Abstract
Renin cells are essential for survival perfected throughout evolution to ensure normal development and defend the organism against a variety of homeostatic threats. During embryonic and early postnatal life, they are progenitors that participate in the morphogenesis of the renal arterial tree. In adult life they are capable of regenerating injured glomeruli, control blood pressure, fluid electrolyte balance, tissue perfusion and in turn, the delivery oxygen and nutrients to cells. Throughout life, renin cell descendants retain the plasticity or memory to regain the renin phenotype when homeostasis is threatened. To perform all of these functions and maintain well-being, renin cells must regulate their identity and fate. Here, we review the major mechanisms that control the differentiation and fate of renin cells, the chromatin events that control the memory of the renin phenotype and the major pathways that determine their plasticity. We also examine how chronic stimulation of renin cells alters their fate leading to the development of a severe and concentric hypertrophy of the intrarenal arteries and arterioles. Lastly, we provide examples of additional changes in renin cell fate that contribute to equally severe kidney disorders.
Keywords: embryonic development, renin angiotensin system, cell identity, cell fate, arteriolar disease, arterial, Hypertension, ACE, Angiotensin Receptors
Introduction
Renin-expressing cells are essential for survival. They emerged in nature over 400 million years ago and have acquired throughout evolution numerous defensive functions as perfect machines to ensure tissue and whole-body homeostasis. They control blood pressure (BP), fluid-electrolyte balance, vascular development, and glomerular regeneration 1. In addition, they share an ancestry association with erythropoietin-synthesizing kidney pericytes and may participate in the regulation of oxygen delivery to tissues1. Outside the kidney, renin cells participate in hematopoietic development and together with other components of the renin angiotensin system (RAS), protect the organism against infections2.
In the adult mammalian kidney, renin-expressing cells are located in the walls of the afferent arterioles at the entrance to the glomeruli and are therefore termed juxtaglomerular (JG) cells3 (Figure 1). JG cells are sensors that manufacture and release the hormone-enzyme renin in response to minute changes in BP and the composition and volume of the extracellular fluid4. Upon reaching the circulation, renin initiates an enzymatic cascade that culminates in the production of angiotensin II, a potent vasoconstrictor which elevates BP and extracellular fluid volume (Figure 1).
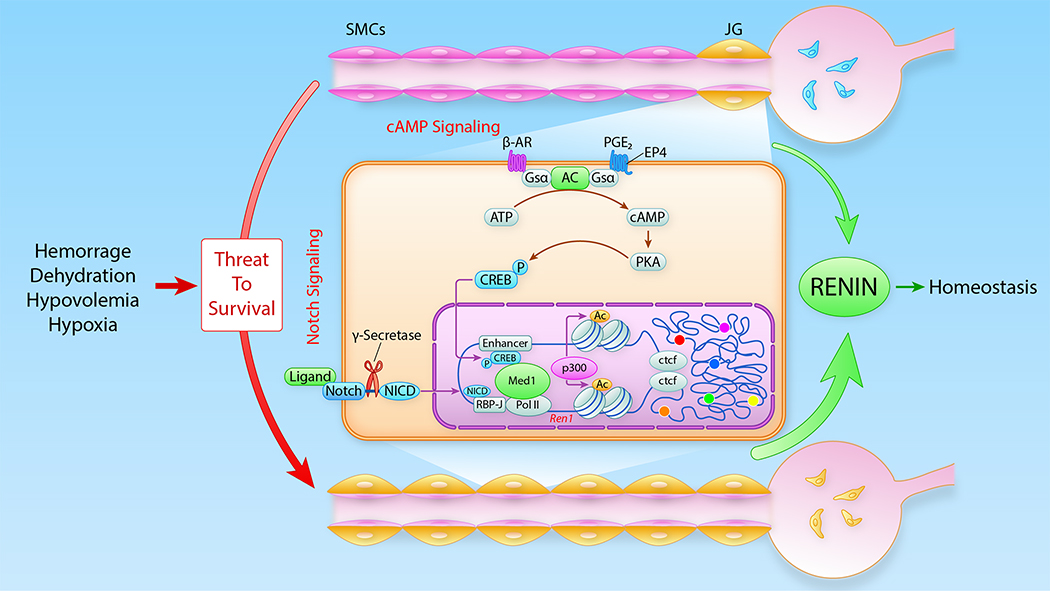
Figure 1: Schematic of the renin angiotensin system cascade and the juxtaglomerular apparatus (JGA).

Renin, the rate-limiting step of the renin angiotensin system, is secreted from juxtaglomerular (JG) cells located at the tip of the afferent arterioles at the entrance to the glomerulus. Renin converts Angiotensinogen into Angiotensin I which is further cleaved by angiotensin converting enzyme (ACE) to generate Angiotensin II. The actions of Angiotensin II are central to maintain homeostasis. The JGA includes the afferent and the efferent arterioles, the extraglomerular mesangium or Polkissen cells, and the macula densa.
The acquisition and maintenance of renin cell identity is directly responsible for the control of homeostasis. Here we review the major mechanisms that define the identity of renin cells, their embryonic origin and how they differentiate to generate the nephron-vascular units ultimately responsible for the generation of a functional kidney prepared for independent extrauterine life. We will examine how renin cells contribute to the morphogenesis and branching of the renal arterial tree and their role in the regeneration of injured glomerular cells. We will discuss how chromatin architecture controls the memory of the renin phenotype and the intrinsic plasticity of renin cells to switch identity to preserve homeostasis. Finally, we describe how permanent changes in renin cell fate lead to the development of a pervasive renal arterial disease and other, equally serious, kidney disorders.
Kidney development: nephrogenesis and vascular progenitors.
The kidney is a highly vascularized organ that in adults receives about 20% of the cardiac output. The unique spatial arrangement of each kidney arteriole with its corresponding nephron is crucial for the regulation of renal blood flow, glomerular filtration rate and other specialized kidney functions that maintain homeostasis. Thus, the proper and timely assembly of these nephron-vascular units is a crucial morphogenetic event leading to the formation of a functioning kidney necessary for independent extrauterine life. The mechanisms that govern the development of the kidney vasculature are poorly understood. In vivo, vascularization of the kidney is synchronized with epithelial nephrogenesis and the differentiation of renin cells as discussed below.
In humans, nephrogenesis of the definitive (metanephric) kidney begins about the 5th week of gestation, culminating in the creation of nearly 1 million (400,000 to 2.4 million) nephrons. In mice, however, nephrogenesis starts around embryonic (E) day E10.5-E11, concluding with the generation of 20,000–30,000 nephrons. In humans, the production of new nephrons stops after 36–38 weeks of gestation. In mice, however, nephrogenesis -and vascular development continues for a week after birth.
The development of the metanephric kidney begins with the reciprocal induction of two crucial structures derived from the intermediate mesoderm, characterized by the expression of the transcription factor Osr15 at different timepoints during early development6. Those two structures are: 1) the ureteric bud, an outgrowth of the Wolffian duct derived from the anterior intermediate mesoderm, and 2) the metanephric mesenchyme, derived from the posterior intermediate mesoderm7 (Figure 2). The ureteric bud invades and branches multiple times within the metanephric mesenchyme. Around each one of the numerous ureteric tips that are generated, the mesenchyme condenses and undergoes mesenchymal to epithelial transformation, differentiating first into a vesicle, then into a comma, followed by an S-shaped body which will differentiate into a mature epithelial nephron7. The macroanatomic structures and inductive processes are conserved in the human and mouse, albeit some individual organization and temporal dynamics with differences in gene expression exist8. Thus, around E11.5 in the early mouse metanephric kidney, there are three morphologically distinct cellular compartments with four major multipotent progenitor cells that will give rise to the whole kidney including its vasculature (Figures 2 and 3). The ureteric bud, which expresses the transcription factor Hoxb7, gives rise to the collecting ducts and ureteric epithelium9. The condensing cap mesenchyme, which expresses Six2 and Cited1, differentiates into the whole epithelial nephron, from podocytes to the distal tubules10, 11. Surrounding the condensed mesenchyme, there is a compartment of loose mesenchymal cells which are precursors for all the components of the renal vessels. As mentioned above, vascular progenitors are present in the embryonic kidney before arterioles are formed. There are two distinct progenitors within this loose mesenchyme: Foxd1 positive stromal cells and endothelial/hematopoietic precursors that express the proto-oncogene c-Kit and the stem cell leukemia/T-cell acute lymphoblastic leukemia protein 1 (SCL)12, 13. Foxd1 positive cells are the progenitors for all the vascular smooth muscle cells (SMCs), pericytes, renin-expressing cells, and mesangial cells7, 12, 14. However, differentiating renin-expressing precursors also give rise to a subset of: 1) arterial SMCs, 2) pericytes, and 3) mesangial cells with the capability, to de-differentiate and re-express renin when homeostasis is threatened15 (Figure 4).
Figure 2: Kidney precursors and their contribution to nephrovascular development.
Schematic containing the four main progenitors that give rise to the whole kidney. The early metanephric mesenchyme is subdivided into condensed (Six2+ precursors, darker area) and loose (Foxd1+ and Endothelial precursors, lighter area) compartments. Highlighted in yellow are epithelial cells/structures that transiently express renin during early embryonic kidney development but do not originate from the same renin precursor that gives rise to mural cells of the vasculature. EC, endothelial cells; HSC, hematopoietic stem cell; SMC, smooth muscle cell. (Illustration credit: Ben Smith)
Figure 3: Renal progenitor cell compartments and the structures they build.

Simplified version of the nephron and its vasculature, stretched for clarity. AA, afferent arteriole; EA, efferent arteriole; JG, juxtaglomerular; SMC, smooth muscle cell.
Figure 4: Distribution of renin expressing cells during kidney development and in response to homeostatic challenges.

In the fetus renin cells (in light green) are present along the afferent arterioles (AA), in the intra and extraglomerular mesangium, and in larger arterioles and arteries. With maturation they become restricted to the juxtaglomerular (JG) area as they differentiate into vascular smooth muscle cells (SMC) and mesangial cells (both in red). In response to homeostatic threats in adult life, such as dehydration, sodium depletion, hypotension and other manipulations there is an increase in the number of renin cells resembling the fetal pattern, by reacquisition of the renin phenotype. EA, efferent arteriole.
The renal endothelium has a distinct origin from the mural cells of the arterioles (Figure 3.) Here, we use the term “mural” to indicate the arteriole layer composed of SMCs, renin cells and pericytes. The endothelial cell layer is excluded.
Using fate tracing in inducible transgenic mice to label explicitly endothelial and hematopoietic precursors, cross-transplantation studies, and hematopoietic colony forming assays, we identified a common progenitor expressing the helix-loop-helix transcription factor SCL that gives rise not only to the renal endothelium but also to blood precursors16. However, the intermediate (downstream from the SCL) precursors for the renal endothelial cells remain more elusive. Some examples follow: a group of cKit positive cells that expresses Flk1 or Tie1 gives rise to renal vascular endothelial cells17, 18. Moreover, using ex vivo re-aggregation assays combined with fate mapping and in vivo and in vitro depletion of specific populations of endothelial precursors, a recent study identified varied subpopulations of endothelial precursors, one of them expressing the melanoma cell adhesion molecule (MCAM/MUC18/CD146) and highlighted the role of CD146+ cells during renal vascular development19. In fact, elimination of the CD146+ cells from the embryonic metanephric mesenchyme prevents the differentiation of endothelial cells19. Thus, it seems that SCL+ progenitors can differentiate into subgroups of intermediate precursors, all culminating in differentiated endothelial cells. The physiological reasons for this heterogeneity remain to be ascertained.
