ABSTRACT
Background
Naturally occurring aflatoxins may contribute to poor growth and nutritional statuses in children.
Objectives
We analyzed the relationship between contemporary and lagged aflatoxin exposure and 1) length-for-age z-score (LAZ); and 2) length, knee-heel length, stunting, weight-for-age z-score (WAZ), and weight-for-length z-score (WLZ).
Methods
We conducted a longitudinal birth cohort study involving 1675 mother-infant dyads in rural Nepal. Participants were repeatedly visited from pregnancy to 2 years of age (2015–2019). One blood sample was collected during pregnancy and 4 samples were collected from the children at 3, 6, 12, and 18–22 months of age to measure concentrations of aflatoxin B1 (AFB1)-lysine adduct. Multivariate linear fixed-effects and logistic models with generalized estimating equations were used to identify associations between child growth and aflatoxin exposure.
Results
AFB1-lysine adducts were detected in the majority of children (at 3 months, 80.5%; at 6 months, 75.3%; at 12 months, 81.1%; and at 18–22 months, 85.1%) and in 94.3% of pregnant women. Changes in contemporary ln child AFB1‐lysine adduct concentrations were significantly associated with changes in LAZ (β, −0.05; 95% CI, −0.09 to −0.02; P = 0.003), length (β, −0.19; 95% CI, −0.29 to −0.10; P < 0.001), knee-heel length (β, −0.09; 95% CI, −0.13 to −0.05; P < 0.001), and WAZ (β, −0.04; 95% CI, −0.07 to −0.005; P = 0.022). Serum aflatoxin concentrations were associated with stunting (OR, 1.18; 95% CI, 1.05–1.32; P = 0.005). Similar results were found in the models using changes in contemporary ln AFB1 adjusted for changes in child weight, with significant associations with changes in WLZ (β, −0.07; 95% CI, −0.10 to −0.03; P < 0.001). Changes in time-lagged ln AFB1 (unadjusted and adjusted for changes in child weight) were associated with changes in length and knee-heel length.
Conclusions
Our results add to the growing body of evidence confirming chronic aflatoxin exposure and suggest that exposure is significantly correlated with various negative growth outcomes, which may vary by child weight status. This trial was registered at clinicaltrials.gov as NCT03312049.
Keywords: aflatoxin, growth, knee-heel length, length-for-age, weight, weight-for-age, weight-for-length
Introduction
Dietary aflatoxins are known for their immunosuppressive, hepatoxic, and growth-limiting effects on animals, and have recently garnered attention as possible contributors to poor in-utero and postnatal child growth (1–4). The 2 most common aflatoxin-producing fungi, Aspergillus flavus and Aspergillus parasiticus, are widely prevalent in dietary staples such as maize and groundnuts. These fungal metabolites are of particular concern in the tropics and subtropics. Contamination with Aspergillus fungi can occur in the field when crops are under stress (e.g., drought, high temperatures) and can also occur postharvest under poor storage conditions (high heat and/or humidity, unclean containers).
Widespread exposure to aflatoxin during pregnancy and early childhood in low-resource settings has been documented (3, 5–10). A relationship between aflatoxin exposure and growth failure has long been seen in animal studies, but it is difficult to study in humans due to its documented toxicity and chronic carcinogenicity. Recent observational evidence suggests that chronic aflatoxin exposure may contribute to growth faltering and/or stunting (a manifestation of poor growth), which are commonly observed in low- and middle-income countries. Prior observational studies have shown a link between aflatoxin exposure during fetal development or early life (5, 8, 9) and postnatal impaired growth (1, 3, 4). More recently, results from a randomized controlled trial suggest aflatoxins may negatively affect linear growth at younger ages (2).
Nepal has a high stunting rate, with a prevalence of 36% (11), and widespread aflatoxin exposure. Studies in Nepal have found aflatoxins in food (12, 13), as well as in human serum samples (14–16). Recognizing the need for region-specific data on aflatoxin exposure and links to impaired child growth, we designed a prospective birth-cohort study to examine the association between aflatoxin B1 exposure and growth impairment in young children in Banke, Nepal. This large cohort study was designed to test the central hypothesis that higher exposures to aflatoxin, measured via concentrations of serum aflatoxin (AFB1)-lysine adducts, result in reduced linear growth in children under 2 years of age. The primary objective was to analyze the statistical association between aflatoxin exposure and children's length-for-age z-scores (LAZs). Secondary outcomes included associations with children's lengths, knee-heel lengths, weight-for-age z-scores (WAZs), weight-for-length z-scores (WLZs), and stunting.
Methods
Subjects, study design, and setting
The AflaCohort Study was an observational, longitudinal, birth-cohort study examining the relationship between aflatoxin exposure and impaired linear growth in early childhood. The study recruited and repeatedly visited 1675 mother-infant dyads from pregnancy through 2 years of age (2015–2019). A rolling recruitment strategy was used to enroll healthy, pregnant women from 17 Village Development Committees in the Banke district (province number 5) of southwestern Nepal. Women between the ages of 16–49 years with a singleton pregnancy, <30 weeks gestation, who intended to deliver and stay in the study area were invited to enroll in the study.
The rationale guiding the sampling has been previously described (17). In brief, the sample size was calculated assuming the following parameters: an alpha of 0.05, a power of 80%, attrition of 20%, an intracluster correlation coefficient of 0.03, and a design effect of 1.5. This allowed for the possible detection of a −0.207 SD difference in postnatal LAZ for every 1-unit increase in log mean maternal AFB1-lysine adduct.
Data collection
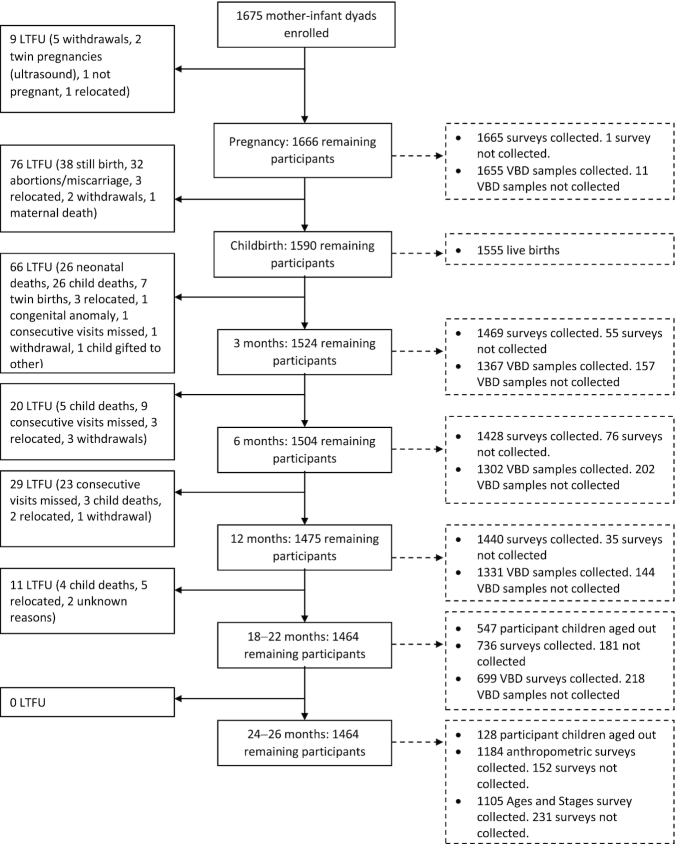
Pregnant women were interviewed immediately after enrollment. Enrollment and prenatal data collection started in July 2015 and was complete by July 2016. Nationwide strikes interrupted recruitment and data collection for 3 months, from August to November 2015. Data collection resumed at the beginning of December 2015. Enumerators attempted to visit mothers and newborns within 72 hours of birth. All children were reassessed with follow-up visits at 3, 6, 9, 12, 18–22, and 24–26 months after birth (Figure 1). Data collection was completed in March of 2019.
