Abstract
Objectives
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. We evaluated radiographic progression in tofacitinib-treated patients with RA for up to 3 years in two pooled long-term extension (LTE) studies (ORAL Sequel; A3921041) (primary analysis), and for up to 5 years using data integrated from one phase (P)2 (A3921068), two P3 (ORAL Start; ORAL Scan) and two LTE studies (exploratory analysis).
Methods
In LTE studies, patients received tofacitinib 5 mg twice daily (BID) or 10 mg BID as monotherapy or with conventional synthetic (cs)DMARDs. Radiographic outcomes up to 3 years: least squares mean (LSM) change from baseline in van der Heijde modified Total Sharp Score (ΔmTSS), erosion score (ΔES) and joint space narrowing (ΔJSN) score; proportion of patients with no radiographic progression (ΔmTSS ≤0.5); proportion of patients with no new erosions (ΔES ≤0.5). ΔmTSS was evaluated for up to 5 years in an exploratory analysis.
Results
For all tofacitinib-treated patients with radiographic data available at LTE month 36 (n = 414), LSM ΔmTSS was 1.14, LSM ΔES was 0.66, LSM ΔJSN was 0.74, and 74.3% and 86.2% of patients showed no radiographic progression and no new erosions, respectively. Similar values were observed regardless of tofacitinib dose, or whether patients received tofacitinib as monotherapy or with csDMARDs. In an exploratory analysis of integrated P2/P3/LTE studies, LSM ΔmTSS was 3.34 at month 60 (n = 269).
Conclusion
Limited progression of structural damage was observed in tofacitinib-treated patients up to 5 years, with similar results for tofacitinib used as monotherapy or combination therapy up to 3 years.
Trial registration
ClinicalTrials.gov (http://clinicaltrials.gov): NCT01164579; NCT01039688; NCT00847613; NCT00413699; NCT00661661.
Keywords: erosion, joint space narrowing, modified Total Sharp Score, progression, radiograph, RA, tofacitinib
Rheumatology key messages
In tofacitinib studies, limited radiographic progression was observed for up to 5 years.
Similar results were observed for patients receiving tofacitinib as monotherapy or in combination with csDMARDs.
Introduction
RA is a chronic inflammatory disease that is characterized by destruction of the cartilage and bone, resulting in irreversible structural damage and joint injury, and leading to substantial disability [1]. Structural damage is traditionally assessed based on radiographs of the hands and feet, including measurements of erosions and joint space narrowing (JSN) [2].
Inflammation in RA leads to structural damage (overall and in the same joint) over time [3]. Therefore, treatments for RA focus on reducing inflammation in order to prevent structural progression and reduce functional disability. DMARDs for RA are distinguished from symptomatic treatments by their ability to inhibit the progression of structural damage and improve physical function [4].
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib 5 and 10 mg twice daily (BID), administered as monotherapy or in combination with conventional synthetic (cs)DMARDs, mainly MTX, in patients with moderately to severely active RA, have been demonstrated in global phase 2 [5–9], phase 3 [10–16], and phase 3b/4 [17] studies of up to 24 months’ duration and in long-term extension (LTE) studies with up to 114 months of observation [18–20]. Limited radiographic progression was also demonstrated in phase 2 and 3 studies of tofacitinib, and these results are summarized in Supplementary Table S1, available at Rheumatology online [13, 14, 21]. For those patients who had radiographic assessments in phase 2 and 3 studies and enrolled into an LTE study, radiographic assessments were continued in the LTE study. The primary aim of this analysis was to evaluate the long-term rates of progression of structural damage in patients with RA treated with tofacitinib for up to 3 years, based on radiographic data from LTE studies.
