Abstract
Gianotti-Crosti syndrome (GCS) is a self-limited condition, mainly affecting children younger than 6 years, less common in adolescents and adults. It consists of a viral exanthema with papular lesions with a flat top and symmetrical distribution, affecting predominantly extremities, gluteal region and extensor surfaces. It is often associated with viral infections but can also be related to bacterial infections, vaccination or be idiopathic. In this report, we present a case of GCS in a 13-year-old healthy female adolescent who presented with fever, odynophagia, prostration and diffuse maculopapular rash. The diagnosis of infectious mononucleosis due to infection by the Epstein-Barr virus was established. On the second week of the disease, a clinical recrudescence occurred, with worsening of the fever and modification of the exanthema characteristics. GCS is often an underdiagnosed entity. The differential diagnosis of viral exanthema can prove to be challenging and clinical suspicion is essential to achieve the diagnosis.
Keywords: paediatrics (drugs and medicines), skin, infectious diseases
Background
Gianotti-Crosti syndrome (GCS), also known as childhood papular acrodermatitis, is a rare and self-limited disorder that predominantly affects the skin.1 It consists of a sudden onset of diffuse and symmetrical papular lesions. Its incidence and prevalence are unknown, but it is estimated to be low, despite the disease being probably underdiagnosed (possible confusion with a viral exanthema).1–3
GCS primarily affects infants and children under 6 years, with no sex predilection. However, it can also occur in older children, adolescents and adults, with the female sex being more affected in this population.1 4
GCS was first described in the 1950s by Ferdinando Gianotti and Agostino Crosti, initially associated with acute hepatitis B virus (HBV) infection.1 3 In the 1960s, similar cases secondary to other etiologies were reported, having been defined as ‘acral papulovesicular syndrome’, which were classified into three types: type A - symmetrical, round, pink, non-coalescent lesions; type B - coalescent lesions, pruritic, with relief, especially in the limbs; type C - symmetrical, prominent and erythematous eruptions, often purpuric, usually non-pruritic, which can last more than 2 months.2
It is now known that GCS is mainly triggered by viral infections, with HBV and Epstein-Barr virus (EBV) being the most commonly involved. EBV is currently the most frequent agent since HBV immunisation was implemented in the national vaccination programme of most countries.4 Other agents such as enterovirus, cytomegalovirus (CMV), parvovirus, coxsackie, parainfluenza, hepatitis A virus, rotavirus, respiratory syncytial virus, herpes simplex virus (HSV), human herpesvirus 6, human papillomavirus and some bacteria (Mycoplasma pneumoniae and beta-haemolytic streptococci) have been associated.5 Postvaccination cases (initially associated with smallpox immunisation) and idiopathic cases have also been described.2 5–7
Typically, cutaneous manifestations have a sudden onset and present as a maculopapular or papulovesicular exanthema (with lichenoid aspect), flattened, with 1–10 mm in diameter, symmetrically distributed.5 The lesions can be normochromic, erythematous or brownish, do not fade under pressure and can be coalescent and form plaques.6 The predominant sites of distribution are the face, buttocks, extensor surfaces of the limbs and feet, but can also involve the trunk.6 It does not affect nails or mucosal surfaces. Anterior chest, palms and soles are normally spared.1 5 Pruritus is usually mild to moderate, but may be absent or become severe. Haemorrhagic lesions may appear, especially in areas more prone to trauma, more often associated with HBV infection.5 6
Regarding extracutaneous manifestations, symptoms of the triggering disease are usually present, namely respiratory, gastrointestinal, malaise and fever (depending on the aetiological agent) in the week before the onset of the lesions. These symptoms can cease before the appearance of the cutaneous lesions or overlap them.5 Lymphadenopathies are present in 25%–35% of the patients, appearing more frequently in the cervical, axillary and inguinal regions.3 6 Liver involvement is also commonly described, with a predominance of hepatomegaly and anicteric hepatitis.5 Splenomegaly is uncommon.
In laboratory findings, lymphocytosis or lymphopenia can be seen, as well as monocytosis (mainly in cases of EBV infection) and elevated transaminases, sometimes more pronounced when there is associated hepatitis (common in EBV and CMV infections).5 6
The anatomopathological examination is not very specific, and the changes may include acanthosis, hyperkeratosis, focal parakeratosis, spongiosis, vesicles, oedema of the papillary dermis with extravasation of erythrocytes and lymphocytic vasculitis.6
We considered this case interesting to share, due to the rarity of GCS, having been the first case in our service. This case report aims to highlight a rare form of exanthem secondary to common viral infections, which can be a diagnostic puzzle in clinical practice.
