Abstract
The rodent ventral and primate anterior hippocampus have been implicated in approach–avoidance (AA) conflict processing. It is unclear, however, whether this structure contributes to AA conflict detection and/or resolution, and if its involvement extends to conditions of AA conflict devoid of spatial/contextual information. To investigate this, neurologically healthy human participants first learned to approach or avoid single novel visual objects with the goal of maximizing earned points. Approaching led to point gain and loss for positive and negative objects, respectively, whereas avoidance had no impact on score. Pairs of these objects, each possessing nonconflicting (positive–positive/negative–negative) or conflicting (positive–negative) valences, were then presented during functional magnetic resonance imaging. Participants either made an AA decision to score points (Decision task), indicated whether the objects had identical or differing valences (Memory task), or followed a visual instruction to approach or avoid (Action task). Converging multivariate and univariate results revealed that within the medial temporal lobe, perirhinal cortex, rather than the anterior hippocampus, was predominantly associated with object-based AA conflict resolution. We suggest the anterior hippocampus may not contribute equally to all learned AA conflict scenarios and that stimulus information type may be a critical and overlooked determinant of the neural mechanisms underlying AA conflict behavior.
Keywords: decision-making, functional MRI, medial temporal lobe, memory, motivational conflict
Introduction
A large body of evidence suggests that the rodent ventral and primate anterior hippocampus (HPC) in the medial temporal lobe (MTL) plays a critical role in arbitrating approach–avoidance (AA) conflict, a scenario that arises when a stimulus is associated simultaneously with reward and punishment, and prompts opposing responses of approach and avoidance behavior (Gray and McNaughton 2000; Bannerman et al. 2014; Ito and Lee 2016). Lesions to the rodent ventral, but not dorsal, HPC have been shown to increase exploration of innately dangerous environments in ethological tests of anxiety such as the illuminated portion of the light–dark box (Bannerman et al. 2002, 2003; Kjelstrup et al. 2002; Trivedi and Coover 2004). Similarly, excitotoxic damage and pharmacological inactivation of the ventral HPC have been reported to increase rodents’ approach behavior towards stimuli associated previously with both positive and aversive outcomes (Schumacher et al. 2016, 2018). Consistent with these rodent findings, human neuropsychological and functional magnetic resonance imaging (fMRI) work has demonstrated anterior HPC involvement in AA decision-making during conditions of high motivational conflict, for instance, when participants forage for virtual tokens in the presence of a predator or make AA decisions to stimuli that could result in either reward or punishment (Bach et al. 2014, 2019; O’Neil et al. 2015a; Loh et al. 2016; see also Oehrn et al. 2015).
Choosing to approach or avoid a stimulus that may result in reward or punishment is a behaviorally complex process that necessitates the retrieval of stimulus valence, detecting the presence of motivational conflict, resolution of this conflict (here defined as arbitrating between approach and avoidance, and selecting a response), and executing a chosen behavior. Existing data provide insufficient insight into whether the ventral/anterior HPC supports one or more of these underlying processes, and thus the precise contributions of this structure to AA conflict processing are unclear. Moreover, it is currently unknown to what extent the suggested role of the ventral/anterior HPC in AA processing of motivational conflict is domain specific or general. A predominant hallmark of AA conflict processing research is the centrality of contextual information, both spatial and nonspatial in nature. Rodent studies that have employed ethological anxiety tests and recent human fMRI tasks have involved exploratory activity or spatial stimuli (Bannerman et al. 2002, 2003; Kjelstrup et al. 2002; Trivedi and Coover 2004; Bach et al. 2014; O’Neil et al. 2015a). Furthermore, learned AA conflict paradigms have employed cues (e.g., cue bars running the extent of a maze arm) (Schumacher et al. 2016, 2018) that may be considered to signal nonspatial, motivational contextual information. Given the role of the HPC in spatial cognition (Hartley et al. 2014; Zeidman and Maguire 2016) and contextual memory (Davachi 2006; Diana et al. 2007), an important question is whether the anterior HPC is involved in processing noncontextual motivational conflict, such as that associated with discrete object stimuli. Indeed, information type has been suggested to influence MTL structure involvement in other cognitive domains such as memory and higher-order perception, with perirhinal cortex (PRC) rather than the HPC being predominantly implicated in object-associated processes (Murray et al. 2007; Graham et al. 2010; Ranganath and Ritchey 2012). It is possible, therefore, that within the MTL, AA conflict processing involving objects may predominantly recruit PRC rather than theHPC.
To address these issues, we designed an object-based paradigm that placed varying demands on the cognitive processes underlying AA conflict processing. Participants first learned whether individual novel objects were associated with the gain or loss of points, and were subsequently presented, during fMRI, with these objects in either conflicting (positive–negative) or nonconflicting (positive–positive or negative–negative) pairs. Across three tasks, participants were required to either decide whether to approach or avoid each pair, indicate the presence or absence of conflicting valence information, or to approach or avoid in response to an on-screen cue. By examining the profiles of activity associated with these different tasks, with a particular interest in the HPC and surrounding MTL cortices, activity related to the resolution and detection of object-associated learned AA conflict could be disentangled.
Materials and Methods
Participants
A total of 24 right-handed individuals took part, with this sample size being based on the number of participants (n = 18) that were involved in a previous MTL-focused fMRI study conducted by our group on AA conflict processing (O’Neil et al. 2015a). All participants self-reported as being neurologically healthy, had no history of mental illness, and had normal or corrected vision. Two participants were excluded for excessive motion and two for poor performance (i.e., beyond 2.0 standard deviations [SDs] of group mean on test phase tasks). The remaining 20 participants (14 female) were aged 18–28 years (mean = 22.95, SD = 2.35). Each participant gave written informed consent prior to testing and received monetary compensation (CAD 50) for their time. Participants also had the opportunity to win a bonus $15 gift card depending on task performance (see Experimental Paradigm). This study was approved by the University of Toronto (#27455) and York University (#2016-291) research ethics boards.
Experimental Paradigm
We designed a novel object-based task inspired by a rodent learned AA conflict task (Schumacher et al. 2016) and adapted from a previous human fMRI AA decision-making paradigm that employed face and spatial scene images and has been demonstrated to elicit AA conflict successfully (O’Neil et al. 2015a). Across a “learn” and “test” phase, participants were instructed to earn points based on their task responses, with the three participants possessing the highest scores at the end of the study winning a $15 gift card. Participants were not informed of other participants’ scores and were thus motivated to accrue as many points as possible in order to maximize their chances of winning a gift card. As point-based systems have previously been used successfully to investigate reward/punishment and AA conflict (Blair et al. 2006; Aupperle and Martin 2010; Mattfeld et al. 2011; Moustafa et al. 2015; O’Neil et al. 2015a), we are confident that our combined use of a points system and a monetary reward was sufficient to motivate participant performance and induce AA conflict. The paradigm was programmed in E-Prime version 2.0 (Psychology Software Tools; https://pstnet.com).
During the learn phase, which was administered prior to entering the magnetic resonance imaging (MRI) scanner, participants were presented with images of individual novel objects (565 × 565 pixels) (courtesy of Yeung et al. 2017) on a 12.5″ LCD screen. There were 40 different objects in total and each object was assigned to be either positive or negative in valence (20 of each). Participants were instructed to maximize their points by correctly approaching or avoiding positive and negative objects, respectively. Each object was presented for 3 s, during which participants could make an approach (press “1”) or avoid (press “2”) response (Fig. 1). Approaching positive objects led to a 100-point gain, whereas approaching negative objects resulted in a 100-point loss. Avoiding an object, irrespective of its valence, resulted in no change in score. The present paradigm, therefore, assessed active rather than passive avoidance, and the use of a single key press to execute an approach or avoid response is consistent with previous work on approach and avoidance behavior (Levita et al. 2012; O’Neil et al. 2015a; Loh et al. 2016; Murty et al. 2016; Schlund et al. 2016). Of note, our decision to have participants start each trial in a “neutral” position from which they must decide whether to approach or avoid is in keeping with the rodent paradigm that inspired the current decision task (Schumacher et al. 2016). In this rodent paradigm, animals were placed in the central hub of a radial maze and could either enter into or move away from a maze arm containing both a rewarding and aversivecue.
Figure 1.
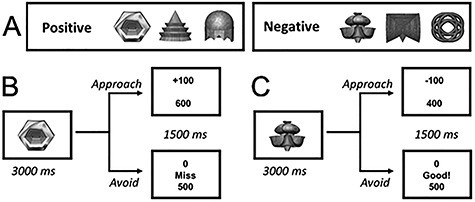
(A) Participants were presented with 40 color objects (shown in black and white here) that were predetermined to be positive or negative (three examples of each shown at top). Participants learned the valences of these objects through trial and error with the goal of maximizing points earned. (B) A schematic illustrating the sequence of events for an example trial for one positive object. Approaching positive objects resulted in point gain, and avoidance resulted in no change in points. (C) The sequence of events for an example trial for one negative object. Approaching negative objects resulted in point loss, and avoidance resulted in no change in points.
Following object presentation, a 1-s feedback screen was presented revealing both the outcome on the current trial (+100, 0, −100) and cumulative score followed by a 1-s interstimulus interval. This feedback allowed participants to learn the correct valence of each object. Of note, the presentation of single rather than pairs of images on each trial differs from our prior work (O’Neil et al. 2015a) and was implemented with the motivation of maximizing participant learning and attenuating previously observed differences in learning between negative- and positive-valenced stimuli. In total, participants completed four learn phase blocks. Each object was presented four times per block and, therefore, was presented 16 times upon completion of the learn phase. At the end of each block, the participant’s cumulative score was reset back to 0.
Following the learn phase, participants completed the test phase while undergoing fMRI scanning (Fig. 2). The objects from the learn phase were combined into three possible types of object pairs: No-Conflict Positive, composed of two positive objects, No-Conflict Negative, composed of two negative objects, and Conflict, consisting of one positive and one negative object (Fig. 2A). These three object pair types were presented across three different tasks administered in a blocked design: Decision, Memory, and Action. Each block consisted of four trials. The type of task was indicated by a 3-s instruction screen that preceded each block as well as a colored border that remained on the screen throughout the block (Decision: blue, Memory: pink, Action: green or red). An object pair was presented for 3 s on each trial, during which participants were required to make a response using a four-button box placed in their right hand, followed by a 1.5-s intertrial interval. During Decision trials, participants were instructed to maximize their points, incentivized by a possible gift card for the three highest scorers (Fig. 2B). Approaching (button press “1”) No-Conflict Positive and No-Conflict Negative pairs was guaranteed to result in point gain and point loss, respectively, whereas for Conflict pairs, an approach response resulted in 50:50 odds of point gain or point loss. In contrast, an avoid response (button press “2”) always had no impact on point score. Participants were told that it was up to them whether they wanted to take a risk by making an approach response on Conflict Decision trials, given the possibility of a point gain or loss outcome. Critically, in order to prevent new learning, participants were neither provided with outcome feedback after each trial nor their cumulative score, although they were told that points would be tracked throughout the session. The Decision task was identical to the paradigm in O’Neil et al. (2015a), which employed face–scene images and observed anterior HPC activity in association with conflict pairs. During Memory trials, participants were required to indicate whether the valences of the objects presented in each pair were identical (i.e., positive–positive or negative–negative, button press “3”) or different (i.e., negative–positive or positive–negative, button press “4”) (Fig. 2C). Finally, during Action trials, participants were asked to respond as accurately as possible according to the color of the border surrounding each object pair, regardless of the valence information conveyed by the objects themselves (Fig. 2D). If the border was green, participants were required to approach (press button “1”) and if the border was red, participants were required to avoid (press button “2”). Participants were always instructed to approach and avoid No-Conflict Positive and Negative Action trial pairs, respectively, and were instructed to approach Conflict Action trial pairs 50% of the time. The three tasks were, therefore, designed in a hierarchical manner to recruit varying cognitive processes in response to the presentation of conflicting and nonconflicting object pairs. Decision trials required participants to retrieve object valence, detect whether the stimuli in each pair were conflicting, make an AA decision, and execute an AA response, whereas the Memory trials only required participants to retrieve stimulus valence and detect the presence of conflicting valence information. In contrast, Action trials did not necessitate the retrieval of stimulus valence, the detection of conflicting valences, or AA decision-making as participants simply had to execute a response in accordance with an on-screen instruction while controlling for visual processing of object pairs. Thus, even though participants made approach/avoidance responses to Action trials, this condition did not place a demand on motivational decision-making processes as participants were told how to respond and no points were at stake. In sum, only the Decision task required the resolution of AA conflict when object pairs of opposing valences were presented, with participants required to arbitrate between approach and avoidance, and select a response.
