Abstract
Objectives
To describe how patients with primary SS (pSS) and systemic organ involvement are classified and clustered in routine practice.
Methods
This multinational, cross-sectional survey of real-world quantitative data was conducted across Europe and the US. Rheumatologists who treated seven or more adult patients per month with pSS and current/past systemic manifestations undertook a survey before completing a patient record form capturing demographic, clinical and treatment information for their next six eligible patients. Patients with a completed patient record form were invited to complete a patient self-completion questionnaire capturing insights into their disease and treatment. Subgroups were defined by physicians’ assessment of disease severity; clusters were derived based on key clinical characteristics using latent class analysis.
Results
Rheumatologists completed 316 physician surveys and 1879 patient record forms; 888 patients completed the patient self-completion questionnaire. pSS severity reflected organ involvement and symptomatology. Latent class analysis produced five clusters distinguished by the organ systems involved and the presence of pain and fatigue symptoms at the time of the survey. A minority of patients [n = 67 (4%)] were categorized with multiple organ involvement and the highest frequency of pain and fatigue. A total of 324 patients (17%) were categorized as ‘low burden’. The remaining three clusters exhibited high frequencies of articular involvement but were distinguished by the extent of other organ system involvement.
Conclusion
Cluster analysis using a real-world cohort of patients with pSS and systemic organ involvement highlights the heterogeneous presentation of patients with pSS and confirms the importance of pain and fatigue as well as organ involvement when determining disease burden.
Keywords: primary Sjögren’s syndrome, systemic autoimmune disease, real-world evidence, latent class analysis, cluster analysis
Rheumatology key messages
The influence of cardinal symptoms vs systemic involvement on pSS burden is not well documented.
Cluster analysis revealed distinct patient subgroups based on organ involvement and the presence of pain/fatigue.
One subgroup has markedly high disease burden, identified by cluster analysis and physician-assessed severity.
Introduction
Primary SS (pSS) affects up to 20 times as many women as men [1], with peak onset at 40–60 years of age [2]. There is substantial variation in published estimates of prevalence due to methodological differences between studies and the choice of classification criteria; a systematic review estimated a pooled prevalence of 0.06% [3]. The condition is highly heterogeneous. Although patients with pSS predominantly present with sicca (dryness) symptoms due to exocrine gland dysfunction, up to 40% of patients also experience extraglandular disease manifestations, including involvement of the musculoskeletal, cutaneous and respiratory systems, with a minority of patients developing serious, life-threatening organ involvement of the CNS or kidneys [1, 4–6]. Most patients also exhibit fatigue [7], which manifests regardless of organ involvement [8] and has been reported to be the most distressing symptom by many patients [9]. The presence of pain is also common and can be related to neuropathy, inflammation or non-specific causes [10]. Consequently, patients with pSS have particularly poor health-related quality of life (HRQoL), similar to that seen in patients with RA or SLE [11]. Furthermore, the mean annual direct and indirect costs for patients with pSS are similar to those of patients with RA [12, 13].
The heterogeneous nature of clinical presentations complicates the current understanding of both the burden of disease and the nature and extent of unmet needs within the pSS population. Some non-specialists perceive pSS as a benign condition, especially when serious organ involvement is limited or absent. Although patients with pSS and organ involvement have been shown to have higher disease costs than those without organ involvement, the degree of systemic disease activity does not correlate well with patient-reported symptoms [14–17]. As such, further differentiation of burden based on disease characteristics is needed. To facilitate this, efforts have focussed on the development of valid tools to assess both the systemic clinical features and subjective manifestations of pSS. The EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) and the EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) were developed for this purpose [18, 19]. While the ESSPRI measures the cardinal symptoms of dryness, pain and fatigue, the ESSDAI was validated as a standardized instrument for the evaluation of systemic activity in pSS in clinical trials [16, 18, 19].
Determining how physicians categorize patients with pSS and systemic organ involvement in routine practice would further inform understanding of the relative impact of symptoms vs organ involvement. Development of more targeted management strategies could be achieved by patient characterization that considers both the type and frequency of organ involvement and the presence of pain and/or fatigue. The Adelphi Disease-Specific Programmes (DSP) are evidence-generation programmes that provide comprehensive real-world data in an individual disease area, which are particularly valuable in diseases with heterogeneous symptoms such as pSS [20]. This study used DSP methodology to identify subgroups of patients with systemic pSS in routine practice based on their disease characteristics and symptoms and to describe the clinical and patient-reported burden of disease and level of treatment satisfaction reported by patients and treating physicians in each of these subgroups. The analysis focuses on patients with current or historical systemic organ involvement.
Methods
Study objectives
The primary study objective was to describe how patients with pSS and systemic organ involvement are classified and clustered in routine practice and how this relates to their clinical and patient-reported burden. Secondary objectives included physician- and patient-reported satisfaction with current treatment.
Study design
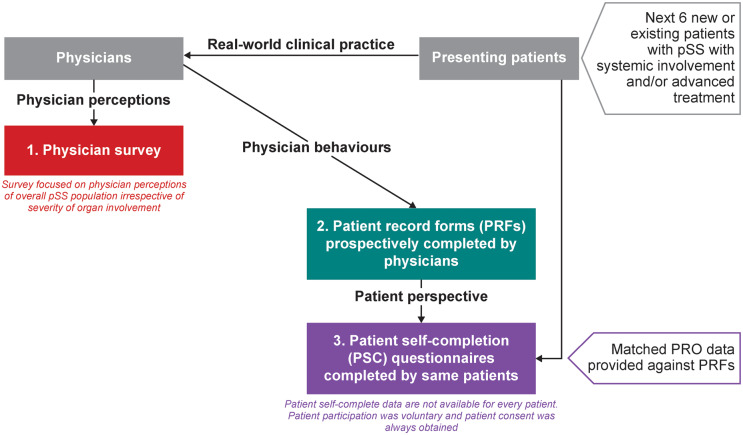
This study was a multinational, cross-sectional survey of real-world quantitative market research data using DSP methodology (Fig. 1) [20]. The pSS DSP was conducted in France, Italy, Spain, Germany and the US. Rheumatologists who made treatment decisions for patients with pSS were identified and data were then collated from three sources: a physician survey, a physician-completed patient record form (PRF) and a patient self-completion (PSC) questionnaire. The analysis was conducted under GlaxoSmithKline (GSK) protocol 207382.