Case presentation
We present the case of a 13-year-old caucasian female adolescent with no significant personal history, except for two hospitalisations for pyelonephritis in the first year of life. She had a healthy weight and height for her age and had adequate psychomotor development. Portuguese immunisation schedule was up to date. Regarding her family history, her mother had allergic rhinitis and her father had venous insufficiency of the lower limbs with no other relevant history.
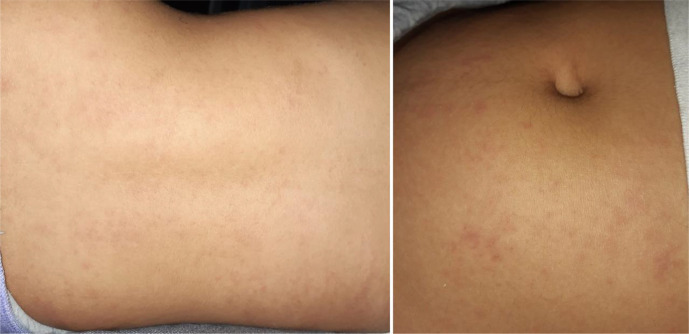
Initial clinical manifestations presented by the patient were odynophagia, fever (38°C–40.5°C), prostration and headache, associated with micropapular exanthema, mostly on the back, abdomen and neck (figure 1). Clinical evaluation on the third day of illness revealed redness of the tonsils with exudate and cervical lymphadenomegaly. To exclude group A beta-haemolytic streptococci infection, a rapid test was performed, and the result was negative. In laboratory findings, Monotest was positive, EBV IgM and IgG were positive. There was a moderate elevation of liver injury biomarkers, which led us to diagnose Infectious Mononucleosis, a frequent condition at this age. Clinical improvement was observed, with defervescence and evanescence of the exanthema on the sixth day of illness.
Figure 1.
Maculopapular exanthema on the abdomen and back in a 13-year-old female adolescent on the third day of illness.
On the seventh day of illness, there was a recrudescence of high fever with a low response to antipyretics drugs, associated with asthenia and coalescent maculopapular exanthema with lichenoid aspect, mainly on the extensor surfaces of the limbs (including the dorsal face of the hands and feet, but saving palms and soles), cervical and dorsal-lumbar regions (figure 2). She had cervical, inguinal and axillary infracentrimetric, mobile, with elastic consistency lymphadenomegalies, as well as mild painful hepatosplenomegaly. The oropharynx and the remaining observation had no relevant findings. Serologies for EBV were repeated and confirmed IgM positive. IgM for HSV type 1 and 2 were negative (with IgG positive for HSV type 1). The patient was empirically medicated with valacyclovir orally for 3 days for possible reactivation of HSV due to immunosuppression.
Figure 2.
Coalescent maculopapular exanthema on the neck, back and dorsal face of the hand in a 13-year-old female adolescent on the eighth day of disease.
For maintaining fever and extensive skin lesions associated with intense pruritus, asthenia and headache, in the 10th day of disease, she was admitted to the hospital for clinical surveillance. At admission, she had a fever of 38.6°C and a coalescent erythematous maculopapulovesicular exanthema, pink-brown coloured, with lichenoid aspect in the previously mentioned areas (figure 3), bilateral painful cervical adenopathies with elastic consistency and mild painful hepatomegaly.
Figure 3.
Coalescent pink-brown papulovesicular exanthema in a 13-year-old female adolescent on the 10th day of disease progression with an established diagnosis of Gianotti-Crosti syndrome.
Investigations
The results of the analytical evaluation done after the onset of the new exanthem (seventh day) showed relative lymphocytosis (64%–6316 lymphocytes), aspartate transaminase 1809 U/L, alanine transaminase 1092 U/L, gamma-glutamyltransferase 380 U/L, lactate dehydrogenase 2017 U/L, erythrocyte sedimentation rate (ESR) 52 mm/1st hour, C reactive protein (CRP) 1.13 mg/dL and peripheral blood smear showed 35% atypical lymphocytes. A respiratory panel (real-time PCR/FilmArray) was performed to exclude some of the most common respiratory agents, including Adenovirus, Coronavirus 229E, HKU1, NL63, MERS and OC43, Human Metapneumovirus, Rhinovirus/Enterovirus, Influenza Virus A/B, Parainfluenza Virus 1/2/3/4, Respiratory Syncytial Virus, Bordetella parapertussis and pertussis, Chlamydia pneumoniae and M. pneumoniae, in which were all negative. Serologies for CMV, hepatitis A, B and C virus, herpes zoster and HSV and enterovirus were negative for acute disease.
