Abstract
Severe coronavirus disease 2019 (COVID-19) is associated with increased risk of venous thromboembolism events (VTE). This study performed a systematic review in PubMed/EMBASE of studies reporting the prevalence of VTE in patients with COVID-19 who were totally screened/assessed for deep vein thrombosis (DVT) and/or for pulmonary embolism (PE). Among 47 candidate studies (n = 6459; 33 in Europe), 17 studies (n = 3973; weighted age 63.0 years, males 60%, intensive care unit (ICU) 16%) reported the prevalence of PE with a pooled estimate of 32% (95% CI: 25, 40%), and 32 studies (n = 2552; weighted age 62.6 years, males 57%, ICU 49%) reported the prevalence of DVT with a pooled estimate of 27% (95% CI: 21, 34%). A total of 36 studies reported the use of at least prophylactic antithrombotic treatment in the majority of their patients. Meta-regression analysis showed that the prevalence of VTE was higher across studies with a higher percentage of ICU patients and higher study population mean D-dimer values, and lower in studies with mixed dosing of anticoagulation in ⩾ 50% of the population compared to studies with standard prophylactic dosing of anticoagulation in < 50% of the population. The pooled odds ratio for death in patients with COVID-19 and VTE versus those without VTE (17 studies, n = 2882) was 2.1 (95% CI: 1.2, 3.6). Hospitalized patients with severe COVID-19 are at high VTE risk despite prophylactic anticoagulation. Further research should investigate the individualized VTE risk of patients with COVID-19 and the optimal preventive antithrombotic therapy. PROSPERO Registration No.: CRD42020185543.
Keywords: deep vein thrombosis (DVT), prevalence, pulmonary embolism (PE), SARS-CoV-2
Introduction
Although coronavirus disease 2019 (COVID-19) has been identified mainly as a viral respiratory tract infection, it has become evident that several complications render a systematic approach to this new infectious disease necessary. Emerging evidence shows that severe COVID-19 is often complicated with coagulopathy, which has prothrombotic effects resulting in high risk of venous thromboembolism events (VTE) and mortality.1–3 However, it appears that there is a significant heterogeneity in the observed VTE phenotypes (isolated deep vein thrombosis (DVT), isolated pulmonary embolism (PE)/thrombosis, combined DVT and PE)2 and the prevalence of VTE among screened patients remains understudied.
Moreover, preliminary evidence suggests that anticoagulant therapy might provide a survival benefit in patients with severe COVID-19.4,5 This issue is being increasingly recognized by international societies that strongly recommend the use of thromboprophylaxis in all hospitalized patients.6–10
This study aimed to review the current evidence regarding the prevalence of VTE in patients with COVID-19 screened/assessed with lower limb ultrasonography or computed tomography pulmonary angiography.
Materials and methods
Data sources and searches
This study protocol was registered in PROSPERO; No.: CRD42020185543.
A systematic literature search of PubMed and EMBASE databases was performed in line with the PRISMA recommendations (www.prisma-statement.org) independently by three investigators (AK, KGK, SL) using the following search keywords: (‘coronavirus 2019’ OR ‘2019-nCoV’ OR ‘SARS-CoV-2’ OR ‘COVID-19’) AND (thrombotic OR thrombosis OR ‘deep vein’ OR ‘pulmonary embolism’ OR thromboemboli*) until September 30, 2020. Articles were also selected from references of relevant articles, by searching in journals’ websites and by hand search. Disagreements were resolved by consensus with a senior author (AK).
Study selection
Eligible studies were full-text articles in English that: (i) reported the prevalence of PE and/or DVT in patients with COVID-19; and (ii) performed screening/assessment in the total sample for DVT (lower limb ultrasonography) or were focused on patients with suspicion for PE (whole study population subjected to computed tomography pulmonary angiography). Case reports and case series studies with ⩽ 10 patients were excluded. The primary endpoint of this analysis was the pooled estimate of PE and DVT prevalence. The secondary endpoint included the pooled estimate of odds ratio for death in patients with COVID-19 with VTE versus non-VTE.
