ABSTRACT
Background
Reduction of vitamin A deficiency (VAD) in Malawi coincided with introduction of vitamin A-fortified staple foods, alongside continued biannual high-dose vitamin A supplementation (VAS).
Objective
We describe coverage of vitamin A interventions and vitamin A status in the 2015–2016 Malawi Micronutrient Survey.
Methods
Food samples and biospecimens were collected within a representative household survey across 105 clusters. Retinol was measured using ultraviolet excitation fluorescence (sugar) and photometric determination (oil). Preschool children (PSC, aged 6–59 mo, n = 1102), school-age children (SAC, aged 5–14 y, n = 758), nonpregnant women (n = 752), and men (n = 219) were initially assessed for vitamin A status using retinol binding protein (RBP) and modified relative dose response (MRDR). Randomly selected fasted MRDR participants (n = 247) and nonfasted women and children (n = 293) were later assessed for serum retinol, retinyl esters, and carotenoids. Analyses accounted for complex survey design.
Results
We tested sugar and oil samples from 71.8% and 70.5% of the households (n = 2,112), respectively. All of the oil samples and all but one of the sugar samples had detectable vitamin A. National mean retinol sugar and oil contents were 6.1 ± 0.7 mg/kg and 6.6 ± 1.4 mg/kg, respectively. Receipt of VAS in the previous 6 mo was reported by 68.0% of PSC. VAD prevalence (RBP equivalent to <0.7µmol retinol/L) was 3.6% in PSC, and <1% in other groups. One woman and no children had MRDR ≥0.060 indicating VAD. Among fasted PSC and SAC, 18.0% (95% CI: 6.4, 29.6) and 18.8% (7.2, 30.5) had >5% of total serum vitamin A as retinyl esters, and 1.7% (0.0, 4.1) and 4.9% (0.0, 10.2) had >10% of total serum vitamin A as retinyl esters. Serum carotenoids indicated recent intake of vitamin A-rich fruits and vegetables.
Conclusions
Near elimination of VAD in Malawi is a public health success story, but elevated levels of vitamin A among children suggests that vitamin A interventions may need modification.
Keywords: vitamin A, overlapping interventions, retinyl esters, carotenoids, fortification
See corresponding editorial on page 769 and article on page 939.
Introduction
Vitamin A is essential for immune function, vision, and proper growth and development (1, 2). Vitamin A deficiency (VAD) is considered the leading cause of preventable childhood blindness and is a major contributor to morbidity and mortality from infections, especially in children and pregnant women (3). To address this burden, the WHO recommends universal vitamin A supplementation (VAS) with 2 annual doses of 200,000 IU preformed vitamin A to preschool children (1 to <5 y of age) or one dose of 100,000 IU vitamin A to infants 6–11 mo of age every 4 to 6 mo in settings where VAD is considered a public health problem (prevalence of night blindness ≥1% or prevalence of serum retinol <0.7 µmol/L ≥20%) (4). As of 2018, approximately 60 low- and middle-income countries (LMICs) have implemented universal VAS programs with a global 2-dose coverage of 61% (5).
The original purpose of VAS was to prevent xerophthalmia (6) and mortality (4), and interventions to reduce VAD have included increasing availability of vitamin A-rich foods through home gardening; fortification of flour, sugar, or oil; biofortification of staple crops, such as orange-flesh sweet potatoes or maize; and micronutrient powders and lipid-based nutrient supplements (7, 8). There are risks associated with excessive intake of preformed vitamin A originating in animal source foods, fortified foods, and supplements. Preformed vitamin A that is not required by tissues will accumulate in the liver “until a pathologic liver condition develops” (9). Vitamin A hepatotoxicity is characterized by stellate cells saturated with vitamin A, which lead to circulating retinyl esters in the fasting state (2, 9). In Zambian children exposed to overlapping vitamin A programs, 16% had >5% retinyl esters in circulation while fasted (10), and in South African children (n = 40) consuming liver twice per month and receiving VAS, 72.5% had >5% retinyl esters circulating while fasted (11). Adequate data on vitamin A-specific intervention coverage, dietary intake, and population vitamin A status are limited. A recent review of 82 countries with VAS programs indicated that most countries had no VAD data or only data that were >10 y old (12).
In Malawi, VAD was a significant public health problem in 2001 and 2009 based on the prevalence of unadjusted serum retinol [or unadjusted retinol binding protein (RBP) as a proxy for retinol] <0.7 µmol/L being >20% in children <5 y old (13, 14). The prevalence of VAD for children <5 y old was 59.2% in 2001 and 22.0% in 2009. There is strong political will and donor support for nutrition interventions in Malawi, including vitamin A interventions (15). Since 2003, Malawi has implemented VAS for preschool children and postpartum women through biannual child health days. Additional policies to improve vitamin A status include mandatory industrial vitamin A fortification of sugar (initiated in 2012), wheat and maize flour, and cooking oil (initiated in 2000); biofortified foods; improved breastfeeding and complementary feeding practices; and prevention and treatment of infections.
Between 2001 and 2009, the relative reduction in VAD in the Malawian population was >60% (13, 14). Speculation as to what caused this decline includes evidence that nearly half of women reported use of vitamin A-fortified cooking oil in a 2009 survey, and a supplementary feeding program included a corn–soya blend fortified with vitamin A in addition to high VAS coverage (14). Despite the potential risk of overlapping vitamin A interventions (16), there have been no analyses on the potential for elevated vitamin A among children and women in Malawi. Using data from the 2015–2016 National Micronutrient Survey (MNS), we described coverage of major vitamin A interventions and vitamin A biomarkers reflecting VAD and elevated vitamin A liver stores.
Subjects and Methods
2015–2016 MNS
Sampling and ethical review procedures
The 2015–2016 Malawi MNS was a subsample of the Malawi Demographic Health Survey (MDHS). The MNS was a cross-sectional study that employed a 2-stage cluster sampling design and has been described in detail elsewhere (17). In the first stage of sampling, enumeration areas (clusters) were randomly selected using probability proportional to population size. Thirty-five clusters per stratum (region) were selected from the 2015–2016 MDHS for the MNS. In the second stage of sampling, within the 105 selected clusters, the subsample of households selected for an HIV component of the MDHS were excluded. A fixed number of 20 households per urban cluster or 22 households per rural cluster were selected using equal probability systematic random sampling. Within selected households, all preschool children 6–59 mo old (PSC) were invited to participate in the MNS. For other population groups, a random subsample of selected households per cluster were invited [9 households for women of reproductive age (WRA), 6 households for school-aged children 5–14 y old (SAC), and 4 households for men]. The first household with an eligible member of each population group for MRDR (PSC, SAC, and WRA) in each cluster was selected at random and invited to participate in the MRDR subsample. Participants selected for analysis of retinol, retinyl esters, and carotenoids were based on inclusion in the MRDR subsample (n = 247), and a random additional representative subsample of women and children (n = 293). The National Health Sciences Research Committee in Malawi granted ethical approval for this survey, and written informed consent was obtained from all eligible participants. Parents provided written consent for their children.
Questionnaire data collection
There were 6 MNS data collection teams that visited clusters 1 to 2 wk following the MDHS data collection teams. MNS data were collected from mid-December 2015 until February 2016; the scheduled biannual VAS campaign was purposefully delayed until completion of the MNS data collection. The most recent VAS campaign prior to the survey was conducted between April and June 2015 (UNICEF communication). During the household visits, a short questionnaire was administered to determine presence of fortified foods and household participation in nutrition-specific and nutrition-sensitive interventions. Vitamin A fortifiable food vehicles (sugar and oil) available for sampling were collected from sampled households. Individuals eligible for the biologic specimen collection were asked to report to the temporary field laboratory, which was constructed in a central location in each cluster.