Methods
Study designs and treatments
LTE data were pooled from the global ORAL Sequel study (NCT00413699) [20] and the Japanese study A3921041 (NCT00661661) [19]. Patients who had participated in qualifying phase 1, 2, or 3 studies of tofacitinib for RA were eligible to enrol into these LTE studies; however, only patients enrolling from studies with radiographic data available, which included one phase 2 study [A3921068 (NCT01164579) [21]] and two phase 3 studies [ORAL Start (NCT01039688) [13]; ORAL Scan (NCT00847613) [14]], were included in the current analysis. At tofacitinib initiation, patients who participated in A3921068 were patients with early-stage (≤2 years disease duration) active RA who were both MTX- and bDMARD-naïve, ORAL Start patients had moderately to severely active RA and were MTX-naïve, and ORAL Scan patients had moderately to severely active RA and were inadequate responders to MTX, but were on a stable dose of MTX. The majority of enrolled patients from the phase 2 study initiated open-label treatment with tofacitinib at 5 mg BID, and the majority of patients from the phase 3 studies initiated open-label treatment with tofacitinib at 10 mg BID, except for patients from China and Korea who initiated with tofacitinib at 5 mg BID as per the protocol. After LTE baseline, the tofacitinib dose could be increased or decreased at the discretion of the investigator. Patients initiated treatment with tofacitinib either as monotherapy or in combination with csDMARDs; patients were allowed to continue or add stable background csDMARDs (MTX, leflunomide, sulfasalazine, anti-malarials, auranofin, and injectable gold preparations at approved doses) and glucocorticoids (≤10 mg prednisone or equivalent/day).
All studies were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines established by the International Conference on Harmonization. The final protocols, amendments and informed consent documentation were reviewed and approved by the Institutional Review Boards and/or Independent Ethics Committee of each study centre, and all patients provided written informed consent.
Primary analysis
For patients with baseline radiographic data [defined as the last assessment from the index study (i.e. A3921068, ORAL Start, or ORAL Scan) prior to entering the LTE study] who enrolled into the LTE studies, additional radiographs were collected at months 6, 12, 24, and 36 in the LTE studies. ORAL Start, ORAL Scan, and ORAL Sequel each contained two reading campaigns (an interim reading and a final reading), and A3921068 and A3921041 each contained one final reading campaign. Radiographs for each patient were independently assessed by two primary reviewers blinded to visit sequence. Adjudication of discrepancies was performed for each patient by a third reader. Average scores of the two reviewers were used for all analyses.
Radiographic assessments included van der Heijde modified Total Sharp Score (mTSS), erosion score (ES) and JSN score at each time point, and are presented as median score values at each visit as well as mean change from baseline (Δ). The mTSS provides an overall score of 0–448, with higher scores indicating greater radiographic progression [2]. ES range from 0 to 280, with higher scores indicating greater erosion [2], and JSN scores range from 0 to 168, with higher scores indicating greater narrowing [2]. The proportion of patients with no radiographic progression (defined as ΔmTSS ≤0.5), and the proportion of patients with no new erosions (defined as ΔES ≤0.5) were also evaluated. In addition, an exploratory analysis evaluated changes in mTSS for up to 5 years based on radiographic data integrated from phase 2, phase 3, and LTE studies (described in full in Supplementary Materials, available at Rheumatology online).
Statistical methods
For the primary analysis, data were pooled across the two LTE studies. Only patients in the LTE studies with baseline (defined as the last assessment from the index study) and at least one post-baseline X-ray assessments were included in this analysis. A mixed-effect model for repeated (longitudinal) measures (MMRM), with treatment, visit, treatment-by-visit interaction, baseline score, geographic region, and disease duration as fixed effects, and patient as a random effect included in the model, was used for continuous variables, while a generalized estimating equation (GEE) model analysis, with the same factors described above, was used for binary endpoints. All analyses were based on observed cases with no imputation.
Data were analysed for all patients with available radiographic data, and are also presented for patients who stayed on monotherapy compared with those who stayed on background csDMARDs (with all tofacitinib doses combined), and for patients who received tofacitinib 5 mg BID compared with 10 mg BID (with monotherapy and combination therapy groups combined). Patients who received tofacitinib monotherapy (i.e. without background csDMARDs) throughout the LTE studies were assigned to the ‘stay-on monotherapy’ group. Those who initiated and remained on tofacitinib with background csDMARDs for the duration of their participation in the LTE studies, or who had one break of ≤28 days from csDMARDs, were assigned to the ‘stay-on background csDMARDs’ group. As dose changes were allowed in the LTE studies, the average total daily dose was used for analyses by tofacitinib dose. Patients with total daily dose <15 mg were assigned to the average ‘tofacitinib 5 mg BID’ group, and patients with total daily dose ≥15 mg were assigned to the average ‘tofacitinib 10 mg BID’ group. The data are also supported by descriptive analyses, without model adjustment. In addition, two sensitivity analyses for changes from baseline in radiographic outcomes used a non-longitudinal ANCOVA model with both trimmed and un-trimmed data, but included only data from ORAL Sequel. The ANCOVA models included baseline score, treatment, region, and disease duration. Statistical methods for the exploratory analysis are described in Supplementary Materials, available at Rheumatology online.
