ABSTRACT
Background
Many studies have addressed effects of dietary supplementation with soy protein, but most have been inconsistent and few have been long-term studies in men.
Objectives
This study was a secondary analysis of body weight, blood pressure, thyroid hormones, iron status, and clinical chemistry in a 2-y trial of soy protein supplementation in middle-aged to older men.
Methods
Data were analyzed as secondary outcomes of a randomized controlled trial of dietary supplementation with 20 g/d soy protein isolate, providing 41 mg/d total isoflavones and 23 mg/d genistein, in 44- to 75-y-old men who were at risk of cancer recurrence following prostatectomy randomized to soy (n = 50) or a casein-based placebo (n = 43). Weight, blood pressure, and blood samples were collected at baseline, every 2 mo in year 1, and every 3 mo in year 2.
Results
Compared with casein, soy supplementation did not affect body weight, blood pressure, serum total cholesterol, calcium, phosphorus, and thyroid hormones. Serum ferritin concentrations doubled over 2 y in both groups (117–129%), whereas hemoglobin and hematocrit increased slightly. In an exploratory subgroup analysis of soy group data, weight increased in subjects producing equol but not in nonproducers. Blood pressure was reduced in nonequol producers but not in producers. Other endpoints were not affected by equol production status.
Conclusions
Soy protein supplementation for 2 y compared with a casein-based placebo did not affect body weight, blood pressure, serum total cholesterol, iron status parameters, calcium, phosphorus, and thyroid hormones. Exploratory analysis suggests that equol production status of subjects on soy may modify effects of soy on body weight and possibly blood pressure. This trial was registered at clinicaltrials.gov as NCT00765479.
Keywords: soy protein isolate, blood pressure, iron status, thyroid function, cholesterol, men
Introduction
The effects of soy protein consumption on cardiovascular risk factors and other endpoints have been studied in a large number of randomized clinical trials. Meta-analyses have firmly demonstrated that total and LDL cholesterol are modestly reduced by consumption of soy-containing foods and soy protein supplementation (1–4). Likewise, meta-analyses have documented that soy protein and soy isoflavones reduce blood pressure modestly (5, 6). However, it is unclear whether these results apply to men as well as women because most studies were conducted in women only or did not examine men and women separately. Studies limited to men only were mostly focused on prostate cancer–related endpoints, such as blood concentration of prostate-specific antigen (PSA) (7).
Clinical studies of soy in men did not exceed 6 mo with the exception of 1 study of a low-fat diet supplemented with soy in prostate cancer survivors that focused on PSA and lasted 48 mo (8). This study, which was randomized but not placebo-controlled and included only 40 subjects, did not find effects on body weight and total cholesterol by soy supplementation. Other studies in men with soy protein were nutritional clinical trials with inconsistent results. Some of these randomized studies observed a significant but modest decrease in serum cholesterol concentrations (9–13), but other studies did not find this (14–17) or reported a decrease in cholesterol in both soy protein and casein-based placebo control groups (18). Any cholesterol-lowering effect of soy in men has been suggested to be attributable to isoflavones, but this is not clear (10, 12, 19, 20).
Effects of soy protein consumption and isoflavones on blood pressure in men are not consistent either. A significant 3–8% reduction in blood pressure was found in men consuming soy protein isolate (containing isoflavones) for 5 wk or 3 mo compared with men on a casein placebo (13, 18). However, this was not observed in a subsequent crossover trial with hypertensive subjects, men and women combined (21), or in a 24-wk-long soy-containing diet study in combined analysis of men and women (17). By contrast, a reduction in blood pressure was observed with soy protein, with or without depletion of isoflavones, in a randomized 3-mo study in type 2 diabetic men with subclinical hypogonadism (19).
We conducted a randomized trial in men who were at risk of prostate cancer recurrence following radical prostatectomy. The trial involved a 2-y intervention in which subjects received either an isoflavone-containing soy protein isolate beverage or a casein-based placebo (22). There were several safety concerns for this clinical trial based on the literature available at the time of conception of this study. These included allergies to soy protein or casein, adverse effects of soy on iron status and thyroid function, and unwanted effects of the high calcium and phosphorus concentrations (∼70% of daily value) in both the casein placebo and the soy protein beverages (22). Here, we report findings on iron status, thyroid function, and serum calcium/phosphorus concentrations, as well as on body weight, blood pressure, and serum cholesterol, in trial participants who were compliant throughout 2 y of the study (22). To our knowledge, this is the longest clinical study of the effects of soy protein in men, apart from the small 48-mo study mentioned previously (8).
Methods
Study design
This study used secondary outcomes from a randomized, double-blind, casein-based placebo-controlled intervention trial with soy protein isolate (registered at clinicaltrials.gov, identifier NCT00765479) previously reported in detail (22). In this trial, subjects were randomized to the intervention or placebo groups (1:1) using a dynamic intervention allocation procedure stratified by hospital, number of high-risk characteristics for recurrence, and race/ethnicity. Dietary supplement use, customary soy consumption, and allergies to milk protein or soy were exclusionary criteria (22).
The intervention agent was a soy protein isolate-based beverage powder, and the placebo was a similar caseinate-based product, produced for this clinical trial by Solae. Subjects were instructed to consume a daily serving of beverage powder (47 g) containing either soy protein isolate (19.2 g as analyzed) or calcium caseinate (19.8 g). The beverage powders were sweetened with a mixture of sucrose and fructose to improve palatability, and artificial strawberry flavoring was added to mask the taste difference between the 2 powders. The soy protein isolate powder contained per serving 70.5 mg of all forms of isoflavones and in aglycone equivalents, 41 mg total isoflavones, 23.8 mg of genistein, and 15.0 mg daidzein, as previously detailed (22). The nutrient composition of the powders is provided in Supplemental Table 1. Subjects were instructed to incorporate the beverage consumption in their daily routine without changing their dietary habits.
Subjects were asked about their medical history and use of medications at baseline and at follow-up visits every 2 mo in year 1 of the study and every 3 mo in year 2. At each study visit, body weight and blood pressure were measured and blood samples were taken. Seven subjects who had blood samples drawn elsewhere and mailed overnight to New York University School of Medicine (NYU) were not included in the current study, except for body weight and blood pressure parameters.
During the first 3 y of the study, blood samples were submitted for hematology and ferritin measurements at each time point for each subject. Once adverse effects on iron status were ruled out and approved by the study's data safety and monitoring board, these analyses were limited to baseline and the 12- and 24-mo time points. Clinical chemistry measurements were conducted at baseline and end of study, except for thyroid hormone measurements, which were conducted on samples from subjects without self-reported thyroid conditions and/or on thyroid medications at baseline and at 2, 4, 6, 12, and 18 mo.
