Abstract
Anti-epidermal growth factor receptor (EGFR) efficacy in patients with microsatellite instability (MSI) metastatic colorectal cancer (mCRC) according to sporadic vs familial origin is unknown. We retrospectively analyzed 128 patients with MSI mCRC treated with first-line chemotherapy ± anti-EGFR. Among them, 61 and 67 patients were respectively categorized as familial and sporadic based on mismatch repair protein immunostaining, BRAF mutational status, and MLH1 promoter methylation status. We observed that addition of anti-EGFR to chemotherapy was associated with a statistically significant improvement of progression-free survival for familial (median = 5.0 vs 10.2 months, hazard ratio [HR] = 0.47, 95% confidence interval [CI] = 0.23 to 0.94; P = .03) but not for sporadic (median = 4.4 vs 5.4 months, HR = 0.80, 95% CI = 0.39 to 1.60; P = .52) MSI mCRC patients. In multivariate analysis, the survival benefit of adding anti-EGFR to chemotherapy remained statistically significant for familial MSI cases (P = .04). These findings deserve to be confirmed in a prospective study and could help decision making in MSI mCRC without access or resistant to immunotherapy.
In colorectal cancer (CRC), DNA microsatellite instability (MSI) or deficient mismatch repair (dMMR) occurs in a familial context from a germline mutation in one of MMR genes (MLH1, MSH2, MSH6, or PMS2) known as Lynch syndrome, or in sporadic cases, by an epigenetic inactivation of MLH1 gene because of the hypermethylation of its promoter (1). The activating somatic V600E mutation in BRAF is frequent (50%-70%) in sporadic MSI tumors but absent (or exceptional) in familial MSI tumors (2).
In metastatic CRC (mCRC), dMMR/MSI is relatively uncommon (<5%) (3,4) but has been shown to be a predictive marker of response to immune checkpoint inhibitors (ICI) (anti-PD-1 alone or combined with anti-CTLA-4) (5,6). However, approximately 30%-40% of MSI mCRC patients do not respond to ICI or will develop resistance to ICI and need alternative treatment options (5,6). The data regarding the effect of chemotherapy +/- targeted therapies in MSI CRC are very scarce, but anti–epidermal growth factor receptor (EGFR) therapy appeared to be ineffective in some studies (7,8). In this work, we aimed to examine the survival outcomes with chemotherapy alone or combined with anti-EGFR of patients with MSI mCRC according to sporadic or familial origin.
We retrospectively included all consecutive patients with MSI/dMMR mCRC treated in first-line by chemotherapy alone or combined with anti-EGFR monoclonal antibody between 2007 and 2017 in 18 French centers. Demographic data, tumor characteristics, treatment responses, and survival were collected. The study was approved by the ethics committee Comité de Protection des Personnes Ouest III, and because of its retrospective nature with a majority of deceased patients, no patient consent was required. MMR tumor status was determined in each center by immunohistochemistry and/or by DNA MSI testing, as recommended (9,10). Methylation of MLH1 promoter and testing for RAS and BRAF (V600E) mutations were performed in genomic DNA extracted from formalin-fixed, paraffin-embedded tumor tissue, as previously described (8,11). The determination of sporadic and familial MSI tumors was based on the MMR protein immunostaining profile, BRAF mutational status, and MLH1 promoter methylation status, as recommended (12).
The efficacy of treatment was assessed by the progression-free survival (PFS) defined as the time elapsed from the beginning of first-line chemotherapy until the date of first progression or death, whichever came first. We also evaluated the overall survival (OS) defined as the time elapsed until death (all causes). Survival curves were drawn with the Kaplan-Meier method. Univariate and multivariate analyses (Cox proportional hazards model) were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). Proportional hazards assumptions were examined graphically by plotting log-minus-log of survival. Variables with P values of no more than 0.10 in univariate analyses were included in a multivariate model, and stepwise selection was then performed. To limit potential bias because of confounding parameters unbalanced between treatment arms in sporadic and familial MSI groups, the inverse probability of treatment weighting method was applied in the Cox regression model using the propensity score derived from multivariate logistic regression (Supplementary Table 2, available online). A P value of less than .05 was considered statistically significant. All statistical tests were 2-sided. All analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC).