Results
Patients
The pooled LTE studies enrolled a total of 1244 patients from A3921068 (n = 69), ORAL Start (n = 658), and ORAL Scan (n = 517), of whom 1169 (94.0%) had baseline and at least one post-baseline radiographic assessments. Of these, 159 patients received average tofacitinib 5 mg BID and 1010 received average tofacitinib 10 mg BID; 422 patients stayed on tofacitinib as monotherapy and 573 stayed on tofacitinib in combination with background csDMARDs (the remaining 174 patients did not remain on their initial DMARD or monotherapy throughout the duration of the study). At month 36, 414/1169 (35.4%) patients had radiographic data available, including 88/1169 (7.5%) patients who stayed on monotherapy and 241/1169 (20.6%) who stayed on combination therapy [the remaining 85/1169 (7.3%) patients switched between monotherapy and combination therapy].
Among patients with baseline radiographic data, in the stay-on background csDMARDs group, a numerically greater proportion of patients were Asian and a numerically smaller proportion of patients were White, RA disease duration was numerically longer, mean CRP was numerically lower, and baseline mTSS and ES were numerically higher, compared with the stay-on monotherapy group (Table 1). In the tofacitinib 5 mg BID group, a numerically greater proportion of patients were Asian and a numerically smaller proportion of patients were White, and mean disease duration and baseline mTSS and ES were numerically higher, compared with the tofacitinib 10 mg BID group. Other patient demographics and baseline disease characteristics were generally similar across groups (Table 1).
Table 1.
Demographics and baseline disease characteristics for patients with radiographic data in the LTE studies
| All patients | Stay-on monotherapy | Stay-on background csDMARDs | Tofacitinib 5 mg BIDa | Tofacitinib 10 mg BIDa | |
|---|---|---|---|---|---|
| n = 1169 | n = 422 | n = 573 | n = 159 | n = 1010 | |
| Age, mean (range), years | 52.7 (20–82) | 52.3 (20–82) | 52.9 (20–82) | 53.8 (20–78) | 52.6 (20–82) |
| Female, n (%) | 946 (80.9) | 322 (76.3) | 471 (82.2) | 130 (81.8) | 816 (80.8) |
| Race, n (%) | |||||
| White | 707 (60.5) | 332 (78.7) | 274 (47.8) | 53 (33.3) | 654 (64.8) |
| Black | 31 (2.7) | 10 (2.4) | 15 (2.6) | 1 (0.6) | 30 (3.0) |
| Asian | 271 (23.2) | 45 (10.7) | 187 (32.6) | 96 (60.4) | 175 (17.3) |
| Other | 160 (13.7) | 35 (8.3) | 97 (16.9) | 9 (5.7) | 151 (15.0) |
| BMI, mean (range), kg/m2 | 26.5 (12.1–55.1) | 26.5 (12.1–50.7) | 26.5 (15.7–51.2) | 24.5 (16.0–50.7) | 26.8 (12.1–55.1) |
| Disease duration, mean (range), years | 5.3 (0.0–44.0) | 3.0 (0.0–34.0) | 6.8 (0.1–44.0) | 6.4 (0.0–28.9) | 5.1 (0.0–44.0) |
| mTSS, mean (s.d.) | 25.4 (44.5) | 19.9 (38.3) | 30.6 (49.6) | 31.0 (49.3) | 24.5 (43.7) |
| ES, mean (s.d.) | 13.6 (24.6) | 10.7 (21.0) | 16.2 (27.4) | 17.1 (28.5) | 13.0 (23.9) |
| HAQ-DI, mean (s.d.) | 1.4 (0.7) | 1.5 (0.6) | 1.4 (0.7) | 1.4 (0.6) | 1.5 (0.7) |
| DAS28-4(ESR), mean (s.d.) | 6.3 (1.1) | 6.5 (1.0) | 6.2 (1.1) | 6.1 (0.9) | 6.4 (1.1) |
| CRP, mean (s.d.), mg/l | 19.9 (25.9) | 23.1 (26.5) | 17.0 (24.2) | 19.7 (23.3) | 19.9 (26.3) |
| ESR, mean (s.d.), mm/h | 51.6 (26.6) | 53.3 (26.9) | 49.6 (25.2) | 51.2 (24.4) | 51.6 (27.0) |
| RF+, n (%) | 930 (79.6) | 346 (82.0) | 442 (77.1) | 122 (76.7) | 808 (80.0) |
| Anti-CPP+, n (%) | 967 (82.7) | 352 (83.4) | 470 (82.0) | 128 (80.5) | 839 (83.1) |
Based on average total daily dose.
