Abstract
Background and Aims
Inflammatory bowel disease (IBD) is a lifelong disease requiring frequent assessment to guide treatment and prevent flares or progression. Multiple tools are available for clinicians to monitor disease activity; however, there are a paucity of data to inform which monitoring tools are most acceptable to patients. The review aims to describe the available evidence for patient preference, satisfaction, tolerance and/or acceptability of the available monitoring tools in adults with IBD.
Methods
Embase, Medline, Cochrane Central and Clinical Trials.gov were searched from January 1980 to April 2019 for all study types reporting on the perspectives of adults with confirmed IBD on monitoring tools, where two or more tools were compared. Outcome measures with summary and descriptive data were presented.
Results
In 10 studies evaluating 1846 participants, monitoring tools included venipuncture, stool collection, gastrointestinal ultrasound, computed tomography, magnetic resonance imaging, wireless capsule endoscopy, barium follow-through and endoscopy. Outcome domains were patient satisfaction, acceptability of monitoring tool and patient preference. Noninvasive investigations were preferable to endoscopy in nine studies. When assessed, gastrointestinal ultrasound was consistently associated with greater acceptability and satisfaction compared with endoscopy or other imaging modalities.
Conclusions
Adults with IBD preferred noninvasive investigations, in particular gastrointestinal ultrasound, as compared to endoscopy for monitoring disease activity. When assessing disease activity, patient perceptions should be considered in the selection of monitoring tools. Further research should address whether adpoting monitoring approaches considered more acceptable to patients results in greater satisfaction, adherence and ultimately more beneficial clinical outcomes.
Keywords: Acceptability, Inflammatory bowel disease, Monitoring tools, Patient preference, Tolerability
Introduction
Inflammatory bowel disease (IBD) is a lifelong disorder with increasing global prevalence (1). Patients are burdened by high rates of surgery, reduced quality of life, and social and occupational dysfunction (2,3). Improving outcomes for patients with IBD necessitates treatment directed toward amelioration of inflammation (4–6). Attainment of mucosal healing in IBD is associated with decreased rates of hospitalization, surgery, steroid use and risk of malignancy (4–8).
In the ‘treat to target’ era of IBD management, objective monitoring of disease activity is necessary to guide treatment decisions (4,7–9). International guidelines recommend frequent endoscopic assessment whilst adjusting therapy (4). However, the burden of endoscopy is substantial in terms of patient risks, need for bowel preparation, inconvenience, cost and loss of productivity.
Noninvasive modalities may be useful to objectively monitor IBD disease activity as a surrogate for endoscopy (10). The role of faecal and serum biomarkers is well-established in IBD practice (10–13). Magnetic resonance imaging (MRI) is a useful noninvasive tool for assessing small bowel involvement and distinguishing active from fibrotic strictures when contrast is used (14). Bowel cleansing is required for MRI and it may be limited by cost and accessibility. Gastrointestinal ultrasound (GIUS) is an accurate tool for assessment of IBD disease activity and extent as well as the presence of complications (15). GIUS closely correlates with endoscopic mucosal healing in both ulcerative colitis (UC) and Crohn’s disease (CD) (16), and holds advantages over other tools because it can be performed at the point of care to expedite clinical decision making. The sensitivity of GIUS ranges from 54 to 93%, however it increases to 94 to 100% when oral contrast enhancement is used, and the specificity ranging from 97 to 100% (17). GIUS has been shown to be comparable to MRI and computed tomography (CT) with regards to disease detection, disease extent, and complications such as abscess, fistulas and strictures (18,19). GIUS is accurate and comparable to MRI and video capsule endoscopy in detecting small bowel disease and accurate in diagnosing postoperative recurrence (20,21). CT imaging is generally reserved for acute presentations because of the risk of repeated radiation exposure (22).
Patients with IBD are subject to multiple diagnostic tests over the course of their chronic illness, yet there are few studies exploring patients’ perspective of monitoring tools in IBD. Acknowledgement of patients as ‘consumers’ is increasingly important as the accuracy and comparability of noninvasive imaging tools for IBD assessment have been demonstrated (15,23). The aim of this study was to systematically review the literature on patient perspectives of tools used to monitor disease activity in adult patients with IBD, regarding preference, tolerance and/or acceptability.
MATERIALS AND METHODS
The review protocol was prospectively registered on PROPERO, CRD42018111311
Information Sources and Searches
This systematic review followed the PRISMA 2009 guidelines. A systematic search of Embase, Medline, Cochrane Central and Clinical Trials.gov from January 1980 to April 2019 was performed. The detailed search strategies are outlined in Supplementary Appendix 1. All identified papers were catalogued using EndNote X8.