Skin biopsy of a lesion in the right thigh was performed, which demonstrated ‘acanthosis of the epidermis with intense superficial and focal perivascular lymphohistiocytic infiltration of the middle dermis; slight focal lichenoid changes with some larger mononuclear cells at the dermoepidermal junction; occasional exocytosis; intense focal oedema of the papillary dermis, outlining a vesicle topped by fibrin and parakeratosis. Unspecified changes that can be found in GCS’ (figure 4).
Figure 4.
Skin biopsy histopathology showing lymphocytic perivascular infiltrate, papillary dermis oedema and parakeratosis.
Differential diagnosis
The patient was examined by different medical specialties (paediatrics, infectious diseases and dermatology) and the diagnosis of GCS was assumed to be the most likely based on clinical findings and laboratory investigation. We considered the following main. The clinical and laboratory study performed excluded the primary differential diagnoses of GSC, like infectious erythema (excluded due to the absence of typical exanthema and the classic erythematous malar rash), erythema multiforme (excluded because the skin lesions were not the characteristic target lesions and IgM for HSV was negative, which is the most frequent etiologic agent), hand-foot-mouth syndrome (excluded since both palms and soles spared), scarlet fever (unlikely since group A beta-haemolytic streptococci rapid test was negative), herpetic infection (negative serologies for an acute HSV infection), scabies and insect bite reactions (excluded due to the absence of epidemiological context and typical appearance of skin lesions), lichen planus (excluded since there was no mucosal involvement), IgA vasculitis (unlikely due to absence of arthralgias, gastrointestinal or renal involvement), molluscum contagiosum (excluded by the presence of critical systemic symptoms and the different appearance of skin lesions) and drug reaction (no history of drugs consumed).1 Other viral exanthems were difficult to exclude. However, the most common respiratory virus was absent from PCR nasopharyngeal secretions testing. Another important differential diagnosis in this age range are sexually transmitted infections, like syphilis, although the patient denied sexual contact or any risky behaviours.
Treatment
The patient received symptomatic treatment with topical corticosteroids, oral antihistamines and antipyretics.
Outcome and follow-up
On the fourth day of hospitalisation (14th day of disease), due to clinical improvement, with better fever control and a slight improvement of the exanthema, associated with a decrease of liver injury biomarkers, she was discharged for ambulatory follow-up.
The adolescent was reassessed 3 days after discharge, maintaining clinical stability, with a considerable improvement of the cutaneous eruptions. After 5 weeks she was asymptomatic, with almost complete regression of the skin manifestations and biomarkers of liver damage practically normalised.
Discussion
The case described corresponds to an adolescent, that presented clinical findings suggestive of infectious mononucleosis due to EBV infection. However, after a transitory clinical improvement, a worsening occurred, with the evolution of an atypical and extensive exanthema.
The GCS was recently classified as a paraviral exanthema, which means that its characteristics are not the same as for a classic viral exanthema but are related.8 In this case, the exanthema appeared about one week after the first diagnosis of Infectious Mononucleosis. The eruptions were distributed on the extensor surfaces of the limbs, cervical and dorsal-lumbar regions and spared the chest, palms and soles, differently from what was initially observed.
Our patient had some extracutaneous manifestations associated with GSC, specifically adenopathies and hepatomegaly, with analytical evaluation showing an increase in transaminases at high levels, similar to viral hepatitis pattern. For many years, these findings were associated with HBV reactivation in CGS patients. Our patient had a complete vaccination schedule for HBV with evidence of seroconversion. Moreover, she had positive serology for acute EBV infection, corroborating the involvement of this virus as a cause for the development of GCS nowadays, as described in the cases published in the literature.4 Less typical is the worsening of fever and asthenia that occurred during the emergence of the second exanthema. The analytical evaluation revealed lymphocytosis, suggesting a viral involvement, compatible with GCS caused by EBV.
The diagnosis of GSC is usually made clinically, however, the anatomopathological study can demonstrate changes that are frequently associated with the condition, like dermal papillary oedema and infiltration by mononuclear cells.9 These findings were present in our case and along with the compatible clinical and analytical findings, the diagnosis of GSC was considered to be definitive, making this the first case of GSC in our service. Also, the favourable clinical evolution with the resolution of the lesions and normalisation of the hepatic inflammatory parameters in a few weeks corroborate this diagnosis.
