Abstract
Background
Health-related quality of life (QoL) is often adversely affected in patients with inflammatory bowel disease (IBD). We aimed to identify factors associated with poor QoL among Canadian patients with IBD in clinical remission.
Methods
We enrolled patients at a single academic tertiary care center with inactive IBD. All eligible patients completed a series of questionnaires that included questions on demographics, disease activity, anxiety, depression and the presence of irritable bowel syndrome (IBS) symptoms. Stool sample for fecal calprotectin (FC) was also collected to assess for subclinical inflammation. The primary outcome measure was QoL assessed by the short inflammatory bowel disease questionnaire (SIBDQ), with planned subgroup comparisons for fatigue, anxiety, depression and IBS symptoms.
Results
Ninety-three patients were eligible for inclusion in this study. The median SIBDQ scores were lower in patients with anxiety (P < 0.001), depression (P = 0.004), IBS symptoms (P < 0.001) and fatigue (P = 0.018). Elevated FC in patients in clinical remission did not impact QoL. These findings were consistent on multivariate linear regression.
Conclusions
Anxiety, depression, fatigue and IBS symptoms are all independently associated with lower QoL in patients with inactive IBD. Clinicians are encouraged to screen for these important factors as they may detrimentally impact QoL in IBD patients even in clinical remission.
Keywords: Crohn’s disease, Inflammatory bowel disease, Irritable bowel syndrome, Quality of life, Ulcerative colitis
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory diseases of the gastrointestinal tract characterized by periods of relapse and remission. Inflammatory bowel disease (IBD) has become increasingly prevalent globally, with accelerating incidence in industrializing countries (1). Several studies have demonstrated that beyond direct medical consequences, IBD can result in considerable physical, mental, emotional and financial repercussions, all of which can have a substantial burden on quality of life (QoL) (2–6).
A previous systematic review on psychological morbidity in patients with IBD identified multiple risk factors for depression and anxiety including increased disease severity, lower socioeconomic status and corticosteroid use (7). Depression and anxiety as well as functional gastrointestinal disorders (FGID) are more common in patients with IBD even in remission as compared to the general population (8–10). This suggests that the adverse impact observed extends beyond disease activity and is likely associated with multiple consequences of this chronic disease. In patients with chronic conditions, depression is linked with reduced adherence to treatment (11,12) and poorer outcomes (13). In Canada, the estimated yearly economic burden of depression is in the order of fifty billion dollars (14).
In patients with IBD, there is a known association between anxiety and depression and poorer QoL (15). One study demonstrated that depression and anxiety are correlated with poorer QoL at the time of diagnosis of IBD and during times of disease relapse (16). Although QoL is negatively correlated with disease severity, it is unclear if QoL is adversely impacted by depression, anxiety and FGID during times of disease remission. Identifying modifiable factors which diminish QoL in patients with inactive IBD could provide clinically relevant avenues for improvement of overall patient care which goes beyond the IBD-specific treatment.
While data exist to support worse overall QoL in patients with IBD in clinical remission compared with those without IBD, there remains a relative paucity of data regarding what specific factors contribute to worse QoL in this specific patient population. We conducted this prospective, cross-sectional study to determine whether depression, anxiety, fatigue, subclinical inflammation and symptoms of FGID were associated with lower QoL in patients with IBD in clinical remission.
METHODS
This study was approved by the St. Michael’s Hospital Research Ethics Board (12–020). The reporting followed recommendations made in the STROBE statement for cross-sectional studies (17). We obtained informed consent from patients at a routine clinic visit. All authors approved the final version of the manuscript.
Participants
We recruited patients with inactive IBD. Inactive IBD was defined using the physician global assessment (PGA) in addition to clinical remission as indicated by either the Harvey-Bradshaw Index (HBI) for CD (18) or the Simple Clinical Colitis Activity Index (SCCAI) for UC (19).
Patients were recruited from the ambulatory clinics of seven gastroenterologists at St. Michael’s Hospital in Toronto, Ontario between December 2012 and November 2013.
Potential participants were informed of the study by telephone approximately 1 week prior to their usual clinic visit.
Patients were eligible for inclusion if they were at least 18 years of age, able to read and write in English and had a diagnosis of IBD. Patients who were willing to participate were asked to bring a first morning stool sample to the clinic for assessment of the fecal calprotectin (FC) protein as a marker of subclinical intestinal inflammation.
