Abstract
Objective
We assessed the incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) in hospitalized patients with coronavirus disease 2019 (COVID-19) compared with that in a matched cohort with similar cardiovascular risk factors and the effects of DVT and PE on the hospital course.
Methods
We performed a retrospective review of prospectively collected data from COVID-19 patients who had been hospitalized from March 11, 2020 to September 4, 2020. The patients were randomly matched in a 1:1 ratio by age, sex, hospital of admission, smoking history, diabetes mellitus, and coronary artery disease with a cohort of patients without COVID-19. The primary end point was the incidence of DVT/PE and the odds of developing DVT/PE using a conditional logistic regression model. The secondary end point was the hospitalization outcomes for COVID-19 patients with and without DVT/PE, including mortality, intensive care unit (ICU) admission, ICU stay, and length of hospitalization (LOH). Multivariable regression analysis was performed to identify the variables associated with mortality, ICU admission, discharge disposition, ICU duration, and LOH.
Results
A total of 13,310 patients had tested positive for COVID-19, 915 of whom (6.9%) had been hospitalized across our multisite health care system. The mean age of the hospitalized patients was 60.8 ± 17.0 years, and 396 (43.3%) were women. Of the 915 patients, 82 (9.0%) had had a diagnosis of DVT/PE confirmed by ultrasound examination of the extremities and/or computed tomography angiography of the chest. The odds of presenting with DVT/PE in the setting of COVID-19 infection was greater than that without COVID-19 infection (0.6% [5 of 915] vs 9.0% [82 of 915]; odds ratio [OR], 18; 95% confidence interval [CI], 8.0-51.2; P < .001). The vascular risk factors were not different between the COVID-19 patients with and without DVT/PE. Mortality (P = .02), the need for ICU stay (P < .001), duration of ICU stay (P < .001), and LOH (P < .001) were greater in the DVT/PE cohort than in the cohort without DVT/PE. On multivariable logistic regression analysis, the hemoglobin (OR, 0.71; 95% CI, 0.46-0.95; P = .04) and D-dimer (OR, 1.0; 95% CI, 0.33-1.56; P = .03) levels were associated with higher mortality. Higher activated partial thromboplastin times (OR, 1.1; 95% CI, 1.00-1.12; P = .03) and higher interleukin-6 (IL-6) levels (OR, 1.0; 95% CI, 1.01-1.07; P = .05) were associated with a greater risk of ICU admission. IL-6 (OR, 1.0; 95% CI, 1.00-1.02; P = .05) was associated with a greater risk of rehabilitation placement after discharge. On multivariable gamma regression analysis, hemoglobin (coefficient, −3.0; 95% CI, 0.03-0.08; P = .005) was associated with a prolonged ICU stay, and the activated partial thromboplastin time (coefficient, 2.0; 95% CI, 0.003-0.006; P = .05), international normalized ratio (coefficient, −3.2; 95% CI, 0.06-0.19; P = .002) and IL-6 (coefficient, 2.4; 95% CI, 0.0011-0.0027; P = .02) were associated with a prolonged LOH.
Conclusions
A significantly greater incidence of DVT/PE occurred in hospitalized COVID-19–positive patients compared with a non–COVID-19 cohort matched for cardiovascular risk factors. Patients affected by DVT/PE were more likely to experience greater mortality, to require ICU admission, and experience prolonged ICU stays and LOH compared with COVID-19–positive patients without DVT/PE. Advancements in DVT/PE prevention are needed for patients hospitalized for COVID-19 infection.
Keywords: COVID-19, Deep venous thrombosis, Hospitalized COVID-19 patients, Pulmonary embolism
Article Highlights.
-
•
Type of Research: A retrospective analysis of prospectively collected data for a multisite health care system review
-
•
Key Findings: The incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) in 915 hospitalized patients with coronavirus disease 2019 (COVID-19) was 9.0%, greater than the 0.6% incidence in the matched non–COVID-19 cohort (P < .01). Patients with COVID-19 who developed DVT/PE had greater mortality (P = .02), more intensive care unit (ICU) admissions (P < .01), a longer ICU stay (P < .001), and longer hospitalization (P < .001) compared with hospitalized COVID-19 patients without DVT/PE.
-
•
Take Home Message: Hospitalized patients with COVID-19 had a greater risk of developing DVT/PE compared with patients without COVID-19. The occurrence of DVT/PE in patients with COVID-19 was associated with greater mortality and prolonged ICU and hospital stays.
Since the emergence of the coronavirus disease 2019 (COVID-19) pandemic, multiple studies have been reported on the high incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) in these patients.1, 2, 3, 4, 5 In response to the surge in ultrasound usage in hospitals overwhelmed by the pandemic, recommendations were made regarding screening for DVT/PE and anticoagulation strategies for patients with positive test results for DVT/PE.6 , 7 We investigated our institution's experience with managing the pandemic. The primary goal of the present study was to assess the incidence of DVT and PE in patients hospitalized for COVID-19 infection and to compare the incidence with that in a non–COVID-19 cohort, matched for cardiovascular risk factors. Our secondary aim was to study the effects of DVT/PE on the hospital course and early outcomes.