Blood and serum assays
Hemoglobin, hematocrit, and red and white blood cell and platelet counts were measured in EDTA blood samples. Serum concentrations were measured for glucose, blood urea nitrogen (BUN), creatinine, electrolytes (sodium, potassium, chloride, and carbon dioxide), total cholesterol, calcium, phosphorus, uric acid, total protein, albumen, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase, and total bilirubin. These measurements were conducted at the NYU Tisch Hospital Clinical Pathology Laboratory using its routine assays. Thyroid hormones were measured in serum samples that had been frozen prior to measurement, according to the assay's requirements, in our research laboratory using an automated immunoenzymometric assay (Tosoh AIA-600; Tosoh Bioscience). These samples were collected between September 2003 and July 2007, and aliquots were stored at –80°C from 2003 to early 2007 and in liquid nitrogen vapor after that time. The intra-assay and interassay CVs of the thyroid hormone assays were 3.4–5.7% and 2.0–6.3%, respectively. Serum ferritin concentrations in frozen serum samples were also measured using a Tosoh assay with an intra-assay CV of 2.3% and an interassay CV of 4.3–4.9%; these measurements were made within 3 wk of blood collection. Serum aglycone genistein and equol concentrations were measured in small batches of aliquoted samples that were stored for no more than 4 y at –80°C or in liquid nitrogen vapor. Isoflavone measurements were done using HPLC with electrochemical detection (CoulArray model 5600A; ESA Biosciences) as described by Gamache and Acworth (23) and Franke et al. (24, 25). The recovery of the internal control (estriol-glucuronide) was 76% with a CV of 17%. The interrun CV was 2.05% and 1.14% for genistein and equol, respectively, and the sensitivity was 3 ng/mL for both analytes.
Statistical analysis
We computed changes in marker values from baseline to various time points (based on the measurement timeline for each marker), examined changes from baseline to the various time points at which each marker was measured, and compared these changes between the 2 treatment groups. These analyses were conducted within each treatment arm using 1-way repeated-measures ANOVA with Bonferroni correction for multiple comparisons and a test for linear trend. Differences between the 2 treatment groups (soy compared with placebo) at the end of the study were analyzed using ANCOVA, with age, height, and (except when weight was the endpoint) weight at baseline as covariates. Interactions between treatment and time were assessed using a 2-way repeated-measures ANOVA. If the D'Agostino–Pearson test was not passed for ≥1 of the samples, a log transformation of the data was applied. For some comparisons between baseline and the 24-mo time point in each of the 2 treatment groups, we used the paired t test or the 2-sided Wilcoxon's matched-pairs signed-rank test. MedCalc software (version 19.4.0) was used for these analyses, except 2-way repeated-measures ANOVA and t and Wilcoxon's tests, for which Prizm software (version 4.03; GraphPad) was used. P values were 2-sided. All data are presented as means and 95% CI unless indicated otherwise.
Ethics
The study was approved by the Institutional Review Boards of New York University School of Medicine and the University of Illinois at Chicago and registered at clinicaltrials.gov (NCT00765479).
Results
Study subjects and baseline characteristics
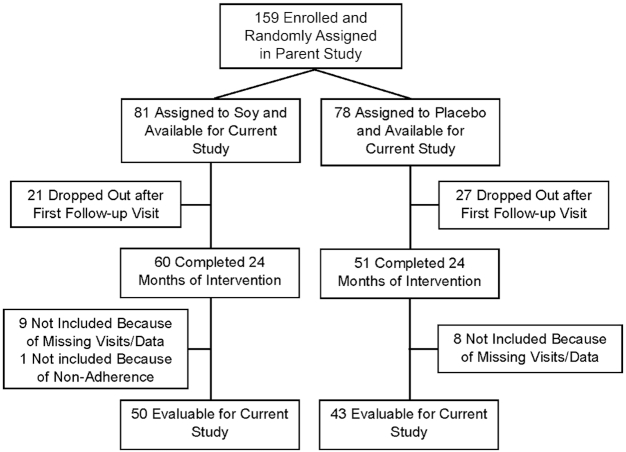
Most participants were enrolled at NYU or the Manhattan VA Medical Center (VA) in New York City. A total of 159 eligible men were enrolled between July 1997 and November 2005 (VA) or May 2009 (NYU), as previously detailed (22). Eighty-one participants assigned to soy protein and 78 to placebo were evaluable for the primary endpoint (biochemical recurrence) (22). Forty-eight (30.1%) stopped intervention for the following reasons: palatability or lack of interest or time, mostly within the first 2 mo (n = 26); PSA recurrence (n = 20); or suspected (but not confirmed) adverse effects (n = 2) (Figure 1). Eighteen subjects were not included because of missing study visits or data (n = 15) or because they had not completed 24 mo on study when it was stopped early (n = 2). One subject self-reported to be noncompliant throughout and was only included in the intent-to-treat analysis [adherence was assessed by the number of beverage powder packets consumed/the number of packets supplied for self-reported compliance (22)].
FIGURE 1.
TYFlow diagram of 44- to 76-y-old US men randomly assigned to a 20 g/d soy protein isolate supplement or a 20 g/d milk protein placebo for 2 y.
A separate analysis was conducted by a modified intent-to-treat approach including subjects who had stopped treatment and 1 who was not compliant. This added 4 subjects to the soy group and 9 subjects to the placebo group, for all of whom we had complete data in body weight, cholesterol, blood pressure, and hematology endpoints. Seven subjects had stopped because of palatability problems, 4 because they “lost interest” or were “too busy,” and 1 because of suspected (but not substantiated) adverse events.
As detailed in Table 1 and Figure 1, 93 subjects (50 subjects on soy protein and 43 subjects on placebo) completed 2 y of intervention, adhered to the treatment, and had blood samples available at baseline, 12 mo, and 24 mo that were of adequate quality for the assays used in the current study. Only 13 (26%) subjects on soy and 10 (23%) subjects on placebo were not on any medication while on the study, and many were on cholesterol-lowering and/or antihypertensive drugs (Table 1, Supplemental Table 2). Both groups were predominantly white (Caucasian) and were comparable in age and BMI. The vast majority of subjects were recruited at Tisch Hospital (Table 1).
TABLE 1.
Demographic and baseline data
| Soy protein | Placebo | |
|---|---|---|
| Evaluable subjects, n | 50 | 43 |
| Age,1 y | 60.6 (58.8, 62.4) | 62.5 (60.4, 64.6) |
| Body weight,1,2 kg | 86.9 (82.1, 89.9) | 90.0 (85.0, 95.1) |
| BMI,1,3 kg/m2 | 27.4 (26.2, 28.5) | 28.2 (26.8, 29.7) |
| Race4 | ||
| White (Caucasian) | 47 (94%) | 38 (89%) |
| African American | 2 (4%) | 2 (5%) |
| Hispanic | 1 (2%) | 1 (2%) |
| Asian | 0 | 1 (2%) |
| Other (Pacific Islander) | 0 | 1 (2%) |
| Hospital | ||
| Tisch Hospital | 44 (88%) | 39 (91%) |
| Manhattan VA | 5 (10%) | 4 (9%) |
| Other | 1 (2%) | 0 |
| Subjects not on cholesterol-lowering medication4 | 34 (68%) | 22 (51%) |
| Subjects not on antihypertensive medication4 | 29 (58%) | 22 (51%) |
| Subjects not on any medication4 | 13 (26%) | 10 (23%) |
Values are means (95% CIs).