Data extraction and risk of bias assessment
Three investigators extracted independently data concerning study design, main characteristics of included populations and data regarding primary and secondary endpoints from included studies where available. The risk of bias was assessed using the Joanna Briggs Institute’s ‘Critical Appraisal Checklist for Analytical Cross Sectional Studies’.11
Data synthesis and analysis
A pooled prevalence estimate was calculated for each outcome, using the numerators and denominators reported and a Freeman–Tukey arcsine transformation12 with the metaan command in Stata.13 Heterogeneity in the meta-analyzed estimates was quantified using the I2 statistic.14 A random effects model was used, and we opted for a nonparametric bootstrapped DerSimonian–Laird approach.15,16 The pooled estimates were back-transformed to percentages and are reported as such in forest plots. Poisson regression models were used to examine associations and potential determinants of high heterogeneity in the primary outcome, in a meta-regression setting. The covariates of interest in these analyses were: age, percentage of male patients, percentage of patients in an intensive care unit (ICU), antithrombotic treatment characteristics (none, prophylaxis in < 50% of subjects, prophylaxis in ⩾ 50% of subjects, prophylaxis and higher doses in ⩾ 50% of subjects), mean D-dimer values of the examined sample, and quality of included studies. Meta-regression bubble plots were obtained to further examine the association between VTE prevalence and displaying the Poisson model regression line over study observations. Since this was a meta-analysis of prevalence values, publication bias could not be assessed through tests or funnel plots. Odds ratios for death in VTE versus non-VTE patients were calculated using appropriate formulas.17 Odds ratios and 95% CI values were logarithmically transformed and SEs were calculated from these values and used in the analysis. Mean values of subgroups were combined where feasible (i.e. when separate values were given for males/females).18 Median values were converted to mean values.19 Missing information about study population characteristics (i.e. age, percentage of males, percentage of patients in ICU, thromboprophylaxis details, D-dimer values, overlapping populations with other studies, etc.) was retrieved through personal communication with the corresponding authors where possible. An alpha level of 5% was used.
Analyses were performed using Stata Statistical Software, Release 16 (StataCorp LLC, College Station, TX, USA).
Results
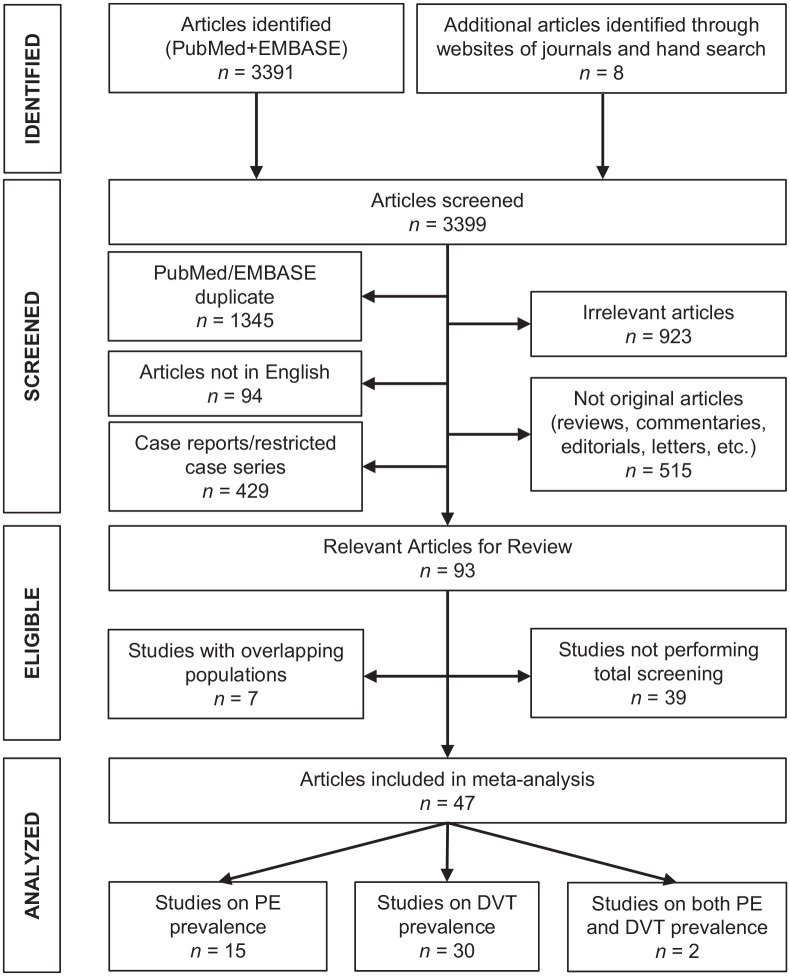
Among 3399 initially identified articles, 47 studies fulfilled the inclusion criteria and were included in the systematic review (Figure 1). The main characteristics of these studies are shown in Table 1.20–66
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart for the selection of studies.
