Abstract
Background
Myocardial injury is a complication of coronavirus disease 2019 (COVID-19). We describe a large multi-center experience of COVID-19 patients with myocardial injury, examining the prognostic role left ventricular function plays on short-term outcomes.
Methods/materials
We included adult COVID-19 patients admitted to our health system with evidence of myocardial injury and who underwent a transthoracic echocardiogram (TTE) during index admission. Patients were dichotomized into those with reduced ejection fraction (EF; <50%) and preserved EF (≥50%).
Results
Across our 11-hospital system, 5032 adult patients were admitted with COVID-19 from March–September 2020. Of these, 235 had evidence of myocardial injury (troponin ≥1 ng/mL). Included were 134 patients who underwent TTE, of whom 43.3% (n = 58) had reduced EF and 56.7% (n = 76) preserved EF. A subset of 6 patients had newly reduced EF, with 5 demonstrating evidence of stress cardiomyopathy and subsequently dying. Overall, mortality was high in those with reduced EF and preserved EF (in-hospital: 34.5% vs. 28.9%; p = 0.494; 6 months: 63.6% vs. 50.0%; p = 0.167; Kaplan-Meier estimates: p = 0.2886). Readmissions were frequent in both groups (30 days: 22.2% vs. 26.0%; p = 0.162; 6 months: 52.0% vs. 54.5%; p = 0.839).
Conclusions
Many COVID-19 patients admitted with evidence of myocardial injury did not undergo TTE. For those who did, short-term mortality was high. Patients who survived hospitalization had frequent readmissions. In patients with newly reduced EF, most had evidence of stress cardiomyopathy and expired. Larger studies are needed to fully evaluate the prognosis of COVID-19 patients with evidence of myocardial injury and left ventricular dysfunction.
Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; CMR, cardiac magnetic resonance; COVID-19, coronavirus disease 2019; EF, ejection fraction; ICU, intensive care unit; LOS, length of stay; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TTE, transthoracic echocardiogram
Keywords: COVID-19, Myocardial injury, Troponin, Left ventricular ejection fraction, Readmission
1. Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has devastated healthcare systems globally [1]. The catastrophic respiratory illness that has ensued, coronavirus disease 2019 (COVID-19), has afflicted over 100 million people worldwide, killing millions [2]. Regardless of preexisting cardiovascular disease, COVID-19 has demonstrated injurious effects on the cardiovascular system, which is associated with worse in-hospital outcomes [[3], [4], [5], [6]]. The ability to quickly identify patients at elevated risk for death from COVID-19 is of great interest to healthcare systems as they balance patient care and allocation of limited resources. There are limited data on the prognostic value of left ventricular function in COVID-19 patients with evidence of myocardial injury [7]. Furthermore, outcomes following discharge in these patients have not been well-described. The present study aimed to better understand the prognostic role left ventricular function plays in patients with evidence of myocardial injury during and after hospitalization for COVID-19.
2. Material and methods
We screened patients who presented to our healthcare system (MedStar Health; 11 acute hospitals in Washington, DC, and Maryland) between March 1 and September 30, 2020. We included adult COVID-19 patients who had evidence of myocardial injury and those who had a transthoracic echocardiogram (TTE) performed during their admission. A diagnosis of COVID-19 required the clinical respiratory syndrome and/or radiological evidence of pulmonary involvement and a positive SARS-CoV-2 polymerase chain reaction. Myocardial injury was defined as a troponin drawn at any point during the admission that was at least 1.0 ng/mL. International Classification of Diseases, 10th Revision (ICD-10) codes were used to help identify patients. We excluded patients who were not admitted, were SARS-CoV-2-negative, had a troponin <1.0 ng/mL, or did not have a TTE during their admission.
Included patients were then dichotomized into those with a reduced ejection fraction (EF) (<50%) and those with a preserved EF (≥50%) based on their in-hospital TTE. The rationale behind using an EF of 50% is derived from the current heart failure guidelines [[8], [9], [10]]. We compared baseline clinical characteristics, laboratory data, rates of acute kidney injury (AKI), respiratory failure requiring intubation, intensive care unit (ICU) length of stay (LOS), mortality (in-hospital, 30-day, 3-month, and 6-month) and readmission (in-hospital, 30-day, 3-month, and 6-month) between the 2 cohorts. Follow-up data were collected, when available, from within our healthcare system. Readmissions were defined as admissions to a hospital ward or ICU. Visits to the emergency department or admission under observation status were not considered readmissions.