After the patients’ regular clinic visit, the patients’ treating gastroenterologist provided the study personnel a PGA of disease activity as well as information pertaining to the presence of an abdominal mass as part of the HBI for patients with CD. Patients who were deemed to have inactive disease by PGA were then asked to complete a series of questionnaires including demographics, the HBI for patients with CD, the SCCAI for patients with UC, the presence of IBS symptoms utilizing the ROME III criteria, their current treatments, QoL and mood. Patients’ medical charts were reviewed to ensure a diagnosis of either CD or UC, based on accepted standard clinical, imaging and pathology parameters (20,21).
The SCCAI consists of six simple items that address bowel habits, extra-abdominal manifestations and general well-being in UC. The HBI includes five questions describing stool frequency, abdominal pain, well-being, extra-intestinal manifestations, as well as the presence of an abdominal mass. We considered patients as having inactive IBD if they had an SCCAI score of ≤ 2 or an HBI score of ≤ 4 for UC and CD, respectively.
Patients were excluded from this study for any of the following reasons: pregnancy, evidence of clinically active disease by PGA or by HBI/SCCAI, did not provide a stool sample, or declined to complete the questionnaires.
QoL Assessment
To assess QoL, the short inflammatory bowel disease questionnaire (SIBDQ) was used which consists of 10 questions that has a total score ranging from 10 (worst health) to 70 (best health) and has been validated amongst patients with CD (22) and with UC (23).
Anxiety and Depression Assessment
Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS), which is a self-assessment scale consisting of 14 items, with subscales for anxiety (7 items) and depression (7 items). Subscores of 0 to 7 are considered normal, 8 to 10 are considered borderline abnormal, and subscores of ≥11 are strongly suggestive of clinical depression and/or anxiety (24). For the purposes of this study, we used a cut-off of >8 as suggestive for the presence of depression or anxiety. Utilizing this cut-off value, we categorized our patients into two groups: (a) possibly having depression, (b) possibly having anxiety, or (c) not having depression/anxiety.
FGID Assessment
FGID were assessed using the Rome III diagnostic criteria for IBS which assesses presence of abdominal pain or discomfort associated with a change in stool frequency and/or form for at least 6 months (25).
Subclinical Inflammation Assessment
Participants were asked to bring a first morning stool sample collected within 7 days of completing the questionnaires which was analyzed for FC. Subclinical inflammation was assessed using the high-range Quantum Blue CHR 20 (26) whereby FC levels greater than 100 µg/g of stool were considered positive for subclinical inflammation. As reported in a meta-analysis, cut-off values of FC of 100 μg/g had a sensitivity and specificity of 0.84 and 0.6 (27). For a subset of patients (n = 30) with undetectable levels of FC by the high-range Quantum Blue CHR 20, stool samples were analyzed using the low range Quantum Blue Rapid Test (26).
Other Data Gathered
Physicians completed the following: a PGA, which is a four-point Likert scale grading their disease as quiescent, or active-and mild, moderate or severe. Study personnel asked patients if they had fatigue symptoms and recorded the patients’ answer as present or absent.
The following data were collected from patients’ electronic medical records: phenotypic IBD characteristics; the Montreal classification parameters for CD or UC which consists of age of onset, site or extent of disease and presence of perianal disease (28); current medication use, including the use of nonsteroidal anti-inflammatory drugs and prior surgeries.
All data collected for study purposes were kept confidential and masked from the health providers in order to avoid influencing management.
Outcome Measures
The primary dependent variable of interest was QoL among patients inactive IBD as assessed by the SIBDQ. Correlations were investigated between QoL and the following variables: presence of fatigue, anxiety, depression and IBS-like symptoms.
QoL as measured by the SIBDQ was compared between patients with and without subclinical inflammation as measured by FC.
Statistical Analysis
Data were analyzed using SPSS 26.0 (SPSS Inc., Chicago, IL). Categorical data were expressed as proportions and continuous data were expressed as median and interquartile range (IQR). We used Mann–Whitney U tests or chi-square tests, as indicated, to determine if there was a difference in SIBDQ among the following groups: patients with anxiety, patients with depression, patients with IBS symptoms, patients with fatigue and FC levels. A multivariable linear regression model was used to investigate the relationships between relevant variables and SIBDQ score. An alpha of 0.05 was set as the cut-off for statistical significance for all analyses.
