Merck.
Glossary
- A
β = β−amyloid
- AD =
Alzheimer’s disease
- ADAS-Cog =
Alzheimer's Disease Assessment Scale—Cognitive subscale
- ADNI =
Alzheimer’s Disease Neuroimaging Initiate
- AUC =
area under curve
- CDR-SB =
Clinical Dementia Rating—Sum of Boxes
- CI =
cognitively impaired
- CSF =
cerebrospinal fluid
- CU =
cognitively unimpaired
- LC-MS/MS =
liquid chromatography tandem mass spectrometry
- LONI =
Laboratory of Neuro Imaging
- MMSE =
Mini–Mental State Examination
- MSD =
Meso Scale Discovery
- NfL =
neurofilament light
- NPV =
negative predictive value
- p-tau181 =
phosphorylated-tau at threonine-181
- PPV =
positive predictive value
- RF =
random forest
- Simoa =
Single molecule array
- SUVR =
standardized uptake value ratio
Data used in preparation of this article was obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
References
- Aisen PS. Editorial: failure after failure. What next in AD drug development? J Prev Alzheimers Dis 2019; 6: 150. [DOI] [PubMed] [Google Scholar]
- Altomare D de Wilde A Ossenkoppele R Pelkmans W Bouwman F Groot C, et al. Applying the ATN scheme in a memory clinic population. The ABIDE Project 2019; 93: e1635–46. [DOI] [PubMed] [Google Scholar]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 2020; 16: 391–460. [DOI] [PubMed] [Google Scholar]
- Ansart M Epelbaum S Gagliardi G Colliot O Dormont D Dubois B, for the Alzheimer’s Disease Neuroimaging Initiative and the INSIGHT-preAD study, et al. Reduction of recruitment costs in preclinical AD trials: validation of automatic pre-screening algorithm for brain amyloidosis. Stat Methods Med Res 2020; 29: 151–64. [DOI] [PubMed] [Google Scholar]
- Ashford MT Veitch DP, Neuhaus J, Nosheny RL, Tosun D Weiner MW. The search for a convenient procedure to detect one of the earliest signs of Alzheimer’s disease: a systematic review of the prediction of brain amyloid status. Alzheimers Dement 2021; 1–22. doi: 10.1002/alz.12253. [DOI] [PubMed]
- Ba M Ng KP Gao X Kong M Guan L Yu L, The Alzheimer’s Disease Neuroimaging Initiative. The combination of apolipoprotein E4, age and Alzheimer’s Disease Assessment Scale—Cognitive Subscale improves the prediction of amyloid positron emission tomography status in clinically diagnosed mild cognitive impairment. Eur J Neurol 2019; 26: 733–e53. [DOI] [PubMed] [Google Scholar]
- Barthélemy NR Horie K Sato C Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med 2020; 217: e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A Karikari TK Ashton N Gazzina S Premi E Benussi L, et al. Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2020; 91: 960–7. [DOI] [PubMed] [Google Scholar]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. Neurotherapeutics 2004; 1: 213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeat P Chételat G Villemagne VL Fripp J Raniga P Pike K, On behalf of the AIBL Research Group, et al. β-Amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology 2010; 74: 121–7. [DOI] [PubMed] [Google Scholar]
- Brunet HE Miller JB Shi J Chung B Munter BT Sabbagh MN. Does informant-based reporting of cognitive symptoms predict amyloid positivity on positron emission tomography? Alzheimers Dement 2019; 11: 424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF Sikkes S Villemagne VL Mormino EC Rabin JS Burnham S, et al. Using subjective cognitive decline to identify high global amyloid in community-based samples: a cross-cohort study. Alzheimer Dement 2019; 11: 670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham SC Bourgeat P Dore V Savage G Brown B Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol 2016; 15: 1044–53. [DOI] [PubMed] [Google Scholar]
- Burnham SC Faux NG Wilson W Laws SM Ames D Bedo J, Alzheimer’s Disease Neuroimaging Initiative, et al. A blood-based predictor for neocortical Abeta burden in Alzheimer’s disease: results from the AIBL study. Mol Psychiatry 2014; 19: 519–26. [DOI] [PubMed] [Google Scholar]
- Cash DM Rohrer JD Ryan NS Ourselin S Fox NC. Imaging endpoints for clinical trials in Alzheimer’s disease. Alz Res Therapy 2014; 6: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P Elmi M Goozee K Shah T Sohrabi HR Dias CB, et al. Ultrasensitive detection of plasma amyloid-beta as a biomarker for cognitively normal elderly individuals at risk of Alzheimer’s disease. J Alzheimer Dis 2019; 71: 775–83. [DOI] [PubMed] [Google Scholar]
