Abstract
Background
There are limited data regarding the impact of body mass index (BMI) on outcomes in advanced breast cancer, especially in patients treated with endocrine therapy (ET) + cyclin-dependent kinase 4/6 inhibitors.
Methods
A pooled analysis of individual patient-level data from MONARCH 2 and 3 trials was performed. Patients were classified according to baseline BMI into underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2) and divided into 2 treatment groups: abemaciclib + ET vs placebo + ET. The primary endpoint was progression-free survival (PFS) according to BMI in each treatment group. Secondary endpoints were response rate, adverse events according to BMI, and loss of weight (≥5% from baseline) during treatment.
Results
This analysis included 1138 patients (757 received abemaciclib + ET and 381 placebo + ET). There was no difference in PFS between BMI categories in either group, although normal-weight patients presented a numerically higher benefit with abemaciclib + ET (Pinteraction = .07). Normal and/or underweight patients presented higher overall response rate in the abemaciclib + ET group compared with overweight and/or obese patients (49.4% vs 41.6%, odds ratio = 0.73, 95% confidence interval = 0.54 to 0.99) as well as higher neutropenia frequency (51.0% vs 40.4%, P = .004). Weight loss was more frequent in the abemaciclib + ET group (odds ratio = 3.23, 95% confidence interval = 2.09 to 5.01).
Conclusions
Adding abemaciclib to ET prolongs PFS regardless of BMI, showing that overweight or obese patients also benefit from this regimen. Our results elicit the possibility of a better effect of abemaciclib in normal and/or underweight patients compared with overweight and/or obese patients. More studies analyzing body composition parameters in patients under treatment with cyclin-dependent kinase 4/6 inhibitors may further clarify this hypothesis.
Breast cancer (BC) is the most frequent form of malignancy among women (1). Although there has been a marked evolution in treatment strategies (2,3), the identification of additional prognostic and predictive factors according to patient’s body composition represents a growing research area aiming to further improve the management of this disease (4–7). In this regard, overweight and obesity, weight gain or loss during treatments, and muscle and adipose tissue measurements have received increasing attention as potential prognostic factors as well as predictors of treatment-related toxicities (8,9).
A substantial body of evidence supports the relationship between being overweight or obese with worse outcomes in patients with early-stage BC, especially in estrogen receptor (ER)-positive BC (10). However, in the metastatic setting, little is known and most data are from retrospective and institutional case series, with conflicting results reported so far (11–16).
The current standard of care for most patients with ER-positive metastatic BC consists of a cyclin-dependent kinase (CDK) 4/6 inhibitor combined with endocrine therapy (ET) (17). Preclinical data suggest that cell-cycle regulators such as CDK 4 and 6 affect cell metabolism and the control of important metabolic processes such as adipogenesis and lipid synthesis, muscle tissue, glucose regulation, and mitochondrial function (18–22). Recent preclinical studies have unveiled CDK4 and 6 as potential targets against diet-induced obesity, suggesting that the use of CDK 4/6 inhibitors could have a direct effect on body fat mass and muscle mass (23,24).
Therefore, we hypothesized that overweight and obese patients could have different efficacy and safety outcomes (in terms of progression-free survival [PFS], response rates [RR], and incidence of adverse events [AEs]) compared with patients with normal body mass index (BMI) when treated with abemaciclib and ET. In addition, because an effect of reducing fat mass was previously reported with the use of abemaciclib in obese mouse models, we investigated whether this treatment regimen could affect body composition parameters (ie, weight loss) compared with patients treated with ET alone.
To answer these research questions, we performed a pooled, individual patient–level analysis of the MONARCH 2 and MONARCH 3 trials.
Methods
Data Source and Patient Selection
This study is a pooled post hoc analysis of individual patient-level data from the MONARCH 2 (NCT02107703) and MONARCH 3 (NCT02246621) clinical trials. Study design and results for the primary analyses of both trials were previously published (25–28). Briefly, MONARCH 2 and MONARCH 3 were randomized, placebo-controlled, phase III trials of abemaciclib combined with ET vs placebo + ET for patients with advanced, ER-positive, HER2-negative BC (25,27).
Deidentified individual patient–level data were made available by Lilly and accessible through the secure Vivli online platform from November 1, 2019, to April 20, 2020 (29). Raw data were extracted and compared with the available published data to ensure accuracy. The institutional review board at each participating site approved the MONARCH 2 and 3 protocols. All patients provided written informed consent as previously reported (25–28).
Predictor and Outcome Definition
The primary outcome of this analysis was PFS according to BMI in each treatment group. For the purpose of this analysis, patients were divided into 2 groups: patients from MONARCH 2 randomly assigned to abemaciclib + fulvestrant and patients from MONARCH 3 randomly assigned to abemaciclib + nonsteroidal aromatase inhibitor were grouped together as abemaciclib + ET, whereas patients randomly assigned to placebo + fulvestrant and placebo + nonsteroidal aromatase inhibitor were grouped together as placebo + ET. Secondary outcomes were RR, treatment-related AEs, and weight changes. PFS, RR, and AEs were defined according to the original study protocols.
Baseline BMI was calculated and recorded at study enrollment or on the first day of treatment. BMI was categorized by World Health Organization criteria: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2) (30). Patients with missing height and/or weight information for the calculation of BMI were excluded from the analysis.
For the primary outcome, patients were classified as underweight and/or normal weight (BMI < 25 kg/m2) vs overweight and/or obese (BMI ≥ 25 kg/m2). Exploratory analyses were also performed according to the 4 BMI categories separately.
Statistical Analysis
The present analysis aimed to determine the prognostic impact of baseline BMI and weight changes at 6, 12, and 18 months after random assignment in patients treated with abemaciclib + ET. Patient weight change was calculated as a percentage (by subtracting weight at 6, 12, or 18 months from random assignment to the baseline value, then dividing the result by baseline weight and finally multiplying the result by 100). According to weight change, patients were classified into 2 categories: at least 5.0% weight loss from baseline compared with less weight loss or weight gain. The 5.0% cutoff point was chosen for consistency with a prior study (31) and considering that this value reflects a clinically significant weight change that accounts for measurement errors or normal fluctuations (32).
