Abstract
Objectives
Patients with APS are at increased risk of thromboembolism. Neutrophils have been shown to play a role in inducing thrombosis. We aimed to investigate differences in neutrophil subpopulations, their potential of activation and neutrophil extracellular trap (NET) formation comparing high and low-density neutrophils (HDNs/LDNs) as well as subpopulations in patients with APS and controls to gain deeper insight into their potential role in thrombotic manifestations in patients with APS.
Methods
HDNs and LDNs of 20 patients with APS and 20 healthy donors were isolated by density gradient centrifugation and stimulated. Neutrophil subpopulations, their activation and NET release were assessed by flow cytometry.
Results
LDNs of both groups showed higher baseline activation, lower response to stimulation (regulation of activation markers CD11b/CD66b), but higher NET formation compared with HDNs. In patients with APS, the absolute number of LDNs was higher compared with controls. HDNs of APS patients showed higher spontaneous activation [%CD11b high: median (interquartile range): 2.78% (0.58–10.24) vs 0.56% (0.19–1.37)] and response to stimulation with ionomycin compared with HDNs of healthy donors [%CD11b high: 98.20 (61.08–99.13) vs 35.50% (13.50–93.85)], whereas no difference was found in LDNs. NET formation was increased in patients’ HDNs upon stimulation.
Conclusion
HDNs and LDNs act differently, unstimulated and upon various stimulations in both healthy controls and APS patients. Differences in HDNs and LDNs between patients with APS and healthy controls indicate that neutrophils may enhance the risk of thrombosis in these patients and could thus be a target for prevention of thrombosis in APS.
Keywords: lupus anticoagulant, antiphospholipid syndrome, neutrophil extracellular traps, low density neutrophils
Rheumatology key messages
Compared with HDNs, LDNs show higher basal activation, lower activatability, but higher NET formation.
Compared with controls, APS patients have more LDNs, which also show higher activation rate.
HDNs of APS patients are easier to activate and show higher NETosis than control HDNs.
Introduction
APS is an autoimmune disorder characterised by the occurrence of arterial and/or venous thromboembolism and/or pregnancy complications and is associated with the presence of antiphospholipid antibodies (aPLAs). Antiphospholipid antibodies, including LA, aCLs and antibodies against β2-glycoprotein I (anti-β2GPI) are autoantibodies against phospholipids and phospholipid-protein complexes [1, 2]. The mechanisms behind the increased activation of the blood coagulation and the high risk to develop thrombosis in aPLA-positive individuals are still under investigation.
Various risk factors for thrombosis, including positivity for all three aPLAs (termed triple positivity) [3] and typical cardiovascular risk factors (such as smoking and diabetes), among others, have been described [4, 5]. Also, neutrophils have been demonstrated to play a role in APS, with increased release of neutrophil extracellular traps (NETs) in these patients [6].
Neutrophils were long considered a homogeneous population. However, in recent years, it became obvious that they are versatile, heterogeneous and have diverse functions in various pathological settings such as cancer and autoimmunity [7, 8]. There are different approaches to characterize neutrophils and their subpopulations, based on differentiation stage and surface marker expression [9, 10]. Pillay et al. described the classification of neutrophils according to their expression of CD16 (FcγRIII) and CD62L (L-selectin) into mature (CD62L high/CD16 high), activated (CD62L low/CD16 high) and newly released neutrophils (CD62L high/CD16 low) [11]. Furthermore, it was reported that neutrophils can be separated according to their density [12]. High density neutrophils (HDNs) – sometimes also termed normal density neutrophils – are found in health and disease, whereas low density neutrophils (LDNs) were mainly described in pathological conditions [7, 8, 13, 14]. In contrast to HDNs, LDNs are found within the peripheral blood mononuclear cell (PBMC) fraction after gradient centrifugation and were identified in blood of patients with cancer, infection and sepsis [8], as well as in APS [15]. They are often described as immature cells [8], prone to undergo NET formation [6], with an immunosuppressive function [16]. Recently, the hypothesis of immunosuppressive LDNs has been challenged in patients with SLE, as it has been shown that LDNs are activated cells with pro-inflammatory effects on other immune cells, like T cells [17].
Neutrophils and especially NETs are of specific interest in diseases with an increased risk of thrombosis. Various studies have shown that NETs contribute to both venous and arterial thromboembolism (VTE, ATE) [18–20], and to the increased thrombotic risk in patients with cancer [21, 22] as well as SLE [23, 24]. NETs are negatively charged chromatin fibres with neutrophil-derived proteases and antimicrobial peptides attached. These fibres provide a scaffold for interaction, trapping and activation of various cells including platelets [25]. Citrullinated histone H3 (H3Cit) is formed during NET formation through the action of PAD4 and is considered as a NET-specific marker [26, 27]. It was shown that neutrophils from APS patients spontaneously release high levels of NETs [19]. Interestingly, stimulation of neutrophils from healthy donors with plasma from APS patients also leads to NET formation [6]. A recent study by our group showed that platelets from patients with APS have an altered proteome, especially proteins involved in platelet functions but also in NET formation, which are changed in this patient cohort with increased thrombotic risk [28].
Considering the distinct functions and NET formation potential of neutrophil subsets, this study investigated the differences of neutrophil populations, their characteristics, their baseline activation and potential of activation upon stimulation as well as their propensity for NET formation in HDNs and LDNs in healthy controls and in patients with APS and a history of arterial and/or venous thrombosis.
Methods
A detailed description of the applied laboratory methods is available in the Supplementary Material, section Methods, available at Rheumatology online.
Study population
The Vienna Lupus Anticoagulant and Thrombosis Study (LATS) is a single-centre, ongoing, prospective observational cohort study including patients persistently positive for LA (confirmed by two tests 12 weeks apart). The study design is described in detail elsewhere [4, 29]. Twenty patients with LA and APS were enrolled in the present study during a regular visit to the centre between December 2018 and August 2019. Investigation of LA and aPLAs levels was performed as described in detail elsewhere [28, 29]. Furthermore, age- and sex-matched healthy controls without infection 6 weeks before study inclusion and no prior history of thrombosis were included. This study was conducted in accordance to the Declaration of Helsinki and was approved by the ethics committee of the Medical University of Vienna (EC No. 1268/2014 and EC No. 039/2006) and each participant provided written informed consent.