Among the 342 patients diagnosed with MSI mCRC, we have excluded those who were not treated with first-line chemotherapy or treated with an anti-angiogenic agent in first-line and those for whom sporadic or familial MSI status was unknown (13). A total of 128 patients with MSI mCRC treated by first-line chemotherapy +/- anti-EGFR monoclonal antibody were analyzed. Among them, 67 patients were categorized as sporadic cases (BRAF mutated and/or with methylation of MLH1 promoter), including 15 treated with anti-EGFR inhibitors and 61 patients as familial cases (BRAF wild-type and unmethylated MLH1 or loss of MSH2, MSH6, and PMS2 protein expression), including 17 treated with anti-EGFR inhibitors (12). The median follow-up was 33.5 months (95% CI = 20.7 to 45). Demographic and pathological characteristics between first-line treatment (chemotherapy alone or with anti-EGFR) and dMMR mechanism (sporadic and familial MSI mCRC) are listed in Table 1. As compared with familial origin, patients with sporadic MSI mCRC were statistically significantly more likely to be older, female, and treated with 5FU alone and to have a cancer localized in the right side, with less liver metastasis, BRAF mutated, and RAS wild-type (Table 1).
Table 1.
Clinical and pathological characteristics of patients with MSI metastatic colorectal cancer treated in first-line with chemotherapy alone or combined with anti-EGFR monoclonal antibodya
| Characteristic | All MSI patients |
MSI sporadic |
MSI familial |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sporadic MSI (n=67) | Familial MSI (n=61) | P b | Chemotherapy alone (n=52) | Chemotherapy + anti-EGFR (n=15) | P b | Chemotherapy alone (n=44) | Chemotherapy + anti-EGFR (n=17) | P b | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Gender | 67 | 61 | <.001 | 52 | 15 | .33 | 44 | 17 | .79 |
| Male | 19 (28.4) | 41 (67.2) | 13 (25.0) | 6 (40.0) | 30 (68.2) | 11 (64.7) | |||
| Female | 48 (71.6) | 20 (32.8) | 39 (75.0) | 9 (60.0) | 14 (31.8) | 6 (35.3) | |||
| Age, y | 67 | 61 | <.001 | 52 | 15 | .32 | 44 | 17 | .13 |
| Median (range) | 71.1 (44.7-90.2) | 49.2 (17.3-78.3) | 71.3 (44.7-90.2) | 68.3 (49.5-85.2) | 46.7 (17.3-78.3) | 51.1 (39.1-74.5) | |||
| ECOG PS | 42 | 36 | .36 | 34 | 8 | .91 | 25 | 11 | .81 |
| 0-1 | 33 (78.6) | 25 (59.4) | 27 (79.4) | 6 (75.0) | 17 (68.0) | 8 (72.7) | |||
| ≥2 | 9 (21.4) | 11 (30.6) | 7 (20.6) | 2 (25.0) | 8 (32.0) | 3 (27.3) | |||
| Primary tumor localization | 67 | 61 | <.001 | 52 | 15 | .74 | 44 | 17 | .22 |
| Right | 58 (86.6) | 33 (54.1) | 44 (84.6) | 14 (93.3) | 21 (47.7) | 12 (70.6) | |||
| Left | 8 (11.9) | 18 (29.5) | 7 (13.5) | 1 (6.7) | 14 (31.8) | 4 (23.5) | |||
| Rectum | 1 (1.5) | 10 (16.4) | 1 (1.9) | 0 (0.0) | 9 (20.5) | 1 (5.9) | |||
| Surgery of primary tumor | 52 | 15 | 44 | 17 | |||||
