Abstract
Objectives
We explored efficacy and safety of IVIg as first-line treatment in patients with an idiopathic inflammatory myopathy.
Methods
In this investigator-initiated phase 2 open-label study, we included 20 adults with a newly diagnosed, biopsy-proven idiopathic inflammatory myopathy, and a disease duration of less than 9 months. Patients with IBM and prior use of immunosuppressants were excluded. The standard treatment regimen consisted of IVIg (Privigen) monotherapy for 9 weeks: a loading dose (2 g/kg body weight) and two subsequent maintenance doses (1 g/kg body weight) with a 3-week interval. The primary outcome was the number of patients with at least moderate improvement on the 2016 ACR/EULAR Total Improvement Score. Secondary outcomes included time to improvement, the number of patients requiring rescue medication and serious adverse events.
Results
We included patients with DM (n = 9), immune-mediated necrotizing myopathy (n = 6), non-specific myositis/overlap myositis (n = 4) and anti-synthetase syndrome (n = 1). One patient was excluded from analyses because of minimal weakness resulting in a ceiling effect. Eight patients (8/19 = 42.0%; Clopper–Pearson 95% CI: 19.6, 64.6) had at least moderate improvement by 9 weeks. Of these, six reached improvement by 3 weeks. Seven patients required rescue medication due to insufficient efficacy and prematurely ended the study. Three serious adverse events occurred, of which one was pulmonary embolism.
Conclusion
First-line IVIg monotherapy led to at least moderate improvement in nearly half of patients with a fast clinical response in the majority of responders.
Trial registration
Netherlands Trial Register identifier, NTR6160.
Keywords: myositis and muscle disease, immunotherapy
Rheumatology key messages
First-line IVIg monotherapy was effective in nearly half of patients with idiopathic inflammatory myopathies.
Treatment response was mostly reached by 3 weeks of IVIg treatment in the responders.
Although generally safe and feasible, caution is advocated in patients with increased thrombosis risk.
Introduction
Glucocorticoids, the first-line treatment in patients with an idiopathic inflammatory myopathy (IIM), have insufficient treatment efficacy. Only a minority of patients reach complete disease remission following glucocorticoid treatment: most patients experience significant residual disability, and disease remission is achieved following a treatment duration of nearly 60 weeks on average [1–3]. Therefore, more effective treatments with a fast mode of action are urgently needed. IVIg has the potential to swiftly reduce disease activity: IVIg acts relatively fast (days–weeks) in other immune-mediated diseases and efficacy has been reported in IIM patients [4–10].
IVIg as first-line treatment in some subtypes of newly diagnosed IIM was described in two small studies, but the results were conflicting [9, 10]. Therefore, we conducted an investigator-initiated phase 2 open-label study to explore efficacy and safety of IVIg as first-line treatment in newly diagnosed IIM.
Methods
Patients
From March 2017 to January 2019, we consecutively included patients diagnosed at three referral centres for IIM in The Netherlands (Amsterdam, Rotterdam, Nijmegen).
Inclusion criteria were: biopsy-proven IIM based on the 2004 European Neuromuscular Centre (ENMC) criteria, age at least 18 years, and disease duration less than 9 months [11]. Based on the 2004 ENMC criteria we excluded diagnosis of IBM, toxic myopathy, active endocrinopathy, amyloidosis or (family history of) muscular dystrophy. Furthermore, we excluded patients with a history of thrombotic episodes within 2 years prior to enrolment, known allergic reactions or other severe reactions to any blood-derived product, known IgA deficiency and anti-IgA serum antibodies, pregnancy (wish), and conditions likely to interfere with either compliance (e.g. critically ill patients) or with the evaluation of efficacy (e.g. other severe pre-existing condition). Prior immunosuppressant treatment was also an exclusion criterion, but patients were eligible if they used prednisolone in a daily dose up to 20 mg for a maximum of 2 weeks without a clinical response; or azathioprine or methotrexate regardless of dose for a maximum of 4 weeks without a clinical response.
The study protocol was amended following the inclusion of the second patient who demonstrated a ceiling effect of the primary outcome measure. We added an inclusion criterion to define minimal disability based on personal communications with R. Aggarwal (one of the developers of this study’s primary outcome measure). Minimal disability was defined as at least 10% loss on manual muscle testing (MMT) and abnormal scores on two other core set measures (CSMs) of the International Myositis Assessment and Clinical Studies (IMACS) Group (see ‘Primary and secondary outcome measures’). Consequently, all included patients had at least a moderate degree of muscle weakness.