Food and biological specimen collection
Within households, approximately 4 tablespoons (50 g) of sugar were collected in a plastic, resealable bag and then placed in a paper bag to avoid exposure to sunlight. Approximately 7 mL of cooking oil were collected in a plastic tube wrapped with foil to prevent light from deteriorating retinol. Food specimens, collected from the households, were replaced.
Medical field staff (nurses and laboratory technicians) worked out of temporary field laboratories. Each selected participant was given a bracelet with a preprinted unique identification number barcode label to ensure matching of the collected specimens with the household questionnaire data. Participants who consented to blood collection had venous blood samples drawn by trained phlebotomists in the field laboratory.
Blood processing in the field
Venous blood was collected into 2 separate tubes. One tube contained the anticoagulant potassium ethylenediaminetetraacetic acid (K2EDTA) and the second royal blue trace metal–free tube contained no additive for serum collection. Whole blood from the first tube containing the anticoagulant EDTA was tested for malaria and anemia using a rapid diagnostic test and the HemoCue® 301. After these 2 field tests were performed, specimens were processed shortly after collection. Each field team was equipped with a portable centrifuge and both venous blood collection tubes were centrifuged at the temporary field laboratory. After centrifugation, the plasma (for MRDR analysis) and serum [for RBP, C-reactive protein (CRP), α1-acid glycoprotein (AGP), carotenoids, retinol, and retinyl esters] were placed into cryovials labeled with preprinted barcodes that contained the participant's unique specimen identification number. The centrifuged serum was separated into aliquots for multiple micronutrient analyses and placed in a portable freezer while in the field laboratory.
Modified relative dose response methodology
Households were selected for the MRDR subsample at random. These households were visited early in the day, and participants were asked to be fasted; if any vitamin A-rich food had been consumed in the past 4 h, then the MRDR dose of retinol analog was delayed for 2 h. An oral dose of 3,4-didehydroretinyl acetate mixed with oil that did not contain vitamin A (to aid in absorption) was administered to consenting individuals (PSC, SAC, and WRA). Approximately 4 h after dosing, the phlebotomist collected a 3-mL venous blood sample using a third blood collection tube containing K2EDTA. The tube was centrifuged in the field and 2 plasma aliquots were created (1 for measurement and 1 for backup). From the time prior to the oral dose and until 4 h after receipt of the dose, the participants were instructed not to consume any vitamin A-rich food sources. The MRDR participants stayed at the temporary field laboratories until the second blood draw and were given nonvitamin A rich foods and beverages to snack on while they waited.
Cold chain and shipping procedures
Each of the 6 teams had portable freezers in the field. The collected biospecimens were kept in cold boxes in the temporary field laboratories with frozen gel packs until they were processed and placed in the portable freezers for overnight storage. Every 1 to 2 d, regional supervisors transported biospecimens to the nearest district or regional-level laboratories where they were temporarily stored (at −20 oC) until being transported to the Community Health Services Unit (CHSU) of the Ministry of Health for long-term storage (at −70 oC) until shipment or analysis.
Storage vials containing serum from the first blood collection tube were shipped frozen on dry ice to VitMin laboratory in Germany in April 2016. Plasma from the MRDR subsample was shipped frozen on dry ice to the Institute of Nutrition of Central America and Panama (INCAP) laboratory in Guatemala in April 2016. Backup storage vials containing serum from the first blood collection tube were shipped frozen on dry ice to the CDC in Atlanta, and then to the University of Wisconsin–Madison for analysis of retinol, retinyl esters, and carotenoids in June 2018.
Laboratory methods
The sugar and oil specimens were analyzed at the CHSU nutrition laboratory. Ultraviolet excitation fluorescence was the method for determining the retinol content of sugar using a portable fluorometer (iCheck FLUORO). Photometric determination of retinol in oil was conducted using a color-generating chemical reaction (Carr-Price) in a portable photometer (iCheck CHROMA). Pretested control samples, prepared in house and of known concentration, were randomly selected and run at regular intervals during the food sample testing for quality control purposes. The range of detection for the portable fluorometer was 0.05–3.0 mg retinol equivalents (RE)/kg; samples with >3.0 mg RE/kg must be diluted. The concentration range limit for the portable photometer was 3–30 mg RE/kg.
The VitMin Lab analyzed serum for RBP, CRP, and AGP using an in-house sandwich ELISA (18). The INCAP laboratory analyzed plasma for 3,4-didehydroretinol and retinol using HPLC. Serum carotenoids, retinol, and retinyl esters were analyzed at the University of Wisconsin–Madison Department of Nutritional Sciences laboratory by UPLC (Waters) according to a previously described solvent system (19), optimized for low sample volumes and desired analytes. Briefly, 150 µL serum, or all available, was divided into aliquots placed into borosilicate glass tubes (Fisher Scientific) to which 100 µL C-23 β-apo-carotenol (internal standard optical density in methanol ∼1.0 at 325 nm) and 225 µL ethanol were added. Organic compounds were extracted 3 times by addition of 500 µL hexanes, mixed with a vortex, and centrifuged at a relative centrifugal force of 1200 × g for 5 min at ambient temperature. Pooled hexane layers were dried in conical-bottom glass tubes under a stream of nitrogen. The dried extract was resuspended in 75 µL of a 75:25 methanol:dichloroethane concentration and transferred to UPLC vials (Waters), and 6 µL was injected onto the UPLC system.
Statistical analysis
All statistical analyses were conducted in SAS, version 9.4 and accounted for the complex survey design variables (weight, cluster, strata). Inclusion criteria for household-level analyses was a response code of a “complete” or “partially complete” interview from MDHS. Descriptive analyses were done using PROC SURVEYFREQ or PROC SURVEYMEANS. Household hunger was measured using a cross-culturally validated 3-question tool (20, 21). Iron deficiency was defined as inflammation-adjusted ferritin <12 µg/L (PSC) and <15 µg/L (SAC and WRA), calculated using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) regression approach with survey-specific coefficients for the relations between ferritin, CRP, and AGP (22, 23). Inflammation was defined as CRP concentration > 5 mg/L or AGP concentration >1 g/L. Where retinol or RBP were adjusted for the influence of inflammation, the BRINDA regression method was used (24). Adjusted and unadjusted retinol and RBP are presented for PSC and SAC. The relation between retinol and RBP was analyzed with simple linear regression using concentrations of biomarkers not adjusted for inflammation to determine RBP concentration equivalent to a concentration of 0.7 µmol retinol/L, which signifies deficiency (25). The cutoff for hypervitaminosis A in humans has been described as >10% elevated retinyl esters in circulation in the fasting state (9), but a recent analysis of cadavers showed correlation between >3 µmol vitamin A/g liver and retinyl esters >7.5% (26), which is why this cutoff was also selected for adults. Based on recent research documenting a high prevalence of hypervitaminosis A (defined as ≥1 µmol vitamin A/g liver) in African children, it was proposed that >5% retinyl esters of total vitamin A in children may be more effective for identifying elevated liver vitamin A when isotope dilution is not feasible (10, 11), which is why this cutoff was also used. Coverages of vitamin A interventions were compared across regions using the Rao-Scott modified chi-square test. Coverage of fortified sugar or oil was defined as a household that had the food sample available and a laboratory value was reported from the sample that indicated RE at or above the limit of detection. At factories, according to fortification standards, the concentration of retinol in sugar and oil should be 4–22 mg RE/kg and 20–40 mg RE/kg, respectively. Mean concentrations of vitamin A (mg RE/kg) in food samples were reported by region and nationally. Concentrations of vitamin A biomarkers were compared across population groups using the median test. Statistical significance of 0.05 was used throughout.