Figure 2.
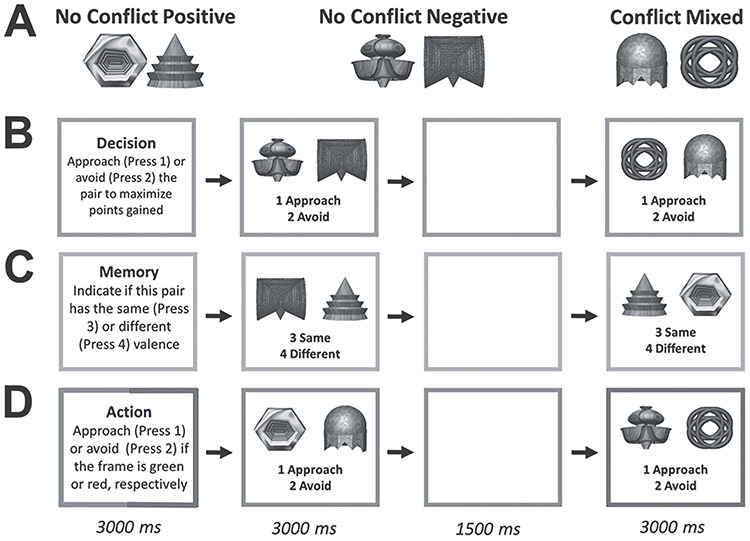
(A) Objects from the learn phase were combined into No-Conflict Positive pairs (both objects positive), No-Conflict Negative pairs (both objects negative), or Conflict pairs (one object positive and one negative) (one example pair of each shown). The three object pair types were presented across three different tasks denoted by a colored border (shown in black and white here): (B) Decision (blue), in which participants were to approach or avoid to maximize points in the absence of point feedback; (C) Memory (pink), in which participants were to indicate whether the valence of the objects was the same or different; and (D) Action (green/red), in which participants were to respond based on the border color (green = approach, red = avoid), irrespective of the information provided by the objects. A sample trial is shown for eachtask.
There were four test phase runs in total, with 10 blocks of Decision, Memory, and Action trials per run and 160 trials per task in total. Within each task, 40 of the trials were No-Conflict Positive, 40 were No-Conflict Negative, and 80 were Conflict, divided equally across all runs. Importantly, each object pair was trial unique and each individual object was repeated the same number of times during the test phase, with left and right positions of valence being counterbalanced. Both block order and the order in which the different object pair types was presented were pseudorandomized.
Participants were given a short practice run in the scanner with a separate set of object pair stimuli to familiarize themselves with the test phase prior to the start of scanning. All stimuli were projected on a screen at the rear of the MRI scanner bore, which participants saw through a mirror attached to the MRI headcoil.
Neuroimaging Data Acquisition
Neuroimaging data were collected at the York MRI Facility, York University, Toronto, Canada, using a 3 T Siemens Tim Trio MRI system. Four runs of blood oxygen level-dependent (BOLD) sensitive echo planar imaging (EPI) data were collected per subject, each composed of 344 volumes (35 slices, voxel size = 3.0 × 3.0 × 3.0, repetition time [TR] = 2000 ms, echo time [TE] = 30 ms, flip angle [FA] = 90°). A T1 anatomical scan (192 slices, voxel size = 1.0 × 1.0 × 1.0 mm, TR = 2300 ms, TE = 2.62 ms, FA = 9°) and fieldmaps (52 slices, voxel size = 1.5 × 1.5 × 3.0 mm, TR = 540 ms, TE 1 = 4.92 ms, TE 2 = 7.38 ms, FA = 60°) were also acquired for each participant, with the latter used to correct scanner magnetic field distortions. As the anterior temporal lobe, including the PRC, is susceptible to BOLD signal dropout, we examined signal-to-noise and contrast-to-noise in the raw EPI data in the PRC, which revealed satisfactory levels of signal (see Supplementary Material).
Behavioral Data Analysis
For the learn phase, performance was measured by the proportion of approach responses and response times. Use of the former is in keeping with our previous work (O’Neil et al. 2015a; Chu et al. 2020) and was adopted to capture both approach and avoidance responding in a single measure, with a high proportion of approach responses reflecting greater approach compared with avoid responses, and a low proportion of avoidance responses reflecting greater avoid compared with approach responses. Separate two-way repeated measures analyses of variance (ANOVA) were conducted for each dependent variable with presentation (1–16) and valence (positive, negative) as within-subject factors. Test phase performance was indexed by the proportion of approach responses (Decision trials only), proportion of correct responses (Memory and Action trials only), and response time (all task conditions). These data were analyzed using three separate two-way repeated measures ANOVAs for each dependent variable with run (1–4) and condition (positive, negative, conflict) as within-subject factors, and significant interaction effects were followed-up with post hoc tests. Proportion correct was chosen as the measure for Memory and Action task performance as there was a clear correct versus incorrect response for each trial in these conditions (i.e., accurately detecting conflicting or nonconflicting valences for Memory trials; responding in accordance with an approach or avoid instruction on Action trials). For Decision trials, No-Conflict trials with nonoptimal responses (e.g., avoiding a No-Conflict Positive object pair) were considered as errors in light of the task goal of maximizing points gained, and thus, response times for these trials were not analyzed. In fact, such trials were infrequent, with a mean of 1.05 and 1.45 trials per participant (out of 40 trials per condition) with nonoptimal responses to No-Conflict Positive and No-Conflict Negative object pairs, respectively.
For statistical robustness, we determined the significance for all ANOVAs and post hoc tests using a permutation-based approach, in keeping with our previous work (Chu et al. 2020) and to account for potential violations of assumptions that are necessary for ANOVAs, for instance, in the case of binomially distributed data such as proportion correct or proportion of approach responses (Jaeger 2008). Parametric statistical values (i.e., F/t values) were first calculated and then recalculated for 10 000 different samples created by label shuffling within participant. The original statistical value was then compared with the permuted (null) distribution, and the probability of obtaining a permuted value greater than the original value was considered the significance of the original statistical test (all t-tests were two tailed by using absolute t-values); 95% confidence intervals (CIs) of all effect sizes (e.g., Cohen’s d, η2) were derived from the permuted data. All P-values for post hoc tests were corrected using the Holm–Bonferroni procedure.
Neuroimaging Data Preprocessing
Prior to statistical analysis, each participant’s run of functional data was subjected to preprocessing using the FMRI Expert Analysis Tool (v. 6.00) component of the FMRIB software library (FSL; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The following preprocessing steps were conducted: (1) removal of the first three volumes due to signal instability, (2) brain extraction of anatomical and field map magnitude images using the Brain Extraction Tool, (3) motion correction using MCFLIRT, (4) application of a 90-s high-pass filter to remove low-frequency signal, (5) B0 unwarping to account for inhomogeneities in the magnetic field, (6) spatial smoothing with a Gaussian kernel of full-width half-maxima (FWHM) 6 mm, (7) coregistration of each participant’s functional data to anatomical space using boundary-based registration (Greve and Fischl 2009), (8) normalization to the Montreal Neurological Institute 152 (MNI-152) 2-mm standard template using the FSL nonlinear registration tool, (9) MELODIC independent component analysis to identify motion and artifact components, and (10) the use of a trained classifier in FSL FIX to identify and remove these noise components (Griffanti et al. 2014; Salimi-Khorshidi et al. 2014).
Neuroimaging Data Statistical Analysis
We first employed a data-driven multivariate statistical method, spatiotemporal partial least squares (PLS) (McIntosh et al. 2004; Krishnan et al. 2011), to examine the fMRI data acquired during the test phase, allowing us to make a qualitative comparison with our previous study (O’Neil et al. 2015a), which used the same approach. Next, given our theoretical focus on the MTL, we conducted a hypothesis-driven univariate statistical analysis that took advantage of the hierarchical nature of our behavioral paradigm.
PLS Analysis
Spatiotemporal PLS is a multivariate approach that, unlike a standard univariate general linear model (GLM) analysis, does not require assumptions to be made about the shape of the hemodynamic response function (HRF) (McIntosh and Lobaugh 2004). A mean-centered, data-driven approach was used to examine the covariance between each voxel at each time point (i.e., TR) for all conditions of interest. This allowed us to identify the most salient patterns of activity associated with our experimental paradigm without specifying a priori contrasts of interest. Considering the different types of object pairs, tasks, and responses, we set up the analysis with the following explanatory variables (EVs): (1) Decision No-Conflict Positive Approach, (2) Decision No-Conflict Negative Avoid, (3) Decision Conflict Approach, (4) Decision Conflict Avoid, (5) Memory No-Conflict Positive, (6) Memory No-Conflict Negative, (7) Memory Conflict, (8) Action No-Conflict Positive Approach, (9) Action No-Conflict Negative Avoid, (10) Action Conflict Approach, and (11) Action Conflict Avoid (only correct trials were included for the Memory and Action conditions, i.e., EVs 5–11). As noted above (see “Behavioral Data Analysis”), trials with nonoptimal responses to No-Conflict Decision trials were infrequent and considered as errors, and were, therefore, not included asEVs.
For each trial of the 11 included conditions, a 14-s time window was specified from stimulus onset (i.e., seven TRs of 2 s each). A covariance matrix was calculated between two matrices: one containing the task conditions (design matrix) and another specifying activation for each voxel, at each time point of the specified time window, for each condition, for all subjects (data matrix). This covariance matrix was then submitted to singular value decomposition to extract latent variables (LVs), each comprised of a linear contrast between the task conditions and a singular image, which represents the pattern of voxels that best reflect the linear contrast. The LVs were ranked according to their singular value, which was the amount of covariance that each LV accounted for. To determine the statistical significance of each LV, nonparametric permutation testing was used with 500 iterations and a significance threshold of P = 0.05. This involved randomly reassigning each subject’s data without replacement to different condition labels, calculating a new set of LVs for each new sample, and determining the probability that the permuted singular values exceeded the original singular values. Similarly, the reliability of the voxel saliences in each LV was assessed using a bootstrap procedure (n = 100), which involved sampling with replacement and recalculating PLS for each sample. A similar bootstrapping procedure was also implemented to derive 95% CIs for the LV brain scores, with CIs that cross zero indicating a condition does not contribute reliably to the associated spatiotemporal pattern, and nonoverlapping CIs indicating a significant difference between the conditions. For the current study, a bootstrap ratio (BSR) (ratio of the salience to bootstrap standard error) of 3.28, corresponding approximately to P = 0.001, with a cluster threshold of 10 voxels, was considered significant. Notably, as the voxel saliences are derived using a single mathematical computation on the whole brain (i.e., singular value decomposition of the data covariance matrix), there is no need to correct for multiple comparisons.