A predefined set of baseline data were recorded: age at onset, sex, ethnicity/ancestry, disease duration, disease subtype, and the presence of dysphagia, extramuscular manifestations, connective tissue disorder, cancer, myositis-specific antibodies and myositis-associated antibodies [12, 13]. Patients were classified in the following disease subtypes: DM, anti-synthetase syndrome, immune-mediated necrotizing myopathy or non-specific/overlap myositis [14, 15]. Serological assessment of myositis-specific antibodies and myositis-associated antibodies was performed by a commercial semi-quantitative line blot essay (Euroimmun, Lubeck, Germany), except for anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibodies, which were analysed using a commercial quantitative ELISA (Inova, San Diego, CA, USA) [16, 17]. An associated connective tissue disorder was considered present if it was diagnosed at any time during the disease. Cancer was considered disease-related if present from 3 years before the diagnosis of IIM or if cancer was diagnosed during follow-up.
The study was conducted with approval of the research protocol by the locally appointed ethics committee and in accordance with the Declaration of Helsinki. This study was registered in the Netherlands Trial Register (Netherlands Trial Register identifier NTR6160). All patients gave verbal and written informed consent prior to inclusion in the study.
Study design
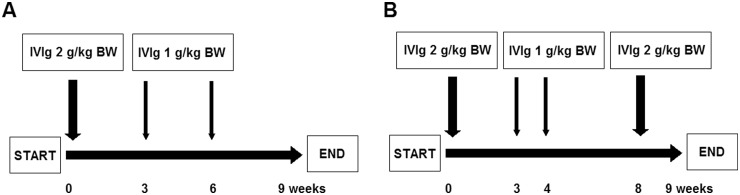
The experimental treatment consisted of IVIg (Privigen, CSL Behring, King of Prussia, PA, USA) monotherapy: a 2 g/kg body weight (BW) loading dose and two subsequent doses of 1 g/kg BW every 3 weeks. This treatment regimen was in accordance with an earlier trial investigating IVIg in patients with chronic inflammatory demyelinating polyradiculoneuropathy (Fig. 1) [18]. Loading dose (2 g/kg BW) was administered over 2–5 days depending on weight and (cardiopulmonal) comorbidity. The maintenance dose of 1 g/kg BW was administered over 1 day. Maximum dose of IVIg administered on a single day was 80 g. After the first 10 patients were enrolled in the study, the treatment protocol was amended. In particular, we observed clinical deterioration following the maintenance doses of 1 g/kg BW in three patients, in whom clinical improvement was initially seen following the loading dose of 2 g/kg BW. According to the amended protocol, patients with insufficient response (Total Improvement Score (TIS) <40, see below) by week 4, could be treated with an amended IVIg treatment regimen consisting of 2 g/kg BW every 4 weeks. This amended IVIg treatment regimen was in accordance with the treatment regimen for refractory myositis [5].
Fig. 1.
Study treatments
(A) Patients received a 2 g/kg BW loading dose of IVIg monotherapy at baseline and thereafter two 1 g/kg BW follow-up infusions every 3 weeks. (B) Patients were converted to IVIg 2 g/kg BW every 4 weeks in cases of insufficient response by week 4 defined as a Total Improvement Score of <40. The patients continued treatment after 9 weeks at the discretion of the treating physician. BW: body weight.
Primary and secondary outcome measures
The primary outcome of this study was the number of patients with at least moderate improvement, defined as at least 40 points on the 2016 ACR/EULAR TIS by 9 weeks compared with baseline [19]. The TIS is a composite, weighted outcome measure of six CSMs: physician global activity, patient global activity, MMT, HAQ, muscle enzyme activities and extramuscular disease activity based on the Myositis Disease Activity Assessment Tool (see Supplementary material, available at Rheumatology online). These CSMs are also evaluated separately as secondary outcome measures of efficacy (see below).
Secondary outcomes of efficacy included time to reach at least moderate improvement, number of patients with at least minimal improvement (TIS at least 20) and change in separate CSMs (including change in extramuscular disease activity). Furthermore, we explored change in quantitative dynamometric muscle strength of the deltoid, biceps and psoas muscles [20], and changes in dysphagia, disability and quality of life, using the following outcome measures, which have not been (sufficiently) validated in IIM patients as of yet: the Rasch modified Medical Research Council Sum Score (Rasch MRC-SS) [21], the amyotrophic lateral sclerosis severity scale-swallowing [22], the modified Rankin Scale [23] and the EuroQol Group Health Questionnaire (EQ-5D-5L; see Supplementary material, available at Rheumatology online) [24, 25].