FIGURE 1.
Participant flowchart. During pregnancy, 1 survey was missing due to a missing upload; 3 VBDs were missing due to participants ending participation, 3 due to refusals, 3 due to severe anemia, and 2 for unknown reasons. At 3 months, 50 surveys were missing due to participants being out of the study area, 4 due to missing uploads, and 1 due to the child being gifted to another family; 5 VBDs were missing due to a child illness, 52 due to refusals, 6 due to severe anemia, 2 due to missing uploads, 2 due to insufficient blood volume, 39 due to participants being out of the study area, 12 due to SAM, 20 due to unsuccessful attempts, and 19 due to participants being unable to reschedule their visit. At 6 months, 39 surveys were missing due to participants being out of the study area, 1 due to a child illness, 1 due to a refusal, and 35 due to missing uploads; 12 VBDs were missing due to a child illness, 84 due to refusals, 2 due to severe anemia, 2 due to an insufficient blood volume, 39 due to participants being out of the study area, 32 due to SAM, 15 due to an unsuccessful attempt, and 16 due to participants being unable to reschedule their visit. At 12 months, 33 surveys were missing due to participants being out of the study area, 1 due to a refusal, and 1 due to an unexpected event; 11 VBDs were missing due to a child illness, 1 due to an unsuccessful attempt, 60 due to refusals, 2 due to severe anemia, 1 due to insufficient blood volume, 33 due to participants being out of the study area, 32 due to SAM, 1 due to an unexpected event, 2 due to participants being unavailable, and 1 for an unknown reason. At 18–22 months, 58 surveys were missing due to participants being out of the study area, 6 due to child illness, 36 due to refusals, 1 due to a missing upload, and 80 due to participants being unable to reschedule their visit; 13 VBDs were missing due to a child illness, 4 due to an unsuccessful attempt, 57 due to refusals, 3 due to severe anemia, 58 due to participants being out of the study area, 3 due to SAM, and 80 due to participants being unable to reschedule their visit. At 24–26 months, 11 anthropometry measurements were missing due to participants being out of the study area, 1 due to a refusal, and 140 due to participants being unable to reschedule their visit; 5 Ages and Stages Questionnaire measurements were missing due to participants being out of the study area and 226 due to participants being unable to reschedule their visit. Abbreviations: LTFU, loss to follow-up; SAM, severe acute malnutrition; VBD, venous blood draw.
The follow-up child visits were scheduled with a ±2-week window from the target age (e.g., ± 2 weeks from the child's first birthday). The tight window of data collection was met for most children in the follow-ups from 3 months through 12 months of age. The original study (Phase I) followed the woman-child dyads from pregnancy through 12 months of age. With additional funding, we were able to add 2 follow-up time points for data collection during the child's second year of life (Phase II), at 18 and 24 months. Exogenous factors caused delays in recommencing data collection, which resulted in a modified design with follow-ups between 18–22 and 24–26 months instead.
All data were collected electronically from participants using Android handheld tablets. With the exception of the 18–22-month visits, which took place at selected community sites (e.g., health centers, local halls, Female Community Health Volunteers’ homes), enumerators (and nurses) visited participants in their homes. During these visits, enumerators administered in-depth, structured surveys to collect data on maternal characteristics (e.g., education, diet, acute morbidity), child characteristics (e.g., diet, morbidity) and household demographics (e.g., number of household members, asset ownership).
Weight and length were collected at birth and additional measurements, such as head circumference, midupper arm circumference (MUAC), and knee-heel length, were collected starting at the 3-month visit. Digital weight scales (Seca 874, Seca North America, Chino, CA) , with 50 gram precision, were used to collect maternal and child weights and height and length, respectively, during the survey visits. The “mother-and-baby” 2-in-1 weighing function was used to measure child weight. Length and knee-heel length were collected using Infant/Child/Adult ShorrBoards and Shorr calipers (also called knemometers) (Shorr Productions LLC, Olney, MD), respectively. MUAC was measured with a standard insertion tape. Triplicate anthropometric measurements were averaged to provide a single measurement, and measurements were recorded to the nearest 0.1 kilogram and 0.1 centimeter. Supervisors and enumerators regularly calibrated scales, height-length boards, and knemometers with standard weights (2 and 5 kg) and length rods (130 cm and 170 cm). Calibrations were done after every 50th mother/child pairwas measured. Lost or damaged MUAC tapes were replaced. The field research team, including enumerators, supervisors, and nurses, met on a weekly basis to report on progress, resolve questions, and plan follow-ups for the week.
Study nurses visited participants in their homes within 7 days of the survey and anthropometric data collection to draw blood samples. One blood sample was collected from each woman during pregnancy and 4 samples were collected from the child at 3, 6, 12, and 18–22 months of age. Nurses first measured hemoglobin concentrations using portable HemoCue Hb 301 Systems (HemoCue America, Brea, CA)to ensure participants were not severely anemic (hemoglobin < 7 g/dL). Participants who were severely anemic [or severely malnourished, defined as children with a WLZ <−3 any time between 3–26 months, MUAC < 11.5 cm (6 months +), or bilateral pitting edema] were excluded from the blood draw for health reasons and were referred to the nearest health facility.
If participants met the blood draw inclusion criteria, nurses then proceeded to collect 3–5 mL of venous blood from the antecubital vein for mothers or 1–3 mL from the dorsal hand vein for children for AFB1-lysine adduct testing. Nurses also collected 25–50 mL of breast milk from lactating mothers when their children were 3 months of age for aflatoxin M1 (AFM1) testing. The breast milk samples were collected using manual breast milk pumps or through manual expression into a 50 mL sterile Falcon tube.