GCS is a benign and self-limiting disease, therefore symptomatic and supportive measures should be privileged. Some sources recommend pruritus control with topical lotions (calamine, pramoxine, menthol, camphor, polidocanol) and an oral antihistamine.2 3 Topical or systemic corticosteroids may be indicated in more severe cases.2 3 5 In our case, treatment with topical corticosteroids and oral antihistamines were performed with appropriate symptomatic control.
Most children with GCS have an excellent prognosis, however, full recovery can take some time until the lesions completely disappear, often leading to anxiety in both patient and family. During the first 2–3 weeks, new lesions may appear, with its distribution becoming progressively more typical. The exanthema usually disappears in 10 days to 6 months (more often 2–8 weeks) but can last up to 12 months.3 After the exanthema resolution, some children maintain hypo or hyperpigmented lesions that can last up to 6 months, but scars usually do not remain.5 Adenopathies or hepatomegaly, if present, tend to regress more slowly than skin lesions. GCS may recur, although it is uncommon.5
GSC is a syndrome whose diagnosis requires a high level of clinical suspicion. The difficulty in the diagnosis, as well as the self-limited and benign evolution may explain the few reports available in the literature. This disease is likely underdiagnosed, and its occurrence could be more frequent than currently described. Therefore, it should always be a diagnosis to consider when investigating atypical exanthemas in paediatric age.
Learning points.
This is a case of Gianotti-Crosti syndrome (GCS) in a healthy female adolescent, which is a rare and self-limited condition that is characterised by symmetrically distributed skin eruptions.
The diagnosis should be made through a high level of clinical suspicion, based on skin and extracutaneous manifestations, findings in the examination, analytical exams and serologies performed, supported by cutaneous biopsy.
GCS is an underdiagnosed entity, which is why it must be considered in the presence of an atypical or extensive exanthema in paediatric age.
Acknowledgments
We would like to thank Dr Margarida Anes, Dermatologist, for the clinical evaluation and realisation of skin biopsy, and to Dr Rui Bajanca, Dermatologist and Dermatopathologist, for the histopathological analysis of the skin biopsy specimen, contributing to the confirmation of the diagnosis, and for kindly conceding the microscopical images.
Footnotes
Contributors: AA: conception of the work, analysis and interpretation of data, drafting the work, final approval of the version published. JC: revising the work critically for important intellectual content, final approval of the version published. VLPJ: revising the work critically for important intellectual content, final approval of the version published. TG: revising it critically for important intellectual content, revising the work critically for important intellectual content, final approval of the version published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pedreira RL, Leal JM, Silvestre KJ, et al. Gianotti-Crosti syndrome: a case report of a teenager. An Bras Dermatol 2016;91:163–5. 10.1590/abd1806-4841.20164410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcassi AP, Piazza CAdeD, Seize MBdeMP, et al. Atypical Gianotti-Crosti syndrome. An Bras Dermatol 2018;93:265–7. 10.1590/abd1806-4841.20186726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Dhaheri HS, Al Kaabi A, Kara Hamo Y, et al. Unusual presentation of Gianotti-Crosti syndrome due to Epstein-Barr virus infection. Case Rep Dermatol Med 2016;2016:1–2. 10.1155/2016/1017524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci G, Patrizi A, Neri I, et al. Gianotti-Crosti syndrome and allergic background. Acta Derm Venereol 2003;83:202–5. 10.1080/00015550310007210 [DOI] [PubMed] [Google Scholar]
- 5.Brandt O, Abeck D, Gianotti R, et al. Gianotti-Crosti syndrome. J Am Acad Dermatol 2006;54:136–45. 10.1016/j.jaad.2005.09.033 [DOI] [PubMed] [Google Scholar]
- 6.Chuh AAT. Md, FRCP f. Gianotti-Crosti syndrome (papular acrodermatitis). Uptodate, 2019. [Google Scholar]
- 7.Chuh A, Zawar V, Lee A, et al. Is Gianotti-Crosti syndrome associated with atopy? A case-control study and a postulation on the intrinsic host factors in Gianotti-Crosti syndrome. Pediatr Dermatol 2016;33:488–92. 10.1111/pde.12886 [DOI] [PubMed] [Google Scholar]
- 8.Lipsker D. Paraviral eruptions in the era of COVID-19: do some skin manifestations point to a natural resistance to SARS-CoV-2? Clin Dermatol 2020;38:757–61. 10.1016/j.clindermatol.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KJ, Skelton H. Histopathologic features seen in Gianotti-Crosti syndrome secondary to Epstein-Barr virus. J Am Acad Dermatol 2000;43:1076–9. 10.1067/mjd.2000.109289 [DOI] [PubMed] [Google Scholar]