| Yes | 63 (94.0) | 53 (86.9) | .17 | 51 (98.1) | 12 (80.0) | .03 | 38 (86.4) | 15 (88.2) | 1.0 |
| Synchronicity of metastases | 67 | 61 | .93 | 52 | 15 | .66 | 44 | 17 | <.01 |
| Synchronous | 39 (58.2) | 36 (59.0) | 31 (59.6) | 8 (53.3) | 31 (70.4) | 5 (29.4) | |||
| Metachronous | 28 (41.8) | 25 (41.0) | 21 (40.4) | 7 (46.7) | 13 (29.6) | 12 (70.6) | |||
| No. metastatic sites | 67 | 61 | .26 | 52 | 15 | .82 | 44 | 17 | .79 |
| 1 | 46 (68.7) | 36 (59.0) | 36 (69.2) | 10 (66.7) | 25 (56.8) | 11 (64.7) | |||
| ≥2 | 21 (31.3) | 25 (41.0) | 16 (30.8) | 5 (33.3) | 19 (43.2) | 6 (35.3) | |||
| Metastases site | 67 | 61 | 52 | 15 | 44 | 17 | |||
| Liver | 18 (26.9) | 31 (50.8) | .01 | 14 (26.9) | 4 (26.7) | 1.0 | 25 (56.8) | 6 (35.3) | .13 |
| Lung | 11 (16.4) | 6 (9.8) | .27 | 9 (17.3) | 2 (13.3) | 1.0 | 4 (9.1) | 2 (11.8) | 1.0 |
| Peritoneal | 29 (43.3) | 29 (47.5) | .63 | 23 (44.2) | 6 (40.0) | 1.0 | 21 (47.7) | 8 (47.1) | .96 |
| Lymph nodes | 26 (38.8) | 18 (29.5) | .27 | 19 (36.5) | 7 (46.7) | .48 | 10 (22.7) | 8 (47.1) | .06 |
| Differentiation | 57 | 54 | .27 | 46 | 11 | .06 | 39 | 15 | .12 |
| Well /moderate | 30 (52.6) | 34 (63.0) | 27 (58.7) | 3 (27.3) | 12 (30.8) | 8 (53.3) | |||
| Poor | 27 (47.4) | 20 (37.0) | 19 (41.3) | 8 (72.7) | 27 (69.2) | 7 (46.7) | |||
| BRAF status | 67 | 57 | <.001 | 52 | 15 | 1.0 | 40 | 17 | .57 |
| Wild-type | 17 (25.4) | 53 (93.0) | 13 (25.0) | 4 (26.7) | 38 (95.0) | 15 (88.2) | |||
| Mutated | 50 (74.6) | 4 (7.0) | 39 (75.0) | 11 (73.3) | 2 (5.0) | 2 (11.8 | |||
| KRAS exon 2 status | 67 | 55 | <.001 | 52 | 15 | .58 | 39 | 16 | <.001 |
| Wild-type | 62 (92.5) | 36 (65.4) | 47 (90.4) | 15 (100) | 20 (51.3) | 16 (100) | |||
| Mutated | 5 (7.5) | 19 (34.6) | 5 (9.6) | 0 (0.0) | 19 (48.7) | 0 (0.0) | |||
| Complete RAS status | 61 | 37 | <.001 | 49 | 12 | .59 | 26 | 11 | <.001 |
| Wild-type | 55 (90.2) | 1 6(43.2) | 43 (87.8) | 12 (100) | 6 (23.1) | 10 (90.9) | |||
| Mutated | 5 (8.8) | 21 (56.8) | 6 (12.2) | 0 (0.0) | 20 (76.9) | 1 (9.1) | |||
| Chemotherapy regimen in first-line | 67 | 61 | <.001 | 52 | 15 | <.001 | 44 | 17 | <.001 |
| 5FU monotherapy-based | 14 (20.9) | 2 (3.3) | 14 (26.9) | 0 (0.0) | 2 (4.6) | 0 (0.0) | |||
| FOLFIRI-based | 14 (20.9) | 19 (31.1) | 6 (11.6) | 8 (53.3) | 7 (15.9) | 12 (70.6) | |||
| FOLFOX-based | 38 (56.7) | 32 (52.5) | 31 (59.6) | 7 (46.7) | 28 (63.6) | 4 (23.5) | |||
| FOLFIRINOX-based | 1 (1.5) | 8 (13.1) | 1 (1.9) | 0 (0.0) | 7 (15.9) | 1 (5.9) | |||
Clinicopathological characteristics at baseline are described as continuous variables with median and range and qualitative variables with frequency and percentages. ECOG PS = Eastern Cooperative Oncology Group performance status; EGFR = epidermal growth factor receptor; 5FU = 5-fluorouracil; MSI = microsatellite instability.