Data were presented as mean ± standard or median with interquartile range for continuous variables and as number (percentage) for categorical variables. Continuous variables were compared using the Student's t-test and categorical variables using the chi-squared test or Fisher's exact test. A p-value <0.05 was considered statistically significant. The Kaplan-Meier estimator was used to estimate the cumulative mortality function, from date of admission, and log rank test was applied to test the equality of the 2 mortality functions. All analyses were performed using SAS version 9.1 (SAS, Cary, North Carolina).
3. Results
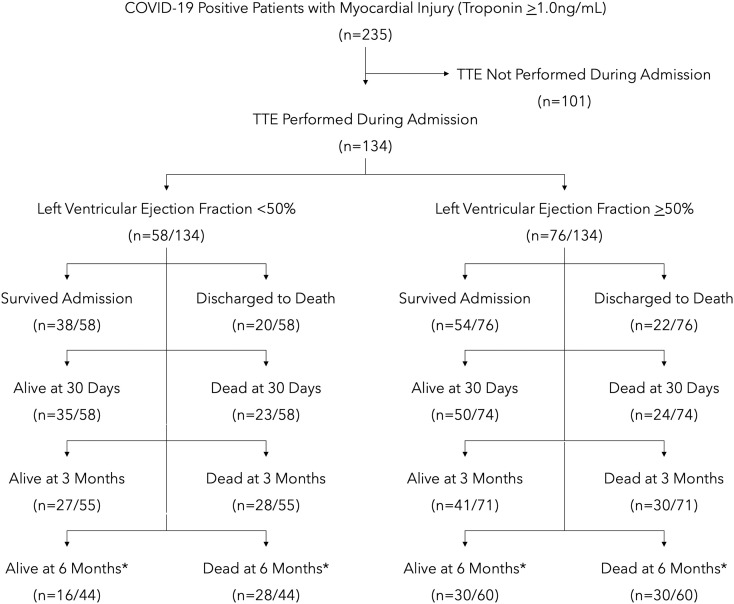
Across the MedStar Health system, a total of 5032 adult patients were admitted and diagnosed with COVID-19 between March 1 and September 30, 2020. Troponin was drawn in 3386, of whom 235 were found to have a troponin of ≥1 ng/mL. Within this group, 134 patients had a TTE performed during their admission and, thus, were included in our study. Included patients had a mean age of 63.9 years, 60.4% were men, and 61.9% were Black. Of those included, 43.3% (n = 58) had a reduced EF (<50%) and 56.7% (n = 76) had a preserved EF (≥50%) (Fig. 1 ). Of the 58 with a reduced EF, 52 had a chronically reduced EF and 6 were found to have a newly reduced EF. A total of 101 patients did not undergo TTE during their hospitalization. Baseline clinical characteristics can be found in Table 1 .
Fig. 1.
Study design and mortality.
*Of the 134 patients included in our analysis, 104 patients had their admission >6 months ago.
Table 1.
Baseline demographics and medical history.
| Reduced EF (<50%) |
Preserved EF (≥50%) |
Unknown EF |
|
|---|---|---|---|
| (n = 58) | (n = 76) | (n = 101) | |
| Age, years | 62.7 ± 15.0 | 64.7 ± 11.6 | 73.6 ± 14.9 |
| Black (%) | 38/58 (65.5%) | 45/76 (59.2%) | 71/99 (71.7%) |
| White (%) | 12/58 (20.7%) | 19/76 (25.0%) | 18/99 (18.2%) |
| Hypertension (%) | 22/58 (37.9%) | 42/76 (55.3%) | 49/101 (48.5%) |
| Hyperlipidemia (%) | 35/58 (60.3%) | 40/76 (52.6%) | 59/101 (58.4%) |
| Diabetes mellitus (%) | 36/58 (62.1%) | 38/76 (50.0%) | 58/101 (57.4%) |
| Cerebrovascular accident (%) | 9/58 (15.5%) | 20/76 (26.3%) | 16/101 (15.8%) |
| Coronary artery disease (%) | 36/58 (%) | 27/76 (35.5%) | 32/101 (31.7%) |
| Left ventricular EF, % | 32.6 ± 10.5 | 60.3 ± 5.4 | – |
| Atrial fibrillation (%) | 9/58 (15.5%) | 14/76 (18.4%) | 19/101 (18.8%) |
| Asthma (%) | 4/58 (6.9%) | 4/76 (5.3%) | 5/101 (5.0%) |
| Chronic obstructive pulmonary disease (%) | 10/58 (17.2%) | 10/76 (13.2%) | 10/101 (9.9%) |
| Chronic kidney disease (%) | 26/58 (44.8%) | 29/76 (38.2%) | 48/101 (47.5%) |
| End stage kidney disease (%) | 17/58 (29.3%) | 16/76 (21.1%) | 17/101 (16.8%) |
Values are mean ± SD or n/N (%).