Comparisons between BMI classes for continuous variables were assessed using t, Mann-Whitney, or Kruskall-Wallis tests; for categorical variables, χ2 or Fisher’s exact tests were used. Comparisons of PFS across BMI and weight change categories were accomplished through Kaplan-Meier curves and log-rank tests and crude and adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CIs), computed using Cox-proportional hazards regression. Patients were stratified according to the trial included and treatment group. Multivariate analyses were adjusted for the factors that differed between BMI groups. Homogeneity tests on the hazard ratios obtained in the planned subgroups were carried out to assess the possible interaction.
Because only patients who survived at least 6 months after random assignment would have information available regarding the 6-month weight change, we performed a 6-month landmark analysis when assessing the weight change as an explanatory variable; the same was performed for weight changes at 12 and 18 months.
The RR endpoint was assessed through the estimation of individual odds ratios (ORs) within each trial or within each treatment group. As per original publications, RR analysis included overall RR (ORR; proportion of patients with complete response [CR] and partial response [PR]) and clinical benefit rate (proportion of patients with CR, PR, and stable disease).
All statistical tests were 2-sided, and P less than .05 was considered statistically significant. No missing data were imputed. Statistical analyses were performed with SAS version 9.4.
Results
Patient Characteristics and Demographics
A total of 1152 patients included in the MONARCH 2 and MONARCH 3 trials (767 randomly assigned to abemaciclib + ET and 385 to placebo + ET) received at least 1 dose of study treatment. Of those, 14 were excluded because height was not recorded, leaving 1138 patients included in this analysis (Supplementary Figure 1, available online).
Of the 757 patients who received abemaciclib + ET, 24 (3.2%) were categorized as underweight, 327 (43.2%) as normal weight, 223 (29.5%) as overweight, and 183 (24.2%) as obese. Of the 381 patients who were treated with placebo + ET, the prevalence of underweight, normal weight, overweight, and obesity was 8 (2.1%), 164 (43.0%), 113 (29.7%), and 96 (25.2%), respectively (Supplementary Table 1, available online).
The presence of overweight and obesity varied statistically significantly according to geographic location, with a higher prevalence in European and North American patients compared with Asian patients. Overweight and/or obese patients were older, were postmenopausal, and more frequently had diabetes (P < .005 for all). Overall, there was no difference in previous ET exposure (P = .19) or ET sensitivity (P = .33) in all included patients according to BMI. In the abemaciclib + ET group, overweight and/or obese patients presented less visceral disease (53.5% vs 59.0%) and more frequently bone-only disease (32.3% vs 23.7%) compared with normal and/or underweight patients (P = .03). Additionally, prior aromatase inhibitor use was more frequent in overweight and/or obese than in normal and/or underweight patients receiving abemaciclib + ET (57.1% vs 48.3%, P = .02). Baseline demographics and clinical characteristics of patients with a BMI less than 25 kg/m2 and at least 25 kg/m2 in the overall population and stratified by treatment group are displayed in Table 1.
Table 1.
Baseline characteristics and demographics of patients with a BMI less than 25 kg/m2 and 25 or greater in the overall population and stratified by treatment group
| Baseline characteristics | Total, No. (%) | Abemaciclib + ET, No. (%) | Placebo + ET, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 1138) |
(n = 757) |
(n = 381) |
|||||||
| BMI < 25 | BMI ≥ 25 | Pa | BMI < 25 | BMI ≥ 25 | Pa | BMI < 25 | BMI ≥ 25 | Pa | |
| kg/m2 (n = 523) | kg/m2 (n = 615) | kg/m2 (n = 172) | kg/m2 (n = 209) | ||||||
| kg/m2 (n = 351) | kg/m2 (n = 406) | ||||||||
| Geographic region | <.001 | <.001 | <.001 | ||||||
| Asia | 241 (46.1) | 114 (18.5) | 169 (48.2) | 79 (19.5) | 72 (41.9) | 35 (16.8) | |||
| Europe | 206 (39.4) | 314 (51.1) | 132 (37.6) | 201 (49.5) | 74 (43.0) | 113 (54.1) | |||
| North America | 76 (14.5) | 187 (30.4) | 50 (14.3) | 126 (31.0) | 26 (15.1) | 61 (29.2) | |||
| Ethnicity | <.001 | <.001 | <.001 | ||||||
| American Indian or Alaska native | 12 (2.5) | 19 (3.3) | 8 (2.5) | 12 (3.2) | 4 (2.5) | 7 (3.5) | |||
