Abstract
Staphylococcus aureus is the leading cause of skin and soft tissue infections. It remains incompletely understood how skin-resident immune cells respond to invading S. aureus and contribute to an effective immune response. Langerhans cells (LCs), the only professional antigen-presenting cell type in the epidermis, sense S. aureus through their pattern-recognition receptor langerin, triggering a proinflammatory response. Langerin recognizes the β-1,4-linked N-acetylglucosamine (β1,4-GlcNAc) but not α-1,4-linked GlcNAc (α1,4-GlcNAc) modifications, which are added by dedicated glycosyltransferases TarS and TarM, respectively, on the cell wall glycopolymer wall teichoic acid (WTA). Recently, an alternative WTA glycosyltransferase, TarP, was identified, which also modifies WTA with β-GlcNAc but at the C-3 position (β1,3-GlcNAc) of the WTA ribitol phosphate (RboP) subunit. Here, we aimed to unravel the impact of β-GlcNAc linkage position for langerin binding and LC activation. Using genetically modified S. aureus strains, we observed that langerin similarly recognized bacteria that produce either TarS- or TarP-modified WTA, yet tarP-expressing S. aureus induced increased cytokine production and maturation of in vitro-generated LCs compared to tarS-expressing S. aureus. Chemically synthesized WTA molecules, representative of the different S. aureus WTA glycosylation patterns, were used to identify langerin-WTA binding requirements. We established that β-GlcNAc is sufficient to confer langerin binding, thereby presenting synthetic WTA molecules as a novel glycobiology tool for structure-binding studies and for elucidating S. aureus molecular pathogenesis. Overall, our data suggest that LCs are able to sense all β-GlcNAc-WTA producing S. aureus strains, likely performing an important role as first responders upon S. aureus skin invasion.
Keywords: Staphylococcus aureus, pattern-recognition receptor, glycosylation, Langerhans cell, wall teichoic acid, langerin
Staphylococcus aureus is a Gram-positive bacterium that transiently colonizes an estimated 20% of the human population at different sites of the body, including the nasopharynx, skin, and gastrointestinal tract.1 The skin is a common entry site for S. aureus, making it the leading cause of skin and soft tissue infections (SSTIs).2 Consequently, efficient and rapid recognition of invading S. aureus by resident skin immune cells is critical for local eradication. When local immune defense fails, bacteria can disseminate into deeper tissues or even cause systemic infections, which are associated with high overall disease burden and mortality. The high recurrence of S. aureus SSTIs indicates that protective immune memory is defective, although the underlying reasons remain elusive. Indeed, there are no clear correlates of protection known for S. aureus, which has been a challenging aspect for vaccine development.3 A complete understanding of the local skin immune response to S. aureus may identify factors that protect the host from (re)infection, thereby providing critical insight for the development of a future S. aureus vaccine.
The skin contains a large arsenal of immune cells, which reside in different compartments within the skin. Langerhans cells (LCs), a highly specialized macrophage subset with dendritic cell-like functions, are the main antigen-presenting cells within the epidermis.4 Human LCs appear to have an important dual role in maintaining skin homeostasis by balancing both tolerogenic responses toward skin commensals as well as pro-inflammatory responses to invading pathogens.5−10 However, the ability of LCs to recognize and respond to invading bacteria remains elusive due to their restricted expression of Toll-like receptors.11,12 C-type lectin receptors (CLRs) constitute a family of pattern-recognition receptors (PRRs), which are dedicated to the recognition of glycans.13 A signature CLR of LCs is langerin (CD207).14 Langerin is a trimeric type II transmembrane receptor with specificity for sulfated and mannosylated glycans as well as β-glucans, which are recognized in a calcium-dependent manner.15−17 The direct downstream effects of receptor activation remain to be determined because langerin only contains a short cytoplasmic tail without classical signaling motifs.14 It is generally assumed that langerin-bound cargo is endocytosed and processed for antigen presentation to CD4 T cells via major histocompatibility complex class II (MHC-II).18−20
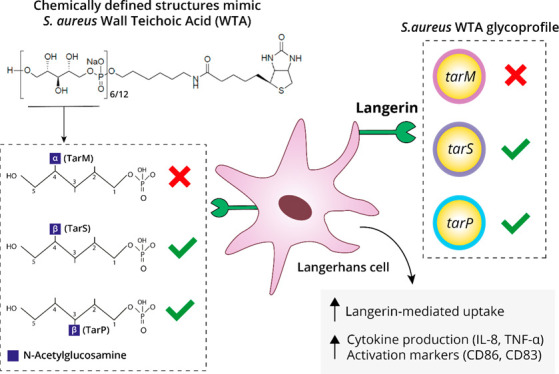
Recent work demonstrated that langerin allows human LCs to discriminate S. aureus from other staphylococci through a specific interaction with glycosylated wall teichoic acid (WTA).21 WTA is a major component of the Gram-positive bacterial cell wall and a well-known immunogenic antigen for opsonic antibodies targeting S. aureus.22−24S. aureus WTA consists of a polymerized ribitol phosphate (RboP) backbone that can be codecorated with positively charged d-alanine and N-acetylglucosamine (GlcNAc) residues. d-Alanylation of WTA is highly regulated and impacts bacterial surface charge, thereby providing protection from host cationic antimicrobial peptides (AMPs) and the lipopeptide antibiotic daptomycin.25−28 WTA glycosylation can be mediated by different glycosyltransferases, resulting in distinct WTA glycoforms. Three different WTA glycoforms have been identified in S. aureus, which differ in the configuration and position of GlcNAc linkage. Langerin binding to S. aureus is conferred by β-1,4-GlcNAc modified WTA, which requires the glycosyltransferase TarS that is present in nearly all S. aureus strains.29,30 Approximately 30% of S. aureus strains derived from nasal isolates coexpress tarM, which encodes a glycosyltransferase that modifies WTA with α-1,4-GlcNAc.29,31 Although α-1,4-GlcNAc did not confer langerin binding, it attenuated langerin binding to β-1,4-GlcNAc WTA, likely as a result of substitution or steric hindrance. This suggests that S. aureus clones coexpressing tarM/tarS can alter WTA glycosylation to evade innate immune activation by LCs.21 Interaction between β-1,4-GlcNAc expressing S. aureus and langerin increased pro-inflammatory cytokine production by in vitro-generated LCs and in the skin of human langerin-transgenic mice after epicutaneous infection, suggesting a contribution to antibacterial host defense.21 Overall, WTA glycosylation impacts the ability of LCs to sense invading S. aureus and mount a local immune response.21
In addition to TarM and TarS, a third glycosyltransferase, TarP, has recently been identified.32 TarP modifies the WTA backbone with β-linked GlcNAc residues similar to TarS but at the C3 position of RboP instead of C4. TarP is always coexpressed with tarS and is associated with, but not limited to, healthcare-associated and livestock-associated MRSA strains belonging to clonal complexes 5 and 398.32,33 TarP can functionally replace TarS with regard to β-lactam resistance and phage susceptibility via the decoration of WTA with β-GlcNAc moieties.30,32 However, whether the same applies to immune recognition remains to be fully elucidated. For example, TarP-modified WTA displayed attenuated immunogenicity in mice compared to TarS-modified WTA and comodification of WTA by TarP may lower S. aureus antibody recognition despite the presence of antibodies to both WTA glycoforms in serum from healthy individuals.24,33
In this study, we assessed the impact of TarP-mediated WTA glycosylation on langerin recognition and responses, i.e. antigen uptake and cytokine production, of in vitro-generated LCs. We describe that langerin-mediated recognition and uptake of S. aureus is similar for strains expressing β-1,3-GlcNAc WTA or β-1,4-GlcNAc WTA. Despite similar recognition and uptake, LC cytokine production was more pronounced upon interaction with tarP-expressing bacteria compared to tarS-expressing bacteria. Finally, employing synthetic WTA molecules with specific GlcNAc modifications,34 we demonstrate that β-GlcNAc WTA is sufficient but not exclusively required for S. aureus binding to langerin-expressing cells. Overall, we provide evidence that LCs are able to sense and respond to all S. aureus strains that produce β-GlcNAc-modified WTA. Furthermore, the use of chemically synthesized WTA structures provides a valuable toolbox to study the interaction between host immune molecules such as CLRs and S. aureus WTA in more detail.