BID: twice daily; csDMARDs: conventional synthetic DMARDs; DAS28-4(ESR): DAS in 28 joints with ESR; ES: erosion score; HAQ-DI: HAQ-Disability Index; LTE: long-term extension; mTSS: modified Total Sharp Score.
Radiographic outcomes in pooled LTE studies up to 3 years
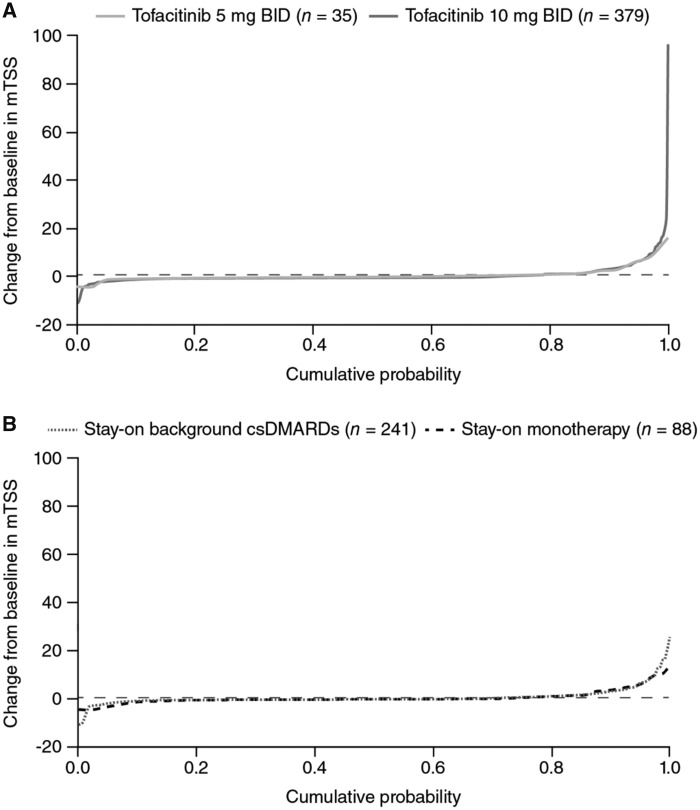
In the pooled LTE studies, the majority of all tofacitinib-treated patients showed no change in mTSS from baseline to month 36 (Fig. 1). Changes from baseline in mTSS were generally similar in patients who stayed on monotherapy or combination therapy, and between tofacitinib doses (Fig. 1). At the earlier time points of months 6, 12, and 24, the majority of patients showed no change from baseline in mTSS, and fewer outliers with large changes from baseline were observed than at month 36 (Supplementary Fig. S2, available at Rheumatology online).
Fig. 1.
Change from baseline in mTSS at month 36
All analyses were based on observed cases. Horizontal reference line represents ΔmTSS equal to 0.5. Δ: change from baseline; BID: twice daily; csDMARDs: conventional synthetic DMARDs; mTSS: modified Total Sharp Score; n: number of patients with baseline and at least one post-baseline radiographic assessments.