In addition to vascular endothelial growth factor (VEGF), Angiopoietins and Notch signaling pathways, one of the most prominent mechanisms controlling the development of endothelial precursors and their subsequent coating by vascular SMCs is the sphingosine 1-phosphate pathway. Sphingosine 1-phosphate (S1P) is a phospholipid released into the circulation by platelets, erythrocytes, neutrophils, and mast cells, which promotes vascular stability and controls endothelium permeability by modulating the function of endothelial tight and adherens junctions20. S1P operates either one of five G-protein coupled receptors (S1PR1–5) that provide functional variations and cell specificity. Supporting the crucial role of S1PR1 in vascular maturation, embryos with global deletion of the S1PR1 die in utero at E14.5 affected by widespread hemorrhages and severe edema due to the absence of SMC coating of the vasculature21. S1P acting on its S1PR1 receptor is necessary for the appropriate assembly of the kidney vasculature, including the mural cell coating of arteries, the development of glomerular capillaries, and lymphatic vessels16. Inducible deletion of S1PR1 resulted in dilatation of the renal arteries and veins, with aberrant coating of SMC and excessive proliferation of endothelial cells, and the formation of glomerular capillary shunts due to the inadequate differentiation of mesangial cells. In addition, the growth of lymphatic vessels in the kidney was stunted16. Using a combination of inducible genetic mosaics, fate mapping, and transcriptomics, a recent and elegant study showed that whereas the VEGF and Notch signaling pathways are crucial for arterialization during vascular development, they act by inhibiting Myc in endothelial cells, thereby suppressing its ability to stimulate cell proliferation and metabolism22. The study suggests that the sole inhibition of Myc in endothelial cells is sufficient to promote arterialization, independent from a genetic predetermination by Notch22.
Whereas the precise site of origin of the embryonic epithelial nephron is firmly established as intrinsic to the differentiating renal anlagen, determining the place of origin (renal versus extrarenal) of the renal vasculature has been more difficult to establish. Early interspecies cross-transplantation experiments of embryonic kidneys into quail or chick chorioallantois membranes suggested that the renal endothelium originated by angiogenic sprouts from outside the kidney23, 24. However, using a variety of transgenic mice and lineage tracing studies, we and others showed that the early embryonic kidney, at a time when the vessels are not yet formed, already possesses all the precursors of the renal vasculature17, 25–27. We hypothesized that those precursors were capable of differentiating and assembling the arterial and arteriolar vessels containing renin, SMCs, and endothelial cells. The hypothesis was experimentally proven by transplanting early nonvascular embryonic kidneys under the renal capsule -or into the anterior chamber of the eye- of adult mice and showing that all the vascular structures-and their constituents cells- developed from the embryonic kidney25–28. Further, if the endothelial precursors of the embryonic kidney were ablated or injured by conditional expression of diphtheria toxin, there was a lack of differentiation of endogenous cells without compensation by endothelial cells from the host16. Those studies highlight the pivotal role of the existing endogenous precursors on vascular formation within the early embryonic kidney. However, if the embryonic kidney is transplanted under the kidney capsule of a newborn mouse -when nephrogenesis is still active- vascularization of the glomeruli within the transplanted kidney is chimeric: endothelial cells originate from both the embryonic kidney and the newborn host17, indicating that during development, endothelial precursors from the developing neonatal cortex can be attracted by angiogenic signals from the embryonic glomeruli. In the developing embryo, before the renal capsule becomes an external barrier, it is almost impossible to separate the developing kidney from the surrounding mesenchyme. Erythroblasts, as well as endothelial precursors, surround the kidney and the ureteric bud. Furthermore, during development, there may also be a contribution from circulating vascular precursors. Cells derived from Foxd1+ precursors extend beyond the early developing kidney, surrounding it with no clear boundaries between the developing kidney and the extrarenal mesenchyme. Therefore, before the renal capsule establishes a physical barrier, there may be a balanced involvement of precursors from the early metanephric mesenchyme and some from the surrounding mesenchyme that enter the developing kidney and contribute to the formation of the whole kidney vasculature, including renin cells. Of note, kidney organoids generated from human embryonic stem cells or induced pluripotent stem (iPS) cells, not connected to a circulation, possess a -rudimentary- endothelial network with lumen formation and with some endothelial cells incorporated into the glomerulus which are progressively lost with time29, 30. The lack of appropriate vascularization of kidney organoids is a major roadblock in developing autologous organs for transplantation. However, kidney organoids subjected to shear stress increase the number of endothelial cells derived from the organoid suggesting that connection to a circulation may facilitate the development of a functional vasculature31. But, when human kidney organoids are transplanted under the kidney capsule of adult immunocompromised mice, the vascularization occurs by the contribution of mouse endothelial cells and not from precursors within the organoids30. Still, even after transplantation, the growth of the organoids may reach a limit due to exhaustion of precursors and/or regression. So far there are no reports of transplanted organoids beyond 28 days30. Understanding how to vascularize organoids would be a huge step in generating kidneys and other organs using the patient’s own iPS cells.
Renin cells and arteriolar development.
Renin cells are essential for the proper morphogenesis of the renal arteriolar tree. Their contribution to the assembly of the arterioles follows a distinctive, fractal-like, developmental pattern. Initially, differentiating renin expressing cells are few and randomly distributed in the stromal-interstitial compartment. As development progresses, the renin cells participate actively in the morphogenesis and branching of the renal arterial tree32. As a new branch is about to form, a few renin cells group as a macula, which then bulges and elongate, maturing into a new arteriolar branch32. As the cells mature further and the vessel consolidates, those renin progenitors differentiate into SMCs. This process lasts several weeks and reiterates multiple times -from the renin cells’ first appearance in mice around E11–12 and postnatally- until maturation and elongation of the arterial tree is completed33. Throughout this process, arterioles and arteries are at different stages of development and maturation. Therefore, during early embryonic and postnatal development, the distribution of renin cells is extensive, heterogeneous, and changes continuously until the arterial tree’s growth is finalized34–36. As the vessels mature and the cells differentiate into SMCs, renin-expressing cells are gradually confined to the JG area, where they remain throughout adult life35. This broad and shifting pattern of renin expression along the arterioles and interlobular arteries has been found in every mammal examined, including rats, mice, pigs, sheep, and humans3, 37–40. Further, the developmental shift in renin expression during mammalian development is reminiscent of its phylogenetic changes: from large arteries in fish to the classical JG localization in mammals41, 42. The factors that control the shift in renin distribution during ontogeny and phylogeny are not well understood but are likely coupled to the mechanisms that control the differentiation of renin cells into SMCs and mesangial cells. It is believed that the function(s) of renin cells in early development are related to tissue morphogenesis rather than to regulation of arterial BP and fluid-electrolyte homeostasis 33, 41, 43, 44. This endocrine function is crucial in extrauterine life.
The main factors that control the differentiation and maintenance of renin cells are the cAMP pathway45–49, the Foxd1 transcription factor, and the Notch/ RBP-J pathway14, 50.
The cAMP pathway is well known to control renin synthesis and release4, 48, 51–53. Beta-adrenergic receptors linked to Gsα and adenylate cyclase are crucial in the generation of cAMP which ultimately regulates the expression and release of renin4, 48, 51–54. JG cells are densely innervated by sympathetic nerve terminals proceeding from the main renal nerve55 (Figure 1). Thus, stimulation of membrane-bound beta adrenergic-receptors via locally released -or circulating- catecholamines lead to the activation of G protein subunit alpha (Gsα) and adenyl cyclase resulting in the generation of intracellular cAMP, activation of protein kinase A, and phosphorylation of the transcription factor Creb (cAMP responsible element binding protein) and/or additional transcription factors important for the renin cell phenotype. In turn, Creb binds to the cAMP-responsive element (CRE) located within the 5’ regulatory region of the renin gene. The most prominent CRE is situated within the traditional renin enhancer. Access to this region and binding of transcription factors is facilitated by histone acetyl-transferases CBP/p300, which introduce acetyl residues in histones, namely Histone H3. Acetylation of lysine 27 of H3 (H3K27ac) promotes the local relaxation of chromatin and the displacement of nucleosomes opening the access for Creb1–3 and other transcription factors, which upon binding to DNA causes transcription of the renin gene. In a similar manner, cAMP also activates other genes characteristic of the renin phenotype, including Akr1b756.
Gene deletion studies have demonstrated that most of the cAMP pathway components are necessary for the differentiation and/or maintenance of the renin cell. Thus, conditional deletion of beta-receptors in renin cells results in a substantial decrease in the number of renin cells in the vasculature accompanied by arteriolar abnormalities in early life46. Similar results are obtained when Gsα is deleted in renin cells49, 57. Moreover, dual homozygous conditional deletion of CBP and p300 leads to an absence of renin cells and severe nephron-vascular abnormalities47, 48. These results unmistakably establish the importance of the cAMP pathway in renin cell development and its central role in maintaining kidney vascular integrity.
The Notch/RBP-J pathway is an ancestral cell-to-cell communication system that regulates cell fate determination during development58, 59. Because renin cells have Notch receptors, their ligands, and the central transcription factor Rbp-J60, which conveys the transcriptional activity of all Notch receptors, we examined the consequences of deleting Rbp-J in cells from the renin lineage and their precursors the Foxd1+ stromal cells. Removal of Rbp-J in renin cells caused a remarkable change in the identity of the renin cells50, 61 with decreased expression of renin and smooth muscle genes accompanied by their ectopic expression of hematopoietic and fibroblast-related genes. When the deletion was performed in the most primitive progenitor, the stromal Foxd1-expressing cell, in addition to fewer renin cells, the arterial vessels were thinner, and the glomeruli were devoid of mesangial cells, which led to the development of glomerular aneurysms14. These findings indicate that the Notch/RBP-J pathway is crucial in the differentiation of renin cells and the morphogenesis of the kidney arterioles and mesangial cells. The studies also suggest that the phenotype is heightened when the deletion of Notch function occurs in cells hierarchically upstream from the renin precursors.
As it can be gathered by the aforementioned reports, the stromal compartment plays a fundamental role in the development of the kidney vasculature and in the differentiation of renin cells. In fact, ablation of Foxd1 cells with diphtheria toxin subunit A, or whole-body deletion of the transcription factor, Foxd1, resulted in similar structural kidney abnormalities characterized by renal hypoplasia due to reduced nephron number and abnormal branching of the ureteric bud62–64. The renal vasculature was markedly abnormal including the absence of the main renal artery and its replacement by multiple branches originating from the aorta. Those ectopic vessels penetrate the kidneys through the renal capsule joining aberrant subcapsular arteries and arterioles in a centripetal fashion rather than the normal centrifugal direction from the renal hilum12. Importantly, removal of Foxd1 or the Foxd1-expressing stromal progenitors resulted in a decreased number of renin cells suggesting that this transcription factor governs a molecular program that controls the origin, number, and orientation of the renal arterioles and the cellular endowment of the renal vasculature12. Given the complexity of the phenotypes elicited by the deletion and cell ablation experiments mentioned above, including ureter and nephron alterations, further work is necessary to dissect how Foxd1 controls guidance cues, cell number, and the fate of the nephron and its vasculature.
Another example of a remarkable change in the phenotype of renin cells was evidenced by the conditional deletion of the von Hippel-Lindau (Vhl) gene in cells of the renin lineage65. JG cells stopped expressing renin and other renin cell specific markers such as Akr1b7 and produced instead erythropoietin, an effect that was stimulated by the accumulation of HIF-2α leading to increased levels of circulating erythropoietin and policytemia66. Whereas, in this case, the preglomerular arterial tree did not seem to be affected65 deletion of Vhl in the Foxd1 progenitors resulted in vascular abnormalities due to impaired differentiation of vascular SMCs, mesangial cells and renin cells67. A recent study in rodents showed that in response to either acute or chronic anemia, some erythropoietin producing renal interstitial fibroblasts also make renin, likely due to a decreae in blood volume and pressure, but not in response solely to hypoxia68.
Cell-to-cell interactions and renin expression
Renin cells are sensors that receive constant mechanical, neurohumoral, and biochemical signals from other cell types and the extracellular matrix where they reside. In fact, when renin cells are cultured, they stop making renin in about 48–72 hours48, 69 and differentiate into SMCs, suggesting that the renin cells depend heavily on their communication with adjacent structures to maintain their phenotype. Although it has not been formally tested, we hypothesize that continuing stimulation by adjacent -smooth muscle, endothelial, mesangial, macula densa- cells, and nerve fibers are important to maintain the identity and function of renin cells. Recent experiments regarding how renin cells communicate with other cell types are briefly discussed below.