Univariate GLM Analysis
Preprocessed functional runs were submitted to an event-related GLM that modeled both object pair valence, task, and participant response. Thus, the paradigm-related EVs in the model were as follows: (1) Decision No-Conflict Positive Approach, (2) Decision No-Conflict Negative Avoid, (3) Decision Conflict Approach, (4) Decision Conflict Avoid, (5) Memory No-Conflict Positive, (6) Memory No-Conflict Negative, (7) Memory Conflict, (8) Action No-Conflict Positive Approach, (9) Action No-Conflict Negative Avoid, (10) Action Conflict Approach, (11) Action Conflict Avoid, and (12) all errors (including nonoptimal responses for No-Conflict Decision trials, and Memory and Action trial errors). Motion parameters were included as confound EV’s in the GLM to account for sudden movements during scanning. All EVs were convolved with a double-gamma HRF, and a 90-s high-pass temporal filter was applied. A parameter estimate image was subsequently created for each EV as well as relevant contrasts of interest (see below). For each participant, all four test phase runs were combined using a second-level fixed-effects analysis, and group-level inference was then made with a higher-level mixed-effects analysis incorporating within-session fixed effects variance and between-session/subject random effects variance (Woolrich et al. 2009).
To further explore the primary finding of interest from the PLS analysis pertaining to the involvement of PRC in object-based learned AA conflict processing (LV1, see Results: Neuroimaging Findings), the following analyses were conducted:
(i) Decision Task Versus Action Task
Given their identical designs with factors of Conflict (Conflict vs. No Conflict) and Response (Approach vs. Avoid), we first compared the Decision task to the Action task in a 2 × 2 × 2 ANOVA. This was conducted at the group level with a mixed-effects analysis by entering the per-subject images for each of the Decision and Action conditions and modeling factors of Task, Conflict, and Response. Of all the possible interaction effects, only the Task by Conflict interaction was significant (see Results: Neuroimaging Findings), and this was explored by examining the effect of Conflict for the Decision and Action tasks independently via contrasts at the individual subject and then group level (i.e., one-sample t-test). We also explored other between-condition effects within the Decision task alone in association with the factors of Conflict and Response via contrasts at the individual subject and then group level, which allowed us to examine the interaction between these two factors, as well as investigate a main effect of Response.
(ii) Decision Task Versus Memory Task
We compared the Decision task to the Memory task in a 2 × 2 design with factors of Task and Conflict (the Response factor was not applicable here as participants only indicated conflicting vs. nonconflicting valences in the Memory task). This was conducted at the group level in a mixed-effects analysis by entering and comparing the per-subject images associated with the contrasts ([Decision Conflict Approach + Decision Conflict Avoid] − [Decision No-Conflict Positive Approach + Decision No-Conflict Negative Avoid]) and ([Memory Conflict] − [Memory No-Conflict Positive + Memory No-Conflict Negative]). A significant interaction between Task and Conflict was explored further by examining the main effect of conflict for the Decision task and Memory task separately (as described in Decision Task Versus Action Task above).
For all the GLM-based analyses described above, a participant-derived group PRC and HPC region of interest (ROI) mask was used. The HPC was delineated in each participant using FIRST (Patenaude et al. 2011), a model-based segmentation and registration tool, and then visually inspected and edited by hand where necessary in accordance with anatomical guidelines (Watson et al. 1992). The PRC was segmented manually according to the Insausti protocol (Insausti et al. 1998). The HPC and PRC masks of each participant were then combined and normalized to MNI-152 space. All participants’ masks were then combined to create a group probabilistic mask, which was thresholded at 50% and binarized (total 1319 and 1309 voxels in the right and left hemispheres, respectively). Significant activity within this mask was determined using a nonparametric approach as implemented by FSL’s Randomise tool (10 000 permutations) (Winkler et al. 2014) with threshold-free cluster enhancement (Smith and Nichols 2009). A significance threshold of P < 0.05, small volume corrected (svc) was applied.
Finally, as the amygdala and striatum have been implicated heavily in decision-making and value processing, we also conducted at the request of a reviewer all of the aforementioned GLM-based analyses in bilateral masks of these regions, as defined by the Harvard–Oxford Subcortical probabilistic atlas and a threshold of 50% (total 520 voxels for the amygdala; total 2847 voxels for the striatum including the caudate, putamen, and nucleus accumbens). The findings from these analyses are provided in the Supplementary Material.
Univariate Percent Signal Change Analysis
Although the voxel dimensions of our fMRI data (3 mm isotropic) and our chosen smoothing kernel (6 mm FWHM) are consistent with existing PLS and univariate fMRI work examining MTL function (including our own studies, e.g., Lee et al. 2008; Watson and Lee 2013; O’Neil et al. 2015b), it is worth considering that individual MTL regions lie in close proximity to each other. Thus, smoothing and normalization procedures may blur the recorded signal between structures, particularly at the borders between regions. To address this, we conducted an additional analysis to further confirm our PLS and GLM-based findings, and investigated whether there was evidence for differential involvement of the PRC and anterior HPC during Decision Conflict versus Decision No-Conflict trials, when examining activity across the entirety of each of these structures. To this end, we used the participant-specific bilateral PRC and anterior HPC masks as ROIs (the latter were created by segmenting participant HPC masks along the longitudinal axis using the disappearance of the uncal apex as a landmark) and extracted percent signal change from each participant’s unsmoothed functional data in native space (left and right hemispheres separately) for the Decision No-Conflict Positive Approach, Decision No-Conflict Negative Avoid, Decision Conflict Approach, and Decision Conflict Avoid trials. These values were then entered into a mixed-effects model in Prism (v 8.4.2; www.graphpad.com) (one for each hemisphere) to investigate the effects of ROI (PRC vs. anterior HPC), Conflict (Conflict vs. No-Conflict), and Response (Approach vs. Avoid) on neural activity. As there was a significant interaction between ROI and Conflict but not between ROI and Response, nor a main effect of Response (see Results: Neuroimaging Findings), percent signal change was collapsed across approach and avoid responses. A follow-up mixed-effects model was run and the effect of Conflict was explored for each ROI separately via pairwise comparisons.
Results
Behavioral Findings
Full details and statistical values of all main omnibus and post hoc tests are reported in Tables 1–4.
Table 1.
Learn phase behavioral results
| Proportion of Approach Responses | ||||
|---|---|---|---|---|
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | P | ηG2 [95% CIs] |
| Valence (Positive vs. Negative) | 1, 19 | 1667.99 | <0.001* | 0.92 [0.0000017, 0.0082] |
| Presentation (1–16) | 1, 19 | 1.76 | 0.04* | 0.04 [0.011, 0.045] |
| Valence × Presentation | 15, 285 | 122.227 | <0.001* | 0.65 [0.01, 0.045] |
| Post hoc pairwise comparisons at each presentation | ||||
| Pair | DOF | t | P | d [95% CIs] |
| Positive versus Negative at Presentation 1 | 19 | 1.99 | 0.063 | 0.2 [−0.632, 0.632] |
| Positive versus Negative at Presentation 2–16 | 19 | All ≥ 3.58 | All ≤ 0.0032* | All ≥ 0.99 [−0.6295 to −0.6234, 0.623–0.655] |
| Response Time | ||||
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | P | ηG2 [95% CIs] |
| Valence (Positive vs. Negative) | 1, 19 | 3.27 | 0.083 | 0.0017 [0.000001345, 0.0062] |
| Presentation (1–16) | 1, 19 | 57.95 | <0.001* | 0.54 [0.0078, 0.0338] |
| Valence × Presentation | 15, 285 | 1.36 | 0.175 | 0.0042 [0.008, 0.034] |
Note: All post hoc P-values are Holm–Bonferroni corrected. *p < 0.05.
Table 4.
Action task behavioral results
| Proportion Correct | |||||
|---|---|---|---|---|---|
| Omnibus 2 × 2 ANOVA | |||||
| Effect | DOF | F | P | ηG2 [95% CIs] | |
| Condition (Positive vs. Negative vs. Conflict Approach vs. Conflict Avoid) | 3, 57 | 6.20 | 0.0002* | 0.045 [0.000589, 0.0231] | |
| Run (1–4) | 3, 57 | 2.75 | 0.043* | 0.0289 [0.00057, 0.0231] | |
| Condition × Run | 9, 171 | 0.91 | 0.52 | 0.018 [0.00703, 0.0472] | |
| Post hoc pairwise comparisons across runs | |||||
| Pair | DOF | t | P | d [95% CIs] | |
| Positive versus Conflict Approach | 19 | 3.74 | 0.0048* | 0.793 [−0.649, 0.646] | |
| All other pairs | 19 | All ≤ 2.94 | All ≥ 0.0792 | All ≤ 0.0084 [−0.635, 0.642] | |
| Post hoc pairwise comparisons across conditions | |||||
| Pair | DOF | t | P | d [95% CIs] | |
| All pairs | 19 | All ≤ 2.29 | All ≥ 0.27 | All ≤ 0.532 [−0.626, 0.6347] | |
| Response Time | |||||
| Omnibus 2 × 2 ANOVA | |||||
| Effect | DOF | F | P | ηG2 [95% CIs] | |
| Condition (Positive vs. Negative vs. Conflict Approach vs. Conflict Avoid) | 3, 57 | 3.8 | 0.013* | 0.0097 [0.000264, 0.011] | |
| Run (1–4) | 3, 57 | 3.41 | 0.020* | 0.0252 [0.00025, 0.011] | |
| Condition × Run | 9, 171 | 2.28 | 0.0184* | 0.014 [0.0032, 0.022] | |
| Post hoc one-way ANOVA at each run | |||||
| Effect | DOF | F | P | ηG2 [95% CIs] | |
| Condition at Run 1, 2, 4 | 3, 57 | All ≤ 2.67 | All ≥ 0.398 | All ≤ 0.031 [0.0005–0.00092, 0.0196–0.0381] | |
| Condition at Run 3 | 3, 57 | 5.38 | 0.0022* | 0.068 [0.00114, 0.0458] | |
| Post hoc pairwise comparisons at Run 3 | |||||
| Pair | DOF | t | P | d [95% CIs] | |
| Negative versus Conflict Avoid at Run 3 | 19 | 3.60 | 0.006* | 0.73 [−0.651, 0.653] | |
| Positive versus Conflict Avoid at Run 3 | 19 | 3.15 | 0.0036* | 0.51 [−0.655, 0.649] | |
| All other pairs | 19 | All ≤ 2.03 | All ≥ 0.398 | All ≤ 0.46 [−0.654 to −0.644, 0.644–0.648] | |
Note: All post hoc P-values are Holm–Bonferroni corrected. *p < 0.05.