Isolation of low- and high-density neutrophils
PBMC and polymorphonuclear cells (PMN) were isolated by density gradient centrifugation of venous blood from patients and healthy volunteers. Neutrophils within the PMN layer were defined as HDNs; neutrophils within the PBMC layer as LDNs.
Characterization of neutrophils by surface markers
PBMCs and PMNs were fixed with paraformaldehyde (PFA) immediately after blood draw or cell isolation as negative controls. For baseline characterization, fixed cells were stained for CD66b, CD62L, CD16 and propidium iodide (PI). Analysis of samples was performed using a CytoFlex S device and FlowJo v10 software.
Short term activation of neutrophils
Cells were incubated in RPMI-1640 containing 10% foetal calf serum (FCS) with the following stimuli for 30 min: HBSS+Ca2+ for unstimulated controls, ionomycin (IO; 4 µM) or phorbol 12-myristate 13-acetate (PMA; 100 nM). Afterwards, samples were fixed with 4% PFA.
Cells (immediately fixed after isolation or stimulation) were stained for CD66b, CD11b and PI. Samples were analysed as described above. Furthermore, immediately fixed samples were used to set a baseline level to define ‘CD11b/CD66b high’ neutrophils.
Long-term activation for NET induction
Cells were incubated in RPMI-1640 (10% FCS) with the same stimuli as indicated above for 3 h. Afterwards, samples were fixed with PFA as described.
Cells were stained with a primary antibody (rabbit anti-H3Cit), a secondary antibody (goat anti-rabbit IgG), CD66b and PI prior to analysis with CytoFlex S. For negative controls, immediately fixed samples were stained with secondary antibody only or remained unstained. Analysis of data was performed as described above.
Statistical analysis
Descriptive statistical analysis was used to describe patient characteristics. Continuous variables are characterized by median values and the respective interquartile ranges (IQR). Wilcoxon-signed rank test (for paired samples) and Mann–Whitney U test (for unpaired samples) were used to analyse differences between two groups. A two-sided P-value <0.05 was defined as significant. Statistical analysis was performed using SPSS version 17.0.2 (IBM). Graphs were generated with GraphPad Prism 6 (GraphPad).
Results
Characteristics of the cohorts
Characteristics of the healthy control and the patient cohort are listed in Table 1. Twenty healthy volunteers (14 female, 70%) with a median age of 49 years (IQR: 36–58 years) were included. Twenty patients with APS (15 female, 75%) with a median age of 47 years (IQR: 36–52 years), were recruited into this study. Each patient had a history of thrombosis, including 11 cases of VTE, four with ATE and five with both. Fourteen patients (70%) were triple positive for all aPLAs, three patients were LA and anti-β2GPI positive and three patients were only positive for LA. Nineteen patients were on antithrombotic agents. Of those, 16 patients received vitamin K antagonist and four low-dose aspirin (one received both). Six patients suffered from concomitant systemic autoimmune rheumatic diseases (ARD), two from diabetes mellitus, five from hyperlipidaemia, eight from hypertension and six received immunosuppressive therapy.
Table 1.
Characteristics of study populations
| Healthy (n = 20) | APS (n = 20) | |
|---|---|---|
| Age at study entry (IQR) | 49 (36–58) | 47 (36–52) |
| Female, n (%) | 14 (70) | 15 (75) |
| Blood count, median (IQR) | ||
| Leukocyte count [G/L] | 5.2 (4.7–6.4) | 5.2 (4.6–7.9) |
| Haemoglobin [g/dL] | 14.2 (12.8–14.4) | 13.5 (12.2–14.5) |
| Platelet count [G/L] | 259 (229–303) | 220 (166–252) |
| History of thromboembolism, n (%) | ||
| Thromboembolism | 0 (0) | 20 (100) |
| Venous thromboembolism | 0 (0) | 11 (55) |
| Arterial thromboembolism | 0 (0) | 4 (20) |
| Both | 0 (0) | 5 (25) |
| Pregnancy complications, n (%) * | 0 (0) | 11 (73) |
| aPLAs positivity, n (%) ** | ||
| LA + anti-β2GPI + aCL antibodies (triple positivity) | 14 (70) | |
| LA + anti-β2GPI antibodies | 3 (15) | |
| LA + aCL antibodies | 0 (0) | |
| LA alone | 3 (15) | |
| Antithrombotic agents, n (%) | 0 (0) | 19 (95) |
| VKA | 0 (0) | 16 (80) |
| LMWH | 0 (0) | 0 (0) |
| DOAC | 0 (0) | 0 (0) |
| LDA | 0 (0) | 4 (20) |
| None | 20 (100) | 1 (5) |
| Concomitant diseases/medication, n (%) | ||
| Concomitant ARD, n (%) | 0 (0) | 6 (30) |
| Hypertension, n (%) | 3 (15) | 8 (40) |
| Hyperlipidaemia, n (%) | 0 (0) | 5 (25) |
| Diabetes mellitus, n (%) | 0 (0) | 2 (10) |
| Immunosuppressive therapy, n (%) | 0 (0) | 6 (30) |
Percentages for pregnancy complications calculated with regard to 15 female patients only.
Cut-offs defining aPLAs parameter positivity: LA positivity: positive aPTT-LA; aCL positivity: >40 GPL/MPL U/ml; anti-β2GPI positivity: >8 GPL/MPL U/ml.
aCL: anticardiolipin antibody; anti-β2GPI: anti-ß2-glycoprotein; aPLA: antiphospholipid antibody; ARD: autoimmune rheumatic disease; DOAC: direct oral anticoagulant; IQR: interquartile range; LA: lupus anticoagulant; LDA: low dose aspirin; LMWH: low molecular weight heparin; VKA: vitamin K antagonist. Patients with concomitant ARD are patients with SLE (n = 4) or lupus-like disease (n = 2).