RESULTS
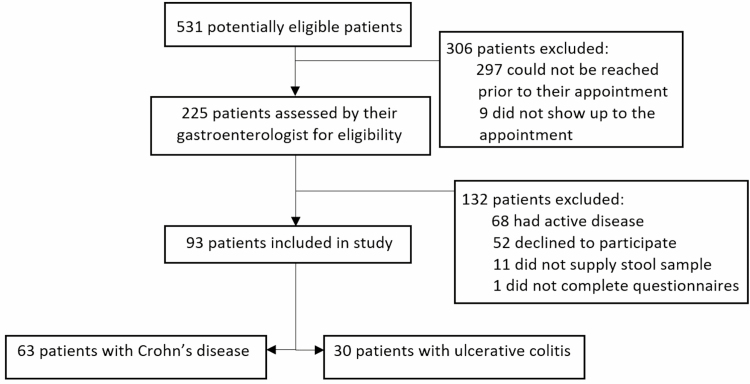
A total of 531 patients were considered for eligibility to participate in the study. Of these, 93 patients were included in the study and data from these patients were considered in the final analysis (63 with CD [67.7% of patients] and 30 with UC [32.3% of cases]). A flow diagram summarizing participant recruitment is illustrated in Figure 1. Demographic and baseline characteristics of study participants are shown in Table 1.
Figure 1.
Flow diagram illustrating number of patients at each stage of recruitment into the study.
Table 1.
Demographic, clinical and psychosocial characteristics of patients
| Characteristic n (%) unless otherwise stated | Crohn’s disease (n = 63) | Ulcerative colitis (n = 30) | Total (n = 93) |
|---|---|---|---|
| Demographic | |||
| Age of IBD diagnosis, years (mean ± SD) | 28.4 ± 13.9 | 32.2 ± 18.6 | 29.5 ± 15.4 |
| Female | 25 (39.7) | 18 (60.0) | 43 (46.2) |
| Ethnicity (European descent) | 50 (79.4) | 18 (60.0) | 68 (73.1) |
| Highest level of education (Technical school, college, university or postgraduate training) | 46 (73.0) | 19 (63.3) | 65 (69.9) |
| Marital status (married or common-law) | 38 (60.3) | 20 (66.7) | 58 (62.4) |
| Employment status (full-time or part-time) | 42 (66.7) | 20 (66.7) | 62 (66.7) |
| Clinical | |||
| Duration of disease (> 2 years) | 60 (95.2) | 24 (80.0) | 84 (90.3) |
| IBS-like symptomsa | 24 (38.1) | 5 (16.7) | 29 (31.2) |
| Positive fecal calprotectinb | 23 (36.5) | 11 (36.7) | 34 (36.6) |
| Psychosocial | |||
| Anxietyc | 10 (15.9) | 9 (30.0) | 19 (20.4) |
| Depression d | 3 (4.8) | 2 (6.7) | 5 (5.4) |
IBD, Inflammatory bowel disease; IBS, Irritable bowel syndrome; SD, Standard deviation.
aEvaluated using Rome III diagnostic criteria.
bPositive cut-off value of FC ≥ 100 µg/g.
cHADS-anxiety subscores of ≥ 8.
dHADS-depression subscores of ≥ 8.
QoL and Anxiety, Depression
The overall prevalence of depression and anxiety in this cohort was 5.4% (n = 5) and 20.4% (n = 19), respectively. Given the relatively small number of patients with depression and anxiety in our sample size, we did not perform comparative analyses for rates of depression and anxiety between patients with CD and patients with UC.
For the SIBDQ, the median score was lower in patients with anxiety compared with those without (55.0 [IQR: 46.0 to 60.0] versus 61.0 [IQR: 57.0 to 65.0], respectively; P < 0.001) and lower in patients with depression compared to those without (50.0 [IQR: 29.5 to 56.0] versus 60.0 [IQR: 56.0 to 64.8], respectively; P = 0.004).
QoL and Irritable Bowel Syndrome
Among 93 patients included in the study, 29 (31.2%) had IBS-like symptoms. The median SIBDQ scores were significantly lower among patients with IBS symptoms compared with the patients without IBS symptoms (55.0 [IQR: 52.0 to 59.5] versus 63.0 [IQR: 59.0 to 65.0], respectively; P < 0.001).