Connexins and Pannexins
Connexins are a family of widely expressed transmembrane proteins that assemble to form channels (gap junctions) between adjacent cells. They play a crucial role in cell-to-cell communication via the intercellular passage of inorganic ions, including Ca2+ and Na+, and secondary messengers such as cAMP and ATP. Within the kidney vasculature, endothelial cells express connexin (Cx)37, Cx40, and Cx4370. SMCs express Cx45 and Cx43, and JG cells express predominantly Cx40 and lesser amounts of Cx37 and Cx4370. Mice with total deletion of Cx40 -the most abundant connexin in JG cells- display severe pathological and, at first sight, paradoxical findings due in great part to the unusual location of renin cells which are displaced from the JG area and are located around the glomeruli71. Because renin cells have the intrinsic ability to detect and respond to mechanical stimuli such as arterial pressure or soluble signals derived from the macula densa, when they are displaced from their context, they cannot possibly sense signals from other components of the JG apparatus (JGA) in the usual fashion and are constantly stimulated to produce large amounts of renin. As a result, the animals are hyperreninemic and have malignant hypertension71. Conditional deletion of Cx40 in renin-expressing cells mimics the phenotype of the total knockout (KO) mouse, whereas when the deletion is targeted to the endothelial cells, BP is not affected72. Moreover, when Cx40 is restored in renin-expressing cells of mice with a global deletion of Cx40, the hypertension is significantly reduced70. On the other hand, lack of Cx37, Cx43, and Cx45 in renin cells does not affect BP or plasma renin activity70, underscoring the specific role of Cx40 in renin cells to control their JG location, renin synthesis, and release.
Pannexins are a family of ubiquitous large transmembrane molecules that aggregate to form non-junctional channels permeable to calcium and purines73, 74. Pannexin 1 (Panx1), which is known for its role in adenosine triphosphate release, is expressed in the vasculature, including the SMCs of the kidney arterial tree75, 76. A recent study investigated whether Panx1 channels in renin cells regulate renin secretion in vivo77. Conditional deletion of Panx1 in cells from the renin lineage resulted in a modest increase in plasma renin and aldosterone and mean arterial BP, which was more pronounced during the “active” phase of the day. This effect was restricted to male mice and only evident in female mice after ovariectomy, providing another example of sex-related differences in BP regulation. This finding is consistent with previous work, which found that estradiol can suppress renin expression and clinical observations of reduced renin levels in patients receiving estrogen replacement therapy78, 79. Intriguingly, mice with a lack of Panx1 in renin cells and wild type controls showed the same response in plasma renin and BP during acute angiotensin II administration or after stimulation of renin secretion by low sodium diet and captopril treatment77. However, in response to a low sodium diet and captopril, the increase in renin positive areas was reduced in mice with deletion of Panx1 in renin cells while still maintaining the same increase in renin mRNA as in mice with an intact Panx1. It has also been reported that mice with concomitant deletion of Cx40 and Panx1 mimic the cardiovascular phenotypes exhibited in Cx40 KO mice but show kidney renin mRNA and plasma renin activity levels higher than the Cx40 null mice80. Further studies will be necessary to understand these intriguing findings and confirm whether Panx1 regulates renin secretion. Many questions remain answered, including whether inactivating mutations of Cx40 in humans result in renin-dependent hypertension. Further, how connexins participate in the periglomerular location of renin cells is also unknown. Equally important from a physiological point of view, the participation of Cx40 in the operation of the baroreceptor control of renin secretion requires more work. Although Cx40 may be important in the transmission of the BP along the afferent arterioles, it is unlikely that Cx40 is the main structural component of the renin cell baroreceptor -as we initially thought- for control of renin release81. The elusive renal baroreceptor is most likely an intrinsic mechanical sensing response located at each individual JG cell. In fact, it seems that cells that lack Cx40 have an intact capacity for sensing a decrease in pressure; because of their miss location, they constantly perceive a reduction in BP and thus hyper secrete renin leading to malignant hypertension.
Integrins
Integrins are a large family of cell adhesion molecules crucial for cell-to-cell and cell-to-matrix interactions. They are transmembrane heterodimers consisting of an alpha (α) and a beta (β) subunit. β1-integrin (Itgb1) is widely expressed in many tissues and is essential for embryonic development. In fact, homozygous deletion of Itgb1 is embryonically lethal during the early post-implantation period82. Itgb1 is the most abundant β-integrin subunit in the kidney83–85. It is necessary for cell adhesion to the ECM, differentiation, migration, apoptosis, and proliferation83. We found that Itgb1 is expressed in renin cells throughout development86. To define whether Itgb1 was necessary for renin cells’ development and function, we generated mice with deletion of Itgb1 in renin-expressing cells and their descendants86. The experiments showed that Itgb1 was required for the maintenance and survival of renin cells and their descendants. Mice without Itgb1 in renin cells showed early signs of failure to thrive, dehydration, hypotension, normocytic normochromic anemia, low circulating renin, and renal failure. Kidneys from mutant mice were small and pale86. Their surface was rough and granular, with cortical concavities86. The histological examination uncovered a marked reduction in the number of renin cells along the renal arterioles, accompanied by vascular alterations86. Further experiments showed that mutant renin cells underwent apoptosis, suggesting that the reduction in the number of renin cells was due to the absence of integrin-mediated survival signals and likely detachment and anoikis of renin cells86.
The lack of Itgb1 resulted in vascular alterations characterized by a reduced number of thin glomerular arterioles suggesting a branching defect86. This phenotype is different from the phenotype found in mice with RAS gene KOs. Whereas in both cases there is a defect in branching, in RAS gene KOs, each arteriole displays concentric hypertrophy due to accumulation of SMCs and renin cells87. In addition, continuously stimulated renin cells in the RAS gene deletions produce a variety of growth and angiogenic factors that may induce the concentric vascular SMC growth87. Absence of cells of the renin lineage seems to confer protection against vascular hypertrophy. Our findings in mice with Itgb1 deletion are reminiscent of those found when diphtheria toxin was knocked into the renin locus to target renin cells’ death. In both cases, vascular hypertrophy did not occur88, indicating that renin cells per se are responsible for the vascular hypertrophy. Overall, the aforementioned studies suggest that integrin function in renin cell precursors is essential for the appropriate differentiation and/or survival of the mural cells of the kidney arterioles.
MicroRNAS, renin and hypertension
MicroRNAs are crucial in the maintenance of the renin phenotype89. Deletion of Dicer the micro-RNA processing enzyme results in absence of renin cells, decreased circulating renin, hypotension and the replacement of the affected renal arterioles with a peculiar form of striped renal fibrosis89. In a recent study, the laboratory of Bernal Mizrachi uncovered a unique link between macrophages, the vitamin D receptor (VDR), production of miR106b-5p and renin synthesis by JG cells90. First the investigators showed that myeloid specific deletion of the VDR induced hypertension. Subsequently, they showed that macrophages lacking the VDR secrete miR-106b-5p. This microRNA enters the JG cells, downregulates the expression of Pde3b and E2f1 leading to increased renin synthesis and release via downstream mediators of the cAMP pathway90. Because the hypertension induced by macrophage VDR deletion is prevented in mice with deletion of miR-106b-5p suggests that miR-106b-5p may be a central player in the mediation of renin-mediated hypertension and may be a molecular target for the management and prevention of hypertension and vascular injury.
Communication with endothelial cells
In addition to receiving signals from other cells in the JGA, renin cells may influence the behavior of adjacent cells. As indicated above, the cAMP pathway is crucial not only for the synthesis and release of renin but also for normal vascular development46, 49. Constitutive deletion of Gsα in renin cells from early life resulted in reduced renin expression, hypotension, rarefaction of the renal arterial tree, and kidney failure57. However, when the same deletion is induced in adult life, affecting Gsα just in JG cells -thus avoiding developmental effects- results only in a transient decrease in renin and hypotension at one month91. This phenotype was spontaneously rescued by the production of renin via dedifferentiation of vascular SMCs of afferent arterioles upstream of the JG areas91. The experiment illustrates how resilience is achieved by an innate ability of the cells to readjust their phenotype and compensate for one another, at least transiently, to preserve well-being. However, after six months, those same mice lacking Gsα in JG cells developed thrombotic microangiopathy with marked reduction of endothelial cells within glomeruli, interstitium, and vasa recta, ultimately leading to chronic kidney damage91, 92. The experiments suggest that in adult life, functional JG cells -with an intact cAMP pathway- are vital for the maintenance of the glomerular and post-glomerular microvessels. Because this effect is independent of circulating renin, the mechanisms whereby JG cells preserve endothelial cells and regulate tissue remodeling are likely to involve cell to cell communication signals and/or secretion of paracrine factors such as angiogenic factors (i.e., VEGF-D) and pro-fibrotic factors produced by JG cells60, 93. Although cells from the renin lineage can repopulate glomerular mesangial cells in models of mesangiolysis/focal glomerular sclerosis as discussed below94, it seems that JG cells are unable to replace glomerular endothelial cells as recently shown in an elegant model of thrombotic microangiopathy95. These interesting experiments open the possibility of discriminating and understanding which mechanisms JG cells possess to repair or not nearby cells.
Renin cells and glomerular regeneration
In addition to their role in kidney vascular morphogenesis and the protection of circulatory and fluid electrolyte homeostasis, renin cells may participate in the repair and/or regeneration of glomerular cells94, 96–98.
Using an experimental model of mesangial proliferative glomerulonephritis94, Starke and colleagues inquired whether renin cells could replace mesangial cells that have undergone mesangiolysis. The investigators found that, in fact, cells from the renin lineage repopulated about sixty percent of the dead glomerular mesangial cells. Once the cells acquired their new identity, they stopped manufacturing renin and expressed markers of mesangial cells. It seems that the newly restored mesangial cells differentiated from renin cells. The actual mechanism and molecules involved remain to be defined, but it seems that renin cells can regain their ancestral progenitor capabilities and constitute a ready niche for the repopulation of injured cells.
In an additional model of focal segmental glomerular sclerosis elicited with an anti-podocyte cytotoxic antibody, Pippin and colleagues97 found that cells from the renin lineage repopulated injured podocytes and a portion of the Bowman’s capsule parietal epithelial cells. The researchers suggested that cells from the JG area replaced the injured podocytes96. Again, upon reaching the glomerulus, the formerly renin-expressing cells stopped expressing renin and instead expressed podocyte markers. This intriguing finding elicits new questions. Given that podocytes descend from Six2 progenitors and JG cells derive from Foxd1 progenitors (Figure 2), it would be critical to define whether adult JG cells have the capability to trans-differentiate into another, lineage-unrelated adult cell type. If transdifferentiation is indeed involved, it will require a massive rearrangement of the transcriptional programs and chromatin architecture to suppress the genes that control renin cell identity and activate those that drive the expression of podocyte identity genes. On the other hand, it should be remembered that epithelial cells of the Bowman’s capsule and proximal tubules derive from Six2 progenitors that during their differentiation transiently express renin during embryonic life at the pretubular aggregate and renal vesicle stages (Figure 2 and The Human Nephrogenesis Atlas). It is reasonable to assume that these epithelial cells closely related by lineage and proximity have the capacity to replace podocytes as well.
The nature of the signals that activate JG cells and the precise mechanisms responsible for such marked cellular transformation remain to be clarified. Because of its potential medical importance, one aspect that should be addressed soon is whether the repair process is a transient phenomenon, a simple reaction to injury or whether it results in sustained improvement of kidney function.
The phenomenon of renin cell recruitment: controlling homeostasis by modulating cell number.