- Chen L Xu S Wu T Shao Y Luo L Zhou L, et al. Abnormal platelet amyloid‐β precursor protein metabolism in SAMP8 mice: evidence for peripheral marker in Alzheimer’s disease. J Cell Physiol 2019; 234: 23528–36. [DOI] [PubMed] [Google Scholar]
- Chételat G Villemagne VL Pike KE Baron J-C Bourgeat P Jones G, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain 2010; 133: 3349–58. [DOI] [PubMed] [Google Scholar]
- DeLong ER DeLong DM Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–45. [PubMed] [Google Scholar]
- de Rojas I Romero J Rodriguez-Gomez O Pesini P Sanabria A Perez-Cordon A, on behalf of the FACEHBI study, et al. Correlations between plasma and PET beta-amyloid levels in individuals with subjective cognitive decline: the Fundacio ACE Healthy Brain Initiative (FACEHBI). Alz Res Therapy 2018; 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTure MA Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegeneration 2019; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC Sperling RA Petersen R Sun CK Weiner MW Aisen PS, for the Alzheimer’s Disease Neuroimaging Initiative. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. J Am Med Assoc 2017; 317: 2305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenau JL Timmers T Wesselman LMP Verberk IMW Verfaillie SCJ Slot RER, et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020; 95: e46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A Harvey DJ Habeck C Golzar A Qureshi IA Zammit AR, for the Alzheimer’s Disease Neuroimaging Initiative, et al. Predicting amyloid-β levels in amnestic mild cognitive impairment using machine learning techniques. J Alzheimers Dis 2020; 73: 1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandos N Pérez-Grijalba V Pesini P Olmos S Bossa M Villemagne VL, AIBL Research Group, et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement 2017; 8: 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q Song Q Wang M Pang P Liao Z Jiang H, et al. Hippocampus radiomic biomarkers for the diagnosis of amnestic mild cognitive impairment: a machine learning method. Front Aging Neurosci 2019; 11: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Delgado M Cernadas E Barro S Amorim DG. Do we need hundreds of classifiers to solve real world classification problems? J Mach Learn Res 2014; 15: 3133–81. [Google Scholar]
- Frisoni GB Fox NC Jack CR Jr Scheltens P Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 2010; 6: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudey B Fung BJ Schieber C Faux NG, Alzheimer’s Disease Metabolomics Consortium. Alzheimer’s Disease Metabolomics C, Alzheimer's Disease Neuroimaging I. A blood-based signature of cerebrospinal fluid Abeta1-42 status. Sci Rep 2019; 9: 4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanczar B Hua J Sima C Weinstein J Bittner M Dougherty ER. Small-sample precision of ROC-related estimates. Bioinformatics 2010; 26: 822–30. [DOI] [PubMed] [Google Scholar]
- Holtzman DM. CSF biomarkers for Alzheimer’s disease: current utility and potential future use. Neurobiol Aging 2011; 32: S4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS Vellas B Woodward M Boada M Bullock R Borrie M, et al. Trial of Solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378: 321–30. [DOI] [PubMed] [Google Scholar]
- Insel PS Palmqvist S Mackin RS Nosheny RL Hansson O Weiner MW, Alzheimer’s Disease Neuroimaging Initiative, et al. Assessing risk for preclinical β-amyloid pathology with APOE, cognitive, and demographic information. Alzheimer Dement 2016; 4: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr Bennett DA Blennow K Carrillo MC Dunn B Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dementia 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr Shiung MM Gunter JL O'Brien PC Weigand SD Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 2004; 62: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr Wiste HJ Therneau TM Weigand SD Knopman DS Mielke MM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. J Am Med Assoc 2019; 321: 2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ Landau SM Koeppe RA Reiman EM Chen K Mathis CA, et al. The Alzheimer’s Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimers Dement 2015; 11: 757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S Mattsson N Palmqvist S Smith R Beach TG Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 2020a; 26: 379–86. [DOI] [PubMed] [Google Scholar]