QoL and Fatigue
The median SIBDQ score in patients with fatigue was significantly lower than those without (57.0 [IQR: 34.5 to 59.5] with fatigue versus 60.0 [55.0 to 64.8], respectively; P = 0.018).
QoL and FC
In our patient cohort with clinically inactive IBD, 34 (36.6%) had positive FC level (≥100 µg/g). Using a FC cut-off of 100 µg/g, there was no significant difference in median SIBDQ scores with active inflammation (i.e., ≥ 100 µg/g) and without active inflammation (i.e., < 100 µg/g) (60.0 [IQR: 56.5 to 64.0] versus 60.0 [IQR: 54.0 to 65.0], respectively; P = 0.838). Twenty-seven (29.0%) patients had a FC greater than 250 µg/g; using this cut-off, there was no difference in the median SIBDQ between those with active inflammation and without active inflammation (<250 µg/g) (60.0 [IQR: 57.0 to 64.0] versus 60.0 [IQR: 54.5 to 64.5]; P = 0.800).
Multivariable Regression
On multivariable linear regression analysis, anxiety, depression, fatigue and IBS symptoms remained independently associated with lower SIBDQ scores (Table 2). Commensurate with the findings on univariate analysis, subclinical inflammation as measured by FC did not have any impact on SIBDQ.
Table 2.
Multivariable linear regression of relevant variables on SIBDQ
| β-coefficient (95% CI) | P value | |
|---|---|---|
| Gender | −0.4 (−2.9, 2.0) | 0.74 |
| Depression | −9.0 (−14.5, −3.5) | 0.002 |
| Anxiety | −6.8 (−9.8, −3.8) | <0.001 |
| Fatigue | −7.2 (−12.6, −1.7) | 0.01 |
| IBS | −5.7 (−8.3, −3.1) | <0.001 |
| Fecal Cal >100 | 1.6 (−1.0, 4.1) | 0.23 |
CI, Confidence interval; IBS, Irritable bowel syndrome.
Discussion
In this cross-sectional study, we found that anxiety, depression, fatigue and IBS-like symptoms were all independently associated with lower QoL among Canadian patients with clinically inactive IBD. In addition, we found that in patients with clinical remission, elevated FC was not associated with worse QoL.
IBD carries a substantial psychosocial burden. In a systematic review and meta-analysis by Knowles et al. pooled mean QoL scores were lower in both adult and paediatric IBD populations compared to age-matched healthy controls (29). Furthermore, they report that QoL is worse in active disease compared with inactive disease (30). Similarly, in a survey study of 314 IBD patients, IBD disease severity was found to be the most important predictor of both physical and mental QoL even with accounting for the presence of other chronic diseases (31). These findings reinforce the importance of good disease control in order to optimize QoL in patients with this chronic and potentially debilitating condition.
While active disease is likely the strongest contributor to poor QoL, several studies have shown that QoL of life is impaired despite well-controlled disease activity, as is the case in the present study (8–10,32,33). Beyond the physical encumbrances associated with poorly controlled IBD, the stigma of chronic disease along with the associated uncertainties and loss of autonomy can contribute to worse QoL. In a qualitative study interviewing patients with CD, several themes were identified that can impact QoL from patients perspectives including, but not limited to hygiene, continence, freedom from infection, security, attractiveness, relationships and autonomy (34). These themes clearly delineate the indirect and widespread consequences of these chronic conditions.
Fatigue, anxiety and depression were also identified as important contributors to poor QoL in our study. Fatigue has been identified in several other studies as an important contributor to health-related QoL in IBD patients (35,36). Previous data using validated measurements of fatigue have shown a high prevalence of fatigue in this patient cohort with 26.4% of subjects reporting fatigue and a strong association with poor health-related QoL (35). Likewise, anxiety and depression occur frequently in IBD with a detrimental effect on QoL even in the absence of luminal symptoms (37). Early identification of anxiety and depression is important as several treatment options are available, though studies in IBD patients have shown variable results. In a meta-analysis inclusive of fourteen randomized control trials, psychological therapies were found to have beneficial effects on depression scores and QoL in IBD, though the benefits appeared to be lost in the long term (38). Likewise, in another meta-analysis by Mikocka-Walus et al., pharmacotherapy for depression and anxiety in IBD patients did not have a clear benefit toward improvement in depression scores (39). Future avenues of research into the how best to approach IBD patients with anxiety and depression as current strategies may not be sufficient.