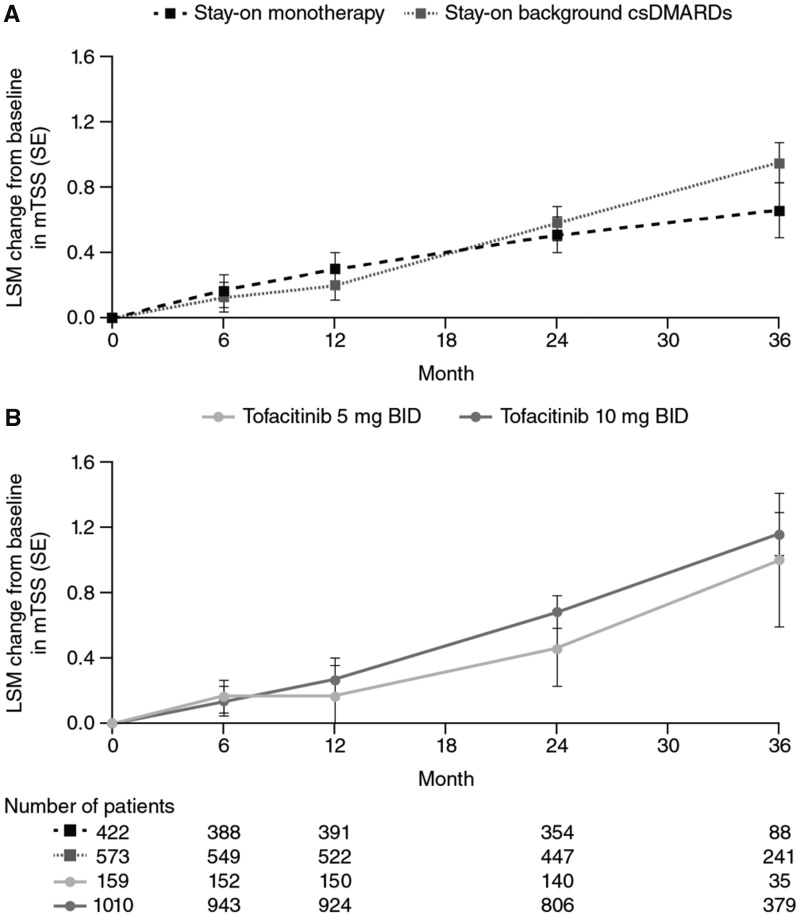
Least squares mean (LSM) changes from baseline in mTSS based on MMRM for all tofacitinib-treated patients showed a small increase over time from 0.14 at month 6, to 1.14 at month 36. Across outcomes, similar changes from baseline were observed between patients who stayed on monotherapy compared with combination therapy (Fig. 2A), and for those who received tofacitinib 5 mg BID compared with 10 mg BID (Fig. 2B), with minimal differences between groups. Similarly, LSM change from baseline in ES increased from 0.13 at month 6, to 0.66 at month 36 (Supplementary Fig. S3A, available at Rheumatology online), and JSN score increased from 0.14 at month 6, to 0.74 at month 36 (Supplementary Fig. S3B, available at Rheumatology online). Values for mean changes from baseline in mTSS, ES, and JSN score were similar when based on observed cases with no imputation and no model adjustment (Supplementary Fig S4, available at Rheumatology online).
Fig. 2.
Least squares mean change from baseline in radiographic assessments over 36 months
All analyses were based on observed cases using MMRM. BID: twice daily; csDMARDs: conventional synthetic DMARDs; LSM: least squares mean; MMRM: mixed-effect model with repeated measures; mTSS: modified Total Sharp Score.
Sensitivity analyses, based on ANCOVA using data from the ORAL Sequel study only, also showed similar results (data not shown). When these data were trimmed to remove 1% of outliers (0.5% at each end of the distribution), the LSM change from baseline to month 36 for all tofacitinib-treated patients was 0.86 for mTSS, 0.45 for ES, and 0.60 for JSN score. When 10% of outliers were removed, the LSM change from baseline to month 36 for all tofacitinib-treated patients was 0.49 for mTSS, 0.21 for ES, and 0.31 for JSN score.
For all tofacitinib-treated patients, median values for mTSS, ES, and JSN score remained relatively stable from baseline throughout 36 months in the LTE studies (Supplementary Fig S5, available at Rheumatology online). Median values at month 6 were 7.0 (inter-quartile range: 1.5–29.8) for mTSS, 4.0 (1.3–15.5) for ES, and 2.5 (0.0–15.3) for JSN score, and at month 36 were 9.8 (2.0–35.5), 5.0 (1.3–17.3), and 3.8 (0.0–18.5), respectively. Across the LTE study duration, including at baseline, median values for mTSS, ES, and JSN score were numerically higher in patients in the stay-on background csDMARDs group vs the stay-on monotherapy group. Median values for mTSS, ES, and JSN score were numerically greater in patients receiving tofacitinib 5 mg BID vs 10 mg BID at baseline through month 36.