- Janelidze S Stomrud E Palmqvist S Zetterberg H van Westen D Jeromin A, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep 2016; 6: 26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S Stomrud E Smith R Palmqvist S Mattsson N Airey DC, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun 2020b; 11: 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ Ossenkoppele R Tijms BM Fagan AM Hansson O Klunk WE, Amyloid Biomarker Study Group, et al. Association of cerebral amyloid-β aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry 2018; 75: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel BM Avants BB Gee JC Arnold SE Wolk DA, for the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Neuroimaging I. neuropsychological testing predicts cerebrospinal fluid amyloid-beta in mild cognitive impairment. J Alzheimers Dis 2015; 46: 901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N Nakamura A Washimi Y Kato T Sakurai T Arahata Y, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B 2014; 90: 353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH Korecka M Figurski MJ Toledo JB Blennow K Zetterberg H, Alzheimer's Disease Neuroimaging Initiative, et al. The Alzheimer’s Disease Neuroimaging Initiative 2 Biomarker Core: a review of progress and plans. Alzheimer Dement 2015; 11: 772–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari TK Pascoal TA Ashton NJ Janelidze S Benedet AL Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020; 19: 422–33. [DOI] [PubMed] [Google Scholar]
- Kim SE Woo S Kim SW Chin J Kim HJ Lee BI, et al. A nomogram for predicting amyloid PET positivity in amnestic mild cognitive impairment. J Alzheimers Dis 2018; 66: 681–91. [DOI] [PubMed] [Google Scholar]
- Klunk WE Engler H Nordberg A Wang Y Blomqvist G Holt DP, et al. Imaging brain amyloid in Alzheimers disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–19. [DOI] [PubMed] [Google Scholar]
- Ko H Ihm JJ Kim HG, for the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Neuroimaging I. Cognitive profiling related to cerebral amyloid beta burden using machine learning approaches. Front Aging Neurosci 2019; 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM Breault C Joshi AD Pontecorvo M Mathis CA Jagust WJ, et al. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med 2013; 54: 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A Weiner MW Tosun D. What can structural MRI tell about A/T/N staging? Alzheimers Dement 2019; 15: 1237–8. [Google Scholar]
- Lantero Rodriguez J Karikari TK Suárez-Calvet M Troakes C King A Emersic A, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol 2020; 140: 267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCun Y Bengio Y Hinton G. Deep learning. Nature 2015; 521: 436–44. [DOI] [PubMed] [Google Scholar]
- Lee JH Byun MS Yi D Sohn BK Jeon SY Lee Y, et al. Prediction of cerebral amyloid with common information obtained from memory clinic practice. Front Aging Neurosci 2018; 10: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A Chiotis K Lemoine L Gillberg P-G Almkvist O Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry 2019; 24: 1112–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-Y Lin K-J Lin P-C Huang C-C Chang C-C Lee Y-C, et al. Plasma amyloid assay as a pre-screening tool for amyloid positron emission tomography imaging in early stage Alzheimer’s disease. Alz Res Therapy 2019; 11: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquie M Normandin MD Vanderburg CR Costantino IM Bien EA Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015; 78: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserejian N Bian S Wang W Jaeger J Syrjanen JA Aakre J, et al. Practical algorithms for amyloid beta probability in subjective or mild cognitive impairmen. Alzheimers Dement 2019; 11: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N Cullen NC Andreasson U Zetterberg H Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2019; 76: 791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM Knopman DS Chertkow H Hyman BT Jack