Body weight data are missing for 2 subjects in the soy protein group and 1 subject in the placebo group.
BMI data are missing for 5 subjects in the soy protein group and 3 subjects in the placebo group because of missing weight and/or height data.
Self-reported.
Serum isoflavone concentrations and equol production
Serum concentrations of genistein were measured in subjects in the soy protein group initially only at 6 and 12 mo and later in the study at 2, 4, 6, 8, 12, and 18 mo and were quite variable, with a mean of 142.4 ng/mL and a median of 110.8 ng/mL (IQR: 48.0–229.0; n = 141 samples). In light of the short half-life of aglycone genistein in men, which is on the order of 3 h (26, 27), this variability was likely due to the fact that we could not standardize the time between soy consumption and time of blood draw because of the often-difficult logistics of study visit scheduling. Genistein was also detectable, but at much lower concentrations, in some of the serum samples from 26 of 31 (84%) placebo subjects for whom we had data (median: 2.0 ng/mL; IQR: 0.4–6.6). Seventeen of 48 (35%) soy subjects for whom we had equol values were equol producers (>20 ng/mL) (28), and there were 15 (31%) subjects who produced equol in low concentrations (>2 to <20 ng/mL), 10 subjects (21%) who did not produce detectable equol (<2 ng/mL), and 6 subjects (13%) who varied between equol production and no equol production at different times (data not shown). We were not able to determine equol production status in the placebo group.
Effects on body weight, blood pressure, and cholesterol
There was a mean weight gain at 2 y of 2.6% in the intervention group and 2.8% in the placebo group. There was no difference in weight gain between the 2 groups in an ANCOVA with height and age at baseline as covariates and no interaction between treatment and time for body weight (Table 2). Data for total serum cholesterol concentrations were only available at baseline and end of study (Table 2). There was no effect of placebo or soy protein on total serum cholesterol in subjects not taking cholesterol-lowering drugs (56% and 42% of all evaluable subjects in the soy and placebo group, respectively). This finding did not change when considering equol production status of subjects in the soy group (data not shown) or conducting the statistical analysis by intent to treat (Supplemental Table 3). There was no difference in 2-y change between the soy and placebo groups in ANCOVA.
TABLE 2.
Body weight and serum total cholesterol at baseline and percentage change from baseline at 12 and 24 mo1
| n | Baseline | Δ at 12 mo, % | Δ at 24 mo, % | ||||
|---|---|---|---|---|---|---|---|
| Body weight,2 kg | P change3 | P trend3 | P ANCOVA4 | ||||
| Soy | 42 | 86.9 (82.1, 89.9) | 1.4 (0.2, 2.5) | 2.6 (1.1, 4.0) | <0.001 | <0.001 | 0.488 |
| Placebo | 38 | 90.0 (85.0, 95.1) | 2.8 (1.4, 4.3) | 2.8 (1.4, 4.3) | <0.001 | <0.001 | |
| Total cholesterol,5 mg/dL | P difference6 | P ANCOVA4 | |||||
| Soy | 29 | 196.9 (186.1, 207.7) | NA | 2.0 (–2.2, 6.2) | 0.351 | 0.316 | |
| Placebo | 18 | 200.5 (186.8, 218.2) | NA | 0.7 (–3.9, 5.4) | 0.904 |
Values are means (95% CIs). NA, not available.
Only subjects with complete data on body weight at baseline, 12 mo, and 24 mo and age and height at baseline are included in this analysis.
One-way repeated-measures ANOVA of change over time.
ANCOVA for difference in percentage change from baseline between the 2 treatments at 24 mo, with age, height, and weight at baseline as covariates.
Only subjects not on cholesterol-lowering medication (self-reported) at any time during the study and with complete data on cholesterol at baseline, 12 mo, and 24 mo and age and height at baseline are included.
Two-sided paired t test.
There was no difference between the soy and placebo groups for systolic and diastolic blood pressure (Table 3). Conducting the statistical analysis by modified intent to treat did not change this result (Supplemental Table 4). There was a slight reduction (2.5–3.5%) in blood pressure from baseline in subjects not on antihypertensive medication (52% and 37% of all evaluable subjects in the soy and placebo group, respectively) at 12 and 24 mo in both groups that was not statistically significant. This reduction may be attributable to possible anxiety related to entering a study that might alter risk of prostate cancer recurrence, similar to the well-established white coat effect on blood pressure. In support of this view, blood pressure was constant between 2 and 24 mo in both groups, and we found the same reduction in blood pressure at 12 and 24 mo compared with baseline (Table 3) and the same constant blood pressure between 2 and 24 mo in subjects taking antihypertensive medication (data not shown).
TABLE 3.
Systolic and diastolic blood pressure at baseline or 2 mo into the study and percentage change from baseline or from 2 mo at 12 and 24 mo1
| n | Baseline | 2 mo | Δ at 12 mo, % | Δ at 24 mo, % | P difference2 | P trend2 | P ANCOVA3 | |
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure, mm Hg | ||||||||
| Soy | ||||||||
| Not on hypertension medication4 | ||||||||
| 26 | 133.7 (126.1, 141.3) | –3.4 (–7.5, 0.8) | –2.7 (–7.0, 1.5) | 0.078 | 0.109 | 0.693 | ||
| 21 | 130.5 (124.3, 136.8) | –1.6 (–4.6, 1.4)5 | –0.6 (–5.4, 4.1)5 | 0.7635 | 0.8295 | 0.8746 | ||
| On hypertension medication6 | ||||||||
| 18 | 135.6 (126.6, 144.9) | –1.9 (–8.2, 4.5) | –1.8 (–8.7, 5.1) | 0.564 | 0.369 | 0.065 | ||
| Placebo | ||||||||
| Not on hypertension medication4 | ||||||||
| 16 | 131.8 (123.4, 140.1) | –3.2 (–9.4, 3.0) | –2.5 (–9.4, 4.4) | 0.269 | 0.296 | |||
| 15 | 128.3 (119.0, 137.6) | 0.4 (–5.9, 6.7)5 | 0.4 (–5.8, 6.6)5 | 0.9675 | 0.8595 | |||
| On hypertension medication6 | ||||||||
| 20 | 138.6 (129.8, 147.4) | –4.1 (–8.5, 0.3) | –7.3 (–12.6, –2.1) | 0.012 | 0.012 | |||
| Diastolic blood pressure, mm Hg | ||||||||
| Soy | ||||||||
| Not on hypertension medication4 | ||||||||
| 26 | 79.6 (75.9, 83.3) | –3.5 (–6.6, 2.5) | –1.4 (–6.1, 3.3) | 0.129 | 0.357 | 0.748 | ||
| 24 | 78.8 (75.5, 82.2) | –2.8 (–5.8, 0.3)5 | –0.8 (–4.6, 3.1)5 | 0.1925 | 0.5335 | 0.4335 | ||
| On hypertension medication6 | ||||||||
| 18 | 81.6 (76.0, 87.1) | –0.8 (–7.3, 5.8) | –6.2 (–19.0, 6.7) | 0.831 | 0.704 | 0.923 | ||
| Placebo | ||||||||
| Not on hypertension medication4 | ||||||||
| 16 | 79.1 (75.0, 83.3) | –3.7 (–8.9, 1.4) | –3.9 (–10.0, 2.2) | 0.165 | 0.159 | |||
| 15 | 76.9 (73.2, 80.5) | –1.4 (–8.3, 5.4)5 | –1.8 (–8.7, 5.1)5 | 0.7435 | 0.5205 | |||
| On hypertension medication6 | ||||||||
| 20 | 79.4 (74.2, 84.5) | –3.6 (–10.9, 3.7) | –5.2 (–10.6, 0.3) | 0.138 | 0.063 | |||
Values are means (95% CIs). Only subjects with complete data on blood pressure at baseline or 2 mo and at 12 and 24 mo and age and height at baseline are included in this analysis.