Methods
Patient selection
The MC NEWS (Mayo Clinic neurological, vascular and neurovascular events with severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) study (institutional review board approval no. 20-003457) is a prospectively collected, retrospective study of all patients affected by the COVID-19 pandemic identified within the three major campuses of Mayo Clinic and the Mayo Clinic Health System, including hospitals in Arizona, Florida, Minnesota, and Wisconsin. Using the shared electronic medical record system (Epic, Verona, Wis), we identified all patients from March 11, 2020 to September 4, 2020 who had tested positive for SARS-CoV-2 using polymerase chain reaction or serology testing and those patients who had been transferred from other facilities to the Mayo Clinic with the COVID-19 diagnosis. From this cohort, the patients who had required hospitalization for SARS-CoV-2 infection were included in our study. To ensure accuracy in the data collection and validity of the cohort, four methods of patient selection were used. First, we identified those patients testing positive for SARS-CoV-2 using Epic (Verona, Wis), which provided hospitalization information. Second, we cross-checked the patients' unique identifiers and their inpatient status after March 11, 2020 using a natural language processing method (Advanced Cohort Explorer) developed by Mayo Clinic. Third, we matched the patients' unique identifiers with the hospital discharge data, which are separately managed by our office of quality management services. Finally, the hospital medical records for each of our patients were manually reviewed to ensure that the hospitalization had been required for SARS-CoV-2 infection. The Mayo Clinic institutional review board and the COVID-19 task force reviewed and approved the study protocol. Written informed consent from the included patients was not required owing to the minimal risk nature of the present study.
Calculation of incidence of DVT/PE and matched controls
We reviewed each patient's hospitalization records, including documentation of venous duplex ultrasound of the upper or lower extremities. Data regarding the presence or absence of DVT were abstracted. Additionally, we reviewed the records for the use of computed tomography angiography (CTA) and recorded the presence or absence of PE. The rate of DVT/PE was calculated using the total number of hospitalized COVID-19 patients as the denominator. Our study group underwent random matching in a 1:1 ratio with patients hospitalized from January 1, 2019 to December 31, 2019, controlling for the following variables: age, sex, hospital of admission, smoking history, diabetes, and coronary artery disease. This matching was facilitated by the office of quality management services. The odds ratios (ORs) for an association with DVT/PE were examined using a conditional logistic regression model, and the ORs for a diagnosis of DVT/PE during hospitalization and the corresponding 95% confidence intervals (CI) were estimated. The intent of our matching was to provide objective comparisons regarding the incidence of DVT/PE between our study population and a comparable control cohort before the pandemic. We did not match for a history of DVT/PE or the presence of thrombophilia.
Outcomes assessment among COVID-19 patients with and without DVT/PE
We collected the demographic data, pertinent medical history, and vital signs at admission; laboratory values at admission and when first measured during hospitalization; and hospital course, including requirement for intensive care unit (ICU), ICU stay, length of hospitalization (LOH), and disposition at discharge from hospitalization (up to September 4, 2020, the end of data collection). Disposition at discharge was characterized as discharged to home and/or a rehabilitation facility, continued hospitalization, or death. We applied multivariable regression models to determine the associations with mortality, ICU requirement, discharge disposition, length of ICU stay, and LOH.
Statistical analysis
Tests of statistical significance for univariate comparisons of demographics and baseline patient risk factors were conducted using the Pearson χ 2 test or the Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Descriptive statistics are presented as the mean ± standard deviation for continuous variables and frequencies and percentages for categorical variables. Multivariable logistic regression analysis was used to assess for the association with mortality, ICU requirement, and rehabilitation requirement after hospital discharge. A gamma regression model was used to assess the associations for ICU duration and LOH. We controlled for patient age and sex in both models. The variables tested included white blood cell count, hemoglobin (Hb), platelet count, activated partial thromboplastin time (aPTT), international normalized ratio (INR), D-dimer, interleukin-6 (IL-6), and procalcitonin levels. Differences were considered significant at P < .05. All statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
Results
Incidence of DVT/PE and conditional logistic regression
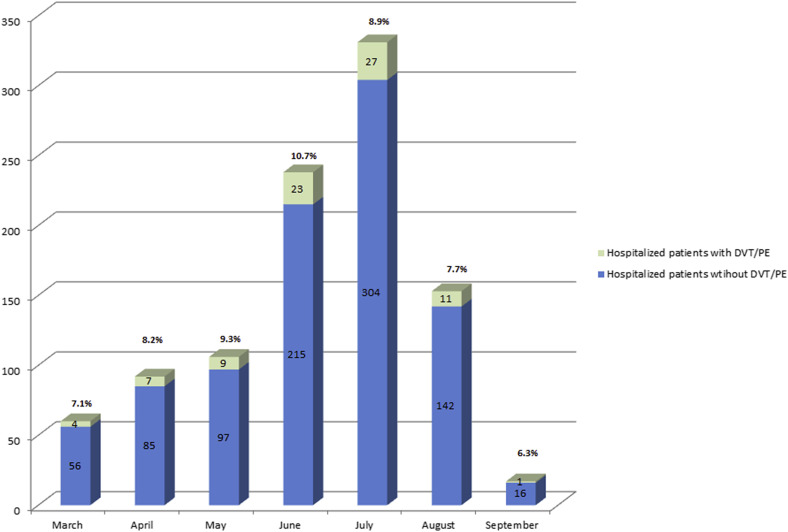
From March 11, 2020 to September 4, 2020, 13,310 patients had tested positive for SARS-CoV-2. Of the 13,310 patients, 915 (6.9%) had required hospitalization for COVID-19. Their mean age was 60.8 ± 17.0 years, and 396 patients (43.3%) were women. Of the 915 hospitalized patients, 82 (9.0%) had had a diagnosis DVT/PE that was confirmed by findings from appropriate imaging studies, including venous duplex ultrasound of the extremities and/or CTA of the chest. The monthly distribution of hospitalizations and patients with DVT/PE is shown in Fig 1 . The baseline characteristics for which the 915 patients were matched were age, sex, hospital of admission, smoking history, diabetes mellitus, and coronary artery disease. The results from the conditional logistic regression analysis are also with Table I . DVT/PE was identified in 82 of the 915 hospitalized patients with COVID-19 (9.0%) at a mean point after admission of 13.1 ± 19.9 days and in 5 of 915 matched control hospitalized patients without COVID-19 (0.6%; OR, 18; 95% CI, 8.0-51.2; P < .0001). The DVT locations are presented in Supplementary Table I (online only). Although race and ethnicity data were collected, these did not play a role in terms of the risk of DVT/PE (Supplementary Table II, online only). Although we did not match for a history of DVT/PE in each compared cohort, 42 patients had had DVT/PE in the COVID-19–positive group and 69 patients had had DVT/PE in the non–COVID-19 group. In addition, two patients in the COVID-19 group and seven in the non–COVID-19 group had a history of thrombophilia.