EF = ejection fraction.
Maximum troponin levels during the admission were higher in patients with a reduced EF than in those with a preserved EF (median 3.5 ng/mL [interquartile range 2.1–15.6 ng/mL] vs. 2.1 ng/mL [interquartile range 1.3–4.9 ng/mL]). Additional laboratory data can be found in Table 2 . In short, all patients (reduced EF, preserved EF, and those with unknown EF) had elevated inflammatory markers during their admission, consistent with COVID-19. Patients with reduced and preserved EF had similar rates of AKI, requiring intubation and ICU LOS. Patients with a reduced EF had more ICU stays on average (1.7 vs. 1.3; p = 0.011). Overall, mortality was high in both those with reduced EF and preserved EF (in-hospital: 34.5% vs. 28.9%; p = 0.494) (Fig. 1, Table 3 ).
Table 2.
Laboratory findings.
| Reduced EF (<50%) |
Preserved EF (≥50%) |
Unknown EF |
|
|---|---|---|---|
| (n = 58) | (n = 76) | (n = 101) | |
| Maximum troponin, ng/mL | 3.5 (IQ, 2.1–15.6) | 2.1 (IQ, 1.3–4.9) | 3.2 (IQ, 1.6–6.2) |
| First creatinine, mg/dL | 1.6 (IQ, 1.1–3.5) | 1.4 (IQ, 1.0–4.0) | 1.7 (IQ, 1.1–2.9) |
| Maximum creatinine, mg/dL | 2.3 (IQ, 1.5–6.5) | 2.8 (IQ, 1.3–6.3) | 2.7 (IQ, 1.5–4.8) |
| First ALT, IU/L | 74 (IQ, 31–146) | 56 (IQ, 30–91) | 59 (IQ, 35–113.5) |
| Maximum ALT, IU/L | 133 (IQ, 68–447) | 121 (IQ, 53–368) | 137 (IQ, 61–433) |
| First AST, IU/L | 42 (IQ, 18–66) | 37 (IQ, 21–58) | 38 (IQ, 21–71) |
| Maximum AST, IU/L | 74 (IQ, 39–303) | 71 (IQ, 40–225) | 66 (IQ, 30–204) |
| First lactate, mmol/L | 1.9 (IQ, 1.3–3.1) | 2.0 (IQ, 1.4–2.7) | 1.9 (IQ, 1.5–3.1) |
| Maximum lactate, mmol/L | 4.4 (IQ, 2.6–6.6) | 3.1 (IQ, 2.0–6.2) | 3.3 (IQ, 2.0–8.7) |
| First white blood cells, cells/mm3 | 10.2 (IQ, 6.7–14.3) | 7.6 (IQ, 5.0–11.8) | 9.4 (IQ, 6.2–12.6) |
| Maximum white blood cells, cells/mm3 | 17.3 (IQ, 12.4–25.3) | 16.0 (IQ, 10.8–24.2) | 14.1 (IQ, 10.5–10.2) |
| First procalcitonin, ng/mL | 0.7 (IQ, 0.1–8.1) | 0.9 (IQ, 0.3–6.7) | 1.6 (IQ, 0.4–8.1) |
| Maximum procalcitonin, ng/mL | 6.3 (IQ, 0.4–16.1) | 2.1 (IQ, 0.6–18.2) | 2.6 (IQ, 0.7–17.8) |
| First D-dimer, μg/mL | 2.3 (IQ, 1.4–9.1) | 3.9 (IQ, 1.9–7.7) | 3.3 (IQ, 1.4–7.5) |
| Maximum D-dimer, μg/mL | 3.5 (IQ, 1.6–17.3) | 6.1 (IQ, 2.6-a) | 4.5 (IQ, 1.9–18.6) |
| Erythrocyte sedimentation rate, mm/h | 113 (IQ, 51-a) | 104 (IQ, 56–138) | 82 (IQ, 49-a) |
| Ferritin, ng/mL | 1519 (IQ, 766–4458) | 1571 (IQ, 767–3560) | 1447 (IQ, 573–6274) |
| Lactate dehydrogenase, U/L | 594 (IQ, 419–993) | 565 (IQ, 386–955) | 658 (IQ, 471–944) |
Values are medians with interquartile ranges.