One-way repeated-measures ANOVA of change over time.
ANCOVA for difference in percentage change from baseline between the 2 treatments at 24 mo, with age, height, and weight at baseline as covariates.
Only subjects not on antihypertensive medication (self-reported) at any time during the study and with complete data on blood pressure at baseline, 12 mo, and 24 mo and age and height at baseline are included.
The 12- and 24-mo data were compared with those of the first study visit occurring 2 mo after baseline.
These are subjects who self-reported to be on antihypertensive medication at any time during the study and for whom there were complete data on blood pressure at baseline and 24 mo and age and height at baseline.
In subgroup analysis of the soy group according to equol production status, body weight increased over 2 y in equol producers and low-equol producers but not in nonequol producers (Table 4). We found the same effects on body weight of equol production status when we included data at all time points during the 2 y of intervention in the analysis (data not shown). Interestingly, body weight at baseline was associated with equol production status (P < 0.001; ANCOVA with age and height as covariates); nonequol producers had lower body weight compared with equol producers (P < 0.001) and low-equol producers (P < 0.001), whereas weights of producers and low-equol producers were not different (P = 0.455).
TABLE 4.
Body weight and blood pressure at baseline and changes compared with baseline at 12 and 24 mo according to equol production status in subjects in the soy protein group1
| n | Baseline | Δ at 12 mo, % | Δ at 24 mo, % | P change2 | P trend2 | P ANCOVA3 | |
|---|---|---|---|---|---|---|---|
| Body weight, kg | |||||||
| Equol producers | 14 | 88.2 (80.6, 95.8)4,5,6 | 2.6 (1.3, 3.9) | 3.1 (1.1, 5.1) | 0.002 | 0.007 | <0.001 |
| Low-equol producers | 15 | 84.3 (78.5, 90.8) 4,6,7 | 1.1 (–1.5, 3.6) | 3.4 (0.4, 6.3) | 0.018 | 0.020 | |
| Nonequol producers | 8 | 81.0 (67.3, 94.8)4,5,7 | –0.04 (–2.9, 2.8) | 1.2 (–1.9, 4.3) | 0.479 | 0.401 | |
| Systolic blood pressure, mm Hg | |||||||
| Equol producers | 13 | 140.2 (127.0, 153.3) | –3.6 (–10.5, 3.2) | –3.9 (–12.5, 4.8) | 0.287 | 0.221 | 0.252 |
| Low-equol producers | 12 | 128.3 (116.5, 140.2) | –0.3 (–7.7, 7.2) | 3.1 (–2.9, 9.2) | 0.416 | 0.166 | |
| Nonequol producers | 10 | 136.8 (126.5, 147.1) | –6.7 (–13.3, –0.1) | –8.2 (–14.4, –1.9) | 0.023 | 0.014 | |
| Diastolic blood pressure, mm Hg | |||||||
| Equol producers | 118 | 82.3 (76.3, 88.3) | –3.4 (–9.5, 2.6) | –3.2 (–10.2, 3.9) | 0.281 | 0.200 | 0.133 |
| Low-equol producers | 119 | 76.8 (69.6, 83.9) | –2.1 (–9.8, 5.5) | 1.1 (–5.2, 7.4) | 0.871 | 0.819 | |
| Nonequol producers | 88 | 82.2 (75.7, 88.7) | –4.8 (–11.0, 1.3) | –9.1 (–13.4, 1.2) | 0.022 | 0.018 | |
Values are means (95% CIs). Equol producers were defined as subjects with serum equol >20 ng/mL and low-producers as subjects with serum equol (2–20 ng/mL), whereas nonproducers had undetectable serum equol.
One-way repeated-measures ANOVA of change over time.
ANCOVA for difference in percentage change from baseline between the 3 groups at 24 mo, with age, height, and (for blood pressure only) weight at baseline as covariates.
P < 0.001; ANCOVA with age and height as covariates for difference among the three groups.
P < 0.001 for difference between equol producers and nonequol producers (ANCOVA with age and height as covariates).
P < 0.001 for difference between equol producers and low-equol producers (ANCOVA with age and height as covariates).
P = 0.455 for difference between low-equol producers nonequol producers (ANCOVA with age and height as covariates).
Two far outliers identified by the Grubbs test were omitted from the analysis; these outlier subjects did not have systolic blood pressure values that were outliers.
One far outlier identified by the Grubbs test was omitted from the analysis; this outlier subject did not have a systolic blood pressure value that was an outlier.
Systolic and diastolic blood pressure were statistically significantly reduced in nonequol producers at 12 and 24 mo but not in low-equol producers, but the difference between the 3 groups in change from baseline at 24 mo was not significant by ANCOVA (Table 4). When we compared these 2 time points with the 2-mo time point, the effect sizes diminished but remained statistically significant in nonequol producers for diastolic pressure (P = 0.036; P trend = 0.023) but not for systolic pressure (P = 0.227; P trend = 0.094) (data not shown).
Effects on iron status and hematology
Ferritin concentrations increased in both soy and placebo groups at all time points, nearly doubling over baseline at end of study (Table 5, showing only the baseline, 12-mo, and 24-mo data). This effect was independent of equol production status of subjects in the soy group (data not shown). Hemoglobin concentrations increased slightly over time in both groups, as did hematocrit values, whereas RBC counts remained stable throughout the study in both groups (Table 5). There was no difference between the 2 groups in the change at 24 mo of these parameters. Limiting the analysis to the small number of subjects who were not on any medication during the study did not alter the results, although they remained statistically significant only for ferritin with an increase in the effect size (data not shown). There was no interaction between treatment and time in 2-way repeated-measures ANOVA for ferritin, hemoglobin, RBC counts, and hematocrit. Conducting the statistical analysis by intent to treat did not change these results (data not shown).
TABLE 5.