| Asian | 244 (50.0) | 117 (20.5) | 171 (52.6) | 80 (21.5) | 73 (44.8) | 37 (18.7) | |||
| Black or African American | 6 (1.2) | 16 (2.8) | 3 (0.9) | 11(3.0) | 3 (1.8) | 5 (2.5) | |||
| Multiple | 1 (0.2) | 3 (0.5) | 1 (0.3) | 3 (0.8) | — | — | |||
| White | 225 (46.1) | 416 (72.9) | 142 (43.7) | 267 (71.6) | 83 (50.9) | 149 (75.3) | |||
| Missing info | 35 | 44 | 26 | 33 | 9 | 11 | |||
| Age | |||||||||
| Median (min-max) | 60 (32 to 87) | 62 (32 to 88) | .004 | 59 (32 to 87) | 62 (34 to 87) | .003 | 63 (32 to 85) | 62 (32 to 88) | .46 |
| <65 y | 330 (63.1) | 351 (57.1) | .04 | 227 (64.7) | 233 (57.4) | .04 | 103 (59.9) | 118 (56.5) | .50 |
| ≥65 y | 193 (36.9) | 264 (42.9) | 124 (35.3) | 173 (42.6) | 69 (40.1) | 91 (43.5) | |||
| ECOG | <.001 | <.001 | .02 | ||||||
| 0 | 349 (67.1) | 337 (54.8) | 231 (66.4) | 218 (53.7) | 118 (68.6) | 119 (56.9) | |||
| 1 | 171 (32.9) | 278 (45.2) | 117 (33.6) | 188 (46.3) | 54 (31.4) | 90 (43.1) | |||
| Missing info | 3 | 3 | — | ||||||
| Menopausal status | <.001 | <0.001 | .03 | ||||||
| Postmenopause | 450 (86.0) | 575 (93.7) | 303 (86.3) | 383 (94.3) | 147 (85.5) | 192 (92.3) | |||
| Pre- or perimenopause | 73 (14.0) | 39 (6.4) | 48 (13.7) | 23 (5.7) | 25 (14.5) | 16 (7.7) | |||
| Missing info | — | 1 | — | — | — | 1 | |||
| Previous ET | .19 | .94 | .03 | ||||||
| No | 113 (21.6) | 153 (24.9) | 83 (23.7) | 97 (23.9) | 30 (17.4) | 56 (26.8) | |||
| Yes | 410 (78.4) | 462 (75.1) | 268 (76.4) | 309 (76.1) | 142 (82.6) | 153 (73.2) | |||
| Sensitivity to ETb | .33 | .51 | .44 | ||||||
| Primary resistance | 85 (27.4) | 82 (24.1) | 54 (27.0) | 56 (24.2) | 31 (28.2) | 26 (23.6) | |||
| Secondary resistance | 225 (72.6) | 259 (76.0) | 146 (73.0) | 175 (76.8) | 79 (71.8) | 84 (76.4) | |||
| Prior AI | .12 | .02 | .48 | ||||||
| No | 255 (49.8) | 274 (45.1) | 178 (51.7) | 172 (42.9) | 77 (45.8) | 102 (49.5) | |||
| Yes | 257 (50.2) | 333 (54.9) | 166 (48.3) | 229 (57.1) | 91 (54.2) | 104 (50.5) | |||
| Missing info | 11 | 8 | 7 | 5 | 4 | 3 | |||
| Prior adj chemo (MONARCH 2) | .97 | .96 | .97 | ||||||
| No | 68 (25.9) | 71 (26.0) | 42 (25.3) | 48 (25.5) | 26 (26.8) | 23 (27.1) | |||
| Yes | 195 (74.1) | 202 (74.0) | 124 (74.7) | 140 (74.5) | 71 (73.2) | 62 (72.9) | |||
| Missing info | 52 | 71 | 38 | 45 | 14 | 26 | |||
| PgR status | .10 | .29 | .17 | ||||||
| Negative | 122 (23.8) | 120 (19.7) | 81 (23.6) | 82 (20.4) | 41 (24.4) | 38 (18.5) | |||
| Positive | 390 (76.2) | 488 (80.3) | 263 (76.5) | 321 (79.7) | 127 (75.6) | 167 (81.5) | |||
| Missing info | 11 | 7 | 7 | 3 | 4 | 4 | |||
| Metastatic sites | .08 | .03 | .22 | ||||||
| Bone only | 134 (25.6) | 187 (30.4) | 83 (23.7) | 131 (32.3) | 51 (29.7) | 56 (26.8) | |||
| Visceral | 296 (56.6) | 342 (55.6) | 207 (59.0) | 217 (53.5) | 89 (51.7) | 125 (59.8) | |||
| Other | 93 (17.8) | 86 (14.0) | 61 (17.4) | 58 (14.3) | 32 (18.6) | 28 (13.4) | |||
| Organs involved, No. | .74 | .19 | .13 | ||||||
| 1 | 178 (34.1) | 223 (36.3) | 118 (33.7) | 157 (38.7) | 60 (34.9) | 66 (31.6) | |||
| 2 | 148 (28.4) | 166 (27.0) | 94 (26.9) | 114 (28.1) | 54 (31.4) | 52 (24.9) | |||
| ≥3 | 196 (37.6) | 226 (36.8) | 138 (39.4) | 135 (33.3) | 58 (33.7) | 91 (43.5) | |||
| Missing info | 1 | — | 1 | — | — | — | |||
| Trial enrolled | .14 | 0.84 | .02 | ||||||
| MONARCH 2 | 315 (60.2) | 344 (55.9) | 204 (58.1) | 233 (57.4) | 111 (64.5) | 111 (53.1) | |||
| MONARCH 3 | 208 (39.8) | 271 (44.1) | 147 (41.9) | 173 (42.6) | 61 (35.5) | 98 (46.9) | |||
| Treatment regimen | .15 | .84 | .02 | ||||||
| Abemaciclib + AI | 147 (28.1) | 173 (28.1) | 147 (41.9) | 173 (42.6) | |||||
| Abemaciclib + Fulv | 204 (39.0) | 233 (37.9) | 204 (58.1) | 233 (57.4) | — | — | |||
| Placebo + AI | 61 (11.7) | 98 (15.9) | — | — | 61 (35.5) | 98 (46.9) | |||
| Placebo + Fulv | 111 (21.2) | 111 (18.1) | 111 (64.5) | 111 (53.1) | |||||
| Diabetes mellitus (medical history) | <.001 | <.001 | <.001 | ||||||
| No | 507 (96.9) | 547 (88.9) | 340 (96.9) | 364 (89.7) | 167 (97.1) | 183 (87.6) | |||
| Yes | 16 (3.1) | 68 (11.1) | 11 (3.1) | 42 (10.3) | 5 (2.9) | 26 (12.4) | |||
P values are from the χ2 test and Kruskal-Wallis test comparing categorical and continuous variables against the 2 BMI categories, respectively (all statistical tests were 2-sided). Adju = adjuvant; AI = aromatase inhibitor; BMI = body mass index; CM = concomitant medication; ECOG = Eastern Cooperative Oncology Group performance scale; ET = endocrine therapy; Fulv = fulvestrant; Prior Adj Chemo = prior adjuvant chemotherapy; PgR = progesterone receptor.
In prior ET patients, only MONARCH 2 patients.