Results
Survey response rates and participant characteristics
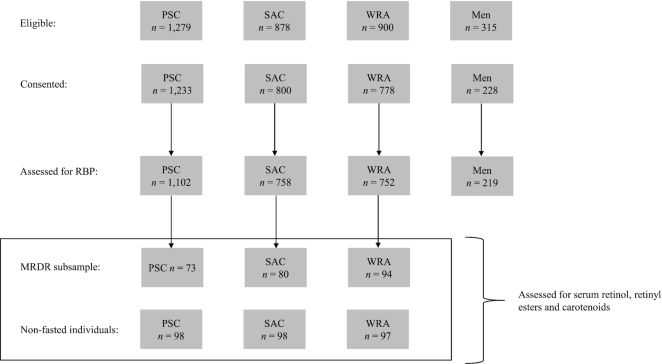
In the 2015–2016 MNS, RBP data were available for 1102 PSC, 758 SAC, 752 nonpregnant WRA, and 219 men sampled from 2112 households (Table 1). The MRDR subsample included 73 PSC, 80 SAC, and 94 nonpregnant WRA (Figure 1). The retinyl ester subsample, which also included assessment of carotenoids and retinol, were from a combination of the fasted MRDR participants (n = 247) and a random representative selection of nonfasted PSC, SAC, and WRA (n = 293, Figure 1).
TABLE 1.
Household and individual characteristics in the 2015–2016 Malawi National Micronutrient Survey1
| Characteristics | n (unweighted) | Percentage (95% CI) |
|---|---|---|
| Household (n = 2112) | ||
| Rural residence | 1791 | 87.2 (75.8, 98.5) |
| Region | ||
| North | 690 | 11.6 (6.2, 17.0) |
| Central | 718 | 42.7 (29.3, 56.1) |
| South | 704 | 45.8 (35.6, 58.9) |
| Reported moderate hunger in past month2 | 1045 | 57.6 (52.0, 63.3) |
| Reported severe hunger in past month2 | 81 | 2.7 (1.6, 3.7) |
| Preschool age children (n = 1102) | ||
| Age group, mo | ||
| 6–23 | 332 | 31.2 (27.1, 35.3) |
| 24–59 | 770 | 68.8 (64.7, 72.9) |
| Male sex | 539 | 50.3 (47.3, 53.3) |
| Stunting3 | 358 | 34.8 (31.0, 38.5) |
| Anemia4 | 342 | 30.6 (26.9, 34.4) |
| Iron deficiency5 | 222 | 21.7 (16.2, 27.2) |
| Iron deficiency anemia6 | 97 | 9.3 (6.4, 12.2) |
| Positive malaria (rapid test) | 290 | 27.9 (19.9, 35.9) |
| CRP concentration >5 mg/L | 279 | 23.9 (19.4, 28.5) |
| AGP concentration >1 g/L | 637 | 56.0 (50.5, 61.4) |
| CRP >5 mg/L or AGP >1 g/L | 651 | 57.0 (51.5, 62.6) |
| School age children (n = 758) | ||
| Age group, y | ||
| 5–10 | 502 | 65.7 (61.9, 69.6) |
| 11–14 | 256 | 34.3 (30.4, 38.1) |
| Male sex | 372 | 50.9 (46.5, 55.3) |
| Anemia4 | 158 | 23.0 (18.4, 27.6) |
| Iron deficiency5 | 34 | 4.9 (2.9, 6.8) |
| Iron deficiency anemia6 | 11 | 1.7 (0.3, 3.1) |
| Positive malaria (rapid test) | 282 | 40.6 (33.8, 47.5) |
| CRP concentration >5 mg/L | 124 | 15.9 (11.9, 19.9) |
| AGP concentration >1 g/L | 251 | 31.8 (27.1, 36.4) |
| CRP >5 mg/L or AGP >1 g/L | 263 | 33.8 (28.7, 38.8) |
| Non-pregnant women of reproductive age (n = 752) | ||
| Age, y | ||
| 15–19 | 159 | 20.2 (16.7, 23.7) |
| 20–29 | 270 | 38.3 (32.3, 44.3) |
| 30–49 | 323 | 41.5 (37.0, 46.0) |
| Weight status | ||
| Underweight (BMI <18.5) | 65 | 9.0 (6.8, 11.3) |
| Normal (18.5 ≤ BMI <25) | 549 | 76.5 (72.1, 80.8) |
| Overweight (25 ≤ BMI <30) | 101 | 10.4 (7.1, 13.6) |
| Obese (BMI ≥30) | 29 | 4.1 (2.2, 6.0) |
| Anemia4 | 160 | 22.4 (18.7, 26.1) |
| Iron deficiency5 | 131 | 15.1 (11.8, 18.5) |
| Iron deficiency anemia6 | 69 | 8.0 (5.5, 10.4) |
| Positive malaria (rapid test) | 109 | 16.6 (12.1, 21.1) |
| CRP concentration >5 mg/L | 67 | 7.3 (5.0, 9.6) |
| AGP concentration >1 g/L | 97 | 11.1 (8.0, 14.2) |
| CRP >5 mg/L or AGP >1 g/L | 122 | 13.2 (9.9, 16.6) |
| Men (15–49 y) (n = 219) | ||
| Age, y | ||
| 15–29 | 75 | 33.9 (25.4, 42.3) |
| 30–54 | 144 | 66.1 (57.7, 74.6) |
| Weight status | ||
| Underweight (BMI <18.5) | 25 | 14.0 (7.1, 21.0) |
| Normal (18.5 ≤ BMI <25) | 177 | 81.0 (73.3, 88.6) |
| Overweight or obesity (BMI ≥25) | 16 | 5.0 (2.2, 7.9) |
| Anemia4 | 11 | 6.3 (1.9, 10.6) |
| Iron deficiency5 | 2 | 1.4 (0.0, 3.4) |
| Iron deficiency anemia6 | 0 | 0 |
| Positive malaria (rapid test) | 26 | 14.6 (6.8, 22.3) |
| CRP concentration >5 mg/L | 23 | 9.3 (3.9, 14.6) |
| AGP concentration >1 g/L | 23 | 10.5 (5.3, 15.6) |
| CRP >5 mg/L or AGP >1 g/L | 35 | 14.1 (8.0, 20.2) |
Data are weighted prevalence estimates and 95% CIs that account for complex survey design variables (strata, cluster, and weight). AGP, α1-acid glycoprotein; CRP, C-reactive protein.
Determined by the Household Hunger Scale (20).
Stunted defined as length or height-for-age z-score <2 (29).
Anemia defined as hemoglobin <11.0 g/dL (<5y) adjusted for altitude, <11.5 g/dL (5–11 y) or <12.0 g/dL (12–14 y) adjusted for altitude, <12.0 g/dL (nonpregnant women) adjusted for altitude and smoking, and <13.0 g/dL (men) adjusted for altitude (smoking data were not available for men) (30).
Iron deficient defined as inflammation-adjusted serum ferritin <12.0 µg/L (<5y) and <15.0 µg/L (5–54y) (22, 23).