Study Selection and Eligibility Criteria
Studies were eligible for inclusion in the systematic review if reporting on the perspectives of adult patients with a formal diagnosis of IBD on diagnostic tools for monitoring disease activity, in which two or more monitoring tools or modalities were compared. All study types including randomized controlled trials, cohort studies and observational studies published in abstract or full text were considered. Outcomes of tolerability, patient preference, satisfaction, acceptability, or perception of clinical utility were evaluated. All studies published between January 1980 and April 2019 were eligible. Only studies with English abstracts were reviewed.
Two researchers (T.M.G. and R.N.) independently screened the titles and abstracts and selected articles for full text review. Full text articles were then reviewed for eligibility, with arbitration by a third author (R.B.) for any differences that could not be resolved by consensus. A manual search of the references of eligible studies was also performed.
Data Extraction and Quality Assessment
Two researchers (T.M.G. and R.N.) independently extracted data from eligible articles. From each article, the first author, journal, year of publication, aim of study, design, funding source, ethical approval, specific outcome measures and numbers in each group, outcomes, and all reported objective results were extracted.
Quality assessment was performed using the Newcastle-Ottawa quality assessment scale for cohort studies (NOS) (Supplementary Appendix 2) (24). The study quality was then graded using the thresholds for converting the NOS to Agency for Health Research and Quality standards as: Good quality (3 or 4 points in selection domain AND 1 or 2 points in comparability domain AND 2 or 3 points in outcome/exposure domain), Fair quality (2 points in selection domain AND 1 or 2 points in comparability domain AND 2 or 3 points in outcome/exposure domain), Poor quality (0 or 1 points in selection domain OR 0 points in comparability domain OR 0 or 1 points in outcome/exposure domain).
Data Synthesis and Analysis
Outcome measures were determined after study selection due to the heterogeneous nature of the data reported. Relevant data were included from each eligible study, subsequently categorised into domains of patient satisfaction with monitoring tools, patient acceptability of monitoring tools, and patient preference for monitoring tools. Appropriate summary data and descriptive analysis are presented as counts and percentages. Overall patient preference was reported using Pearson’s Chi2 test. All analysis was performed using Stata 14.2 (StataCorp, USA).
RESULTS
Search Results and Included Studies
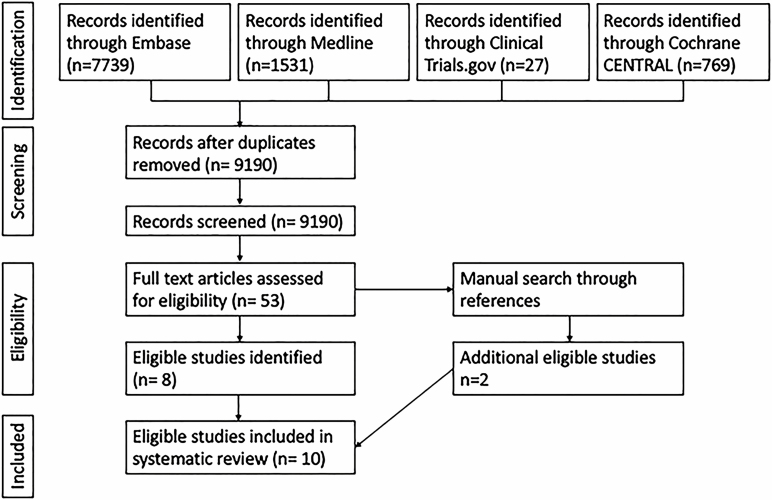
The systematic search yielded 10,073 studies including 883 duplicates, leaving 9190 studies for screening. After the initial screening 53 abstracts were selected for full text review and eight studies were determined to be eligible. A manual review of references yielded a further two eligible studies (25,26) (Figure 1).
Figure 1.
Prisma diagram.
Study Characteristics
Ten studies published between 2005 and 2019, evaluating 1846 participants, were identified (Table 1). All studies were observational in nature. The study size was heterogeneous, ranging from 18 to 916 participants (median 88.5, interquartile range 31–210; mean 185 ± standard deviation 270). One study reported on patients with suspected colorectal cancer, among whom only those with IBD (10/18) were included in the analysis (26). Four studies were available in abstract form only. One Spanish study only had an English abstract and the original study was irretrievable (27). Further information was provided by the authors of two abstracts (28,29). Five studies compared two monitoring tools, two studies compared multiple imaging modalities (28,30) and three studies compared multiple monitoring tools in CD and UC populations (29,31,32).