Differences between HDNs and LDNs in healthy controls
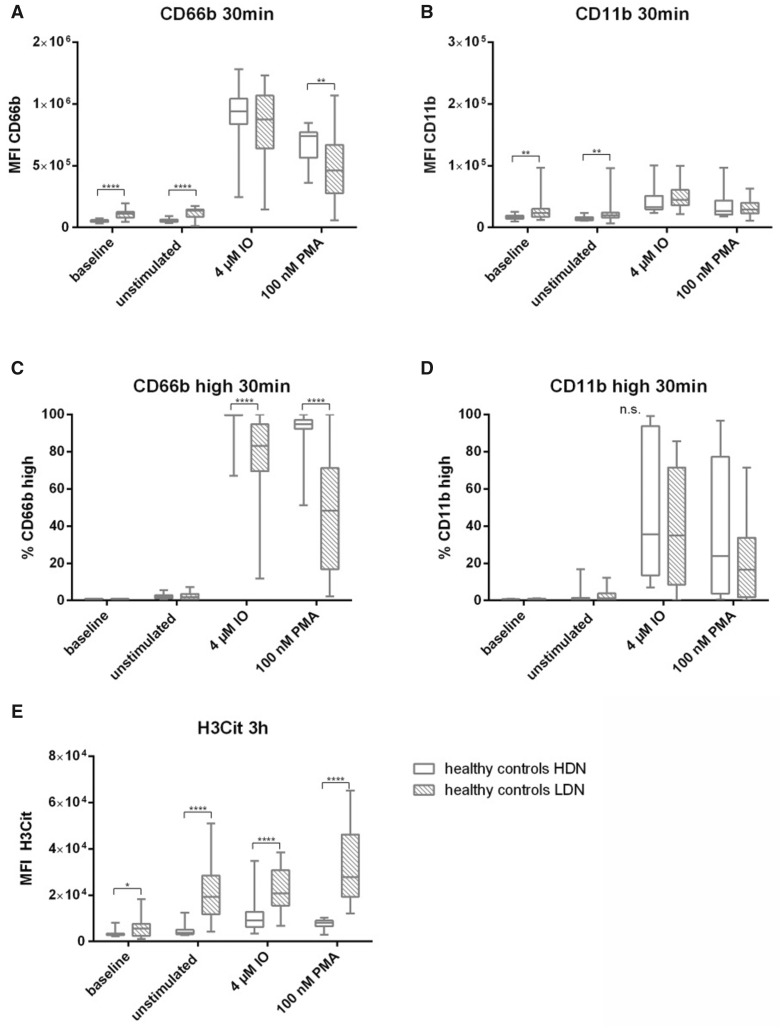
In order to investigate whether HDNs and LDNs are differentially pre-activated (baseline) and show spontaneous activation, the mean fluorescent intensity (MFI) of activation markers CD66b and CD11b were analysed. LDNs showed a significantly higher expression of these activation markers already at baseline (immediately fixed samples after isolation) compared with HDNs (MFI CD66b: 2.4-fold, P < 0.0001; MFI CD11b: 1.4-fold, P = 0.0045). In line, spontaneous upregulation of activation markers (within 30 min of in vitro incubation without additional stimulus) was higher in LDNs compared with HDNs in the control cohort (MFI CD66b: 2.8-fold, P < 0.0001; MFI CD11b: 1.5-fold, P = 0.0012) as shown in Fig. 1A and B (Supplementary Table S1A and B, available at Rheumatology online). Upon addition of an in vitro stimulus, LDNs showed a comparable upregulation in the percentage of CD11b positive cells as HDNs (Fig. 1D, Supplementary Table S1D, available at Rheumatology online) whereas significant differences were observed for upregulation of CD66b. Higher frequencies of CD66b high cells were seen in stimulated HDNs (IO: median 99.80%; PMA: 94.75%), compared with LDNs (IO: median 83.20%; PMA: 48.25%; both P < 0.0001) as shown in Fig. 1C and Supplementary Table S1C, available at Rheumatology online.
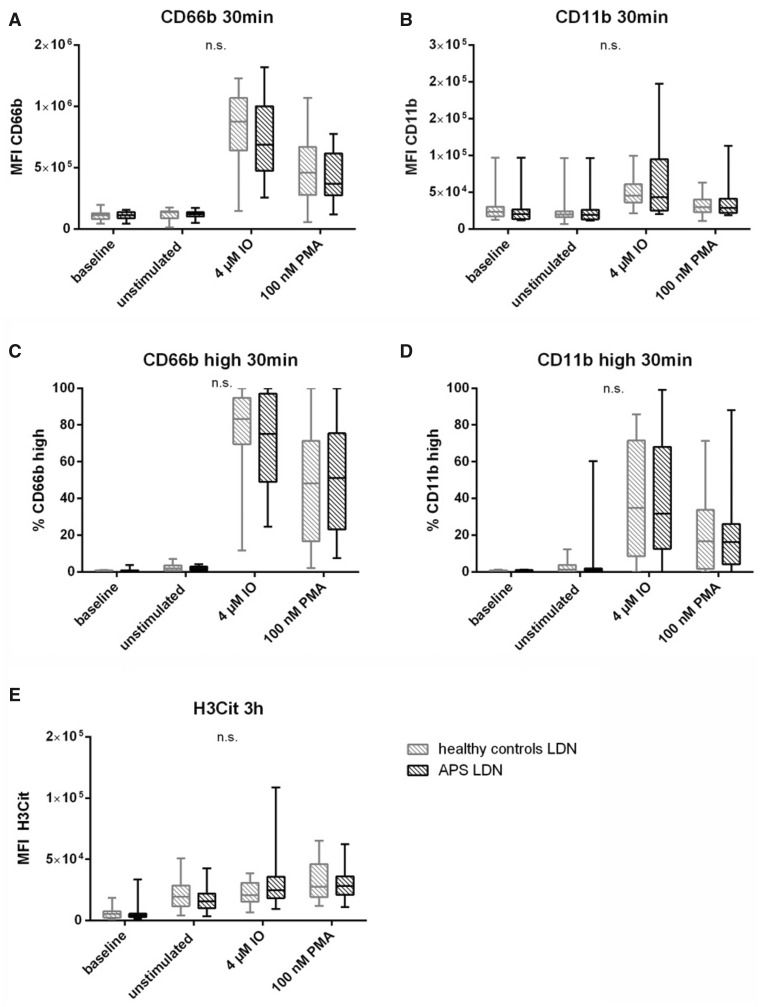
Fig. 1.