CR Jr Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM Hagen CE Xu J Chai X Vemuri P Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018; 14: 989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM Syrjanen JA Blennow K Zetterberg H Vemuri P Skoog I, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 2019; 93: e252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM Wiste HJ Weigand SD Knopman DS Lowe VJ Roberts RO, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 2012; 79: 1570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL Besser LM Beekly D Blennow K Kukull W Morris JC. Factors influencing successful lumbar puncture in Alzheimer research. Alzheimer Dis Assoc Disord 2017; 31: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A Kaneko N Villemagne VL Kato T Doecke J Doré V, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018; 554: 249–54. [DOI] [PubMed] [Google Scholar]
- Ovod V Ramsey KN Mawuenyega KG Bollinger JG Hicks T Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017; 13: 841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S Insel PS Zetterberg H Blennow K Brix B Stomrud E, the Alzheimer’s Disease Neuroimaging Initiative, et al. Accurate risk estimation of beta-amyloid positivity to identify prodromal Alzheimer’s disease: cross-validation study of practical algorithms. Alzheimers Dement. 2019a; 15: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S Janelidze S Quiroz YT Zetterberg H Lopera F Stomrud E, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. J Am Med Assoc 2020; 324: 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S Janelidze S Stomrud E Zetterberg H Karl J Zink K, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related beta-amyloid status. JAMA Neurol 2019b; 76: 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S Mattsson N Hansson O, for the Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain 2016; 139: 1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC Han SH Cho HJ Byun MS Yi D Choe YM, et al. Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Therapy 2017; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC Han SH Lee H Jeong H Byun MS Bae J, et al. Prognostic plasma protein panel for Abeta deposition in the brain in Alzheimer’s disease. Prog Neurobiol 2019; 183: 101690. [DOI] [PubMed] [Google Scholar]
- Payami H Grimslid H Oken B Camicioli R Sexton G Dame A, et al. A prospective study of cognitive health in the elderly (Oregon Brain Aging Study): effects of family history and apolipoprotein E genotype. Am J Hum Genet 1997; 60: 948–56. [PMC free article] [PubMed] [Google Scholar]
- Perez-Grijalba V Arbizu J Romero J Prieto E Pesini P Sarasa L, The AB255 Study Group, et al. Plasma Abeta42/40 ratio alone or combined with FDG-PET can accurately predict amyloid-PET positivity: a cross-sectional analysis from the AB255 Study. Alz Res Therapy 2019; 11: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind E Nordberg A Darreh-Shori T Soininen H. Safety of lumbar puncture procedures in patients with Alzheimer’s disease. Curr Alzheimers Res 2009; 6: 290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC Aisen PS Beckett LA Donohue MC Gamst AC Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010; 74: 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone PM Casamitjana A Falcon C Artigues M Operto G Cacciaglia R, for the Alzheimer’s Disease Neuroimaging Initiative, et al. Prediction of amyloid pathology in cognitively unimpaired individuals using voxel-wise analysis of longitudinal structural brain MRI. Alzheimer Res Therapy 2019; 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar S Velgos SN Dueck AC Geda YE Mitchell JR, The Alzheimer’s Disease Neuroimaging Initiative. Brain MR radiomics to differentiate cognitive disorders. J Neuropsychiatry Clin Neurosci 2019; 31: 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM Fagan AM Grant EA Hassenstab J Moulder KL Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 2013; 80: 1784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE Esh CL Kokjohn TA Castaño EM Van Vickle GD Kalback WM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement 2009; 5: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga M Bittner T Batrla R Karl J. Preanalytical sample handling recommendations