Fig 1.
Monthly distribution in 2020 of hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and diagnosed with deep vein thrombosis (DVT)/pulmonary embolism (PE) (with respective percentages). The incidence of DVT/PE among patients with SARS-CoV-2 did not change significantly across the months reviewed.
Table I.
Baseline patient characteristics stratified by COVID-19a
| Characteristic | Non–COVID-19 matched controlsb (n = 915) | COVID-19–positive patients (n = 915) | P value |
|---|---|---|---|
| DVT/PE diagnosis | 5 (0.6) | 82 (9.0) | <.0001 |
| Age, years | 62 (50.0-73.0) | 62 (50.0-73.0) | .934 |
| Male sex | 520 (56.8) | 520 (56.8) | 1.000 |
| Hospital admission | 1.000 | ||
| Arizona campus | 317 (34.6) | 317 (34.6) | |
| Florida campus | 172 (18.8) | 172 (18.8) | |
| Wisconsin and Minnesota | 189 (20.7) | 189 (20.7) | |
| Rochester campus | 237 (25.9) | 237 (25.9) | |
| Diabetes mellitus | 324 (35.4) | 325 (35.4) | 1.000 |
| Coronary artery disease | 119 (13.0) | 119 (13.0) | 1.000 |
| Variables accounted for but not matched | |||
| History of DVT/PE | 69 (7.5) | 42 (4.6) | .01 |
| History of thrombophilia | 7 (0.77) | 2 (0.22) | .18 |
COVID-19, Coronavirus disease 2019; DVT, deep vein thrombosis; PE, pulmonary embolism.
Data presented as number (%) or median (interquartile range).
Conditional logistic regression analysis: variable, DVT/PE; level, positive vs negative severe acute respiratory syndrome coronavirus 2 status; Pr>χ2, <0.0001; odds ratio, 18.0; 95% confidence interval, 8.0-51.2.
Controls matched for age, sex, hospital location, diabetes mellitus, and coronary artery disease.
Outcomes among COVID-19 patients stratified by DVT/PE
The baseline patient demographic data and medical history were not different among the COVID-19 patients with and without DVT/PE, including age (P = .764) and sex (P = .485; Table II ). The Hb level was significantly lower (P = .022) and the white blood cell count (P < .001) and platelet count (P = .002) were significantly higher in the patients with DVT/PE. The proportion of patients who required intubation (P = .015) and the proportion with two or more systemic inflammatory response syndrome criteria (P = .010) were also significantly greater in the DVT/PE cohort. The prothrombin time (P = .006), aPTT (P = .025), INR (P = .003), D-dimer (P < .001), probrain natriuretic peptide (P < .001), IL-6 (P = .002), and procalcitonin (P = .005) were also significantly higher in the DVT/PE cohort. All patients with a diagnosis of DVT/PE were treated with therapeutic anticoagulation in the form of weight-based intravenous heparin during hospitalization and a direct-acting oral anticoagulant after hospital discharge (Table II).
Table II.