Iron deficiency anemia defined as iron deficiency plus anemia.
FIGURE 1.
Participant flow chart from the 2015–2016 Malawi Micronutrient Survey and supplementary vitamin A biomarker analysis. Men (20–54 y); MRDR, modified relative dose response; PSC, preschool age children (6–59 mo); RBP, retinol binding protein; SAC, school age children (5–14 y); WRA, women of reproductive age (15–49 y).
Most households had a rural residence, and moderate household hunger was reported in over half (57.6%) of households. Data collection from mid-December to February corresponded to the lean (hungry) season (27, 28). Inflammation affected more than half of PSC and approximately one-third of SAC. More than a quarter (27.9%) of PSC and 40.6% of SAC had a positive malaria rapid diagnostic test. Elevated inflammatory proteins and positive malaria were less common among adults than children, yet 16.6% of WRA and 14.6% of men had positive malaria test results. Anemia and iron deficiency anemia affected 30.6% and 9.3% of PSC, 23.0% and 1.7% of SAC, and 22.4% and 8.0% of nonpregnant WRA, respectively (Table 1). No men had iron deficiency anemia, and 6.3% of men were anemic.
Coverage of vitamin A interventions in Malawi
Vitamin A-fortified cooking oil was present in 70.5% (95% CI: 63.1, 77.9) of households in the MNS, and vitamin A-fortified sugar was present in 71.8% (64.1, 79.6) of households (Table 2). Households in the Southern region were least likely to have oil or sugar in the household available for sampling. Among households that provided food samples and the laboratory-reported results, the presence of any detectable RE in sugar and oil was nearly universal. One sugar sample had undetectable vitamin A. The mean content of retinol in oil (6.6 ± 1.4 mg RE/kg, Table 2) was much lower than the standard for oil at the factory level (20–40 mg RE/kg).
TABLE 2.
Household coverage and mean concentration of vitamin A-fortified sugar and oil in Malawi 2015–2016 by region
| Region | |||||
|---|---|---|---|---|---|
| n (unweighted) | Overall (n = 2112) | North (n = 690) | Central (n = 718) | South (n = 704) | |
| Cooking oil1 | |||||
| No oil in household | 484 | 23.2 (17.3, 29.2) | 20.3 (14.3, 26.3) | 20.5 (11.7, 29.4) | 26.4 (17.2, 35.7) |
| Oil present in household (not collected or tested) | 178 | 6.3 (3.4, 9.2) | 16.1 (7.3, 24.9) | 3.4 (1.3, 5.6) | 6.5 (1.2, 11.8) |
| Vitamin A in oil >0 mg RE/kg2 | 1441 | 70.5 (63.1, 77.9) | 63.6 (51.2, 76.0) | 76.0 (66.5, 85.6) | 67.1 (54.7, 79.4) |
| Sugar3 | |||||
| No sugar in household | 504 | 25.3 (18.9, 31.7) | 20.6 (14.1, 27.1) | 20.4 (10.1, 30.7) | 30.9 (21.9, 40.0) |
| Sugar present in household (not collected or tested) | 30 | 2.9 (0.0, 6.8) | 1.4 (0.2, 2.6) | <0.1 (0.0, 0.1) | 5.9 (0.0, 14.1) |
| Vitamin A in sugar >0 mg RE/kg4 | 1566 | 71.8 (64.1, 79.6) | 78.0 (71.1, 84.9) | 79.6 (69.3, 89.9) | 63.2 (51.1, 75.2) |
| Concentration, mg/kg | |||||
| Retinol in cooking oil | 1441 | 6.6 (5.3, 8.0) | 8.6 (6.3, 10.8) | 4.1 (3.3, 4.9) | 8.8 (6.3, 11.4) |
| Retinol in sugar | 1567 | 6.1 (5.4, 6.9) | 9.2 (8.3, 10.1) | 6.9 (5.7, 8.1) | 4.4 (3.7, 5.1) |
Values are numbers of households or means (95% CIs). Nine households had missing data on cooking oil. Concentration range limit for the portable photometer, used to measure vitamin A in oil, was 3–30 mg RE/kg. Rao–Scott modified chi-square found no difference across regions comparing vitamin A in oil >0 mg RE/kg. RE, retinol equivalents;
There were n = 937 oil samples equal to 3 mg RE/kg.
Eleven households had missing data on sugar. Concentration range limit for the portable fluorometer, used to measure vitamin A in sugar, was 0.5–3.0 mg RE/kg; samples with RE above 3 mg/kg were diluted. Rao–Scott modified chi-square found no difference across regions comparing vitamin A in sugar >0 mg RE/kg.
One sugar sample had undetectable vitamin A; the maximum recorded concentration of retinol in sugar was 33 mg/kg.
According to parental report in the MDHS, 68.0% (95% CI: 62.5, 73.5) of children 6–59 mo old participating in the MNS had received a high dose of VAS in the previous 6 mo. There was no variation in prevalence of VAS receipt by region (data not shown). Receipt of high-dose VAS postpartum among WRA was not reported in the MDHS.
Biomarkers of VAD, elevated vitamin A, and vitamin A intake
The prevalence of VAD was low among all population groups. VAD, defined as RBP (not adjusted for inflammation) <0.46 µmol/L, was 3.6% (95% CI: 1.7, 5.4) for PSC, 0.9% (0.1, 1.7) for SAC, 0.3% (0.0, 0.8) for nonpregnant WRA, and 0.1% (0.0, 0.3) for men (Table 3). The prevalence of inflammation-adjusted RBP <0.46 µmol/L was 0.4% (0.0, 0.9) for PSC, and no SAC had inflammation-adjusted RBP <0.46 µmol/L. VAD, defined by the prevalence of inflammation-adjusted serum retinol <0.7 µmol/L, was 1.8% (0.0, 4.6) for PSC and 4.4% (0.0, 9.7) for SAC. The prevalence of serum retinol <0.7 µmol/L was 1.9% (0.0, 4.4) for nonpregnant WRA (Table 3).
TABLE 3.
Summary of vitamin A biomarkers reflective of deficiency or elevated status among PSC, SAC, nonpregnant WRA, and men in the 2015–2016 MNS1
| Biomarker and cutoffs for vitamin A status | RBP <0.46 µmol/L2 | Serum retinol <0.7 µmol/L3,4 | MRDR ratio3 | Fasted serum retinyl esters >5% (children) or 7.5% (women)3,5 | Fasted serum retinyl esters >10% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | mean ± SE | n | % (95% CI) | n | % (95% CI) | |
| PSC (6–59 mo) | 1102 | 3.6 (1.7, 5.4) | 171 | 1.8 (0.0, 4.6) | 73 | 0.018 ± 0.001 | 73 | 18.0 (6.4, 29.6) | 73 | 1.7 (0.0, 4.1) |
| SAC (5–14 y) | 758 | 0.9 (0.1, 1.7) | 178 | 4.4 (0.0, 9.7) | 80 | 0.011 ± 0.001 | 80 | 18.8 (7.2, 30.5) | 80 | 4.9 (0.0, 10.2) |
| Nonpregnant WRA (15–49 y) | 752 | 0.3 (0.0, 0.8) | 181 | 1.9 (0.0, 4.4) | 94 | 0.010 ± 0.001 | 94 | 1.3 (0.0, 2.9) | 94 | 0.9 (0.0, 2.3) |
| Men | 219 | 0.1 (0.0, 0.3) | NA | NA | NA | NA | NA | NA | NA | NA |
RBP and MRDR results adapted with permission from the 2015–2016 Malawi micronutrient survey report (19). Serum retinol results adapted with permission from the Malawi Micronutrient Survey 2015–2016 Addendum: Additional Vitamin A Biomarker Results, February 2020 (35). MDHS, Malawi Demographic and Health Survey; MNS, Malawi National Micronutrient Survey; MRDR, modified relative dose response; NA, not applicable; PSC, preschool age children; RBP, retinol binding protein; SAC, school age children; WRA, women of reproductive age.