Secondary outcomes of safety included number of (serious) adverse events [(S)AEs], number of patients with clinically relevant deterioration and/or the number of patients needing rescue medication at the discretion of the treating physician. The number of patients with clinically relevant deterioration was defined in accordance with the IMACS clinical trial design tools (see: Supplementary material, available at Rheumatology online) [26].
One investigator (J.L.) assessed primary and secondary outcomes of all patients at baseline and all subsequent study visits. Outcomes were assessed at each admission for study treatment and at 9 weeks or, if appropriate, if a premature end point was reached. Patients eligible for the amended treatment regimen underwent an additional assessment at 4 weeks.
Statistical analysis
We assumed that the proportion of patients with at least moderate improvement of at least 40 points on the TIS was 0.60. A sample size of 20 patients had an exact (Clopper–Pearson method) two-sided 95% CI with a total width of 0.448 (0.361–0.809) assuming a sample proportion of 0.60.
Patient baseline characteristics and the primary and secondary outcomes, including efficacy and safety measures, were summarized using simple descriptive statistics. Statistical uncertainty with regard to the primary outcome estimate was expressed in a two-sided 95% CI. Change scores from baseline to follow-up at 9 weeks on the continuous secondary outcome parameters were analysed using the Wilcoxon signed rank test. A two-sided P-value <0.05 was considered statistically significant. In view of the explorative nature of this pilot study we did not correct for multiple comparisons [27]. All analyses were performed in SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
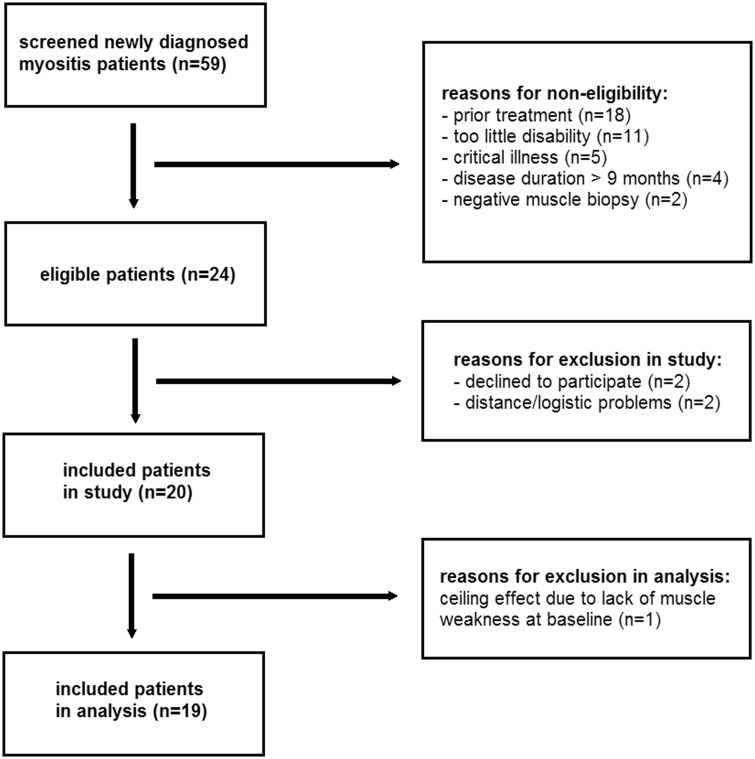
We consecutively screened 59 patients with newly diagnosed IIM and included 20 patients (Fig. 2). The most common reasons for non-eligibility were the use of prior immunosuppressants at a higher dose than predefined (n = 18), not meeting the minimal disability criterion (n = 11) and a disease duration of >9 months (n = 4). Two patients were treated with immunosuppresssants prior to study enrolment, which were stopped 1 month prior to study enrolment in both. Patient 5 had had prednisolone 20 mg/day for 5 days and patient 15 had had methotrexate for 3 weeks with a maximum dose of 20 mg/week, both without clinical benefit at time of enrolment. The second included patient was excluded from analysis. Namely, the patient’s high baseline MMT score did not allow at least moderate improvement on the TIS to reach the threshold of improvement due to a ceiling effect. Of note, the patient gained near-normal functioning following 9 weeks of IVIg, illustrated by the fact that he resumed mountain hiking again.
Fig. 2.
Schematic representation of screening and inclusion of patients
Note that patients may have more than one reason to be non-eligible.