Table 1.
Characteristics and results of included studies
| Study (year) | Study type | Participant number (disease phenotype) | Participant characteristics | Age—years (mean ± SD) | Monitoring tools compared | Outcome measures | Assessment tool |
|---|---|---|---|---|---|---|---|
| Buisson et al. (2017) (31) | Cohort | 916 (618 CD, 298 UC) | French adults with established IBD. Male 40.4% | CD 38.2 (20) UC 42.1 (14.5) | Venipuncture Stool collection Colonoscopy Rectosigmoidoscopy MRE† Ultrasound† WCE† | Acceptability | 100 mm VAS |
| Camara Viudez et al. (2014) (27) | Cohort—abstract only | 48 CD | Spanish adults with CD. Male 50% | 43 (13.8) | Colonoscopy MRI colonography | Preference | Binary ranking |
| Chang et al. (2011) (33) | Cohort—abstract only | 27 Majority CD | Australian adults with IBD requiring colonoscopy Male 63% | 39 | Colonoscopy FC | Satisfaction Preference | Likert scale and binary preference ranking. |
| Florie et al. (2005) (25) | Cohort | 31 CD | Dutch adults with suspected CD relapse. Male 71% | 36 (12) | Colonoscopy MRE | Preference | Likert scale and binary preference ranking. |
| Friedman et al. (2018) (28) | Cohort—abstract and unpublished data only | 260 (GIUS = 73, non-GIUS = 187) | Australian adults with IBD. Male 53.8% | GIUS 38.8 (13.8) Non-GIUS 40 (12.4) | GIUS Colonoscopy CT MRI | Satisfaction Preference | 100 mm VAS and preference ranking |
| Hafeez et al. (2012) (26) | Cohort | 18 (10 IBD [2 CD, 8 UC], 8 non-IBD and excluded from analysis) | English adults enrolled in a trial comparing MRI colonoscopy with colonoscopy. Male 61% | 41 (range 17–65) | Colonoscopy MRI colonoscopy | Preference | Binary ranking |
| Lahat et al. (2016) (34) | Cohort | 56 CD | Israeli adults with small bowel CD, mild disease or in remission Male 59% | 32 (11) | WCE MRE | Preference | Binary ranking |
| Miles et al. (2019) (30) | Cohort | 159 CD | UK patients aged 16 years and over with a new diagnosis of CD or established CD with suspected flare. Male 40.9% | 38.2 (16.4) | Colonoscopy Rectosigmoidoscopy MRE GIUS Hydro-GIUS CT-Enterography BaFT | Acceptability Burden Preference | Likert scale and binary preference ranking for MRE and GIUS |
| Noiseux et al. (2019) (32) | Cohort | 210 (145 CD, 65 UC) | Canadian adults with IBD and members of Crohn’s and Colitis Canada. Male 18.6% | Unclear | General blood test Stool test Colonoscopy Colon biopsy Medical imaging | Level of comfort | Likert scale |
| Rajagopalan et al. (2018) (29) | Cohort—abstract and unpublished data only | 121 (79 CD, 42 UC) | Australian adults with IBD undergoing GIUS during routine clinical care. Male 45% | 42 (17) | Blood sampling Stool sampling Colonoscopy Sigmoidoscopy GIUS Imaging† | Acceptability | 10-point VAS |
BaFT, barium follow through; CD, Crohn’s disease; FC, faecal calprotectin; GIUS, gastrointestinal ultrasound; IBD, inflammatory bowel disease; MRE, magnetic resonance enterography; MRI, magnetic resonance imaging; WCE, wireless capsule endoscopy; SD, standard deviation; UC, ulcerative colitis.
†=CD cohort only.
Study Quality
One study was classified as good quality, four studies as fair quality and five studies as poor quality (Table 2). Common domains for low quality assessment were ascertainment of exposure and assessment of outcome as all, but one study used a self-written survey. In all studies, the participants had a risk of prior exposure to the outcome. Two studies had inadequate follow-up with 48% and 22.5% response rates (30,32).
Table 2.