Fig. 1.
Summary of DSP methodology
DSP: Disease-Specific Programme; PRO: patient reported outcome; pSS: primary Sjögren’s syndrome.
Study population
Physician eligibility criteria
To take part in the study, physicians’ primary specialty had to be rheumatology and they had to treat seven or more patients with pSS in a typical month. Additionally, they had to be actively involved in and personally responsible for management and treatment decisions for patients with pSS.
Patient eligibility criteria
Adults with pSS and current or past systemic disease activity were included in the study. Specifically, eligible patients were ≥18 years of age with a confirmed diagnosis of pSS, in the opinion of the rheumatologist, in the absence of SLE, RA or SSc, and were currently exhibiting or had previously exhibited disease activity in one or more of the categories described in the ESSDAI (found in the Supplementary material, available at Rheumatology online).
This study complied with the Declaration of Helsinki. Ethics approval was granted for all countries by the Western Institutional Review Board (study number 20181321, tracking number 1186583). Prior to the completion of any study-related activities for the DSP, patients provided informed consent.
Data collection
Physician survey
Physicians completed an online survey prior to commencement of the PRFs.
PRFs
Rheumatologists completed PRFs for each of their next six eligible patients with pSS, capturing clinical, objective and subjective data on each patient, including demographics, pSS history and diagnosis, symptomatology, medication use and effects and treatment satisfaction. Organ manifestation [21] categories were based on the ESSDAI domains; physicians were able to select a specific condition reported in each domain or ‘other’.
PSCs
Physicians invited eligible patients, for whom the physician had completed a PRF, to complete the optional PSC questionnaire (independent of their physician). It captured patient insights, including demographic information, disease and symptom severity, impact of pSS on health status [assessed using the EuroQol 5-dimension 3-level (EQ-5D-3L, range 0–1, with higher scores indicating better QoL) and the EuroQol 5-dimension visual analogue scale (EQ-5D-VAS, range 0–100, with higher scores indicating better QoL)] [21], impact of pSS on work performance [assessed using the Work Productivity and Activity Impairment questionnaire (WPAI)] [22], impact of pSS on fatigue [assessed using the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-fatigue, range 0–4, with lower scores indicating worse fatigue)] [23], medication use and treatment satisfaction.
Data analysis
The DSP was conducted with no prior hypotheses specified, therefore formal sample size calculations were not performed.
Responses from the physician interview were used to explore the most common perceptual categorizations of patients with pSS for disease severity (mild, moderate or severe), systemic involvement [non-systemic (sicca only) or systemic (organ involvement)], glandular involvement (glandular or non-glandular) and disease activity (inactive, mildly active, very active).
Latent class analysis (LCA) was used to derive clusters of patients. Patient demographics, disease and treatment history, clinical characteristics and physician-assessed symptomology and treatment experience reported in the PRF were assessed for the degree of missingness and were included in the first model (see Supplementary material, available at Rheumatology online). Of these, the type of organ involvement, presence of pain and presence of fatigue at the time of the survey were found to be the most important factors determining the probability of cluster assignment. The final analysis was conducted using these variables and the duration of the pSS diagnosis, which was retained given the cross-sectional nature of the data, to aid interpretation. Models were generated, which varied in the number of derived clusters (from one to six clusters); the choice of cluster solution was based on the Bayesian information criterion and clinical input.
Following cluster assignment, descriptive statistics were used to describe cluster characteristics, including demographics, clinical characteristics and humanistic burden. Categorical variables are presented as frequency and percentage distributions when appropriate. Ordinal variables are reported as frequencies and percentages and/or medians and interquartile ranges (IQRs), as appropriate for the individual variables. Continuous variables (e.g. age, time since diagnosis and questions with numeric rating scale responses) are presented as mean (s.d.), median (IQR) and score ranges.
Results
Study population
Physicians completed a total of 316 physician surveys and 1879 PRFs (Table 1). Physicians reported that they see around half (49%) of their ambulatory care pSS patients in the public hospital setting, with expected wide variation by country (6% in the US to 81% in France). In the online survey, physicians were also asked to attribute weights to their preferred method of classifying patients with pSS (all patients, including those without systemic involvement). Among those who used classifications (84%), the greatest weight was attributed to the presence of systemic manifestations (non-systemic/sicca only vs systemic), followed by severity levels (mild, moderate, severe), then the level of disease activity (inactive, mildly active, very active) and finally glandular involvement (glandular vs non-glandular).
Table 1.