Results
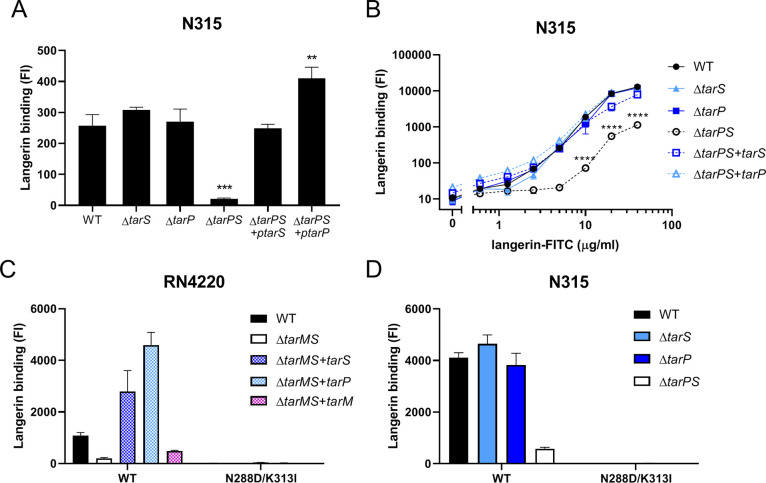
TarP and TarS Both Confer Binding of Human Langerin to S. aureus
TarP can replace several key functions of TarS, including resistance to β-lactam antibiotics and susceptibility to siphophage infection.32 In contrast, decoration of WTA with β-1,3-GlcNAc in addition to or instead of β-1,4-GlcNAc may impact immune detection by antibodies.24,32 We recently identified that β-1,4-GlcNAc WTA is specifically detected by the human innate receptor langerin.21 To assess whether human langerin was also able to detect tarP-expressing S. aureus strains, we employed a FITC-labeled recombinant construct of the extracellular carbohydrate domain (ECD) of human langerin (langerin-FITC).35 Using S. aureus strain N315 that naturally expresses both tarS and tarP,32 we observed that langerin binding was significantly impaired upon deletion of both glycosyltransferases (ΔtarPS), but not in either of the single mutant strains (Figure 1A). Subsequent complementation of the ΔtarPS double mutant with a plasmid containing either tarS or tarP restored the binding to recombinant langerin-FITC (Figure 1A). This observation in differential langerin binding among the N315 mutant panel persisted over a 100-fold concentration range of langerin-FITC, although at higher concentrations, langerin-FITC also showed significant binding to the ΔtarPS strain (Figure 1B). Binding to the N315 ΔtarPS strain was also dependent on the langerin carbohydrate recognition domain (CRD) because the interaction could be blocked by addition of mannan (Supporting Figure 1B). Similar binding experiments were additionally performed in S. aureus strain RN4220, which naturally coexpresses tarS and tarM, but not tarP. As previously reported,21 langerin binding to RN4220 wild-type was significantly reduced in the ΔtarMS double mutant (Figure 1C). Binding could be restored by complementation with either tarS or tarP but not tarM (Figure 1C). For the N315 and RN4220 strain panels, expression of the correct WTA glycoform was confirmed through binding of specific mAbs (Supporting Figure 1A24). Overall, langerin binds to TarP-modified WTA independent of strain background.
Figure 1.
WTA β-GlcNAcylation by TarS and TarP confers langerin binding to S. aureus. Binding of recombinant human langerin-FITC (A) to N315 WT, ΔtarS, ΔtarP, ΔtarPS, ΔtarPS + ptarS, and ΔtarPS + ptarP at a fixed concentration of 5 μg/mL and (B) to the indicated N315 strain panel using a concentration range of langerin-FITC (0.6–40 μg/mL). (C, D) Binding of FITC-labeled recombinant human langerin wild-type and N288D/K313I double SNP variant (10 μg/mL) to (C) RN4220 WT, ΔtarMS, ΔtarMS + ptarS, ΔtarMS + ptarP, and ΔtarMS + ptarM and (D) the N315 mutant panel (mentioned above). Data are depicted as geometric mean fluorescence intensity (FI) + standard error of mean (SEM) of biological triplicates. **p < 0.01, ***p < 0.001, ****p < 0.0001.
While it was apparent that langerin binding to S. aureus required either TarP or TarS, it was not clear whether the receptor bound the two different modifications in a similar way. Previously, we showed that langerin binding to S. aureus was abrogated when a naturally occurring double SNP was introduced into the human langerin ECD.36 Using these same langerin SNP constructs, we observed a similar loss of binding to TarP-expressing S. aureus (Figure 1C, D). These data suggest that the WTA β-1,3-GlcNAc moiety created by TarP is similarly dependent on these two residues in the CRD of langerin compared to the β-1,4-GlcNAc moiety on WTA generated by TarS.
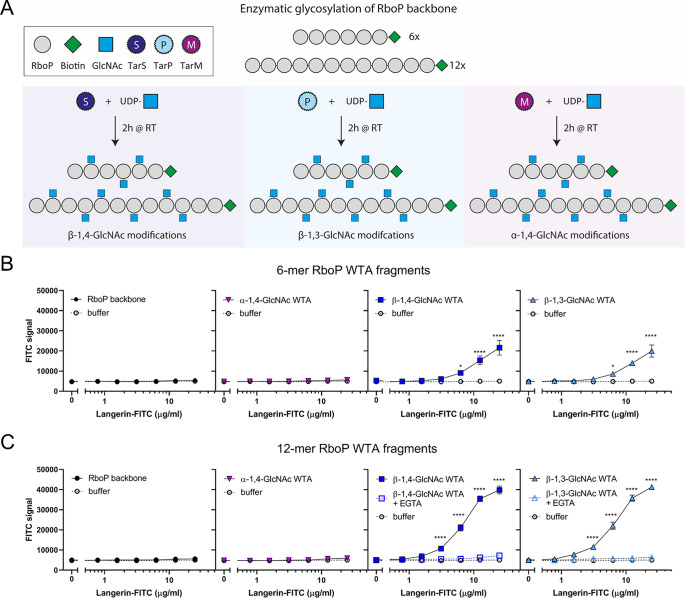
WTA β-GlcNAc is Sufficient to Confer Langerin Binding
TarP-expressing S. aureus can bind langerin in a similar way to S. aureus expressing TarS. However, we also observed significant residual binding in the ΔtarPS background at higher langerin concentrations (Figure 1B). We therefore asked whether WTA-β-GlcNAc is sufficient to confer binding to S. aureus or whether additional bacterial cofactors are required. The isolation of WTA from the bacterial cell wall is challenging; the procedure is labor intensive, but moreover, the instability and variation in isolated WTA creates difficulties for assay reproducibility. Therefore, we used our previously developed system,24 where chemically synthesized WTA backbone fragments of defined length are glycosylated by specific recombinant Tar enzymes in vitro (Figure 2A). With this robust system, we have previously studied the interaction of specific WTA glycoforms and antibodies in a reproducible and low background manner.24 In this study, we used both hexameric and dodecameric RboP backbones to assess the influence of WTA chain length on langerin binding. Differently glycosylated biotinylated WTA structures were coated on streptavidin-coated ELISA plates and incubated with a concentration range of recombinant langerin-FITC. Only wells coated with β-1,4-GlcNAc- and β-1,3-GlcNAc-glycosylated WTA structures mediated concentration-dependent binding to langerin and no binding was observed to the RboP backbone or α-1,4-GlcNAc-glycosylated WTA (Figure 2B, C). In addition, langerin binding was increased when the WTA backbone was extended from 6- to 12-RboP units (Figure 2B, C). Interaction between recombinant langerin-FITC and synthetic WTA was completely abolished in the presence of EGTA (Figure 2C), which scavenges calcium ions required for receptor binding. Langerin binding likely requires more than two β-GlcNAc residues, because we could not detect binding to a fully synthetic WTA molecule consisting of hexameric RboP backbone and β-1,4-GlcNAc coupled to the third and terminal RboP subunit (Supporting Figure 2A, B). In contrast, monoclonal antibodies specific for either α-GlcNAc-WTA or β-GlcNAc-WTA were able to bind the fully synthetic WTA structures (Supporting Figure 2C). This does not only indicate that fully synthetic structures were coated correctly to the wells but also underlines the differences in minimal binding requirements to glycosylated WTA between antibodies and langerin. Overall, these data confirm that β-GlcNAc WTA is sufficient to confer interaction with langerin and does not require the presence of d-alanine residues on WTA nor additional bacterial factors.
Figure 2.
β-GlcNAc-modified WTA is sufficient to confer langerin binding. (A) Schematic overview of the synthetic WTA structures and in vitro glycosylation by recombinant TarS, TarP, or TarM. (B) Binding of recombinant human langerin-FITC (0.4–25 μg/mL) to RboP hexamers alone (RboP backbone) or after in vitro glycosylation by TarS, TarP, or TarM. (C) Binding of recombinant human langerin-FITC (0.4–25 μg/mL) to RboP dodecamers alone (RboP backbone) or after in vitro glycosylation similar to RboP hexamers. Binding to β-GlcNAc WTA was assessed in the absence and presence of EGTA (10 mM). Data for panel B and C are shown as fluorescence signal + SEM of three independent experiments and were compared with the negative control (buffer). *p < 0.05, ****p < 0.0001.