Serum ferritin, hemoglobin, hematocrit, and RBC counts at baseline and at 12 and 24 mo1
| n | Baseline | Δ at 12 mo, % | Δ at 24 mo, % | P change2 | P trend2 | P ANCOVA3 | |
|---|---|---|---|---|---|---|---|
| Ferritin, ng/mL | |||||||
| Soy | 38 | 48.3 (37.8, 58.9) | 78.3 (53.2, 103.4) | 128.8 (61.9, 195.6) | <0.001 | <0.001 | 0.608 |
| Placebo | 33 | 60.3 (42.4, 78.3) | 70.2 (45.4, 95.0) | 117.1 (79.9, 157.2) | <0.001 | <0.001 | |
| Hemoglobin, g/dL | |||||||
| Soy | 45 | 14.5 (14.2, 14.8) | 2.7 (1.0, 4.3) | 2.7 (0.8, 4.3) | <0.001 | 0.003 | 0.722 |
| Placebo | 37 | 14.7 (14.3, 15.0) | 1.7 (0.0, 3.5) | 3.3 (1.5, 5.1) | 0.002 | <0.001 | |
| Hematocrit, % | |||||||
| Soy | 46 | 43.6 (42.7, 44.6) | 1.7 (–0.2, 3.6) | 2.6 (0.8, 4.4) | 0.015 | 0.008 | 0.445 |
| Placebo | 37 | 44.1 (43.0, 45.2) | 1.6 (–0.2, 3.5) | 3.6 (1.4, 5.7) | 0.002 | 0.002 | |
| RBCs, 1012/L | |||||||
| Soy | 46 | 5.00 (4.88, 5.08) | –0.9 (–2.2, 0.5) | –0.1 (–1.7, 1.4) | 0.323 | 0.816 | 0.969 |
| Placebo | 38 | 4.99 (4.85, 5.13) | –1.9 (–3.8, 0.0) | 0.2 (–1.9, 2.3) | 0.041 | 0.934 | |
Values are means (95% CIs). Only subjects with complete data at baseline, 12 mo, and 24 mo and age, weight, and height at baseline are included in this analysis.
One-way repeated-measures ANOVA of change over time.
ANCOVA for percentage difference between the 2 treatments at 24 mo, with age, height, and weight at baseline as covariates.
Effects on thyroid hormones
There were no differences between the soy and placebo groups at baseline and between baseline and 2–18 mo on study for the thyroid hormones measured [triiodothyronine (T3), free triiodothyronine (fT3), thyroxine (T4), and free thyroxine (fT4)]. There was no difference at baseline between the soy and placebo groups in thyrotropin [thyroid-stimulating hormone (TSH)] concentration, which increased over time in the placebo group (by 34–37%) but not in the soy group, whereas there was no significant difference between the 2 groups in the change from baseline at 18 mo by ANCOVA (Table 6). Because most of the subjects (8/11) in the thyroid hormone analysis were equol producers, we could not evaluate the effect of equol production status. There was no interaction between treatment and time in 2-way repeated-measures ANOVA for any of these endpoints (T3: P = 0.208; fT3: P = 0.604; T4: P = 0.571; fT4: P = 196; TSH: P = 0.677).
TABLE 6.
Thyroid hormone concentrations at baseline and change from baseline at 2–18 mo of study1
| Δ, % | ANOVA2P | Trend2P | ANCOVA3P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Group | Baseline | 2 mo | 4 mo | 8 mo | 12 mo | 18 mo | |||
| Triiodothyronine, ng/mL | Soy | 1.02 (0.87, 1.17) | 6.56 (–2.23, 15.53) | 4.05 (–3.49, 11.59) | 3.79 (–6.59, 14.19) | 6.38 (–6.59, 19.34) | 5.99 (–1.89, 13.88) | 0.696 | 0.430 | 0.948 |
| Placebo | 1.01 (0.83, 1.19) | 4.47 (–5.79, 14.72) | 1.68 (–7.74, 11.10) | 5.64 (–5.88, 17.15) | 4.67 (–5.44, 14.79) | 4.00 (–6.33, 14.34) | 0.861 | 0.471 | ||
| Free triiodothyronine, pg/mL | Soy | 2.99 (2.64, 3.34) | 7.53 (1.22, 13.85) | 7.42 (0.23, 14.60) | 5.52 (–2.37, 13.41) | 4.00 (–3.53, 11.53) | 8.65 (1.16, 16.14) | 0.189 | 0.221 | 0.940 |
| Placebo | 2.74 (2.34, 3.14) | 7.48 (–4.52, 19.47) | 3.55 (–11.04, 18.13) | 7.40 (–3.34, 18.14) | 4.02 (–5.95, 14.00) | 9.20 (–2.84, 21.24) | 0.438 | 0.158 | ||
| Thyroxine, µg/dL | Soy | 6.39 (5.80, 7.98) | –1.07 (–7.00, 4.86) | –6.66 (–25.03, 11.71) | 1.52 (–6.91, 9.95) | 6.88 (–2.92, 16.69) | 5.83 (–4.70, 16.35) | 0.396 | 0.085 | 0.6484 |
| Placebo | 6.26 (5.42, 7.11) | –1.83 (–11.54, 7.88) | 1.57 (–7.51, 10.66) | 0.28 (–9.85, 10.40) | 1.86 (–7.09, 10.81) | 6.43 (–4.22, 12.91) | 0.595 | 0.207 | ||
| Free thyroxine, ng/dL | Soy | 1.25 (1.11, 1.40) | 2.53 (–3.48, 8.53) | 2.79 (–5.27, 10.84) | 1.92 (–4.02, 7.86) | 4.42 (–3.66, 12.49) | 5.41 (0.11, 10.71) | 0.616 | 0.124 | 0.2144 |
| Placebo | 1.17 (1.05, 1.30) | 0.35 (–5.91, 6.61) | 1.30 (–4.20, 6.81) | 1.37 (–7.37, 10.11) | –0.77 (–7.58, 6.04) | 2.14 (–5.22, 9.50) | 0.944 | 0.874 | ||
| Thyrotropin, µIU/mL | Soy | 2.13 (1.30, 3.00) | –1.3 (–17.6, 14.0) | 4.9 (–16.5, 26.2) | 9.4 (–3.0, 21.9) | 10.1 (–11.4, 31.6) | 11.3 (–12.5, 35.1) | 0.674 | 0.130 | 0.856 |
| Placebo | 1.82 (1.21, 2.43) | 10.5 (–10.0, 31.1) | 22.2 (–6.1, 50.4) | 21.0 (–11.2, 53.3) | 33.6 (13.6, 53.6) | 37.1 (10.0, 64.2) | 0.022 | 0.005 | ||
Values are means (95% CIs). Only subjects without self-reported thyroid disease and/or on thyroid medication and with complete data at all time points are included. Each group consisted of 11 men. The age (mean ± SD) of the soy group was 59.3 ± 6.1 y, and that of the placebo group was 61.6 ± 8.3 y (P = 0.472; 2-sided t test).
Two-way repeated-measures ANOVA of change over time after log transformation of the data.
ANCOVA for difference in percentage change from baseline between the 2 treatments at 18 mo, with age at baseline as covariate on log-transformed data unless indicated otherwise.
The data for this ANCOVA analysis were not transformed to avoid violating required assumptions.