Table 1.
Main characteristics and findings of studies.
| Study | Setting, country | n | ICU (%) |
Male (%) |
Age, years mean ± SD |
PCR-based COVID-19 diagnosis (%) |
Event for which all patients were evaluated | Symptomatic (%) |
Anticoagulation treatment (%) |
Prevalence (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | DVT | ||||||||||
| Ventura-Díaz et al.20 | Radiology Dept, Spain | 242 | NR | 62 | 66 ± 15 | NR | PE | 100 | NR | 30 | |
| Avruscio et al.21 | General ward, ICU, Italy | 85 | 48 | 72 | 67 ± 13 | 100 | DVT | 28 | Prophylactic 69 Intermediate 31 |
42 | |
| Longhitano et al.22 | General ward, ICU, Italy | 74 | 24 | 59 | 69 ± 15 | NR | DVT | NR | Prophylactic 37 Intermediate 31 Therapeutic 32 |
16 | |
| Pavoni et al.23 | ICU, Italy | 42 | 100 | 64 | 64 ± 12 | 100 | DVT | NR | Intermediate 52 Therapeutic 48 |
31 | |
| Dujardin et al.24 | ICU, The Netherlands | 127 | 100 | 77 | 62 ± 11 | 100 | DVT | NR | Prophylactic 100 | 21 | |
| Espallargas et al.25 | Radiology Dept, Spain | 47 | 49 | 64 | 64 ± 14 | 100 | PE | 100 | Prophylactic 38 Intermediate 36 Therapeutic 2 |
34 | |
| Mueller-Peltzer et al.26 | ICU, Germany | 16 | 100 | 69 | 62 ± 8 | NR | PE | 100 | Therapeutic 25 | 56 | |
| Ramadan et al.27 | Emergency Dept, general ward, ICU, USA | 367 | NR | 62 | 61 ± NR | NR | PE | NR | NR | 26 | |
| Torres-Machorro et al.28 | ICU, Mexico | 30 | 100 | 77 | 57 ± 33 | NR | DVT | 0 | Intermediate 43 Therapeutic 57 |
30 | |
| Jimenez-Guiu et al.29 | General ward, Spain | 57 | 0 | 51 | 71 ± 13 | 100 | DVT | 2 | Prophylactic 65 Intermediate 21 Therapeutic 14 |
11 | |
| Mouhat et al.30 | General ward, ICU, France | 162 | 42 | 67 | 66 ± 13 | 100 | PE | 100 | Prophylactic or Therapeutic 87 | 27 | |
| Yu et al.31 | Radiology Dept, China | 142 | 58 | 57 | 62 ± 12 | NR | DVT | NR | None 74 | 35 | |
| Alonso-Fernández et al.32 | General ward, ICU, Spain | 30 | 37 | 63 | 64 ± 12 | 100 | PE | NR | Prophylactic 87 | 50 | |
| Giorgi-Pierfranceschi et al.33 | General ward, Italy | 66 | 0 | 70 | 72 ± 11 | 100 | DVT | 0 | Prophylactic 80 Intermediate 14 |
14 | |
| Le Jeune et al.34 | General ward, France | 42 | 0 | 55 | 65 ± 19 | 79 | DVT | 0 | Prophylactic 59 Intermediate 24 Therapeutic 17 |