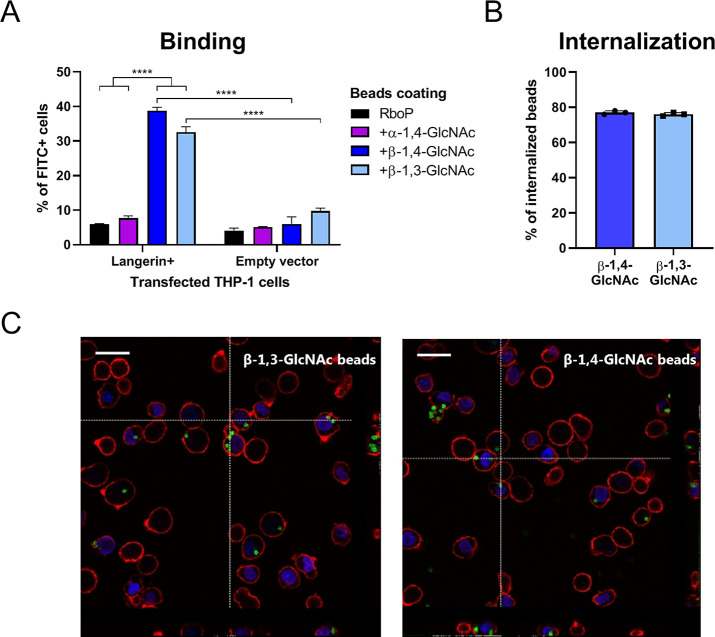
We also assessed binding of beads, coated with the differently glycosylated WTA oligomers, to surface-expressed langerin on transfected THP-1 cells. FITC-labeled beads were coated with synthetic glycosylated WTA hexamers, and coating was verified by binding of monoclonal antibodies specific for either α-GlcNAc or β-GlcNAc WTA (Supporting Figure 3). We observed strong binding of β-GlcNAc WTA beads, modified by either TarS or TarP, to langerin-expressing THP-1 cells but not empty vector control cells (Figure 3A). In addition to binding, Langerin + THP-1 cells internalized the majority of adhered beads as assessed by flow cytometry (Figure 3B) and confocal microscopy (Figure 3C). No apparent differences in receptor binding or cellular uptake were observed for TarS- and TarP-modified WTA beads in this system, suggesting that both modifications confer a similar function with regard to langerin interaction.
Figure 3.
Binding and internalization of β-GlcNAc-WTA-coated beads by langerin-expressing THP-1 cells. (A) Binding of FITC-labeled beads, coated with unglycosylated or in vitro glycosylated RboP hexamers, to THP-1 cells transfected with human langerin or empty vector at a bead-to-cell ratio of 1. Adherence is represented by percent of FITC+ cells. (B) Proportion of adherent β-GlcNAc WTA beads that is internalized by Langerin + THP-1 cells. (C) Confocal microscopy images (40×) of β-GlcNAc WTA beads (FITC-labeled: green) bound to and internalized by Langerin+THP-1 cells (WGA-Alexa 647: red, DAPI: blue). Vertical lines correspond to cross section of z-stack on the right, horizontal lines to cross section below, scale bars correspond to 25 μm. For panels A and B, graphs represent mean + SEM of biological triplicates, ****p < 0.001.
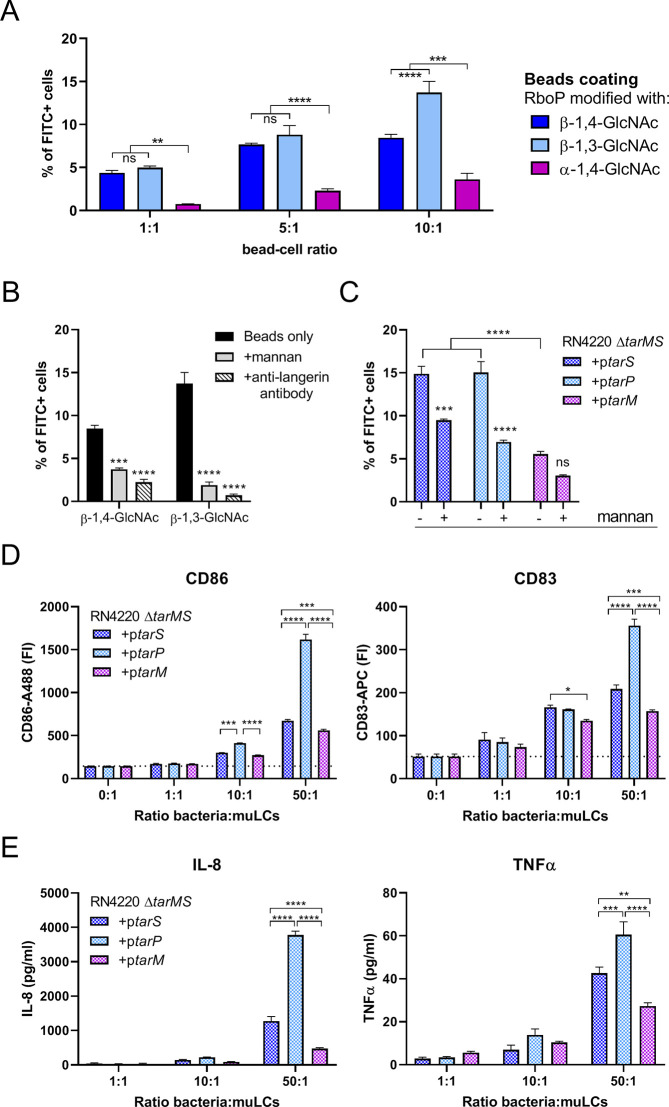
Expression of β-GlcNAc WTA Contributes Significantly to the Interaction between S. aureus and LCs
We have recently shown that langerin significantly contributes to the interaction between S. aureus and primary human LCs.21 In addition, in vitro-generated muLCs were used as an LC cell model to demonstrate the impact of langerin recognition on activation of APCs.21 Here, we again used muLCs to study the binding of surface-expressed langerin to β-GlcNAc WTA modifications mediated by TarS or TarP. In line with the THP-1 binding experiments, muLCs also specifically bound to β-GlcNAc WTA beads, irrespective of linkage to C3 (TarP) or C4 (TarS) (Figure 4A). At a bead-to-cell ratio of 10, beads decorated with β-1,3-GlcNAc WTA adhered significantly better compared to beads decorated with β-1,4-GlcNAc WTA (Figure 4A). This observed binding was mediated by the presence of langerin, as we were able to block the binding of muLCs to β-GlcNAc WTA beads by addition of mannan, a ligand for langerin, or specific langerin-blocking monoclonal antibodies (Figure 4B). These data show that β-GlcNAcylated WTA is sufficient to confer binding to muLCs and does not require bacterial cofactors.
Figure 4.
S. aureus WTA glycoform affects binding to and activation of in vitro-generated LCs. (A) Binding of FITC-labeled beads, coated with in vitro glycosylated RboP dodecamers, to muLCs at bead-to-cell ratios of 1, 5, and 10. Bead adherence is displayed as percent of FITC+ cells. (B) Binding of FITC-labeled beads coated with TarS- or TarP-modified RboP dodecamers to muLCs at a bead-to-cell ratio of 10 in the absence (similar to A) or presence of mannan (20 μg/mL) or anti-langerin blocking antibody (20 μg/mL). (C) Binding of FITC-labeled RN4220 ΔtarMS complemented with plasmid-expressed tarS, tarP, or tarM to muLCs at a bacteria-to-cell ratio of 1. Bacterial binding is represented by percent of FITC+ cells. (D) Surface expression of activation marker CD86 and maturation marker CD83 by muLCs after 24 h of stimulation with γ-irradiated RN4220 ΔtarMS complemented with plasmid-expressed tarS, tarP, or tarM at bacteria-to-cell ratios of 1, 10, and 50. (E) Concentration of IL-8 and TNFα in the supernatant of muLCs described in D. The data for all panels represent mean + SEM of biological triplicates. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Next, we assessed whether β-GlcNAc WTA was necessary for S. aureus binding to muLCs. For these experiments we used the RN4220 ΔtarMS background where tarM, tarS and tarP are individually and constitutively expressed from a complementation plasmid. We observed an approximately 3-fold higher binding to muLCs by S. aureus strains expressing β-GlcNAc WTA compared to α-GlcNAc-WTA producing S. aureus (Figure 4C). However, even in the absence of β-GlcNAc WTA, S. aureus was able to adhere to muLCs. Furthermore, binding of β-GlcNAc WTA producing S. aureus, but not α-GlcNAc producing S. aureus, to muLCs was significantly blocked by addition of mannan (Figure 4C). These results indicate that the interaction between langerin and β-GlcNAc WTA is an important determinant, although not exclusively required, for S. aureus binding to LCs.