Study population and patient demographics and clinical characteristics
| Study population, n (%) | Physician survey | Physician- reported PRFs | Patient- reported PSCsa | |
|---|---|---|---|---|
| Total | 316 (100) | 1879 (100) | 888 (100) | |
| France | 52 (16.5) | 287 (15.3) | 95 (10.7) | |
| Germany | 60 (19.0) | 360 (19.2) | 226 (25.5) | |
| Italy | 61 (19.3) | 360 (19.2) | 79 (8.9) | |
| Spain | 60 (19.0) | 361 (19.2) | 179 (20.2) | |
| US | 83 (26.3) | 511 (27.2) | 308 (34.7) | |
|
Patient demographics and clinical characteristics based on physician-reported PRFs | ||||
|---|---|---|---|---|
| Overall (N = 1879) | Mild (n = 914) | Moderate (n = 895) | Severe (n = 70) | |
| Age, years, mean (s.d.) | 53.2 (12.2) | 52.2 (12.9) | 54.1 (11.2) | 55.3 (13.3) |
| Female, n (%) | 1677 (89.2) | 813 (88.9) | 801 (89.5) | 63 (90.0) |
| Years since diagnosis, mean (s.d.) | 4.5 (5.1) | 4.3 (5.2) | 4.6 (4.9) | 6.1 (6.5) |
| Ethnicity, n (%) | ||||
| White/Caucasian | 1678 (89.3) | 814 (89.1) | 805 (89.9) | 59 (84.3) |
| Non-white/non-Caucasian | 201 (10.7) | 100 (10.9) | 90 (10.1) | 11 (15.7) |
| Employment status, n (%) | ||||
| Working full time | 762 (40.6) | 431 (47.2) | 312 (34.9) | 19 (27.1) |
| Working part time | 231 (12.3) | 101 (11.1) | 121 (13.5) | 9 (12.9) |
| On long-time sick leave | 39 (2.1) | 13 (1.4) | 22 (2.5) | 4 (5.7) |
| Homemaker | 414 (22.0) | 168 (18.4) | 236 (26.4) | 10 (14.3) |
| Student | 18 (1.0) | 14 (1.5) | 4 (0.4) | 0 (0.0) |
| Retired | 316 (16.8) | 149 (16.3) | 148 (16.5) | 19 (27.1) |
| Unemployed | 55 (2.9) | 21 (2.3) | 28 (3.1) | 6 (8.6) |
| Don’t know | 44 (2.3) | 17 (1.9) | 24 (2.7) | 3 (4.3) |
| Organ involvement at diagnosis, n (%) | ||||
| Any | 1589 (84.6) | 795 (87.0) | 729 (81.5) | 65 (92.9) |
| Organ involvement at time of PRF completion, n (%) | ||||
| Any | 1816 (96.6) | 867 (94.9) | 880 (98.3) | 69 (98.6) |
| Articular | 1366 (72.7) | 636 (69.6) | 680 (76.0) | 50 (71.4) |
| Glandular | 714 (38.0) | 294 (32.2) | 376 (42.0) | 44 (62.9) |
| Cutaneous | 376 (20.0) | 143 (15.6) | 217 (24.2) | 16 (22.9) |
| Muscular | 341 (18.1) | 110 (12.0) | 218 (24.4) | 13 (18.6) |
| Pulmonary | 299 (15.9) | 109 (11.9) | 163 (18.2) | 27 (38.6) |
| Haematological | 350 (18.6) | 166 (18.2) | 167 (18.7) | 17 (24.3) |
| Lymphadenopathy | 190 (10.1) | 96 (10.5) | 83 (9.3) | 11 (15.7) |
| Peripheral nervous system | 155 (8.2) | 61 (6.7) | 83 (9.3) | 11 (15.7) |
| Renal | 100 (5.3) | 36 (3.9) | 54 (6.0) | 10 (14.3) |
| CNS | 96 (5.1) | 38 (4.2) | 51 (5.7) | 7 (10.0) |
| Current treatment classes, n (%) | ||||
| OTC | 1188 (63.2) | 555 (60.7) | 592 (66.1) | 41 (58.6) |
| Corticosteroids | 885 (47.1) | 377 (41.2) | 471 (52.6) | 37 (52.9) |
| csDMARDs | 816 (43.4) | 329 (36.0) | 455 (50.8) | 32 (45.7) |
| Secretagogues | 765 (40.7) | 313 (34.2) | 425 (47.5) | 27 (38.6) |
| Antimalarials | 696 (37.0) | 354 (38.7) | 317 (35.4) | 25 (35.7) |
| Biologic/biosimilar | 223 (11.9) | 80 (8.8) | 126 (14.1) | 17 (24.3) |
| Prescription eye drops | 172 (9.2) | 56 (6.1) | 108 (12.1) | 8 (11.4) |
| Other treatments | 1344 (71.5) | 643 (70.4) | 652 (72.8) | 49 (70.0) |
| Patient-reported outcomes (collected in the PSCs) | ||||
|---|---|---|---|---|
| Overall (N = 888) | Mild (n = 381) | Moderate (n = 475) | Severe (n = 32) | |
| Health status, mean (s.d.)b | ||||
| EQ-5D-3L | N = 877, 0.7 (0.24) | n = 378, 0.8 (0.19) | n = 468, 0.7 (0.24) | n = 31, 0.5 (0.33) |
| EQ-5D-VAS | N = 874, 63.5 (19.02) | n = 373, 70.3 (18.01) | n = 469, 59.2 (17.41) | n = 32, 46.3 (23.84) |
| Fatigue, mean (s.d.) b | ||||
| FACIT-fatigue | N = 879, 31.3 (10.91) | n = 377, 35.6 (10.06) | n = 470, 28.5 (10.22) | n = 32, 21.9 (11.45) |
| Work productivity and activity impairment due to pSS, mean (s.d.)c | ||||
| WPAI: percent activity impairment | N = 841, 37.8 (21.65) | n = 357, 29.3 (20.47) | n = 453, 42.8 (19.57) | n = 31, 61.6 (23.82) |
| WPAI: percent overall work impairment | N = 375, 31.4 (21.93) | n = 177, 25.7 (21.35) | n = 192, 36.1 (20.78) | n = 6, 50.8 (31.05) |
| WPAI: percent impairment while working | N = 464, 29.8 (21.07) | n = 228, 25.2 (20.66) | n = 229, 34.0 (20.38) | n = 7, 42.9 (25.63) |
| WPAI: percent work time missed | N = 383, 5.0 (15.55) | n = 181, 4.4 (15.15) | n = 196, 5.3 (15.75) | n = 6, 12.5 (20.92) |
A total of 888 patients completed a PSC, a lower value indicates that patients did not complete the question.