In adult mammals, the number of JG cells is small: 0.01 % of the kidney cells.50, 61, 87, 99 Under normal circumstances, the release of renin by those few cells usually suffice to maintain BP and fluid-electrolyte balance. However, if an adult animal is subjected to manipulations that threaten homeostasis, such as hypotension, dehydration, sodium depletion, or treatment with RAS blockers, additional cells along the renal arterioles are “recruited” to synthesize and release renin (Figure 4). Recruited cells are SMCs that de-differentiate and become renin-expressing cells once again15. The ability to produce ample quantities of renin allows animals to restore their BP, extracellular fluid volume and composition, tissue perfusion and oxygen delivery to critical organs, thus ensuring survival. During such homeostatic threats the three major mechanisms that control renin synthesis and release -the renal baroreceptor, the macula densa, and the β-adrenergic receptors- are activated and potentiate each other. Central to the operation of these mechanisms is the regulation of cAMP generation, ultimately the major intracellular effector of renin expression, renin release and renin cell identity45–49. During salt depletion activation of Cyclooxygenase 2 results in the production of prostaglandin E2 which -via EP4 receptors- stimulates production of cAMP in nearby JG cells and presumably in newly transformed SMCs100. Likewise, deletion of transporters such as CLCK2results in a form of salt losing nephropahy101 and deletion of NKCC2 resembles Bartter’s syndrome102. In both cases there is marked recruitment of renin cells along the renal arterioles. Thus, induction of volume-electrolyte depletion by salt restriction, dehydration, and/or genetic interference with molecules devoted to salt conservation results in marked renin cell recruitment along the renal vessels.
During high salt conditions, the opposite is observed: a decrease in the number of renin-expressing cells. Whether this can be linked to the release of adenosine by macula densa cells affecting intracellular cAMP in nearby cells of the renal arterioles remains to be determined103.
How a decrease in pressure induces the recruitment of renin cells has not been clearly delineated. In fact, the nature of the pressure-sensing mechanism controlling renin synthesis and release (the baroreceptor) is unknown. The role of TRPV4 and piezo channels remains to be independently corroborated given that the expression of these molecules is minimal to non-existent in native renin cells99.
In addition to mechanisms that increase the number of renin cells, angiotensin II exerts a negative feedback on the amount of renin released to the circulation, thereby preventing hypertension. Although, in vitro, angiotensin II has a direct effect on the number of cells secreting renin, in vivo the ability of angiotensin II to suppress renin cell recruitment, and renin release seems to depend heavily on the BP levels51, 103–105. As discussed below, in this process, there is no proliferation or cell migration,106–108 SMCs along the arterioles, sometimes mesangial cells and interstitial peritubular pericytes and are transformed to synthesize renin1, 15, 109–111. Thus, the ability to reestablish homeostasis is in large part determined by the number of cells that synthesize the hormone37, 111. The transformation of these cells is not limited to the expression of Renin: the cells adopt an epithelioid appearance, they produce granules to store renin, their endoplasmic reticulum and Golgi become prominent, their glycogen content increases and the number of myofibrils is diminished3, 60, 107. Underlying such remarkable morphological changes, a whole molecular program is activated: expression of Renin is accompanied by enhanced production of Akr1b7, an aldo-keto-reductase that detoxifies harmful aldehydes produced during the high synthetic period of the cells56, 60. Expression of Akr1b7 follows that of renin throughout development and when newly transformed cells acquire the renin phenotype. In fact, expression of Akr1b7 is now considered a novel and highly reliable, renin-independent marker of cells programmed for the renin phenotype56, 60. The newly transformed cells express miR-330, transcription factors such as Nkx3.1, KLF2, and a variety of genes involved in granulopoiesis, secretion, and angiogenesis60, 87. Once homeostasis is reestablished, the transformed cells stop making renin and become SMCs again. At this stage, the cells are still plastic and can reversibly switch back and forth between phenotypes. However, if the physiological challenge is not overcome, the cells continue to transform from a reversible SMC←→Endocrine state to an Embryonic-Invasive phenotype. This progressive, pathological transformation of the cells is at the core of a concentric arterial disease described below.
This phenomenon -the increase in renin cell number- has received various -confusing- names such as “recruitment”, “metaplastic transformation”, or “JG cell hyperplasia”. The first term continues to be used in part because it suggests the notion that additional cells participate in the process. However, it generates the erroneous, implicit concept that the “recruited” cells originate and migrate from another site other than the arterioles. The process is reversible and does not require migration3, 15. The term “metaplastic transformation” is rarely used as it conveys the idea that there is a pathological irreversible transformation of one cell to another. Finally, the term “JG cell hyperplasia” indicates that the JG cells proliferate. Although there is cell hypertrophy, there is no DNA replication or cell division, even in chronic cases108. Because proliferation does not occur, the term hyperplasia should therefore be avoided. We know that the increase in renin cell number occurs by de-differentiation or retransformation of SMCs -which, having descended from renin progenitors, are capable of switching back from a contractile to an endocrine phenotype resembling the embryonic pattern of renin expression described above15 (Figure 4). A small percentage of renin-expressing cells may be generated by a process denominated neogenesis, defined as de novo expression of renin by cells that never expressed it98. Thus, most of the arteriolar SMCs acquire and retain the memory of the renin phenotype. Such memory resides in the cells’ chromatin and is driven by a set of epigenetic marks at the locus of the renin gene and additional coordinated loci throughout the genome99. It is possible that during embryonic development, the early expression of renin throughout the renal arteriolar SMCs imprint these cells – epigenetically – for de-differentiation and renin expression during serious threats to homeostasis. The ability to respond to such homeostatic threats emerged early in evolution, having been observed in zebrafish43, 44, 112, and is retained throughout phylogeny. Whether this plasticity to transform bestows an evolutionary advantage remains to be studied. The capability to re-express renin is not limited to the kidney and has also been observed in the adrenal gland. In embryonic life, the fetal zone of the adrenal cortex expresses renin113 well before renin cells appear in the kidney25. As the renin-expressing cells from the adrenal gland differentiate into other cell types and the adrenal gland is remodeled, they cease to express renin. Still, the organ retains radial stripes of cells derived from the renin lineage15. As in the kidney, those cells from the renin lineage maintain the plasticity to re-express renin, becoming overly manifest in mice subjected to global deletion of the aldosterone synthase gene114. Similarly, mice subjected to bilaterally nephrectomy and excision of the submandibular glands exhibit strong renin expression in the adrenal glands’ inner cortex113, 115. It would be interesting to understand whether the transformation of adrenal cells to synthesize renin is controlled by the same epigenetic and transcriptional mechanisms that control renal arteriolar SMCs’ transformation. In addition to the kidney and adrenal glands, the recruitment phenomenon has been observed in other tissues, including insulin-producing beta cells, atrial natriuretic peptide-producing myocardial cells, follicular cells of the thyroid, erythropoietin producing cells, and albumin-synthesizing hepatocytes116–119. Thus, increasing the number of cells -by the transformation of pre-existing cells- to perform a specific function (such as the synthesis and release of a particular hormone) in response to a physiological challenge is a phenomenon of major biological importance conserved among different cell types and species as a mechanism to preserve homeostasis. It would be important to ascertain whether these various cell types share the same molecular-epigenetic mechanisms that control recruitment.
Chromatin architecture of renin cells and the memory of the renin phenotype.
The plastic, reversible transformation of SMCs to the renin phenotype occurs not only in vivo but also in vitro in cells derived from the renin progeny.99 This suggests that the reacquisition of the renin phenotype is an intrinsic, cell-autonomous process whereby each cell preserves the ability to sense and respond.37, 51, 120 The ability to switch on and off the renin cell identity depends on the developmental history of the cells. As mentioned above, renin cells are progenitors for arteriolar SMCs, mesangial cells, and interstitial pericytes15 (Figure 2). Those descendants retain the memory to synthesize renin when a physiological or pathological challenge necessitates that more renin be produced to recover homeostasis.15 Preservation of such molecular memory is a fundamental mechanism to respond to homeostatic threats. Discovering where in the genome the memory of the renin phenotype resides, how it is constructed, and how it is retained or erased when the cells differentiate or their physiological status changes is crucial to understand how renin cells control homeostasis or lead to cardiovascular and/or renal pathology. Investigating these questions, we found that the molecular memory of the renin phenotype resides in specific chromatin sites and configurations that control the identity of renin cells99.Using genome-wide ChIP-Seq for Mediator complex 1 (Med1), H3K27Ac (active enhancers), Pol II (genomic areas undergoing transcription)121 and chromatin accessibility using ATAC-seq122, 123 we mapped the chromatin landscape of native renin cells isolated from Ren1d-GFP mice, Ren1c-YFP mice, and As4.1 cells, a tumoral JG cell line that expresses and secretes renin constitutively. The results obtained from these renin cells were compared to those from twenty-one cell types that do not express the Renin gene. As shown in Figure 5 the chromatin of renin cells at the regulatory locus of the Renin gene is open for transcription and it is marked by the deposition of H3K27ac, a histone mark characteristic of active enhancers and Med1, a member of the macromolecular transcriptional complex whose conditional deletion in renin cells resulted in fewer renin cells, decrease circulating renin and hypotension99.
Figure 5: Renin cells possess a unique set of SEs that differentiate them from other cell types.
Top: Renin cells share ninety-one super-enhancers, including the renin super-enhancer, which are absent in other 21 tissue types. Bottom: The renin super-enhancer (grey bar) located upstream of the coding region of the renin (Ren1) gene shows overlap of the ATAC-Seq profile (JG cells, recruited cells, and As4.1 cells) with the ChIP-Seq signals for H3K27Ac (recruited cells and As4.1 cells), MED1 (As4.1 cells) and Pol II (As4.1 cells). Modified from99
Renin cells share numerous open chromatin regions along the genome (Figure 5). Of those, ninety-one regions including the one corresponding to the Renin gene are classified as super enhancers (SEs), clusters of large genomic regulatory regions, composed of multiple enhancers driving the expression of genes that define cell identity124, 125. In addition, SEs are characterized by high density of transcription factor (TF) binding to DNA, deposition of Med1, and histone acetyltransferase p300, which acetylates H3K27 and open chromatin. Usually, CCCTC-binding factor (Ctcf), a genome organizer, demarcates SE boundaries (Figure 6)126. SEs can be distinguished from traditional enhancers using the Rank Order of Super Enhancers program and more recently by the combination of the several of the marks mentioned above, their transcription of enhancer RNA and their property for phase separation, the formation of non-membranous aggregates that facilitate the concentration of the transcriptional machinery and thus their function125, 127. SEs and the expression of the genes associated with them, mostly those that encode candidate master TFs tend to be cell specific99,125, 128 and relevant to the plasticity of renin cells, they are dynamic: they can assemble or dissipate as cells change fate or in response to physiological stimuli or disease129, 130.
Figure 6: Super-enhancers act as chromatin sensors that control the identity and memory of renin cells to maintain homeostasis.
Schematic summarizing the main signaling pathways and chromatin changes involved in the maintenance of JG cell identity and reacquisition of the renin phenotype by SMCs in response to physiological demands. Activation of the cAMP and/or the Notch pathways leads to profound epigenetic changes at the renin locus regulatory region characterized by deposition of H3K27ac by p300, sliding of nucleosomes and opening of chromatin which facilitate the access of numerous transcription factors including but not limited to Med1, Creb1, and RBP-J. Loop formation is maintained by Ctcf (CCCTC-binding factor). The colored dots indicate the presence of additional SEs throughout the genome that also regulate renin cell identity. β-AR, beta adrenergic receptor; PGE2, prostaglandin E2; AC, adenylate cyclase; Gsα, activating G protein–coupled subunit; PKA, protein kinase A; CREBP, phosphorylated cAMP responsive element binding protein. Modified from99. (Illustration credit: Ben Smith)
To determine whether the memory of the renin phenotype can be reenacted by epigenetic rewriting, we used CRISPR/Cas to introduce the catalytic moiety of p300 -which acetylates H3K27- in SMCs from the renin lineage99. These cells express CFP constitutively indicating they derived from renin-expressing arteriolar cells. They also carry a transgene that report YFP when the renin promoter is activated. Although those cells were able to express renin and YFP in vivo, in culture their expression was silent. However, introduction of p300 into the renin regulatory locus resulted in transcription of renin and YFP indicating that the expression of the renin gene can be manipulated by epigenetic re-writing in a locus specific manner99. The experiments also suggest that the memory of the renin phenotype resides at least in part within the regulatory region of the renin gene. As we discussed above p300, a histone acetyl transferase, elicited by the cAMP pathway is instrumental in the activation of this genetic program that confers the identity of the renin cell99.