To assess the downstream effects of langerin-mediated binding of S. aureus to muLCs and potential differences herein between β-1,4-GlcNAc-WTA versus β-1,3-GlcNAc-WTA producing S. aureus, we stimulated muLCs for 24 h with gamma-irradiated RN4220 ΔtarMS, complemented with either plasmid-expressed tarS, tarP or tarM. Surface expression of activation markers CD86 and CD83 increased in a dose-dependent manner in response to all three strains. Expression of CD86 and CD83 was highest in response to tarP-complemented S. aureus and differed significantly from tarS-complemented S. aureus (Figure 4D). The production of IL-8 and TNF-α showed a similar pattern, where all three strains induced a dose-dependent cytokine response with highest cytokine levels in response to tarP-complemented S. aureus (Figure 4E). In line with previous results, tarM-complemented S. aureus showed the lowest activation of muLCs, both in surface expression of CD86 and CD83, as well as cytokine production. This data suggests that besides the known effect between α-GlcNAc-WTA and β-GlcNAc-WTA, there could be additional differences in langerin-mediated LC activation between β-1,3-GlcNAc-WTA and β-1,4-GlcNAc-WTA.
Discussion
LCs are among the first responders upon invasion of S. aureus into the skin, contributing to early initiation of pro-inflammatory responses and recruitment of neutrophils. At the molecular level, langerin is an important sensor of specific S. aureus cell wall constituents, i.e. β-GlcNAcylated WTA, which can be mediated by the housekeeping glycosyltransferase TarS and the accessory enzyme TarP.21,32 Using a combination of recombinant langerin and langerin-transfected cell lines, genetically-modified S. aureus strains and in vitro generated LCs, we demonstrate that the interaction between langerin and tarP-expressing S. aureus results in similar binding but quantitatively different immunological responses. Moreover, comparing the binding of beads coated with synthetic glycosylated WTA oligomers and S. aureus modified strains emphasized that the interaction between LCs and S. aureus is largely, but not solely, dependent on the expression of β-GlcNAc WTA.
Binding of recombinant langerin to S. aureus was abrogated in bacteria that lack WTA glycosyltransferases, i.e. N315ΔtarPS and RN4220ΔtarMS bacteria. However, at higher concentrations, residual langerin binding to these WTA-deglycosylated strains was still observed, suggesting the presence of a second, currently unidentified minor ligand for langerin on the S. aureus surface. This observed binding was specific, as the binding was saturable and was inhibited by addition of mannan (Supporting Figure 1B). S. aureus expresses a wide variety of surface proteins that contribute to skin colonization and infection.37 Interestingly, some of these proteins, such as the serine-aspartate repeat (SDR) proteins and SraP, are heavily glycosylated,38−40 thereby representing potential targets for langerin in addition to β-GlcNAc WTA.
The toolbox of synthetic WTA fragments allowed us to gain more insights into the binding requirements of langerin to glycosylated WTA. Following current consensus, the WTA backbone consists of up to 40 repeating units of RboP that can be co-decorated with d-alanine and GlcNAc residues.41 The synthetic RboP polymers used here are only modified with GlcNAc and do not contain d-alanine residues. Consequently, we conclude that d-alanylation of WTA is dispensable for langerin binding in our assays, although we cannot rule out that the interaction would be affected by the presence of d-alanine. Also, when expressed by S. aureus, the absence or presence of d-alanine does not seem to impact langerin binding (Supplementary Figure 1C). In addition, we observed a strong impact of GlcNAc abundance on langerin binding; doubling the length of the synthetic WTA backbone enhanced langerin binding, which is most likely explained by an increased number of GlcNAc moieties following in vitro glycosylation. Furthermore, we did not observe langerin binding to fully defined WTA structures, which only contained two β-GlcNAc modifications (Supporting Figure 2B). This could be due to a limited sensitivity of our assay. Alternatively, it may indicate that langerin requires more than two β-GlcNAc moieties or differently spaced β-GlcNAc moieties to interact. In contrast, two GlcNAc moieties are sufficient for antibodies to interact with WTA (Supporting Figure 2C). Currently, not much is known about the regulation of WTA biosynthesis and glycosylation, although both the length of the WTA backbone as well as the expression of glycosyltransferases are believed to be affected by environmental cues. In the skin, activation of the Agr regulon results in increased WTA expression on the surface.42 Additionally, TarS-mediated WTA glycosylation increases under infection conditions at the expense of TarM- or TarP-mediated glycosylation, which dominate WTA glycosylation under in vitro growth conditions.32,43,44 Consequently, more β-1,4-GlcNAc moieties are produced in vivo,43 which would greatly enhance receptor avidity of langerin and impact its function.15
TarP can replace TarS in several key processes, including β-lactam resistance.30,32 However, whether the same applies to immune recognition still remains to be fully clarified. In mice, TarP-modified WTA appeared less immunogenic as compared to TarS-modified WTA.32 Previous work has shown the existence of cross-reactive human antibodies to both β-GlcNAc moieties, while other antibodies seem to be more exclusively directed toward β-1,4-GlcNAc.24 Until now, no studies have assessed the potential discrimination between tarS- and tarP-expressing S. aureus strains by innate immune cells. From our cell-based assays, β-1,3-GlcNAc-modified WTA has a similar ability to bind langerin compared to β-1,4-GlcNAc-modified WTA. However, LC activation as detected by cytokine production appears to be higher in response to tarP- versus tarS-expressing S. aureus strains. This observed difference in LC activation between TarS- and TarP-modified WTA was only observed at higher bacteria to cell ratios. Given the estimated density of LCs of approximately 1000 cells per mm2 in human skin,45 this ratio does not seem impossible to reach in vivo, especially when bacteria are able to grow out although it remains difficult to judge which conditions are most reflecting physiologically relevant conditions. Nevertheless, this finding potentially underlines an important difference in the stimulatory capacity of both modifications, where β-1,3-GlcNAc is more immunostimulatory for innate responses, whereas β-1,4-GlcNAc is dominant for adaptive antibody recognition. One explanation for this could be the difference in glycosylation between both glycosyltransferases. TarP modifies the RboP backbone with GlcNAc moieties at a higher efficiency than TarS, which could subsequently enhance receptor clustering and internalization by LCs. Moreover, glycosylation by TarS or TarP differentially affects d-alanylation of WTA, resulting in overall charge differences.32 As a consequence, TarP-mediated glycosylation might negatively affect antigen-presentation by APCs due to decreased zwitterionic charge properties. As a result, T cell responses and T cell-dependent B cell responses to TarP-modified WTA may be hampered. Furthermore, T cell-independent B cell responses to TarP-modified WTA could be affected as well, via decreased cross-linking of the B cell receptor. However, more research is needed to support this hypothesis, and the synthesis of WTA oligomers with added d-alanine modifications will serve as an excellent tool to study this.
Our results underline the ability of muLCs to detect and internalize S. aureus that express β-GlcNAc on their surface. In line with previous work, we observed that S. aureus-langerin interaction increased surface expression of activation markers CD86 and CD83 and enhanced the production of pro-inflammatory cytokines such as IL-8. Cytokine production was also increased upon epicutaneous infection of human langerin transgenic mice with tarS-expressing S. aureus.21 Although an increased IL-8 response would generally serve to recruit neutrophils to the site of infection to promote rapid eradication of invading S. aureus, we did not observe a significant reduction in bacterial load at the experimental conditions tested using this model.21 It therefore remains to be elucidated whether and how the interaction between human langerin and WTA would contribute to LC-mediated immunity against S. aureus. Besides processes such as antigen uptake and presentation to CD4+ T cells, little is known about direct downstream responses of langerin.18−20,46 Moreover, a lack of robust models, including limited access to human skin explants, differences in langerin ligand specificity17 and immune cell subsets in commonly used experimental animals,47 represent significant challenges to study immature LC function. The synthetic WTA oligomers used here could represent a robust tool to specifically study downstream effects of langerin receptor binding, and could even be used in combination with appropriate TLR stimulation to unravel LC responses in response to specific langerin-TLR triggers.48
Overall, langerin senses all β-GlcNAc WTA-producing S. aureus strains, which contributes to but is not exclusively required for recognition by LCs. In addition, we suspect the existence of a second langerin ligand on the surface of S. aureus. It is currently difficult to dissect the functional consequences of LCs responses in more relevant biological systems. In addition, we also lack knowledge on in vivo expression of WTA glycosyltransferases, the resulting WTA glycoform and the spatial distribution across the bacterial cell wall, which all impact interaction and responses triggered by CLRs such as langerin. Future research will need to elucidate the impact of the S. aureus WTA glycoform on the ability of LCs in situ to sense invading S. aureus in the skin, a frequent point of entry, and whether this interaction aids in prevention of bacterial dissemination by mounting an effective local response.