Differences between HDNs and LDNs in healthy controls
Neutrophil subpopulations isolated from 20 healthy volunteers at baseline (immediately fixed after isolation), untreated and after stimulation with IO and PMA. (A) MFI of CD66b and (B) CD11b as well as (C) % of CD66b high and (D) % of CD11b high of HDNs (clear boxes) and LDNs (scattered boxes) after treatment for 30 min at 37°C. (E) MFI of H3Cit after stimulation for 3 h at 37°C. Statistical analysis was performed using Mann–Whitney U test. H3Cit: citrullinated histone H3; IO: ionomycin; LDN: low density neutrophil; MFI: mean fluorescent intensity; n.s.: not significant; PMA: phorbol 12-myristate 13-acetate. *P ≤0.05; **P ≤0.01; ****P≤0.0001.
Differences in NET formation were investigated using H3Cit as a NET marker. Already at baseline, LDNs from healthy volunteers showed significantly higher H3Cit positivity compared with HDNs (1.4-fold, P =0.048). Spontaneous NET formation was highly increased in LDNs compared with HDNs (4.8-fold, P <0.0001) as well as after stimulation with IO (2.7-fold, P <0.0001) and PMA (3.8-fold, P <0.0001) as shown in Fig. 1E.
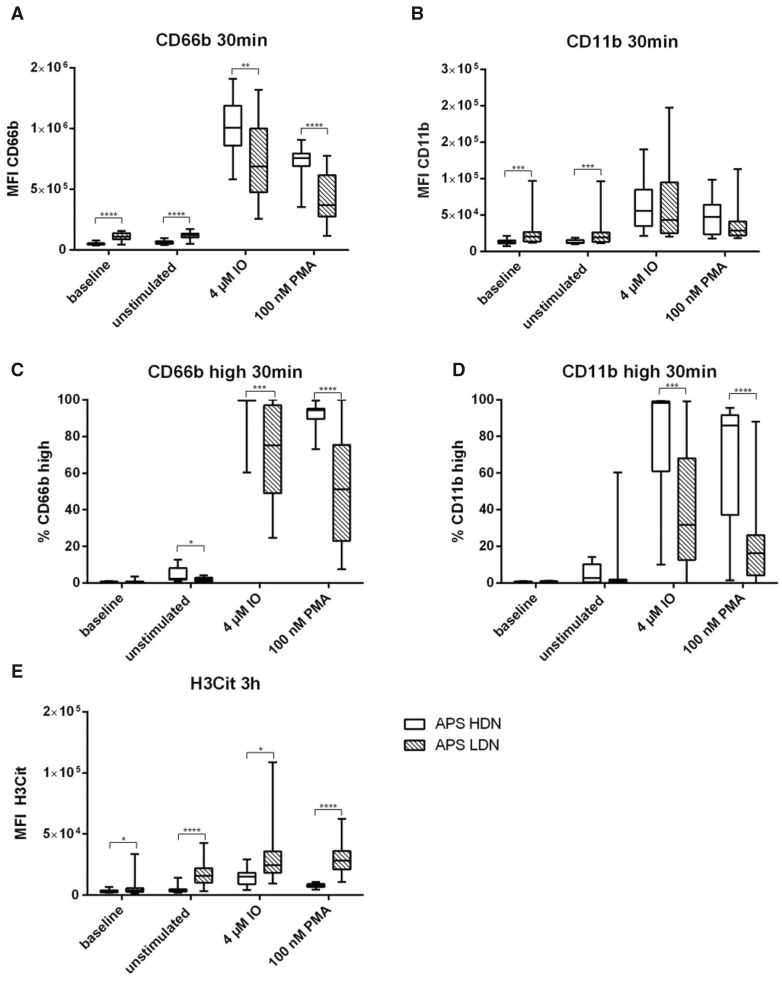
Differences between HDNs and LDNs in patients with APS
Similarly to our healthy cohort, differences between HDNs and LDNs were investigated in patients with APS. As found for healthy individuals, LDNs showed both, higher baseline and spontaneous activation compared with HDNs. MFI of CD66b at baseline was 2.3-fold and after being left untreated for 30 min, 2.2-fold increase in LDNs compare to HDNs (both P <0.0001) (Fig. 2A, Supplementary Table S2A, available at Rheumatology online). A similar pattern was seen regarding the MFI dynamics I of CD11b. At baseline, LDNs had a 1.5-fold higher and untreated a 1.6-fold higher CD11b expression compared with the respective HDN samples (both P <0.001) (Fig. 2B and Supplementary Table S2B, available at Rheumatology online). When looking at the potential of activation, both activation markers revealed significant higher upregulation after stimulation in HDNs as compared with LDNs (MFI, Fig. 2C and D). Higher percentages of CD66b high cells were seen in stimulated HDNs [IO: median 99.75% (IQR: 99.63–99.88); PMA: 94.35% (89.53–95.20)] compared with stimulated LDNs [IO: 75.15% (49.05–97.00), P <0.001; PMA: 51.20% (23.10–75.43), P <0.0001] as shown in Fig. 2C. Comparable data is seen in Fig. 2D for the frequencies of CD11b high cells. HDNs showed higher response to stimulation with IO [98.20% (61.08–99.13)] and PMA [86.05% (37.10–91.55)] compared with LDNs [IO: 31.70% (12.65–68.03), P<0.001; PMA: 16.20% (4.22–26.18), P <0.0001].
Fig. 2.
Differences between HDNs and LDNs in patients with APS
Neutrophil subpopulations isolated from 20 patients with APS at baseline (immediately fixed after isolation), untreated and after stimulation with IO and PMA. (A) MFI of CD66b and (B) CD11b as well as (C) % of CD66b high and (D) % of CD11b high of HDNs (clear boxes) and LDNs (scattered boxes) after treatment for 30 min at 37°C. (E) MFI of H3Cit after stimulation for 3 h at 37°C. Statistical analysis was performed using Mann–Whitney U test. H3Cit: citrullinated histone H3; IO: ionomycin; LDN: low density neutrophil; MFI: mean fluorescent intensity; PMA: phorbol 12-myristate 13-acetate. *P ≤0.05; **P ≤0.01; ***P ≤0.001; ****P ≤0.0001.