Blood and breast milk samples were immediately stored on wet ice in cool boxes and transported to a laboratory in Kohalpur, Banke, within 5 hours of collection. At the lab, blood was left to clot for 30 minutes at room temperature and then centrifuged at <5000 revolutions per minute for 10 minutes. A minimum of 400 μL of serum was frozen in a −20°C (or lower) freezer. Within a month of collection, samples were air-shipped to a −80°C (or lower) freezer at the Patan Academy for Health Sciences in Nepal's capital, Kathmandu.
Laboratory analyses
Serum samples were air-shipped on dry ice to the University of Georgia in the United States for serum AFB1-lysine adduct analysis within 6 months of collection. Serum aflatoxin B1-lysine adduct concentrations were measured using a validated method of HPLC with fluorescence detection. This included measurements of albumin and total protein concentrations for each sample, digestion with protease to release amino acids, concentration and purification of the AFB1‐lysine adduct, and finally separation and quantification by HPLC (18).
In short, thawed serum samples were inactivated for possible infectious agents via heating at 56°C for 30 minutes. Albumin and total protein concentrations were measured using modified procedures, as previously described (18). Approximately 150 μL was digested by pronase (pronase:total protein, 1:4, w:w) at 37°C for 3 hours to release AFB1‐lysine. AFB1‐lysine in digests were further extracted and purified by passing through a Waters mixed-mode anion exchange sorbent solid phase extraction cartridge, which was preprimed with methanol and equilibrated with water. The loaded cartridge was sequentially washed with 2 mL water, 1 mL 70% methanol, and 1 mL 1% ammonium hydroxide in methanol at a flow rate of 1 mL/min. Purified AFB1‐lysine was eluted with 1 mL 2% formic acid in methanol. The eluent was vacuum dried with a Labconco Centrivap concentrator and reconstituted for HPLC‐fluorescence detection.
An Agilent 1200 HPLC‐fluorescence system was used for the analysis of AFB1‐lysine adduct. The mobile phases consisted of buffer A (20 mM NH4H2PO4, pH 7.2) and buffer B (100% methanol). The Zorbax Eclipse XDB‐C18 reverse phase column (5 microns, 4.6 × 250 mm), equipped with a guard column, was used (Agilent). The column temperature was maintained at 25°C during analysis, and a volume of 100 μL was injected at a flow rate of 1 mL/min.
A gradient was generated to separate the AFB1‐lysine adduct within 25 minutes of injection. Adduct was detected by fluorescence at maximum excitation and emission wavelengths of 405 and 470 nm, respectively. Calibration curves of an authentic standard were generated weekly, and the standard AFB1‐lysine was eluted at approximately 13.0 minutes. The limit of detection was 0.4 pg/mg albumin, with a mean recovery rate of 90%. The AFB1‐lysine concentration was adjusted by albumin concentration.
Quality control and assurance procedures were maintained during analyses. These included simultaneous analyses of 1 authentic standard in every 10 samples and 2 quality control samples daily. Following completion of the laboratory analysis, sets of 3 samples were selected and pooled into 11 intraday reproducibility samples and 11 interday reproducibility samples. The former were analyzed twice on the same day by the same analyst, and the latter were analyzed on different days by different analysts to demonstrate laboratory precision and sampling reproducibility. Aflatoxin M1 concentrations in breast milk samples (from when the children were 3 months of age) were also measured using a validated method of HPLC with fluorescence detection.
Statistical analyses
The 2 primary exposures for this analysis were log-transformed aflatoxin exposure, measured as pg of aflatoxin B1-lysine adduct per mg albumin, and log-transformed aflatoxin B1-lysine adduct adjusted for body weight, measured as pg of aflatoxin B1-lysine adduct per mg of albumin over kg body weight. A constant value of half the limit of detection (LOD) of 0.2 pg/mg albumin was used for values under the LOD of 0.4 pg/mg albumin (19). Appropriate robustness checks were incorporated to ensure that adjusting for difference in the LOD indicator did not modify the regression estimates.
The primary outcome was child LAZ. Secondary outcomes were length, stunting, WAZ, WLZ, and knee-heel length, a useful measure for short-term linear growth (20–22). WHO standards were used to calculate z-scores (23). Stunting was defined as an LAZ <−2 standard deviations below the WHO reference LAZ median standardized z-scores. In accordance with WHO recommendations, z-score outliers (<−6 or >5 for WAZ, <−5 or >5 for WLZ, and <−6 or >6 for LAZ) were excluded prior to analysis.
All analyses were conducted with Stata statistical software (version 15; StataCorp LLC). A P value ≤ 0.05 was considered statistically significant. Chi-squared and Student's t tests were used to test for differences in proportions and continuous variables, and ANOVA tests were used for categorical variables. The relationship between length and knee-heel length was examined using a Pearson correlation analysis. Multivariate, linear, child-level fixed-effects models, with cluster-robust SEs, were used to identify associations between changes in AFB1-lysine adduct concentrations and changes in continuous growth outcomes (i.e., attained length, LAZ, knee-heel length, WAZ, or WLZ). Multivariate logistic regression models with generalized estimating equations (GEEs) were used to identify associations between AFB1-lysine adduct concentrations and stunting.
The SEs in all fixed-effects models were adjusted for the lack of independence between observations in the same child using regressions with robust SEs (using the cluster option in Stata). In the GEE models, robust SEs were adjusted for misspecification in the assumed covariance structure. The longitudinal nature of the data and the use of child-level fixed-effects regressions provides confidence that we have minimized any potential omitted-variable bias. This method allowed us to measure changes in linear growth for the child over time while holding unobserved time-invariant and mean observed child-specific characteristics constant.
Accumulating evidence indicates that environmental factors can affect children's health quite differently from adults’ health. Young children may experience worse adverse effects of aflatoxin exposure because of their larger intake per body weight ratio and their lower capacity to detoxify (24, 25). Therefore, in addition to exploring the relationship between AFB1-lysine adduct concentrations and growth outcomes, we conducted an exploratory analysis examining the relationship between changes in AFB1-lysine adduct concentrations, adjusted for deviations in the child's weight from their average weight, and changes in the aforementioned growth outcomes.
The AFB1-lysine adduct is considered a useful biomarker that reflects long-term exposure to aflatoxins. While serum adduct measurements reflect prolonged exposure over periods of 3–6 months (26, 27), there is no a priori published evidence on time-varying relationships between aflatoxin exposure and adverse growth outcomes. Therefore, both contemporary and time-lagged fixed-effects models were used to test the relationship between the change in growth and the change in aflatoxin exposure. Contemporary aflatoxin models included aflatoxin exposure during the most recent months, while time-lagged models were used to predict changes in growth based on the change in time-lagged (past period) values of the aflatoxin B1-lysine adduct concentration. Time-lagged models adjusted for the difference in the time-lagged value and the child-level time-lagged mean.
Covariates included in the models were: child age (in months), season of measurement (autumn, early winter, winter, spring, summer, and rainy/monsoon), and a dichotomous indicator of having detectable aflatoxin adduct concentrations in blood. This dichotomous variable indicating whether or not the child had detectable aflatoxin adduct concentrations in blood was included as a robustness check for including children with undetectable values in the models.