The current study has several identifiable limitations which impact the generalizability of our findings. First, this study involved a relatively small cohort of patients treated in a single tertiary academic center, wherein the majority of the patients included in this study were of European descent with at least some postgraduate education. In particular, there were relative few patients with UC who met the criteria to be included in this study, which may limit the generalizability of our findings. Second, although many of our findings met statistical significance, the question of clinical significance remains. The SIBDQ was initially validated against the health-related QoL questionnaire which is largely based on clinical assessment of disease activity (22). It was later found to be responsive to clinically important change in UC disease activity (23). In the responsiveness study, the authors reported a difference in mean SIBDQ score of 31.9 between patients in remission and severe relapse and 14.6 between patients in remission and mild relapse. For comparison, the decrease in SIBQD found on our multivariate analysis was less but approached the decrease in SIBDQ score in patients with mild relapse in disease. Third, study personnel assessed fatigue by asking patients regarding symptoms of fatigue. In our study, this was a subjective assessment and interpretation may be limited by lack of objective validated fatigue questionnaire.
In conclusion, this cross-sectional study identified fatigue, depression, anxiety and IBS symptoms are all associated with poor QoL among Canadian patients with inactive IBD. Importantly, subclinical inflammation as defined by a positive FC in the absence of clinical symptoms did not have an adverse effect on QoL. The findings of this study suggest that patients with IBD would likely benefit from screening for depression, anxiety, fatigue and IBS even in the setting of clinical remission. Further research is warranted to determine if targeted treatment of these conditions, specifically in patients with quiescent IBD would lead to improved outcomes.
Funding
None declared.
Conflict of Interest
S.C.G. has received research grants and personal fees from AbbVie and Ferring Pharmaceuticals, personal fees from Takeda, education grants from Janssen, and has equity in Volo Healthcare. All other authors have no relevant conflicts of interest.
Author Contributions
Study conception and design: E.J.I., S.C.G.; Data acquisition: A.N., D.C.L., N.G., K.B., R.G.; Analysis and interpretation of data: A.N., K.B., R.G., S.C.G.; Drafting of the manuscript: A.N., K.B., R.G., M.A.S., R.K., S.C.G.; Study supervision: E.J.I., S.C.G. Critical revision of the manuscript for important intellectual content: all authors. Final manuscript approval: all authors.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018;390(10114):2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Jones JL, Nguyen GC, Benchimol EI, et al. The impact of inflammatory bowel disease in Canada 2018: Quality of life. J Can Assoc Gastroenterol 2019;2(Suppl 1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernklev T, Jahnsen J, Lygren I, et al. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: Psychometric assessments and a comparison with general population norms. Inflamm Bowel Dis 2005;11(10):909–18. [DOI] [PubMed] [Google Scholar]
- 4. Hoivik ML, Moum B, Solberg IC, et al. ; IBSEN Study Group . Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: Results from the IBSEN study. Inflamm Bowel Dis 2012;18(8):1540–9. [DOI] [PubMed] [Google Scholar]
- 5. Casellas F, Arenas JI, Baudet JS, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: A Spanish multicenter study. Inflamm Bowel Dis 2005;11(5):488–96. [DOI] [PubMed] [Google Scholar]
- 6. Sainsbury A, Heatley RV. Review article: Psychosocial factors in the quality of life of patients with inflammatory bowel disease. Aliment Pharmacol Ther 2005;21(5):499–508. [DOI] [PubMed] [Google Scholar]
- 7. Brooks AJ, Rowse G, Ryder A, et al. Systematic review: Psychological morbidity in young people with inflammatory bowel disease - risk factors and impacts. Aliment Pharmacol Ther 2016;44(1):3–15. [DOI] [PubMed] [Google Scholar]
- 8. Farrokhyar F, Marshall JK, Easterbrook B, et al. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: Prevalence and impact on health. Inflamm Bowel Dis 2006;12(1):38–46. [DOI] [PubMed] [Google Scholar]
- 9. Simrén M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 2002;97(2):389–96. [DOI] [PubMed] [Google Scholar]