Effects on other parameters
Statistically significant differences between baseline and end of study were found for BUN, uric acid, AST, LDH, and alkaline phosphatase (Supplemental Table 5). BUN concentrations at 24 mo were increased over baseline in both intervention groups. These differences were no longer significant when considering only subjects not on any medication. Uric acid concentrations were slightly elevated at 24 mo compared with baseline in the soy group only; this was not found when limiting the analysis to subjects who were not on any medication. AST concentrations were elevated at end of study over baseline, and this difference remained when considering only subjects not on any medication, but it was only significant in the soy group. LDH concentrations were elevated at end of study over baseline in both intervention groups, and this difference increased in the soy group when considering only subjects not on any medication. Alkaline phosphatase concentrations were slightly elevated at 24 mo over baseline, but not when considering only subjects not on any medication. Although we do not have an explanation for these observations other than the aging process, the concentrations of these analytes were well within the normal reference range and their elevations thus do not constitute adverse effects.
There was no difference between baseline and 24 mo for serum calcium and phosphorus concentrations when considering all evaluable subjects or only those who were not on any medication, and serum concentrations of these 2 analytes remained well within the normal reference range in both study groups (Supplemental Table 5). There were no differences between baseline and end of study in either intervention group for white blood cell and platelet counts and for serum concentrations of glucose, creatinine, electrolytes (sodium, potassium, chloride, and carbon dioxide), uric acid, total protein, albumen, and total bilirubin (data not shown). Of note, no effects of isolated soy isoflavones on any of these parameters were reported in a single-dose and 12-wk study (26, 27).
Discussion
In this 2-y randomized intervention study with ∼20 g/d soy protein isolate providing ∼70 mg/d total isoflavones and ∼24 mg/d genistein compared with a casein-based placebo, serum concentrations of genistein in the soy group were comparable to those of other studies with soy protein isolate at doses that were similar or somewhat higher than we used (9, 29, 30). The percentage of equol producers in the soy group was similar to those reported in the literature (28, 31).
We observed an increase in body weight over 2 y in both the soy group and the placebo group, which may reflect a weight gain with aging. Alternatively, one could speculate that this weight gain is related to the energetic effect of adding the protein supplementation to the customary dietary habits of the study subjects, but adding subjects who had stopped treatment in intent-to-treat analysis did not change the result. No changes in body weight were found in all similar previous studies of shorter duration (8, 10, 13, 15–18, 21).
No effect of soy was found on blood pressure, consistent with the results of a 24-wk-long study (15) but not with those of two 3-mo studies with interventions comparable to the current trial that found decreases in blood pressure (17, 18). These contrasting results may suggest that duration of intervention is an important factor. However, in a meta-analysis, the blood-pressure–lowering effects of soy were greater in interventions lasting 12–24 wk than in shorter-duration studies (6). Because the current 2-y study showed no such effect, duration of soy intervention may have a biphasic or transient effect on blood pressure. We observed higher blood pressure at baseline than at all later time points in both study groups, whereas blood pressure was constant between 2 and 24 mo. A possible explanation is that anxiety or excitement related to entering a clinical study may lead to higher blood pressure at baseline than at subsequent study visits. Consistent with this hypothesis, we observed higher blood pressure at baseline than at all later time points in both study groups, whereas blood pressure was constant between 2 and 24 mo, regardless of whether subjects were on antihypertensive medication. Many other studies, in both men and women (e.g., see 10, 15, 17, 21), have similarly reported higher blood pressure at baseline than at later time points, which may explain inconsistent results across studies. Equol production may be a factor as well for effects on blood pressure. Subjects in the soy protein group who did not produce equol experienced a reduction in systolic pressure, whereas this did not occur in low-equol producers, an observation for which we do not have a mechanistic explanation. However, this difference in systolic pressure between producers and nonproducers was not statistically significant. No effect of equol production status on blood pressure was found in a 4-wk study with whole soy foods (32).
We did not find effects on total cholesterol of soy protein isolate, which is in line with some studies in men (8–12) but not others (13–16), regardless of the duration of those studies. However, a meta-analysis identified that the cholesterol-lowering effect of soy was stronger in studies with interventions of 10 wk to 1 y than in shorter-duration studies, but data from studies in men and women were not analyzed separately (4). To the best of our knowledge, whether a sex difference exists in cholesterol responses to soy is not well established. Collectively, these findings suggest that duration of soy intervention may have a biphasic or transient effect on total serum cholesterol.
Goitrogenic effects of soy have been reported in rats (33), raising concerns about possible adverse effects of soy on thyroid function (34–36). Some studies with soy supplementation in women found effects on thyroid function (37), but other more recent studies did not (38–40). In 1 study of soy in young men, no effects were found on thyroid hormones and TSH of 8-wk supplementation with soy protein isolate that either contained isoflavones or was depleted or with milk protein in a crossover design (41); this is in line with our findings in older men. However, in a 3-mo randomized study with older hypogonadal men with type 2 diabetes (19), consumption of soy snack bars caused a decrease in fT4 and reverse T3, but not T3, whereas TSH concentrations were elevated (19, 42). We observed neither of these effects in healthy men after much longer periods of soy consumption. Supplementation with soy protein isolate depleted of isoflavones did not have these effects, which were thus attributed to the isoflavones (19, 42–44).
The effects of soy and its isoflavones on thyroid hormones remain somewhat unclear and may be different in men and women and affected by comorbidities. The elevation in TSH concentrations over time that we observed in the placebo group was well within the normal range and may be due to chance. Our observation of a lack of effects of soy protein isolate on thyroid hormones and TSH is consistent with the conclusions of extensive evaluations of the literature on soy and thyroid function (35, 36) and a recent meta-analysis (45).
The only established potential adverse effect of soy identified in humans is an inhibition of intestinal iron absorption in situations of dietary imbalance (46–50). Surprisingly, we found over the course of the study a marked improvement in iron status as assessed by serum ferritin concentrations in both intervention groups as well as slight increases in hemoglobin and hematocrit. This may suggest that many subjects had a low iron status at baseline, but a more likely explanation for this effect may be the iron content of the intervention materials, which was 45% of the Recommended Daily Allowance by the Institute of Medicine Food and Nutrition Board (50).
A major strength of this study is the long duration of its intervention; however, it also has several limitations. It was not powered to evaluate effects on the secondary endpoints included in this report, and the blood sampling time points and most endpoint analyses were dictated by the protocol of the original clinical trial (22). The subgroup analyses of the modifying effects of equol production status and the effects of the interventions in subjects who were not on any medication during the study were particularly limited by small sample sizes and should be considered exploratory. Possible modifying effects of equol production status were only studied in subjects on soy, and urinary output of isoflavones and metabolites was not determined. The effect of sample storage on thyroid hormone and isoflavone assays was not determined, but thyroid hormones are stable in serum samples stored at –25°C for ≥10 y (51) and isoflavones are extraordinarily stable even at temperatures >0°C; our samples were stored at –80°C or in liquid nitrogen vapor. The soy and milk protein doses were at the low end of doses studied in some other clinical trials, but they were selected to produce palatability that would limit loss of adherence, which was a low 5% in the parent trial (22).