RBP equivalent to 0.7 µmol retinol/L was calculated using linear regression to estimate a survey-specific retinol binding protein cutoff to reflect vitamin A deficiency. Retinol binding protein concentrations for women and children were not adjusted for inflammation in the 2015–2016 Malawi micronutrient survey report, although guidance suggests that child concentrations be adjusted. The decision not to adjust for inflammation was based on the prevalence of deficiency being very low. There were 5 PSC [weighted estimate of 0.4 (0.0–0.9)] and no SAC with inflammation adjusted RBP <0.46 µmol/L.
The serum retinol (fasting and nonfasting), MRDR (fasting only), and serum retinyl esters (fasting only) were collected from a random subsample designed to be representative at the national level.
The 2015–2016 serum retinol was inflammation-adjusted using the BRINDA regression method for children but was not inflammation-adjusted for women (24). No other biomarkers in the table were adjusted for inflammation.
VAD, defined as an MRDR ratio ≥0.060 (2) among a random subsample, identified no VAD among children; 1 nonpregnant WRA had an elevated MRDR (ratio = 0.14). The mean ± SE MRDR concentration was 0.018 ± 0.001, 0.011 ± 0.001 and 0.010 ± 0.001 for PSC, SAC, and nonpregnant WRA, respectively.
Using the cutoff of >10% serum retinyl esters in circulation, the prevalence of PSC, SAC, and WRA with >10% retinyl esters in circulation was 1.7% (95% CI: 0.0, 4.1), 4.9% (0.0, 10.2), and 0.9% (0.0, 2.3), respectively (Table 3). Using a more sensitive cutoff for probable elevated vitamin A (serum retinyl esters in circulation >5% for children and >7.5% for adults), 18.0% (95% CI: 6.4, 29.6) of fasted PSC and 18.8% (7.2, 30.5) of fasted SAC had circulating esters >5% of total vitamin A in circulation (Table 3). The prevalence of fasted nonpregnant WRA with circulating esters >7.5% of total vitamin A in circulation was 1.3% (95% CI: 0.0, 2.9) (Table 3). The nonfasted subsample of women and children analyzed for retinyl esters had higher concentrations of retinyl esters in circulation (Supplementary Table 1). Weighted median (quartile 1, quartile 3) retinyl ester concentrations were 0.03 (0.01, 0.05) µmol/L among fasted PSC, and 0.04 (0.02, 0.08) µmol/L among nonfasted PSC. Similarly, the median (quartile 1, quartile 3) concentrations were 0.04 (0.02, 0.06) µmol/L for fasted SAC and 0.05 (0.02, 0.08) µmol/L for nonfasted SAC; and 0.02 (0.01, 0.05) µmol/L for fasted nonpregnant WRA and 0.04 (0.02, 0.07) µmol/L for nonfasted nonpregnant WRA (Supplementary Table 1).
Carotenoid data provided quantitative evidence of recent consumption of vitamin A-rich fruits and vegetables among PSC, SAC, and WRA. The median (quartile 1, quartile 3) concentration of serum lycopene was 0.02 (0.004, 0.03) µmol/L for PSC, 0.03 (0.00, 0.06) µmol/L for SAC, and 0.04 (0.02, 0.07) µmol/L for WRA (Table 4); of serum α-carotene was 0.01 (0.01, 0.02) µmol/L for PSC, 0.03 (0.02, 0.05) µmol/L for SAC, and 0.03 (0.02, 0.05) µmol/L for WRA; and of serum β-carotene was 0.24 (0.12, 0.48) µmol/L for PSC, 0.75 (0.42, 1.26) µmol/L for SAC, and 0.69 (0.38, 1.15) µmol/L for WRA. The median (quartile 1, quartile 3) concentration of serum lutein and zeaxanthin combined was 0.46 (0.29, 0.72) µmol/L for PSC, 0.66 (0.40, 1.09) µmol/L for SAC, and 0.65 (0.38, 1.04) µmol/L for WRA; and of serum β-cryptoxanthin was 0.08 (0.05, 0.14) µmol/L for PSC, 0.13 (0.08, 0.21) µmol/L for SAC, and 0.12 (0.08, 0.17) µmol/L for WRA (Table 4).
TABLE 4.
Serum carotenoids and additional vitamin A indicators from a representative subsample of women and children in the 2015–2016 Malawi Micronutrient Survey1
| PSC (n = 171)1 | SAC (n = 178)1 | Nonpregnant WRA (n = 191)1 | ||||
|---|---|---|---|---|---|---|
| Median (Q1, Q3) | Min, Max | Median (Q1, Q3) | Min, Max | Median (Q1, Q3) | Min, Max | |
| Retinol, µmol/L | 1.0 (0.8, 1.2) | 0.4, 1.9 | 1.2 (0.9, 1.4) | 0.4, 2.6 | 1.6 (1.3, 1.9) | 0.4, 2.9 |
| Retinyl esters among fasted, µmol/L | 0.03 (0.01, 0.05) | 0.0, 0.3 | 0.04 (0.02, 0.06) | 0.0, 0.3 | 0.02 (0.01, 0.05) | 0.0, 0.3 |
| Retinyl esters among nonfasted, µmol/L | 0.04 (0.02, 0.08) | 0.0, 0.4 | 0.05 (0.02, 0.08) | 0.0, 0.4 | 0.04 (0.02, 0.07) | 0.0, 0.4 |
| Total circulating retinol as retinyl esters among fasted, % | 2.9 (1.5, 4.7) | 0.0, 22.9 | 3.0 (1.6, 4.5) | 0.0, 16.5 | 1.6 (0.8, 3.1) | 0.0, 15.7 |
| Total circulating retinol as retinyl esters among nonfasted, % | 4.2 (1.7, 8.3) | 0.0, 35.8 | 3.5 (2.1, 5.7) | 0.0, 40.4 | 2.1 (1.3, 4.2) | 0.5, 21.2 |
| Lycopene, µmol/L | 0.02 *** (0.004, 0.03) | 0.0, 0.1 | 0.03 *** (0.0, 0.06) | 0.0, 1.2 | 0.04 *** (0.02, 0.07) | 0.0, 0.2 |
| α-carotene, µmol/L | 0.01 ** (0.01, 0.02) | 0.0, 0.8 | 0.03 ** (0.02, 0.05) | 0.0, 1.0 | 0.03 ** (0.02, 0.05) | 0.0, 1.7 |
| β-carotene, µmol/L | 0.24 *** (0.12, 0.48) | 0.0, 2.3 | 0.75 *** (0.42, 1.26) | 0.02, 4.6 | 0.69 *** (0.38, 1.15) | 0.0, 4.2 |
| Lutein and zeaxanthin, µmol/L | 0.46 *** (0.29, 0.72) | 0.03, 2.3 | 0.66 *** (0.40, 1.09) | 0.07, 2.6 | 0.65 *** (0.38, 1.04) | 0.0, 2.3 |
| β-cryptoxanthin, µmol/L | 0.08 *** (0.05, 0.14) | 0.01, 0.7 | 0.13 *** (0.08, 0.21) | 0.02, 0.7 | 0.12 *** (0.08, 0.17) | 0.0, 1.0 |
| RBP, µmol/L | 0.9 (0.7, 1.1) | 0.4, 1.9 | 1.05 (0.84, 1.26) | 0.52, 2.20 | 1.41 (1.13, 1.70) | 0.27, 2.59 |
| MRDR ratio | 0.012 (0.006, 0.021) | 0.003, 0.052 | 0.010 (0.007, 0.014) | 0.001, 0.047 | 0.008 (0.005, 0.012) | 0.002, 0.137 |
Sample size for MRDR and retinyl esters, fasted: n = 73 PSC, 80 SAC, 94 WRA and nonfasted: n = 98 PSC, 98 SAC, 97 WRA. MRDR did not include nonfasted individuals. Biomarker concentrations were compared across population groups using the median test, controlling for cluster, strata and weight. ***P < 0.0001; **P = 0.0005. BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; Q, quartile; MRDR, modified relative dose response; PSC, preschool age children; RBP, retinol binding protein; SAC, school age children; WRA, women of reproductive age.