Conclusion
Here, we show that LCs, the main antigen-presenting cells in the skin, sense all S. aureus strains that express β-GlcNAc WTA, which is conferred by glycosyltransferases TarS as well as the recently described TarP through the C-type lectin receptor langerin. Langerin binding increased bacterial uptake, LC maturation, and the production of pro-inflammatory cytokines such as neutrophil chemoattractant IL-8. Despite similar interaction with langerin, LC activation is more pronounced in response to β1,3-GlcNAc-expressing versus β1,4-GlcNAc-expressing S. aureus, suggesting different activation pathways related to specific glycan linkage. Future studies may be able to unravel this linkage-specific activation using chemically synthesized WTA oligomers, which we demonstrated to be a valuable novel glycobiology tool to study langerin-WTA binding requirements. Furthermore, these stable WTA oligomers may pave the way for future crystallography studies to further characterize WTA-langerin interaction at the atomic level. In summary, our study provides insight into the relevance of unique S. aureus WTA glycoforms for immune interactions in specific human tissues. Future studies will undoubtedly benefit from the chemically synthesized WTA oligomers used here to further our understanding of S. aureus molecular pathogenesis.
Methods
Bacterial Strains and Culture Conditions
All plasmids and strains used in this study are listed in Table S1. Bacteria were grown overnight in 5 mL of Todd-Hewitt broth (THB; Oxoid) at 37 °C with agitation. Growth medium was supplemented with 10 μg/mL chloramphenicol (Sigma) for plasmid-complemented S. aureus strains. Overnight cultures were subcultured the next day in fresh THB and grown to a midexponential growth phase, corresponding to an optical density of 0.6–0.7 at 600 nm (OD600).
Generation of Complemented N315 ΔtarPS Strains
Plasmids containing the shuttle vector RB474 with full-length copies of tarS or tarP as inserts were isolated from complemented RN4220 ΔtarMS strains,49 and transformed into Escherichia coli DC10B by heat shock. Competent S. aureus N315 ΔtarPS cellswere transformed with pRB474-tarS or pRB474-tarP (isolated from E. coli DC10B) through electroporation with a Bio-Rad Gene Pulser II (100 ohm, 25 μF, 2.5 kV). After recovery, bacteria were plated on Todd-Hewitt agar supplemented with 10 μg/mL chloramphenicol to select plasmid-complemented colonies. The presence of tarS or tarP was confirmed by PCR analysis, using the primers for TarP (up) 5′-CTTCACGAAAGAGCACTAGAAG-3′ and TarP (dn) 5′-TTCCCGGCAAGTTGGTG-3′ and for TarS (up) 5′- GTGAACATATGAGTAGTGCGTA-3′ and TarS (dn) 5′-CATAATGTCCTTCGCCAATCAT-3′. The corresponding WTA glycoform of complemented strains was also verified by bacterial staining with WTA-specific Fab fragments, followed by staining with goat F(ab’)2 anti-human kappa-Alexa Fluor 647 (5 μg/mL, Southern Biotech) (Supporting Figure 1A).
Bacterial Binding to Recombinant Human Langerin
Bacteria were grown to midexponential phase as described above and collected by centrifugation (10 min, 4000 rpm). Supernatant was discarded, and bacteria were resuspended to an OD600 of 0.4, which corresponds to approximately 108 colony forming units (CFU)/mL in TSM buffer (2.4 g/L Tris (Roche), 8.77 g/L NaCl (Sigma-Aldrich), 294 mg/L CaCl2·2H20 (Merck), 294 mg/L MgCl2·6H20 (Merck), pH 7.4) containing 0.1% bovine serum albumin (BSA, Merck). Next, bacteria were incubated at 37 °C for 30 min with FITC-labeled human langerin-extracellular domain (ECD) constructs, referred to as human langerin-FITC, as previously described.21,35 Bacteria were washed once with TSM 0.1% BSA, fixed in 1% formaldehyde in PBS, and analyzed by flow cytometry on a FACSverse (BD Biosciences). Per sample, 10 000 gated events were collected, and data were analyzed using FlowJo 10 (FlowJo, LLC).
Recombinant Expression of Monoclonal Antibodies and Fab Fragments
For monoclonal antibody expression, we cloned the human IgG1 heavy chain (hG) and kappa light chain (hK) constant regions (sequences as present in pFUSE-CHIg-hG1 and pFUSE2-CLIg-hk; Invivogen) in the XbaI-AgeI cloning site of the pcDNA34 vector (Thermo Fisher). VH and VL sequences from monoclonal antibodies specific for α-GlcNAc-WTA (4461), β-GlcNAc-WTA (4497) and β-1,4-GlcNAc-WTA (6292) were derived from patent WO 2014/193722 A1.50 As the VL of anti-WTA antibody 6292 resulted in precipitation problems, it was adapted toward a Vκ3, leaving the CDR regions (in bold) intact (VL(6292-Vκ3: EIVLTQSPATLSLSPGERATLSCRASQGIRNGLGWYQQKPGQAPRLLIYPASTLESGVPARFSGSGSGTDFTLTISSLEPEDFAVYYCLQDHNYPPTFGQGTKVEIK). The VH and VL sequences, preceded by a Kozak sequence (ACCACC) and the HAVT20 signal peptide (MACPGFLWALVISTCLEFSMA), were codon optimized for human expression and ordered as gBlocks (IDT). We cloned VH and VL gBlocks into the pcDNA34 vector, upstream of the IgG1 heavy chain (hG) and kappa light chain (hK) constant regions, respectively, by Gibson assembly (New England Biolabs) according to the manufacturer’s instructions. NheI and BsiWI were used as the 3′ cloning sites for VH and VL, respectively, to preserve the immunoglobulin heavy and kappa light chain amino acid sequence. The constructs were transformed in E. coli TOP10F′ by heat shock, and clones were verified by PCR and Sanger sequencing (Macrogen). Plasmids were isolated by NucleoBond Xtra Midi kit (Macherey-Nagel) and sterilized using 0.22 μm Spin-X centrifuge columns (Corning). We used EXPI293F cells (Thermo Fisher), grown in EXPI293 Expression medium (Thermo Fisher) at 37 °C, 8% CO2 in culture filter cap conical flasks (Sigma) on a rotation platform (125 rotations/min) for protein production. One day before transfection, cells were diluted to 2 × 106 cells/mL, and 100 mL cell culture was used for transfection the next day. In 10 mL of Opti-MEM (Thermo Fisher), 500 μL PEI-max (1 μg/μL; Polysciences) was mixed with DNA (1 μg/mL cells) in a 3:2 ratio of hK and hG vectors. After 20 min of incubation at room temperature, this DNA/PEI mixture was added dropwise to 100 mL of EXPI293F cells. After 5 days, we verified IgG expression by SDS-PAGE and harvested cell supernatant by centrifugation and subsequent filtration through a 0.45 μM filter. IgG was purified using a HiTrap Protein A column (GE Healthcare) and Äkta Pure (GE Healthcare). Protein was eluted in 0.1 M citric acid, pH 3.0, and neutralized with 1 M Tris, pH 9.0. The IgG fraction was dialyzed overnight against PBS at 4 °C. Purified monoclonal antibodies were stored at −20 °C. Fab fragments specific for α-GlcNAc-WTA (4461), β-GlcNAc-WTA (4497), and β-1,4-GlcNAc-WTA (6292) were cloned and expressed similar as the full-length monoclonal antibodies, except that the Fab heavy chain ends with 211VEPKSC216. A flexible linker (GGGGS), an LPETG, and a 6xHIS tag were added at the C-terminus of each Fab. EXPI293F expression supernatant was dialyzed against 50 mM Tris, 500 mM NaCl; pH 8.0, before Fab purification on a HISTrap FF column (GE Healthcare). Fab fragments were dialyzed against 50 mM Tris, 300 mM NaCl; pH 8.0 and stored at −20 °C.
Production of Biotinylated Ribitolphosphate (RboP) Hexamer (6-) and Dodeca (12-)mer
Biotinylated RboP hexamers were synthesized
as described previously.24,32 The synthesis of biotinylated
RboP dodecamers and chemically defined glycosylated RboP hexamers
will be described in detail elsewhere (S. Ali et al, paper in preparation).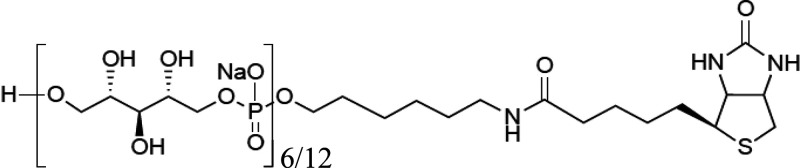
Enzymatic Glycosylation of RboP Oligomers
Recombinant TarP protein and transformed E. coli TOP10F’ strains with pBAD-tarM or pBAD-tarS were kindly provided by Prof. Thilo Stehle (University of Tübingen, Germany).32,51 Biotinylated RboP oligomers (0.17 mM) were incubated with recombinant glycosyltransferases TarS, TarP or TarM (6.3 μg/mL) for 2 h at room temperature with UDP-GlcNAc (2 mM, Merck) in glycosylation buffer (15 mM HEPES, 20 mM NaCl, 1 mM EGTA, 0.02% Tween 20, 10 mM MgCl2, 0.1% BSA, pH 7.4). Glycosylated RboP hexamers were coupled to beads by adding 5 × 107 Dynabeads M280 Streptavidin (Thermo Fisher Scientific) to the individual glycosylation reaction mixtures. After incubation for 15 min at room temperature, the coated beads were washed three times with PBS 0.1% BSA 0.05% Tween-20 using a magnetic sample rack and stored at 4 °C.