In terms of H3Cit expression, a similar trend was observed in healthy controls was visible. Again, at baseline but also unstimulated and after stimulation with IO or PMA, LDNs from patients with APS showed a higher ability of NET production compared with HDNs (baseline: 1.3-fold, P =0.025; unstimulated: 4.4-fold, P <0.0001; IO: 1.5-fold, P =0.014; PMA: 3.6-fold, P <0.0001) (Fig. 2E, Supplementary Table S2E, available at Rheumatology online).
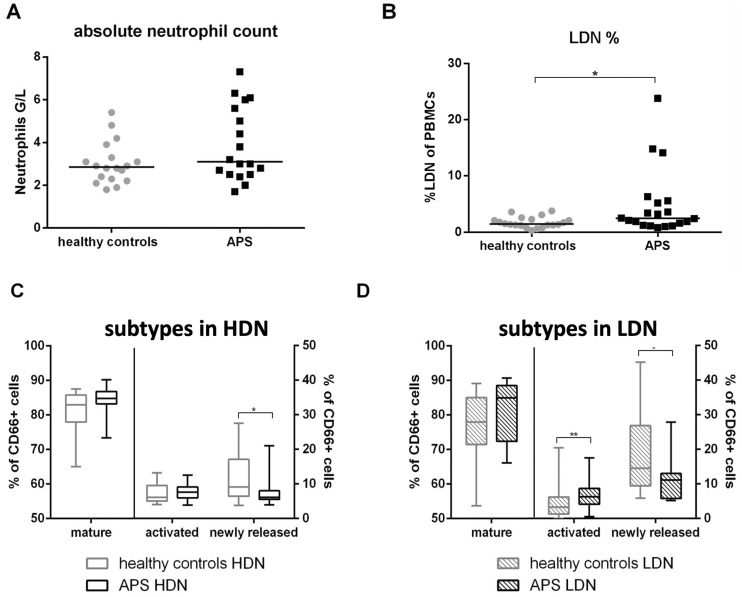
Neutrophil counts and subsets in healthy controls compared with patients with APS
No difference in absolute neutrophil counts between healthy controls [median: 2.9 G/l, IQR (2.3–3.5)] and patients with APS [3.1 G/l (2.5–5.7)] was found (Fig. 3A, Supplementary Table S3A, available at Rheumatology online). After PBMC isolation, percentage of LDNs was investigated. Healthy controls [1.45% (1.23–2.25)] had a significantly lower percentage of LDNs in their PBMC fraction as compared with patients [2.45% (1.3–5.5), P =0.037] (Fig. 3B, Supplementary Table S3B, available at Rheumatology online).
Fig. 3.
Absolute neutrophil counts, LDN percentage and subsets in patients with APS compared with healthy controls
(A) Absolute neutrophil counts and (B) % of LDNs in PBMCs of healthy controls (grey circles) and patients with APS (black squares). Flow cytometric characterization of neutrophil subsets in (C) HDNs and (D) LDNs. The proportions of CD62L↑/CD16↑ (mature), CD62L↓/CD16↑ (activated) and CD62L↑/CD16↓ (newly released) neutrophils to CD66b positive cells are indicated. Statistical analysis was performed using Mann–Whitney U test. HDN: high density neutrophil; LDN: low density neutrophil; PBMC: peripheral blood mononuclear cell. *P ≤0.05; **P≤0.01.
Investigation of neutrophil maturation state according to the expression of CD62L and CD16 revealed differences between healthy controls and APS patients. Regarding HDNs, similar percentages of mature and activated neutrophils and slightly but significantly lower amounts of newly released neutrophils were found in patients with APS [healthy: median: 9.15% (IQR: 6.44–17.10), APS: 6.16% (5.51–8.01), P =0.042] (Fig. 3C, Supplementary Table S3C, available at Rheumatology online). Analysis of LDNs revealed similar percentages of mature neutrophils but higher amounts of activated [healthy: 3.33% (1.33–6.23), APS: 6.25% (4.15–8.73), P =0.008] and lower amounts of newly released neutrophils in APS patients [healthy: 14.55% (9.42–26.88), APS: 11.15% (5.78–13.00), P =0.037] (Fig. 3D, Supplementary Table S3D, available at Rheumatology online).