3D organization of the chromatin and the renin cell.
The spatial organization of chromatin in the nucleus is essential for gene expression and may determine -together with the factors mentioned above- the fate of renin cells during development and disease. To accommodate six feet of DNA within the tiny space of each nucleus, the cells must accomplish the remarkable architectural feat of -tightly- packing the genome while maintaining appropriate time and cell specific gene function. Within such tight space, the chromatin is packed in territories which are delimitated by the establishment of topological associated domains that are circumscribed and marked by the Ctcf, one of the best studied chromatin architectural proteins5. Ctcf binds DNA and plays a fundamental role in the regulation of gene expression. It is an 11-zinc finger protein, highly conserved throughout the evolution of vertebrates. It binds numerous intergenic sites throughout the genome regulating transcriptional activity through the different combinations of its zinc fingers6,7. Although initially thought to be a repressor8, it also functions as an activator. It is a well-known insulator, preventing undesired gene activation9 and mediates long-range chromatin interactions bringing into proximity enhancers to the promoter of target genes10. Using Chip-PCR and data from the ENCODE project we found that Ctcf binds several sites within and around the renin locus and hypothesized that it might be involved in regulating renin cell identity by controlling the chromatin architecture of the renin cell. Because total body KO of Ctcf is embryonically lethal, we performed conditionally deletion of Ctcf in cells of the renin lineage. Mutant mice had a severe reduction in the endowment of renin-expressing cells, decreased circulating renin, hypotension, compromised renal arteriolar branching, renal fibrosis, hyposthenuria, and ultimately renal failure126. Thus, Ctcf is necessary for the control of renin cell number, renin expression, and the structural integrity of the kidney. Without Ctcf the transcriptional machinery that governs the identity of the renin cells is unable to function. Contrary to other models of RAS gene deletions characterized by arterial hypertrophy, lack of Ctcf in renin cells results in stunted branching but not concentric arterial hypertrophy indicating that lack of Ctcf abolishes the ability of renin cells to preserve the molecular program that characterizes their phenotype, thus explaining the lack of arteriolar hypertrophy -discussed below- heavily dependent on the presence of overly functioning stimulated renin cells126.
Changes in renin cell fate and arterial disease.
Although the evolutionary conserved ability to recruit renin-producing cells -via the transformation of SMCs to renin cells- serves the organism well in response to physiological challenges, it can result in arterial pathology when homeostatic balance is not achieved, as it occurs when the inciting stimulus (hypotension, fluid depletion, and/or inhibition of the RAS) is not removed or brought under control37, 87, 131. Renin cells will continue to produce renin until homeostasis is achieved or until the animal dies. The ability to produce renin has an infinite gain. In our own unpublished experiments in mice treated chronically with captopril, circulating renin levels never stop to increase from 40 thousand pg/ml in the basal state to 2 million pg/ml after only six months of treatment. When the cells are constantly stimulated to produce renin, they undergo a progressive and relentless transformation, ultimately resulting in severe arterial disease, as discussed below. Experimental or spontaneous mutations of any of the RAS genes in animals and humans and treatment of mice, rats, monkeys, and humans with inhibitors of the RAS lead to the development of a silent, progressive, and severe thickening of the intrarenal arterial tree characterized by the concentric hypertrophy of the kidney arterioles and interlobular arteries 87, 109, 111, 131–135 (Figure 7). Numerous hypertrophic renin cells encircle the vessels and insert themselves higgledy-piggledy within the arteriolar walls. In addition, numerous SMCs accumulate inwardly and concentrically, partially -or totally- obstructing the vessel lumens, leading to hypoperfusion, focal ischemia, fibrosis, and kidney failure. The significance of this vascular disease cannot be emphasized enough, considering that more than 30% of the United States population suffer from hypertension136, 137, which is frequently treated with RAS inhibitors. Because hypertension is a major risk factor for mortality, myocardial infarction, stroke, heart and renal failure137, 138, the American College of Cardiology and the American Heart Association have established newer and stricter BP control guidelines136. In fact, the findings of the SPRINT and ACCORD trials indicate that intensive lowering of systolic BP below 120 mmHg versus standard lowering below 140 mmHg reduces the risk of death and adverse cardiovascular events in hypertensive individuals139, 140. However, the SPRINT data also suggest that intensive BP lowering might increase the risk (by 3.5-fold) of incident chronic kidney disease (CKD), defined as a ≥30% decrease in estimated glomerular filtration rate (eGFR) to ≤60 ml/min/1.73 m2. A secondary analysis of data from the SPRINT and ACCORD trials focused on participants who did not have evidence of CKD at baseline showed that after three years, the cumulative incidence of CKD was 10% in the intensive treatment group versus 4.1% in the standard treatment group. The researchers concluded that more intensive BP lowering may increase the risk of kidney disease in patients with or without diabetes and advised that close monitoring of renal function is needed141. Further, in 2011, Novartis halted its clinical trial with the renin inhibitor Aliskiren due to increased incidence of strokes and renal complications in patients with diabetes and renal impairment. Interestingly, the patients had decreased proteinuria, which in retrospect was not only a poor predictor but gave a false sense of reassurance when, in fact, a silent kidney pathology was evolving142. Because these patients do not routinely receive a kidney biopsy, morphological alterations of the kidneys may be missed. Unfortunately, we continue to rely on dubious markers such as proteinuria, as mentioned above. Incisive commentaries on these and other trials are reviewed in142–144. Remarkably, in essential hypertension, although renin levels are in most cases low or normal, RAS inhibitors are used widely. Whereas these pharmacological agents are extremely beneficial in the prevention and management of cardiovascular events in hypertensive individuals145, 146 nevertheless, inhibition of renin (aliskiren), angiotensin-converting enzyme (ACE inhibitors), or Angiotensin II actions (angiotensin receptor blockers, ARBs) together with their pressure-lowering effect cause profound changes in renin cells131. The reasons for the development of Concentric Arterial and Arteriolar Hypertrophy (CAAH) are not fully understood, but results from animal and retrospective human studies are illuminating: we examined the hemodynamic and renal consequences of inhibiting the RAS in rats with severe hypertension treated for (only) 14 days with the ARB valsartan, the direct renin inhibitor aliskiren or both.133 Although all treatments markedly improved BP, cardiac hypertrophy, and proteinuria, blind histological examination of the kidneys at fourteen days unexpectedly detected tubular dilatation and tubular cell proliferation, interstitial and periglomerular fibrosis, and vascular thickening of arterioles for all treatments compared with vehicle-treated hypertensive rats133. Remarkably, these alterations were most pronounced in rats that received dual treatment. Importantly, we found that -as in humans- proteinuria, which is commonly used as a marker of renal injury, improved in the treated rats, giving the false initial impression that no renal damage existed.133 Clearly, there is a need to identify markers that accurately reflect the structural integrity of the kidneys. Recently, Nagai and colleagues assessed the morphological changes of the renal arterioles induced by ARBs and ACE inhibitors in 44 patients (ages 45–74 years old) from Japan whose kidney were resected because of renal/urothelial carcinomas147. Kidney samples from the healthy portions were examined. Patients with diabetes, ESRD, systemic sclerosis, or kidney atrophy were excluded from the analysis147. The investigators found that patients treated with ARBs or ACE inhibitors displayed severe changes in the structure of the glomerular afferent arterioles consisting of increased numbers of renin cells and increased layers of distorted and larger SMCs147. This led to the thickening of the vessel wall and narrowing of the arteriolar lumens. Because the authors found an increase in the number of SMC nuclei, they assumed that the cells underwent proliferation, although none of the usual markers of proliferation were included in their study. In fact, in our own assessment of this pathology, although there is an increase in the number of SMCs and renin cells within the renal arterioles87, we have not been able to find evidence of actual proliferation using a variety of markers of cell proliferation or assessment of cell cycle genes87. Those findings are remarkably similar to those we found in young normotensive rats treated with losartan for fourteen days148 and then corroborated by similar studies in Zucker rats149. Although the aforementioned studies in humans are not prospective and have obvious limitations, they suggest the distinct possibility that the CAAH may develop in humans providing additional impetus and the need to explore the mechanisms underlying this disease. It has been proposed that because renin levels are so high in patients receiving ARBs or ACE inhibitors, perhaps the prorenin receptor is activated, inducing the accumulation of SMCs147. However, the fact that a similar lesion develops in animals devoid of renin (total body renin deletion135 or deletion of renin in the kidney vasculature150) suggests that the cause of the arteriolar hypertrophy is not due to the enzyme/hormone renin per se. In fact, all evidence points at the renin cell per se. Under constant, relentless stimulation -as it occurs under constant inhibition of the RAS and hypotension- renin cells continue to transform, adopting embryonic invasive characteristics and the production of secreted factors that may induce the concentric accumulation of adjacent SMCs. In that regard, and as mentioned above, the vascular hypertrophy does not occur if we ablate the renin cells using either diphtheria toxin or conditional deletion of Itgb186, 88. These results indicate that renin cells either by direct contact interactions with adjacent SMCs and/or by the production of secreted factors, induce the concentric accumulation of SMCs. These last two possibilities, physical encroachment, and secretion of angiogenic/growth factors by renin cells are not mutually exclusive. In fact, during the development of CAAH, renin cells synthesize and secrete a variety of proteins, including osteopontin (Spp1), Matrix gla protein, Byglycan (Bgn), Sparc, fibrillary collagens, and other proteins that constitute the biochemical correlates of the disease (unpublished). Interestingly, the levels of these proteins also increase in the circulation. It will be exciting to explore whether Spp1 and Bgn can be used to follow the progression or regression of CAAH in response to the pharmacological manipulations to prevent the arterial disease in hypertensive patients.
Figure 7: Chronic inactivation of the renin–angiotensin system (RAS) leads to concentric vascular hypertrophy of the kidney arterial tree.