A lower score indicates a higher burden. cA higher score indicates a higher burden.
csDMARD: conventional synthetic DMARD; EQ-5D-3L: EuroQol-5 Dimension-3 Level; EQ-5D-VAS: EuroQol-Visual Analogue Scale; OTC: over the counter; PRF: patient record form; PSC: patient self-completion questionnaire; pSS: primary Sjögren’s Syndrome; WPAI: Work Productivity and Activity Impairment questionnaire.
Based on the physician-completed PRFs, 914 (49%) patients were classified as having mild pSS, 895 (48%) with moderate disease and 70 (4%) with severe disease at the time of the survey (Table 1). Most patients were female (89%) and white/Caucasian (89%). The overall average age was 53.2 years (s.d. 12.2). Overall, 97% of patients had organ involvement, with 73% having articular involvement and 38% having glandular involvement at PRF completion (Table 1 and Supplementary Table S1, available at Rheumatology online). Physicians reported that 63% of patients were using medication that is available over-the-counter (OTC; e.g. eye drops, nasal sprays) and 47% were using corticosteroids. Of those patients receiving any OTC medication, 83% received artificial tears. Fatigue was experienced by most patients (75–89%), regardless of overall severity, as were dry eyes and dry mouth.
Few patients were reported with serious organ manifestations according to the ESSDAI score [24]: 6% of patients had moderate–severe myositis, 3% had moderate–severe interstitial lung disease and ≤1% had malignant B cell disorder, haematuria or renal failure or highly active CNS involvement.
Of the 1879 PRFs, the optional PSC was completed by 888 patients (47%). The mean EQ-5D-3L score for the overall population was 0.7 (s.d. 0.24) and the mean EQ-5D-VAS score was 63.5 (s.d. 19.02), with scores worsening with increasing disease severity (Table 1). Overall, patients reported a mean FACIT-fatigue score of 31.3 (s.d. 10.91) and, again, the FACIT score became worse as disease severity increased. Among these patients, WPAI impairment was 38% and 31% for activity and work impairment, respectively, with higher proportions of patients in the severe disease group reporting impairment than in the mild and moderate categories.
Cluster analysis
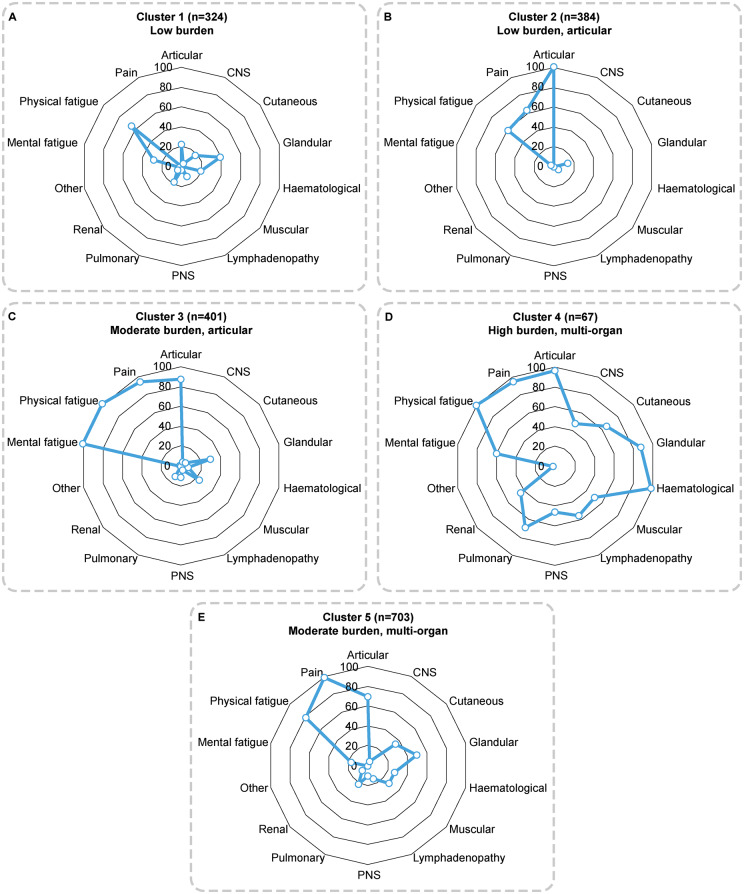
When the PRF data on organ involvement and the presence of pain and fatigue were analysed to identify clusters of patients, a four-cluster solution demonstrated the best statistical fit based on the Bayesian information criterion. However, a five-cluster solution was considered the most clinically meaningful strategy, as it added greater granularity than the four-cluster solution’s somewhat undifferentiated clusters. Organ involvement and symptoms across these five clusters are shown in Fig. 2 and demographics and characteristics in Supplementary Table S2, available at Rheumatology online. Patients included in cluster 1 [n = 324 (17%)] presented with low levels of organ involvement and experienced low levels of mental fatigue but higher levels of physical fatigue and did not present with pain symptoms (‘low burden’). Patients included in cluster 2 [n = 384 (20%)] presented predominantly with articular involvement and few experienced mental fatigue; however, they did demonstrate some level of physical fatigue and pain (‘low burden, articular’). Patients in cluster 2 had been diagnosed for the shortest time [3.8 years (s.d. 4.4)]. Patients included in cluster 3 [n = 401 (21%)] presented with low levels of organ involvement other than in the articular domain, the highest frequency of fatigue and a high frequency of pain (‘moderate burden, articular’). Patients included in cluster 4 [n = 67 (4%)] presented with a high degree of organ involvement across multiple systems, experienced a moderate frequency of mental fatigue and very high frequencies of physical fatigue and pain (‘high burden, multi-organ’). Patients in cluster 4 had been diagnosed for the longest time. Patients included in cluster 5 [n = 703 (37%)] presented with some organ involvement across domains, particularly articular and glandular, and experienced infrequent mental fatigue, a high frequency of physical fatigue and the highest frequency of pain (‘moderate burden, multi-organ’).