Demographic and clinical characteristics of COVID-19–positive patients stratified by DVT and PE
| Characteristic | DVT/PE |
P value | |
|---|---|---|---|
| No (n = 833) | Yes (n = 82) | ||
| Male sex | 469 (56.3) | 50 (61.0) | .485 |
| Age, years | 61.0 (50.0-73.0) | 61.5 (50.2-73.8) | .764 |
| Congestive heart failure | 89 (10.7) | 10 (12.2) | .827 |
| Hypertension | 440 (52.9) | 39 (47.6) | .414 |
| Coronary artery disease | 108 (13.0) | 11 (13.4) | 1.000 |
| Myocardial infarction | 27 (3.3) | 5 (6.2) | .195 |
| Diabetes mellitus | 297 (35.8) | 27 (32.9) | .074 |
| Chronic kidney disease | 125 (15.0) | 13 (15.9) | .973 |
| Peripheral vascular disease | 33 (4.0) | 2 (2.4) | .784 |
| Ischemic stroke | 40 (4.8) | 1 (1.2) | .310 |
| Transient ischemic attack | 14 (1.7) | 0 (0) | .692 |
| Intracerebral hemorrhage | 2 (0.2) | 0 (0) | 1.000 |
| Atrial fibrillation | 116 (10.0) | 11 (13.4) | .310 |
| Hyperlipidemia | 337 (40.6) | 32 (39.0) | .873 |
| Antihypertensive medication | 403 (50.8) | 33 (43.4) | .359 |
| Lipid-lowering medication | 328 (41.2) | 23 (30.3) | .148 |
| Antiplatelet medication | 250 (31.5) | 15 (19.7) | .076 |
| CHA2DS2VASc score | 2.00 (1.00-3.00) | 2.00 (1.00-3.00) | .299 |
| Endotracheal mechanical ventilation | 44 (6.0) | 8 (15.7) | .015 |
| BMI, kg/m2 | 28.7 (25.2-35.3) | 28.2 (25.3-34.6) | .587 |
| ≥2 criteria of SIRS | 213 (23.3) | 34 (45.3) | .010 |
| WBC count,a 109/L | 6.30 (4.70-8.70) | 8.40 (5.7-11.2) | <.001 |
| Hemoglobin, g/dL | 13.1 (11.5-14.3) | 12.4 (10.5-14.0) | .022 |
| Hematocrit, % | 39.1 (35.2-42.8) | 37.6 (32.5-42.2) | .029 |
| Platelet count,b 109/L | 199 (151-255) | 230 (168-304) | .002 |
| Albumin, g/dL | 3.60 (3.20-3.90) | 3.40 (2.98-3.80) | .012 |
| Creatinine, mg/dL | 0.90 (0.73-1.21) | 0.89 (0.75-1.29) | .523 |
| PT, seconds | 13.3 (12.4-14.8) | 14.3 (12.9-17.6) | .006 |
| aPTT, seconds | 31.0 (29.0-35.0) | 33.0 (30.0-54.0) | .025 |
| INR | 1.2 (1.10-1.30) | 1.30 (1.10-1.50) | .003 |
| D-dimer,c ng/mL | 675 (466-1141) | 2112 (969-13,286) | <.001 |
| Ferritin,d μg/L | 514 (226-968) | 448 (238-1076) | .927 |
| C-reactive protein, mg/L | 70.7 (34.0-128) | 74.4 (41.7-159) | .170 |
| Probrain natriuretic peptide, pg/mL | 230 (68.0-938) | 754 (207-3155) | <.001 |
| Interleukin-6, pg/mL | 17.9 (8.50-34.5) | 25.1 (14.3-150) | .002 |
| Procalcitonin, ng/mL | 0.15 (0.09-0.35) | 0.23 (0.13-0.51) | .005 |
| Anticoagulation therapy | 0 (0) | 82 (100) | NA |
aPTT, Activated partial thromboplastin time; BMI, body mass index; CHA2DS2VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65-74 years, sex category; COVID-19, coronavirus disease 2019; DVT, deep vein thrombosis; INR, international normalized ratio; NA, not applicable; PE, pulmonary embolism; PT, prothrombin time; SIRS, systemic inflammatory response syndrome; WBC, white blood cell.
Data presented as number (%) or median (interquartile range).
Normal range, 3.4-9.6 × 109/L.
Normal range, 135-317 × 109/L.
Normal range, ≤500 mg/mL.
Normal range, 24-337 μg/L.
Mortality (P = .02), an ICU requirement (P < .001), duration of ICU stay (P < .001), and LOH (P < .001) were greater in the DVT/PE cohort. The discrepancy noted mortality and the number of patients dying in the hospital resulted from the number of patients who had not died in hospital but had been to hospice care. A greater proportion of patients in the DVT/PE cohort had been discharged to a rehabilitation facility (P = .001). On multivariable logistic regression analysis, low Hb levels (OR, 0.71; 95% CI, 0.46-0.95; P = .04) and high D-dimer levels (OR, 1.0; 95% CI, 0.33-1.56; P = .03) were associated with greater mortality. High aPTT (OR, 1.1; 95% CI, 1.00-1.12; P = .03) and IL-6 (OR, 1.0; 95% CI, 1.01-1.07; P = .05) levels were associated with a greater risk of ICU admission. High IL-6 levels (OR, 1.0; 95% CI, 1.00-1.02; P = .05) was associated with a greater risk of discharge to a rehabilitation facility. On multivariable gamma regression analysis, low Hb levels (coefficient, −3.0; 95% CI, 0.03-0.08; P = .005) was associated with a prolonged ICU stay. High aPTT (coefficient, 2.0; 95% CI, 0.003-0.006; P = .05), INR (coefficient, −3.2; 95% CI, 0.06-0.19; P = .002), and IL-6 (coefficient, 2.4; 95% CI, 0.0011-0.0027; P = .02) levels were associated with a prolonged LOH (Tables III and IV ).
Table III.