Discussion
VAD in Malawi was very low in 2015–2016. The low prevalence of VAD appears to be a continuation from the decline in VAD observed between 2001 and 2009 (13, 14), and could be due to the new staple foods (sugar and flour) mandated to be fortified with vitamin A after 2009 (13, 14). However, the coverage and performance of maize and wheat flour fortification was not assessed in this survey, and the Global Fortification Data Exchange shows low fortification quality/compliance of flour in Malawi (31). This decline in VAD suggests effective vitamin A interventions in an environment that continues to see a high prevalence of infection and inflammation. RBP is a negative acute-phase protein and is depressed during the inflammatory response, leading to low serum retinol concentrations (2). While these findings are a public health success story, elevated concentrations of vitamin A in preschool and school-aged children were detected in the 2015–2016 MNS, raising concerns for potential unintended negative effects of excessive vitamin A interventions that have also been raised in other settings (16). These findings are supported by multiple biomarkers of vitamin A status. The primary biomarkers used in the survey to understand vitamin A status were RBP and MRDR, which suggested very low or no deficiency in all population groups (PSC, SAC, WRA, and men) and adequate liver reserves (PSC, SAC, WRA), similar to those found in the US population for well-nourished children (32). Post hoc additional laboratory analysis of serum retinol and retinyl esters further confirmed that VAD was not a public health problem in Malawi. The serum retinyl esters among fasting respondents suggested elevated vitamin A among almost 1 in 5 PSC or SAC. Carotenoid data indicated regular dietary intake of vitamin A from provitamin A-rich fruits and vegetables.
The preponderance of evidence from National surveys indicating that there has been a large decline in VAD over time in Malawi (Supplementary Table 2) suggests that vitamin A interventions have been successful. Although there has not been a formal evaluation of vitamin A interventions, the 2015–2016 MNS assessed vitamin A intervention coverage. Although not all households had food samples available when surveyed, 68.8% of households that had PSC living in them had both fortified oil and sugar available (data not shown). Although the national mean contents of 6.6 ± 1.4 mg RE/kg in oil and 6.1 ± 0.7 mg RE/kg in sugar were lower (oil) than those set for standards at the factory level or at the lower end of the range (sugar); those mean contents equate to 24.4 µg RE/teaspoon (4 g) sugar and 31.0 µg RE/teaspoon (4.7 g) oil. Vitamin A fortification of cooking oil began in 2000, and vitamin A fortification of sugar began in 2012. In 2009, the MNS report indicated that nearly half of women reported cooking with a brand of oil that was fortified with vitamin A (13) and a decline in VAD was already underway. Vitamin A-fortified oil and sugar are 2 of many interventions, in addition to high-dose VAS, biofortified orange-flesh sweet potatoes, multiple micronutrient powders, promotion of breastfeeding, and dietary diversification, that deliver vitamin A to the Malawian population. The 2015–2016 MDHS indicated that 79% of children aged 6–23 mo consumed foods rich in vitamin A the day prior to the interview (33).
Carotenoids provide a biomarker of dietary intake of plant-based foods, and include α-carotene, β-carotene, and lutein among others. Although carotenoid data are not often available in population-based surveys, they were assessed to estimate the intake of provitamin A-rich fruit and vegetable consumption among women and children. Median β-carotene concentration among PSC was similar to that of a US population aged 6–16 y, with a median concentration of 0.25 µmol/L, while SAC in Malawi had median β-carotene above the 95th percentile of US children (34). Median lutein and zeaxanthin concentrations in Malawi were at the >85th percentile for PSC and >95th percentile for SAC compared with US children (34). The median concentrations of α-carotene and β-cryptoxanthin were below the median concentrations among US children (34). The US concentrations were also used as a yard stick to compare a Zambian population of preschool children that had 16% elevated retinyl esters (>5% of total vitamin A) (10). In Zambian children, 13% had total carotenoid concentration >3.7 µmol/L, which in combination with elevated retinyl esters and saturated RBP was suggestive of elevated vitamin A liver stores (10).
There are numerous challenges when interpreting RBP and retinol. Although serum retinol measured by HPLC is the recommended biomarker for assessing VAD in populations (25), RBP is more easily measured with the ELISA technique. However, the physiologic 1:1 molar ratio of RBP:retinol is not consistent across populations. Unbound apo-RBP has been reported in circulation among adults with obesity (36). However, the Malawian population did not have a high prevalence of obesity in the 2015–2016 survey. An alternate explanation of the high retinol-to-RBP ratio that was observed in the 2015–2016 survey could be analytic error if high retinol concentrations were extrapolated above the standard curve from the HPLC. Within the 2 most recent national micronutrient surveys in Malawi, the RBP–retinol relation changed to the extent that the calculated cutoff varied from 0.78 µmol/L (2009) to 0.46 µmol/L (2015–2016) (13, 14). The same laboratory analyzed the RBP for both surveys, while different laboratories analyzed the retinol for each survey. Given the large deviation in the survey-specific RBP cutoff of 0.46 µmol/L from the 0.7 µmol/L retinol, we relied on the triangulation of biomarkers to make an assessment about VAD in the 2015–2016 survey. The inflammation-adjusted retinol, retinyl esters, MRDR, and carotenoid data provided the evidence to make a definitive statement about VAD that would have been challenging given RBP alone.
The influence of inflammation on vitamin A biomarkers, i.e., RBP and retinol, also makes vitamin A assessment challenging (37). Although there is no WHO recommendation on how to adjust RBP or retinol for inflammation, we applied the BRINDA regression approach to adjust RBP and retinol concentrations for PSC and SAC (24), and we present adjusted as well as unadjusted prevalence estimates (Supplementary Table 3). Among the subsample in which retinyl esters were assessed, the prevalences of inflammation-adjusted retinol <0.7 µmol/L were 1.8 (95% CI: 0.0, 4.6) and 4.4 (0.0, 9.7) for PSC (n = 171) and SAC (n = 178), respectively. The BRINDA recommendation is not to correct retinol or RBP for inflammation among WRA, based on the weak and inconsistent relation between inflammatory biomarkers and retinol and RBP (24); the prevalence of retinol <0.7 µmol/L was 1.9 (95% CI: 0.0, 4.4) for nonpregnant WRA (n = 181). The WHO uses categories of VAD prevalence to define a public health problem that is based on noninflammation adjusted retinol <0.7 µmol/L (25). However, there are critical gaps in WHO guidance on how to interpret retinol (and RBP) in the context of inflammation and how to define a public health problem using inflammation-adjusted data.