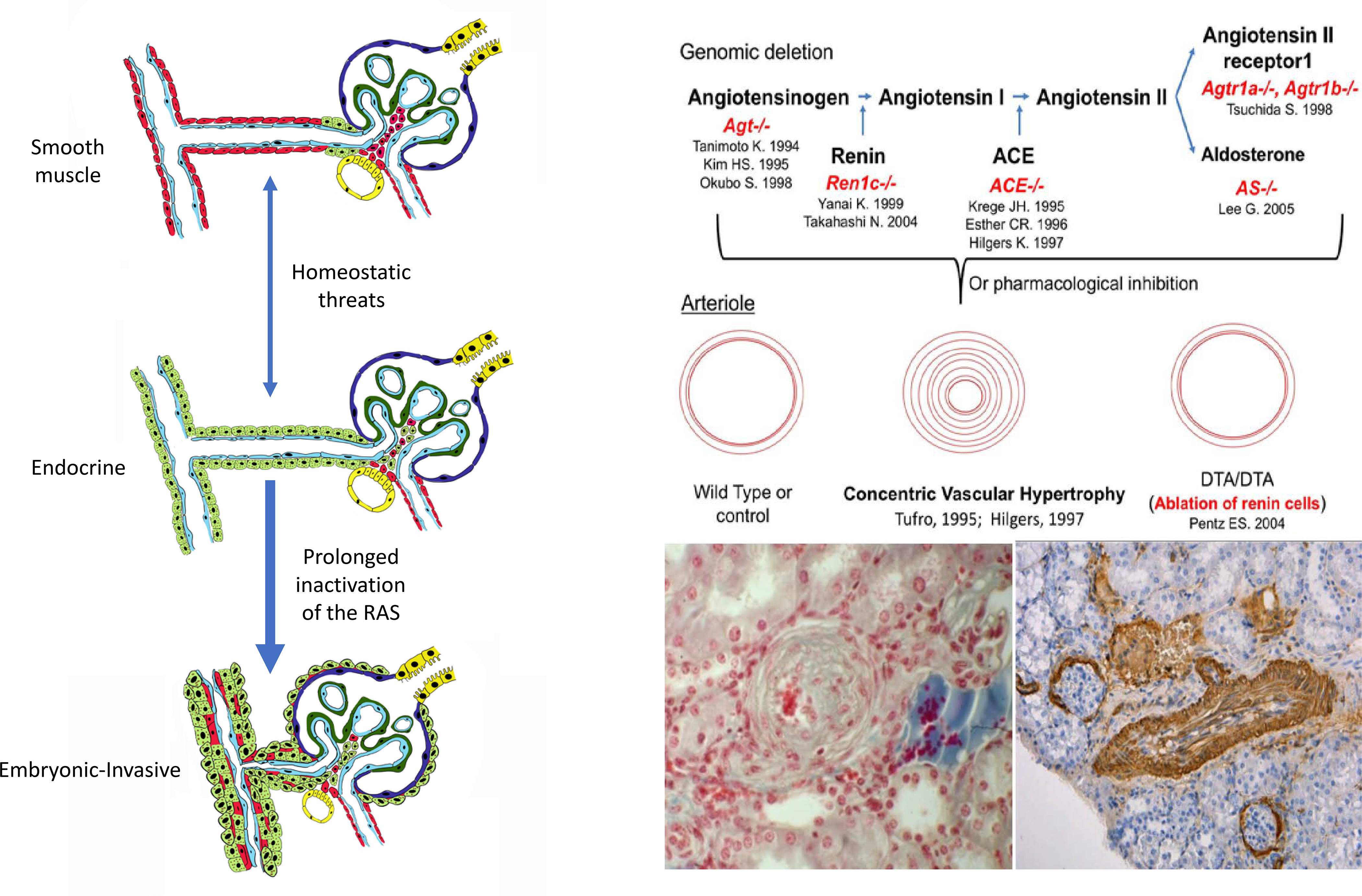
Left: Schematic showing the different stages of morphological changes of the renal arterioles due to stimulation of renin cells. Short-term stimulation as in acute dehydration or hypotension leads to transformation of SMCs to an endocrine, renin-expressing phenotype along the arterioles. At this stage, the transformation is reversible. Thus, when the crisis passes and homeostasis is regained, the cells return to their previous smooth muscle contractile phenotype. However, when homeostasis is not regained (constant hypotension, NaCl depletion, etc.) as it occurs with mutations of the RAS genes or chronic inhibition of the RAS) there is constant relentless and progressive transformation of the arteriolar cells which acquire an embryonic-invasive phenotype and the production of angiogenic/growth factors leading to the development of concentric arteriolar hypertrophy.
Right Top: Deletion of any of the RAS genes or pharmacological inhibition of the system in humans and other animals, results in concentric arteriolar hypertrophy. Because this pathognomonic lesion does not occur when the renin cells are ablated with diphtheria toxin subunit A (DTA) targeted to the Renin gene, suggests that renin cells per se are responsible for the pathological changes. Humans with renin gene mutations show the same vascular alteration. −/−, total gene deletion; ACE, angiotensin converting enzyme; Agt, angiotensinogen; Agtr1a and Agtr1b, angiotensin receptors subtypes A and B. AS, aldosterone synthase; Ren1c, renin. Reprinted from131. Bottom: high magnification of a transversal cut of an hypertrophic arteriole from an ACE−/− mouse kidney (left, reprinted from153) and lower magnification of a longitudinal cut of a hypertrophic interlobular artery from a Ren1c−/− mouse kidney immunostained for α-smooth muscle actin (brown, right, reprinted from150). Similar lesions occur when Renin is deleted solely in the kidney vasculature, or with the protracted use of RAS inhibitors in humans.
The dynamics, epigenetic mechanisms, and regulatory networks that transform the renin cells into an aggressive cell capable of migrating inside the arterial wall and inducing the concentric hypertrophy of nearby cells are poorly understood. We are currently mapping the chromatin domains and transcriptional events that determine the identity and fate trajectories that the vascular cells adopt as the arterial pathology evolves. Understanding the chromatin structure of the diseased arteriolar cells will define the genomic hotspots that imprint the initiation, evolution, and persistence of the disease. This knowledge will be vital to identify the major therapeutic targets of this pervasive arteriolar disease. Given the clear beneficial effects of RAS inhibitors in diminishing mortality and cardiovascular events, understanding the mechanisms underlying CAAH may help in the development of targeted therapies so that patients can receive RAS inhibitors without sacrificing kidney health151, 152.
Finally, it should be noted that in addition to the aforementioned arterial disease, given the fundamental roles of renin cells in maintaining well-being, changes in renin cell fate may cause additional renal and extrarenal diseases. Figure 8 summarizes some of these, mostly experimental, disorders resulting from the ablation of key factors that under normal circumstances maintain the vitality and identity of renin cells.
Figure 8: Changes in renin cell fate result in disease.
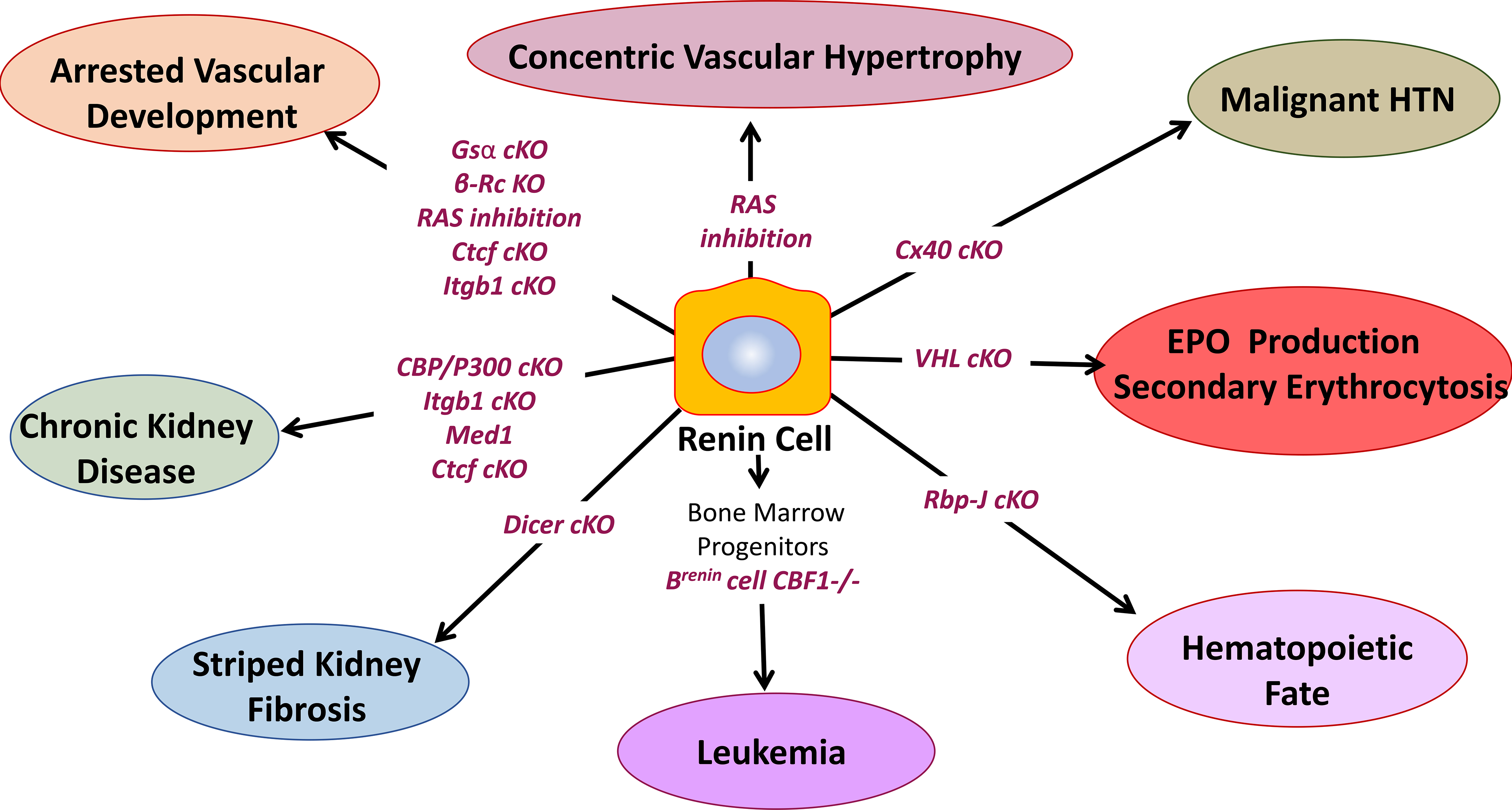
Conditional deletion (cKO) of crucial genes within renin cells, and genetic or pharmacological inhibition of the renin angiotensin system (RAS) result in cell fate changes ultimately leading to renal and systemic pathology. β-Rc, β-adrenergic receptor; Gsα, G protein α subunit; RBP-J, recombination signal binding protein for immunoglobulin Kappa J; CBF1, human homolog of RBP-J; Cx40, connexin 40; VHL, Von Hippel–Lindau gene; Ctcf, CCCTC-binding factor; Itgb1, integrin beta 1; Med1, Mediator complex subunit 1.
Acknowledgments
Sources of Funding: NIH grants P50 DK-096373 and R01 DK-116718 to RAG, DK-116196, DK-096373 and HL-148044 to MLSSL.
Non-standard Abbreviations and Acronyms
- ACE
angiotensin converting enzy,e
- ARB
angiotensin receptor blocker
- Bgn
byglycan
- BP
blood pressure
- CAAH
Concentric Arterial and Arteriolar Hypertrophy
- CCCTC
binding factor Ctcf
- Cx
connexin
- CRE
cAMP-responsive element
- E
embryonic
- Gsα
G protein subunit alpha
- H3K27ac
Acetylation of lysine 27 of Histone 3
- Itgb1
β1-integrin
- JG
juxtaglomerular
- KO
knockout
- Panx1
Pannexin 1
- RAS
renin angiotensin system
- SCL
stem cell leukemia/T-cell acute lymphoblastic leukemia protein 1
- SMC
smooth muscle cell
- S1P
Sphingosine 1-phosphate
- SE
super enhancer
- Spp1o
steopontin
- TF
transcription factor
- VEGF
vascular endothelial growth factor
- VDR
vitamin D receptor
- Vhl
von Hippel-Lindau
Footnotes
Disclosures: None.