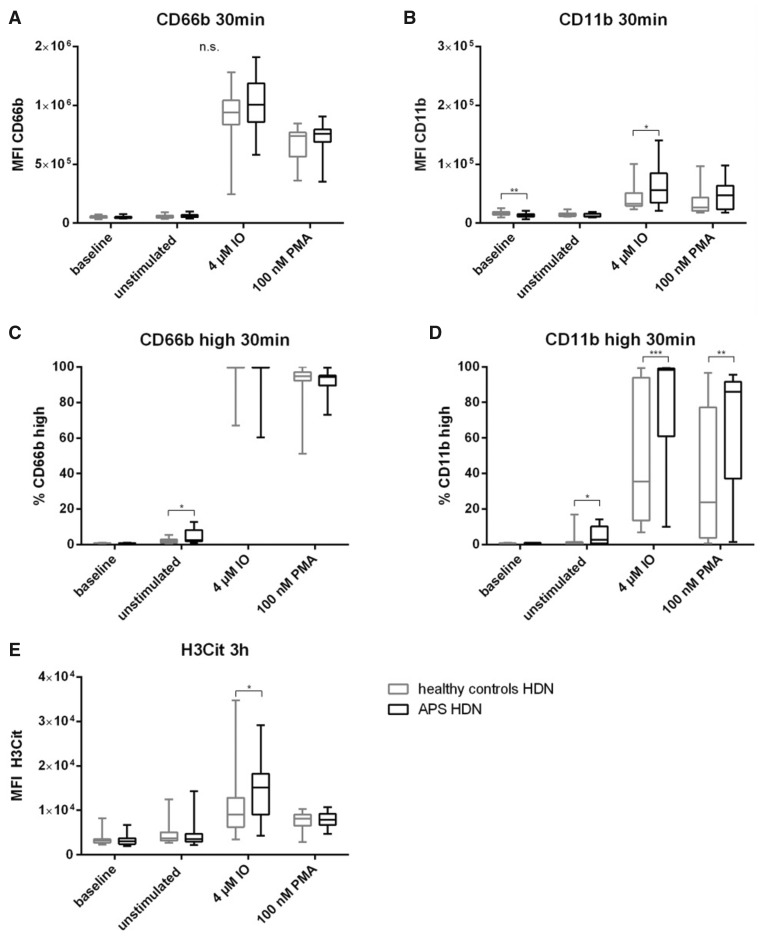
Differences between HDNs and LDNs of healthy controls and patients with APS
HDNs of healthy controls had significantly higher baseline CD11b (1.25-fold, P = 0.0012) but similar CD66b expression (Fig. 4A and B, Supplementary Table S4A/B, available at Rheumatology online) as APS patients. Spontaneous activation was higher in HDNs from patients with APS compared with those isolated from healthy controls. This was seen with respect to frequencies of CD66b high [healthy: median: 1.93% (IQR: 1.16–2.89), APS: 2.49% (1.90–8.21), P =0.044] as well as CD11b positive cells [healthy: 0.56% (0.19–1.37), APS: 2.78% (0.58–10.24), P =0.01] in Fig. 4C and D and Supplementary Table S4C/D, available at Rheumatology online. Furthermore, stimulation with IO [healthy: 35.50% (13.50–93.89), APS: 98.20% (61.08–99.13), P <0.0001] and PMA [healthy: 23.85% (3.74–77.28), APS: 86.05% (37.10–91.55), P =0.007] revealed a significantly increased reactivity of HDNs isolated from patients compared with healthy controls. The ability of HDNs to undergo NET formation was comparable in both cohorts. Only stimulation with IO showed increased ability of HDNs of APS patients to form NETs (1.8-fold, P =0.023) (Fig. 4E, Supplementary Table S4E, available at Rheumatology online).
Fig. 4.
HDNs in APS patients compared with healthy controls
HDNs isolated from 20 healthy controls and 20 patients with APS at baseline (immediately fixed after isolation), untreated and after stimulation with IO and PMA. (A) MFI of CD66b and (B) CD11b as well as (C) % of CD66b high and (D) % of CD11b high of healthy controls (grey boxes) and patients with APS (black boxes) after treatment for 30 min at 37°C. (E) MFI of H3Cit after stimulation for 3 h at 37°C. Statistical analysis was performed using Mann–Whitney U test. H3Cit: citrullinated histone H3; HDN: high density neutrophil; IO: ionomycin; MFI: mean fluorescent intensity; n.s.: not significant; PMA: phorbol 12-myristate 13-acetate. *P ≤0.05; **P ≤0.01; ***P ≤0.001.
The comparison between LDNs isolated from healthy controls and patients with APS showed no significant differences, in expression of their activation markers (Fig. 5A–D). Also, no differences in NET production were detected (Fig. 5E). Additional information is given in Supplementary Table S5, available at Rheumatology online.
Fig. 5.
LDNs in APS patients compared with healthy controls
LDNs isolated from 20 healthy controls and 20 patients with APS at baseline (immediately fixed after isolation), untreated and after stimulation with IO and PMA. (A) MFI of CD66b and (B) CD11b as well as (C) % of CD66b high and (D) % of CD11b high of healthy controls (grey boxes) and patients with APS (black boxes) after treatment for 30 min at 37°C. (E) MFI of H3Cit after stimulation for 3 h at 37°C. Statistical analysis was performed using Mann–Whitney U test. H3Cit: citrullinated histone H3; IO: ionomycin; LDN: low density neutrophil; MFI: mean fluorescent intensity; n.s.: not significant; PMA: phorbol 12-myristate 13-acetate.
Discussion
In this study, we investigated neutrophil subpopulations and their features in healthy controls and patients with APS. In both groups, HDNs showed a stronger response to inflammatory stimuli as reflected by the expression of the adhesion molecules CD11b and CD66b. In contrast, LDNs had a greater tendency to undergo spontaneous and induced NET formation. A higher response of HDNs in terms of increased ability to upregulate CD66b and CD11b as well as elevated NET formation was revealed for patients with APS. Patients with APS had higher numbers of LDNS, which also showed an enhanced activation state compared with sex and age matched healthy controls, which may further support their NETosis capacity.
In the present study, we investigated the activation potential of different neutrophil subpopulations based on density (HDN/LDN). To our knowledge, such data are not available in healthy individuals yet. We found that LDNs from healthy volunteers have increased basal activation and show lower ability to further elevate the expression of activation markers (CD66b and CD11b). Furthermore, LDNs have higher basal H3Cit positivity which is also significantly upregulated upon activation compared with HDNs. There is ongoing discussion whether LDNs detected in the PBMC layer of healthy individuals are just residues of HDNs that have been stuck in the PBMC layer upon isolation and that healthy individuals do not have LDNs at all. However, our results clearly indicate that LDNs also exist in healthy individuals, but their function is still unclear.
The review by Scapini et al. [8] summarized available data on human LDNs, which were mostly derived from patients with cancer. LDNs in autoimmune diseases such as SLE showed less phagocytic activity, but enhanced cytokine secretion and NET formation [30]. Van der Linden et al. [6] investigated patients with SLE, and also included patients with APS in their study. They found that neutrophils from healthy controls release more NETs when treated with serum from patients with SLE but no significant difference was found after treatment with APS patient-derived serum. Furthermore, increased IFNα is associated with enhanced NET formation and higher LDN counts in patients with SLE but not in patients with APS [6]. However, the role of LDNs in APS is still unclear. Our investigations of LDNs and HDNs of APS patients showed a pattern that is largely comparable to that found in neutrophils from healthy controls. LDNs of APS patients have a higher basal activation state (CD11b and CD66b) but are less reactive to stimulation with IO and PMA than HDNs of APS patients. Compared with HDNs, H3Cit positivity was highest in LDNs at baseline but also elevated after stimulation with IO and PMA.