Patient outcomes stratified by DVT and PE
| Characteristic | DVT/PE |
P value | |
|---|---|---|---|
| No (n = 833) | Yes (n = 82) | ||
| Mortality | 68 (8.2) | 13 (15.9) | .03 |
| ICU admission | 230 (27.6) | 48 (58.5) | <.001 |
| ICU duration, days | 6.00 (3.00-15.0) | 17.0 (5.00-23.0) | <.001 |
| LOH, days | 6.00 (4.00-10.0) | 10.0 (7.00-23.8) | <.001 |
| Discharge disposition | .001 | ||
| Home | 655 (78.6) | 52 (63.4) | |
| Rehabilitation facility | 93 (11.2) | 18 (22.0) | |
| Died in hospital | 50 (6.0) | 6 (7.3) | |
| Still hospitalized | 35 (4.2) | 4 (4.0) | |
DVT, Deep vein thrombosis; ICU, intensive care unit; LOH, length of hospitalization; PE, pulmonary embolism.
Data presented as number (%) or median (interquartile range).
Table IV.
Multivariable logistic regression analysis for ICU stay and gamma regression analysis for ICU stay and LOH for DVT/PE
| Variablea | Pr>χ2 | OR | 95% CI |
|---|---|---|---|
| Multivariable logistic regression | |||
| Mortality | |||
| Hb | 0.04 | 0.71 | 0.46-0.95 |
| D-dimer | 0.03 | 1.0 | 0.33-1.56 |
| aPTT | 0.62 | 1.0 | 0.94-1.01 |
| INR | 0.84 | 1.1 | 0.33-1.60 |
| IL-6 | 0.34 | 1.0 | 1.00-1.00 |
| ICU stay | |||
| Hb | 0.95 | 1.00 | 0.88-1.11 |
| D-dimer | 0.98 | 1.00 | 1.00-1.00 |
| aPTT | 0.03 | 1.1 | 1.00-1.12 |
| INR | 0.36 | 0.42 | 0.03-0.97 |
| IL-6 | 0.05 | 1.0 | 1.00-1.07 |
| Admission to rehabilitation facility | |||
| Hb | 0.27 | 0.87 | 0.66-1.07 |
| D-dimer | 0.65 | 1.00 | 1.00-1.00 |
| aPTT | 0.38 | 1.02 | 1.00-1.07 |
| INR | 0.60 | 0.77 | NA-1.24 |
| IL-6 | 0.05 | 1.0 | 1.00-1.02 |
| Multivariable gamma regression | |||
| ICU stay | |||
| Hb | 0.005 | −3.0 | 0.03-0.08 |
| D-dimer | 0.43 | 0.80 | 0.000012-0.000015 |
| aPTT | 0.78 | 0.23 | 0.00084-0.0030 |
| INR | 0.85 | 0.19 | 0.091-0.47 |
| IL-6 | 0.78 | 0.29 | 0.0039-0.015 |
| LOH | |||
| Hb | 0.32 | −1.0 | 0.02-0.02 |
| D-dimer | 0.87 | −0.2 | 0.000002-0.000013 |
| aPTT | 0.05 | 2.0 | 0.003-0.006 |
| INR | 0.002 | −3.2 | 0.06-0.19 |
| IL-6 | 0.02 | 2.4 | 0.0011-0.0027 |
aPTT, activated partial thromboplastin time; CI, confidence interval; DVT, deep vein thrombosis; Hb, hemoglobin; ICU, intensive care unit; IL-6, interleukin-6; INR, international normalized ratio; LOH, length of hospitalization; OR, odds ratio; PE, pulmonary embolism.
All variables were continuous.
Discussion
Multiple studies to date have reported a rate of DVT/PE in hospitalized patients with COVID-19 of 25% to 58%.8, 9, 10 Within our multisite, single health care system, we found a DVT/PE rate of 9.0%. Few comprehensive studies have been reported regarding the incidence of DVT/PE in an American health care system. In a similar review from a Philadelphia hospital, Rali et al11 found a 17% incidence of DVT/PE in COVID-19–positive patients. In New Orleans, Hill et al reported a 7.2% rate of DVT/PE in COVID-19 patients requiring mechanical ventilation.12 In a recent meta-analysis, Lu et al13 reported a high incidence of 21% venous thromboembolic events in SARS-CoV-2 hospitalized patient population. Furthermore, Longchamp et al14 reported a systematic assessment using ultrasound examinations of all patients in the ICU because of SARS-Co-V-2 infection. They found a 32% rate of DVT and 20% rate of PE, and all the patients received therapeutic anticoagulation. Finally, Edler et al15 reported in their autopsy data of 80 patients, a high prevalence of fulminant PE combined with a 40% prevalence of DVT.
Our review included all patients with COVID-19 who had required hospitalization within our multisite health care system. Our data provide an important perspective on the incidence of DVT/PE in COVID-19 patients, in part owing to our strategic geographic locations across the United States. In our system, we have treated a large cohort of SARS-CoV-2–positive patients; however, only those who had required intensive treatment or closer monitoring were hospitalized. Those with mild symptoms were cared for by our connected care team using a remote patient monitoring program.16 Hospitalized patients have the theoretical advantage of the early diagnosis of DVT/PE owing to the daily monitoring for signs and symptoms and the increased awareness of hospital staff of the unusual parainfectious complications becoming apparent in this patient population.17 Our calculated rate of DV/PE was 9.0%, lower than that reported in other studies.8, 9, 10 , 14 This could be related to our aggressive management approach, which includes prophylactic treatment of all hospitalized patients with anticoagulation using 5000 U or 7500 U of heparin three times daily according to the patient's weight.18 It is also possible that a less virulent mutation of the virus was present in North America than in other parts of the world, and differences exist in social behaviors and cultures compared with other countries.19, 20, 21 These social differences were explored by Yamamoto and Bauer,21 who provided evidence that the initial spread of COVID-19 disease in Europe was slowed by a more distanced social behavior of greeting people such as in Asian countries. However, comparing our study patients with a patient cohort matched for age, sex, admission hospital, diabetes mellitus, and coronary artery disease, we observed a significantly greater risk of DVT/PE in patients hospitalized with SARS-CoV-2 infection.