for Alzheimer’s disease plasma biomarkers. Alzheimers Dement 2019; 11: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling LP Zimmer ER Shin M Leuzy A Pascoal TA Benedet AL, et al. Imaging Alzheimer’s disease pathophysiology with PET. Dement Neuropsychol 2016; 10: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE Bollinger JG Ovod V Mawuenyega KG Li Y Gordon BA, et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019; 93: e1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll M Ossenkoppele R Strandberg O Palmqvist S Jögi J Ohlsson T, The Swedish BioFINDER study, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain 2017; 140: 2286–94. [DOI] [PubMed] [Google Scholar]
- Sørensen L Igel C Liv Hansen N Osler M Lauritzen M Rostrup E, for the Alzheimer’s Disease Neuroimaging Initiative and the Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing, et al. Early detection of Alzheimer’s disease using MRI hippocampal texture. Hum Brain Mapp 2016; 37: 1148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA Rentz DM Johnson KA Karlawish J Donohue M Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014; 6: 228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T Alafuzoff I Herukka SK Parkkinen L Hartikainen P Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009; 66: 382–9. [DOI] [PubMed] [Google Scholar]
- Ten Kate M Redolfi A Peira E Bos I Vos SJ Vandenberghe R, et al. MRI predictors of amyloid pathology: results from the EMIF-AD Multimodal Biomarker Discovery study. Alz Res Therapy 2018; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen EH La Joie R Wolf A Strom A Wang P Iaccarino L, Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 2020; 26: 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D Chen YF Yu P Sundell KL Suhy J Siemers E, Alzheimer’s Disease Neuroimaging Initiative, et al. Amyloid status imputed from a multimodal classifier including structural MRI distinguishes progressors from nonprogressors in a mild Alzheimer’s disease clinical trial cohort. Alzheimers Dement 2016; 12: 977–86. [DOI] [PubMed] [Google Scholar]
- Tosun D Joshi S Weiner MW, for the Alzheimer’s Disease Neuroimaging Initiative. Neuroimaging predictors of brain amyloidosis in mild cognitive impairment. Ann Neurol 2013; 74: 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D Joshi S Weiner MW, for the Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging I. Multimodal MRI-based imputation of the Abeta+ in early mild cognitive impairment. Ann Clin Transl Neurol 2014; 1: 160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D Schuff N Mathis CA Jagust W Weiner MW, Alzheimer’s Disease NeuroImaging Initiative. Initiative AsDN. Spatial patterns of brain amyloid-β burden and atrophy rate associations in mild cognitive impairment. Brain 2011; 134: 1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanschoren J Blockeel H Pfahringer B Holmes G. Experiment databases. Mach Learn 2012; 87: 127–58. [Google Scholar]
- Verberk IMW Slot RE Verfaillie SCJ Heijst H Prins ND van Berckel BNM, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018; 84: 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergallo A Megret L Lista S Cavedo E Zetterberg H Blennow K, and the INSIGHT-preAD study group, et al. Plasma amyloid beta 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement 2019; 15: 764–75. [DOI] [PubMed] [Google Scholar]
- Weiner MW Veitch DP Aisen PS Beckett LA Cairns NJ Green RC, Alzheimer’s Disease Neuroimaging Initiative, et al. The Alzheimer’s Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimers Dement 2017; 13: 561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood S Baird AL Hye A Ashton NJ Nevado-Holgado AJ Anand SN, et al. Plasma protein biomarkers for the prediction of CSF amyloid and tau and [(18)F]-flutemetamol PET scan result. Front Aging Neurosci 2018; 10: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H. Blood-based biomarkers for Alzheimer’s disease-An update. J Neurosci Methods 2019; 319: 2–6. [DOI] [PubMed] [Google Scholar]
- Zetterberg H Blennow K. Blood biomarkers: democratizing Alzheimer’s diagnostics. Neuron 2020; 106: 881–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study has been made publicly available by the ADNI in the Laboratory of Neuro Imaging (LONI) database.