Learn Phase
Participants successfully learned the valences of the object stimuli across the four blocks of the learn phase (16 presentations of each object in total) (Fig. 3 and Table 1). Valence (P < 0.001) and the number of presentations (P = 0.04) had a significant impact on the proportion of approach responses. There was also a significant interaction between these two factors (P < 0.001), which reflects the varying differences between positive and negative pairs across presentations (e.g., P = 0.063 at presentation 1 vs. P ≤ 0.0032 at all other presentations) as well as the faster rate at which participants learned the negative pairs. Indicative of successful learning, participants reliably approached positive objects (mean proportion approach = 0.99, SD = 0.24) and avoided negative objects (mean proportion approach = 0.00, SD = 0.00) by the final presentation of the learn phase. Importantly, analysis of proportion correct during the final presentation of the learn phase revealed no significant difference between positive and negative objects (t(19) = 1.37, P = 0.48, d = 0.43 [95% CI: −0.433, 0.433]), suggesting that valence did not impact learning success. Mean response time was influenced by the number of presentations (P < 0.001) but not valence (P = 0.083), and there was no interaction between these two factors (P = 0.175). The significant effect of presentation reflects the fact that participants responded faster with increased exposure to the object stimuli irrespective of valence, with no significant difference in response times between positive and negative objects during final stimulus presentation (t(19) = 0.31, P = 0.75, d = 0.026 [95% CI: −0.628, 0.64]).
Figure 3.
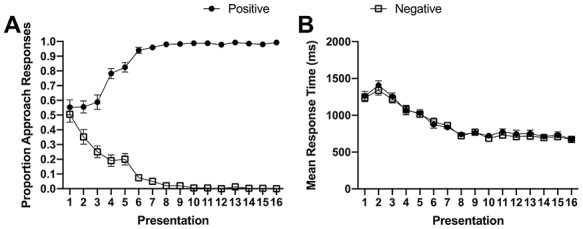
Learn phase behavioral results across all 16 object presentations for (A) mean proportion of approach responses and (B) mean response times. Error bars indicate ±SE.
Test Phase
Decision Task
The proportion of approach responses differed significantly across conditions (P < 0.001), but not run (P = 0.70), with no interaction effect between condition and run (P = 0.77). Across runs, participants approached positive pairs more than negative pairs (P < 0.001) and conflict pairs (P < 0.001), and approached conflict pairs more than negative pairs (P < 0.001). On the other hand, there were significant effects of condition (P < 0.001) and run (P = 0.032) on mean response times for decision trials. There was also an interaction effect between these two factors (P = 0.016), with a significant effect of condition at each run (all P < 0.001). In general, response times to No-Conflict Positive Approach trials were quickest, followed by No-Conflict Negative Avoid, Conflict Avoid, and finally Conflict Approach, with slight variations in significance between conditions at different runs (Fig. 4A,B and Table 2).
Figure 4.
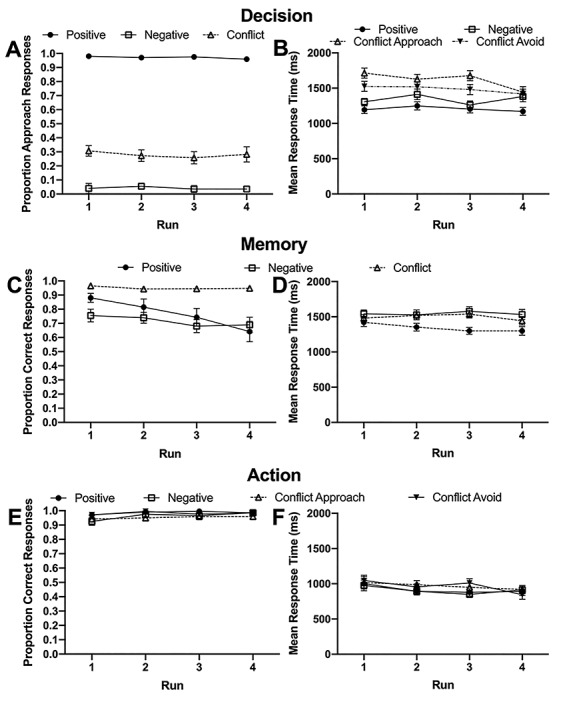
Test phase behavioral results across all four fMRI data acquisition runs for (A) mean proportion of approach responses and (B) mean response times for the Decision task, (C) mean proportion of correct responses and (D) mean correct response times for the Memory task, and finally, (E) mean proportion of correct responses and (F) mean correct response times for the Action task. Error bars indicate ±SE.
Table 2.
Decision task behavioral results
| Proportion of Approach Responses | |||||
|---|---|---|---|---|---|
| Omnibus 2 × 2 ANOVA | |||||
| Effect | DOF | F | P | ηG2 [95% CIs] | |
| Condition (Positive vs. Negative vs. Conflict) | 2, 38 | 312.34 | <0.001* | 0.896 [0.000233, 0.034] | |
| Run (1–4) | 3, 57 | 0.49 | 0.70 | 0.032 [0.0011, 0.043] | |
| Condition × Run | 6, 114 | 0.55 | 0.77 | 0.0051 [0.0057, 0.065] | |
| Post hoc pairwise comparisons at each run | |||||
| Pair | DOF | t | P | d [95% CIs] | |
| Positive versus Negative | 19 | 57.95 | <0.001* | 10.26 [−0.319, 0.308] | |
| Positive versus Conflict | 19 | 30.39 | <0.001* | 4.7 [−0.315, 0.312] | |
| Conflict versus Negative | 19 | 9.18 | <0.001* | 1.49 [−0.316, 0.322] | |
| Response Time | |||||
| Omnibus 2 × 2 ANOVA | |||||
| Effect | DOF | F | P | ηG2 [95% CIs] | |
| Condition (Positive vs. Negative vs. Conflict Approach vs. Conflict Avoid) | 3, 45 | 50.61 | <0.001* | 0.26 [0.00045, 0.0193] | |
| Run (1–4) | 3, 45 | 3.28 | 0.032* | 0.016 [0.00042, 0.0198] | |
| Condition × Run | 9, 135 | 2.39 | 0.016* | 0.175 [0.0057, 0.0389] | |
| Post hoc one-way ANOVA at each run | |||||
| Effect | DOF | F | P | ηG2 [95% CIs] | |
| Condition at Run 1, 2, 3, 4 | 3, 45 | All ≥ 15.61 | All < 0.001* | All ≥ 0.162 [0.00152–0.0021, 0.0595–0.081] | |
| Post hoc pairwise comparisons within each run | |||||
| Pair | DOF | t | P | d [95% CIs] | |
| Positive versus Negative at Run 1, 2, 4 | 15 | All ≥ 3.078 | All ≤ 0.0486* | All ≥ 0.492 [−0.633 to −0.721, 0.652–0.715] | |
| Positive versus Negative at Run 3 | 17 | 1.62 | 0.508 | 0.237 [−0.6933, 06752] | |
| Positive versus Conflict Approach at Run 1, 2, 3, 4 | 15 | All ≥ 5.203 | All ≤ 0.0015* | All ≥ 1.14 [−0.724 to −0.644, 0.538–0.711] | |
| Positive versus Conflict Avoid at Run 1, 2, 3, 4 | 19 | All ≥ 5.17 | All ≤ 0.001* | All ≥ 0.98 [−0.711 to −0.685, 0.682–0.727] | |
| Negative versus Conflict Approach at Run 1, 3 | 17 | All ≥ 6.94 | All ≤ 0.001* | All ≥ 1.375 [−0.682 to −0.653, 0.641–0.682] | |
| Negative versus Conflict Approach at Run 2, 4 | 15 | All ≤ 2.468 | All ≥ 0.183 | All ≤ 0.459 [−0.725 to −0.664, 0.663–0.711] | |
| Negative versus Conflict Avoid at Run 1, 3 | 17 | All ≥ 3.987 | All ≤ 0.0098* | All ≥ 0.795 [−0.687 to −0.641, 0.647–0.684] | |
| Negative versus Conflict Avoid at Run 2, 4 | 15 | All ≤ 1.76 | All ≥ 0.486 | All ≤ 0.289 [−0.725 to −0.658, 0.664–0.721] | |
| Conflict Approach versus Conflict Avoid at Run 1, 3 | 17 | All ≥ 3.29 | All ≤ 0.046* | All ≥ 0.59 [−0.683 to −0.64, 0.641–0.684] | |
| Conflict Approach versus Conflict Avoid at Run 2, 4 | 15 | All ≥ 1.38 | All ≥ 0.508 | All ≤ 0.169 [−0.712 to −0.652, 0.677–0.729] | |
Note: All post hoc P-values are Holm–Bonferroni corrected. *p < 0.05.
Memory Task
Performance accuracy varied significantly across conditions (P < 0.001) and runs (P = 0.014), with a significant interaction between these factors (P = 0.002). At each of the four runs, there was a significant effect of condition (all P < 0.001), and there were varying differences in accuracy between the conditions across runs. Broadly speaking, performance on Conflict trials was highest followed by that on No-Conflict Positive and No-Conflict Negative trials. There were significant differences between Conflict and No-Conflict Negative trials in all four runs (all ≤ 0.001), and between Conflict and No-Conflict Positive trials at the first, third, and fourth runs (all ≤ 0.035). Accuracy was not significantly different between No-Conflict Positive and No-Conflict Negative trials in all runs (all P ≥ 0.25) except the first run (P = 0.020). Response times were significantly impacted by condition (P < 0.001) but not run (P = 0.164). There was a significant interaction effect between these two factors (P = 0.045), and there was a significant effect of condition at all runs (all P ≤ 0.001) except for the first run (P = 0.075). Participants responded significantly faster on No-Conflict Positive trials compared with No-Conflict Negative trials at runs 2–4 (all P ≤ 0.004), and similarly on No-Conflict Positive trials compared with Conflict trials at runs 2–4 (all P ≤ 0.036). Response times were similar on No-Conflict Negative compared with Conflict trials in all runs (all P ≥ 0.33) (Fig. 4C,D and Table 3).
Table 3.
Memory task behavioral results
| Proportion Correct | ||||
|---|---|---|---|---|
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | P | ηG2 [95% CIs] |
| Condition (Positive vs. Negative vs. Conflict) | 2, 38 | 22.09 | <0.001* | 0.216 [0.00018, 0.0256] |
| Run (1–4) | 3, 57 | 3.6 | 0.014* | 0.044 [0.008, 0.032] |
| Condition × Run | 6, 114 | 3.6 | 0.002* | 0.034 [0.0045, 0.0492] |
| Post hoc one-way ANOVA at each run | ||||
| Effect | DOF | F | P | ηG2 [95% CIs] |
| Condition at Run 1, 2, 3, 4 | 2, 38 | All ≥ 11.13 | All ≤ 0.001* | All ≥ 0.18 [−0.00095 to −0.00074, 0.092–0.123] |
| Post hoc pairwise comparisons within each run | ||||
| Pair | DOF | t | P | d [95% CIs] |
| Positive versus Negative at Run 1 | 19 | 3.15 | 0.020* | 0.71 [−0.646, 0.646] |
| Positive versus Negative at Run 2, 3, 4 | 19 | All ≤ 1.78 | All ≥ 0.252 | All ≤ 0.34 [−0.633 to −0.634, 0.6332–0.6549] |
| Positive versus Conflict at Run 2 | 19 | 2.5 | 0.067 | 0.67 [−0.6395, 0.611] |
| Positive versus Conflict at Run 1, 3, 4 | 19 | All ≥ 2.82 | All ≤ 0.035* | All ≥ 0.792 [−0.6357 to −0.6315, 0.6315–0.6357] |
| Negative versus Conflict at Run 1, 2, 3, 4 | 19 | All ≥ 4.51 | All ≤ 0.001* | All ≥ 1.46 [−0.677 to −0.6315, 0.6315–0.6418] |
| Response Time | ||||
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | P | ηG2 [95% CIs] |
| Condition (Positive vs. Negative vs. Conflict) | 2, 38 | 39.13 | <0.001* | 0.1 [0.00008896, 0.01153] |
| Run (1–4) | 3, 57 | 1.73 | 0.164 | 0.071 [0.000341, 0.0145] |
| Condition × Run | 6, 114 | 2.21 | 0.045* | 0.14 [0.00201, 0.02187] |
| Post hoc one-way ANOVA at each run | ||||
| Effect | DOF | F | P | ηG2 [95% CIs] |
| Condition at Run 1 | 2, 38 | 4.83 | 0.075 | 0.041 [0.00047, 0.0623] |
| Condition at Run 2, 3, 4 | 2, 38 | All ≥ 10.08 | All ≤ 0.001* | All ≥ 0.092 [0.000397–0.000576, 0.0546–0.0732] |
| Post hoc pairwise comparisons within Runs 2, 3, 4 | ||||
| Pair | DOF | t | P | d [95% CIs] |
| Positive versus Negative at Run 2, 3, 4 | 19 | All ≥ 4.19 | All ≤ 0.004* | All ≥ 0.616 [−0.64 to −0.637, 0.645–0.649] |
| Positive versus Conflict at Run 2, 3, 4 | 19 | All ≥ 3.20 | All ≤ 0.036* | All ≥ 0.52 [−0.639 to −0.638, 0.646–0.651] |
| Negative versus Conflict at Run 2, 3, 4 | 19 | All ≤ 1.96 | All ≥ 0.33 | All ≤ 0.3 [−0.641 to −0.633, 0.639–0.652] |
Note: All post hoc P-values are Holm–Bonferroni corrected. *p < 0.05.