Recombinant Langerin Binding to Synthetic WTA
Maxisorb plates (Nunc) were coated with 10 μg/mL his-tetrameric-streptavidin-LPETG overnight at 4 °C, which was expressed and isolated from a pColdl-Stav-LPETG vector kindly provided by Tsutomu Tanaka (Kobo University, Japan). The plates were washed three times with TSM 0.05% Tween-20 (TSMT) and subsequently blocked with TSM 1% BSA for 1 h at 37 °C. After three washing steps with TSMT, a 50-fold dilution of the glycosylation mixture described above (corresponding to 3 uM RboP 6-mer or 12-mer) was added to the plates and incubated for 1 h at 37 °C. Next, the plates were washed with TSMT and further incubated with a concentration range of recombinant human langerin-FITC for 30 min at 37 °C. For blocking experiments, mannan (20 μg/mL) or EGTA (10 mM) were added immediately prior to addition of recombinant human langerin-FITC. Finally, after three washing steps, the plates were analyzed for langerin binding using a Clariostar plate reader (BMG Labtech; excitation 495 nm, emission 535 nm, gain 2000).
Cell Culture and muLC Differentiation
MUTZ-3 cells (ACC-295, DSMZ) were provided by Prof. T. de Gruijl (Amsterdam UMC, The Netherlands). Cells were maintained at a cell density of 0.5–1 × 106 cells/mL in 12-well tissue culture plates (Corning) in MEM-alpha (Gibco) with 20% FBS (Hyclone), 1% glutaMAX (Gibco), 10% spent medium from the renal carcinoma cell line 5637 (ACC-35, DSMZ) and 100 U/mL penicillin–streptomycin (Gibco). Cells were routinely cultured at 37 °C with 5% CO2. Differentiation of MUTZ-3 cells into MUTZ-3-derived LCs (muLCs) was performed according to described protocols.52,53 In short, MUTZ-3 cells were differentiated in the presence of 100 ng/mL GM-CSF (Genway Biotech), 10 ng/mL TGF-β (R&D Systems), and 2.5 ng/mL TNF-α (R&D Systems) for 11 days. Twice a week, half of the medium was replaced with fresh medium and double concentration of cytokines. To verify the differentiated muLC phenotype, cells were analyzed by flow cytometry for expression of CD207 (clone DCGM4, Beckman Coulter) and CD1a (clone Hl149, BD Biosciences) as well as the absence of CD34 (clone 581, BD Biosciences).
THP-1 cells, transfected with a lentiviral human langerin construct or empty vector, were cultured in RPMI-1640 (Lonza) supplemented with 10% heat-inactivated FBS and 100 U/mL penicillin-streptomycin (Gibco) as described in.21
Binding and Internalization of WTA Beads or S. aureus by Langerin-Expressing Cells
Dynabeads-M280 Streptavidin (Thermo Fisher Scientific) and midexponential S. aureus (OD600 = 0.6–0.7) were labeled with 0.5 mg/mL FITC (Sigma) in PBS for 30 min at 4 °C. After extensive washing and coating of the beads with glycosylated RboP hexamers as described above, beads and bacteria were resuspended in RPMI 0.1% BSA at a concentration of 5 × 107 beads/mL or 1 × 108 CFU/mL (OD600 = 0.4), respectively. Bacteria were stored at −20 °C and beads at 4 °C in the dark. For binding experiments, 1 × 105 cells (THP-1 cells or muLCs) were incubated with FITC-labeled WTA beads or FITC-labeled S. aureus at different ratios in RPMI 0.1% BSA for 30 min at 4 °C. Cells were washed (300g for 10 min at 4 °C), fixed in PBS 1% formaldehyde, and analyzed by flow cytometry as described above. To quantify internalization of β-GlcNAc WTA beads by THP-1 cells, we incubated WTA beads with 2 × 105 cells in RPMI 0.1% BSA at a bead-to-cell ratio of 1 for 30 min at 4 °C. Cells were washed twice to remove unbound beads, and the sample was divided over two separate tubes. Both samples were incubated for an additional 30 min, one at 4 °C and the other at 37 °C with 5% CO2 to allow phagocytosis. Cells were washed, and Fc-receptors were blocked with recombinant FLIPR-like (6 μg/mL) for 15 min at 4 °C.54 Next, monoclonal antibodies specific for β-GlcNAc or α-GlcNAc WTA (4497/4461-IgG1, respectively) were added to all samples at 3 μg/mL for 20 min at 4 °C, followed by goat antihuman kappa-Alexa Fluor 647 (5 μg/mL, Southern biotech) for another 20 min at 4 °C to allow discrimination between cell adherent (FITC+/Alexa fluor 647+) and internalized beads (FITC+/Alexa fluor 647-). Finally, cells were washed and fixed in 1% formaldehyde in PBS. The internalized fraction was calculated from the loss of Alexa Fluor 647 signal of FITC+ cells by flow cytometry, as previously described.36
To confirm bead internalization by confocal microscopy, cells were stained with WGA-Alexa Fluor 647 (Thermo Fisher Scientific) and DAPI (Sigma) following incubation for 30 min at 37 °C with FITC-labeled WTA beads and coated on 8 well chamber slides glass slides (Ibidi) before analysis by confocal laser scanning microscopy (SP5, Leica).
muLC Stimulation
Gamma-irradiation of S. aureus and stimulation of muLCs was performed as previously described.21 Briefly, S. aureus strains were grown to exponential phase, washed with PBS, concentrated 10-fold in PBS with 17% glycerol, and stored at −80 °C. Gamma irradiation of bacteria was performed at Synergy Health Ede B.V., a STERIS company (Ede, The Netherlands). The loss of viability was confirmed by plating, and the bacterial concentrations were calculated using the MACSQuant Analyzer 10.
muLCs (1 ×105) were stimulated with γ-irradiated RN4220 ΔtarMS+ptarS, RN4220 ΔtarMS+ptarP, or RN4220 ΔtarMS+ptarM at bacteria to cell ratios of 0, 1, 10, and 50 for 24 h at 37 °C with 5% CO2 in IMDM containing 10% FBS. Supernatants for cytokine analysis were collected after centrifugation (300g, 10 min at 4 °C), and stored at −80 °C until further analysis. Cells were washed with PBS 0.1% BSA, stained with CD83 (clone HB15e) and CD86 (clone IT2.2, Sony Biotechnology), fixed, and analyzed by flow cytometry. Cytokine production was analyzed by ELISA for IL-8 (Sanquin) and TNFα (Thermo Fisher) following manufacturer’s instructions.
Statistical Analysis
Flow cytometry data were analyzed using FlowJo 10 (FlowJo, LLC). All data were analyzed using GraphPad Prism 8.3 (GraphPad Software) with a two-way ANOVA followed by a Dunnett’s multiple comparison test except for bacterial binding to langerin-FITC at one fixed concentration for which one-way ANOVA was performed with Dunnett’s multiple comparison test. p-Values are depicted in the figures, and p < 0.05 was considered significant.
Acknowledgments
We thank Dani Heesterbeek and Lisanne de Vor for technical assistance with confocal microscopy.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00822.
Table S1, bacterial strains used in this study; Figure S1, bacterial binding of Fab fragments specific for α-GlcNAc-WTA (4461), β-GlcNAc-WTA (4497), and β-1,4-GlcNAc-WTA (6292); Figure S2, binding of langerin-FITC and monoclonal antibodies specific for α-GlcNAc-WTA (4461) and β-GlcNAc-WTA (4497) to fully synthetic WTA oligomers; Figure S3, validation of beads coating with enzymatically glycosylated RboP oligomers (PDF)
Author Present Address
○ R.v.D.: Interfaculty Institute of Microbiology and Infection Medicine, University of Tübingen, 72074 Tübingen, Germany
Author Present Address
△ N.M.v.S.: Amsterdam University Medical Center, location AMC, University of Amsterdam, Department of Medical Microbiology and Infection Prevention and Netherlands Reference Center for Bacterial Meninigitis, Meibergdreef 9 (IA3-0159), 1105 AZ Amsterdam, The Netherlands
Author Present Address
▽ F.F.F.: Department of Pharmaceutical Chemistry, University of Vienna, Althanstrasse 14, 1080 Vienna, Austria; Department of Microbiology, Immunobiology and Genetics, Max F. Perutz Laboratories, Campus Vienna Biocenter 5, 1030 Vienna, Austria
Author Present Address
⬡ C.R.: Department of Pharmaceutical Chemistry, University of Vienna, Althanstrasse 14, 1080 Vienna, Austria; Department of Microbiology, Immunobiology and Genetics, Max F. Perutz Laboratories, Campus Vienna Biocenter 5, 1030 Vienna, Austria
Author Contributions
□ R.v.D. and S.A. contributed equally.