PFS According to BMI
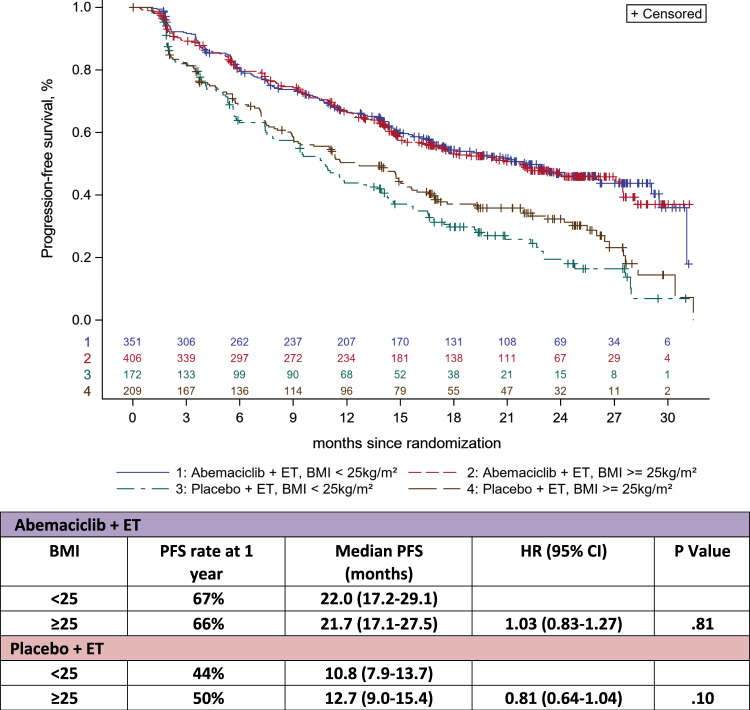
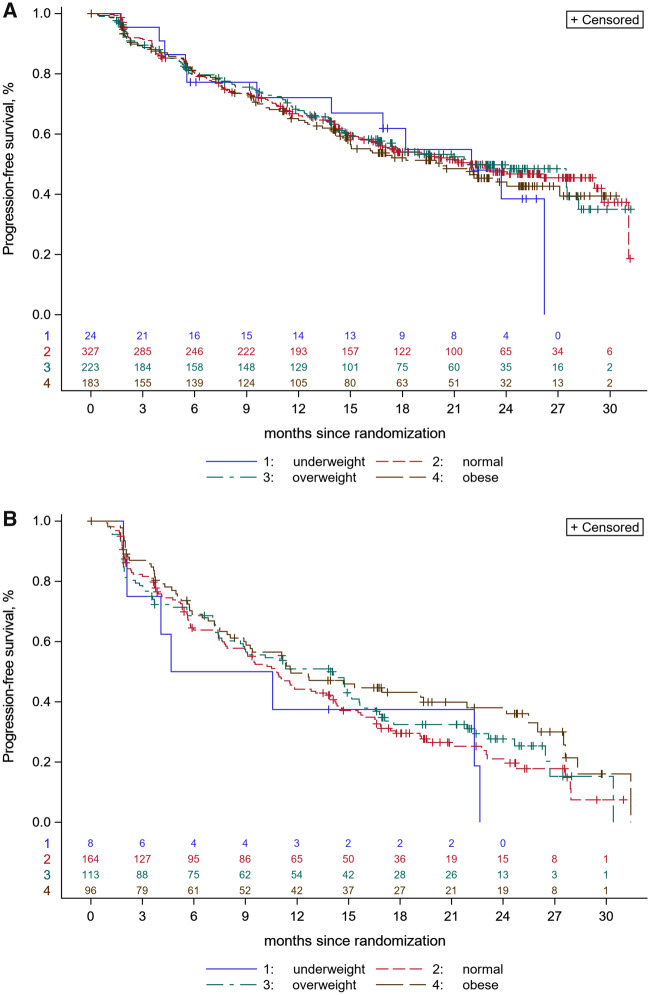
There was no statistically significant difference in PFS between patients with BMI less than 25 and BMI at least 25 kg/m2 in the abemaciclib + ET group: median PFS was 22.0 months (range = 17.2-29.1) vs 21.7 months (range = 17.1-27.5), respectively, for a hazard ratio of 1.03 (95% CI = 0.83 to 1.27, P = .81). Similar results were observed in the placebo + ET group according to BMI less than 25 kg/m2 and BMI at least 25 kg/m2: median PFS was 10.8 months (range = 7.9-13.7) vs 12.7 months (range = 9.0-15.4) for a hazard ratio of 0.81 (95% CI = 0.64 to 1.04, P = .10) (Figure 1). No statistical differences in PFS were found when categorizing the patients in the 4 BMI categories in both treatment groups (Figure 2, A and B). Patients receiving abemaciclib + ET presented a higher PFS than those receiving placebo + ET across all BMI categories.
Figure 1.
Kaplan-Meier curves for progression-free survival (PFS) according to body mass index (BMI; <25 kg/m2 vs ≥25 kg/m2). PFS according to BMI: median PFS according to 2 main BMI categories in patients treated with abemaciclib + endocrine therapy (ET) and in patients treated with placebo + ET. CI = confidence interval; HR = hazard ratio.
Figure 2.
Kaplan-Meier curves for progression-free survival (PFS) according to body mass index (BMI) categories (underweight, normal weight, overweight, and obese). A) PFS according to the 4 BMI categories in patients receiving abemaciclib + endocrine therapy (ET) (P = .91; 2-sided log-rank test). B) PFS according to the 4 BMI categories in patients receiving placebo + ET (P = .19; 2-sided log-rank test).
In an exploratory analysis, there was a numerically higher magnitude of benefit for the addition of abemaciclib to ET for patients with normal weight (21.9 vs 10.8 months, HR = 0.48, 95% CI = 0.38 to 0.61) compared with patients with overweight (22.0 vs 14.0 months, HR = 0.54, 95% CI = 0.40 to 0.73) and obesity (20.2 vs 11.6 months, HR = 0.70, 95% CI = 0.50 to 0.97, P = .03) with an Pinteraction = .07 (Supplementary Figure 2 A–C, available online).
Multivariable analysis adjusting for factors that differed between BMI categories (age, Eastern Cooperative Oncology Group performance scale, prior ET, prior aromatase inhibitor, menopausal status, number of metastatic sites, and type of ET) demonstrated no impact of BMI (<25 or ≥25 kg/m2) in PFS in both treatment groups (HR = 1.0, 95% CI = 0.81 to 1.25, P = .98 for abemaciclib + ET and HR = 0.80, 95% CI = 0.62 to 1.04, P = .09 for placebo + ET) (Supplementary Table 2, available online).