19 | |
| Ierardi et al.35 | ICU, Italy | 234 | 100 | 30 | 62 ± 14 | NR | DVT | 0 | Prophylactic or higher doses 100 | 11 | |
| Alharthy et al.36 | ICU, Saudi Arabia | 89 | 100 | 84 | 43 ± 16 | 100 | DVT | NR | Prophylactic 100 | 17 | |
| Pizzolo et al.37 | General ward, Italy | 43 | 0 | 67 | 65 ± 22 | NR | DVT | 0 | Prophylactic 100 | 28 | |
| Cho et al.38 | General ward, ICU, USA | 158 | 58 | 54 | 67 ± 15 | 100 | DVT | NR | Prophylactic > 90 | 33 | |
| Monfardini et al.39 | General ward, ICU, Italy | 34 | 32 | 76 | 62 ± 9 | 100 | PE DVT |
100 | Patients with PE Prophylactic 31 |
76 | 12 |
| Freund et al.40 | Emergency Dept, France | 974 | 0 | 59 | 61 ± 19 | 62 | PE | 100 | NR | 15 | |
| Chen et al.41 | Radiology Dept, China | 25 | NR | 60 | 64 ± 11 | 60 | PE | 100 | Therapeutic 80 | 40 | |
| Longchamp et al.42 | ICU, Switzerland | 25 | 100 | 64 | 68 ± 11 | 100 | DVT | 24 | Prophylactic 100 | 24 | |
| Whyte et al.43 | General ward, ICU, UK | 214 | 36 | 60 | 61 ± 2 | NR | PE | 100 | Prophylactic 100 | 37 | |
| Marone et al.44 | Vascular Units, Italy | 101 | 27 | 58 | 70 ± 10 | NR | DVT | 100 | Patients with DVT Prophylactic > 90 |
42 | |
| Fauvel et al.45 | General ward, ICU, France | 1240 | 15 | 58 | 64 ± 17 | 91 | PE | 100 | Prophylactic 63 Intermediate 8 Therapeutic 11 |
8 | |
| Santoliquido et al.46 | General ward, Italy | 84 | 0 | 73 | 68 ± 14 | 100 | DVT | 2 | Prophylactic 100 | 12 | |
| Trigonis et al.47 | ICU, USA | 45 | 100 | NR | 61 ± 15 | NR | DVT | NR | Prophylactic 38 Intermediate 53 |
42 | |
| Larsen et al.48 | General ward, ICU, Reunion Island | 35 | 11 | 77 | 67 ± 17 | 100 | PE | NR | Prophylactic 80 | 14 | |
| Chen et al.49 | ICU, China | 88 | 100 | 61 | 63 ± 12 | NR | DVT | 13 | Prophylactic 100 | 45 | |
| Koleilat et al.50 | General ward, USA | 135 | 0 | 53 | 63 ± 15 | 100 | DVT | NR | None 19 Prophylactic 63 Therapeutic 18 |
13 | |
| Bavaro et al.51 | General ward, ICU, Italy | 20 | 30 | 40 | 65 ± 23 | NR | PE | NR | Prophylactic 85 | 40 | |
| Grandmaison et al.52 | General ward, ICU, Switzerland | 58 | 50 | NR | ICU patients 62 ± 31 |
100 | DVT | NR | Prophylactic 100 | 36 | |
| Mazzaccaro et al.53 | General ward, Italy | 32 | 0 | 72 | 69 ± 12 | 100 | PE DVT |