Autumn in this region is typically characterized by wet, cool weather, while the early winter and winter are cooler and drier. The spring and summer are warm and dry. The summer months are the hottest, while the rainy/monsoon months are the most humid. A previous analysis of historical annual rainfall data collected from the Department of Hydrology and Meteorology in Nepal showed no significant differences in monthly means between the survey period and prior years (January 1999–December 2014) (16).
Models examining aflatoxin exposure adjusted for changes in child weight as the predictor included an additional weight-for-length control variable, except for the model examining WLZ as the outcome. As part of an additional robustness check, we fitted an additional version of the models specified above, with 2 added variables: 1) severity of household food insecurity, as measured by the Household Food Insecurity Access Scale (28); and 2) child dietary diversity, measured using the WHO minimum dietary diversity score for children 6–23 months old (29). Inclusion of these variables did not change the results, and they were thus excluded from the final reported models.
Ethical oversight
Written informed consent was obtained in accordance with the Nepal Health Research Council Ethical Review Board (295/2014) and the Tufts University Health Sciences Institutional Review Board (11535) prior to enrollment and data collection.
Results
Demographic data for the households, pregnant women, and children are presented in Table 1. Most children came from households composed of a mean of 6 individuals, and most came from homes that were not experiencing food insecurity. The majority of the women enrolled were at a mean of 19.4 weeks (SD, 6.2 weeks) weeks of gestation. Around 37% of mothers had not completed primary education, and 20% had some primary education. Approximately 20% of the children were classified as having a low birth weight (<2500 grams). The majority of the birth visits occurred within 72 hours of delivery and within a ±2-week window from the target age.
TABLE 1.
Descriptive characteristics of the household, mother and child
| n | Mean ± SD or % | |
|---|---|---|
| Household characteristics | ||
| Household size | 1664 | 5.8 ± 3.0 |
| HFIAS at prenatal visit | ||
| Food secure | 1165 | 70.4 |
| Mildly food insecure | 282 | 17.0 |
| Moderately food insecure | 170 | 10.3 |
| Severely food insecure | 39 | 2.4 |
| Maternal characteristics | ||
| Gestational age at recruitment,1 weeks | 1664 | 19.4 ± 6.2 |
| Education level | ||
| None | 615 | 37.0 |
| Some primary, 1–5 | 322 | 19.4 |
| Some secondary, 6–10 | 583 | 35.0 |
| More than secondary, >10 | 144 | 8.7 |
| Child characteristics | ||
| Sex,2 female | 1568 | 50.6 |
| Low birth weight,3 <2500 grams | 1484 | 20.8 |
| Age, in months | ||
| Birth, all | 1595 | 0.05 ± 0.04 |
| Birth, <72 hours after birth | 1490 | 0.04 ± 0.03 |
| 3 mo | 1469 | 2.98 ± 0.18 |
| 6 mo | 1428 | 5.96 ± 0.19 |
| 9 mo | 1448 | 8.92 ± 0.21 |
| 12 mo | 1440 | 12.02 ± 0.20 |
| 18–22 mo | 736 | 21.28 ± 1.03 |
| 24–26 mo | 1184 | 25.58 ± 0.87 |
| Child anemia, Hb < 11 g/dL | ||
| Birth | — | — |
| 3 mo | 1435 | 65.1 |
| 6 mo | 1379 | 59.9 |
| 9 mo | 1387 | 68.6 |
| 12 mo | 1370 | 69.1 |
| 18–22 mo | 712 | 68.3 |
| 24–26 mo | — | — |
Abbreviations: Hb, hemoglobin; HFIAS, Household Food Insecurity Access Scale.
Based on last menstrual period.
Live births only.
Infants measured <72 hours after birth.
Child anthropometric data are presented in Table 2. At birth, children enrolled in the AflaCohort Study were 1.3 cm shorter than the median expected length for their age and sex (23); this deficit increased to 3.7 cm and 4.9 cm at 12 months and 24–26 months of age, respectively. Furthermore, at birth, children were 0.3 kg lighter than the median expected length for their age and sex. This weight deficit increased to 1.4 kg and 1.7 kg at 12 months and 24–26 months of age, respectively. The proportion of children defined as stunted increased between birth and 24–26 months of age (from 14.6% at birth to 27.3% at 12 months and 41.2% at 24–26 months). The mean age at the last visit (24–26 months) was 25.6 months; therefore, deficits in attained length and weight may be slightly underestimated when compared to 24-month-old children.
TABLE 2.
Child anthropometry
| Length, cm | Weight, kg | Knee-heel length, cm | Length-for-age z-score | Weight-for-age z-score | Weight-for-length z-score | Stunting1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | % | |
| Birth | 1483 | 48.19 ± 2.15 | 1484 | 2.83 ± 0.46 | — | — | 1483 | −0.82 ± 1.12 | 1484 | −1.01 ± 1.05 | 1389 | −0.78 ± 1.02 | 1483 | 14.6 |
| 3 mo | 1464 | 58.97 ± 2.51 | 1469 | 5.52 ± 0.83 | 1469 | 15.48 ± 0.94 | 1463 | −0.77 ± 1.16 | 1464 | −0.87 ± 1.16 | 1462 | −0.30 ± 1.17 | 1464 | 13.1 |
| 6 mo | 1427 | 64.55 ± 2.57 | 1428 | 6.89 ± 1.00 | 1428 | 17.20 ± 0.93 | 1425 | −0.92 ± 1.10 | 1410 | −0.90 ± 1.18 | 1409 | −0.36 ± 1.17 | 1427 | 15.1 |
| 9 mo | 1447 | 68.29 ± 2.67 | 1448 | 7.47 ± 1.04 | 1448 | 18.33 ± 0.93 | 1442 | −1.12 ± 1.08 | 1403 | −1.22 ± 1.15 | 1404 | −0.76 ± 1.14 | 1447 | 19.6 |
| 12 mo | 1437 | 71.33 ± 2.72 | 1440 | 7.94 ± 1.06 | 1440 | 19.24 ± 0.96 | 1422 | −1.43 ± 1.04 | 1386 | −1.44 ± 1.11 | 1386 | −0.98 ± 1.07 | 1437 | 27.3 |
| 18–22 mo | 733 | 79.30 ± 3.53 | 736 | 9.34 ± 1.20 | 736 | 21.90 ± 1.26 | 732 | −1.76 ± 1.11 | 732 | −1.63 ± 1.02 | 733 | −1.03 ± 0.94 | 733 | 42.1 |
| 24–26 mo | 1182 | 82.05 ± 3.72 | 1184 | 10.06 ± 1.28 | 1184 | 23.08 ± 1.33 | 1162 | −1.75 ± 1.08 | 1177 | −1.65 ± 1.03 | 1176 | −0.99 ± 0.94 | 1182 | 41.2 |
Stunting was defined as having a length-for-age z-score more than two standard deviations below the WHO Child Growth Standards median.