Our cohort of patients with and without DVT/PE was similar in age, overall health risk factors, and cardiovascular comorbidities. However, the patients with DVT/PE had a greater rate of mortality, required more frequent ICU admission, and had both prolonged ICU and hospital stays. The median LOH was 10 days for the patients with DVT/PE compared with 6 days for the patients without DVT/PE. Our results have confirmed that patients with COVID-19 are vulnerable to the development DVT/PE, which contributes to the high morbidity and mortality. Low Hb and high D-dimer levels were also associated with a greater mortality risk. Regarding the need for ICU and rehabilitation center admission, it would seem that high IL-6 levels are associated with a greater risk of more severe infection.22
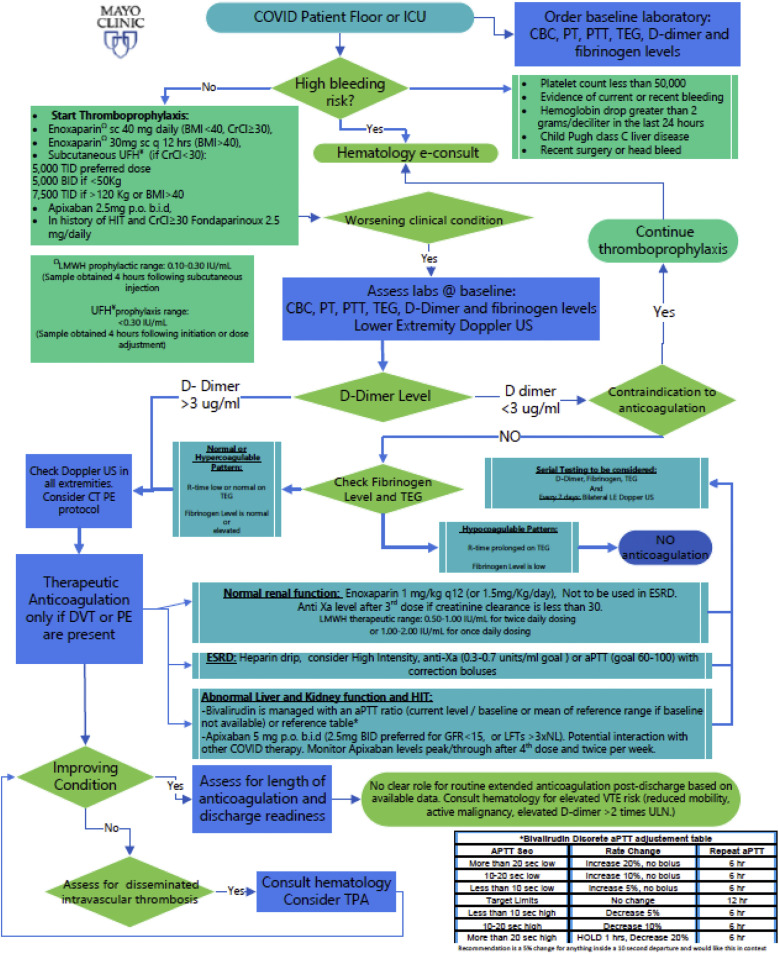
Our multidisciplinary COVID-19 treatment review panel identified the need for an evidence-based decision support tool to help guide anticoagulation therapy for this complex patient population.18 This panel provides continually updated therapeutic and research recommendations for SARS-CoV-2–positive patients. Its mission includes three main aspects: a refractory hypoxemia algorithm, a thromboprophylaxis and therapeutic anticoagulation algorithm, and general pharmacologic management with antiviral and anti-inflammatory medications in accordance with the currently recommended guidelines.7 After general consensus was reached, the latest (updated July 27, 2020) coagulopathy algorithm protocol was developed (Fig 2 ). The protocol is updated and/or discussed weekly across the campuses, especially because of the pauses in clinical trials evaluating anticoagulation therapy for all COVID-19–positive patients.23 In general, the anticoagulation recommendations consider, not only the presence or absence of DVT/PE, but also the D-dimer and fibrinogen levels and thromboelastography findings.
Fig 2.
Intensive care unit (ICU) algorithm for management of the hypercoagulable state in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). @, At; aPTT, APTT, activated partial thromboplastin time; BID, b.i.d., twice daily; BMI, body mass index; CBC, complete blood count; COVID, coronavirus disease 2019; CrCl, creatinine clearance; CT, computed tomography; e-consult, electronic/virtual consultation; DVT, deep vein thrombosis; ESRD, end-stage renal disease; GFR, glomerular filtration rate; HIT, heparin-induced thrombocytopenia; ICU, intensive care unit; labs, laboratory test results; LE, lower extremity; LFTs, liver function tests; LMWH, low-molecular-weight heparin; NL, normal limit; PE, pulmonary embolism; p.o., oral; PT, prothrombin time; PTT, partial thromboplastin time; q 12 hrs, every 12 hours; sc, subcutaneous; TEG, thromboelastography; TID, three times daily; TPA, tissue plasminogen activator; UFH, unfractionated heparin; ULN, upper limit of normal; US, ultrasound (examination); VTE, venous thromboembolism.