Defining elevated vitamin A using retinyl esters in circulation is challenging because there is no global guidance nor has the vitamin A community reached a consensus regarding cutoffs (2). We chose to use a more sensitive cutoff for probable elevated vitamin A (>5% or >7.5% serum retinyl esters in circulation) than what has been used in some publications (>10% serum retinyl esters in circulation) in order to identify all individuals at risk. Published work from national surveys in the US used a cutoff of >10% of total vitamin A as retinyl esters to reflect excess retinol stores and potential toxicity and using this cutoff identified a high prevalence of adults with elevated esters (37, 38). However, the presence of elevated esters was not associated with the hypothesized downstream negative health consequences from excess vitamin A in the US adult population (38, 39). More recent assessment of US adults postmortem suggested that >7.5% of total vitamin A as retinyl esters was associated with high liver retinol and abnormal liver histology (26). In children, more recent work in Zambia and South Africa suggested that levels of circulating retinyl esters >5% in circulation may not be sensitive enough to detect total liver reserves ≥1 µmol/g liver because more than half of children in the Zambia and South Africa studies had ≥1 µmol/g liver, while 16% (Zambia) or 5% (South Africa) had elevated retinyl esters, defined as >5% in circulation (10, 11).
The strength of this study is the use of multiple biomarkers for vitamin A collected simultaneously, including RBP, MRDR, retinol, retinyl esters, and carotenoids, which allowed for triangulation of biomarker data alongside descriptions of vitamin A-specific programs in a national survey. A purposeful decision was made to ensure the availability of adequate biospecimens and budgetary resources to assess retinyl esters given the expectation in the 2015–2016 survey planning phase that VAD may be very low. Countries that have multiple vitamin A interventions and plan to assess vitamin A may be best suited to assess retinol, retinyl esters, and carotenoids in addition to MRDR or RBP to enable a similar triangulation of data. An additional strength is the assessment of vitamin A status across multiple population groups (children, women, and men). However, the variation in the primary vitamin A indicator to define deficiency across years precluded a trend analysis. Moreover, data on inflammation were not available in 2001. Another limitation was self-reported fasting status in the 2015–2016 MNS. Increased retinyl esters may be in circulation for 3–5 h postprandially (2), and the survey protocol followed a 2-h recommendation for fasting before taking the blood sample for MRDR. Therefore, some increase in circulating retinyl esters may be due to recent dietary consumption, especially when considering breastfed children that may be nursing more frequently than every 2 h.
Although the 2015–2016 Malawi national survey provided evidence that VAD is no longer a public health problem, the new challenge facing policy makers is to determine the optimal mix of interventions in Malawi. Future assessments of vitamin A in Malawi may benefit from collecting data on the purchasing and consumption patterns of industrially fortified vitamin A staple foods within the household, complemented by the regulatory monitoring data from vitamin A fortification programs. Excess vitamin A in children suggests that universal VAS among preschool children may need to be reconsidered in Malawi.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate the survey participants, and the dedication the micronutrient survey field staff and coordinators who ensured the data were collected and managed according to the protocol.
The authors’ contribution were as follows—AMW, ECR, CM, BK, FP, DDK, VO, KT, PSS: designed and conducted the research; JS, SAT: contributed to the laboratory analysis and interpretation of results; all authors: contributed to the final version of the manuscript; AMW, PSS: wrote the paper; AMW: had full access to all data and final responsibility for final content; and all authors: read and approved the final manuscript. Financial support for the Malawi Micronutrient Survey was provided by the government of Malawi, the United States Agency for International Development (USAID), the United Nations Children's Fund (UNICEF), Irish Aid, the World Bank, and the Emory Global Health Institute. The Centers for Disease Control and Prevention provided in kind technical assistance. ECR received salary support from the NIH Fogarty International Center (grant numbers R25TW09337 and D43TW009337).
Author disclosures: The authors report no conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Notes
Supported by the Government of Malawi, USAID, UNICEF, Irish Aid, the World Bank, and Emory University
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AGP, α1-acid glycoprotein; APP, acute phase protein; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; CHSU, Community Health Services Unit; CRP, C-reactive protein; LMIC, low- and middle-income country; MDHS, Malawi Demographic and Health Survey; MNS, Malawi National Micronutrient Survey; MRDR, modified relative dose response; K2EDTA, potassium ethylenediaminetetraacetic acid; PSC, preschool age children; RBP, retinol binding protein; RE, retinol equivalents; SAC, school age children; VAD, vitamin A deficiency; VAS, vitamin A supplementation; WRA, women of reproductive age
Contributor Information
Anne M Williams, McKing Consulting Corporation, Atlanta, GA USA; Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA; Division of Nutrition, Physical Activity and Obesity, US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Sherry A Tanumihardjo, Department of Nutritional Sciences, University of Wisconsin-Madison, Madison, WI, USA.
Elizabeth C Rhodes, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Carine Mapango, Division of Nutrition, Physical Activity and Obesity, US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Benson Kazembe, United Nations Children's Fund, UNICEF Malawi, Lilongwe, Malawi.
Felix Phiri, Department of Nutrition, HIV and AIDS, Ministry of Health, Lilongwe, Malawi.
Dalitso D Kang'ombe, Department of Nutrition, HIV and AIDS, Ministry of Health, Lilongwe, Malawi.
Jesse Sheftel, Department of Nutritional Sciences, University of Wisconsin-Madison, Madison, WI, USA.
Violet Orchardson, United States Agency for International Development, USAID, Malawi, Lilongwe, Malawi.
Katie Tripp, Division of Nutrition, Physical Activity and Obesity, US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
Parminder S Suchdev, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA; Division of Nutrition, Physical Activity and Obesity, US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA; Department of Pediatrics and Emory Global Health Institute, Emory University, Atlanta, GA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval directed to corresponding author.