Table 3 shows AFB1 and AFM1 concentrations across the different study visits. AFB1-lysine adduct concentrations were detected in the majority of serum samples from pregnant women (94.3%; range, 0.4–147.32 pg/mg albumin) and children [3 months, 80.5% (range, 0.4–24.72pg/mg albumin); 6 months, 75.3% (range, 0.4–41.60 pg/mg albumin); 12 months, 81.1% (range, 0.4–84.65 pg/mg albumin); and 18–22 months, 85.1% (range, 0.4–128.07 pg/mg albumin)]. Mean AFB1-lysine adduct concentrations were highest in the pregnant women (geometric mean 1.54 pg/mg; 95% CI, 1.46–1.62 pg/mg albumin) and lowest in children at 3 months of age (0.83 pg/mg; 95% CI, 0.80–0.86 pg/mg albumin). Child AFB1-lysine adduct concentrations increased with age. AFM1 was detected in the majority of breast milk samples (94.1%; range, 0.04–315.99 ng/L).
TABLE 3.
Concentrations of aflatoxin B1-lysine adduct in serum and aflatoxin M1 in breast milk
| n | % detectable | Min > LOD | Maximum | Mean (SD) | Median (IQR) | Geometric mean (95% CI) | |
|---|---|---|---|---|---|---|---|
| Serum aflatoxin B1,1 pg/mg albumin | |||||||
| Pregnancy | 1652 | 94.3 | 0.4 | 147.32 | 3.38 (8.48) | 1.20 (0.74–2.45) | 1.54 (1.46–1.62) |
| 3 mo | 1363 | 80.5 | 0.4 | 24.72 | 1.01 (1.06) | 0.72 (0.54–1.15) | 0.83 (0.80–0.86) |
| 6 mo | 1297 | 75.3 | 0.4 | 41.6 | 1.18 (2.09) | 0.75 (0.53–1.16) | 0.86 (0.83– 0.90) |
| 12 mo | 1329 | 81.1 | 0.4 | 84.65 | 2.02 (4.57) | 0.87 (0.52–1.66) | 1.08 (1.02–1.14) |
| 18–22 mo | 699 | 85.1 | 0.4 | 128.07 | 2.41 (7.88) | 1.11 (0.64–1.98) | 1.27 (1.18–1.36) |
| Breast milk aflatoxin M1,2 ng/L | |||||||
| 3 mo | 1355 | 94.1 | 0.04 | 315.99 | 4.74 (19.30) | 0.96 (0.24–3.10) | 0.99 (0.90–1.08) |
Abbreviation: LOD, limit of detection.
LOD < 0.4 pg/mg albumin.
LOD < 0.04 ng/L. Measurements of central tendency include detectable samples only.
Table 4 shows results for the multivariate logistic regression models with GEE. It also shows results for linear fixed-effects models in which changes in growth outcomes are regressed on changes in contemporary and time-lagged child aflatoxin concentrations (each of which were also adjusted for changes in child weight).
TABLE 4.
Multivariate child-level fixed-effects linear and generalized estimating equation panel data logistic models regressing growth outcomes on contemporary and time-lagged aflatoxin B1-lysine adduct concentrations
| Length,1 cm | LAZ1 | Stunting2 | Knee-heel length,1 cm | WAZ1 | WLZ1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | OR | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| Contemporary AFB1 models | ||||||||||||||||||
| (ln) AFB13 | −0.19 | −0.29 to −0.10 | <0.001 | −0.05 | −0.09 to −0.02 | 0.003 | 1.18 | 1.05–1.32 | 0.005 | −0.09 | −0.13 to −0.05 | <0.001 | −0.04 | −0.07 to −0.005 | 0.022 | −0.01 | −0.05 to 0.03 | 0.565 |
| Observations | 4637 | — | 4631 | — | 4649 | — | 4656 | — | 4582 | — | 4578 | — | ||||||
| Number of children | 1480 | — | 1476 | — | 1477 | — | 1481 | — | 1477 | — | 1477 | — | ||||||
| (ln) AFB1/weight,4kg | −0.26 | −0.33 to −0.18 | <0.001 | −0.08 | −0.11 to −0.05 | <0.001 | 1.22 | 1.12–1.32 | <0.001 | −0.08 | −0.11 to −0.04 | <0.001 | −0.06 | −0.08 to −0.04 | <0.001 | −0.07 | −0.10 to −0.03 | <0.001 |
| Observations | 4563 | — | 4559 | — | 4576 | — | 4580 | — | 4580 | — | 4580 | — | ||||||
| Number ofchildren | 1478 | — | 1476 | — | 1477 | — | 1479 | — | 1479 | — | 1479 | — | ||||||
| Lagged AFB1 models | ||||||||||||||||||
| (ln) AFB1,3 1 lag | −0.15 | −0.27 to −0.02 | 0.024 | −0.03 | −0.07 to 0.02 | 0.254 | 1.09 | 0.96–1.23 | 0.189 | −0.07 | −0.13 to −0.01 | 0.015 | −0.04 | −0.08 to 0.003 | 0.052 | −0.02 | −0.08 to 0.033 | 0.439 |
| Observations | 3197 | — | 3196 | — | 4731 | — | 3223 | — | 3161 | — | 3161 | — | ||||||
| Number ofchildren | 1399 | — | 1398 | — | 1548 | — | 1399 | — | 1394 | — | 1395 | — | ||||||
| (ln) AFB1/weight,4kg, 1 lag | −0.14 | −0.24 to −0.03 | 0.011 | −0.03 | −0.07 to 0.002 | 0.070 | 1.26 | 1.11–1.40 | <0.001 | −0.07 | −0.12 to −0.02 | 0.003 | −0.02 | −0.05 to 0.01 | 0.186 | −0.01 | −0.05 to 0.04 | 0.718 |
| Observations | 3172 | — | 3171 | — | 3197 | — | 3198 | — | 3149 | — | 3161 | — | ||||||
| Number ofchildren | 1399 | — | 1398 | — | 1398 | — | 1399 | — | 1393 | — | 1395 | — | ||||||
Multivariate child-level fixed effects and GEE panel data logistic regression models with cluster-adjusted robust SEs were used to account for clustering at the child level. The outcomes were changes in growth parameters [i.e., length (cm), LAZ, stunting, knee-heel length (cm), WAZ, and WLZ]. Abbreviations: AFB1, aflatoxin B1; GEE, generalized estimating equation; LAZ, length-for-age z-score; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score.
Values are child-level fixed-effects regression-adjusted coefficients and 95% CIs.
Values are GEE regression adjusted ORs and 95% CIs.
Covariates: age (months), season of measurement, and detectable AFB1 concentrations (yes/no).
Covariates: age (months), WLZ, season of measurement, and detectable AFB1 concentrations (yes/no), with the exception of the WLZ models, which did not include WLZ as a covariate.