Fig. 2.
A five-cluster solution illustrating the patient proportion (%) in each cluster with organ involvement, pain and fatigue
PNS: peripheral nervous system.
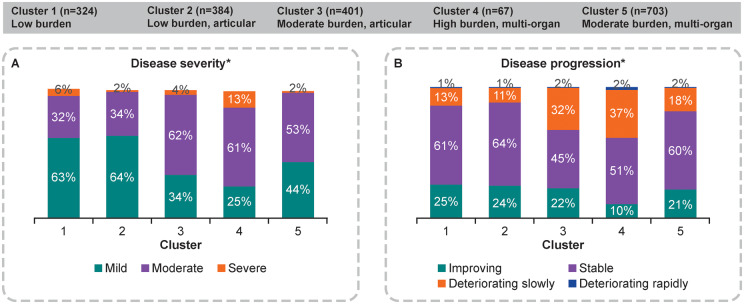
When considering disease severity, as reported by physicians, patients in cluster 4 presented as the most clinically burdened, with the highest proportion of patients classified as severe (13%), the highest proportion of deteriorating patients (39%) and the lowest proportion of patients whose disease was improving (10%) (Fig. 3). Additionally, patients in cluster 4 were reported by their physician to have the most severe symptoms, including oral dryness, ocular dryness, physical fatigue, mental fatigue and pain, followed by patients in cluster 3. Patients in cluster 3 had the second lowest proportion of patients with mild disease (34%) and had the second highest proportion of patients who were deteriorating slowly (32%). Cluster 3 patients were also reported to have the second most severe oral dryness, ocular dryness, physical fatigue and pain symptoms.
Fig. 3.
Cluster analysis of physician-reported disease and symptom severity (n = 1879)
*Based on physician’s subjective assessment at the time of the survey.
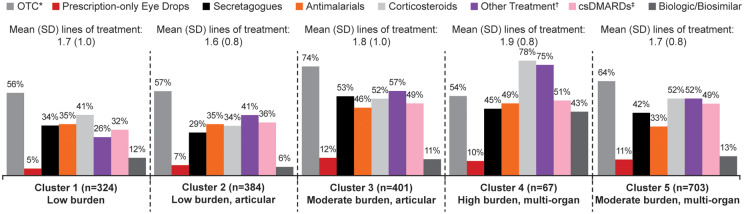
With regard to current pSS therapy, cluster 4 had the highest proportions of patients using corticosteroids (78%), biologics (43%) and conventional synthetic DMARDs (51%) (Fig. 4). Additionally, around half of patients in cluster 4 were using antimalarials (49%) or secretagogues (45%). Patients in cluster 3 had relatively low levels of biologic use (11%), similar to clusters 1 (12%) and 5 (13%).
Fig. 4.
Cluster analysis of current therapy classes (n = 1879)
*OTC treatments include artificial tears, artificial saliva, nasal spray, anti-bacterial mouthwash, vaginal lubricant and NSAIDs.
†Other treatments include DHEA, NSAIDs, COX-2, gabapentin, pregabalin, omega-6, antidepressants, ciprofloxacin, metronidazole and IVIG.
‡csDMARDs in mild subgroup: MTX 17%, AZA 2%; moderate subgroup: MTX 31%, AZA 11%, MMF 3%, CYC 2%, cyclosporine 1%; severe subgroup: MTX 37%, AZA 7%, MMF 5%, CYC 2%, cyclosporine 2%.
COX: cyclooxygenase; csDMARDs: conventional synthetic DMARD; OTC: over the counter.
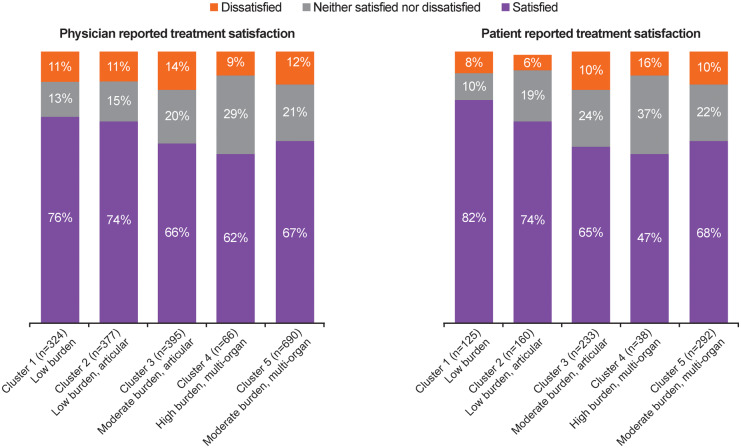
Cluster 1 contained the highest proportion of patients who were satisfied with their treatment (82%) and the lowest proportion of patients who were dissatisfied with their treatment (8%), while the converse was true for cluster 4 (Fig. 5). This pattern was similar for physician-reported satisfaction with treatment, with physicians reporting the highest level of satisfaction for patients in cluster 1 (76%) and the lowest level of satisfaction in cluster 4 (62%).