RR According to BMI
There were statistically significant differences in ORR (CR + PR) according to BMI. Among patients receiving abemaciclib + ET, ORR was statistically significantly lower in overweight and/or obese patients compared with underweight and/or normal-weight patients: 41.6% vs 49.4% (OR = 0.73, 95% CI = 0.54 to 0.99, P = .04). For patients receiving placebo + ET, the opposite was observed: ORR was higher in overweight or obese patients compared with underweight or normal-weight patients (30.7% vs 21.1%, OR = 1.65, 95% CI = 1.02 to 2.67, P = .04). The clinical benefit rate (CR + PR + stable disease) did not differ statistically according to BMI in either treatment group. Abemaciclib + ET was superior in terms of ORR and CBR compared with placebo + ET in both underweight and/or normalweight patients and in overweight and/or obese patients (P < .05) (Table 2).
Table 2.
RRs to BMI (<25 and ≥25 kg/m2) in patients receiving abemaciclib + ET and placebo + ET
| RRs | Abemaciclib + ET | Placebo + ET | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 757) |
(n = 381) |
|||||||
| BMI <25 kg/m2 | BMI ≥25 kg/m2 | OR (95% CI) | P a | BMI <25 kg/m2 | BMI <25 kg/m2 | OR (95% CI) | P a | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||||
| Best overall response | 351 (46.4) | 406 (53.6) | — | — | 172 (45.1) | 209 (54.9) | — | — |
| CR | 6 (1.8) | 17 (4.5) | — | — | 2 (1.2) | 0 (0) | — | — |
| PR | 155 (47.6) | 140 (37.1) | — | — | 33 (19.9) | 61 (30.7) | — | — |
| Stable disease | 145 (44.5) | 189 (50.1) | — | — | 104 (62.7) | 108 (54.3) | — | — |
| PD | 20 (6.1) | 31 (8.2) | — | — | 27 (16.3) | 30 15.1) | — | — |
| NE | 25 | 29 | — | — | 6 | 10 | — | — |
| ORR (CR + PR) | 161 (49.4) | 157 (41.6) | 0.73 (0.54 to 0.99) | .04 | 35 (21.1) | 61 (30.7) | 1.65 (1.02 to 2.67) | .04 |
| Clinical benefit rate (CR + PR + Stable disease) | 306 (93.9) | 346 (91.8) | 0.73 (0.41 to 1.31) | .28 | 139 (83.7) | 169 (84.9) | 1.09 (0.62 to 1.93) | .76 |
P values are from the χ2 test (all statistical tests were 2-sided). BMI = body mass index; CI = confidence interval; CR = complete response; ET = endocrine therapy; NE = nonevaluable; OR = odds ratio; ORR = overall response rate; PR = partial response; RR = response rate.
Treatment-Related Toxicities According to BMI
For patients receiving abemaciclib + ET, the incidence of neutropenia of any grade was statistically significantly lower in overweight and/or obese patients compared with underweight and/or normal-weight patients (40.4% vs 51.0%, P = .004) as well as the incidence of neutropenia grade 3 or higher neutropenia (21.7% vs 29.3%, P = .02). No differences in other toxicities were observed between the 2 BMI categories, including diarrhea. For patients under treatment with placebo + ET, toxicities were similar between the 2 BMI categories (Supplementary Table 3, available online). There were no differences regarding dose adjustment, reduction, or omission, as well as treatment discontinuation according to BMI (Supplementary Table 4, available online).
Weight Changes According to Regimen of Treatment
At the landmark of 6 months (n = 820), the rate of patients with at least a 5% weight loss was almost threefold higher in the abemaciclib + ET group compared with the placebo + ET group (27.1% vs 10.3%, OR = 3.23, 95% CI = 2.08 to 5.01, P < .001). This difference was increased at 12 months (n = 608) (26.2% vs 8.1%, OR = 4.03, 95% CI = 2.24 to 7.25, P < .001) and at 18 months (n = 400) (22.3% vs 6.4%, OR = 4.19, 95% CI = 1.86 to 9.46, P < .001) (Table 3). In the abemaciclib + ET group, weight changes were not associated with grade 3 or higher diarrhea (OR = 1.48, 95% CI = 0.86 to 2.55, P = .16) or any grade 3 or higher AEs (OR = 1.20, 95% CI = 0.81 to 1.7, P = .37).
Table 3.
Weight changes during therapy with abemaciclib + ET and placebo + ET
| Treatment group | <5% weight change (loss or increase) | ≥5% weight loss | OR (95% CI) | P a |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Weight change b/w baseline and 6 mo | ||||
| Abemaciclib + ET | 407 (72.9) | 151 (27.1) | 3.23 (2.08 to 5.01) | <.001 |
| Placebo + ET | 235 (89.7) | 27 (10.3) | ||
| Weight change b/w baseline and 12 mo | ||||
| Abemaciclib + ET | 321 (73.9) | 114 (26.2) | 4.03 (2.24 to 7.25) | <.001 |
| Placebo + ET | 159 (91.9) | 14 (8.1) | ||
| Weight change b/w baseline and 18 mo | ||||
| Abemaciclib + ET | 226 (77.7) | 65 (22.3) | 4.19 (1.86 to 9.46) | <.001 |
| Placebo + ET | 102 (93.6) | 7 (6.4) |
P values are from the χ2 test (all statistical tests were 2-sided). b/w = between; CI = confidence interval; ET = endocrine therapy; OR = odds ratio.
There was no association between PFS and weight loss at the 3 time-points in either treatment group (Supplementary Figure 3, available online).
Discussion
Obesity has long been considered a growing public health issue (33–36), and its relation with BC has been extensively studied in the early setting (10,11,37–39), whereas fewer studies investigated its impact in patients with metastatic disease (11–13,40). To our knowledge, this analysis, which pooled individual patient–level data from 2 randomized trials of abemaciclib, is the largest study to evaluate the association between BMI and outcomes with CDK 4/6 inhibitors in advanced BC. Our results show that more than one-half of the patients included in the MONARCH 2 and MONARCH 3 trials were classified as overweight or obese. This reflects the high prevalence of overweightness and obesity among patients with advanced BC who are currently living longer because of better disease control and for whom research investigating the impact of BMI during treatment is insufficient.