Data on numbers of LDNs in patients with APS are conflicting. Van Hoogen and van der Linden described higher counts of low-density granulocytes in patients with APS compared with healthy controls and even stratified according to disease activity [15]. This is similar to our results, as we observed significantly increased percentages of LDNs in patients with APS compared with sex- and age-matched healthy controls. Contrary to those results, Yalavarthi et al. reported no significant difference between low density granulocyte counts in control individuals and patients with APS [19].
Irrespective of characterization by neutrophil density, Pillay et al. [31] subdivided neutrophils according to their expression of CD62L and CD16. They showed that almost 100% of neutrophils in healthy controls were CD62Lhigh/CD16high neutrophils (often referred to as mature and quiescent). Upon lipopolysaccharide stimulation in vivo, levels of CD62Llow/CD16high neutrophils (identified as activated) as well as CD62Lhigh/CD16low neutrophils (characterised as newly released from bone marrow or spleen) increased in this cohort. CD62Lhigh/CD16low neutrophils are also considered as immunosuppressive neutrophils, given their T-cell inhibitory functions and are found in different diseases including cancer, HIV, infection and sepsis [16]. Furthermore, data show that LDNs may suppress T-cell responses such as proliferation and IFN production. Despite these results, data from neutrophils isolated from patients with SLE showed LDNs with a more activated and pro-inflammatory phenotype [17]. Millrud et al. [10] reported increased levels of activated neutrophils in patients with cancer. We investigated neutrophil membrane expression of CD62L and CD16 for discrimination of maturation states in HDNs and LDNs. We found that the majority of neutrophils in both APS and healthy individuals are mature neutrophils. Antibodies against β2-glycoprotein I (anti-β2GPI) also induce a proinflammatory response, as it has been reported that those antibodies trigger MyD88-dependent nuclear factor κB (NFκB) translocation upon endothelial cell binding [32]. Also, monocyte activation by anti-β2GPI leads to a phenotypical change of the cell towards a more proinflammatory and procoagulant monocyte [33]. The vast majority of patients with APS in our cohort (85%) have elevated anti-β2GPI antibody levels. Comparable to observations by Sagiv et al. [12] we saw increased percentages of activated neutrophils within LDNs, as also shown for SLE patients by Rahman et al. [17], but not in HDNs. Furthermore, in our study, patients with APS did not show elevated percentages of newly released neutrophils compared with matched healthy controls. Nevertheless, there are differences between the basal activation states of HDNs and LDNs from patients with APS and healthy controls. This might strengthen the hypothesis that aPLAs present in the blood of patients with APS might lead to pre-activation of neutrophils.
The investigation of activation potential and NET formation capability of neutrophil subpopulations in patients with APS compared with healthy controls was the major aim of this study. H3Cit positive NETs were shown to be involved in different pathologies [34–36] as well as thrombus formation [21, 22]. One hypothesis for the increased risk to develop thrombosis in APS is that neutrophils release more NETs as a result of a primed state due to aPLAs exposure. It was already shown that aPLAs, especially anti-β2GPI antibodies, lead to activation of innate immune cells [37]. Yalavarthi et al. compared neutrophils derived from patients with APS with healthy controls [19]. They showed that plasma levels of cell-free DNA are increased in patients with APS and that their neutrophils have higher spontaneous NET formation after 2 h incubation in serum-free media. Furthermore, treatment of control neutrophils with APS serum resulted in increased NET formation. Interestingly, experiments by van der Linden et al. [6] did not show a significantly increased NET production. This was only true when control neutrophils were treated with plasma from SLE patients. We also checked for differences between patients with and without ARD and did not see any significant differences (data not shown). In our experiments we did not detect higher spontaneous NET release by neutrophils isolated from patients with APS, neither in HDNs nor in LDNs. We also cultured neutrophils in serum-free media, even for 3 h, but only a small difference in NET release after HDN stimulation with IO was observed. Besides the higher NET formation in neutrophils from patients with APS, it was shown by Leffler et al. [38] that serum from patients with APS was not able to degrade NETs as fast as serum from healthy controls. This supports the evidence that NETs play an important role in APS and the increased thrombotic risk.
Investigation of LDN counts revealed that some patients had increased LDN levels. In a subgroup analysis, we investigated differences between those patients with increased LDN levels (above the 75th percentile, cut-off: 5.5%; n = 6) and below the cut-off (n = 14). We saw that four out of six patients in the LDNhigh group received immunosuppressive therapy and that those patients also had increased absolute neutrophil counts (P =0.039, 1.54-fold). No difference between age and sex were found between the groups.
To our knowledge, activation studies with HDNs and LDNs investigating CD11b and CD66b expression have not been published yet. Basal marker expression levels could help to interpret activation states even more precisely than a single CD62L investigation does. In our studies we could not detect higher activation states of HDNs and LDNs in our cohorts when assessing CD11b and CD66b. Nevertheless, HDNs of patients with APS showed higher spontaneous upregulation of both markers and were increased CD11b to a higher extent compared with control HDNs upon activation with both applied stimuli.
Some limitations of this study have to be discussed. First, the sample size is rather small; however, APS is a rare disease and the investigations had to be performed with freshly collected blood samples. Due to the relatively low number of participants, we were not able to evaluate the influence of comorbidities. Second, only one mechanism of NET formation including the citrullination of histone H3 was investigated. Apart from histone citrullination, additional mechanisms of NET formation exist [39]; thus, it would be interesting to analyse other NETosis pathways in this context. This is, however, hampered by the lack of parameters specific for NET formation and distinguishing to cell death programs. Currently, measurement of H3Cit is one of the most widely used and accepted methods to measure NET formation. Furthermore, detection of NET formation could be performed by other methods where neutrophils and NETs can adhere to the surface and may not be washed away, which is a frequent problem with staining procedures. Establishment of alternative ad reliable methods to detect NETs and NET-forming neutrophils is crucial in the future.