Baseline characteristics of the 19 patients included in the analyses are summarized in Table 1. All patients had muscle weakness. Three patients had CTD-associated IIM at time of diagnosis: one patient with non-specific/overlap myositis and mixed connective tissue disease, one patient with non-specific/overlap myositis and Sjögren’s syndrome, and one patient with immune-mediated necrotizing myopathy and systemic sclerosis. No patients had cancer at time of diagnosis, but one patient with DM was diagnosed with ovarian cancer during the follow-up of the study. Ten patients had myositis-specific antibodies, two patients had myositis-associated antibodies only, seven patients did not have myositis-specific antibodies nor myositis-associated antibodies, and none of the patients were seropositive for multiple myositis-specific antibodies.
Table 1.
Baseline characteristics of 19 included patients in the analysis
| Characteristic | Patients (n = 19) | DM (n = 8) | IMNM (n = 6) | NM/OM (n = 4) | ASS (n = 1) |
|---|---|---|---|---|---|
| Age at onset, median (IQR), years | 59 (37–69) | 44 (31–61) | 67 (62–69) | 60 (35–77) | 53 |
| Females, n (%) | 12 (63) | 5 (63) | 3 (50) | 3 (75) | 1 |
| European/Caucasian ancestry, n (%) | 13 (68) | 6 (75) | 4 (67) | 2 (50) | 1 |
| Disease duration, median (IQR), months | 5 (3–6) | 5 (3–6) | 5 (3–7) | 6 (4–8) | 4 |
| Dysphagia, n (%) | 14 (74) | 6 (75) | 4 (67) | 3 (75) | 1 |
| Extramuscular disease activity, n (%) | |||||
| Skin | 11 (58) | 8 (100) | 1 (17) | 1 (25) | 1 |
| Arthritis | 7 (37) | 2 (25) | 2 (33) | 2 (50) | 1 |
| Raynaud | 3 (16) | 1 (13) | 0 (0) | 1 (25) | 1 |
| Cardiaca | 3 (16) | 0 (0) | 2 (33) | 1 (25) | 0 |
| Pulmonaryb | 2 (11) | 0 (0) | 0 (0) | 2 (50) | 0 |
| Other, i.e. subcutaneous oedema | 13 (68) | 8 (100) | 3 (50) | 1 (25) | 1 |
| Connective tissue disorder, n (%) | 3 (16) | 0 | 1 | 2 | 0 |
| Cancer, n (%) | 0 (0) | 0 | 0 | 0 | 0 |
| Myositis-specific antibodies and myositis-associated antibodies, n (%) | |||||
| Anti-HMGCR+ | 3 (16) | 3 (50) | |||
| Anti-NXP2+ | 3 (16) | 3 (38%) | |||
| Anti-Jo1+ | 1 (5) | 1 | |||
| Anti-MDA5+ | 1 (5) | 1 (13) | |||
| Anti-SRP+ | 1 (5) | 1 (17) | |||
| Anti-TIF1γ+ | 1 (5) | 1 (13) | |||
| Seronegative | 3 (38) | 1 (17) | 3 (75) | ||
| Myositis-associated antibodies only | 2 (11) | 1 (17) | 1 (25) | ||
| Myositis-specific antibodies | 0 (0) | ||||
| Absent myositis-specific antibodies or myositis-associated antibodies | 7 (37) |
Cardiac extramuscular disease activity consisted of peri/myocarditis as diagnosed by the treating cardiologist based on cardiac magnetic resonance imaging. bPulmonary extramuscular disease activity consisted of interstitial lung disease as confirmed by high-resolution chest computer tomography. ASS: anti-synthetase syndrome; IMNM: immune-mediated necrotizing myopathy; IQR: interquartile range; NM/OM: non-specific/overlap myositis.
Primary outcome
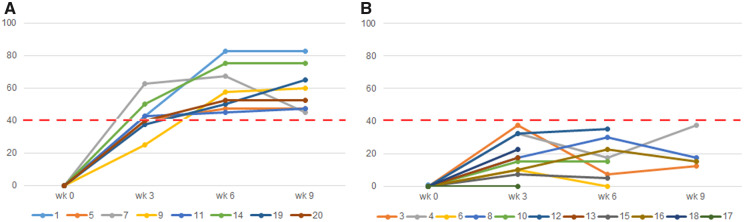
Eight patients (8/19 = 42.0%; Clopper–Pearson 95% CI: 19.6, 64.6) had at least moderate improvement by week 9 (Fig. 3).
Fig. 3.