NR | Prophylactic or therapeutic 100 | 66 | 3 |
| Gervaise et al.54 | Radiology Dept, France | 72 | NR | 75 | 62 ± 18 | 80 | PE | 100 | NR | 18 | |
| Voicu et al.55 | ICU, France | 56 | 100 | 74 | 60 ± 12 | NR | DVT | NR | Prophylactic 100 | 46 | |
| Nahum et al.56 | ICU, France | 34 | 100 | 74 | 62 ± 9 | 76 | DVT | NR | Prophylactic 100 | 79 | |
| Artifoni et al.57 | General ward, France | 71 | 0 | 61 | 62 ± 22 | NR | DVT | 3 | Prophylactic 100 | 21 | |
| Zhang et al.58 | General ward, China | 143 | 0 | 52 | 63 ± 14 | NR | DVT | NR | Prophylactic 37 | 46 | |
| Ren et al.59 | ICU, China | 48 | 100 | 54 | 71 ± 14 | NR | DVT | NR | Prophylactic 98 | 85 | |
| Poyiadji et al.60 | Radiology Dept, USA | 328 | 25 | 46 | 61 ± 16 | 100 | PE | NR | Prophylactic 37 | 22 | |
| Demelo-Rodríguez et al.61 | General ward, Spain | 156 | 0 | 65 | 68 ± 15 | 85 | DVT | 0 | Prophylactic 98 | 15 | |
| Bompard et al.62 | Radiology Dept, France | 135 | 18 | 70 | 65 ± 17 | NR | PE | 100 | ICU patients Prophylactic or intermediate 100 |
24 | |
| Criel et al.63 | General ward, ICU, Belgium | 82 | 37 | 59 | 64 ± 13 | NR | DVT | 0 | Prophylactic or intermediate 95 | 7 | |
| Cattaneo et al.64 | General ward, Italy | 64 | 0 | 55 | 68 ± 14 | NR | DVT | 0 | Prophylactic 100 | 0 | |
| Llitjos et al.65 | ICU, France | 26 | 100 | 77 | 65 ± 18 | 100 | DVT | NR | Prophylactic 31 Therapeutic 69 |
54 | |
| Cui et al.66 | ICU, China | 81 | 100 | 46 | 60 ± 14 | 100 | DVT | NR | None | 25 | |
COVID-19, coronavirus disease 2019; DVT, deep vein thrombosis; ICU, intensive care unit; NR, not reported; PCR, polymerase chain reaction; PE, pulmonary embolism.
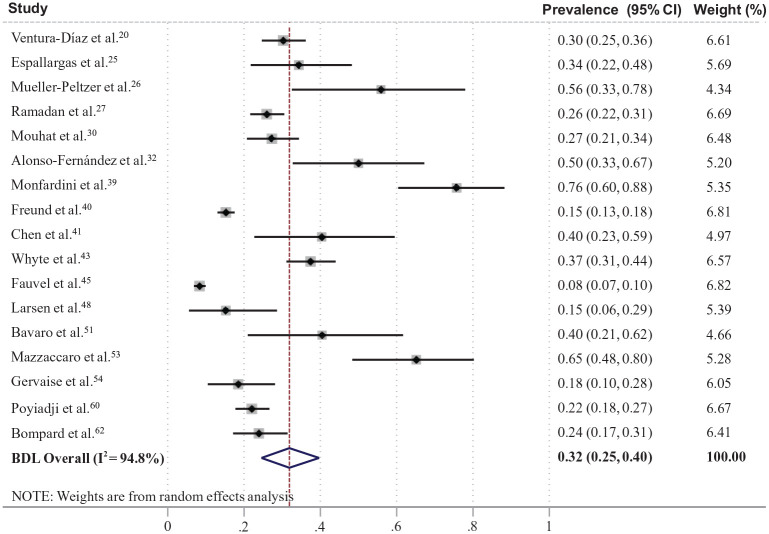
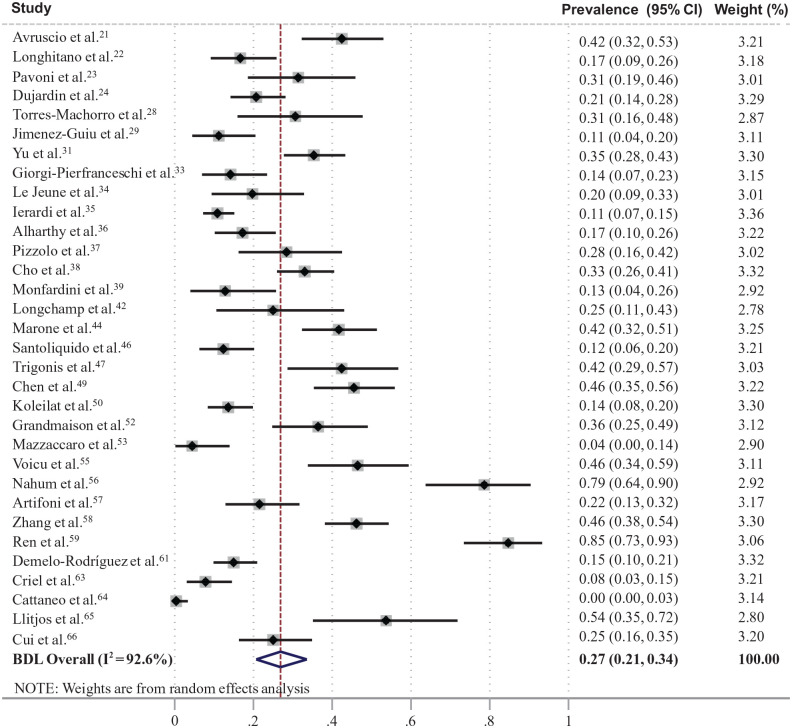
A total of 47 studies (n = 6459; 33 in Europe) reported the prevalence of VTE in totally screened/assessed patients with COVID-19.20–66 Among them, 17 studies (n = 3973; weighted age 63.0 years, males 60%, ICU 16%) reported the prevalence of PE with a pooled estimate of 32% (95% CI: 25, 40%) (Figure 2),20,25–27,30,32,39–41,43,45,48,51,53,54,60,62 and 32 studies (n = 2552; weighted age 62.6 years, males 57%, ICU 49%) reported the prevalence of