EF = ejection fraction, IQ = interquartile range, ALT = alanine aminotransferase, AST = aspartate transaminase.
Certain tests could not report values above or below a certain limit.
Table 3.
In-hospital outcomes of patients included in our study.
| Reduced EF (<50%) (n = 58) |
Preserved EF (≥50%) (n = 76) |
P-value | |
|---|---|---|---|
| Acute kidney injury (%) | 19/41 (46.3) | 34/64 (53.1) | 0.498 |
| Intubated (%) | 36/58 (62.1) | 42/76 (55.3) | 0.429 |
| Intensive care unit stays | 1.7 ± 0.5 | 1.3 ± 0.5 | 0.011 |
| Intensive unit length of stay, days | 12.9 ± 14.2 | 14.9 ± 20.9 | 0.577 |
| Death (%) | 20/58 (34.5) | 22/76 (28.9) | 0.494 |
Values are mean ± SD or n/N (%).
EF = ejection fraction.
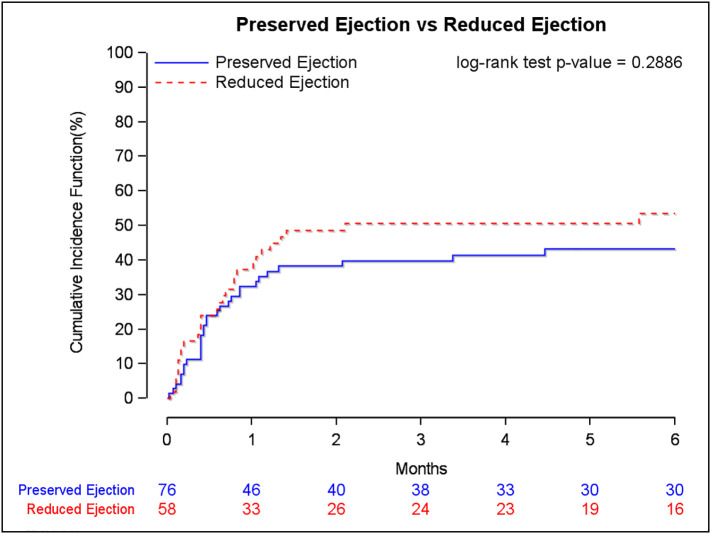
Follow-up data after discharge were available in 100% of patients at 30 days and 98.5% of patients at 3 months. Of the 134 patients included in our analysis, 30 patients had not yet reached 6-month follow-up. Following discharge, mortality was high in those with reduced EF and preserved EF (30 days: 39.7% vs. 32.4%; p = 0.1057; 6 months: 63.6% vs. 50.0%; p = 0.167; Kaplan-Meier estimates: p = 0.2886) (Fig. 1, Fig. 2 , Table 3, Table 4 ). Readmissions were frequent in those with reduced EF and preserved EF (30 days: 22.2% vs. 26.0%; p = 0.162; 6 months: 52.0% vs. 54.5%; p = 0.839) (Table 4).
Fig. 2.
Mortality curve and the Kaplan-Meier estimates for COVID-19 patients with a positive troponin who underwent echocardiography during their admission.
Table 4.
Post-discharge outcomes of patients included in our study.
| Reduced EF (<50%) | Preserved EF (≥50%) | P-value | |
|---|---|---|---|
| 30-day | |||
| Readmission (%) | 8/36 (22.2) | 13/50 (26.0) | 0.1618 |
| Death (%) | 23/58 (39.7) | 24/74 (32.4) | 0.1057 |
| Cumulative 3-month | |||
| Readmission (%) | 13/33 (39.4) | 22/46 (47.8) | 0.4568 |
| Death (%) | 28/55 (50.9) | 30/71 (42.3) | 0.3337 |
| Cumulative 6-montha | |||
| Readmission (%) | 13/25 (52.0) | 24/44 (54.5) | 0.8385 |
| Death (%) | 28/44 (63.6) | 30/60 (50.0) | 0.1666 |
Values are n/N (%).