Treatment response of the 19 included patients in the analysis
Treatment response was assessed by the 2016 ACR/EULAR TIS. Improvement was defined as a TIS of at least 40 by 9 weeks of IVIg treatment (dotted red line). Eight patients reached at least moderate improvement by 9 weeks of treatment (A), while 11 patients did not (B). TIS: Total Improvement Score.
Secondary outcomes
Of the eight responders, six patients reached at least moderate improvement by week 3. The two other responders reached at least moderate improvement by week 4 and by week 6. Nine patients reached at least minimal improvement (TIS at least 20) by week 9. Minimal improvement was seen in another three patients after the 2 g/kg BW loading dose, but this improvement was not maintained thereafter. Three patients had insufficient treatment response (TIS <40) by week 4 and were eligible for the amended IVIg treatment regimen for refractory disease. Of these patients, one patient prematurely ended the study due to lack of efficacy and severe muscle weakness (bed-ridden). In the two other patients, treatment according to the amended IVIg regimen did not lead to further improvement. No clear differences in treatment response between disease and/or serological subtypes were found, with the possible exception of immune-mediated necrotizing myopathy patients with anti-HMGCR antibodies in whom a favourable treatment response appeared to be associated with recent exposure to statins (see Supplementary Table S1, available at Rheumatology online).
There were statistically significant improvements between baseline and week 9 on the following outcome measures: physician global activity, PGA, HAQ, muscle enzymes/serum creatine kinase activity, extramuscular disease activity and EQ-5D-5L (Table 2). There were no statistically significant changes between baseline and week 9 with regard to MMT, Rasch MRC-SS, dynamometric muscle strength of the deltoid/biceps/psoas muscles, amyotrophic lateral sclerosis severity scale-swallowing or modified Rankin Scale.
Table 2.
Treatment response on secondary outcome measures after IVIg treatment expressed in median change scoresa as calculated by the non-parametric Wilcoxon signed rank test
| Outcome measure | Baseline, median (IQR) | End, median (IQR) | Δ Base-end, median (IQR) | P-value |
|---|---|---|---|---|
| Core set measures | ||||
| PhGA | 3.8 (3.2–4.0) | 2.3 (1.0–4.0) | −1.3 (−2.0 to −0.1) | < 0.01 |
| PaGA | 6.1 (5.3–7.6) | 4.6 (2.0–6.6) | −2.7 (−4.1 to −0.7) | 0.03 |
| MMTb | 211 (185–225) | 227 (191–241) | 10 (−7.0 to 29) | 0.12 |
| HAQ | 2.0 (1.5–2.5) | 1.6 (0.8–2.1) | −0.6 (−1.1 to 0.0) | 0.03 |
| sCK activity, U/l | 1199 (179–6500) | 196 (83–3877) | −103 (−3066 to −12) | < 0.01 |
| Extramuscular disease activity | 2.2 (0.6–3.0) | 1.0 (0.3–2.3) | −0.3 (−1.2 to 0.0) | 0.04 |
| Exploratory outcome measures of muscle function | ||||
| Rasch MRC-SS | 27 (24–28) | 28 (24–30) | 0.0 (−2.0 to 2.0) | 0.78 |
| Dynamometric muscle strengthc | ||||
| Deltoid muscles | 52 (41–72) | 53 (30–88) | 3.0 (−16 to 14) | 0.78 |
| Biceps muscles | 77 (46–95) | 73 (52–124) | 4.5 (−18 to 32) | 0.28 |
| Psoas muscles | 141 (124–162) | 174 (128–209) | 28 (−16 to 61) | 0.10 |
| ALSSS-SW | 9.0 (8.0–10) | 9.0 (8.0–10) | 0.0 (−1.0 to 1.0) | 0.40 |
| Exploratory outcome measures of disability | ||||
| mRS | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) | 0.0 (−1.0 to 0.0) | 0.06 |
| Exploratory outcome measures of quality of life | ||||
| EQ-5D-5L | 40 (35–55) | 60 (40–70) | 15 (−5.0 to 30) | 0.03 |
The median change score was calculated as the 50th percentile of all individual differences between baseline and outcome assessment.
MMT according to Kendall.
Dynamometric muscle strength expressed as mean of three measurements per side by a hand-held Citec dynamometer in Newton. Of note, dynamometric muscle strength of the psoas muscles from one patient with DM was not available at baseline. ALSSS-SW: amyotrophic lateral sclerosis severity scale swallowing; EMA: extramuscular disease activity; EQ-5D-5L: EuroQol Group Health Questionnaire; IQR: interquartile range; MMT: manual muscle testing; mRS: modified Rankin Scale; PaGA: patient global activity; PhGA: physician global activity; Rasch MRC-SS: Rasch modified Medical Research Sum Score; sCK: serum creatine kinase.