DVT with a pooled estimate of 27% (95% CI: 21, 34%) (Figure 3).21–24,28,29,31,33–39,42,44,46,47,49,50,52,53,55–59,61,63–66 A total of 36 studies reported the use of at least prophylactic antithrombotic treatment in the majority of their patients (Table 1).21–25,28–30,32–38,41–43,45–53,55–57,59,61–65 The assessment of the risk of bias is presented in online Supplementary Figure S1. In plots of prevalence versus study sample size, there was a trend for higher PE prevalence in smaller studies, but there was no apparent trend in DVT prevalence (online Supplementary Figure S2).
Figure 2.
Forest plot of prevalence of pulmonary embolism in patients with coronavirus disease (COVID-19). BDL, Bootstrapped DerSimonian-Laird’ model.
Figure 3.
Forest plot of prevalence of deep vein thrombosis in patients with coronavirus disease (COVID-19). BDL, Bootstrapped DerSimonian-Laird’ model.
Meta-regression analysis did not reveal any significant associations between mean age, percentage of males, or quality of the included studies and the prevalence of PE/DVT. However, the prevalence of PE was higher across studies with higher mean D-dimer values (prevalence ratio 1.3 per 1000 ng/mL increase; 95% CI: 1.11, 1.50, p = 0.002) and higher percentage of ICU patients (1.02 per 1% increase; 95% CI: 1.01, 1.03, p < 0.001). In addition, prevalence of DVT was higher across studies with higher mean D-dimer values (1.04 per 1000 ng/mL increase; 95% CI: 1.01, 1.07, p = 0.022) and lower in studies with mixed dosing of anticoagulation in ⩾ 50% of the population compared to studies with standard prophylactic dosing of anticoagulation in < 50% of the population (0.49; 95% CI: 0.31, 0.78, p = 0.003). Meta-regression bubble plots for noncategorical variables are shown in online Supplementary Figure S3. The above-mentioned estimates regarding the associations of PE prevalence were almost identical when a small study outlier was removed (online Supplementary Figure S4).
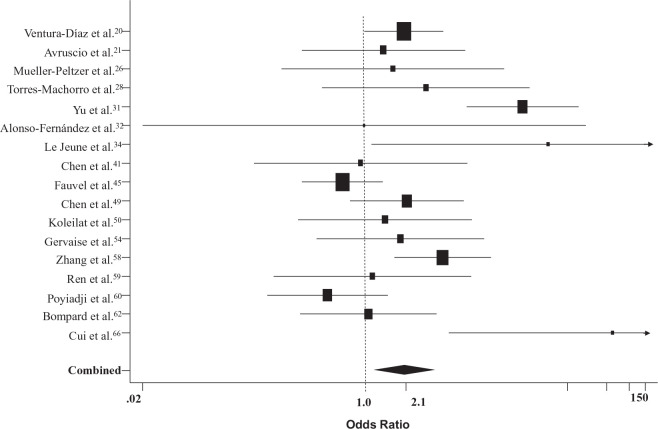
The pooled odds ratio for death in patients with COVID-19 and VTE versus those without VTE (17 studies, n = 2882)20,21,26,28,31,32,34,41,45,49,50,54,58–60,62,66 was 2.1 (95% CI: 1.2, 3.6) (Figure 4).