In conclusion, these experiments are the first to systematically investigate the potential of activation and H3Cit production of HDNs and LDNs in different stimulation settings in healthy controls and patients with APS. Our findings strengthen the hypothesis that neutrophils play a role in APS and the increased thrombotic risk in this patient cohort. LDNs from patients with APS showed a primed or exhausted phenotype that might be due to pre-activation by aPLAs. HDNs of patients with APS are easier to activate and show higher NET production than HDNs from matched controls. These results could help to further unravel underlying mechanisms of this rare, but in some cases serious, immunologic disease and to potentially introduce novel treatment options for patients with APS.
Supplementary Material
Acknowledgements
We would like to thank the Core Facility Flow Cytometry, Medical University of Vienna for their support.
Funding: This work was supported by the Austrian Science Fund (FWF), Special Research Program (SFB) 54 on ‘Inflammation and Thrombosis’.
Disclaimer: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Cervera R, Piette JC, Font J. et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. [DOI] [PubMed] [Google Scholar]
- 2. de Groot PG, Lutters B, Derksen RH. et al. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost 2005;3:1993–7. [DOI] [PubMed] [Google Scholar]
- 3. Pengo V, Ruffatti A, Legnani C. et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010;8:237–42. [DOI] [PubMed] [Google Scholar]
- 4. Posch F, Gebhart J, Rand JH. et al. Cardiovascular risk factors are major determinants of thrombotic risk in patients with the lupus anticoagulant. BMC Med 2017;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willis R, Pierangeli SS.. Pathophysiology of the antiphospholipid antibody syndrome. Auto Immun Highlights 2011;2:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Linden M, van den Hoogen LL, Westerlaken GHA. et al. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology 2018;57:1228–34. [DOI] [PubMed] [Google Scholar]
- 7. Jablonska J, Granot Z.. Neutrophil, quo vadis? J Leukoc Biol 2017;102:685–8. [DOI] [PubMed] [Google Scholar]
- 8. Scapini P, Marini O, Tecchio C, Cassatella MA.. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev 2016;273:48–60. [DOI] [PubMed] [Google Scholar]
- 9. da Silva FM, Massart-Leen AM, Burvenich C.. Development and maturation of neutrophils. Vet Q 1994;16:220–5. [DOI] [PubMed] [Google Scholar]
- 10. Millrud CR, Kagedal A, Kumlien Georen S. et al. NET-producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer 2017;140:2557–67. [DOI] [PubMed] [Google Scholar]
- 11. Pillay J, Ramakers BP, Kamp VM. et al. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J Leukoc Biol 2010;88:211–20. [DOI] [PubMed] [Google Scholar]
- 12. Sagiv JY, Michaeli J, Assi S. et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015;10:562–73. [DOI] [PubMed] [Google Scholar]
- 13. Villanueva E, Yalavarthi S, Berthier CC. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Denny MF, Yalavarthi S, Zhao W. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 2010;184:3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Hoogen LL, van der Linden M, Meyaard L, Fritsch-Stork RDE, van Roon JA, Radstake TR.. Neutrophil extracellular traps and low-density granulocytes are associated with the interferon signature in systemic lupus erythematosus, but not in antiphospholipid syndrome. Ann Rheum Dis. 2019. doi:10.1136/annrheumdis-2019-215781. [DOI] [PubMed] [Google Scholar]
- 16. Hong CW. Current understanding in neutrophil differentiation and heterogeneity. Immune Netw 2017;17:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rahman S, Sagar D, Hanna RN. et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann Rheum Dis 2019;78:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mauracher LM, Posch F, Martinod K. et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost 2018;16:508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yalavarthi S, Gould TJ, Rao AN. et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 2015;67:2990–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofbauer TM, Mangold A, Scherz T, Seidl V. et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction. Basic Res Cardiol 2019;114:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brill A, Fuchs TA, Savchenko AS, Thomas GM. et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 2012;10:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinod K, Wagner DD.. Thrombosis: tangled up in NETs. Blood 2014;123:2768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hakkim A, Furnrohr BG, Amann K. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 2010;107:9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rao AN, Kazzaz NM, Knight JS.. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J Cardiol 2015;7:829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brinkmann V, Reichard U, Goosmann C. et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. [DOI] [PubMed] [Google Scholar]
- 26. Demers M, Krause DS, Schatzberg D. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 2012;109:13076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thalin C, Demers M, Blomgren B. et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res 2016;139:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hell L, Lurger K, Mauracher LM. et al. Altered platelet proteome in lupus anticoagulant (LA)-positive patients-protein disulfide isomerase and NETosis as new players in LA-related thrombosis. Exp Mol Med 2020;52:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gebhart J, Posch F, Koder S. et al. Increased mortality in patients with the lupus anticoagulant: the Vienna Lupus Anticoagulant and Thrombosis Study (LATS). Blood 2015;125:3477–83. [DOI] [PubMed] [Google Scholar]
- 30. Carmona-Rivera C, Kaplan MJ.. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol 2013;35:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pillay J, Kamp VM, van Hoffen E. et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 2012;122:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raschi E, Testoni C, Bosisio D. et al. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood 2003;101:3495–500. [DOI] [PubMed] [Google Scholar]
- 33. Sorice M, Longo A, Capozzi A. et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum 2007;56:2687–97. [DOI] [PubMed] [Google Scholar]
- 34. Kaplan MJ, Radic M. NETosis: at the interface of cell biology, microbiology, and immunology. Frontiers E-books 2013. [DOI] [PMC free article] [PubMed]
- 35. Paues Goranson S, Thalin C, Lundstrom A. et al. Circulating H3Cit is elevated in a human model of endotoxemia and can be detected bound to microvesicles. Sci Rep 2018;8:12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong SL, Demers M, Martinod K. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015;21: 815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rauch J, Dieude M, Subang R, Levine JS.. The dual role of innate immunity in the antiphospholipid syndrome. Lupus 2010;19:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leffler J, Stojanovich L, Shoenfeld Y. et al. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin Exp Rheumatol 2014;32:66–70. [PubMed] [Google Scholar]
- 39. Hamam HJ, Khan MA, Palaniyar N.. Histone acetylation promotes neutrophil extracellular trap formation. Biomolecules 2019;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.