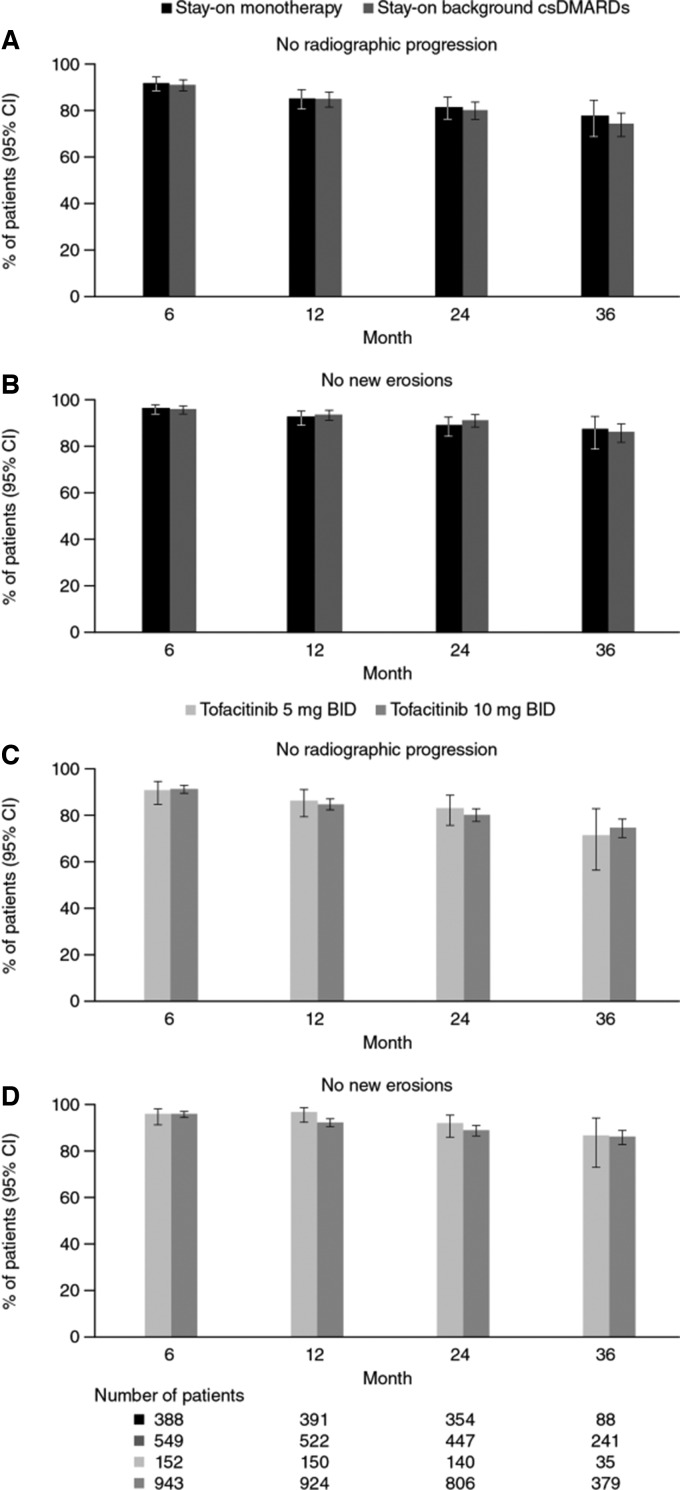
Among all tofacitinib-treated patients, the majority of patients showed no radiographic progression and no new erosions over 36 months based on the GEE model, with 74.3% of patients showing no progression and 86.2% of patients with no new erosions at month 36. No differences in rates of non-progression or no new erosions were observed between patients who stayed on monotherapy or background therapy (Fig. 3A and B), or between tofacitinib doses (Fig. 3C and D). Similar percentages of patients showed no progression and no new erosions based on observed cases with no imputation or model adjustment (data not shown).
Fig. 3.
Percentage of patients with no radiographic progression or no new erosions
All analyses were based on observed cases. BID: twice daily; csDMARDs: conventional synthetic DMARDs.
In addition, progression of mTSS in phase 2, phase 3, and LTE studies up to 5 years was assessed in the exploratory integrated analysis. An increase from baseline of 3.34 was observed for LSM mTSS over 60 months (Supplementary Fig S6A, available at Rheumatology online). Additionally, 51.1% of patients did not experience progression in mTSS at month 60 (Supplementary Fig S6B, available at Rheumatology online).