Newcastle-Ottawa Scale assessment of study quality
| Study (year) | Selection | Comparability | Outcome | Total score (/9) | AHRQ Standard | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representative-ness of the exposed cohort (/1) | Selection of the non-exposed cohort (/1) | Ascertainment of exposure (/1) | Demonstration that outcome of interest was not present at the start of study (/1) | Comparability of cohorts on the basis of the design or analysis (/2) | Assessment of outcome (/1) | Was follow-up long enough for outcomes to occur (/1) | Adequacy of follow-up (/1) | |||
| Buisson et al. (2017) (31) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 6 | Fair |
| Camara Viudez et al. (2014) (27) | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | Poor |
| Chang et al. (2011) (33) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 6 | Fair |
| Florie et al. (2005) (25) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 6 | Fair |
| Friedman et al. (2018) (28) | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 4 | Poor |
| Hafeez et al. (2012) (26) | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 7 | Good |
| Lahat et al. (2016) (34) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 6 | Fair |
| Miles et al. (2019) (30) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 5 | Poor |
| Noiseux et al. (2019) (32) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 5 | Poor |
| Rajagopalan et al. (2018) (29) | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 6 | Fair |
Satisfaction with IBD Monitoring Tools
Overall, two studies evaluating 287 patients with IBD both reported higher satisfaction with noninvasive tools. Chang et al. (2011) reported overall satisfaction ratings from 27 patients undergoing FC (faecal calprotectin) testing and colonoscopy on a standardized five-point scoring scale, with a trend towards greater satisfaction for FC (4.11) over colonoscopy (3.51), although the difference was not significant (P = 0.069) (33). Friedman et al. (2018) reported satisfaction scores with IBD imaging techniques (GIUS, CT, MRI and colonoscopy) from 260 IBD patients in clinic using a 100 mm visual analogue scale (VAS). GIUS was rated the highest level of satisfaction (90.9) (28) (Table 3).
Table 3.
Patient satisfaction, acceptability and preference for monitoring tools in IBD
| Study | Monitoring tools compared | Measurement tool | Domain reported | ||
|---|---|---|---|---|---|
| Patient satisfaction | Acceptability of monitoring tool | Patient preference | |||
| Buisson et al. (2017) | Colonoscopy GIUS MRE Rectosigmoidoscopy Stool collection Venipuncture WCE | 100 mm VAS | - | CD: GIUS (9.3) and venepuncture (9.3) most acceptable, WCE (8.5), M RE (8.0), and stool collection (7.7) all similar, colonoscopy (6.7) and rectosigmoidoscopy (4.4) least acceptable (P < 0.0001†). UC: Colonoscopy (7.5), stool collection (8.1) and venipuncture (9.4) similar, rectosigmoidocopy least acceptable (6.7) (P < 0.001) | - |
| Camara Viudez et al. (2014) | Colonoscopy MRI colonography | Binary ranking | - | - | Trend for preference of Colonoscopy (48%) over MRI (33%) (P = 0.13) |
| Chang et al. (2011) | Colonoscopy FC | Likert scale and binary ranking | Trend towards greater satisfaction with FC compared with colonoscopy (P = 0.69) | - | FC preferred over colonoscopy by 92% (P < 0.001) |
| Florie et al. (2005) | Ileocolonoscopy MRE | Likert scale and binary ranking | - | MRE preferred over ileocolonoscopy by 94% (P < 0.001) | |
| Friedman et al. (2018) | Colonoscopy CT GIUS MRI | 100 mm VAS and preference ranking | GIUS rated highest level of satisfaction. Rating for GIUS in experienced patients (90.9) higher than in treatment naïve (83.7) (P = 0.033) | - | GIUS preferred over other modalities by 65% and 62% of GIUS experienced and naïve patients‡ |
| Hafeez et al. (2012) | Colonoscopy MRI Colonography | Binary ranking | - | - | Trend for preference of MRI colonography (50%) over colonoscopy (30%) (P = 0.36) |
| Lahat et al. (2016) | MRE WCE | Binary ranking | - | - | WCE preferred over MRE by 78% (P < 0.0001) |
| Miles et al. (2019) | BaFT Colonoscopy CTE Hydro-GIUS GIUS MRE Rectosigmoidoscopy | Likert scale and binary ranking for GIUS and MRE | - | GIUS acceptable in 99% compared with MRE 88% (P < 0.001) and lower scan burden (P < 0.001). Colonoscopy less acceptable than other tools (P < 0.001) Willingness to repeat a test highest for GIUS (99%) compared with MRE (91%) (P = 0.012) or colonoscopy (75%) (P = 0.017). BaFT, CTE, Hydro-GIUS not different to MRE. | GIUS preferred over MRE by 80% (P < 0.0001) |
| Noiseux et al. (2019) | General blood test Stool test Colonoscopy Colon biopsy Medical imaging | Likert Scale | Percentage reporting high level of comfort for stool test 61.4%, Medical imaging 60.8%, colon biopsy 54.1%, colonoscopy 24.5%, general blood test 9.8%. No statistical comparison available. | ||
| Rajagopalan et al. (2018) | Blood sampling Stool sampling Colonoscopy Sigmoidoscopy GIUS Imaging | 100mm VAS | Overall acceptability of GIUS (9.21) was significantly greater than acceptability of blood sampling (8.87), imaging (8.67 CD only), stool sampling (8.17), sigmoidoscopy (8.0 UC only) or colonoscopy (7.94) (P < 0.01 for all comparisons). | ||
BaFT, barium follow through; CD, Crohn’s disease; FC, faecal calprotectin; GIUS, gastrointestinal ultrasound; IBD, inflammatory bowel disease; MRE, magnetic resonance enterography; MRI magnetic resonance imaging; UC, Ulcerative colitis; WCE, wireless capsule endoscopy.