Our findings indicate that the combination of abemaciclib + ET is superior to placebo + ET independently of BMI categories, showing that this regimen is also effective for overweight and obese patients. The results of this post hoc analysis are aligned with results of a previous one conducted by our group in a small retrospective cohort (n = 50), in which we found no difference in PFS in patients treated with palbociclib or ribociclib + ET as first or second-line therapy for advanced BC, according to BMI (40).
Moreover it is important to mention, as previously demonstrated, that BMI alone can be a poor surrogate for obesity because of its inability to differentiate fat and lean muscle mass, precluding the diagnosis of sarcopenia as well as body fat distribution (41–43). With all the caveats of a small retrospective study, our previous work identified baseline sarcopenia (measured by computed tomography-scan body composition analysis) as a potential marker of poor prognosis in patients receiving CDK4/6 inhibitors (palbociclib or ribociclib) + ET, regardless of BMI, line of treatment, or disease burden (40). Moreover, in this previous report, sarcopenia was present in 40% of the patients despite the early course of their metastatic disease and good performance status. Additional studies in larger cohorts, preferentially with a prospective design, analyzing body composition parameters in patients receiving CDK4/6 inhibitors is needed and could lead to a deeper understanding of our findings.
In our analysis, overweight and obese patients were older, had a slightly worse performance status, and were more frequently postmenopausal compared with underweight and normal-weight patients. The prevalence of overweightness and obesity differed statistically significantly according to geographic region and ethnicity, with a lower prevalence among Asian patients compared with Europeans and North Americans. This is an interesting finding considering previous data showed that pharmacokinetic and pharmacogenomic profiles of some treatments for BC [eg, fluodropirimidines (44,45), tamoxifen (46,47), everolimus (48)] differ between Asian and non-Asians patients.
Although the efficacy of CDK4/6 inhibitors has already been established in Asian patients (49–51), previous data from the PALOMA-3 trial and MONALEESA-2 trial showed higher rates of grade 3 or greater neutropenia, an AE-specific drug class effect, in Asian patients compared with non-Asians (92% vs 58%) (49). Additionally, in a phase I study of palbociclib plus letrozole in Japanese patients, 83% had grade 3 or higher neutropenia (52). Similar results were also seen with ribociclib in the MONALEESA-2 trial in which grade 3 or higher neutropenia was documented in 71% of the Asians patients treated with ribociclib and letrozole (50). In our study, lower BMI correlated with higher rates of neutropenia, which was also seen in a recent pooled analysis of 2 trials testing palbociclib (53). Perhaps besides interethnic variabilities, differences in BMI could be one of the mechanistic reasons why Asian patients present higher neutropenia rates with these CDK 4/6 inhibitors. A possible explanation for the lower neutropenia rates in overweight or obese patients could refer to the fact that higher blood neutrophil counts might be a potential inflammatory biomarker of overweightness or obesity, as already shown in noncancer patients (54–57). Moreover, the distribution of abemaciclib in fat tissue could explain these findings, similar to what is seen with cytotoxic chemotherapy agents (58); thus, the differentiation between fat and muscle mass in these patients would be paramount to understand this phenome.
Interestingly, an additional exploratory analysis demonstrated that the magnitude of benefit with abemaciclib + ET was numerically higher in normal-weight patients compared with overweight or obese patients. Additionally, overweight or obese patients presented lower ORRs when treated with abemaciclib + ET. This difference was not present in the placebo + ET group, reflecting that the lower magnitude of benefit in obese patients is not related with endocrine resistance caused by obesity. These results may suggest a potential suboptimal dose intensity in this group, though this hypothesis would need to be carefully confirmed, preferably through a prospective trial in the setting of obesity. In addition, encouraging metastatic BC patients to maintain a healthy weight can be beneficial for those receiving abemaciclib + ET besides the well-known advantages such as the control of metabolic, cardiovascular, muscular, and degenerative joint and bone diseases.
Important metabolic functions coregulated by CDK 4/6 have been described, such as adipogenesis in white adipose tissue, insulin secretion and ß-cell function at the pancreatic level, gluconeogenesis and mitochondrial regulation in the liver, and control of insulin sensitivity and oxidative metabolism in the muscular compartment (18–22). Data also suggest that CDKs could be implicated and hyperactivated in obesity (23,24). Our previous exploratory study did not detect changes in weight and in body composition parameters during treatment with CDK 4/6 inhibitors (40). In our current analysis, loss of weight was at least 3 times more frequent in patients receiving abemaciclib compared with placebo; it was not correlated with the presence of diarrhea, suggesting a possible effect of abemaciclib on reducing fat mass as previously described in mouse models (23). Because weight loss can be interpreted as a sign of active disease by physician and patients, this finding is clinically important.
We should, however, be cautious when extrapolating our results to the entire “class” of CDK4/6 inhibitors, because palbociclib and ribociclib differ from abemaciclib in several aspects (59). Palbociclib and ribociclib present a greater lipophilicity and different binding sites compared with abemaciclib. Also, abemaciclib is more potent and presents target activity against CDK9, whereas palbociclib and ribociclib only inhibit CDK4 and CDK6. Additionally, abemaciclib may potentially cross the blood-brain barrier. Of note, there are few but consistent differences in terms of treatment-related toxicities between the 3 CDK4/6 inhibitors, including higher rates of diarrhea with abemaciclib compared with an increased incidence of neutropenia with palbociclib and ribociclib (59). For all these reasons, future studies focusing on the impact of BMI among patients treated with palbociclib and ribociclib are also warranted.
Results from this analysis should be considered as exploratory, not preplanned, and therefore warrant confirmation. The interaction test between BMI and PFS did not reach statistical significance. Moreover, as previously mentioned, BMI is not the most accurate method to assess obesity, and future studies integrating muscle and fat measures are highly desired and should be pursued. Importantly, in the this dataset, no overall survival data were yet available. Last, no data were available regarding diet and physical activity, which could influence loss of weight.