In conclusion, 2 y of consumption of 19.2 g/d of whole soy protein isolate containing 24 mg genistein by middle-aged to older men did not affect major cardiovascular risk factors (body weight, blood pressure, and cholesterol) and did not exert any adverse effects on thyroid function or clinical chemistry compared with the casein-based placebo. Iron status improved considerably in both intervention groups, and exploratory data of subjects on soy suggest that equol production status may modify soy effects on body weight and possibly blood pressure.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Herbert Lepor and Samir Taneja (NYU), Pablo Torre (VA), and Michael Howard (Howard, Leitner & Perlmutter Urologic Associates) for their help recruiting subjects to this study. We also acknowledge Nikola Baumann for help with assay validation, Jaap Joles for help interpreting the blood pressure results, and Hiroko Meserve-Watanabe and Joanne A. Davies for their expert technical assistance.
The authors’ responsibilities were as follows—MCB, AZ-J, and IK: designed the research (project conception, development of overall research plan, and study oversight); MCB, EE, JS, MJS, CR, AZ-J, and IK: conducted the research (hands-on conduct of the clinical trial, ferritin and thyroid hormone assays, and data collection); MCB, EE, RJD, and HX: analyzed the data and/or performed statistical analysis; MCB: drafted the manuscript and had primary responsibility for final content; and all authors: involved in critical review and revision of the manuscript and read and approved the final manuscript. MCB received funding for the clinical trial from NIH, the Prevent Cancer Foundation, and the United Soybean Board. Solae provided the intervention materials in part as a donation (initially) and later at cost. None of the funding agencies or Solae had any influence on the design of the study nor on the analyses, interpretation, or implementation of the data, although the NIH was involved in the first 5 y under a U01 mechanism, but this involved only regular reporting of progress and results to NIH. All other authors report no conflicts of interest.
Notes
Financial support was provided by NIH grants U01 CA072290 and R01 CA166195, as well as NIH grants P50 CA16087 and UL1 TR000050, with minor support from the Prevent Cancer Foundation and the United Soybean Board. Solae provided the intervention materials. None of the funding agencies or Solae had any influence on the design of the study nor on the analyses, interpretation, or implementation of the data.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AST, aspartate aminotransferase; BUN, blood urea nitrogen; fT3, free triiodothyronine; fT4, free thyroxine; LDH, lactate dehydrogenase; NYU, New York University School of Medicine; PSA, prostate-specific antigen; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; VA, Manhattan VA Medical Center.
Contributor Information
Maarten C Bosland, Department of Pathology, College of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Erika Enk, Department of Pathology, College of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Joanne Schmoll, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Michael J Schlicht, Department of Pathology, College of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Carla Randolph, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Ryan J Deaton, Department of Pathology, College of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Hui Xie, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, IL, USA.
Anne Zeleniuch-Jacquotte, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Ikuko Kato, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA; Departments of Oncology and Pathology, Wayne State University, Detroit, MI, USA.
Data Availability
Data described in the manuscript, protocol, code book, and analytic code will be made available upon request to the corresponding author.
References
- 1. Simental-Mendía LE, Gotto AM Jr., Atkin SL, Banach M, Pirro M, Sahebkar A. Effect of soy isoflavone supplementation on plasma lipoprotein(a) concentrations: a meta-analysis. J Clin Lipidol. 2018;12:16–24. [DOI] [PubMed] [Google Scholar]
- 2. Blanco Mejia S, Messina M, Li SS, Viguiliouk E, Chiavaroli L, Khan TA, Srichaikul K, Mirrahimi A, Sievenpiper JL, Kris-Etherton Pet al. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J Nutr. 2019;149:968–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jenkins DJA, Blanco Mejia S, Chiavaroli L, Viguiliouk E, Li SS, Kendall CWC, Vuksan V, Sievenpiper JL. Cumulative meta-analysis of the soy effect over time. J Am Heart Assoc. 2019;8:e012458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tokede OA, Onabanjo TA, Yansane A, Gaziano JM, Djoussé L. Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br J Nutr. 2015;114:831–43. [DOI] [PubMed] [Google Scholar]
- 5. Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, Wang P, Melby MK, Hooper L, Kurzer MSet al. Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens. 2010;28:1971–82. [DOI] [PubMed] [Google Scholar]
- 6. Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, Hui RT. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22:463–70. [DOI] [PubMed] [Google Scholar]
- 7. van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113:E119–30. [DOI] [PubMed] [Google Scholar]
- 8. Li Z, Aronson WJ, Arteaga JR, Hong K, Thames G, Henning SM, Liu W, Elashoff R, Ashley JM, Heber D. Feasibility of a low-fat/high-fiber diet intervention with soy supplementation in prostate cancer patients after prostatectomy. Eur J Clin Nutr. 2008;62:526–36. [DOI] [PubMed] [Google Scholar]
- 9. Teixeira SR, Potter SM, Weigel R, Hannum S, Erdman JW Jr., Hasler CM. Effects of feeding 4 levels of soy protein for 3 and 6 wk on blood lipids and apolipoproteins in moderately hypercholesterolemic men. Am J Clin Nutr. 2000;71:1077–84. [DOI] [PubMed] [Google Scholar]
- 10. Urban D, Irwin W, Kirk M, Markiewicz MA, Myers R, Smith M, Weiss H, Grizzle WE, Barnes S. The effect of isolated soy protein on plasma biomarkers in elderly men with elevated serum prostate specific antigen. J Urol. 2001;165:294–300. [DOI] [PubMed] [Google Scholar]
- 11. West SG, Hilpert KF, Juturu V, Bordi PL, Lampe JW, Mousa SA, Kris-Etherton PM. Effects of including soy protein in a blood cholesterol-lowering diet on markers of cardiac risk in men and in postmenopausal women with and without hormone replacement therapy. J Womens Health (Larchmt). 2005;14:253–62. [DOI] [PubMed] [Google Scholar]
- 12. McVeigh BL, Dillingham BL, Lampe JW, Duncan AM. Effect of soy protein varying in isoflavone content on serum lipids in healthy young men. Am J Clin Nutr. 2006;83:244–51. [DOI] [PubMed] [Google Scholar]
- 13. Sagara M, Kanda T, NJelekera M, Teramoto T, Armitage L, Birt N, Birt C, Yamori Y. Effects of dietary intake of soy protein and isoflavones on cardiovascular disease risk factors in high risk, middle-aged men in Scotland. J Am Coll Nutr. 2004;23:85–91. [DOI] [PubMed] [Google Scholar]
- 14. Wong WW, Smith EO, Stuff JE, Hachey DL, Heird WC, Pownell HJ. Cholesterol-lowering effect of soy protein in normocholesterolemic and hypercholesterolemic men. Am J Clin Nutr. 1998;68(6 Suppl):1385S–9S. [DOI] [PubMed] [Google Scholar]