†=For each comparison. ‡=No statistical comparison available.
Acceptability of IBD Monitoring Tools
Overall, four studies evaluating 1406 patients reported on the acceptability of IBD monitoring tools (Table 3).
Buisson et al. (2017) asked 916 participants with IBD (67% CD) to compare multiple monitoring tools and rate their acceptability on a VAS with 0 the lowest and 10 the highest score. In patients with CD, GIUS and venepuncture were ranked as the most acceptable tools to monitor IBD disease activity (median VAS scores of 9.3 and 9.3, respectively) and were significantly more acceptable than all other tools (P < 0.0001). Wireless capsule endoscopy (WCE) (VAS 8.5), magnetic resonance enterography (MRE) (VAS 8.0) and stool collection (VAS 7.7) were all significantly more acceptable than colonoscopy (VAS 6.7) (P < 0.0001). Rectosigmoidoscopy was the least acceptable tool (VAS 4.4, P < 0.0001). In patients with UC, venepuncture (VAS 9.4), stool collection (VAS 8.1) and colonoscopy (VAS 7.5) were not significantly different in acceptability, however rectosigmoidoscopy was least acceptable (VAS 6.7), P < 0.001 (31). GIUS, MRE and WCE were not assessed in the UC cohort.
Miles et al. (2019) also compared multiple monitoring tools on a four-point scale (‘not at all acceptable’ to ‘very acceptable’) among 146 patients with CD. GIUS was considered very or fairly acceptable by 144/146 (99%) of patients, while only 128/145 patients (88%) considered MRE very or fairly acceptable (P < 0.001). There was no significant difference in ‘very or fairly acceptable’ rates between MRE, barium follow-through (20/24, 83%), CT enterography (29/31, 94%) or hydro-sonography (41/46, 89%). Colonoscopy rates of ‘very or fairly acceptable’ were significantly lower than other tools (60/100, 60%) (P < 0.001). Similarly, the proportion of patients willing to repeat a test was greater for GIUS (133/135, 99%) than for MRE (127/140, 91%, P = 0.012) or colonoscopy (68/91, 75%, P = 0.017). Willingness to repeat barium follow through, CT enterography and hydro-sonography were not significantly different to MRE (30). Miles 2019 also reported a significantly lower scan burden (composite measure of satisfaction, worry and discomfort derived from a seven-point Likert scale) for GIUS (1.66) compared with MRE (2.72) (P < 0.001) (30).
Noiseux et al. (2019) surveyed a Canadian online IBD community. Two hundred and ten out of nine hundred and thirty-three (22.5%) of members responded (69% CD). The six category Likert scale ranged from ‘not at all comfortable’ to ‘very comfortable’ to undergo a test. These were then grouped into three levels of high, medium and low comfort. In order of decreasing rates of comfort, the reported diagnostic and monitoring tests were: stool testing (61.4% high), medical imaging (60.8% high), colon biopsy (54.1% high), colonoscopy (24.5% high) and general blood test (9.8% high). No statistical comparison was made between comfort levels for the different diagnostic and monitoring tests.
Rajagopalan et al. (2018) surveyed 121 patients with IBD (65% CD) undergoing point of care GIUS using a 0 to 10 VAS to rank comparative acceptability of monitoring tools in IBD. Overall, acceptability was greatest for GIUS (9.21), followed by blood sampling (8.87), imaging (8.67, CD cohort only), stool sampling (8.17), colonoscopy (7.94) or sigmoidoscopy (8.0, UC cohort only) (P < 0.01 for all comparisons).
Patient Preference for IBD Monitoring Tools
Seven studies evaluating 432 patients with IBD reported on patient preference of IBD monitoring tools (Table 3). Overall, there was a consistent preference for noninvasive imaging techniques over endoscopy with 75/107 (70.1%, P < 0.0001) preferring noninvasive tools (25–28,33). GIUS was also preferred over MRI (28,30). Three studies reported preference for MRI or colonoscopy (25–27) with a total preference for MRI (50/77, 65%) over colonoscopy (27/77, 35%) (P < 0.0001).