Discussion
The short-term limitation of progression of structural damage has previously been reported in patients with moderate to severe RA treated with tofacitinib [13, 14, 21]. Given the chronic nature of RA, the present post hoc analysis was undertaken to determine the longer-term impact of tofacitinib treatment for up to 3 years on the progression of structural damage in patients with RA based on data from LTE studies, and for up to 5 years based on radiographic data integrated from phase 2, phase 3, and LTE studies in an exploratory analysis.
The current analysis extends the findings previously reported in phase 2 and 3 studies of tofacitinib, which reported minimal changes from baseline in mTSS, ES, or JSN scores, with the majority of patients showing no radiographic progression and no new erosions up to month 24. In the LTE studies, limited radiographic progression was sustained up to 3 years in the majority of patients, with over 70% of patients who received tofacitinib 5 mg BID or 10 mg BID showing no radiographic progression and no new erosions over this period. Small increases in the mean change from baseline in mTSS, ES, and JSN score were observed over time. However, these differences were minimal, with an ∼1-point increase in mTSS at month 36. In addition, cumulative probability plots indicated that the increase in mean values is likely to be a result of a small proportion of patients who had a higher rate of progression of structural damage. This is supported by the sensitivity analysis for the ORAL Sequel data, which showed substantially smaller mean changes from baseline in mTSS, ES, and JSN score at month 36 when outliers were trimmed from the analysis set.
There were no observed differences in LSM changes from baseline in mTSS, ES, and JSN score, or in rates of non-progression, and no new erosions between patients who received tofacitinib as monotherapy or with background csDMARDs over 3 years in the LTE studies. However, median values were numerically higher at baseline, across all radiographic outcomes, in patients receiving tofacitinib with background csDMARDs compared with monotherapy, suggesting the possibility of structural damage in the beginning of the study. Also, patients who stayed on background csDMARDs had a longer duration of RA than those who stayed on monotherapy. These differences may reflect the different patient populations enrolled and treatments received in the qualifying studies. The majority of patients who received tofacitinib as monotherapy in the LTE studies used in the primary analysis had previously participated in ORAL Start and were MTX-naïve, whereas most patients who received background csDMARDs in the LTE studies had previously participated in ORAL Scan and had a prior inadequate response to MTX. In addition, patients in ORAL Start had a shorter mean disease duration and lower mean baseline mTSS, compared with those in ORAL Scan. Radiographic outcomes were numerically similar between patients who received tofacitinib 5 mg BID or 10 mg BID in the LTE study. However, fewer patients received tofacitinib as monotherapy, compared with those receiving tofacitinib with background csDMARDs, and this may confound interpretation of these results. Likewise, the small number of patients in the tofacitinib 5 mg BID group, especially at month 36, limits the conclusions that can be drawn from these data.
Despite a high baseline median mTSS (25.4) in phase 2/3 studies, an exploratory integrated analysis of radiographic data across phase 2, 3, and LTE studies also showed minimal radiographic progression (mean ΔmTSS of 3.34; 51.1% of patients with no radiographic progression) for patients receiving tofacitinib for up to 5 years. The changes in radiographic outcomes over 3 years in the LTE studies reported here (mean ΔmTSS of 1.14; 74.3% of patients with no radiographic progression) and over 5 years in the integrated analysis of phase 2/3/LTE studies (mean ΔmTSS of 3.34) are similar to those reported for TNF inhibitors in patients with RA, although direct comparisons are limited by the differences in study populations, time that the studies were conducted, and methodology used. In patients treated with adalimumab with MTX in a randomized controlled trial, mean ΔmTSS was 1.2 at year 2, which increased to 2.9 by year 5 in an open-label LTE study (i.e. 1.7 increase from LTE baseline over 3 years) [22]. At year 5, 53% of patients treated with adalimumab with MTX showed no radiographic progression from baseline of the randomized controlled trial (defined as change in mTSS ≤ 0.5) [22].