- 15. Mackey R, Ekangaki A, Eden JA. The effects of soy protein in women and men with elevated plasma lipids. Biofactors. 2000;12:251. [DOI] [PubMed] [Google Scholar]
- 16. Higashi K, Abata S, Iwamoto N, Ogura M, Yamashita T, Ishikawa O, Ohslzu F, Nakamura H. Effects of soy protein on levels of remnant-like particles cholesterol and vitamin E in healthy men. J Nutr Sci Vitaminol (Tokyo). 2001;47:283–8. [DOI] [PubMed] [Google Scholar]
- 17. Hermansen K, Hansen B, Jacobsen R, Clausen P, Dalgaard M, Dinesen B, Holst JJ, Pedersen E, Astrup A. Effects of soy supplementation on blood lipids and arterial function in hypercholesterolaemic subjects. Eur J Clin Nutr. 2005;59:843–50. [DOI] [PubMed] [Google Scholar]
- 18. Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–60. [DOI] [PubMed] [Google Scholar]
- 19. Sathyapalan T, Rigby AS, Bhasin S, Thatcher NJ, Kilpatrick ES, Atkin SL. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: a randomized controlled study. J Clin Endocrinol Metab. 2017;102:425–33. [DOI] [PubMed] [Google Scholar]
- 20. Hamilton-Reeves JM, Banerjee S, Banerjee SK, Holzbeierlein JM, Thrasher JB, Kambhampati S, Keighley J, Van Veldhuizen P. Short-term soy isoflavone intervention in patients with localized prostate cancer: a randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8:e68331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teede HJ, Giannopoulos D, Dalais FS, Hodgson J, McGrath BP. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am Coll Nutr. 2006;25:533–40. [DOI] [PubMed] [Google Scholar]
- 22. Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E, Melamed J, Kong MX, Macias V, Kajdacsy-Balla A, Lumey LHet al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. 2013;310:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gamache PH, Acworth IN. Analysis of phytoestrogens and polyphenols in plasma, tissue, and urine using HPLC with coulometric array detection. Exp Biol Med. 1998;217(3):274–80. [DOI] [PubMed] [Google Scholar]
- 24. Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Exp Biol Med. 1995;208:18–26. [DOI] [PubMed] [Google Scholar]
- 25. Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Exp Biol Med. 1998;217:263–73. [DOI] [PubMed] [Google Scholar]
- 26. Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JAet al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–36. [DOI] [PubMed] [Google Scholar]
- 27. Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BE, Valentine JL, Stinchcombe T, Boan J, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48:160–70. [DOI] [PubMed] [Google Scholar]
- 28. Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136:2188–93. [DOI] [PubMed] [Google Scholar]
- 29. Adams KF, Chen C, Newton KM, Potter JD, Lampe JW. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:644–8. [PubMed] [Google Scholar]
- 30. Ma Y, Chiriboga D, Olendzki BC, Nicolosi R, Merriam PA, Ockene IS. Effect of soy protein containing isoflavones on blood lipids in moderately hypercholesterolemic adults: a randomized controlled trial. J Am Coll Nutr. 2005;24(4):275–85. [DOI] [PubMed] [Google Scholar]
- 31. Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol: a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 32. Wong JM, Kendall CW, Marchie A, Liu Z, Vidgen E, Holmes C, Jackson CJ, Josse RG, Pencharz PB, Rao AVet al. Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am J Clin Nutr. 2012;95:564–71. [DOI] [PubMed] [Google Scholar]
- 33. Chang HC, Doerge DR. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol Appl Pharmacol. 2000;168:244–52. [DOI] [PubMed] [Google Scholar]
- 34. Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(Suppl 3):349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–58. [DOI] [PubMed] [Google Scholar]
- 36. Hüser S, Guth S, Joost HG, Soukup ST, Köhrle J, Kreienbrock L, Diel P, Lachenmeier DW, Eisenbrand G, Vollmer Get al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: a comprehensive safety evaluation. Arch Toxicol. 2018;92:2703–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Persky VW, Turyk ME, Wang L, Freels S, Chatterton R Jr., Barnes S, Erdman J Jr., Sepkovic DW, Bradlow HL, Potter S. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr. 2002;75:145–53. [DOI] [PubMed] [Google Scholar]
- 38. Bruce B, Messina M, Spiller GA. Isoflavone supplements do not affect thyroid function in iodine-replete postmenopausal women. J Med Food. 2003;6:309–16. [DOI] [PubMed] [Google Scholar]
- 39. Sathyapalan T, Manuchehri AM, Thatcher NJ, Rigby AS, Chapman T, Kilpatrick ES, Atkin SL. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2011;96:1442–9. [DOI] [PubMed] [Google Scholar]
- 40. Alekel DL, Genschel U, Koehler KJ, Hofmann H, Van Loan MD, Beer BS, Hanson LN, Peterson CT, Kurzer MS. Soy Isoflavones for Reducing Bone Loss Study: effects of a 3-year trial on hormones, adverse events, and endometrial thickness in postmenopausal women. Menopause. 2015;22:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dillingham BL, McVeigh BL, Lampe JW, Duncan AM. Soy protein isolates of varied isoflavone content do not influence serum thyroid hormones in healthy young men. Thyroid. 2007;17:131–7. [DOI] [PubMed] [Google Scholar]
- 42. Sathyapalan T, Köhrle J, Rijntjes E, Rigby AS, Dargham SR, Kilpatrick ES, Atkin SL. The effect of high dose isoflavone supplementation on serum reverse T3 in euthyroid men with type 2 diabetes and post-menopausal women. Front Endocrinol. 2018;9:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sathyapalan T, Javed Z, Rigby AS, Kilpatrick ES, Atkin SL. Soy protein improves cardiovascular risk in subclinical hypothyroidism: a randomized double-blinded crossover study. J Endocr Soc. 2017;1:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sathyapalan T, Dawson AJ, Rigby AS, Thatcher NJ, Kilpatrick ES, Atkin SL. The effect of phytoestrogen on thyroid in subclinical hypothyroidism: randomized, double blind, crossover study. Front Endocrinol. 2018;9:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Otun J, Sahebkar A, Östlundh L, Atkin SL, Sathyapalan T. Systematic review and meta-analysis on the effect of soy on thyroid function. Sci Rep. 2019;9:3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cook JD, Morck TA, Lynch SR. The inhibitory effect of soy products on nonheme iron absorption in man. Am J Clin Nutr. 1981;34:2622–9. [DOI] [PubMed] [Google Scholar]
- 47. Macfarlane BJ, van der Riet WB, Bothwell TH, Baynes RD, Siegenberg D, Schmidt U, Tal A, Taylor JR, Mayet F. Effect of traditional oriental soy products on iron absorption. J Clin Nutr. 1990;51:873–80. [DOI] [PubMed] [Google Scholar]
- 48. Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr. 1992;56:573–8. [DOI] [PubMed] [Google Scholar]
- 49. Lynch SR, Dassenko SA, Cook JD, Juillerat MA, Hurrell RF. Inhibitory effect of a soybean–protein–related moiety on iron absorption in humans. Am J Clin Nutr. 1994;60:567–72. [DOI] [PubMed] [Google Scholar]
- 50. Food and Nutrition Board, Institute of Medicine . Dietary Reference Intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 51. Männistö T, Surcel HM, Bloigu A, Ruokonen A, Hartikainen AL, Järvelin MR, Pouta A, Vääräsmäki M, Suvanto-Luukkonen E. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53:1986–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, protocol, code book, and analytic code will be made available upon request to the corresponding author.