Camara Viudez et al. (2014) reported that 48 CD patients preferred colonoscopy (23/48, 48%) to MRI colonography (16/48 33%); however, this was not statistically significant (P = 0.1344) (27). Chang et al. (2011) found that the majority of n = 27 patients (92%) would favour FC over colonoscopy if clinical benefits were identical (P < 0.001) (33). Florie et al. (2005) evaluated 31 CD patients with regard to preference for MRE or ileocolonoscopy with sedation, finding that 29/31 (94%) patients preferred MRE over ileocolonoscopy (P < 0.001). Significant preference for MRE was consistent across domains of preparation, pain, discomfort and embarrassment (25). Friedman et al. (2018) reported that GIUS was the preferred imaging modality amongst 260 patients with IBD (61% CD) when compared with CT, MRI and colonoscopy (P = 0.033), with 65% of patients preferring this modality (28). Hafeez et al. (2012) evaluated 10 patients with IBD (20% CD) demonstrating a nonsignificant preference for MRI colonography (5/10, 50%) over colonoscopy with procedural sedation (3/10 30%) (P = 0.3613) (26). Lahat et al. (2016) evaluated 56 CD patients and found a preference for capsule endoscopy over MRE in 44/56 (78%), P < 0.0001 (34). Miles et al. (2019) evaluated 159 CD patients and found a preference for GIUS over MRE for small bowel imaging in 100/125 patients (80%), P < 0.0001 (30).
Discussion
This is the first systematic review to evaluate patient perceptions of monitoring tools used in IBD. The key finding was that noninvasive techniques such as FC and GIUS were preferred by patients, as compared to other imaging tools or endoscopy. The small number of quality studies exposes an underappreciation of patients as health care ‘consumers’.
These findings are likely a reflection of the burden that invasive tests place on patients. Colonoscopy necessitates time off work for preparation, procedure and recovery, and can be associated with embarrassment and discomfort, as well as a risk of complication (31). Similarly, the acceptability of CTE and MRE is decreased by the need for intravenous contrast injection, as well as polyethylene glycol preparation, which is associated with faecal urgency and fear of intraprocedural incontinence (31,34). CTE is associated with ionising radiation exposure, which may be cumulative in a young cohort exposed to serial imaging over their disease course (22). WCE requires bowel preparation, carries risk of obstruction and need for surgery, and remuneration is variable between health jurisdictions. The rate of capsule retention and obstruction in established IBD is 8.2% and the burden of a patency capsule test must also be considered when selecting this modality (35).
GIUS was associated with a significantly higher level of satisfaction and acceptability when compared to other imaging modalities, endoscopy or laboratory tests by 63% (1158/1846) of participants in this review (28–31). GIUS is unique in that it can be performed at the point of care, meaning that results are immediately available. GIUS has been shown to have comparable accuracy to both ileocolonoscopy and other imaging modalities in assessing disease activity and extent for both UC and CD (17–21,36,37). Patients can communicate with the examining physician during GIUS, which provides an opportunity for education and generation of rapport (28,29). Two included studies found that IBD patients undergoing GIUS demonstrated significantly greater understanding and knowledge of their disease, with an associated increase in adherence to therapy over time (28,29).
Patients’ perceptions of tools for monitoring IBD are often overlooked yet are an important consideration in therapeutic decision making. Where there is similar accuracy between tests, physician should engage patients in monitoring their IBD. Perhaps the greatest barrier to less invasive/more acceptable monitoring tool use is concern regarding reduced clinical utility.
Clinician perceptions of the utility of monitoring tools in IBD are important and influence the choice of investigations ordered for patients. In centres that utilize routine GIUS and FC, clinicians perceive utility of these tests as equal to MRE and only slightly lower than colonoscopy (31). GIUS is also cheaper than MRE or colonoscopy (38). The uptake of GIUS outside of continental Europe has been slow due in part to a perception of limited clinical utility, operator dependence and limited research data (15,38). FC has been shown to be a valuable early predictor of relapse and disease flares and rises in FC may precede mucosal change (39,40).
This systematic review has some limitations. First, the heterogeneous measurement tools assessed, and different outcomes used in individual studies prevented valid pooling of the data. This partly reflects the absence of any single validated and standardised measurement tool. Buisson et al. (2017) (31) developed an externally reviewed tool which could be further validated for this purpose in the future. Second, paucity of data necessitated a broad capture search strategy. As a result, data of limited quality was evaluated in this systematic review, which may limit generalizability of the findings. Third, the findings are limited to patient perception and do not include clinical utility.