Fig. 5.
Cluster analysis of physician- and patient-reported satisfaction with treatment
Discussion
This first analysis of the pSS DSP provides relevant, real-world information about the way that physicians categorize their patients in routine clinical practice and allowed clustering of patients with pSS based on a broad spectrum of disease characteristics. The patient population comprised predominantly female patients. Based on physician reports, 49% of the population had mild disease, 48% had moderate disease and 4% had severe disease. The inclusion criteria meant that all patients in the sample presented here had current or past systemic involvement, although few patients aligned with an ESSDAI score that is classified as ‘high activity’ at the time of PRF completion, consistent with the small proportion of patients whose disease was classified as severe by their physicians. While frequency of organ involvement was not markedly different between patients categorized as mild and moderate/severe, the physician-assessed severity of conditions within each organ system was more frequently assessed as moderate/severe, thus reflecting the physicians’ overall severity categorization. Most patients experienced fatigue and patients reported reduced HRQoL and impairment in activity and work, which was present regardless of disease severity but did increase as physician-assessed disease severity increased.
Importantly, the distribution of organ involvement across ESSDAI domains was similar to the distribution seen in a multicentre pSS registry in Spain (SJOGRENSER) [25], although the Spanish registry includes all patients with pSS, i.e. with or without systemic organ involvement, whereas the present study is skewed towards those with current systemic organ involvement. In both populations, ≥70% of patients had articular involvement and 30–40% had glandular involvement, with ≤10% experiencing renal, CNS or peripheral nervous system involvement. In a UK registry, the Spanish GEAS-SS registry and the international Big Data Sjögren Consortium registry, patients had somewhat lower rates of organ involvement than in the current study [26–28], which is again to be expected given the inclusion criteria of the current study and also methodological differences between registries. In contrast to the current study, which provides a cross-sectional view of patient burden in a cohort with a mean time since diagnosis of 4.5 years, the international registry reflects the systemic phenotype at diagnosis [27]. In the UK registry, patients had a slightly lower mean EQ-5D utility value of 0.62 (against a UK general population value of 0.86) compared with 0.7 in the current study, which may reflect regional differences, including those arising from different preference-based scoring functions [29]. When considering the relative distribution of manifestations across organ systems, these comparisons to clinical registry cohorts highlight the generalizability of our results.
Given the extensive variation in the clinical spectrum of pSS, accurate classification of patients is vital for effective management pathways and drug development. As such, ACR/EULAR classification criteria were published in 2016 [30] and ESSDAI and ESSPRI (systemic and symptomatic disease measures) are increasingly being used to define inclusion criteria and endpoints in pSS clinical trials [8]. The Innovative Medicines Initiative NECESSITY (New clinical endpoints in primary Sjögren’s syndrome: an interventional trial based on stratifying patients) project aims to build on this and further develop outcome measures [31]. Also, a better understanding of disease phenotypes could facilitate more targeted selection of patients with pSS into clinical trials with therapies assessed by organ-specific endpoints. This study provides important insights into pSS phenotypes with high unmet need.
The current study provides important information regarding the factors physicians consider when categorizing patients with pSS. Physicians reported that they mostly categorize their patients based on systemic involvement, followed by their level of disease severity (mild, moderate or severe), then the level of disease activity (inactive, mildly active or very active) and most infrequently, glandular involvement. Comparison of patient characteristics categorized according to severity shows that the physicians’ overall assessment of disease severity is holistic. Nonetheless, given such heterogeneous patient presentations, severity categorizations cannot reflect the full extent of the disease burden experienced by patients.
Latent class analysis allowed the categorization and examination of patients according to their disease presentation using a data-driven approach. Based on physician-reported organ involvement and symptoms, two potential solutions were revealed: a four-cluster solution and a five-cluster solution. Although the four-cluster solution was the best statistical fit, the five-cluster solution was more clinically meaningful based on its provision of more defined individual clusters in terms of disease severity and level of systemic involvement. Furthermore, there was a clearer separation between the clusters, which is likely a reflection of differences in the impact of the disease on HRQoL, thus better reflecting treatment needs. The five clusters were differentiated based on the level of organ involvement and the presence of mental and physical fatigue and pain symptoms.
In general, the cluster analysis highlighted the importance of considering both systemic and symptomatic manifestations of pSS when classifying patients and deciding upon appropriate management strategies. Cluster 1 represented patients with low disease severity, who had little organ involvement and minimal levels of fatigue and pain at the time of the survey. More than 50% of patients in this cluster used OTC medications and 41% used systemic corticosteroids; this cluster had the highest levels of patient- and physician-reported treatment satisfaction. Is it noteworthy, however, that based on physicians’ assessment, no patients in this group experienced pain whereas slightly more than half (52%) of patients with PSC data in this cluster completed the pain numerical response scale, with a mean score of 3.6 (s.d. 1.8). A statistical analysis of the level of agreement between physician and patient ratings of symptoms was not planned as part of this current analysis but represents a key area of future research. Patients in cluster 2 were a distinct group who had articular involvement, little organ involvement and experienced considerable levels of physical fatigue and pain. Treatment patterns and levels of patient- and physician-reported treatment satisfaction paralleled those in cluster 1.