BIBLIOGRAPHY
- 1.Gomez RA, Sequeira-Lopez MLS. Renin cells in homeostasis, regeneration and immune defence mechanisms. Nat Rev Nephrol. 2018;14:231–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyea BCS, Vasconez Araceli E. Nagalakshmi Wilson A., Mehalic Vidya K., Sequeira- Lopez Theodore C., Gomez Maria Luisa S., Ariel R A primitive type of renin-expressing lymphocyte protects the organism against infections. 2019 [DOI] [PMC free article] [PubMed]
- 3.Taugner R The juxtaglomerular apparatus: Structure and function. 1989:104–126
- 4.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227 [PubMed] [Google Scholar]
- 5.Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67 [DOI] [PubMed] [Google Scholar]
- 7.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindström NO, McMahon JA, Guo J, Tran T, Guo Q, Rutledge E, Parvez RK, Saribekyan G, Schuler RE, Liao C, Kim AD, Abdelhalim A, Ruffins SW, Thornton ME, Baskin L, Grubbs B, Kesselman C, McMahon AP. Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol. 2018;29:785–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: A new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–251 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using cited1-creert2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008;313:234–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol. 2015;308:R138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekel B, Hochman E, Sanchez MJ, Maharshak N, Amariglio N, Green AR, Izraeli S. Kidney, blood, and endothelium: Developmental expression of stem cell leukemia during nephrogenesis. Kidney Int. 2004;65:1162–1169 [DOI] [PubMed] [Google Scholar]
- 14.Lin EE, Sequeira-Lopez ML, Gomez RA. Rbp-j in foxd1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol. 2014;306:F249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728 [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Li M, Gothert JR, Gomez RA, Sequeira-Lopez ML. Hemovascular progenitors in the kidney require sphingosine-1-phosphate receptor 1 for vascular development. J Am Soc Nephrol. 2016;27:1984–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert B, St John PL, Hyink DP, Abrahamson DR. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol. 1996;271:F744–753 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. C-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249 [DOI] [PubMed] [Google Scholar]
- 19.Halt KJ, Pärssinen HE, Junttila SM, Saarela U, Sims-Lucas S, Koivunen P, Myllyharju J, Quaggin S, Skovorodkin IN, Vainio SJ. Cd146(+) cells are essential for kidney vasculature development. Kidney Int. 2016;90:311–324 [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor s1p1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667 [DOI] [PubMed] [Google Scholar]
- 22.Luo W, Garcia-Gonzalez I, Fernández-Chacón M, Casquero-Garcia V, Sanchez-Muñoz MS, Mühleder S, Garcia-Ortega L, Andrade J, Potente M, Benedito R. Arterialization requires the timely suppression of cell growth. Nature. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekblom P, Sariola H, Karkinen-Jääskeläinen M, Saxén L. The origin of the glomerular endothelium. Cell Differ. 1982;11:35–39 [DOI] [PubMed] [Google Scholar]
- 24.Sariola H, Ekblom P, Lehtonen E, Saxén L. Differentiation and vascularization of the metanephric kidney grafted on the chorioallantoic membrane. Dev Biol. 1983;96:427–435 [DOI] [PubMed] [Google Scholar]
- 25.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol. 2001;281:F345–356 [DOI] [PubMed] [Google Scholar]
- 26.Hyink DP, Tucker DC, St John PL, Leardkamolkarn V, Accavitti MA, Abrass CK, Abrahamson DR. Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol. 1996;270:F886–899 [DOI] [PubMed] [Google Scholar]
- 27.Loughna S, Hardman P, Landels E, Jussila L, Alitalo K, Woolf AS. A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis. 1997;1:84–101 [DOI] [PubMed] [Google Scholar]
- 28.Robert B, St John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in flk1 heterozygous mutant mice. Am J Physiol. 1998;275:F164–172 [DOI] [PubMed] [Google Scholar]
- 29.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human ips cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568 [DOI] [PubMed] [Google Scholar]
- 30.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ. Renal subcapsular transplantation of psc-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 2018;10:751–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol. 1998;9:63–71 [DOI] [PubMed] [Google Scholar]
- 33.Gomez RA, Sequeira-Lopez ML. Novel functions of renin precursors in homeostasis and disease. Physiology (Bethesda). 2016;31:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mrna and its protein in the developing kidney. Am J Physiol. 1989;257:F850–858 [DOI] [PubMed] [Google Scholar]
- 35.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ace inhibition. Am J Physiol. 1988;254:F900–906 [DOI] [PubMed] [Google Scholar]
- 36.Gomez RA. Molecular biology of components of the renin-angiotensin system during development. Pediatr Nephrol. 1990;4:421–423 [DOI] [PubMed] [Google Scholar]
- 37.Gomez RA, Lopez ML. Plasticity of renin cells in the kidney vasculature. Curr Hypertens Rep. 2017;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celio MR, Groscurth P, Inagami T. Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl). 1985;173:149–155 [DOI] [PubMed] [Google Scholar]
- 39.Celio MR, Inagami T. Renin in the human kidney. Immunohistochemical localization. Histochemistry. 1981;72:1–10 [DOI] [PubMed] [Google Scholar]
- 40.Egerer G, Taugner R, Tiedemann K. Renin immunohistochemistry in the mesonephros and metanephros of the pig embryo. Histochemistry. 1984;81:385–390 [DOI] [PubMed] [Google Scholar]
- 41.Nishimura H Renin-angiotensin system in vertebrates: Phylogenetic view of structure and function. Anat Sci Int. 2017;92:215–247 [DOI] [PubMed] [Google Scholar]
- 42.Nishimura H, Ogawa M, Sawyer WH. Renin-angiotensin system in primitive bony fishes and a holocephalian. Am J Physiol. 1973;224:950–956 [DOI] [PubMed] [Google Scholar]
- 43.Rider SA, Mullins LJ, Verdon RF, MacRae CA, Mullins JJ. Renin expression in developing zebrafish is associated with angiogenesis and requires the notch pathway and endothelium. Am J Physiol Renal Physiol. 2015;309:F531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rider SA, Christian HC, Mullins LJ, Howarth AR, MacRae CA, Mullins JJ. Zebrafish mesonephric renin cells are functionally conserved and comprise two distinct morphological populations. Am J Physiol Renal Physiol. 2017;312:F778–F790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML. Cbp and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol. 2009;296:H1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic amp pathway. Am J Physiol Renal Physiol. 2009;296:F1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pentz ES, Cordaillat M, Carretero OA, Tucker AE, Sequeira Lopez ML, Gomez RA. Histone acetyl transferases cbp and p300 are necessary for maintenance of renin cell identity and transformation of smooth muscle cells to the renin phenotype. Am J Physiol Heart Circ Physiol. 2012;302:H2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pentz ES, Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by camp and chromatin remodeling: An in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008;294:H699–707 [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with cre recombinase-mediated deletion of g protein gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–37 [DOI] [PubMed] [Google Scholar]
- 50.Castellanos-Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, Sequeira-Lopez ML, Gomez RA. Recombination signal binding protein for ig-kappaj region regulates juxtaglomerular cell phenotype by activating the myo-endocrine program and suppressing ectopic gene expression. J Am Soc Nephrol. 2015;26:67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Everett AD, Carey RM, Chevalier RL, Peach MJ, Gomez RA. Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest. 1990;86:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klar J, Sandner P, Muller MW, Kurtz A. Cyclic amp stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 2002;444:335–344 [DOI] [PubMed] [Google Scholar]
- 53.Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F. Cis-regulatory elements and trans-acting factors directing basal and camp-stimulated human renin gene expression in chorionic cells. Circ Res. 1994;74:764–773 [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Kim SM, Eisner C, Oppermann M, Huang Y, Mizel D, Li L, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Schnermann J, Briggs JP. Stimulation of renin secretion by angiotensin ii blockade is gsalpha-dependent. J Am Soc Nephrol. 2010;21:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pupilli C, Gomez RA, Tuttle JB, Peach MJ, Carey RM. Spatial association of renin-containing cells and nerve fibers in developing rat kidney. Pediatr Nephrol. 1991;5:690–695 [DOI] [PubMed] [Google Scholar]
- 56.Lin EE, Pentz ES, Sequeira-Lopez ML, Gomez RA. Aldo-keto reductase 1b7, a novel marker for renin cells, is regulated by cyclic amp signaling. Am J Physiol Regul Integr Comp Physiol. 2015;309:R576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Renal failure in mice with gsalpha deletion in juxtaglomerular cells. Am J Nephrol. 2010;32:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232 [DOI] [PubMed] [Google Scholar]
- 59.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776 [DOI] [PubMed] [Google Scholar]
- 60.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;22:2213–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez ML, Gomez RA. Transcriptional regulator rbp-j regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539 [DOI] [PubMed] [Google Scholar]
- 63.Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L. Foxd1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged helix transcription factor bf-2. Genes Dev. 1996;10:1467–1478 [DOI] [PubMed] [Google Scholar]
- 65.Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez ML, Bachmann S, Gomez RA, Eckardt KU, Kurtz A. Deletion of von hippel-lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol. 2013;24:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurt B, Gerl K, Karger C, Schwarzensteiner I, Kurtz A. Chronic hypoxia-inducible transcription factor-2 activation stably transforms juxtaglomerular renin cells into fibroblast-like cells in vivo. J Am Soc Nephrol. 2015;26:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerl K, Steppan D, Fuchs M, Wagner C, Willam C, Kurtz A, Kurt B. Activation of hypoxia signaling in stromal progenitors impairs kidney development. Am J Pathol. 2017;187:1496–1511 [DOI] [PubMed] [Google Scholar]
- 68.Miyauchi K, Nakai T, Saito S, Yamamoto T, Sato K, Kato K, Nezu M, Miyazaki M, Ito S, Yamamoto M, Suzuki N. Renal interstitial fibroblasts coproduce erythropoietin and renin under anaemic conditions. EBioMedicine. 2021;64:103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karginova EA, Pentz ES, Kazakova IG, Norwood VF, Carey RM, Gomez RA. Zis: A developmentally regulated gene expressed in juxtaglomerular cells. Am J Physiol. 1997;273:F731–738 [DOI] [PubMed] [Google Scholar]
- 70.Kurtz A Connexins, renin cell displacement and hypertension. Curr Opin Pharmacol. 2015;21:1–6 [DOI] [PubMed] [Google Scholar]
- 71.Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563 [DOI] [PubMed] [Google Scholar]
- 72.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A. Selective deletion of connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, Prevarskaya N. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol. 2006;174:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lohman AW, Billaud M, Isakson BE. Mechanisms of atp release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE. Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res. 2012;49:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22 [DOI] [PubMed] [Google Scholar]
- 77.DeLalio LJ, Masati E, Mendu S, Ruddiman CA, Yang Y, Johnstone SR, Milstein JA, Keller TCS, Weaver RB, Guagliardo NA, Best AK, Ravichandran KS, Bayliss DA, Sequeira-Lopez MLS, Sonkusare SN, Shu XH, Desai B, Barrett PQ, Le TH, Gomez RA, Isakson BE. Pannexin 1 channels in renin-expressing cells influence renin secretion and blood pressure homeostasis. Kidney Int. 2020;98:630–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45 [DOI] [PubMed] [Google Scholar]
- 79.Scammell JG, Fregly MJ. Attenuation of isoproterenol-stimulated plasma renin activity by chronic estrogen treatment. Proc Soc Exp Biol Med. 1981;167:117–121 [DOI] [PubMed] [Google Scholar]
- 80.Novielli-Kuntz NM, Jelen M, Barr K, DeLalio LJ, Feng Q, Isakson BE, Gros R, Laird DW. Ablation of both cx40 and panx1 results in similar cardiovascular phenotypes exhibited in cx40 knockout mice. Biosci Rep. 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez RA, Sequeira Lopez ML. Who and where is the renal baroreceptor?: The connexin hypothesis. Kidney Int. 2009;75:460–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895 [DOI] [PubMed] [Google Scholar]
- 83.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687 [DOI] [PubMed] [Google Scholar]