Our study found that low Hb and INR and elevated aPTT and IL-6 were associated with prolonged ICU and hospital stays. Evidence has suggested that higher levels of inflammatory markers correlate with a greater risk of death and a worse prognosis.24, 25, 26, 27 A low Hb level has been well studied as a factor prolonging ICU stays for multiple groups of medical and surgical patients.28 , 29 At present, the body of evidence regarding COVID-19 is in flux; therefore, we must monitor our patients' progression during their hospitalization and after discharge. We must remain open to multiple solutions to manage this complex disease process. Further evidence is required to understand and recommend the best model of care for the hypercoagulable condition of patients with COVID-19. As new data have emerged and we have become more aware of the complicated hospitalizations of all patients with COVID-19, our management protocols have undergone multiple iterations to adjust for the hypercoagulable and possible coagulopathic state.
Our study had several limitations. The most important was the retrospective nature of our analysis. Another limitation was that we had to use historic controls to define the incidence of DVT/PE because during the first phase of the pandemic almost all hospital activity was directed to COVID-19 patients and a sufficient number of controls for appropriate matching was not available. Second, in the electronic medical records, we do not have access to the socioeconomic status of individual patients that could have made access to the health care needed for the management of complications of COVID-19 either possible or challenging. It is also very possible that during the first few months of the pandemic, awareness was lacking of the greater risk of this patient population to DVT/PE; thus, we most likely failed to diagnose all thromboembolic events that occurred in patients with COVID-19.
Conclusions
We found a significantly greater incidence of DVT/PE in hospitalized patients with COVID-19 than in matched hospitalized patients without COVID-19. COVID-19 patients who develop DVT/PE during their hospitalization have a greater risk of mortality, ICU admission, and prolonged ICU and hospital stays. Our inpatient anticoagulation prevention protocols were adjusted owing to the greater incidence of DVT/PE. However, the number of COVID-19 patients affected by DVT/PE has remained high. Further studies analyzing each patient's inflammatory and procoagulant factors might be necessary to establish the best management protocol for this high-risk patient population.
Author contributions
Conception and design: YE
Analysis and interpretation: YE
Data collection: YE, CF, PG, WS, AQ, AM, ML, MG, OH, ZD, BT, CR, CL, RD, JS, HF, AH, DS, YL, CR, PM, NO, NG, CM, JH, MK, RS, MP, TG, RM, MP, JH, LP, JO, JM
Writing the article: YE
Critical revision of the article: YE, CF, PG, WS, AQ, AM, ML, MG, OH, ZD, BT, CR, CL, RD, JS, HF, AH, DS, YL, CR, PM, NO, NG, CM, JH, MK, RS, MP, TG, RM, MP, JH, LP, JO, JM
Final approval of the article: YE, CF, PG, WS, AQ, AM, ML, MG, OH, ZD, BT, CR, CL, RD, JS, HF, AH, DS, YL, CR, PM, NO, NG, CM, JH, MK, RS, MP, TG, RM, MP, JH LP, JO, JM
Statistical analysis: YL
Obtained funding: Not applicable
Overall responsibility: YE
Acknowledgments
We thank the Office of Quality Management Services for their support and willingness to participate in the improvement of care for all our patients across all our sites.
The present study was supported by the National Institutes of Health (grants R35 NS097273 and P01 NS084974 to L.P.; grant P01 NS099114 to T.G. and L.P.; grants U01 NS080168 and U19 NS115388 to J.F.M.; and grant R01 GM 126086-03 to M.S.P.), the Donald G. and Jodi P. Heeringa Family Foundation (to L.P.), the Earl and Nyda Swanson Neurosciences Research Fund (to J.F.M.), and the Harley N. and Rebecca N. Hotchkiss Endowed Fund in Neuroscience Research, Honoring Ken and Marietta (to J.F.M.).
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvsvenous.org.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix (online only).
Supplementary Table I (online only).
DVT location (n = 45)
| DVT location | No. (%) |
|---|---|
| Calf vein | 17 (38) |
| Femoral vein | 10 (23) |
| Popliteal vein | 5 (11) |
| Axillary vein | 5 (11) |
| Internal jugular vein | 5 (11) |
| Brachial vein | 1 (2) |
| External iliac vein | 1 (2) |
| Subclavian vein | 1 (2) |
DVT, Deep vein thrombosis.
Supplementary Table II (online only).
Multivariable logistic regression analysis of race adjusting for risk of DVT/PE
| Racea | Pr>χ2 | OR | 95% CI |
|---|---|---|---|
| Black/African American | 0.08 | 1.8 | 0.36-0.63 |
| Native American/Hawaiian | 0.15 | 1.4 | 0.43-0.62 |
| Asian | 0.64 | 0.5 | 0.23-0.49 |
| Other | 0.79 | 0.3 | 0.13-0.49 |
CI, Confidence interval; DVT, deep vein thrombosis; OR, odds ratio; PE, pulmonary embolism.
Categorical variable.