EF = ejection fraction.
Of the 134 patients included in our analysis, 104 had their index admission >6 months ago.
Of the 58 COVID-19 patients with evidence of myocardial injury and a reduced EF, 52 had a prior history of heart failure with reduced EF. The remaining 6 had a newly reduced EF. All of them presented with, and were confirmed to have, COVID-19 pneumonia and subsequently required intubation and vasopressors. Their mean age was 66.2 years (ranging from 45 to 85 years), and 4 were men. Wall-motion analysis on TTE was consistent with stress cardiomyopathy in 5 of the patients, with most receiving a combination of hydroxychloroquine, dexamethasone, convalescent plasma, tocilizumab, or remdesivir. None of them were taken for cardiac catheterization, and all of them died during the admission. These 5 had a maximum troponin range between 1.03 and 11.5 ng/mL. The final patient with a newly reduced EF was a 75-year-old male with a history of coronary artery disease (CAD) whose admission was complicated by a plaque-rupture non-ST-segment elevation myocardial infarction (NSTEMI) and cardiogenic shock requiring intra-aortic balloon pump. His troponin peaked at 81 ng/mL, prompting cardiac catheterization, which demonstrated an ulcerated 99% proximal left anterior descending artery lesion, resulting in percutaneous coronary intervention (PCI), and he was discharged several days later.
Cardiac magnetic resonance (CMR) imaging was performed in 2 patients following discharge. The first was a 55-year-old male with prior CAD who had initially presented in July 2020 with dyspnea and chest pain. He was found to be COVID-19-positive with an NSTEMI and a preserved EF. His troponin peaked at 8.85 ng/mL, but given his COVID-19 status, he was treated medically. Cardiac catheterization was electively performed several weeks later, revealing multivessel CAD. Subsequent CMR demonstrated an EF of 34%, diffuse pericardial gadolinium enhancement suggestive of pericardial inflammation, and transmural enhancement indicative of prior myocardial infarction. He was initially scheduled for coronary artery bypass grafting but suffered a second NSTEMI and underwent uncomplicated multivessel PCI. The second patient was a 41-year-old male without significant history who initially presented with acute encephalopathy in the setting of COVID-19 complicated by acute pulmonary embolism. He was found to have mildly reduced biventricular function and a secundum atrial septal defect (ASD) on TTE during the admission. Following discharge, he remained asymptomatic and underwent CMR, which demonstrated a left ventricular EF of 53% without abnormal myocardial enhancement, a secundum ASD, and a severely dilated right ventricle with a right ventricular EF of 48%.
4. Discussion
This study is one of only a few to examine the short-term prognostic implications of left ventricular function and to describe the post-discharge outcomes of COVID-19 patients with myocardial injury. The principal findings of this study are summarized as follows: 1) In COVID-19 patients with evidence of myocardial injury, nearly half did not undergo TTE and mortality was extremely high, both in-hospital and out to 6 months, with a trend toward higher mortality in patients with a reduced EF; 2) COVID-19 patients with evidence of myocardial injury saw exceedingly high rates of AKI (requiring intubation) and in-hospital death; 3) For those who survived their initial COVID-19 admission, many had subsequent admissions and died; 4) Finally, in the subset of patients with a newly reduced EF, the majority demonstrated multi-organ system failure with evidence of stress cardiomyopathy, dying during the initial hospitalization.