Three serious adverse events occurred. One patient with DM was diagnosed with ovarian cancer during the study and had a life-threatening pulmonary embolism at week 3, deemed (partially) treatment-related. The same patient was later also hospitalized for anaemia related to chemotherapy and dehydration due to gastro-enteritis. The third serious adverse event occurred in a patient with DM who was hospitalized for folliculitis with suspected abscess formation. All patients experienced AEs, mostly mild and transient flu-like symptoms, e.g. malaise, fatigue, mild temperature increase and headache. In none of the patients were (S)AEs a reason to discontinue the study medication.
None of the patients met the predefined definition for clinically relevant deterioration. Seven patients prematurely ended the study and switched to rescue medication due to insufficient treatment response.
Discussion
We found that first-line IVIg monotherapy induced at least moderate improvement in nearly half of patients with newly diagnosed IIM. The IMACS has defined different categories of response but there is as of yet no consensus on which level of improvement should be considered as clinically relevant. For this study we used a rather conservative cut off of at least moderate improvement (TIS of at least 40) to define a responder in the primary outcome. Using a less stringent definition of TIS >20 as currently used in some randomized clinical trials (NCT02728752 and NCT04044690), the proportion of responders in our study would be 55% (including patient 2, who was excluded from further analysis due to a ceiling effect, and patient 4). Therefore, future studies may define being a responder as having at least minimal improvement (TIS of at least 20) to identify additional patients who might benefit from treatment with IVIg.
The proportion of responders in our study (42%) is higher than reported in an open-label study in 11 DM/PM patients that showed a clear benefit following IVIg treatment in only three patients (27%) [9]. However, the proportion of responders in our study is lower than reported in another open-label study that included three IIM patients with a history of statin exposure and anti-HMGCR antibodies who all showed favourable responses to IVIg [10]. Differences might be explained by publication bias and by differences in the studied populations. The open-label study in 11 DM/PM patients used the 1975 Bohan and Peter criteria, which lack diagnostic accuracy as illustrated by the inclusion of a patient with suspected drug-induced myopathy. Similarly, the use of these criteria may have led to the inclusion of patients with (as of yet untreatable) IBM. Our study applied the highly specific 2004 ENMC criteria [14]. The open-label study that included three IIM patients with a history of statin exposure and anti-HMGCR antibodies investigated a specific subset of IIM patients. One might speculate about a variation in treatment response related to the varying proportions of the IIM subtypes between the studies [15, 28–34]. Interestingly, treatment response in our patients with anti-HMGCR antibodies appeared to be favourable in those with recent exposure to statins, contrary to those without recent exposure to statins. However, our pilot study was underpowered for any formal subgroup analyses.
Another important finding is that at least moderate improvement was reached by 3 weeks of IVIg treatment in six of eight responders, indicating a fast mode of action. In a previous randomised clinical trial (RCT) that compared oral dexamethasone pulse treatment with daily prednisolone treatment in patients with newly diagnosed IIM, improvement in muscle strength was mostly seen between 4 and 12 weeks after start of glucocorticoids [3]. Although these studies cannot be compared directly, our study results suggest that improvement in responders to IVIg might come earlier compared with improvement in responders to glucocorticoids.
With regard to safety, one patient with DM and ovarian cancer developed a life-threatening pulmonary embolism. Currently, there is no consensus whether patients with two risk factors for thrombosis (malignancy and IVIg treatment) should receive prophylactic anticoagulant treatment. Our patient with cancer-associated DM showed clear improvement by 3 weeks of IVIg treatment and anticoagulant treatment following the pulomary embolism warranted thromboprophylaxis henceforth. Therefore, we believe that a malignancy should not necessarily be an exclusion criterion. In all other patients, IVIg was well tolerated. The occurrence of (S)AEs did not necessitate premature ending of the study in any of the patients.
The strength of our study is the prospective design and inclusion of well-defined subtypes of IIM. We minimized the risk of bias and confounding by consecutively including patients and by using the highly specific 2004 ENMC criteria [14]. The main limitations of this study are the small sample size, the heterogeneity of the studied patients, the lack of blinding and the lack of a control group. However, we considered this phase 2 study as a proof of principle study. As such, one of the study aims was to explore treatment effect and time to improvement, prior to engaging in a larger phase 3 double-blinded placebo-controlled RCT.