Action Task
The proportion of correct responses was impacted by condition (P = 0.0002) and run (P = 0.043), but there was no interaction between condition and run (P = 0.52). The effect of condition was driven by greater accuracy on No-Conflict Positive trials compared with Conflict Approach trials (P = 0.005), with no other significant differences in accuracy found (P ≥ 0.079). There were no significant pairwise comparisons driving the main effect of run (all P ≥ 0.27). Mean response times varied significantly by condition (P = 0.013) and run (P = 0.020). There was also a significant interaction between condition and run (P = 0.018), which reflected a significant effect of condition at run 3 (P = 0.002) but not runs 1, 2, or 4 (P ≥ 0.398). At run 3, participants were significantly slower during Conflict Avoid trials compared with No-Conflict Negative Avoid trials (P = 0.006) and No-Conflict Positive Approach trials (P = 0.0036). There were no other significant differences in response speed between conditions in run 3 (all P ≥ 0.4) (Fig. 4E,F and Table 4).
Neuroimaging Findings
Multivariate (PLS) Analysis
Data-driven PLS analyses revealed three significant LVs that together accounted for 72.70% of the covariance between the design and brain data matrices. Given our a priori interest in the MTL, defined here as the HPC and surrounding cortices, we focus our report and subsequent discussion on findings within the MTL. For reference, full details of significant activity beyond this region (as depicted in Figs 5–6) are listed in Supplementary Tables S1–S3.
Figure 5.
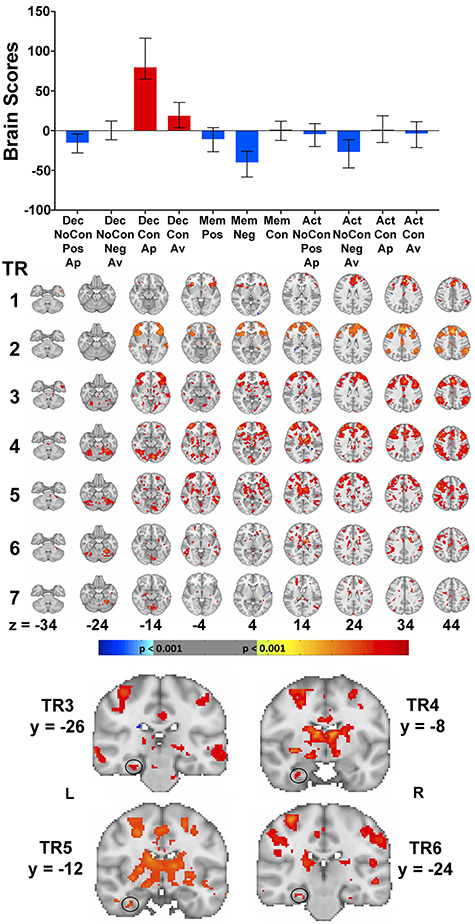
LV1 reflecting an effect of AA conflict resolution, in particular when an approach response is made. The brain scores (error bars depict 95% CIs) and associated spatiotemporal pattern of activity are shown in the top and middle panels, respectively. Warm colors indicate patterns of increased activity during task conditions with positive brain scores and decreased activity during task conditions with negative brain scores. Cool colors reflect the opposing pattern. Each row reflects each TR during the 14-s time window from the start of stimulus presentation. Increased PRC activity during Decision Conflict Approach and Avoid trials was observed at TRs 3–6 (bottom panel). For the activity maps, activation is thresholded at a bootstrap ratio of 3.28 (P = 0.001) and rendered on the MNI-152 standard template. Significant regions of activity beyond the MTL are listed in Supplementary Table S1. Key: Dec, Decision; Mem, Memory; Act, Action; NoCon, No Conflict; Con, Conflict; Ap, Approach; Av, Avoid; Pos, Positive; Neg, Negative; L, Left; R, Right.
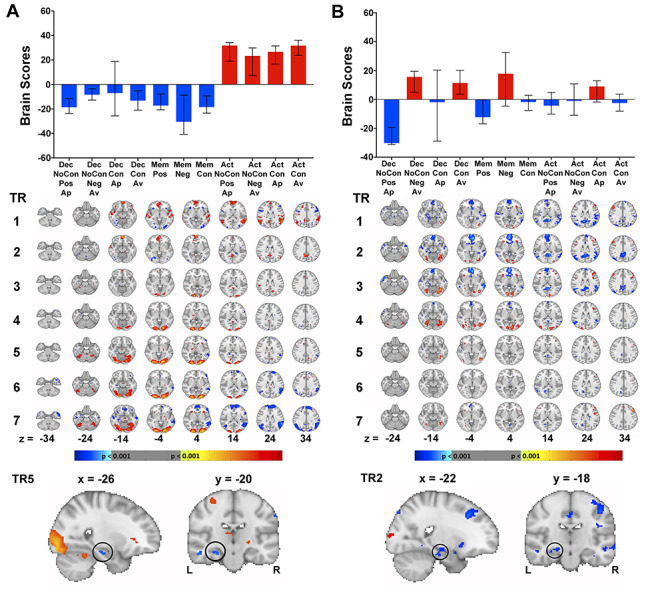
Figure 6.
(A) LV2 reflecting an effect of cognitive and perceptual demands. (B) LV3 possibly reflecting potential point loss versus point gain. For both LVs, the brain scores (error bars depict 95% CIs) and associated spatiotemporal patterns of activity are shown in the top and middle panels, respectively. Warm colors indicate patterns of increased activity during task conditions with positive brain scores and decreased activity during task conditions with negative brain scores. Cool colors reflect the opposing pattern. Each row reflects each TR during the 14-s time window from the start of stimulus presentation. Significant HPC activity is highlighted for LV2 at TR5 and for LV3 at TR2 on sagittal and coronal slices (bottom panel). For all activity maps, activation is thresholded at a bootstrap ratio of 3.28 (P = 0.001) and rendered on the MNI-152 standard template. Significant regions of activity beyond the MTL are listed in Supplementary Tables S2 and S3. Key: Dec, Decision; Mem, Memory; Act, Action; NoCon, No Conflict; Con, Conflict; Ap, Approach; Av, Avoid; Pos, Positive; Neg, Negative; L, Left; R, Right.
LV1: Effect of Resolving AA Conflict (40.77% of Covariance, P < 0.001)
The LV that accounted for the largest portion of covariance distinguished broadly between conflict trials during the decision task, in particular those associated with an approach response, and all other task conditions (Fig. 5A). Specifically, as indicated by the nonoverlapping 95% CI bars, Decision Conflict Approach trials were distinct from the Decision No-Conflict trials (i.e., Decision No-Conflict Positive Approach and Decision No-Conflict Negative Avoid), the Memory trials (i.e., Memory No-Conflict Positive, Memory No-Conflict Negative, and Memory Conflict), and the Action trials (i.e., Action No-Conflict Positive Approach, Action No-Conflict Negative Avoid, Action Conflict Approach, and Action Conflict Avoid). On the other hand, Decision Conflict Avoid trials were distinct from Decision No-Conflict Approach trials, Memory No-Conflict Positive and Negative trials, and Action No-Conflict Negative Avoid trials. Notably, there was also a significant difference between the two response types (i.e., approach or avoid) during Decision conflict trials, with approach responses being associated with a significantly greater brain score compared with avoid responses. In light of the varying cognitive demands of the different task conditions, with the Decision task uniquely requiring participants to make an AA decision, LV1 can be interpreted as reflecting the resolution of AA conflict, in particular when an approach response is made to two objects of opposing valence.
Examination of the singular value image (Fig. 5B) revealed that there was greater activity in a widespread network of regions during the Decision Conflict and Approach trials (warm colors), with reduced activity in a substantially smaller number of areas (cool colors). Notably, within the MTL, elevated HPC activity was not observed during the 14-s time period from stimulus presentation. Instead there was unique increased left PRC involvement, with a posterior located cluster during TR3 (peak voxel: −30, −26, −20; BSR = 4.27; P < 0.0001; 27 voxels) (Fig. 5C) and more anterior clusters during TR4 (peak voxel: −30, −8, −30; BSR = 4.01; P = 0.0001; 12 voxels) (Fig. 5C) and TR5 (peak voxel: −34, −12, −32; BSR = 4.41, P < 0.0001; 18 voxels), as well as TR6 (peak voxel: −30, −24, −20; BSR = 4.14, P < 0.0001; 12 voxels). There were no regions of reduced activity in the MTL during the Decision Conflict trials. Beyond the MTL, the Decision Conflict trials were associated with greater activity in the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate, posterior cingulate, caudate, putamen, thalamus, amygdala, and frontal pole, and decreased activity in the insular (Supplementary Table S1).
LV2: Effect of Cognitive and Perceptual Demands (23.81% Covariance, P < 0.001)
Inspection of the LV2 brain scores revealed a general distinction between Action trials from Decision and Memory trials. Specifically, the Action trials differed significantly from all Decision and Memory trials, with the exception of Decision Conflict Approach trials (Fig. 6A). There were no significant differences between the brain scores of the four Action trial types or between the different Decision and Memory trials. LV2 can be interpreted as distinguishing between the varying levels of cognitive and perceptual demands associated with the different tasks. More specifically, while Decision and Memory trials required a range of memory, and decision-making-related processes, Action trials necessitated only the perceptual identification of border color to make a behavioral response.