Radiographic outcomes are presented here using a number of different statistical methods. While observed data provide an understanding of the true values observed in the study, this method does not take into account those patients who may have had radiographic progression and discontinued from the study or did not have radiographic data at later time points. Patients who continue in LTE studies are typically those who have a good response, and may show less progression of structural damage, and can tolerate the treatment. At month 36, a relatively small number of patients had radiographic data (414/1169; 35.4%), which reflects both drop-outs from the study over time, variation in treatment exposure time, and an amendment to the study protocol, where collection of radiographs was stopped at month 36 (even though some patients remained in the study). Therefore, radiographic outcomes are also presented using longitudinal models (MMRM for continuous data and GEE for binary outcomes), which may provide more representative values of the overall LTE study population, and not only those who continued treatment and had a full set of radiographic assessments. The results of these different statistical analyses were similar to one another, suggesting that in these LTE studies, those patients who discontinued were not necessarily those with greater progression of structural damage. In addition, as outliers are common for mTSS data, we conducted a sensitivity analysis using trimmed data. Trimming of mTSS data has been shown to be a useful analysis to establish whether any significant inhibition of structural damage is being driven by extremes of data or if this is a true effect [23]. In the exploratory analysis, only 269/1607 (16.7%) of patients had radiographic data at year 5, resulting in less precision for the estimate of radiographic progression over 5 years in the integrated P2/3/LTE cohort analysis.
In summary, as shown previously in phase 2 and 3 studies, LTE studies show limited radiographic progression in the majority of patients receiving longer tofacitinib treatment, and limited progression of structural damage during long-term treatment with tofacitinib was observed. Results were similar, irrespective of whether tofacitinib was used as monotherapy or in combination with csDMARDs, although limited conclusions can be drawn, due to the low number of patients receiving tofacitinib monotherapy.
Supplementary Material
Acknowledgements
The authors would like to thank Ryan DeMasi for contributions to the manuscript and Vara Bandi and Irina Lazariciu for statistical analysis support, and to thank the study patients and investigators. This study was sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Jennifer Stewart, PhD, MBA, and Alice MacLachlan, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163:461–4).
Funding: This work was sponsored by Pfizer Inc.
Disclosure statement: D.vdH. is the Director of Imaging Rheumatology BV; and has acted as a consultant for AbbVie, Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer Inc, Regeneron, Roche, Sanofi, Takeda, and UCB. R.B.M.L. is the Director of Rheumatology Consultancy BV, which is a registered company under Dutch law; and has acted as a consultant, participated in advisory boards, or has received honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer Inc, and UCB. J.W. has acted as a consultant for, and has received speaker fees and honoraria from, Pfizer Inc. S.S., K.T., K.K., and L.W. are employees and shareholders of Pfizer Inc. S.C. has acted as a consultant for, and has received speaker fees and honoraria from, Pfizer Inc.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. van der Heijde D, Landewé R, van Vollenhoven R, Fatenejad S, Klareskog L.. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis 2007;67:1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 3. Navarro-Compán V, Gherghe AM, Smolen JS. et al. Relationship between disease activity indices and their individual components and radiographic progression in RA: a systematic literature review. Rheumatology (Oxford) 2015;54:994–1007. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 5. Fleischmann R, Cutolo M, Genovese MC. et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 6. Kremer JM, Cohen S, Wilkinson BE. et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 7. Kremer JM, Bloom BJ, Breedveld FC. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka Y, Takeuchi T, Yamanaka H. et al. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 10. Burmester GR, Blanco R, Charles-Schoeman C. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 11. Fleischmann R, Kremer J, Cush J. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 12. Kremer J, Li Z-G, Hall S. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 13. Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 14. van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 15. van Vollenhoven RF, Fleischmann R, Cohen S. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 16. van der Heijde D, Strand V, Tanaka Y. et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: clinical efficacy, radiographic, and safety outcomes from a twenty-four-month, phase III study. Arthritis Rheumatol 2019;71:878–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleischmann R, Mysler E, Hall S. et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 18. Wollenhaupt J, Silverfield J, Lee EB. et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 19. Yamanaka H, Tanaka Y, Takeuchi T. et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther 2016;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wollenhaupt J, Lee EB, Curtis JR. et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conaghan PG, Østergaard M, Bowes MA. et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis 2016;75:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Heijde D, Breedveld FC, Kavanaugh A. et al. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol 2010;37:2237–46. [DOI] [PubMed] [Google Scholar]
- 23. Landewé RB, Connell CA, Bradley JD. et al. Is radiographic progression in modern rheumatoid arthritis trials still a robust outcome? Experience from tofacitinib clinical trials. Arthritis Res Ther 2016;18:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.