Cluster 4 included patients whose disease was clearly the most severe, with a high frequency of fatigue and pain and the highest treatment burden, including the highest use of corticosteroids, biologics and antimalarials across the five clusters. Patients in cluster 4 also had the lowest patient- and physician-reported treatment satisfaction, highlighting a significant unmet treatment need in this cluster. Cluster 3 was of particular interest, as patients had a high burden of pain and fatigue despite relatively low levels of organ involvement (except for articular involvement). Findings for this cluster demonstrated a dissociation between organ involvement and physician-reported pain and fatigue. This concept is supported by numerous studies that have shown the considerable impact of subjective symptoms such as fatigue and pain on patients’ experience of pSS and their related QoL [7, 10, 32–37] but also the lack of correlation between systemic and patient-reported facets of pSS [38]. Our findings complement those of a recent cluster analysis of pSS based on patient-reported outcomes, which identified a pain-dominant cluster with fatigue as well as three other clusters: low symptom burden, high symptom burden and dryness dominant with fatigue [21].
This study has numerous strengths that provide assurance of the quality of the data produced and the relevance of the findings in clinical practice. First, the minimal inclusion criteria ensured a broad inclusion of physicians and patients, producing a study cohort that was representative of the presenting population with systemic pSS. In addition, physicians prospectively provided data for a consecutive series of patients that avoided selection bias, known to be problematic when retrospective patient selection is carried out.
However, the study also had some notable limitations. The cross-sectional nature of the data limited the ability to use cluster analysis techniques to understand the characteristics that may influence longer-term outcomes. Furthermore, the DSP sample is not completely random, as the selection of a patient was influenced by the patient’s propensity to consult his/her physician and his/her current disease severity. Therefore the sample should be considered a pragmatic sample that is not fully representative of the overall population of patients with pSS. It should also be emphasized that these data are reflective of the pSS with systemic involvement population, therefore these findings do not inform patients with pSS and dryness only. The potential influence of country health system differences on the subgroups identified here cannot be completely ruled out and further analysis at a country level may be informative. However, given the absence of proven efficacious treatments for systemic pSS, we do not consider that healthcare access influenced these findings. Additionally, definitions of disease severity were not anchored and there was no definition for mental fatigue on the surveys, leaving participants to interpret this term as pertaining to cognitive impairment or other manifestations. Some of these definitions need to be anchored before correlations with items featured in the ESSPRI and ESSDAI indices can be made. Nonetheless, this analysis relies on a large multinational sample with matched physician- and patient-reported data. The data are valuable because they provide a real-world reflection of the pSS burden of disease. Future work could aim to validate the clusters identified in this study with rheumatologists and using other datasets.
This study confirms that pSS disease burden is determined by fatigue and pain levels as well as organ involvement. Cluster analysis highlights the heterogeneous presentation of patients with pSS while identifying subsets of patients who may benefit from different treatment strategies.
Supplementary Material
Acknowledgements
The authors would like to thank the physicians and their patients who participated in this study. They would also like to thank James Hetherington, who contributed to protocol development, survey design and data analysis. Raj Punwaney, Jennifer Moore and Kiran Nistala gave clinical input into determining the appropriate cluster solution. Medical writing support was provided by Casmira Brazaitis, PhD, and Jennie McLean, PhD, of Fishawack Indicia (Knutsford, UK) and was funded by GlaxoSmithKline. The data that support the findings of this study are available from Adelphi Real World, but restrictions apply to the availability of these data, which were used under license for the current study and thus are not publicly available. However, data are available from the authors upon reasonable request and with permission from Adelphi Real World.
Funding: This study (GSK 207382) was funded by GlaxoSmithKline. Ownership of the data is retained by Adelphi Real World.
Disclosure statement: KG is an employee of GlaxoSmithKline and holds stocks and shares in the company. CK, PA and BH are all employees of Adelphi Real World.
Supplementary data
Supplementary data are available at Rheumatology online
References
- 1. Garcia-Carrasco M, Mendoza-Pinto C, Jimenez-Hernandez C. et al. Serologic features of primary Sjögren’s syndrome: clinical and prognostic correlation. Int J Clin Rheumtol 2012;7:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borchers AT, Naguwa SM, Keen CL, Gershwin ME.. Immunopathogenesis of Sjögren’s syndrome. Clin Rev Allergy Immunol 2003;25:89–104. [DOI] [PubMed] [Google Scholar]
- 3. Qin B, Wang J, Yang Z. et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:1983–9. [DOI] [PubMed] [Google Scholar]