In summary, there is a paucity of data evaluating patients’ perceptions of diagnostic tests in IBD. Existing studies indicate that patients prefer noninvasive and less burdensome diagnostic modalities. Further studies are needed to compare the acceptability of monitoring tools in IBD, as well as their impact on disease-related and health-economic outcomes.
Author Contributions
Study concept and design was conducted by T.MG., V.J. and R.V.B. Data acquisition was performed by T.M.G., R.N., V.J., T.M.N. and R.V.B.. Data analysis and interpretation was performed by T.M.G., R.N. and R.V.B. Manuscript drafting was performed by T.M.G., S.P.C. and R.V.B. Manuscript editing was performed by T.M.G., R.N., V.J., T.M.N., S.P.C. and R.V.B. Study supervision was performed by R.V.B.
Conflict of Interest
T.M.G. has no conflicts of interest to declare; R.N. has no conflicts of interest to declare; T.M.N. is an employee of Robarts Clinical Trials Inc; S.P.C. has received grants from NHMRC and research support/consulting fees from Ferring, Janssen, Microbiotica, Shire; Vipul Jairath has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials Inc., Topivert, Celltrion; Speaker fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer; Robert V Bryant is employed by CALHN/TQEH; Grant/research support/speaker fees (all paid to employer for research support) from AbbVie, Ferring, Janssen, Shire, Takeda, Emerge Health.
Supplementary Material
References
- 1. Wilson J, Hair C, Knight R, et al. High incidence of inflammatory bowel disease in Australia: A prospective population-based Australian incidence study. Inflamm Bowel Dis 2010;16(9):1550–6. [DOI] [PubMed] [Google Scholar]
- 2. Lo B, Prosberg MV, Gluud LL, et al. Systematic review and meta-analysis: Assessment of factors affecting disability in inflammatory bowel disease and the reliability of the inflammatory bowel disease disability index. Aliment Pharmacol Ther 2018;47(1):6–15. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105(2):289–97. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110(9):1324–38. [DOI] [PubMed] [Google Scholar]
- 5. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014;63(1):88–95. [DOI] [PubMed] [Google Scholar]
- 6. Gracie DJ, Williams CJ, Sood R, et al. Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol 2016;111(4):541–51. [DOI] [PubMed] [Google Scholar]
- 7. Henriksen M, Jahnsen J, Lygren I, et al. ; IBSEN Study Group . C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008;57(11):1518–23. [DOI] [PubMed] [Google Scholar]
- 8. Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflamm Bowel Dis 2017;23(9):1600–4. [DOI] [PubMed] [Google Scholar]
- 9. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015;148(5):938–47.e1. [DOI] [PubMed] [Google Scholar]
- 10. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016;13(10):567–79. [DOI] [PubMed] [Google Scholar]
- 11. Yoon J, Park SJ, Hong SP, et al. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci 2014;59:829–37. [DOI] [PubMed] [Google Scholar]
- 12. Osada T, Ohkusa T, Okayasu I, et al. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J Gastroenterol Hepatol 2008;23 (Suppl 2):S262–7. [DOI] [PubMed] [Google Scholar]
- 13. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut 1996;38(6):905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rimola J, Panés J, Ordás I. Magnetic resonance enterography in Crohn’s disease: Optimal use in clinical practice and clinical trials. Scand J Gastroenterol 2015;50(1):66–73. [DOI] [PubMed] [Google Scholar]
- 15. Bryant RV, Friedman AB, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: An underused resource with potential paradigm-changing application. Gut 2018;67(5):973–85. [DOI] [PubMed] [Google Scholar]
- 16. Dong J, Wang H, Zhao J, et al. Ultrasound as a diagnostic tool in detecting active Crohn’s disease: A meta-analysis of prospective studies. Eur Radiol 2014;24(1):26–33. [DOI] [PubMed] [Google Scholar]
- 17. Bollegala N, Griller N, Bannerman H, et al. Ultrasound vs endoscopy, surgery, or pathology for the diagnosis of small bowel Crohn’s disease and its complications. Inflamm Bowel Dis 2019;25(8):1313–38. [DOI] [PubMed] [Google Scholar]
- 18. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease. A review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016;22(5):1168–83. [DOI] [PubMed] [Google Scholar]