- 84.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pozzi A, Zent R. Integrins in kidney disease. J Am Soc Nephrol. 2013;24:1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohamed TH, Watanabe H, Kaur R, Belyea BC, Walker PD, Gomez RA, Sequeira-Lopez MLS. Renin-expressing cells require beta1-integrin for survival and for development and maintenance of the renal vasculature. Hypertension. 2020;76:458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oka M, Medrano S, Sequeira-Lomicronpez MLS, Gomez RA. Chronic stimulation of renin cells leads to vascular pathology. Hypertension. 2017;70:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;286:R474–483 [DOI] [PubMed] [Google Scholar]
- 89.Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microrna-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oh J, Matkovich SJ, Riek AE, Bindom SM, Shao JS, Head RD, Barve RA, Sands MS, Carmeliet G, Osei-Owusu P, Knutsen RH, Zhang H, Blumer KJ, Nichols CG, Mecham RP, Baldán Á, Benitez BA, Sequeira-Lopez ML, Gomez RA, Bernal-Mizrachi C. Macrophage secretion of mir-106b-5p causes renin-dependent hypertension. Nat Commun. 2020;11:4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lachmann P, Hickmann L, Steglich A, Al-Mekhlafi M, Gerlach M, Jetschin N, Jahn S, Hamann B, Wnuk M, Madsen K, Djonov V, Chen M, Weinstein LS, Hohenstein B, Hugo CPM, Todorov VT. Interference with cAMP-mediated signaling by inducible knockout of Gs-alpha (Gsα) in RPCs of adult mice resulted in a complex adverse kidney phenotype. J Am Soc Nephrol. 2017;28:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steglich A, Kessel F, Hickmann L, Gerlach M, Lachmann P, Gembardt F, Lesche M, Dahl A, Federlein A, Schweda F, Hugo CPM, Todorov VT. Renin cells with defective gsα/camp signaling contribute to renal endothelial damage. Pflugers Arch. 2019;471:1205–1217 [DOI] [PubMed] [Google Scholar]
- 93.Steglich A, Hickmann L, Linkermann A, Bornstein S, Hugo C, Todorov VT. Beyond the paradigm: Novel functions of renin-producing cells. Rev Physiol Biochem Pharmacol. 2020;177:53–81 [DOI] [PubMed] [Google Scholar]
- 94.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol. 2015;26:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruhnke L, Sradnick J, Al-Mekhlafi M, Gerlach M, Gembardt F, Hohenstein B, Todorov VT, Hugo C. Progenitor renin lineage cells are not involved in the regeneration of glomerular endothelial cells during experimental renal thrombotic microangiopathy. PLoS One. 2018;13:e0196752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaverina NV, Kadoya H, Eng DG, Rusiniak ME, Sequeira-Lopez ML, Gomez RA, Pippin JW, Gross KW, Peti-Peterdi J, Shankland SJ. Tracking the stochastic fate of cells of the renin lineage after podocyte depletion using multicolor reporters and intravital imaging. PLoS One. 2017;12:e0173891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol. 2013;183:542–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hickmann L, Steglich A, Gerlach M, Al-Mekhlafi M, Sradnick J, Lachmann P, Sequeira-Lopez MLS, Gomez RA, Hohenstein B, Hugo C, Todorov VT. Persistent and inducible neogenesis repopulates progenitor renin lineage cells in the kidney. Kidney Int. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez MF, Medrano S, Brown EA, Tufan T, Shang S, Bertoncello N, Guessoum O, Adli M, Belyea BC, Sequeira-Lopez MLS, Gomez RA. Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. J Clin Invest. 2018;128:4787–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nüsing RM, Skøtt O, Jensen BL. Prostaglandin e2 ep2 and ep4 receptor activation mediates camp-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol. 2005;289:F989–997 [DOI] [PubMed] [Google Scholar]
- 101.Grill A, Schießl IM, Gess B, Fremter K, Hammer A, Castrop H. Salt-losing nephropathy in mice with a null mutation of the clcnk2 gene. Acta Physiol (Oxf). 2016;218:198–211 [DOI] [PubMed] [Google Scholar]
- 102.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of bartter’s syndrome. Proc Natl Acad Sci U S A. 2000;97:5434–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bader M Of mice and renin. Hypertension. 2017;70:35–37 [DOI] [PubMed] [Google Scholar]
- 104.Neubauer B, Schrankl J, Steppan D, Neubauer K, Sequeira-Lopez ML, Pan L, Gomez RA, Coffman TM, Gross KW, Kurtz A, Wagner C. Angiotensin ii short-loop feedback: Is there a role of ang ii for the regulation of the renin system in vivo? Hypertension. 2018;71:1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsusaka T, Nishimura H, Utsunomiya H, Kakuchi J, Niimura F, Inagami T, Fogo A, Ichikawa I. Chimeric mice carrying ‘regional’ targeted deletion of the angiotensin type 1a receptor gene. Evidence against the role for local angiotensin in the in vivo feedback regulation of renin synthesis in juxtaglomerular cells. J Clin Invest. 1996;98:1867–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y. Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, and arterioles of the ischemic (endocrine) kidney. An ultrastructural-cytochemical and autoradiographic study. Am J Pathol. 1977;87:581–602 [PMC free article] [PubMed] [Google Scholar]
- 107.Araujo-Nascimento M, Desormeaux Y, Cantin M. Ultrastructural cytochemistry of the ischemic (endocrine) kidney. Am J Pathol. 1976;82:527–548 [PMC free article] [PubMed] [Google Scholar]
- 108.Guessoum O, Zainab M, Sequeira-Lopez MLS, Gomez RA. Proliferation does not contribute to murine models of renin cell recruitment. Acta Physiologica. 2020:1–14 [Google Scholar]
- 109.Makhanova N, Sequeira-Lopez ML, Gomez RA, Kim HS, Smithies O. Disturbed homeostasis in sodium-restricted mice heterozygous and homozygous for aldosterone synthase gene disruption. Hypertension. 2006;48:1151–1159 [DOI] [PubMed] [Google Scholar]
- 110.Berg AC, Chernavvsky-Sequeira C, Lindsey J, Gomez RA, Sequeira-Lopez ML. Pericytes synthesize renin. World J Nephrol. 2013;2:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217 [DOI] [PubMed] [Google Scholar]
- 112.Hoffmann S, Mullins L, Buckley C, Rider S, Mullins J. Investigating the ras can be a fishy business: Interdisciplinary opportunities using zebrafish. Clin Sci (Lond). 2018;132:2469–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naruse M, Naruse K, Inagaki T, Inagami T. Immunoreactive renin in mouse adrenal gland. Localization in the inner cortical region. Hypertension. 1984;6:275–280 [PubMed] [Google Scholar]
- 114.Lee G, Makhanova N, Caron K, Lopez ML, Gomez RA, Smithies O, Kim HS. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656 [DOI] [PubMed] [Google Scholar]
- 115.Naruse M, Inagami T. Markedly elevated specific renin levels in the adrenal in genetically hypertensive rats. Proc Natl Acad Sci U S A. 1982;79:3295–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lattion AL, Michel JB, Arnauld E, Corvol P, Soubrier F. Myocardial recruitment during anf mrna increase with volume overload in the rat. Am J Physiol. 1986;251:H890–896 [DOI] [PubMed] [Google Scholar]
- 118.Lin CT, Palmer W, Wu JY, Chan L. Estrogen induction of very low density apolipoprotein ii synthesis, a major avian liver yolk protein, involves the recruitment of hepatocytes. Endocrinology. 1986;118:538–544 [DOI] [PubMed] [Google Scholar]
- 119.Gerber H, Peter HJ, Bachmeier C, Kaempf J, Studer H. Progressive recruitment of follicular cells with graded secretory responsiveness during stimulation of the thyroid gland by thyrotropin. Endocrinology. 1987;120:91–96 [DOI] [PubMed] [Google Scholar]
- 120.Sequeira Lopez ML, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep. 2010;12:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin JW, Wang G. The mediator complex: A master coordinator of transcription and cell lineage development. Development. 2014;141:977–987 [DOI] [PubMed] [Google Scholar]
- 122.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, dna-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martinez MF, Martini AG, Sequeira-Lopez MLS, Gomez RA. Ctcf is required for renin expression and maintenance of the structural integrity of the kidney. Clin Sci (Lond). 2020;134:1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A phase separation model for transcriptional control. Cell. 2017;169:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vahedi G, Kanno Y, Furumoto Y, Jiang K, Parker SC, Erdos MR, Davis SR, Roychoudhuri R, Restifo NP, Gadina M, Tang Z, Ruan Y, Collins FS, Sartorelli V, O’Shea JJ. Super-enhancers delineate disease-associated regulatory nodes in t cells. Nature. 2015;520:558–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Niederriter AR, Varshney A, Parker SC, Martin DM. Super enhancers in cancers, complex disease, and developmental disorders. Genes (Basel). 2015;6:1183–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown JD, Lin CY, Duan Q, Griffin G, Federation AJ, Paranal RM, Bair S, Newton G, Lichtman AH, Kung AL, Yang T, Wang H, Luscinskas FW, Croce KJ, Bradner JE, Plutzky J. Nf-κb directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gomez RA. Fate of renin cells during development and disease. Hypertension. 2017;69:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gribouval O, Moriniere V, Pawtowski A, Arrondel C, Sallinen SL, Saloranta C, Clericuzio C, Viot G, Tantau J, Blesson S, Cloarec S, Machet MC, Chitayat D, Thauvin C, Laurent N, Sampson JR, Bernstein JA, Clemenson A, Prieur F, Daniel L, Levy-Mozziconacci A, Lachlan K, Alessandri JL, Cartault F, Riviere JP, Picard N, Baumann C, Delezoide AL, Belar Ortega M, Chassaing N, Labrune P, Yu S, Firth H, Wellesley D, Bitzan M, Alfares A, Braverman N, Krogh L, Tolmie J, Gaspar H, Doray B, Majore S, Bonneau D, Triau S, Loirat C, David A, Bartholdi D, Peleg A, Brackman D, Stone R, DeBerardinis R, Corvol P, Michaud A, Antignac C, Gubler MC. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33:316–326 [DOI] [PubMed] [Google Scholar]
- 133.Moniwa N, Varagic J, Ahmad S, VonCannon JL, Simington SW, Wang H, Groban L, Brosnihan KB, Nagata S, Kato J, Kitamura K, Gomez RA, Lopez ML, Ferrario CM. Hemodynamic and hormonal changes to dual renin-angiotensin system inhibition in experimental hypertension. Hypertension. 2013;61:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1a and 1b receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takahashi N, Lopez ML, Cowhig JE Jr., Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132 [DOI] [PubMed] [Google Scholar]
- 136.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 137.Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the united states. Circulation. 2011;123:1737–1744 [DOI] [PubMed] [Google Scholar]
- 138.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among united states adults 1999–2004. Hypertension. 2007;49:69–75 [DOI] [PubMed] [Google Scholar]
- 139.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beddhu S, Greene T, Boucher R, Cushman WC, Wei G, Stoddard G, Ix JH, Chonchol M, Kramer H, Cheung AK, Kimmel PL, Whelton PK, Chertow GM. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: Secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luft FC. Perspective on combination ras blocking therapy: Off-target, dis-cord, map-to-nowhere, low altitude, and nephron-d. Am J Nephrol. 2014;39:46–49 [DOI] [PubMed] [Google Scholar]
- 143.Savoia C, Touyz RM. Hypertension, diabetes mellitus, and excess cardiovascular risk: Importance of baseline systolic blood pressure. Hypertension. 2017;70:882–883 [DOI] [PubMed] [Google Scholar]
- 144.Mancia G Target blood pressure and kidney protection. Lancet Diabetes Endocrinol. 2018 [DOI] [PubMed] [Google Scholar]
- 145.Schiffrin EL. Vascular and cardiac benefits of angiotensin receptor blockers. Am J Med. 2002;113:409–418 [DOI] [PubMed] [Google Scholar]
- 146.Schiffrin EL. Vascular changes in hypertension in response to drug treatment: Effects of angiotensin receptor blockers. Can J Cardiol. 2002;18 Suppl A:15A–18A [PubMed] [Google Scholar]
- 147.Nagai Y, Yamabe F, Sasaki Y, Ishii T, Nakanishi K, Nakajima K, Shibuya K, Mikami T, Akasaka Y, Urita Y, Yamanaka N. A study of morphological changes in renal afferent arterioles induced by angiotensin ii type 1 receptor blockers in hypertensive patients. Kidney Blood Press Res. 2020;45:194–208 [DOI] [PubMed] [Google Scholar]
- 148.Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin ii regulates nephrogenesis and renal vascular development. Am J Physiol. 1995;269:F110–115 [DOI] [PubMed] [Google Scholar]
- 149.Nakanishi K, Nagai Y, Honglan Piao, Akimoto T, Kato H, Yanakieva-Georgieva N, Ishikawa Y, Yoshihara K, Ito K, Yamanaka N, Oite T. Changes in renal vessels following the long-term administration of an angiotensin ii receptor blocker in zucker fatty rats. J Renin Angiotensin Aldosterone Syst. 2011;12:65–74 [DOI] [PubMed] [Google Scholar]
- 150.Sequeira-Lopez ML, Nagalakshmi VK, Li M, Sigmund CD, Gomez RA. Vascular versus tubular renin: Role in kidney development. Am J Physiol Regul Integr Comp Physiol. 2015;309:R650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fu EL, Evans M, Clase CM, Tomlinson LA, van Diepen M, Dekker FW, Carrero JJ. Stopping renin-angiotensin system inhibitors in patients with advanced ckd and risk of adverse outcomes: A nationwide study. J Am Soc Nephrol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sequeira-Lopez MLS, Gomez RA. Preserving kidney health during intensive blood pressure control. Nat Rev Nephrol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA. Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension. 1997;29:216–221 [DOI] [PubMed] [Google Scholar]