Contemporary aflatoxin exposure and attained growth measurements
In adjusted fixed-effect regression models, changes in contemporary log‐transformed child AFB1‐lysine adduct concentrations were associated with changes in length (β, −0.19; 95% CI, −0.29 to −0.10; P < 0.001), LAZ (β, −0.05; 95% CI, −0.09 to −0.02; P = 0.003), knee-heel length (β, −0.09; 95% CI, −0.13 to −0.05; P < 0.001), and WAZ (β, −0.04; 95% CI, −0.07 to −0.005; P = 0.022). Contemporary log‐transformed child AFB1‐lysine adduct concentrations were also associated with the odds of stunting (OR, 1.18; 95% CI, 1.05–1.32; P = 0.005). Changes in aflatoxin concentrations were not associated with changes in WLZ.
Similarly, adjusted fixed-effect regression models with changes in log‐transformed child AFB1‐lysine adduct concentrations, adjusted for changes in child weight, were significantly associated with changes in length (β, −0.26; 95% CI, −0.33 to −0.18; P < 0.001), LAZ (β, −0.08; 95% CI, −0.11 to −0.05; P < 0.001), knee-heel length (β, −0.08; 95% CI, −0.11 to −0.04; P < 0.001), WAZ (β, −0.06; 95% CI, −0.08 to −0.04; P < 0.001), and WLZ (β, −0.07; 95% CI, −0.10 to −0.03; P < 0.001). Log‐transformed child AFB1‐lysine adduct concentrations, adjusted for changes in child weight, were significantly associated with the odds of stunting (OR, 1.22; 95% CI, 1.12–1.32; P < 0.001).
Time-lagged aflatoxin exposure and attained growth measurements
In adjusted fixed-effect regression models, changes in lagged log‐transformed serum AFB1‐lysine adduct concentrations were significantly associated with changes in length (β, −0.15; 95% CI, −0.27 to −0.02; P = 0.024) and knee-heel length (β, −0.07; 95% CI, −0.13 to −0.01; P = 0.015).
Similarly, changes in lagged log-transformed serum AFB1‐lysine adduct concentrations, adjusted for changes in child weight, were significantly associated with changes in length (β, −0.14; 95% CI, −0.24 to −0.03; P = 0.011) and knee-heel length (β, −0.07; 95% CI, −0.12 to −0.02; P = 0.003). Lagged log-transformed serum AFB1‐lysine adduct concentrations, adjusted for child weight, were significantly associated with odds of stunting (OR, 1.26; 95% CI, 1.11–1.40; P < 0.001). No significant associations were observed between changes in time-lagged aflatoxin concentrations (both unadjusted and adjusted for changes in child weight) and changes in LAZ, WAZ, or WLZ.
Discussion
In this 4-year prospective cohort study, we examined the relationship between AFB1 exposure and child growth. We observed widespread, low-dose aflatoxin concentrations in a sample of pregnant women and in their young children. A higher aflatoxin concentration was significantly associated with impaired child linear and long-bone growth and with an increased likelihood of stunting.
The high prevalence of exposure is consistent with data from previous studies in this region (14, 30, 31). Consistent with previous literature, aflatoxin B1 concentrations gradually increased as children got older, presumably as their diets began to more closely resemble adult diets (31, 32). Although we assessed aflatoxin M1 in the breast milk, we did not find a relationship between those levels and infant blood serum aflatoxin B1 levels.
Findings from the contemporary models strengthen the available evidence linking serum AFB1-lysine adduct concentrations with growth-faltering rates among children (1,3). The observed levels of aflatoxin exposure translated into a length deficit among children of 1.6 cm. There are several possible explanations for the general lack of associations in time-lagged models. One is that the lag between exposure and the effect is temporally too distant, making it difficult to differentiate the effect of aflatoxin from other factors that could affect growth. Other reasons may include the biomarker used (its half-life and differing fractions of aflatoxin metabolites), the outcome variables of longitudinal growth (given the potential saltatory nature of linear growth among infants) (33, 34), and the relatively low absolute concentrations of aflatoxin in the serum samples.
Regarding the potential saltatory nature of linear growth in early life (35), children typically experience varying rates of linear growth over time, with faster growth during the first months of infancy and with deceleration later (36). Lampl and colleagues (33, 35, 37) describe this saltatory process in terms of amplitude pulsatile growth events (or saltations), followed by intervals of stasis with no growth (33, 35). Lampl et al. (37) note that this pattern may reflect population-based differences in growth pulse patterns, and that it is likely to have wide variability in the timing or amount of discrete growth linked to genetics, metabolic availability, or age-related developmental changes in growth dynamics.
There are challenges associated with measuring recumbent length (e.g., appropriate stretching before measurement, time needed). Therefore, a main and novel component of this study was to longitudinally measure knee-heel length as an additional measure of short-term long-bone growth. We showed that the knee-heel length measurement is strongly and negatively correlated with aflatoxin exposure and positively correlated with length. The latter finding is in line with previous literature showing segmental body measures are well correlated with stature in children when height measurements are difficult to attain (e.g., severe contractures) (38), and are also correlated with stature in adults (39, 40). Measuring knee-heel length is a promising yet underutilized technique. In combination with child height, it may provide a more complete assessment of short-term growth (20–22).
Evidence indicates the degree of toxicity can be higher in children than in adults, because children have a larger intake proportional to body weight and a lower capacity to detoxify (24, 25). The effects of aflatoxins are known to vary across animals and within species (41). In our study, we found evidence that aflatoxin toxicity and its effects on child growth have an association with child weight. The small difference in the magnitude of this association leads to important questions. A recent randomized trial with humans in Kenya detected an age-dependent relationship of aflatoxin on linear growth (2). These variations represent an important avenue for further research on causal pathways.
As with any complex study design, our analysis was subject to several limitations. Our design was observational, meaning that aflatoxin exposure might have been associated with confounding factors. To address this, we conducted sensitivity analyses including participants with undetectable aflatoxin values, and controlled for known confounders. Moreover, because of the long duration of the study, loss to follow-up was inevitable. Efforts to minimize attrition included sharing results of the study and having the same study personnel visit each household over time. In the end, data were collected from 86% and 70% of children at the 12- and 24–26-month visits, respectively. Once funding for the second phase of the study was awarded, some of the children had aged out of the 18–22- and/or the 24–26-month visits, resulting in smaller sample sizes than the Phase I follow-up visits. Lastly, a limitation of fixed-effects regression modeling is the inability to control for unmeasured time-varying confounding or potential reverse causation.