Comparisons of characteristics in MSI patients between sporadic and familial and between first-line treatment with or without anti-EGFR were performed with Wilcoxon test for continuous variables and χ2 test or Fisher exact test for qualitative variables.
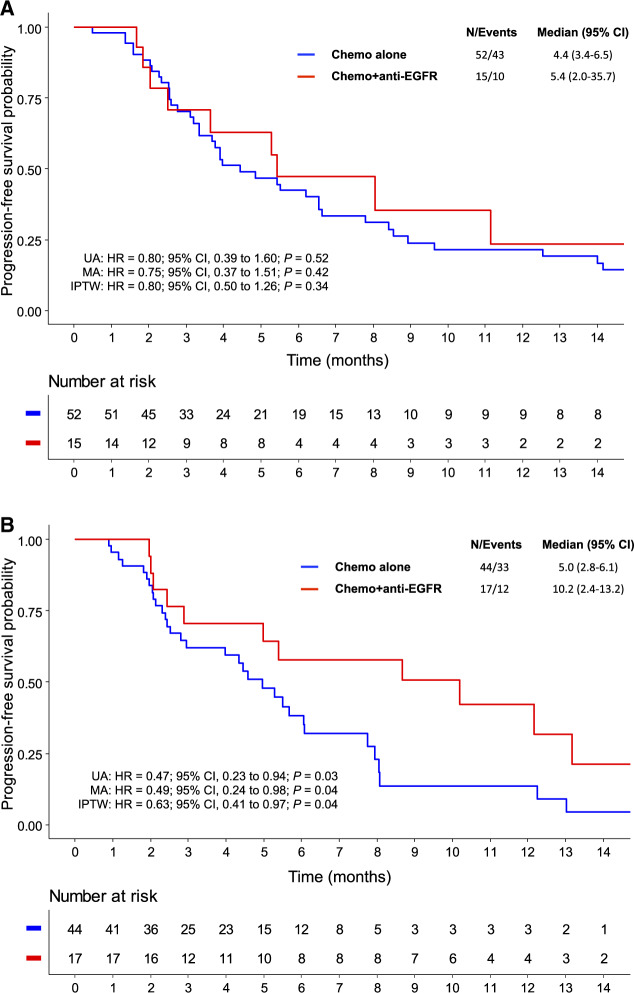
The median PFS of sporadic and familial MSI mCRC patients were 5.3 and 5.4 months, respectively (HR = 1.10, 95% CI = 0.73 to 1.64; P = .65). In patients with sporadic MSI mCRC, there was no statistically significant difference in terms of PFS between chemotherapy alone and anti-EGFR–based treatment (median = 4.4 vs 5.4 months, HR = 0.80, 95% CI = 0.39 to 1.60; P = .52) (Figure 1A). However, in patients with familial MSI mCRC, the addition of anti-EGFR to chemotherapy was associated with a statistically significant improvement of PFS (median = 5.0 vs 10.2 months, HR = 0.47, 95% CI = 0.23 to 0.94; P = .03) (Figure 1B). The survival benefit of adding anti-EGFR remained statistically significant for familial MSI cases in both multivariate analysis (HR = 0.49, 95% CI = 0.24 to 0.98; P = .04) (Supplementary Table 1, available online) and inverse probability of treatment weighting Cox analysis (HR = 0.63, 95% CI = 0.41 to 0.97; P = .04) (Supplementary Table 2, available online). For OS, the addition of anti-EGFR to first-line chemotherapy did not statistically significantly improve outcomes for patients with sporadic (HR = 0.88, 95% CI = 0.42 to 1.84; P = .73) and familial (HR = 0.93, 95% CI = 0.37 to 2.34; P = .88) MSI tumors. These OS results should be interpreted with caution because of different treatments received beyond the first-line chemotherapy, including immunotherapy.