This work was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant 675106 coordinated by Dr. Fabio Bagnoli (GSK, Siena, Italy) and by Vidi (91713303) and Vici (09150181910001) grants from The Netherlands Organisation for Health Research and Development (ZonMW) to N.M.v.S. A.H. is a Ph.D. fellow and is enrolled in the Infection and Immunity Ph.D. program, part of the graduate school of Life Sciences at Utrecht University and participated in a post graduate studentship program at GSK.
Upon request, the data supporting these findings are available from the corresponding author.
The authors declare no competing financial interest.
Supplementary Material
References
- Wertheim H. F.; Melles D. C.; Vos M. C.; van Leeuwen W.; van Belkum A.; Verbrugh H. A.; Nouwen J. L. (2005) The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5, 751–762. 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- Ray G. T.; Suaya J. A.; Baxter R. (2013) Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 76, 24–30. 10.1016/j.diagmicrobio.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Bagnoli F.; Bertholet S.; Grandi G. (2012) Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front. Cell. Infect. Microbiol. 2, 16. 10.3389/fcimb.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebel T.; Voisin B.; Nagao K. (2017) Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends Immunol. 38, 817–828. 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Seneschal J.; Clark R. A.; Gehad A.; Baecher-Allan C. M.; Kupper T. S. (2012) Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36, 873–884. 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber F.; De Gregorio C.; Steckholzer S.; Ferreira F. M.; Dolowschiak T.; Ruchti F.; Kirchner F. R.; Mertens S.; Prinz I.; Joller N.; Buch T.; Glatz M.; Sallusto F.; LeibundGut-Landmann S. (2019) The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe 25, 389–403. 10.1016/j.chom.2019.02.002. [DOI] [PubMed] [Google Scholar]
- de Witte L.; Nabatov A.; Pion M.; Fluitsma D.; de Jong M. A.; de Gruijl T.; Piguet V.; van Kooyk Y.; Geijtenbeek T. B. (2007) Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13, 367–371. 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Igyarto B. Z.; Haley K.; Ortner D.; Bobr A.; Gerami-Nejad M.; Edelson B. T.; Zurawski S. M.; Malissen B.; Zurawski G.; Berman J.; Kaplan D. H. (2011) Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272. 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T.; Glatz M.; Horiuchi K.; Kawasaki H.; Akiyama H.; Kaplan D. H.; Kong H. H.; Amagai M.; Nagao K. (2015) Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 42, 756–766. 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M. A.; Vriend L. E.; Theelen B.; Taylor M. E.; Fluitsma D.; Boekhout T.; Geijtenbeek T. B. (2010) C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol. Immunol. 47, 1216–1225. 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aar A. M.; Sylva-Steenland R. M.; Bos J. D.; Kapsenberg M. L.; de Jong E. C.; Teunissen M. B. (2007) Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J. Immunol. 178, 1986–1990. 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- Flacher V.; Bouschbacher M.; Verronese E.; Massacrier C.; Sisirak V.; Berthier-Vergnes O.; de Saint-Vis B.; Caux C.; Dezutter-Dambuyant C.; Lebecque S.; Valladeau J. (2006) Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J. Immunol. 177, 7959–7967. 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- Brown G. D.; Willment J. A.; Whitehead L. (2018) C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 18, 374–389. 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- Valladeau J.; Ravel O.; Dezutter-Dambuyant C.; Moore K.; Kleijmeer M.; Liu Y.; Duvert-Frances V.; Vincent C.; Schmitt D.; Davoust J.; Caux C.; Lebecque S.; Saeland S. (2000) Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81. 10.1016/S1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Feinberg H.; Powlesland A. S.; Taylor M. E.; Weis W. I. (2010) Trimeric structure of langerin. J. Biol. Chem. 285, 13285–13293. 10.1074/jbc.M109.086058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H.; Taylor M. E.; Razi N.; McBride R.; Knirel Y. A.; Graham S. A.; Drickamer K.; Weis W. I. (2011) Structural basis for langerin recognition of diverse pathogen and mammalian glycans through a single binding site. J. Mol. Biol. 405, 1027–1039. 10.1016/j.jmb.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanske J.; Schulze J.; Aretz J.; McBride R.; Loll B.; Schmidt H.; Knirel Y.; Rabsch W.; Wahl M. C.; Paulson J. C.; Rademacher C. (2017) Bacterial Polysaccharide Specificity of the Pattern Recognition Receptor Langerin Is Highly Species-dependent. J. Biol. Chem. 292, 862–871. 10.1074/jbc.M116.751750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepaut M.; Valladeau J.; Nurisso A.; Kahn R.; Arnou B.; Vives C.; Saeland S.; Ebel C.; Monnier C.; Dezutter-Dambuyant C.; Imberty A.; Fieschi F. (2009) Structural studies of langerin and Birbeck granule: a macromolecular organization model. Biochemistry 48, 2684–2698. 10.1021/bi802151w. [DOI] [PubMed] [Google Scholar]
- Mc Dermott R.; Ziylan U.; Spehner D.; Bausinger H.; Lipsker D.; Mommaas M.; Cazenave J. P.; Raposo G.; Goud B.; de la Salle H.; Salamero J.; Hanau D. (2002) Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol. Biol. Cell 13, 317–335. 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vlist M.; de Witte L.; de Vries R. D.; Litjens M.; de Jong M. A.; Fluitsma D.; de Swart R. L.; Geijtenbeek T. B. (2011) Human Langerhans cells capture measles virus through Langerin and present viral antigens to CD4(+) T cells but are incapable of cross-presentation. Eur. J. Immunol. 41, 2619–2631. 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- van Dalen R.; De La Cruz Diaz J. S.; Rumpret M.; Fuchsberger F. F.; van Teijlingen N. H.; Hanske J.; Rademacher C.; Geijtenbeek T. B. H.; van Strijp J. A. G.; Weidenmaier C.; Peschel A.; Kaplan D. H.; van Sorge N. M. (2019) Langerhans Cells Sense Staphylococcus aureus Wall Teichoic Acid through Langerin To Induce Inflammatory Responses. mBio 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstel V.; Xia G.; Peschel A. (2014) Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus. Int. J. Med. Microbiol. 304, 215–221. 10.1016/j.ijmm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Lehar S. M.; Pillow T.; Xu M.; Staben L.; Kajihara K. K.; Vandlen R.; DePalatis L.; Raab H.; Hazenbos W. L.; Hiroshi Morisaki J.; Kim J.; Park S.; Darwish M.; Lee B. C.; Hernandez H.; Loyet K. M.; Lupardus P.; Fong R.; Yan D.; Chalouni C.; Luis E.; Khalfin Y.; Plise E.; Cheong J.; Lyssikatos J. P.; Strandh M.; Koefoed K.; Andersen P. S.; Flygare J. A.; Wah Tan M.; Brown E. J.; Mariathasan S. (2015) Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 527, 323–328. 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- van Dalen R.; Molendijk M. M.; Ali S.; van Kessel K. P. M.; Aerts P.; van Strijp J. A. G.; de Haas C. J. C.; Codee J.; van Sorge N. M. (2019) Do not discard Staphylococcus aureus WTA as a vaccine antigen. Nature 572, E1–E2. 10.1038/s41586-019-1416-8. [DOI] [PubMed] [Google Scholar]
- Simanski M.; Glaser R.; Koten B.; Meyer-Hoffert U.; Wanner S.; Weidenmaier C.; Peschel A.; Harder J. (2013) Staphylococcus aureus subverts cutaneous defense by D-alanylation of teichoic acids. Exp Dermatol 22, 294–296. 10.1111/exd.12114. [DOI] [PubMed] [Google Scholar]
- Bayer A. S.; Mishra N. N.; Cheung A. L.; Rubio A.; Yang S. J. (2016) Dysregulation of mprF and dltABCD expression among daptomycin-non-susceptible MRSA clinical isolates. J. Antimicrob. Chemother. 71, 2100–2104. 10.1093/jac/dkw142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Lasek-Nesselquist E.; Lu J.; Schneider R.; Shah R.; Oliva G.; Pata J.; McDonough K.; Pai M. P.; Rose W. E.; Sakoulas G.; Malik M. (2018) Characterization of genetic changes associated with daptomycin nonsusceptibility in Staphylococcus aureus.. PLoS One 13, e0198366 10.1371/journal.pone.0198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivnjak T.; Peschel A.; Gelb M. H.; Liang N. S.; Weiss J. P. (2002) Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277, 47636–47644. 10.1074/jbc.M205104200. [DOI] [PubMed] [Google Scholar]