Myocardial injury, as evidenced by a troponin elevation, in COVID-19 patients has been well-described as a risk factor for worse outcomes during the pandemic [[3], [4], [5], [6]]. The concern initially was that the majority of COVID-19 patients with elevated troponin were experiencing myocarditis, some even fulminant myocarditis, which was contributing to poor outcomes in these patients. However, as the death toll rose, so did post mortem investigations into this diagnosis, eventually disproving initial concerns surrounding myocarditis-related deaths in COVID-19 [11,12]. Evidence of cardiac histopathological findings, such as macro/microvascular thrombi, inflammation, or intraluminal megakaryocytes, were much more common, discovered in nearly half of autopsies [11]. Despite these post mortem findings, clinical evaluation of these patients during admission has been extremely challenging during the pandemic. In the pre-pandemic world of cardiovascular disease, diagnosis and management of patients with troponin elevation was driven by cardiovascular imaging, with nearly every patient undergoing echocardiography. During the pandemic, infection-control measures and reallocated resources have greatly reduced the utilization of echocardiography, computed tomography, magnetic resonance imaging, and angiography in these patients [13]. Myocardial biopsy and CMR for the diagnosis of myocarditis have become exceedingly uncommon during the pandemic. Thus, there are limited data on the impact of left ventricular function on outcomes in COVID-19 patients with myocardial injury. A small retrospective study reported that COVID-19 patients with a reduced EF had higher in-hospital mortality [7]. However, this study was limited to 39 patients, examined only in-hospital outcomes, and included patients admitted with COVID-19 regardless of troponin [7]. Our experience of 235 COVID-19 patients admitted across our 11-hospital healthcare system during the pandemic was similar in that nearly half of COVID-19 patients with myocardial injury did not undergoing TTE. Furthermore, only 2 patients underwent CMR in the outpatient setting following discharge, with no evidence of myocarditis. That being said, the included cohort of COVID-19 patients with evidence of myocardial injury suffered a high incidence of adverse outcomes, with approximately half experiencing multiorgan involvement and only ⅔ surviving their COVID-19 admission. Arguably, the worse outcomes occurred in those with newly reduced left ventricular function. All of these patients required intubation and vasopressor support. Of the 6 patients with newly reduced EF, just 1 was found to have true plaque-rupture NSTEMI, underwent successful PCI, and then was discharged home. The others had echocardiographic findings suggestive of stress cardiomyopathy, with mild troponin elevation, but they were never stable enough for cardiac catheterization, eventually succumbing to multiorgan system failure and death.
In-hospital survival may represent the largest hurdle in these patients; however, the consequences of COVID-19 appear to extend well past discharge. One study of 1733 COVID-19 patients demonstrated that 76% of patients remained debilitated to some degree at 6 months [14]. Moreover, more than half demonstrated persistent pulmonary abnormalities on imaging with disease severity during the acute phase predicting the extent of lung diffusion impairment at follow-up [14]. Finally, in this study of consecutive COVID-19 patients admitted, 1.3% of patients died during the follow-up period due to exacerbations of underlying cardiovascular, pulmonary, and renal disease. Our highly selective study of COVID-19 patients with evidence of myocardial injury is the first to offer insight into post-discharge outcomes in this high-risk population, demonstrating persistent debilitation, regardless of EF. Patients who survived their admission remained at risk for readmissions and death following discharge for their acute COVID-19 illness. Thus, efforts should focus on early recognition of these patients prior to discharge and close post-discharge follow-up in the months that follow.
Cytokine storm and cytokine release syndrome are life-threatening complications that have been demonstrated in patients with COVID-19, leading to disastrous complications [15,16]. Stress cardiomyopathy is one such complication whose proposed mechanism of action is catecholamine surge. During the pandemic, several studies have described an increase in the occurrence of stress cardiomyopathy due to the cytokine response in COVID-19 [15,16]. However, the diagnosis of stress cardiomyopathy is one of exclusion, requiring newly reduced EF on echocardiography and invasive coronary angiography to rule out obstructive CAD [17]. Unfortunately, such ordinarily routine evaluations cannot be regularly performed during the pandemic as overwhelmed healthcare systems struggle to safely provide care to innumerable critically ill patients. As such, data regarding outcomes in COVID-19 patients with stress cardiomyopathy are scarce. Only a handful of case series have been reported, demonstrating that many suffer complications, such as cardiac tamponade, heart failure, myocarditis, hypertensive crisis, and cardiogenic shock [15,16]. Our findings support these reports, as the subset of patients with a newly reduced EF, albeit small, represented the highest-risk group, with 5 of 6 demonstrating echocardiographic evidence of stress cardiomyopathy. These moribund patients all presented with COVID-19 pneumonia requiring intubation, complicated by multiorgan system failure, shock requiring vasopressor support, and ultimately died.