Figure 1.
Progression-free survival in patients with sporadic or familial MSI metastatic colorectal cancer treated with first-line by chemotherapy alone or combined with anti-EGFR monoclonal antibody. For sporadic MSI cases (A), multivariate analysis was adjusted on variables with P values of no more than .10 in univariate analyses (ie, number of metastatic sites and peritoneal metastases). The IPTW was applied in the Cox regression model using the propensity score based on tumor differentiation and chemotherapy regimen. For familial MSI cases (B), multivariate analysis was adjusted on variables with P values of no more than .10 in univariate analyses (ie, gender and surgery of primary tumor). The IPTW was applied in the Cox regression model using the propensity score based on synchronicity of metastases, lymph nodes metastases, and chemotherapy regimen. All statistical tests were 2-sided. Anti-EGFR = anti-epidermal growth factor receptor; chemo = chemotherapy; CI = confidence interval; HR = hazard ratio; IPTW = inverse probability of treatment weighting; MA = multivariate analysis; MSI = microsatellite instability; UA = univariate analysis.
A post hoc analysis of the randomized CALGB/SWOG 80405 trial showed that OS from the first-line chemotherapy (FOLFOX or FOLFIRI) for MSI mCRC patients (n = 37) was shorter for those receiving cetuximab as compared with bevacizumab (median OS = 11.9 vs 30.0 months). In contrast, median OS was similar between cetuximab and bevacizumab treatment arms for MSS mCRC patients (n = 586; median OS = 30.7 vs 30.3 months) (7). The low number of MSI mCRC patients included in this study, without classification between sporadic and familial cases, did not help to evaluate the efficacy of anti-EGFR–based chemotherapy according to the dMMR mechanism. More recently, the post hoc analysis of PETACC8 and N0147 trials showed in patients with sporadic MSI stage III colon cancer (n = 255) that the addition of anti-EGFR to FOLFOX was associated with shorter DFS, whatever the BRAF mutational status (8). In our study, we observed that adding anti-EGFR to chemotherapy in sporadic MSI cases was not associated with a statistically significant improvement of PFS in BRAF wild-type (HR = 1.63, 95% CI = 0.42 to 6.38; P = .48) or in BRAF mutated (HR = 0.63, 95% CI = 0.28 to 1.44; P = .26) tumors. Some studies have suggested that the resistance to anti-EGFR could be linked to genomic alterations involved in sporadic MSI CRC, such as HER2 or MET amplification, PTEN or PIK3CA mutations, and methylator phenotype (CIMP) (14–16).
To our knowledge, our report is the largest series of patients with MSI mCRC evaluating anti-EGFR efficacy according to the dMMR mechanism. Study limitations include first the retrospective nature but with few missing data, and second the categorization of dMMR/MSI tumors into suspected familial origin that was not systematically confirmed by germline MMR mutation testing but was done using already validated algorithms (12). Our findings deserve to be confirmed in a prospective study and could help decision making in MSI mCRC patients without access or resistant to ICI.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Notes