Inspection of the singular value image (Fig. 6A) revealed that while there was no significant increase in MTL activity during the Action trials at any time point, there was reduced left anterior HPC activity at TR2 (peak voxel: −34, −18, −18; BSR = −3.94; P = 0.0001; 10 voxels), TR5 (peak voxel: −26, −20, −18; BSR = −4.38; P < 0.0001; 43 voxels), TR6 (peak voxel: −28, −24, −14; BSR = −3.82; P = 0.0001; 23 voxels), and TR7 (peak voxel: −26, −18, −18; BSR = −6.95; P < 0.0001; 207 voxels; TR7), as well as decreased right PRC activity at TR1 (peak voxel: 28, −10, −30; BSR = −4.02; P = 0.0001; 28 voxels). The Decision and Memory trials were associated with the opposite pattern. Beyond the MTL, increased activity was observed during the Action trials in the anterior cingulate and medial prefrontal cortex directly after stimulus presentation, as well as the occipital pole and lateral occipital cortex in later TRs, whereas activity was decreased in the orbitofrontal cortex, insula, amygdala, and medial prefrontal cortex (Supplementary Table S2). The reverse was true of the Decision and Memory trials. Of note, the increased visual cortex activity during the Action trials at later TRs may reflect the fact that the participants were presented with a changing colored border on these trials (associated with an “approach” or “avoid” instruction) rather than a fixed color on Decision and Action trials. Moreover, this visual cortex activity may have also been driven by the relatively shorter response times on Action trials, leading to greater post-response visual exploration of the presented stimuli.
LV3: Effect of Potential Point Loss Versus Point Gain (8.12% Covariance, P = 0.046)
Finally, a third LV was identified, which primarily distinguished Decision No-Conflict Negative Avoid and Decision Conflict Avoid trials from Decision No-Conflict Positive Approach and Memory No-Conflict Positive trials (Fig. 6B). The interpretation of this LV is not clear as there is no obvious explanation that accounts fully for the complex pattern of brain scores observed across the different task conditions. We speculate that one possibility is that it may reflect, at least in part, dissociation between potential point loss and gain. Specifically, participants may have chosen to avoid pairs of negative objects (Decision No-Conflict Negative Avoid) or objects with conflicting valences (Decision Conflict Avoid) due to the possibility of losing task points, whereas deciding to approach pairs of positive objects (Decision No-Conflict Positive Approach) and detecting nonconflicting positive objects (Memory No-Conflict Positive) may have been accompanied with a greater sense of potential reward in the form of pointgain.
Examination of the singular value image (Fig. 6B) revealed that there were no regions of greater MTL activity during the Decision No-Conflict Negative Avoid and Decision Conflict Avoid trials, whereas reduced activity was found in the bilateral HPC (TR1—peak voxel: 32, −16, −22; BSR: −5.01, P < 0.0001, 62 voxels; TR2—right peak voxel: 22, −16, −26; BSR: −4.60, P < 0.0001, 39 voxels; left peak voxel: −22, −18, −12; BSR: −4.30, P < 0.0001, 39 voxels; TR3—peak voxel: −30, −16, −16; BSR: −3.59, P = 0.0003, 11 voxels; TR5—peak voxel: −32, −18, −16; BSR: −3.91, P = 0.0001, 13 voxels) and left parahippocampal cortex (TR2—peak voxel: −18, −36, −12; BSR: −5.24, P < 0.0001, 79 voxels; TR3—peak voxel: −18, −38, −10; BSR: −4.59, P < 0.0001, 31 voxels). Beyond the MTL, there was greater activity in the medial prefrontal cortex, insula, orbitofrontal cortex, and frontal pole, and reduced involvement of the medial prefrontal cortex, posterior cingulate, anterior cingulate, frontal pole, thalamus, insula, and orbitofrontal cortex (Supplementary Table S3). Converse patterns of activity were seen within and beyond the MTL for the Decision No-Conflict Positive Approach and Memory No-Conflict Positive trials (Supplementary Table S3).
Univariate GLM-Based Analysis
To confirm our primary PLS finding of interest that the PRC, rather than anterior HPC, is implicated in the resolution of object-based learned AA conflict (LV1), we conducted a series of GLM-based analyses within a participant-derived group MTL mask incorporating the HPC and PRC to compare the Decision task with the Action task and the Decision task with the Memory task (findings pertaining to identical analyses using amygdala and striatum masks are reported in the Supplementary Material).
Decision Task Versus Action Task
A 2 × 2 × 2 ANOVA with Task (Decision vs. Action), Conflict (Conflict vs. No-Conflict), and Response (Approach vs. Avoid) revealed a nonsignificant three-way interaction, a nonsignificant two-way interaction between Task × Response, and a nonsignificant two-way interaction between Conflict × Response, even when a liberal threshold was applied (P < 0.1 svc). There was, however, significant activity in the left PRC for a two-way interaction between Task × Conflict (peak voxel: −28, −10, −32, P = 0.046, 3 voxels) (Fig. 7B). Follow-up analyses revealed that this significant Task × Conflict interaction was driven by significant activation in the left PRC for Conflict in the Decision task (Decision Conflict > Decision No-Conflict; peak voxel: −28, −10, −32, P = 0.010, 59 voxels) (Fig. 7B). There was a nonsignificant effect for No-Conflict in the Decision Task (Decision No-Conflict > Decision Conflict) as well as nonsignificant effects in the Action Task for both Conflict (Action Conflict > Action No-Conflict) and No-Conflict (Action No-Conflict > Action Conflict), even when a liberal threshold was applied (P < 0.1 svc). Exploring the Decision task data further using a 2 × 2 factorial design revealed a nonsignificant interaction between Conflict and Response, even at a liberal threshold (P < 0.1 svc). Thus, even though the main effect of Conflict was associated with numerically greater left PRC activity in association with approach as opposed to avoid responses (Fig. 7C), the difference between Conflict and No-Conflict Approach trials was not significantly different than that between Conflict and No-Conflict Avoid trials. Interestingly, there was also a significant main effect of Response for the Decision task, in which Approach compared with Avoid responses were associated with greater left PRC activity (peak voxel: −36, −18, −24, P = 0.036, 4 voxels) as well as bilateral HPC activity (left—peak voxels: −20, −30, −6, P = 0.028, 39 voxels; −20, −36, 2, P = 0.035, 17 voxels; −26, −18, −20, P = 0.048, 3 voxels; right—peak voxel: 22, −28, −12, P = 0.018, 12 voxels). A main effect of Approach was not seen for the Action task, although given the absence of an overall Task × Response interaction, a strong claim cannot be made that the observed effect of Approach is unique to the Decisiontask.
Figure 7.
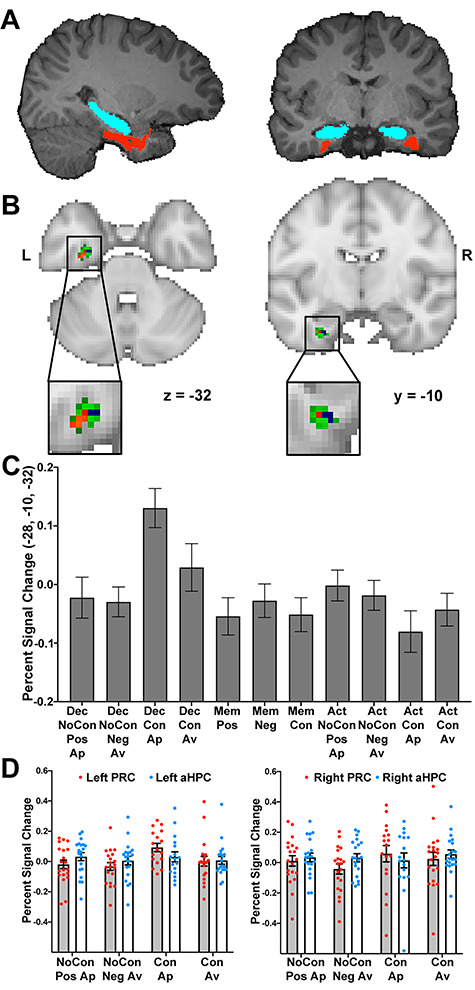
(A) Example delineation of PRC (red) and the HPC (blue) in native space for one participant. (B) Significant left PRC activity in association with a two-way interaction between Task and Conflict when comparing the Decision and Action tasks (blue voxels), a two-way interaction between Task and Conflict when comparing the Decision and Memory tasks (red voxels), and a significant main effect of Conflict for the Decision task (green voxels). Activity is thresholded at P < 0.05 svc and rendered onto the MNI-152 standard template. (C) Percent signal change at the peak voxel for the main effect of Conflict for the Decision task. (D) Percent signal change associated with the Decision task trials across the entire PRC and anterior HPC (aHPC) in each hemisphere. Individual participant data points are shown in addition to the group mean. Error bars in all graphs indicate ±SE. Key: Dec, Decision; Mem, Memory; Act, Action; NoCon, No Conflict; Con, Conflict; Ap, Approach; Av, Avoid; Pos, Positive; Neg, Negative; L, Left; R, Right.
Decision Task Versus Memory Task
A 2 × 2 ANOVA with factors of Task (Decision vs. Memory) and Conflict (Conflict vs. No-Conflict) revealed a significant interaction effect ([Decision Conflict − Decision No-Conflict] > [Memory Conflict − Memory No-Conflict]) in the left PRC (peak voxel: −32, −14, −30, P = 0.033, 14 voxels), which overlapped with the left PRC activity observed for the Decision versus Action analyses (Fig. 7B). Follow-up analyses suggest that the left PRC effect is specific to Decision Conflict trials (see above Decision vs. Action results), with nonsignificant effects of both Conflict (Memory Conflict > Memory No-Conflict) and No-Conflict (Memory No-Conflict > Memory Conflict) in the Memory task, even when a liberal threshold was applied (P < 0.1svc).
Univariate Percent Signal Change Analysis
To examine neural activity across the entirety of the PRC and anterior HPC during Decision Task trials, percent signal change values were extracted from functional data in native space using participant-specific PRC and anterior HPC masks. We fit two mixed models to the data for the left and right hemispheres separately (Fig. 7D), with fixed effects of ROI, Conflict and Response, and a random effect of subject. A mixed model fit to the left hemisphere data revealed a sole significant interaction between ROI and Conflict (F(1,19) = 6.99, P = 0.016, η2 = 0.27), with none of the main effects (all F < 3.67, P > 0.07, η2 < 0.17) or other interactions (all F < 3.02, P > 0.10, η2 < 0.19) reaching significance. To examine the significant interaction effect, we implemented a mixed model with fixed effects of ROI and Conflict and a random effect of Subject. As expected, this revealed nonsignificant main effects of ROI (F(1,19) = 3.26, P = 0.087, η2 = 0.15) and Conflict (F(1,19) = 0.47, P = 0.31, η2 = 0.024), but a significant interaction effect (F(1,19) = 14.24, P = 0.0013, η2 = 0.43). Planned pairwise comparisons within the left PRC revealed significantly greater activity for Conflict compared with No-Conflict trials (t(38) = 2.53, P = 0.016, d = 0.59), with a nonsignificant difference between Conflict and No-Conflict trials in the left anterior HPC (t(38) = 1.24, P = 0.22, d = 0.39). Similarly, a mixed model fit to the right hemisphere data also revealed a significant effect of ROI × Conflict (F(1,19) = 5.47, P = 0.03, η2 = 0.22), with no main effects (all F < 1.078, P > 0.31, η2 < 0.055) nor other interactions (all F(1,19) < 1.99, P > 0.17, η2 < 0.095) reaching significance. A mixed model with fixed effects of ROI and Conflict and a random effect of subject revealed nonsignificant main effects of ROI (F(1,19) = 1.305, P = 0.27, η2 = 0.064) and Conflict (F(1,19) = 0.79, P = 0.39, η2 = 0.04), but a significant interaction effect (F(1,19) = 5.84, P = 0.026, η2 = 0.24). Planned pairwise comparisons within Conflict trials revealed, however, nonsignificant differences between Conflict and No-Conflict trials in both the right PRC (t(38) = 2.58, P = 0.12, d = 0.35) and right anterior HPC (t(38) = 0.11, P = 0.91, d = 0.034). In sum, the results from this percent signal change analysis provide confirmation of the findings from the data-driven PLS and univariate GLM-based analyses, and undermine the possibility that the observation of predominant PRC rather than anterior HPC involvement can be attributed to the use of spatial smoothing and the close proximity of these MTL structures.