In conclusion, this pooled analysis of individual patient–level data from the MONARCH 2 and 3 trials showed that the combination of abemaciclib + ET is effective and superior to ET alone irrespective of BMI. Weight loss was more frequent for patients using abemaciclib + ET compared with placebo + ET. An apparent increased benefit was observed for normal and underweight patients than for obese patients treated with abemaciclib + ET. Additionally, lower RRs and lower rates of neutropenia were seen in overweight and obese patients. Because palbociclib, ribociclib, and abemaciclib differ in several aspects, further research regarding the use of other CDK 4/6 inhibitors in this representative subpopulation is desired. Moreover, a future study integrating body composition parameters could more precisely analyze the impact of overweight and obesity on the outcomes of patients treated with abemaciclib plus ET.
Funding
This study has received no funding.
Notes
Disclosures: MAF, LA, CD, NK: none. DE: Funding for his ESMO fellowship (2018–2019): Novartis. RC: speaker honoraria from Boehringer Ingelheim, AstraZeneca and Janssen, travel grants from AstraZeneca and Pfizer. MB: travel grant and speaker honoraria from Roche/GNE. AA: Advisory role, speaker fees and research funding for his institute from: Roche, Lilly, Amgen, EISAI, BMS, Pfizer, Novartis, MSD, Genomic Health, Ipsen, AstraZeneca, Bayer, Leo Pharma. SC: recipient of the IG 20774 grant from Fondazione Associazione Italiana Ricerca sul Cancro. Speaker bureau from Novartis and fees for advisory board from Pierre-Fabre, outside this work. M.P.: Board Member (Scientific Board): Oncolytics, Radius; Consultant (honoraria): AstraZeneca, Camel-IDS, Crescendo Biologics, Debiopharm, G1 Therapeutics, Genentech, Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Oncolytics, Periphagen, Pfizer, Roche, Seattle Genetics; Research grants to her Institute: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier, Synthon; Speakers bureau/stock ownership: none; EA: honoraria and advisory board: Roche/GNE, Novartis, Seattle Genetics; travel grants: Roche/GNE, GSK/Novartis; co-principal investigator of the LORELEI trial (NCT02273973). Research grant for his institute: Roche/GNE, Astra-Zeneca, Novartis, and Servier. NP: Fees from Eli Lilly and Novartis and travel grants from Lilly, Novartis and Pfizer. ML: advisory role for Roche, speaker honoraria from Roche, Lilly, Theramex and Takeda.
Role of the authors: DE: Visualization; Writing—original draft; Writing—review and editing. LA: Formal analysis; Methodology; Software; Validation. NP: Validation; Writing—original draft; Writing—review and editing. RC: Visualization; Writing—original draft; Writing—review and editing. CDA: Writing—original draft; Writing—review and editing. MB: Visualization; Writing—original draft; Writing—review and editing. CD: Validation; Visualization; Writing—original draft; Writing—review and editing. SDC: Conceptualization; Validation; Writing—original draft; Writing—review and editing. NK: Writing—original draft; Writing—review and editing. ML: Writing—original draft; Writing—review and editing. AA: Supervision; Writing—original draft; Writing—review and editing. MP: Supervision; Validation; Visualization; Writing—original draft; Writing—review and editing. EdA: Conceptualization; Investigation; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft; Writing—review and editing.
Acknowledgments: This manuscript is based on research using data from Lilly that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Prior presentations: Partial results of this manuscript were presented as a Poster at ESMO Breast 2020.
Data availability
This manuscript is based on research using data from Lilly that has been made available through Vivli—Data request ID: 4319 ( research proposal available at: https://vivli.org/clinical-implications-of-body-mass-index-and-weight-in-metastatic-breast-cancer-patients-receiving-abemaciclib-a-combined-individual-patient-level-data-sub-analysis-of-monarch-2-and-monarch-3-trials/). Data may be available to other researchers through submission and approval of a research proposal at the Vivli website: https://vivli.org/ and a signed data access agreement.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Prim. 2019;5(1):66. [DOI] [PubMed] [Google Scholar]
- 3. Waks AG, Winer EP.. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. [DOI] [PubMed] [Google Scholar]
- 4. Rybinska I, Agresti R, Trapani A, Tagliabue E, Triulzi T.. Adipocytes in breast cancer, the thick and the thin. Cells. 2020;9(3):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: continuous update project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183–1200. [DOI] [PubMed] [Google Scholar]
- 6. Aleixo GFP, Williams GR, Nyrop KA, Muss HB, Shachar SS.. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat. 2019;177(3):569–579. [DOI] [PubMed] [Google Scholar]
- 7. Jiralerspong S, Goodwin PJ.. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203–4216. [DOI] [PubMed] [Google Scholar]
- 8. Feliciano EMC, Chen WY, Lee V, et al. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol. 2020;6(2):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lehuédé C, Li X, Dauvillier S, et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res. 2019;21(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trestini I, Carbognin L, Monteverdi S, et al. Clinical implication of changes in body composition and weight in patients with early-stage and metastatic breast cancer. Crit Rev Oncol Hematol. 2018;129:54–66. [DOI] [PubMed] [Google Scholar]
- 12. Zewenghiel L, Lindman H, Valachis A.. Impact of body mass index on the efficacy of endocrine therapy in patients with metastatic breast cancer-a retrospective two-center cohort study. Breast. 2018;40:136–140. [DOI] [PubMed] [Google Scholar]
- 13. Pizzuti L, Natoli C, Gamucci T, et al. Anthropometric, clinical and molecular determinants of treatment outcomes in postmenopausal, hormone receptor positive metastatic breast cancer patients treated with fulvestrant: results from a real word setting. Oncotarget. 2017;8(40):69025–69037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gevorgyan A, Bregni G, Galli G, et al. Body mass index and clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer. Tumor. 2016;102(4):e11–e14. [DOI] [PubMed] [Google Scholar]
- 15. Ioannides SJ, Barlow PL, Elwood JM, Porter D.. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res Treat. 2014;147(2):237–248. [DOI] [PubMed] [Google Scholar]
- 16. Martel S, Poletto E, Ferreira AR, et al. Impact of body mass index on the clinical outcomes of patients with HER2-positive metastatic breast cancer. Breast. 2018;37:142–147. [DOI] [PubMed] [Google Scholar]
- 17. Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A.. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395(10226):817–827. [DOI] [PubMed] [Google Scholar]
- 18. Lagarrigue S, Lopez-Mejia IC, Denechaud PD, et al. CDK4 is an essential insulin effector in adipocytes. J Clin Invest. 2015;126(1):335–348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Abella A, Dubus P, Malumbres M, et al. Cdk4 promotes adipogenesis through PPARγ activation. Cell Metab. 2005;2(4):239–249. [DOI] [PubMed] [Google Scholar]
- 20. Fajas L. Re-thinking cell cycle regulators: the cross-talk with metabolism. Front Oncol. 2013;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopez-Mejia IC, Castillo-Armengol J, Lagarrigue S, Fajas L.. Role of cell cycle regulators in adipose tissue and whole body energy homeostasis. Cell Mol Life Sci. 2018;75(6):975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguilar V, Fajas L.. Cycling through metabolism. EMBO Mol Med. 2010;2(9):338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iqbal NJ, Lu Z, Liu SM, Schwartz GJ, Chua S, Zhu L.. Cyclin-dependent kinase 4 is a preclinical target for diet-induced obesity. JCI Insight. 2018;3(17):e123000. doi:10.1172/jci.insight.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hou X, Zhang Y, Li W, et al. CDK6 inhibits white to beige fat transition by suppressing RUNX1. Nat Commun. 2018;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. [DOI] [PubMed] [Google Scholar]
- 26. Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. [DOI] [PubMed] [Google Scholar]
- 28. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohmann C, Banzi R, Canham S, et al. Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open. 2017;7(12):e018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO/Europe | Nutrition—Body mass index—BMI. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed April 9, 2020.