- 4. Both T, Dalm VA, van Hagen PM, van Daele PL.. Reviewing primary Sjögren’s syndrome: beyond the dryness – from pathophysiology to diagnosis and treatment. Int J Med Sci 2017;14:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramos-Casals M, Brito-Zeron P, Seror R. et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 2015;54:2230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holdgate N, St Clair EW.. Recent advances in primary Sjögren’s syndrome. F1000Res 2016;5:1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segal B, Thomas W, Rogers T. et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum 2008;59:1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Papa N, Vitali C.. Management of primary Sjögren’s syndrome: recent developments and new classification criteria. Ther Adv Musculoskelet Dis 2018;10:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stefanski AL, Tomiak C, Pleyer U. et al. The diagnosis and treatment of Sjögren’s syndrome. Dtsch Arztebl Int 2017;114:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Segal BM, Pogatchnik B, Henn L, Rudser K, Sivils KM.. Pain severity and neuropathic pain symptoms in primary Sjögren’s syndrome: a comparison study of seropositive and seronegative Sjögren’s syndrome patients. Arthritis Care Res (Hoboken) 2013;65:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotsis K, Voulgari PV, Tsifetaki N. et al. Illness perceptions and psychological distress associated with physical health-related quality of life in primary Sjögren’s syndrome compared to systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int 2014;34:1671–81. [DOI] [PubMed] [Google Scholar]
- 12. Callaghan R, Prabu A, Allan RB. et al. Direct healthcare costs and predictors of costs in patients with primary Sjögren’s syndrome. Rheumatology (Oxford) 2007;46:105–11. [DOI] [PubMed] [Google Scholar]
- 13. Bowman SJ, St Pierre Y, Sutcliffe N. et al. Estimating indirect costs in primary Sjögren’s syndrome. J Rheumatol 2010;37:1010–5. [DOI] [PubMed] [Google Scholar]
- 14. Bowman SJ, Booth DA, Platts RG. et al. Validation of the Sicca Symptoms Inventory for clinical studies of Sjögren’s syndrome. J Rheumatol 2003;30:1259–66. [PubMed] [Google Scholar]
- 15. Hay EM, Thomas E, Pal B. et al. Weak association between subjective symptoms or and objective testing for dry eyes and dry mouth: results from a population based study. Ann Rheum Dis 1998;57:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seror R, Theander E, Bootsma H. et al. Outcome measures for primary Sjögren’s syndrome: a comprehensive review. J Autoimmun 2014;51:51–6. [DOI] [PubMed] [Google Scholar]
- 17. Perera S, Ma L, Punwaney R, Ramachandran S.. Clinical and cost burden of primary Sjögren’s syndrome: descriptive analysis using a US Administrative Claims Database. J Health Econ Outcomes Res 2018;5:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seror R, Ravaud P, Bowman SJ. et al. EULAR Sjögren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann Rheum Dis 2010;69:1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seror R, Ravaud P, Mariette X. et al. EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjögren’s syndrome. Ann Rheum Dis 2011;70:968–72. [DOI] [PubMed] [Google Scholar]
- 20. Anderson P, Benford M, Harris N, Karavali M, Piercy J.. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr Med Res Opin 2008;24:3063–72. [DOI] [PubMed] [Google Scholar]
- 21. Tarn JR, Howard-Tripp N, Lendrem DW. et al. Symptom-based stratification of patients with primary Sjögren’s syndrome: multi-dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol 2019;1:e85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah DK, Betts AM.. Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs 2013;5:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FACIT.org. Questionnaires. The Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) Scale: Summary of development and validation. www.facit.org/FACITOrg/Questionnaires (July2019, date last accessed).
- 24. Seror R, Bowman SJ, Brito-Zeron P. et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open 2015;1:e000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez Castro M, Sanchez-Piedra C, Andreu JL. et al. Factors associated with severe dry eye in primary Sjögren’s syndrome diagnosed patients. Rheumatol Int 2018;38:1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eligibility for clinical trials in primary Sjögren’s syndrome: lessons from the UK Primary Sjögren’s Syndrome Registry [supplemental appendix]. https://academic.oup.com/rheumatology/article/55/3/544/1793616#supplementary-data (10 June 2019, date last accessed).
- 27. Brito-Zeron P, Acar-Denizli N, Ng WF. et al. Epidemiological profile and north-south gradient driving baseline systemic involvement of primary Sjögren’s syndrome. Rheumatology 2020;59:2350–9. [DOI] [PubMed] [Google Scholar]
- 28. Ramos-Casals M, Brito-Zeron P, Solans R. et al. Systemic involvement in primary Sjögren’s syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology (Oxford) 2014;53:321–31. [DOI] [PubMed] [Google Scholar]
- 29. Lendrem D, Mitchell S, McMeekin P. et al. Health-related utility values of patients with primary Sjögren’s syndrome and its predictors. Ann Rheum Dis 2014;73:1362–8. [DOI] [PubMed] [Google Scholar]
- 30. Shiboski CH, Shiboski SC, Seror R. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Innovative Medicines Initiative. NECESSITY project factsheet. https://www.imi.europa.eu/projects-results/project-factsheets/necessity (June 2020, date last accessed).
- 32. Hackett KL, Newton JL, Frith J. et al. Impaired functional status in primary Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2012;64:1760–4. [DOI] [PubMed] [Google Scholar]
- 33. Mengshoel AM, Norheim KB, Omdal R.. Primary Sjögren’s syndrome: fatigue is an ever-present, fluctuating, and uncontrollable lack of energy. Arthritis Care Res (Hoboken) 2014;66:1227–32. [DOI] [PubMed] [Google Scholar]
- 34. Newton JL, Frith J, Powell D. et al. Autonomic symptoms are common and are associated with overall symptom burden and disease activity in primary Sjögren’s syndrome. Ann Rheum Dis 2012;71:1973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seror R, Gottenberg JE, Devauchelle-Pensec V. et al. European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index and European League Against Rheumatism Sjögren’s Syndrome Patient-Reported Index: a complete picture of primary Sjögren’s syndrome patients. Arthritis Care Res (Hoboken) 2013;65:1358–64. [DOI] [PubMed] [Google Scholar]
- 36. Westhoff G, Dorner T, Zink A.. Fatigue and depression predict physician visits and work disability in women with primary Sjögren’s syndrome: results from a cohort study. Rheumatology (Oxford) 2012;51:262–9. [DOI] [PubMed] [Google Scholar]
- 37. Priori R, Iannuccelli C, Alessandri C. et al. Fatigue in Sjögren’s syndrome: relationship with fibromyalgia, clinical and biologic features. Clin Exp Rheumatol 2010;28:S82–6. [PubMed] [Google Scholar]
- 38. Seror R, Theander E, Brun JG. et al. Validation of EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis 2015;74:859–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.