- 10. Hoekman, DR, Zeevenhooven, J, D’Haens, GR, Benninga, MA. The prevalence of irritable bowel syndrome-type symptoms in inflammatory bowel disease patients in remission. Eur J Gastroenterol Hepatol. 2017;29(9):1086–90. [DOI] [PubMed] [Google Scholar]
- 11. Susin N, de Melo Boff R, Ludwig MW, et al. Predictors of adherence in a prevention program for patients with metabolic syndrome. J Health Psychol 2016;21(10):2156–67. [DOI] [PubMed] [Google Scholar]
- 12. Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: A systematic review. Diabet Med 2015;32(6):725–37. [DOI] [PubMed] [Google Scholar]
- 13. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet 2007;370(9590):851–8. [DOI] [PubMed] [Google Scholar]
- 14. Lim KL, Jacobs P, Ohinmaa A, et al. A new population-based measure of the economic burden of mental illness in Canada. Chronic Dis Can 2008;28(3):92–8. [PubMed] [Google Scholar]
- 15. Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, et al. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis 2014;20(1):92–102. [DOI] [PubMed] [Google Scholar]
- 16. Goodhand JR, Wahed M, Mawdsley JE, et al. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis 2012;18(12):2301–9. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 18. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1(8167):514. [DOI] [PubMed] [Google Scholar]
- 19. Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J Crohns Colitis 2012;6(10):965–90. [DOI] [PubMed] [Google Scholar]
- 21. Van Assche G, Dignass A, Panes J, et al. ; European Crohn’s and Colitis Organisation (ECCO) . The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis 2010;4(1):7–27. [DOI] [PubMed] [Google Scholar]
- 22. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91(8):1571–8. [PubMed] [Google Scholar]
- 23. Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol 2001;96(10):2921–8. [DOI] [PubMed] [Google Scholar]
- 24. Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 25. Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15(3):237–41. [PubMed] [Google Scholar]
- 26. Waugh N, Cummins E, Royle P, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: Systematic review and economic evaluation. Health Technol Assess 2013;17(55):xv–xix, 1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis 2014;20(8):1407–15. [DOI] [PubMed] [Google Scholar]
- 28. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55(6):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knowles SR, Graff LA, Wilding H, et al. Quality of life in inflammatory Bowel disease: A systematic review and meta-analyses-Part I. Inflamm Bowel Dis 2018;24(4):742–51. [DOI] [PubMed] [Google Scholar]
- 30. Knowles SR, Keefer L, Wilding H, et al. Quality of life in inflammatory Bowel disease: A systematic review and meta-analyses-Part II. Inflamm Bowel Dis 2018;24(5):966–76. [DOI] [PubMed] [Google Scholar]
- 31. Pizzi LT, Weston CM, Goldfarb NI, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis 2006;12(1):47–52. [DOI] [PubMed] [Google Scholar]
- 32. Chan W, Shim HH, Lim MS, et al. Symptoms of anxiety and depression are independently associated with inflammatory bowel disease-related disability. Dig Liver Dis 2017;49(12):1314–9. [DOI] [PubMed] [Google Scholar]
- 33. Leone D, Gilardi D, Corrò BE, et al. Psychological characteristics of inflammatory bowel disease patients: A comparison between active and nonactive patients. Inflamm Bowel Dis 2019;25(8):1399–407. [DOI] [PubMed] [Google Scholar]
- 34. Wilburn J, Twiss J, Kemp K, et al. A qualitative study of the impact of Crohn’s disease from a patient’s perspective. Frontline Gastroenterol 2017;8(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen BL, Zoëga H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther 2014;39(8):811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jelsness-Jørgensen LP, Bernklev T, Henriksen M, et al. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther 2011;33(1):106–14. [DOI] [PubMed] [Google Scholar]
- 37. Naliboff BD, Kim SE, Bolus R, et al. Gastrointestinal and psychological mediators of health-related quality of life in IBS and IBD: A structural equation modeling analysis. Am J Gastroenterol 2012;107(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gracie DJ, Irvine AJ, Sood R, et al. Effect of psychological therapy on disease activity, psychological comorbidity, and quality of life in inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2(3):189–99. [DOI] [PubMed] [Google Scholar]
- 39. Mikocka-Walus A, Prady SL, Pollok J, et al. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev 2019;4:CD012680. [DOI] [PMC free article] [PubMed] [Google Scholar]