- 31. Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML.. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst. 2015;107(12):djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. [DOI] [PubMed] [Google Scholar]
- 34. Hruby A, Hu FB.. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilding JPH, Mooney V, Pile R.. Should obesity be recognised as a disease? BMJ. 2019;366:I4258. [DOI] [PubMed] [Google Scholar]
- 36. Ayton A, Ibrahim A.. Obesity is a public health emergency. BMJ. 2019;366:l5463. doi:10.1136/bmj.l5463. [DOI] [PubMed] [Google Scholar]
- 37. Biganzoli E, Desmedt C, Fornili M, et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer. 2017;87:10–20. [DOI] [PubMed] [Google Scholar]
- 38. De Azambuja E, McCaskill-Stevens W, Francis P, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119(1):145–153. [DOI] [PubMed] [Google Scholar]
- 39. Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1(5):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franzoi MA, Vandeputte C, Eiger D, et al. Computed tomography-based analyses of baseline body composition parameters and changes in breast cancer patients under treatment with CDK 4/6 inhibitors. Breast Cancer Res Treat. 2020;181(1):199–209. [DOI] [PubMed] [Google Scholar]
- 41. Caan BJ, Cespedes Feliciano EM, Kroenke CH.. The importance of body composition in explaining the overweight paradox in cancer. Cancer Res. 2018;78(8):1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prado CM, Cristina Gonzalez M, Heymsfield SB.. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care. 2015;18(6):535–551. [DOI] [PubMed] [Google Scholar]
- 43. Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (c-scans study). Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marsh S, Collie-Duguid ESR, Li T, Liu X, McLeod HL.. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999;58(3):310–312. [DOI] [PubMed] [Google Scholar]
- 45. Haller DJ, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26(13):2118–2123. doi:10.1200/J Clin Oncol.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- 46. Lim HS, Lee HJ, Lee KS, Lee ES, Jang IJ, Ro J.. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25(25):3837–3845. [DOI] [PubMed] [Google Scholar]
- 47. Bradford LDA. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3(2):229–243. [DOI] [PubMed] [Google Scholar]
- 48. Noguchi S, Masuda N, Iwata H, et al. Efficacy of everolimus with exemestane versus exemestane alone in Asian patients with HER2-negative, hormone-receptor-positive breast cancer in BOLERO-2. Breast Cancer. 2014;21(6):703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iwata H, Im S-A, Masuda N, et al. PALOMA-3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer that progressed on prior endocrine therapy—safety and efficacy in Asian patients. J Glob Oncol. 2017;3(4):289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yap YS, Tsang LM, Blackwell KL, et al. First line ribociclib + letrozole in portemenopausal Asian women with hormone receptor-positive (HR+) human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): a subgroup analysis from MONALEESA 2. Ann Oncol. 2016;27(suppl 9):ix190–ix191.
- 51.Toi M, Huang C, Im Y, et al. MONARCH 2: abemaciclib in combination with fulvestrant in Asian women with HR+, HER2- advanced breast cancer who progressed on endocrine therapy (96O) | OncologyPRO. Ann Oncol. 2017;28(suppl 10):x26–x34.
- 52. Tamura K, Mukai H, Naito Y, et al. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016;107(6):755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ettl J, Im SA, Ro J, et al. Hematologic adverse events following palbociclib dose reduction in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: pooled analysis from randomized phase 2 and 3 studies. Breast Cancer Res. 2020;22(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu X, Su S, Wang X, et al. Obesity is associated with more activated neutrophils in African American male youth. Int J Obes. 2015;39(1):26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herishanu Y, Rogowski O, Polliack A, Marilus R.. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76(6):516–520. [DOI] [PubMed] [Google Scholar]
- 56. Yilmaz H, Ucan B, Sayki M, et al. Usefulness of the neutrophil-to-lymphocyte ratio to prediction of type 2 diabetes mellitus in morbid obesity. Diabetes Metab Syndr Clin Res Rev. 2015;9(4):299–304. [DOI] [PubMed] [Google Scholar]
- 57. Lampalo M, Ferara N, Jukić I, et al. Blood neutrophils correlate with obesity in asthmatic patients. Eur Respir J. 2019:PA4274. doi:10.1183/13993003.congress-2019.pa4274. [Google Scholar]
- 58. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553–1561. [DOI] [PubMed] [Google Scholar]
- 59. Marra A, Curigliano G.. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer. 2019;5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript is based on research using data from Lilly that has been made available through Vivli—Data request ID: 4319 ( research proposal available at: https://vivli.org/clinical-implications-of-body-mass-index-and-weight-in-metastatic-breast-cancer-patients-receiving-abemaciclib-a-combined-individual-patient-level-data-sub-analysis-of-monarch-2-and-monarch-3-trials/). Data may be available to other researchers through submission and approval of a research proposal at the Vivli website: https://vivli.org/ and a signed data access agreement.