- 19. Greenup AJ, Bressler B, Rosenfeld G. Medical imaging in small bowel Crohn’s disease-computer tomography enterography, magnetic resonance enterography, and ultrasound: “Which one is the best for what?”. Inflamm Bowel Dis 2016;22(5):1246–61. [DOI] [PubMed] [Google Scholar]
- 20. Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: Systematic review and meta-analysis. Dig Liver Dis 2017;49(8):854–63. [DOI] [PubMed] [Google Scholar]
- 21. Rispo A, Imperatore N, Testa A, et al. Diagnostic accuracy of ultrasonography in the detection of postsurgical recurrence in Crohn’s disease: A systematic review with meta-analysis. Inflamm Bowel Dis 2018;24(5):977–88. [DOI] [PubMed] [Google Scholar]
- 22. Swanson G, Behara R, Braun R, et al. Diagnostic medical radiation in inflammatory bowel disease: How to limit risk and maximize benefit. Inflamm Bowel Dis 2013;19(11):2501–8. [DOI] [PubMed] [Google Scholar]
- 23. Taylor SA, Mallett S, Bhatnagar G, et al. ; METRIC Study Investigators . Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): A multicentre trial. Lancet Gastroenterol Hepatol 2018;3(8):548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells G, Shea B, O’Connell D, et al. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 25. Florie J, Horsthuis K, Hommes DW, et al. Magnetic resonance imaging compared with ileocolonoscopy in evaluating disease severity in Crohn’s disease. Clin Gastroenterol Hepatol 2005;3(12):1221–8. [DOI] [PubMed] [Google Scholar]
- 26. Hafeez R, Wagner CV, Smith S, et al. Patient experiences of MR colonography and colonoscopy: A qualitative study. Br J Radiol 2012;85(1014):765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cámara Viudez G, Toro Flores R, Villafruela Cives M, et al. [Crohn’s disease patients’ satisfaction with colonoscopy versus entero Magnetic Resonance Imaging (MRI)]. Rev Enferm 2014;37(12):43–6. [PubMed] [Google Scholar]
- 28. Friedman A, Asthana A, Knowles S, et al. Gastroenterologist-performed point-of-care gastrointestinal ultrasound improves patient understanding of disease activity, symptomatology, management decisions, and clinical outcomes. J Gastroenterol Hepatol 2018;33:107. [Google Scholar]
- 29. Rajagopalan A, Sathananthan D, Van De Ven L, et al. Gastrointestinal ultrasound in routine inflammatory bowel disease care: Acceptability, tolerability, and impact on illness-related knowledge. J Gastroenterol Hepatol 2018;33 (Supplement 2):111–112.28960448 [Google Scholar]
- 30. Miles A, Bhatnagar G, Halligan S, et al. ; METRIC investigators . Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: Patient acceptability and perceived burden. Eur Radiol 2019;29(3):1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buisson A, Gonzalez F, Poullenot F, et al. ; ACCEPT study group . Comparative acceptability and perceived clinical utility of monitoring tools: A nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23(8):1425–33. [DOI] [PubMed] [Google Scholar]
- 32. Noiseux I, Veilleux S, Bitton A, et al. Inflammatory bowel disease patient perceptions of diagnostic and monitoring tests and procedures. BMC Gastroenterol 2019;19(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang J, Kouzios D, Janu M, et al. Cost benefit and patient satisfaction of calprotectin versus colonoscopy in inflammatory bowel disease. J Gastroenterol Hepatol 2011;4:60–1. [Google Scholar]
- 34. Lahat A, Kopylov U, Amitai MM, et al. Magnetic resonance enterography or video capsule endoscopy - what do Crohn’s disease patients prefer? Patient Prefer Adherence 2016;10:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rezapour M, Amadi C, Gerson LB. Retention associated with video capsule endoscopy: Systematic review and meta-analysis. Gastrointest Endosc 2017;85(6):1157–68.e2. [DOI] [PubMed] [Google Scholar]
- 36. Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis 2009;27(4):482–93. [DOI] [PubMed] [Google Scholar]
- 37. Panés J, Bouzas R, Chaparro M, et al. Systematic review: The use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011;34(2):125–45. [DOI] [PubMed] [Google Scholar]
- 38. Asthana AK, Friedman AB, Maconi G, et al. Failure of gastroenterologists to apply intestinal ultrasound in inflammatory bowel disease in the Asia-Pacific: A need for action. J Gastroenterol Hepatol 2015;30(3):446–52. [DOI] [PubMed] [Google Scholar]
- 39. Vos MD, Hindryckx PM, Baert FJ, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis 2013;19:2111–2117. [DOI] [PubMed] [Google Scholar]
- 40. Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm Bowel Dis 2012;18(10):1894–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.