Discussion
Using a novel paradigm designed to disentangle the different processes underpinning AA conflict processing, we have demonstrated that within the MTL, there is predominant PRC, rather than anterior HPC, involvement in the resolution of learned, object-based AA conflict. Our findings provide early evidence that stimulus type may determine, at least in part, the extent to which the anterior HPC and other MTL structures contribute to learned AA conflict processing.
By modifying a previous paradigm that utilized face–scene image pairs (O’Neil et al. 2015a), we were able to elicit and examine the neural correlates associated with AA conflict in response to discrete objects devoid of pre-existing contextual information. Participants first successfully learned the valences of individual novel objects (Fig. 3). A hierarchical task design was then employed during scanning, with only the Decision task requiring participants to resolve motivational conflict. Indicative of the manifestation of AA conflict, participants’ performance profile on Decision conflict trials differed significantly from that on no-conflict trials. Specifically, while participants largely approached and avoided no-conflict positive and negative object pairs, respectively (>96% trials), they responded in a mixed fashion to conflict trials with a greater proportion of avoid responses (70%) reflecting a risk-adverse attitude towards stimuli that could result in point reward or loss if approached. Moreover, responses to conflict, particularly approach, were associated with greater response times compared with no conflict trials, suggesting increased decision time when AA conflict was high. Thus, the present Decision data replicate O’Neil et al. (2015a), in which AA decisions were made to face–scene pairs of conflicting or nonconflicting valences. Unexpectedly, participants were more accurate at indicating the presence of conflicting, compared with nonconflicting, valences during the Memory task, and participants’ accuracy on no-conflict positive Memory trials decreased across scanning runs. The reason behind this is uncertain as participants clearly possessed excellent valence knowledge, as demonstrated by asymptotic learning phase performance, and optimal responding to nonconflicting Decision object pairs. One possibility is that participants struggled with the response button mapping that was unique to the Memory task (“same/different” vs. “approach/avoid” in other tasks), with No-Conflict Positive and Negative object pairs sharing the same optimal key response in the Memory task (“same”) but not the other tasks (“approach” vs. “avoid”). Notably, however, accuracy for detecting conflicting valences was close to ceiling. Regardless, given the profiles of learning phase and Decision task performance, plus our adopted neuroimaging analysis approach, the performance profile on Memory task no-conflict pairs does not impact the interpretation of the fMRIdata.
Our key neural finding is that within the MTL, the PRC, rather than anterior HPC, was predominantly involved in the resolution of learned object-associated AA conflict, in the context of a wider network of regions previously associated with AA conflict and value-based decision-making (Rushworth et al. 2011; Kirlic et al. 2017), including the amygdala, anterior cingulate, orbitofrontal cortex, lateral prefrontal cortex, putamen, and accumbens. LV1, which accounted for the greatest proportion of variance in the data-driven PLS analysis, revealed that Decision Conflict Approach and Decision Conflict Avoid were the only two conditions associated with significant left PRC activity, with the former coupled with significantly greater PRC involvement compared with the latter. Crucially, targeted univariate analyses yielded convergent findings. A 2 × 2 × 2 factorial analysis to compare the Decision task with the Action task revealed a significant interaction between Task and Conflict in the left PRC, which was driven by an effect of Conflict in the Decision, but not Action, task. Moreover, a 2 × 2 analysis to compare the Decision and Memory tasks also revealed a significant interaction between Task and Conflict in the left PRC, with no significant effect of Conflict in association with the Memory task. Lastly, examination of whole structure PRC and anterior HPC activity during Decision task trials using participant-specific anatomical masks yielded a complementary pattern of activity, particularly in the left hemisphere.
The observation of predominant PRC rather than HPC activity during high AA conflict is at odds with the existing literature’s focus on the rodent ventral and primate anterior HPC (Ito and Lee 2016). Given our previous work, in which high AA conflict-related anterior HPC activity was observed during a paradigm that was identical to the present Decision task with the exception of the involvement of face–scene image pairs rather than novel objects (O’Neil et al. 2015a), the most parsimonious explanation for this PRC activation is the present use of object stimuli. Studies that have implicated the ventral/anterior HPC in high AA conflict processing, including studies of anxiety, have typically employed paradigms that place a demand on spatial cognition (Ito and Lee 2016). These not only include tasks that incorporate an explicit spatial component such as a requirement to process and/or navigate a spatial environment (Bach et al. 2014) or perceive scene images (O’Neil et al. 2015a), but potentially also paradigms in which engagement of spatial contextual processing may be more subtle or implicit, for instance, a decision-making task in which participants must decide whether to forage for energy in a series of abstract “forests” associated with varying threats of predation (Korn and Bach 2019). In addition, although some studies have minimized the importance of spatial information by using nonspatial cues to signal learned high AA conflict, these cues, which include texture bars running the length of the arms of a radial maze (Schumacher et al. 2016, 2018) and different background colors on a computer monitor (Loh et al. 2016), may signal motivational contextual information that participants utilize to guide their behavior. Given the role of the HPC in spatial cognition and contextual memory, it is conceivable, therefore, that ventral/anterior HPC involvement in AA conflict processing is dependent on the engagement of contextual information (spatial or nonspatial) and that HPC recruitment is diminished when AA conflict arises in association with discrete objects with which participants must interact directly. Indeed, in a recent study in which marmosets learned to touch or avoid touching green circles on a touchscreen to receive reward or avoid punishment, it was found that inactivation of anterior HPC did not alter AA conflict behavior (Wallis et al. 2019). Notably, a number of fMRI studies have employed AA conflict tasks with a clear spatial component and yet failed to observe HPC involvement (Aupperle et al. 2015; Gonen et al. 2016). Although the precise reasons for this are difficult to ascertain, it is worth noting that these studies did not conduct a region-specific examination of HPC activity, which is often necessary to observe task-related changes in HPC BOLD signal with univariate statistical approaches, given the higher susceptibility to magnetic resonance signal loss (Greicius et al. 2003) and relatively small magnitude of BOLD signal fluctuations in this region.
That the PRC is predominantly associated with learned object-associated AA conflict processing aligns with theoretical views that emphasize the importance of information type in governing the contribution of MTL structures to cognition (Bussey and Saksida 2007; Graham et al. 2010; Ranganath and Ritchey 2012; Zeidman and Maguire 2016). For instance, representational accounts of MTL function posit that the HPC and PRC subserve complex scene/context and object representations, respectively, which can be recruited across a wide range of cognitive tasks within and beyond the domain of memory (Bussey and Saksida 2007; Cowell et al. 2010; Graham et al. 2010; Lee et al. 2012; Kent et al. 2016). According to this viewpoint, PRC functions as the apex of the ventral visual stream to represent conjunctions of object features (whole objects), whereas the HPC sits at the top of the hierarchy and processes conjunctions of features (e.g., multiple objects) that compose a place/context. In support, a body of cross-species research has demonstrated differential involvement of the HPC and PRC in both memory and complex visual perception tasks (Buckley et al. 2001; Lee et al. 2005, 2008; Barense et al. 2007; Bartko et al. 2007; Taylor et al. 2007; Watson et al. 2012) depending on the involvement of scene or object stimuli, respectively. Beyond a representational viewpoint, other theoretical accounts and studies have emphasized a role for the PRC in item memory (Davachi et al. 2003; Davachi 2006; Staresina and Davachi 2008), and, moreover, it has been shown that active nonmotivational decision-making concerning object stimuli can increase PRC involvement in association with greater memory encoding (Murty et al. 2019). The present data are broadly consistent with this literature and the role of the PRC in object-related processing and constitute early evidence that stimulus type may also impact the involvement of MTL structures in AA conflict.
Notably, AA conflict resolution, which was unique to the Decision task, is likely to involve various contributing processes including but not limited to the consideration of outcome uncertainty and potential risk, response deliberation, which may include the perception of response conflict (i.e., between approach and avoidance), and decision. Indeed, demands on these processes may vary according to the response chosen during high AA conflict, potentially accounting for the observed difference in PRC activity between Decision Conflict Approach and Avoid trials, with the former being associated with greater PRC involvement across our different analysis approaches. For instance, outcome uncertainty and response type are often intricately linked to AA conflict in real world and experimental scenarios as approaching a high AA conflict stimulus is typically associated with greater outcome uncertainty (possibility of reward or punishment) compared with an avoid response (leading to a null outcome) or conditions lacking AA conflict (e.g., certain reward when approaching a positive valenced stimulus or punishment in the case of a negative stimulus). In fact, in the present study, it is possible that participants chose to avoid high AA conflict stimuli due to an intolerance of the uncertainty associated with an approach response and unwillingness to take a risk, rather than the motivation to avoid potential punishment. Indeed, many of the neural regions implicated in high AA conflict processing (particularly those beyond the MTL such as the anterior cingulate and orbitofrontal cortex) have also been associated with risky decision-making (Cohen et al. 2005; Krain et al. 2006). Although the current paradigm was not designed to disentangle these different processes, we have recently examined the relationship between outcome uncertainty and MTL activity during object-associated AA conflict. The current finding of predominant PRC, rather than HPC, activity during object-associated AA conflict was replicated and, importantly, found not to be driven by risk taking that is driven by outcome uncertainty. Ongoing computational modeling work will aim to reveal whether this PRC involvement during object-associated AA conflict resolution relates specifically to decision-making or nondecision-making processes (Chu, Hutcherson, Ito, Lee, unpublished results).
The current findings have potential implications for our understanding of the neural substrates underlying AA conflict behavior and the notion that within the MTL, only the anterior HPC is critically involved (McNaughton and Gray 2000; Bannerman et al. 2014). For instance, one long-standing view is that the HPC compares incoming goal information and plays a key role in adjusting behavior in the face of motivational conflict (McNaughton and Gray 2000). Moreover, it has been suggested recently that distinct HPC subfields, including the CA3, CA1, and dentate gyrus, and their associated circuits may play differential roles in mediating approach and avoidance behavior (Schumacher et al. 2018; Yeates et al. 2020). Our observance of predominant PRC rather than anterior HPC activity does not contradict these ideas, but rather suggests that our understanding of MTL involvement in AA conflict behavior may require expanding, with the need to consider the potential involvement of other MTL structures. Importantly, our failure to observe significant HPC involvement during object-based AA conflict processing cannot dismiss entirely the involvement of this structure, something that only further research can do. Rather, we suggest the HPC may not be involved equally across all AA scenarios and that when AA conflict arises in the absence of contextual information, the involvement of other MTL structures may rise to the fore, in this case the PRC during object-based AA conflict.
Interestingly, a main effect of Response was also observed in association with the Decision task, with approach trials being associated with significantly greater left PRC as well as bilateral HPC activity compared with avoid trials, irrespective of conflict. Given the lack of interaction between Task and Response when comparing the Decision and Action tasks, suitable caution must be exercised when interpreting this finding. It is worth noting, however, that our earlier study also found increased HPC activity during No-Conflict Positive and Conflict Approach decision trials compared with trials in which participants avoided (O’Neil et al. 2015a), albeit at a more anterior location than that observed here. We previously interpreted this HPC involvement as reflecting the facilitation of monitoring/updating mechanisms in response to the potential outcomes associated with approach but not avoid responses (with the latter always leading to zero point gain or loss), and it is not inconceivable that a similar interpretation applies here, with the PRC and HPC activity reflecting such mechanisms at the object level and higher-conjunction level, respectively.