The response to IVIg treatment in our patients with IIM is divergent. One group showed fast and moderate to major improvement, while the other group showed insufficient improvement to no improvement. Without absence of predictors of treatment response to IVIg, one could question whether IVIg should be used as first-line monotherapy in future studies. Therefore, we suggest that IVIg might be considered as a concomitantly administered add-on treatment to standard glucocorticoids in the induction phase of the treatment to swiftly achieve clinically relevant improvement. Another possibility could be a 3-week run-in on IVIg monotherapy, with concomitantly administered add-on glucocorticoids at that point in cases of no or minimal treatment response. Indeed, earlier RCTs studying IVIg or subcutaneous immunoglobulins as add-on to concomitantly administered glucocorticoids in patients with refractory IIM have shown favourable, albeit conflicting, results [5, 6, 35]. Thus, potential benefit of IVIg as run-in or add-on treatment in patients with newly diagnosed IIM needs to be proven in a subsequent RCT that also addresses safety of combining IVIg treatment with glucocorticoids.
In conclusion, our pilot study indicates that first-line IVIg treatment in IIM leads to clinically relevant improvement in nearly half of patients with a fast clinical response in the majority of responders. Although generally safe and feasible, caution is advocated in patients with increased risk of thrombosis, such as patients with concomitant malignancy. We recommend further studies to assess the efficacy of add-on IVIg treatment in combination with glucocorticoids.
Supplementary Material
Acknowledgements
We thank R. Aggarwal for his help regarding the implementation of the 2016 ACR/EULAR TIS and M.D.J. Wolvers for her help with the statistical analyses.
Funding: This study was funded by a grant (Interlaken Leadership Award) from CSL Behring. Also, CSL Behring kindly provided IVIg (Privigen, CSL Behring, King of Prussia, PA, USA) free of charge for this study. The funder of the study had no role in the design of the study, the analysis, collection, interpretation of the data, and writing of the manuscript.
Disclosure statement: J.L. reports financial support from Sanquin for attending a conference. F.E. has received grants from the Prinses Beatrix Spierfonds and the Netherlands Organization for Health Research and Development for studies in CIDP, is the principal investigator of INCbase, an international CIDP registry which is co-funded by CSL Behring, Takeda, Kedrion, and Terumo BCT based on investigator-initiated proposals. F.E. has also received consulting fees from CSL Behring, UCB Pharma, and Aserta Pharma. All grants and consulting fees were paid to the Amsterdam UMC and not related to the submitted work. C.V. received a consulting fee from Inflectis paid to the Amsterdam UMC, and not related to the submitted work. I.N.vS. chairs a steering committee for CSL Behring and received departmental honoraria for serving on scientific advisory boards for CSL Behring and Baxter, is a member of the Scientific Board of the Kreuth III meeting on the optimal use of plasma-derived medicinal products, especially coagulation factors and normal immunoglobulins organised under the auspices of the European Directorate for the Quality of Medicines & HealthCare. M.dV. is a member of the Data Safety Monitoring Board with Avexis and member of the Adjucation Board with Bristol-Myers Squibb, and received financial support from Sanquin to attend a conference, not related to the submitted work. A.J.vdK. reports grants from CSL Behring and non-financial support (IVIg free of charge) from CSL Behring, during the conduct of the study. The other authors have declared no conflicts of interest.