Nevertheless, this study has several notable strengths. It is 1 of the only studies to assess the aflatoxin-growth association longitudinally (2) and 1 of the few community-based studies in Asia examining how exposure to this food-borne hazard affects growth. The longitudinal design offered a rare opportunity to observe a large group of pregnant women and children, with 4 repeat measurements of aflatoxin B1-lysine adduct concentrations for the study children. It also allowed for adjustments for seasonal variation in aflatoxin exposure. This is also the first study to collect blood samples at 3 months of age, when most infants are still exclusively breastfed, and at multiple follow-ups as the children's diets gradually begin to resemble adult diets. Moreover, the serum AFB1‐lysine adduct measure is considered the most reliable biomarker of aflatoxin exposure, and the team analyzing the samples was blinded to both the study outcomes and data analysis. Lastly, in addition to the traditional methods to evaluate child growth, this study collected knee-heel length measurements, a novel measurement of long-bone growth.
In conclusion, these findings fill a gap in the available longitudinal evidence on links between concentrations of AFB1 exposure and impaired child growth. Given aflatoxin's toxicity and carcinogenicity, our results highlight the urgent need to better understand and implement strategies to reduce aflatoxin exposure. Interventions to reduce dietary exposure to aflatoxin may have positive effects on child growth in low- and middle-income countries.
Moving forward, there is a need for larger, more robust studies that examine the mediating biological pathways by which aflatoxin exposure may affect linear growth. Future studies should explore potential weight-varying effects of the relationship between aflatoxin and growth. This should be done across populations with varying likelihoods of exposure. This type of research will help determine the existence of a threshold dose of aflatoxin that induces child growth impairment.
ACKNOWLEDGEMENTS
We thank the Feed the Future Innovation Lab for Nutrition, which is funded by the United States Agency for International Development, for supporting this research. We express special gratitude to Dr. Maura Mack and Mr. Debendra Adhikari, without whom this research would not have been possible. We thank the Child Health Division of the Department of Health Services, Ministry of Health and Population, in the Government of Nepal; the Banke District Public Health Office; and the Nepalgunj Medical College. We are particularly grateful for the work of the AflaCohort Study enumerators, field supervisors, field guides, nurses, lab technicians, and administrative/office staff. Finally, we sincerely thank the families from participating households who graciously gave their time to this study.
The authors’ responsibilities were as follows – JYA-T, KB, PW, SG: designed the research; GS, JYA-T, SG: analyzed the data; JYA-T: wrote the paper; AK, DD, KP, RS: contributed to the design; AP: supervised the implementation of the study and all study protocols; SA: cleaned the data; J-SW, KSX: conducted the analysis of aflatoxin-exposure markers; and all authors: read and approved the final manuscript.
Author disclosures: JYA-T, PW, GS, AK, KB, DD, KP, RS, AP, SA, J-SW, KSX, and SG, no conflicts of interest.
Notes
This study was supported by the Feed the Future Innovation Lab for Nutrition, which is funded by the US Agency for International Development under grant ID AID-OAA-L-10- 00006.
The views and opinions expressed in this paper are those of the authors and not necessarily the views and opinions of the United States Agency for International Development.
Abbreviations used: AFB1, aflatoxin B1; AFM1, aflatoxin M1; GEE, generalized estimating equation; LAZ, length-for-age z-score; LOD, limit of detection; MUAC, midupper arm circumference; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score.
Contributor Information
Johanna Y Andrews-Trevino, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Patrick Webb, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Gerald Shively, Department of Agricultural Economics, Purdue University, West Lafayette, IN, USA.
Ahmed Kablan, Bureau of Resilience and Food Security, United States Agency for International Development, Washington, DC, USA.
Kedar Baral, Department of Community Health Sciences, Patan Academy of Health Sciences, Lalitpur, Nepal.
Dale Davis, Helen Keller International-Nepal, Kathmandu, Nepal.
Krishna Paudel, Kanti Children's Hospital, Kathmandu, Nepal.
Robin Shrestha, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Ashish Pokharel, Helen Keller International-Nepal, Kathmandu, Nepal.
Sudikshya Acharya, Helen Keller International-Nepal, Kathmandu, Nepal.
Jia-Sheng Wang, Department of Environmental Health Science, University of Georgia, Athens, GA, USA.
Kathy S Xue, Department of Environmental Health Science, University of Georgia, Athens, GA, USA.
Shibani Ghosh, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Data Availability
Data described in the manuscript, codebook, and analytic code will be made available upon request pending approval. Data will be made publicly and freely available without restriction at https://data.usaid.gov/ once all manuscripts related to the study's original research questions have been published in peer-reviewed journals.
References
- 1. Gong YY, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ Health Perspect. 2004;112:1334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann V, Jones K, Leroy JL. The impact of reducing dietary aflatoxin exposure on child linear growth: A cluster randomised controlled trial in Kenya. BMJ Glob Health. 2018;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner PC, Collinson AC, Cheung YB, Gong Y, Hall AJ, Prentice AM, Wild CP. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 2007;36:1119–25. [DOI] [PubMed] [Google Scholar]
- 4. Watson S, Chen G, Moore SE, Darboe MK, Prentice AM, Tu Y-K, Huang Y-T, Eriksen KG, Bernstein RM, Wild CPet al. Impaired growth in rural Gambian infants exposed to aflatoxin: A prospective cohort study. BMC Public Health. 2018;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrews-Trevino JY, Webb P, Shively G, Rogers BL, Baral K, Davis D, Paudel K, Pokharel A, Shrestha R, Wang Jet al. Relatively low maternal aflatoxin exposure is associated with small-for-gestational-age but not with other birth outcomes in a prospective birth cohort study of Nepalese infants. J Nutr. 2019, 149(10);1–8. [DOI] [PubMed] [Google Scholar]
- 6. Castelino JM, Dominguez-Salas P, Routledge MN, Prentice AM, Moore SE, Hennig BJ, Wild CP, Gong YY. Seasonal and gestation stage associated differences in aflatoxin exposure in pregnant Gambian women. Trop Med Int Health. 2014;19(3):348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gong Y, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study, BMJ. 2002;325:20–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauer JM, Duggan CP, Ausman LM, Griffiths JK, Webb P, Wang JS, Xue KS, Agaba E, Nshakira N, Ghosh S. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern Child Nutr. 2019;15:e12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shuaib FMB, Jolly PE, Ehiri JE, Yatich N, Jiang Y, Funkhouser E, Person SD, Wilson C, Ellis WO, Wang JSet al. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop Med Int Heal. 2010;15:160–7. [DOI] [PubMed] [Google Scholar]