Conflicts of interest: AZ funded by Amgen, Roche; Advisory board: Baxter, Merck Serono, MSD, Servier, Sanofi, Lilly; Honoraria: Baxter, Roche, Merck Serono, MSD, Amgen, Servier, Sanofi, Lilly; Travel: Amgen, Merck, Roche, Servier. RC: Honoraria: AMGEN, SANOFI, Servier; Travel: Sanofi. DS: Honoraria: Servier, BIONEST Partners, Ipsen, AMGEN, SANOFI; Travel: Sandoz, Ipsen, Pfizer, Servier, Novartis, AMGEN. CDLF funded by Bayer, Roche; Advisory board: Astra Zeneca, Bayer, BMS, MSD, Roche, Servier, SANOFI; Honoraria: AMGEN, Bayer, BMS, Eisai, Merck, Roche, SANOFI, Servier; Travel: AMGEN, Servier, SANOFI, Roche, BMS. TL: Advisory board: AMGEN, Servier, SANOFI, Merck Serono; Honoraria: AMGEN, Servier, SANOFI; Travel: AMGEN, Servier. TA funded by Merck, Novartis, Bayer; Advisory board: BMS, Halio DX; Honoraria: Amgen, BMS, Servier, Roche, Ipsen; Travel: Roche, Bayer, Ipsen, Hospira. MS: Advisory board: Bristol-Myers Squibb, Astellas, MSD Oncology, Sanofi; Travel: Bristol-Myers Squibb, Roche/Ventana. JT: Advisory board: Merck, SANOFI, Roche, MSD, Lilly, Celgene, Servier, Pierre Fabre, Amgen, Sirtex; Honoraria: Merck, SANOFI, Roche, MSD, Lilly, Celgene, Servier, Pierre Fabre, Amgen, Sirtex; Travel: Merck, SANOFI, Roche, MSD, Lilly, Celgene, Servier, Pierre Fabre, Amgen. TA: Advisory board: Astra Zeneca, BMS, Gritstone, Halio DX, MSD oncology, Roche, Tesaro/GSK; Servier; Honoraria: Amgen, BMS, Chugai, Pierre Fabre, Roche/Ventana, Sanofi, Servier, Yakult; Travel: Roche/Ventana, MSD Oncology, BMS. DT funded by Merck Serono, Novartis, BMS; Advisory board: BMS, Astra Zeneca, Servier, SANOFI, Roche, MSD; Honoraria: Amgen, BMS, Servier, Roche, Ipsen, SANOFI, Astra Zeneca, Novartis, Merck Serono; Travel: Roche, MSD, BMS, Amgen, Servier, SANOFI.
All other authors declare no other potential conflict(s) of interest.
Supplementary Material
References
- 1. Kawakami H, Zaanan A, Sinicrope FA.. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Domingo E, Niessen RC, Oliveira C, et al. BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene. 2005;24(24):3995-3998. [DOI] [PubMed] [Google Scholar]
- 3. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taieb J, Shi Q, Pederson L, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol. 2019;30(9):1466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. [DOI] [PubMed] [Google Scholar]
- 7. Innocenti F, Ou FS, Qu X, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaanan A, Shi Q, Taieb J, et al. Clinical outcomes in patients with colon cancer with microsatellite instability of sporadic or familial origin treated with adjuvant FOLFOX with or without cetuximab: a pooled analysis of the PETACC8 and N0147 trials. J Clin Oncol Precision Oncol. 2020;4(4):116-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boland CR, Goel A.. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073-2087.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suraweera N, Duval A, Reperant M, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123(6):1804-1811. [DOI] [PubMed] [Google Scholar]
- 11. Taieb J, Zaanan A, Le Malicot K, et al. Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. JAMA Oncol. 2016;2(5):643-653. [DOI] [PubMed] [Google Scholar]
- 12. Sinicrope FA. Lynch syndrome-associated colorectal cancer. N Engl J Med. 2018;379(8):764-773. [DOI] [PubMed] [Google Scholar]
- 13. Tougeron D, Sueur B, Zaanan A, et al. ; for the Association des Gastro‐entérologues Oncologues (AGEO). Prognosis and chemosensitivity of deficient MMR phenotype in patients with metastatic colorectal cancer: an AGEO retrospective multicenter study. Int J Cancer. 2020;147(1):285-296. [DOI] [PubMed] [Google Scholar]
- 14. Smith CG, Fisher D, Claes B, et al. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy +/- cetuximab. Clin Cancer Res. 2013;19(15):4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cremolini C, Morano F, Moretto R, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28(12):3009-3014. [DOI] [PubMed] [Google Scholar]
- 16. Gallois C, Taieb J, Le Corre D, et al. Prognostic value of methylator phenotype in stage III colon cancer treated with oxaliplatin-based adjuvant chemotherapy. Clin Cancer Res. 2018;24(19):4745-4753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.