References
- 1.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marone E.M., Bonalumi G., Curci R. Characteristics of venous thromboembolism in COVID-19 patients: a multicenter experience from Northern Italy. Ann Vasc Surg. 2020;68:83–87. doi: 10.1016/j.avsg.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8:694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducharme J. World Health Organization Declares COVID-19 a “Pandemic”: Here's What That Means. Time Magazine. https://time.com/5791661/who-coronavirus-pandemic-declaration/ Available at:
- 6.Obi A.T., Barnes G.D., Wakefield T.W., Brown S., Eliason J.L., Arndt E. Practical diagnosis and treatment of suspected venous thromboembolism during COVID-19 pandemic. J Vasc Surg Venous Lymphat Disord. 2020;8:526–534. doi: 10.1016/j.jvsv.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C., Shen L., Le K.J., Pan M.M., Kong L.C., Gu Z.C. Incidence of venous thromboembolism in hospitalized coronavirus disease 2019 patients: a systematic review and meta-analysis. Front Cardiovasc Med. 2020;7:151. doi: 10.3389/fcvm.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rali P., O'Corragain O., Oresanya L., Yu D., Sheriff O., Weiss R. Incidence of venous thromboembolism in coronavirus disease 2019: an experience from a single large academic center [e-pub ahead of print] https://doi.org/10.1016/j.jvsv.2020.09.006 J Vasc Surg Venous Lymphat Disord. [DOI] [PMC free article] [PubMed]
- 12.Hill J.B., Garcia D., Crowther M., Savage B., Peress S., Chang K., Deitelzweig S. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4:5373–5377. doi: 10.1182/bloodadvances.2020003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y.F., Pan L.Y., Zhang W.W., Cheng F., Hu S.S., Zhang X. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J Infect Dis. 2020;100:34–41. doi: 10.1016/j.ijid.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longchamp A., Longchamp J., Manzocchi-Besson S., Whiting L., Haller C., Jeanneret S. Venous thromboembolism in critically Ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4:842–847. doi: 10.1002/rth2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edler C., Schroder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oestreich K. Remote patient monitoring provides patients with comprehensive care at home. Mayo Clinic. https://newsnetwork.mayoclinic.org/discussion/remote-patient-monitoring-provides-patients-with-comprehensive-care-at-home/ Available at:
- 17.American College of Cardiology Feature: Thrombosis and COVID-19: FAQs for current practice. Cardiology Magazine. https://www.acc.org/latest-in-cardiology/articles/2020/04/17/14/42/thrombosis-and-coronavirus-disease-2019-covid-19-faqs-for-current-practice Available at:
- 18.McBane R.D., II, Torres Roldan V.D., Niven A.S., Pruthi R.K., Franco P.M., Linderbaum J.A. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clin Proc. 2020;95:2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdullahi I.N., Emeribe A.U., Ajayi O.A., Oderinde B.S., Amadu D.O., Osuji A.I. Implications of SARS-CoV-2 genetic diversity and mutations on pathogenicity of the COVID-19 and biomedical interventions. J Taibah Univ Med Sci. 2020;15:258–264. doi: 10.1016/j.jtumed.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sardar R., Satish D., Birla S., Gupta D. Integrative analyses of SARS-CoV-2 genomes from different geographical locations reveal unique features potentially consequential to host-virus interaction, pathogenesis and clues for novel therapies. Heliyon. 2020;6:e04658. doi: 10.1016/j.heliyon.2020.e04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto N., Bauer G. Apparent difference in fatalities between Central Europe and East Asia due to SARS-COV-2 and COVID-19: four hypotheses for possible explanation. Med Hypotheses. 2020;144:110160. doi: 10.1016/j.mehy.2020.110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mojtabavi H., Saghazadeh A., Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw. 2020;31:44–49. doi: 10.1684/ecn.2020.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutkowski C. International trials of blood thinners in critically ill COVID-19 patients pause due to futility. UM Today News, University of Manitoba. https://news.umanitoba.ca/international-trials-of-blood-thinners-in-critically-ill-covid-19-patients-pause-due-to-futility/ Available at:
- 24.Liu Q., Dai Y., Feng M., Wang X., Liang W., Yang F. Associations between serum amyloid A, interleukin-6, and COVID-19: a cross-sectional study. J Clin Lab Anal. 2020;34:e23527. doi: 10.1002/jcla.23527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:e2141. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maes B., Bosteels C., De Leeuw E., Declercq J., Van Damme K., Delporte A. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayina C.A., Haritha D., Soni L., Behera S., Nair P.R., Gouri M. Epidemiological & clinical characteristics & early outcome of COVID-19 patients in a tertiary care teaching hospital in India: a preliminary analysis. Indian J Med Res. 2020;152:100–104. doi: 10.4103/ijmr.IJMR_2890_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapadohos T., Angelopoulos E., Vasileiadis I., Nanas S., Kotanidou A., Karabinis A. Determinants of prolonged intensive care unit stay in patients after cardiac surgery: a prospective observational study. J Thorac Dis. 2017;9:70–79. doi: 10.21037/jtd.2017.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellert L., Schrader F., Ringleb P., Steiner T., Bosel J. The impact of low hemoglobin levels and transfusion on critical care patients with severe ischemic stroke: stroke: relevant impact of hemoglobin, hematocrit and transfusion (STRAIGHT)—an observational study. J Crit Care. 2014;29:236–240. doi: 10.1016/j.jcrc.2013.11.008. [DOI] [PubMed] [Google Scholar]