- 10. Passarelli S, Bromage S, Darling AM, Wang JS, Aboud S, Mugusi F, Griffiths JK, Fawzi W. Aflatoxin exposure in utero and birth and growth outcomes in Tanzania. Matern Child Nutr. 2019;16:e12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nepali S, Simkhada P, Davies I. Trends and inequalities in stunting in Nepal: A secondary data analysis of four Nepal demographic health surveys from 2001 to 2016. BMC Nutr. 2019;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kafle P, Sedai D, Rai KP, Pokharel BB. Study on the level of aflatoxin m1 contamination in raw and processed milk marketed in Kathmandu valley. J Food Sci Technol Nepal. 2014;7:52–6. [Google Scholar]
- 13. Koirala KS, BK Y, KC P. Occurrence of aflatoxin in some of the food and feed in Nepal. Indian J Med Sci. 2005;59:331–6. [PubMed] [Google Scholar]
- 14. Groopman JD, Egner PA, Schulze KJ, Wu LSF, Merrill R, Mehra S, Shamim AA, Ali H, Shaikh S, Gernand Aet al. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B1-lysine albumin biomarkers. Food Chem Toxicol. 2014;74:184–9.. doi: 10.1016/j.fct.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell NJ, Riley RT, Egner PA, Groopman JD, Wu F. Chronic aflatoxin exposure in children living in Bhaktapur, Nepal: Extension of the MAL-ED study. J Expo Sci Environ Epidemiol. 2016;27:106–11.. doi:10.1038/jes.2015.87. [DOI] [PubMed] [Google Scholar]
- 16. Andrews-Trevino JY, Webb P, Shively G, Rogers B, Baral K, Davis D, Paudel K, Pokharel A, Shrestha R, Wang JSet al. Dietary determinants of aflatoxin B1-lysine adduct in pregnant women consuming a rice-dominated diet in Nepal. Eur J Clin Nutr. 2020, 74, 732–40.. doi: 10.1038/s41430-019-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrews-Trevino J, Webb P, Baral K, Davis D, Shrestha R, Pokharel A, Acharya S, Lamichhane A, Shively G, Paudel Ket al. Early life exposure to mycotoxins and child linear growth in Nepal: Methods and design of a prospective birth cohort study. J Food Secur. 2020;8:1–10. [Google Scholar]
- 18. Qian G, Tang L, Wang F, Guo X, Massey ME, Williams JH, Phillips TD, Wang JS. Physiologically based toxicokinetics of serum aflatoxin B1-lysine adduct in F344 rats. Toxicology. 2013;303:147–51.. doi: 10.1016/j.tox.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin Y, Hein MJ, Deddens JA, Hines CJ. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using SAS. Ann Occup Hyg. 2011;55:97–112. [DOI] [PubMed] [Google Scholar]
- 20. Davies HA, Pickering M, Wales JKH. A portable knemometer: A technique for assessment of short-term growth. Ann Hum Biol. 1996;23(2):149–57. [DOI] [PubMed] [Google Scholar]
- 21. University of Michigan. Highway Safety Research Institute, Snyder RG. Anthropometry of infants, children, and youths to age 18 for product safety design: Final report May 31, 1977, Washington DC. US Govt. Print. Off; 1977. [Google Scholar]
- 22. Michaelsen K, Skov L, Henrik Badsberg J, Jørgensen M. Short-term measurement of linear growth in preterm infants: Validation of a hand-held knemometer. Pediatr Res. 1991;30(5):464–8. [DOI] [PubMed] [Google Scholar]
- 23. WHO Multicentre Growth Reference Study Group . WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 24. WHO , Principles for evaluating health risks in children associated with exposure to chemicals, Environmental Health Criteria 237. in, (Geneva Switzerland: WHO, 2006), 237. [Google Scholar]
- 25. Sherif SO, Salama EE, Abdel-Wahhab MA. Mycotoxins and child health: The need for health risk assessment. Int J Hyg Environ Health. 2009;212:347–68. [DOI] [PubMed] [Google Scholar]
- 26. Leroy JL, Sununtnasuk C, García-Guerra A, Wang JS. Low level aflatoxin exposure associated with greater linear growth in southern Mexico: A longitudinal study. Matern Child Nutr. 2018;14:e12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levitt DG, Levitt MD. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: Indicator guide. Washington, DC, Food and Nutrition Technical Assistance Project, Academy for Educational Development: 2007. [Google Scholar]
- 29. World Health Organization . Indicators for assessing infant and young child feeding practices: Conclusions of a consensus meeting held 6–8 November 2007 in Washington DC, USA, Geneva Switzerland. WHO; 2008. [Google Scholar]
- 30. Mitchell NJ, Hsu HH, Chandyo RK, Shrestha B, Bodhidatta L, Tu YK, Gong YY, Egner PA, Ulak M, Groopman JDet al. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: An extension of the MAL-ED study. PLOS One. 2017;12:e0172124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahfuz M, Alam MA, Fahim SM, Gazi MA, Raihan MJ, Hossain M, Egner PA, Bessong PO, Petri WA, Groopman JDet al. Aflatoxin exposure in children living in Mirpur, Dhaka: Data from MAL-ED companion study. J Expo Sci Environ Epidemiol. 2019;29:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, Wild CP. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: The critical role of weaning. Int J Epidemiol. 2003;32(4):556–62. [DOI] [PubMed] [Google Scholar]
- 33. Lampl M. Evidence of saltatory growth in infancy. Am J Hum Biol. 1993;5(6):641–52. [DOI] [PubMed] [Google Scholar]
- 34. Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80(5):1106–22. [DOI] [PubMed] [Google Scholar]
- 35. Lampl M, Veldhuis JD, Johnson ML. Saltation and stasis: A model of human growth. Science. 1992;258(5083):801–3. [DOI] [PubMed] [Google Scholar]
- 36. Barker DJ, Bergmann RL, Ogra PL. Concluding remarks. The window of opportunity: Pre-pregnancy to 24 months of age. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:255–60. [DOI] [PubMed] [Google Scholar]
- 37. Lampl M, Ashizawa K, Kawabata M, Johnson ML. An example of variation and pattern in saltation and stasis growth dynamics. Ann Hum Biol. 1998;25:203–19. [DOI] [PubMed] [Google Scholar]
- 38. Samson-Fang L, Bell KL. Assessment of growth and nutrition in children with cerebral palsy. Eur J Clin Nutr. 2013;67:S5–8. [DOI] [PubMed] [Google Scholar]
- 39. García-Peña C, Pérez-Zepeda MU. Validity of knee-estimated height to assess standing height in older adults: A secondary longitudinal analysis of the Mexican health and aging study. J Nutr Health Aging. 2017;21(3):262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ritz P, Acher S, Beaufrère B, Blondé-Cynober F, Boulier A, Bouthier F, Bouthier-Quintard F, Constans T, Dardaine V, Desport JCet al. Validity of measuring knee-height as an estimate of height in diseased French elderly persons. J Nutr Heal Aging. 2004;8(5):386–8. [PubMed] [Google Scholar]
- 41. Dhanasekaran D, Shanmugapriya S, Thajuddin N, Panneerselvam A. Aflatoxins and aflatoxicosis in human and animals. Guevara-Gonzalez RG, in Aflatoxins - Biochemistry and Molecular Biology, Intech Open, 2011, 221–254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, codebook, and analytic code will be made available upon request pending approval. Data will be made publicly and freely available without restriction at https://data.usaid.gov/ once all manuscripts related to the study's original research questions have been published in peer-reviewed journals.