- Winstel V.; Kuhner P.; Salomon F.; Larsen J.; Skov R.; Hoffmann W.; Peschel A.; Weidenmaier C. (2015) Wall Teichoic Acid Glycosylation Governs Staphylococcus aureus Nasal Colonization. mBio 6, 6. 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.; Xia G.; Luhachack L. G.; Campbell J.; Meredith T. C.; Chen C.; Winstel V.; Gekeler C.; Irazoqui J. E.; Peschel A.; Walker S. (2012) Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. U. S. A. 109, 18909–18914. 10.1073/pnas.1209126109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G.; Maier L.; Sanchez-Carballo P.; Li M.; Otto M.; Holst O.; Peschel A. (2010) Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J. Biol. Chem. 285, 13405–13415. 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach D.; Guo Y.; De Castro C.; Kim S. H.; Schlatterer K.; Xu F. F.; Pereira C.; Seeberger P. H.; Ali S.; Codee J.; Sirisarn W.; Schulte B.; Wolz C.; Larsen J.; Molinaro A.; Lee B. L.; Xia G.; Stehle T.; Peschel A. (2018) Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 563, 705–709. 10.1038/s41586-018-0730-x. [DOI] [PubMed] [Google Scholar]
- Xiong M.; Zhao J.; Huang T.; Wang W.; Wang L.; Zhao Z.; Li X.; Zhou J.; Xiao X.; Pan Y.; Lin J.; Li Y. (2020) Molecular Characteristics, Virulence Gene and Wall Teichoic Acid Glycosyltransferase Profiles of Staphylococcus aureus: A Multicenter Study in China. Front. Microbiol. 11, 2013. 10.3389/fmicb.2020.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Es D.; Hogendorf W. F.; Overkleeft H. S.; van der Marel G. A.; Codee J. D. (2017) Teichoic acids: synthesis and applications. Chem. Soc. Rev. 46, 1464–1482. 10.1039/C6CS00270F. [DOI] [PubMed] [Google Scholar]
- Wamhoff E. C.; Schulze J.; Bellmann L.; Rentzsch M.; Bachem G.; Fuchsberger F. F.; Rademacher J.; Hermann M.; Del Frari B.; van Dalen R.; Hartmann D.; van Sorge N. M.; Seitz O.; Stoitzner P.; Rademacher C. (2019) A Specific, Glycomimetic Langerin Ligand for Human Langerhans Cell Targeting. ACS Cent Sci. 5, 808–820. 10.1021/acscentsci.9b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dalen R.; Fuchsberger F. F.; Rademacher C.; van Strijp J. A. G.; van Sorge N. M. (2020) A Common Genetic Variation in Langerin (CD207) Compromises Cellular Uptake of Staphylococcus aureus. J. Innate Immun. 12, 191–200. 10.1159/000500547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey K. A.; Mulcahy M. E.; Towell A. M.; Geoghegan J. A.; McLoughlin R. M. (2019) Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target. PLoS Pathog. 15, e1007713 10.1371/journal.ppat.1007713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenbos W. L.; Kajihara K. K.; Vandlen R.; Morisaki J. H.; Lehar S. M.; Kwakkenbos M. J.; Beaumont T.; Bakker A. Q.; Phung Q.; Swem L. R.; Ramakrishnan S.; Kim J.; Xu M.; Shah I. M.; Diep B. A.; Sai T.; Sebrell A.; Khalfin Y.; Oh A.; Koth C.; Lin S. J.; Lee B. C.; Strandh M.; Koefoed K.; Andersen P. S.; Spits H.; Brown E. J.; Tan M. W.; Mariathasan S. (2013) Novel staphylococcal glycosyltransferases SdgA and SdgB mediate immunogenicity and protection of virulence-associated cell wall proteins. PLoS Pathog. 9, e1003653 10.1371/journal.ppat.1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiziffer I.; Eikmeier J.; Pohlentz G.; McAulay K.; Xia G.; Hussain M.; Peschel A.; Foster S.; Peters G.; Heilmann C. (2017) The Plasmin-Sensitive Protein Pls in Methicillin-Resistant Staphylococcus aureus (MRSA) Is a Glycoprotein. PLoS Pathog. 13, e1006110 10.1371/journal.ppat.1006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siboo I. R.; Chambers H. F.; Sullam P. M. (2005) Role of SraP, a Serine-Rich Surface Protein of Staphylococcus aureus in binding to human platelets. Infect. Immun. 73, 2273–2280. 10.1128/IAI.73.4.2273-2280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G.; Kohler T.; Peschel A. (2010) The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 300, 148–154. 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Wanner S.; Schade J.; Keinhorster D.; Weller N.; George S. E.; Kull L.; Bauer J.; Grau T.; Winstel V.; Stoy H.; Kretschmer D.; Kolata J.; Wolz C.; Broker B. M.; Weidenmaier C. (2017) Wall teichoic acids mediate increased virulence in Staphylococcus aureus. Nat. Microbiol 2, 16257. 10.1038/nmicrobiol.2016.257. [DOI] [PubMed] [Google Scholar]
- Mistretta N.; Brossaud M.; Telles F.; Sanchez V.; Talaga P.; Rokbi B. (2019) Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions. Sci. Rep. 9, 3212. 10.1038/s41598-019-39929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Gerlach D.; Du X.; Larsen J.; Stegger M.; Kuhner P.; Peschel A.; Xia G.; Winstel V. (2015) An accessory wall teichoic acid glycosyltransferase protects Staphylococcus aureus from the lytic activity of Podoviridae. Sci. Rep. 5, 17219. 10.1038/srep17219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J.; Bahmer F. A.; Worl J.; Neuhuber W.; Schuler G.; Fartasch M. (2001) A strikingly constant ratio exists between Langerhans cells and other epidermal cells in human skin. A stereologic study using the optical disector method and the confocal laser scanning microscope. J. Invest. Dermatol. 116, 313–318. 10.1046/j.1523-1747.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- McDermott R.; Bausinger H.; Fricker D.; Spehner D.; Proamer F.; Lipsker D.; Cazenave J. P.; Goud B.; De La Salle H.; Salamero J.; Hanau D. (2004) Reproduction of Langerin/CD207 traffic and Birbeck granule formation in a human cell line model. J. Invest. Dermatol. 123, 72–77. 10.1111/j.0022-202X.2004.22728.x. [DOI] [PubMed] [Google Scholar]
- Pasparakis M.; Haase I.; Nestle F. O. (2014) Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 14, 289–301. 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- Li R. E.; Hogervorst T. P.; Achilli S.; Bruijns S. C.; Arnoldus T.; Vives C.; Wong C. C.; Thepaut M.; Meeuwenoord N. J.; van den Elst H.; Overkleeft H. S.; van der Marel G. A.; Filippov D. V.; van Vliet S. J.; Fieschi F.; Codee J. D. C.; van Kooyk Y. (2019) Systematic Dual Targeting of Dendritic Cell C-Type Lectin Receptor DC-SIGN and TLR7 Using a Trifunctional Mannosylated Antigen. Front. Chem. 7, 650. 10.3389/fchem.2019.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstel V.; Liang C.; Sanchez-Carballo P.; Steglich M.; Munar M.; Broker B. M.; Penades J. R.; Nubel U.; Holst O.; Dandekar T.; Peschel A.; Xia G. (2013) Wall teichoic acid structure governs horizontal gene transfer between major bacterial pathogens. Nat. Commun. 4, 2345. 10.1038/ncomms3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J. (2015) Anti-wall teichoic antibodies and conjugates; Genentech, Inc., United States. [Google Scholar]
- Koc C.; Gerlach D.; Beck S.; Peschel A.; Xia G.; Stehle T. (2015) Structural and enzymatic analysis of TarM glycosyltransferase from Staphylococcus aureus reveals an oligomeric protein specific for the glycosylation of wall teichoic acid. J. Biol. Chem. 290, 9874–9885. 10.1074/jbc.M114.619924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson A. J.; Sombroek C. C.; De Gruijl T. D.; Graus Y. M.; van der Vliet H. J.; Lougheed S. M.; van den Eertwegh A. J.; Pinedo H. M.; Scheper R. J. (2002) MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood 100, 701–703. 10.1182/blood.V100.2.701. [DOI] [PubMed] [Google Scholar]
- Santegoets S. J.; Masterson A. J.; van der Sluis P. C.; Lougheed S. M.; Fluitsma D. M.; van den Eertwegh A. J.; Pinedo H. M.; Scheper R. J.; de Gruijl T. D. (2006) A CD34(+) human cell line model of myeloid dendritic cell differentiation: evidence for a CD14(+)CD11b(+) Langerhans cell precursor. J. Leukocyte Biol. 80, 1337–1344. 10.1189/jlb.0206111. [DOI] [PubMed] [Google Scholar]
- Stemerding A. M.; Kohl J.; Pandey M. K.; Kuipers A.; Leusen J. H.; Boross P.; Nederend M.; Vidarsson G.; Weersink A. Y.; van de Winkel J. G.; van Kessel K. P.; van Strijp J. A. (2013) Staphylococcus aureus formyl peptide receptor-like 1 inhibitor (FLIPr) and its homologue FLIPr-like are potent FcgammaR antagonists that inhibit IgG-mediated effector functions. J. Immunol. 191, 353–362. 10.4049/jimmunol.1203243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.