References
- 1. Sommer A, Davidson FR.. Assessment and control of vitamin A deficiency: the Annecy Accords. J Nutr. 2002;132(9 Suppl):2845S–50S. [DOI] [PubMed] [Google Scholar]
- 2. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr. 2016;146(9):1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . Global prevalence of vitamin A deficiency in populations at risk 1995–2005. Geneva: World Health Organization; 2009. [Google Scholar]
- 4. WHO . Guideline: vitamin A supplementation in infants and children 6–59 months of age. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 5. United Nations Children's Fund (UNICEF) global nutrition database, 2020; [Internet]. Available from: https://data.unicef.org/topic/nutrition/vitamin-a-deficiency/, accessed October 21, 2020. [Google Scholar]
- 6. WHO, UNICEF, IVACG Task Force . Vitamin A supplements: a guide to their use in the treatment and prevention of vitamin A deficiency and xeropthalmia, 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 7. Klemm RD, Palmer AC, Greig A, Engle-Stone R, Dalmiya N. A changing landscape for vitamin A programs: implications for optimal intervention packages, program monitoring, and safety. Food Nutr Bull. 2016;37(2 Suppl):S75–86. [DOI] [PubMed] [Google Scholar]
- 8. Tanumihardjo SA, Ball AM, Kaliwile C, Pixley KV. The research and implementation continuum of biofortified sweet potato and maize in Africa. Ann NY Acad Sci. 2017;1390(1):88–103. [DOI] [PubMed] [Google Scholar]
- 9. Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83(2):191–201. [DOI] [PubMed] [Google Scholar]
- 10. Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory JF, Tanumihardjo SA. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am J Clin Nutr. 2015;102(2):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Stuijvenberg ME, Dhansay MA, Nel J, Suri D, Grahn M, Davis CR, Tanumihardjo SA. South African preschool children habitually consuming sheep liver and exposed to vitamin A supplementation and fortification have hypervitaminotic A liver stores: a cohort study. Am J Clin Nutr. 2019;110(1):91–101. [DOI] [PubMed] [Google Scholar]
- 12. Wirth JP, Petry N, Tanumihardjo SA, Rogers LM, McLean E, Greig A, Garrett GS, Klemm RDW, Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin a deficiency. Nutrients. 2017;9(3):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Department of Nutrition HIV and AIDS, Ministry of Health (MOH), National Statistical Office (NSO), United Nations Children's Fund (UNICEF), U.S. Centers for Disease Control and Prevention (CDC) . A report for the National Micronutrient Survey 2009. Atlanta, GA, USA; 2011. [Google Scholar]
- 14. United Nations Children's Fund (UNICEF), US Centers for Disease Control and Prevention (CDC) . Report of the Malawi National Micronutrient Survey 2001. Atlanta, GA, USA; 2003. [Google Scholar]
- 15. Government of Malawi Department of Nutrition HIV and AIDS . National Multi-Sector Nutrition Strategic Plan 2018–2022. Malawi; 2018; [Internet]. Available from: https://www.fantaproject.org/sites/default/files/resources/Malawi-National-Nutrition-Strategic%20Plan-2018-2022.pdf, accessed Oct 16, 2020. [Google Scholar]
- 16. Tanumihardjo SA, Kaliwile C, Boy E, Dhansay MA, van Stuijvenberg ME. Overlapping vitamin A interventions in the United States, Guatemala, Zambia, and South Africa: case studies. Ann NY Acad Sci. 2019;1446(1):102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Statistical Office (NSO), Community Health Sciences Unit (CHSU) [Malawi], U.S. Centers for Disease Control and Prevention (CDC), and EmoryUniversity. 2017 . Malawi Micronutrient Survey 2015–16. Atlanta, GA, USA: NSO, CHSU, CDC and Emory University; [Internet]. Available from: https://dhsprogram.com/pubs/pdf/FR319/FR319.m.final.pdf, accessed Oct 16. 2020. [Google Scholar]
- 18. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134(11):3127–32. [DOI] [PubMed] [Google Scholar]
- 19. Gannon BM, Valentine AR, Davis CR, Howe JA, Tanumihardjo SA. Duration of retinol isotope dilution studies with compartmental modeling affects model complexity, kinetic parameters, and calculated vitamin A stores in US women. J Nutr. 2018;148(8):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deitchler M, Ballard T, Swindale A, Coates J. Validation of a measure of household hunger for cross cultural use. Food and Nutrition Technical Assistance II Project (FANTA-2). Washington, D.C. 2010 [Internet]. Available from: https://www.fantaproject.org/sites/default/files/resources/HHS_Validation_Report_May2010_0.pdf, accessed Oct 16, 2020.
- 21. Deitchler M, Ballard T, Swindale A, Coates J. Introducing a simple measure of household hunger for cross-cultural use. In: Food and Nutrition Technical Assistance II Project. Washington, D.C. 2011 [Internet]. Available from: https://www.fantaproject.org/sites/default/files/resources/TN12-HHS-Feb2011.pdf, accessed Oct 16, 2020.
- 22. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJet al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO . WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Geneva: World Health Organization; 2020. [PubMed] [Google Scholar]
- 24. Larson LM, Guo J, Williams AM, Young MF, Ismaily S, Addo OY, Thurnham D, Tanumihardjo SA, Suchdev PS, Northrop-Clewes CA. Approaches to assess vitamin A status in settings of inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Nutrients. 2018;10(8):1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. WHO . Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: World Health Organization; 2011. [Google Scholar]
- 26. Olsen K, Suri DJ, Davis C, Sheftel J, Nishimoto K, Yamaoka Y, Toya Y, Welham NV, Tanumihardjo SA. Serum retinyl esters are positively correlated with analyzed total liver vitamin A reserves collected from US adults at time of death. Am J Clin Nutr. 2018;108(5):997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Government of Malawi . The Malawi Vulnerability Assessment Committee National Food Security Forecast, April 2016 to March 2017. . Lilongwe (Malawi): Government of Malawi; 2015; [Internet]. Available from: https://www.wfp.org/publications/malawi-national-food-and-nutrition-security-forecast-april-2016-march-2017, accessed Oct. 16, 2020. [Google Scholar]
- 28. UNICEF . Malawi Humanitarian Situation Report. Lilongwe (Malawi: ); 2016; [Internet]. Available from: https://reliefweb.int/report/malawi/unicef-malawi-humanitarian-situation-report- 2-2-march-2016, accessed Oct 16, 2020. [Google Scholar]
- 29. WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 30. WHO . Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. VMNIS, ed. WHO/NMH/NHD/MNM/11.1 ed. Geneva: World Health Organization; 2011. [Google Scholar]
- 31. Global Fortification Data Exchange. [Internet]. Available from: https://fortificationdata.org/country-fortification-dashboard/?alpha3_code=MWI&lang=en, accessed Nov 30, 2020. [Google Scholar]
- 32. Tanumihardjo SA, Koellner PG, Olson JA. The modified relative-dose-response assay as an indicator of vitamin A status in a population of well-nourished American children. Am J Clin Nutr. 1990;52(6):1064–7. [DOI] [PubMed] [Google Scholar]
- 33. ICF International, National Statistical Office . Malawi Demographic and Health Survey 2015–16. Calverton, Maryland; 2017; [Internet]. Available from: https://dhsprogram.com/pubs/pdf/FR319/FR319.pdf, accessed Oct. 16, 2020. [Google Scholar]
- 34. Ford ES, Gillespie C, Ballew C, Sowell A, Mannino DM. Serum carotenoid concentrations in US children and adolescents. Am J Clin Nutr. 2002;76(4):818–27. [DOI] [PubMed] [Google Scholar]
- 35. National Statistics Office (NSO), Community Health Sciences Unit (CHSU, Ministry of Health, Malawi), Centers for Disease Control and Prevention (CDC), and Emory University . 2020 Malawi Micronutrient Survey 2015–16 Addendum: Additional Vitamin A Biomarker Results. Atlanta, GA, USA: NSO, CHSU, CDC and Emory University, [Google Scholar]
- 36. Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood). 2008;233(10):1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams AM, Ladva CN, Leon JS, Lopman BA, Tangpricha V, Whitehead RD, Armitage AE, Wray K, Morovat A, Pasricha Set al. Changes in micronutrient and inflammation serum biomarker concentrations after a norovirus human challenge. Am J Clin Nutr. 2019;110(6):1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballew C, Bowman BA, Russell RM, Sowell AL, Gillespie C. Serum retinyl esters are not associated with biochemical markers of liver dysfunction in adult participants in the third National Health and Nutrition Examination Survey (NHANES III), 1988—1994. Am J Clin Nutr. 2001;73(5):934–40. [DOI] [PubMed] [Google Scholar]
- 39. Ballew C, Galuska D, Gillespie C. High serum retinyl esters are not associated with reduced bone mineral density in the Third National Health And Nutrition Examination Survey, 1988–1994. J Bone Miner Res. 2001;16(12):2306–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval directed to corresponding author.