Our study has several limitations. This was a retrospective observational study that partially relied on ICD-10 codes to help identify our cohort of interest, which introduces selection bias. Only a fraction of patients admitted for COVID-19 had a troponin drawn, and an even smaller number of patients underwent TTE during their admission; therefore, it is likely that this retrospective study did not capture the true incidence of myocardial injury or reduced left ventricular function in COVID-19 patients. Many patients had laboratory values that extended past the upper/lower limits of detection, rendering a statistical comparison difficult; thus, we reported medians and interquartile ranges instead. Additionally, our cohort was relatively small, limiting our primary analysis of short-term outcomes in patients with a reduced EF compared to those with a preserved EF. The size of our cohorts also limited adequate subgroup analysis of outcomes in patients with newly reduced EF compared to those with chronically reduced EF. The results of this study are hypothesis-generating and will require prospective validation in a large cohort of patients.
5. Conclusion
Many COVID-19 patients admitted with evidence of myocardial injury did not undergo TTE. For those who did, short-term mortality was extremely high. Patients who survived their hospitalization had frequent readmissions. In the subset with newly reduced EF, the majority had evidence of stress cardiomyopathy and expired. Larger studies will be needed to fully evaluate the prognosis of COVID-19 patients with evidence of myocardial injury and left ventricular dysfunction.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
William S. Weintraub – Research support: Amarin Corporation, National Institutes of Health; Consultant: Amarin Corporation, AstraZeneca, Janssen, SC Pharma, The Medicines Company.
Ron Waksman – Advisory Board: Abbott Vascular, Amgen, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips, Pi-Cardia Ltd.; Consultant: Abbott Vascular, Amgen, Biotronik, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips, Pi-Cardia Ltd., Transmural Systems; Grant Support: AstraZeneca, Biotronik, Boston Scientific, Chiesi; Speakers Bureau: AstraZeneca, Chiesi; Investor: MedAlliance; Transmural Systems.
All other authors – None.
References
- 1.Shadmi E., Chen Y., Dourado I., Faran-Perach I., Furler J., Hangoma P., et al. Health equity and COVID-19: global perspectives. Int J Equity Health. 2020;19:104. doi: 10.1186/s12939-020-01218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus update. https://www.worldometers.info/coronavirus/
- 3.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoval Y., Januzzi J.L., Jr., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalid N., Chen Y., Case B.C., Shlofmitz E., Wermers J.P., Rogers T., et al. COVID-19 (SARS-CoV-2) and the heart - an ominous association. Cardiovasc Revasc Med. 2020;21:946–949. doi: 10.1016/j.carrev.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case B.C., Yerasi C., Forrestal B.J., Shea C., Rappaport H., Medranda G.A., et al. Comparison of characteristics and outcomes of patients with acute myocardial infarction with versus without coronarvirus-19. Am J Cardiol. 2021;144:8–12. doi: 10.1016/j.amjcard.2020.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soulat-Dufour L., Lang S., Ederhy S., Adavane-Scheuble S., Chauvet-Droit M., Nhan P., et al. Left ventricular ejection fraction: an additional risk marker in COVID-19. Arch Cardiovasc Dis. 2020;113:760–762. doi: 10.1016/j.acvd.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Colvin M.M., et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 10.Hollenberg S.M., Warner Stevenson L., Ahmad T., Amin V.J., Bozkurt B., Butler J., et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966–2011. doi: 10.1016/j.jacc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapkiewicz A.V., Mai X., Carsons S.E., Pittaluga S., Kleiner D.E., Berger J.S., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoghbi W.A., DiCarli M.F., Blankstein R., Choi A.D., Dilsizian V., Flachskampf F.A., et al. Multimodality cardiovascular imaging in the midst of the COVID-19 pandemic: ramping up safely to a new normal. JACC Cardiovasc Imaging. 2020;13:1615–1626. doi: 10.1016/j.jcmg.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S., Desai R., Gandhi Z., Fong H.K., Doreswamy S., Desai V., et al. Takotsubo syndrome in patients with COVID-19: a systematic review of published cases. SN Compr Clin Med. 2020:1–7. doi: 10.1007/s42399-020-00557-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai H.D., Sharma K., Jadeja D.M., Desai H.M., Moliya P. COVID-19 pandemic induced stress cardiomyopathy: a literature review. Int J Cardiol Heart Vasc. 2020;31:100628. doi: 10.1016/j.ijcha.2020.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komamura K., Fukui M., Iwasaku T., Hirotani S., Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602–609. doi: 10.4330/wjc.v6.i7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]