Finally, unlike LV1, HPC activity was observed in LV2 and LV3 of the PLS analysis during Decision and Memory trials as well as trials associated with potential reward, respectively. Regarding LV2, HPC engagement may reflect higher-level conjunctive/relational processes that were required in the Decision and Memory tasks (e.g., pertaining to the features and valences of two objects in the context of an overarching goal), but not Action task. As noted earlier, the HPC is thought to process the relationships between items (Lee et al. 2012; Olsen et al. 2012) contributing to a richer hierarchical representation. Regarding LV3, it is unknown why HPC (as well as parahippocampal cortex) was differentially involved in potential point loss versus gain, although it is important to note that this LV was difficult to interpret with confidence, given the complex pattern of associated brain scores.
In summary, we demonstrate that PRC is involved in resolving motivational conflict associated with discrete objects. This finding suggests that stimulus type may be an important determinant of MTL structure involvement in AA conflict processing and has potential implications for theoretical perspectives that conceptualize the anterior HPC as serving a domain-general role in AA conflict processing (Gray and McNaughton 2000; Ito and Lee 2016).
Notes
The authors thank all participants for their time, Jonathan Tay for help with task programming and data collection, Joy Williams for MRI scanning assistance at the York MRI Centre, and Dr Zhemeng Wu and Prof Florian Jaeger for advice on behavioral statistical analyses. Conflict of Interest: None declared.
Funding
Canadian Institutes of Health Research (CIHR) CGS-M and CGS-D (93007347) Awards to S.C., a Natural Sciences and Engineering Research Council of Canada (NSERC) PGS-D (504464-2017) to S.T., a NSERC Postdoctoral Fellowship (471806-2015) to E.B.O., and a CIHR Project Grant (156070) to R.I. and A.C.H.L.
Supplementary Material
Contributor Information
Sonja Chu, Department of Psychological Clinical Science, University of Toronto, Toronto, Ontario, Canada.
Matthew Margerison, Department of Psychology (Scarborough), University of Toronto, Toronto, Ontario, Canada.
Sathesan Thavabalasingam, Department of Psychology (Scarborough), University of Toronto, Toronto, Ontario, Canada.
Edward B O’Neil, Department of Psychology (Scarborough), University of Toronto, Toronto, Ontario, Canada.
Yuan-Fang Zhao, Department of Psychology (Scarborough), University of Toronto, Toronto, Ontario, Canada.
Rutsuko Ito, Department of Psychological Clinical Science, University of Toronto, Toronto, Ontario, Canada; Department of Psychology (Scarborough), University of Toronto, Toronto, Ontario, Canada; Department of Cell and Systems Biology, University of Toronto, Toronto, Ontario, Canada.
Andy C H Lee, Department of Psychological Clinical Science, University of Toronto, Toronto, Ontario, Canada; Department of Psychology (Scarborough), University of Toronto, Toronto, Ontario, Canada; Rotman Research Institute, Baycrest Centre, Toronto, Ontario, Canada.
References
- Aupperle RL, Martin PP. 2010. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci. 12:517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. 2015. Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp. 36:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Guitart-Masip M, Packard PA, Miró J, Falip M, Fuentemilla L, Dolan RJ. 2014. Human hippocampus arbitrates approach-avoidance conflict. Curr Biol. 24:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Hoffmann M, Finke C, Hurlemann R, Ploner CJ. 2019. Disentangling hippocampal and amygdala contribution to human anxiety-like behavior. J Neurosci. 39:8517–8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D, Deacon R, Offen S, Friswell J, Grubb M, Rawlins J. 2002. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 116:884. [DOI] [PubMed] [Google Scholar]
- Bannerman D, Grubb M, Deacon R, Yee B, Feldon J, Rawlins J. 2003. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 139:197–213. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JNP, Monyer H, Seeburg PH. 2014. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 15:181–192. [DOI] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. 2007. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 45:2963–2974. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. 2007. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem. 14:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Marsh AA, Morton J, Vythilingam M, Jones M, Mondillo K, Pine DC, Drevets WC, Blair JR. 2006. Choosing the lesser of two evils, the better of two goods: specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate in object choice. J Neurosci. 26:11379–11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Booth MC, Rolls ET, Gaffan D. 2001. Selective perceptual impairments after perirhinal cortex ablation. J Neurosci. 21:9824–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey T, Saksida L. 2007. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus. 17:898–908. [DOI] [PubMed] [Google Scholar]
- Chu S, Thavabalasingam S, Hamel L, Aashat S, Tay J, Ito R, Lee ACH. 2020. Exploring the interaction between approach-avoidance conflict and memory processing. Memory. 28:141–156. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. 2005. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cogn Brain Res. 23:61–70. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. 2010. Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus. 20:1245–1262. [DOI] [PubMed] [Google Scholar]
- Davachi L. 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 16:693–700. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. 2003. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 100:2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. 2007. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 11:379–386. [DOI] [PubMed] [Google Scholar]
- Gonen T, Soreq E, Eldar E, Ben-Simon E, Raz G, Hendler T. 2016. Human mesostriatal response tracks motivational tendencies under naturalistic goal conflict. Soc Cogn Affect Neurosci. 11:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee ACH. 2010. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 48:831–853. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. 2000. The neuropsychology of anxiety. 2. New York: Oxford University Press. [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. 2003. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 13:164–174. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE. 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 95:232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. 2014. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc B Biol Sci. 369:20120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partenen K, Vainio P, Laakso MP, Pitkanen A. 1998. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Ito R, Lee ACH. 2016. The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav Brain Res. 313:345–357. [DOI] [PubMed] [Google Scholar]
- Jaeger TF. 2008. Categorical data analysis: away from ANOVAs (transformation or not) and towards logit mixed models. J Mem Lang. 59:434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent B, Hvoslef-Eide M, Saksida LM, Bussey TJ. 2016. The representational–hierarchical view of pattern separation: not just hippocampus, not just space, not just memory? Neurobiol Learn Mem. 129:99–106. [DOI] [PubMed] [Google Scholar]
- Kirlic N, Young J, Aupperle RL. 2017. Animal to human translational paradigms relevant for approach avoidance conflict decision making. Behav Res Ther. 96:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach H-A, Murison R, Moser EI, Moser M-B. 2002. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci. 99:10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Bach DR. 2019. Minimizing threat via heuristic and optimal policies recruits hippocampus and medial prefrontal cortex. Nat Hum Behav. 3:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Xavier Castellanos F, Milham MP. 2006. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 32:477–484. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. 2011. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 56:455–475. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Hodges JR, Graham KS. 2005. Perceptual deficits in amnesia: challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia. 43:1–11. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Scahill VL, Graham KS. 2008. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb Cortex. 18:683–696. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Yeung L-K, Barense MD. 2012. The hippocampus and visual perception. Front Hum Neurosci. 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hoskin R, Champi S. 2012. Avoidance of harm and anxiety: a role for the nucleus accumbens. Neuroimage. 62:189–198. [DOI] [PubMed] [Google Scholar]
- Loh E, Kurth-Nelson Z, Berron D, Dayan P, Duzel E, Dolan R, Guitart-Masip M. 2016. Parsing the role of the hippocampus in approach–avoidance conflict. Cereb Cortex. 27:201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CE. 2011. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learn Mem. 18:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A, Chau W, Protzner A. 2004. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 23:764–775. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. 2004. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 23:S250–S263. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. 2000. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord. 61:161–176. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Gluck MA, Herzallah MM, Myers CE. 2015. The influence of trial order on learning from reward vs. punishment in a probabilistic categorization task: experimental and computational analyses. Front Behav Neurosci. 9:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. 2007. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 30:99–122. [DOI] [PubMed] [Google Scholar]
- Murty VP, DuBrow S, Davachi L. 2019. Decision-making increases episodic memory via postencoding consolidation. J Cogn Neurosci. 31:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, FeldmanHall O, Hunter LE, Phelps EA, Davachi L. 2016. Episodic memories predict adaptive value-based decision-making. J Exp Psychol Gen. 145:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil EB, Newsome RN, Li IH, Thavabalasingam S, Ito R, Lee ACH. 2015a. Examining the role of the human hippocampus in approach–avoidance decision making using a novel conflict paradigm and multivariate functional magnetic resonance imaging. J Neurosci. 35:15039–15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil EB, Watson HC, Dhillon S, Lobaugh NJ, Lee ACH. 2015b. Multivariate fMRI and eye tracking reveal differential effects of visual interference on recognition memory judgments for objects and scenes. J Cogn Neurosci. 27:1708–1722. [DOI] [PubMed] [Google Scholar]
- Oehrn CR, Baumann C, Fell J, Lee H, Kessler H, Habel U, Hanslmayr S, Axmacher N. 2015. Human hippocampal dynamics during response conflict. Curr Biol. 25:2307–2313. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. 2012. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 6:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. 2011. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. 2012. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 13:713–726. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. 2011. Frontal cortex and reward-guided learning and decision-making. Neuron. 70:1054–1069. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 90:449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund MW, Brewer AT, Magee SK, Richman DM, Solomon S, Ludlum M, Dymond S. 2016. The tipping point: value differences and parallel dorsal–ventral frontal circuits gating human approach–avoidance behavior. Neuroimage. 136:94–105. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Villaruel FR, Ussling A, Riaz S, Lee ACH, Ito R. 2018. Ventral hippocampal CA1 and CA3 differentially mediate learned approach-avoidance conflict processing. Curr Biol. 28:1–7. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Vlassov E, Ito R. 2016. The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus. 26:530–542. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44:83–98. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. 2008. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 20:1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KJ, Henson RN, Graham KS. 2007. Recognition memory for faces and scenes in amnesia: dissociable roles of medial temporal lobe structures. Neuropsychologia. 45:2428–2438. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. 2004. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 81:172–184. [DOI] [PubMed] [Google Scholar]
- Wallis CU, Cockcroft GJ, Cardinal RN, Roberts AC, Clarke HF. 2019. Hippocampal interaction with area 25, but not area 32, regulates marmoset approach–avoidance behavior. Cereb Cortex. 29:4818–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. 1992. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 42:1743–1750. [DOI] [PubMed] [Google Scholar]
- Watson HC, Lee ACH. 2013. The perirhinal cortex and recognition memory interference. J Neurosci. 33:4192–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HC, Wilding EL, Graham KS. 2012. A role for perirhinal cortex in memory for novel object–context associations. J Neurosci. 32:4473–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. 2014. Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. 2009. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Yeates DCM, Ussling A, Lee ACH, Ito R. 2020. Double dissociation of learned approach-avoidance conflict processing and spatial pattern separation along the dorsoventral axis of the dentate gyrus. Hippocampus. 30:596–609. [DOI] [PubMed] [Google Scholar]
- Yeung L-K, Olsen RK, Bild-Enkin HE, D’Angelo MC, Kacollja A, McQuiggan DA, Keshabyan A, Ryan JD, Barense MD. 2017. Anterolateral entorhinal cortex volume predicted by altered intra-item configural processing. J Neurosci. 37:5527–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. 2016. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 17:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