Data availability statement
Any data not published within the article will (after anonymisation) be shared upon request from any qualified investigator.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Van de Vlekkert J, Hoogendijk JE, de Visser M.. Long-term follow-up of 62 patients with myositis. J Neurol 2014;261:992–8. [DOI] [PubMed] [Google Scholar]
- 2. Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep 2012;14:275–85. [DOI] [PubMed] [Google Scholar]
- 3. Van de Vlekkert J, Hoogendijk JE, de Haan RJ. et al. Oral dexamethasone pulse therapy versus daily prednisolone in sub-acute onset myositis, a randomised clinical trial. Neuromuscul Disord 2010;20:382–9. [DOI] [PubMed] [Google Scholar]
- 4. Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 2004;291:2367–75. [DOI] [PubMed] [Google Scholar]
- 5. Dalakas MC, Illa I, Dambrosia JM. et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 1993;329:1993–2000. [DOI] [PubMed] [Google Scholar]
- 6. Miyasaka N, Hara M, Koike T. et al. Effects of intravenous immunoglobulin therapy in Japanese patients with polymyositis and dermatomyositis resistant to corticosteroids: a randomised double-blind placebo-controlled trial. Mod Rheumatol 2012;22:382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moriguchi M, Suzuki T, Tateishi M, Hara M, Kashiwazaki S.. Intravenous immunoglobulin therapy for refractory myositis. Intern Med 1996;35:663–7. [DOI] [PubMed] [Google Scholar]
- 8. Saito E, Koike T, Hashimoto H. et al. Efficacy of high-dose intravenous immunoglobulin therapy in Japanese patients with steroid-resistant polymyositis and dermatomyositis. Mod Rheumatol 2008;18:34–44. [DOI] [PubMed] [Google Scholar]
- 9. Cherin P, Piette JC, Wechsler B. et al. Intravenous gamma globulin as first line therapy in polymyositis and dermatomyositis: an open study in 11 adult patients. J Rheumatol 1994;21:1092–7. [PubMed] [Google Scholar]
- 10. Mammen AL, Tiniakou E.. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015;373:1680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoogendijk JE, Amato AA, Lecky BR. et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord 2004;14:337–45. [DOI] [PubMed] [Google Scholar]
- 12. Miller FW, Rider LG, Chung YL. et al. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology 2001;40:1262–73. [DOI] [PubMed] [Google Scholar]
- 13. Isenberg DA, Allen E, Farewell V. et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology 2004;43:49–54. [DOI] [PubMed] [Google Scholar]
- 14. Lundberg IE, Tjarnlund A, Bottai M. et al. 2017 European League against Rheumatism/American College of Rheumatology Classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol 2017;69:2271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mariampillai K, Granger B, Amelin D. et al. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol 2018;75:1528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavazzana I, Fredi M, Ceribelli A. et al. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J Immunol Methods 2016;433:1–5. [DOI] [PubMed] [Google Scholar]
- 17. Shovman O, Gilburd B, Chayat C. et al. Anti-HMGCR antibodies demonstrate high diagnostic value in the diagnosis of immune-mediated necrotizing myopathy following statin exposure. Immunol Res 2017;65:276–81. [DOI] [PubMed] [Google Scholar]
- 18. Hughes RA, Donofrio P, Bril V. et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008;7:136–44. [DOI] [PubMed] [Google Scholar]
- 19. Aggarwal R, Rider LG, Ruperto N. et al. 2016 American College of Rheumatology/European League against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol 2017;69:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanhoutte EK, Faber CG, van Nes SI. et al. Modifying the Medical Research Council grading system through Rasch analyses. Brain 2012;135:1639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baschung Pfister P, de Bruin ED, Sterkele I. et al. Manual muscle testing and hand-held dynamometry in people with inflammatory myopathy: an intra- and interrater reliability and validity study. PLoS One 2018;13:e0194531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hillel AD, Miller RM, Yorkston K. et al. Amyotrophic lateral sclerosis severity scale. Neuroepidemiology 1989;8:142–50. [DOI] [PubMed] [Google Scholar]
- 23. Farrell B, Godwin J, Richards S, Warlow C.. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991;54:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janssen MF, Pickard AS, Golicki D. et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herdman M, Gudex C, Lloyd A. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The National Institute of Environmental Health Sciences (NIEHS). IMACS Form 00: Clinical Trial Design Features. https://www.niehs.nih.gov/research/resources/assets/docs/00_imacs_clinical_trial_design_tool_pdf_format_508.pdf (3 October 2016, date last accessed).
- 27. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
- 28. Cao H, Pan M, Kang Y. et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res 2012;64:1602–10. [DOI] [PubMed] [Google Scholar]
- 29. Hall JC, Casciola-Rosen L, Samedy LA. et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res 2013;65:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamaguchi Y, Kuwana M, Hoshino K. et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol 2011;147:391–8. [DOI] [PubMed] [Google Scholar]
- 31. Troyanov Y, Targoff IN, Tremblay JL. et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine 2005;84:231–49. [DOI] [PubMed] [Google Scholar]
- 32. Lim J, Rietveld A, De Bleecker JL. et al. Seronegative patients form a distinctive subgroup of immune-mediated necrotizing myopathy. Neurol Neuroimmunol Neuroinflamm 2019;6:e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi J, Li S, Yang H. et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 2017;44:1051–7. [DOI] [PubMed] [Google Scholar]
- 34. Hamaguchi Y, Fujimoto M, Matsushita T. et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 2013;8:e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Danieli MG, Gelardi C, Pedini V. et al. Subcutaneous immunoglobulin in inflammatory myopathies: efficacy in different organ systems. Autoimmun Rev 2020;19:102426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data not published within the article will (after anonymisation) be shared upon request from any qualified investigator.