Figure 4.
Forest plot of odds ratios for death in patients with COVID-19 and VTE versus those without VTE. COVID-19, coronavirus disease 2019; VTE, venous thromboembolism.
Discussion
The main findings of this analysis were the following: (i) the overall prevalence of PE/DVT in hospitalized patients with COVID-19 subjected to assessment was about 30% but with considerable observed heterogeneity; (ii) VTE prevalence was high, even in patients receiving thromboprophylaxis, and appeared to be higher in studies with < 50% of patients anticoagulated; and (iii) patients with COVID-19 and VTE compared to those without VTE had higher risk for death.
It is well recognized that all hospitalized patients with acute medical illness are at high VTE risk. Critically ill patients admitted to ICUs are at very high VTE risk because of ICU-specific risk factors (immobilization, sedation, vasopressors or central venous catheters), but also individual patient-related risk factors (age, obesity, immobilization, history of personal or familial VTE, cancer, sepsis, respiratory or heart failure, pregnancy, stroke, trauma, or recent surgery).1,2,67 Thus, all hospitalized patients, and especially those in ICUs, are routinely assessed for VTE risk and often administered thromboprophylaxis.
At present, whether COVID-19 is associated with a higher VTE risk than other infections remains unclear. Initial case reports of VTE events in patients with COVID-19 were followed by case series studies, mainly conducted in an ICU setting, and showed high VTE prevalence, particularly in patients with severe COVID-19.20–66,68 It has been suggested that SARS-Cov-2 in severe forms of the disease induces an excessive inflammatory state via cytokine storm combined with endothelial injury and pulmonary vascular microthrombosis, which could considerably increase the risk for VTE, mainly PE.1–10 In recent autopsy studies, it has been found that the lungs of the infected patients are characterized by a widespread thrombosis with microangiopathy, whereas a high incidence of DVT has been recognized with PE being identified as a direct cause of death.69,70
This review of the current evidence indicates that the course of hospitalized patients with COVID-19 is complicated with DVT/PE in about 30% of cases, irrespective that most of them have received thromboprophylaxis. Unfortunately, studies providing direct head-to-head comparison and using the same assessment methodology between patients with COVID-19 and patients hospitalized for other reasons in terms of VTE prevalence are lacking. However, data from two studies that compared patients with COVID-19 and other (non-COVID-19) patients hospitalized in the same ICU but at different time points, showed higher prevalence of VTE in COVID-19.71,72 These data might support the notion that COVID-19 is associated with a higher risk of thrombosis than other diseases requiring ICU admission but future, well-designed studies should confirm this finding.
Another important finding was that patients with COVID-19 and VTE had a higher risk for death compared to those without VTE. Unfortunately, the exact cause of death (all-cause versus VTE-related) in these patients has not been reported in the included studies. On the other hand, data on the bleeding complications were scarce. However, in three of these studies reporting such information, bleeding complications were uncommon and minor.21,28,49
The findings of the current meta-analysis showed a relatively high prevalence of DVT and PE in the range of about 30%. Previous relevant meta-analyses have shown a pooled prevalence ranging from about 13%73,74 to 20%.75,76 However, these have included studies with a large methodological heterogeneity, with the results being dependent on the percentage of the study sample assessed for VTE. Shi et al. showed that the pooled prevalence of PE was increased from 8% to 28% when the assessment was performed in the total population.77 In addition, in line with the meta-regression analysis of this study showing that hospitalization in ICU determines a higher prevalence of PE, previous reports have shown a higher pooled prevalence in studies including patients in ICU versus those hospitalized in general wards.77,78 Thus, the current analysis reported higher prevalence of VTE compared to the existing literature and this was driven by the methodology of the included studies (screening/assessment in the total sample). The clinical relevance of this methodology is highlighted by the fact that most cases of DVT were reported as asymptomatic in many studies.
It should be noted that the high prevalence of VTE among patients with COVID-19 was observed despite using thromboprophylaxis in the majority of the included studies. In the meta-regression analysis, the prevalence of DVT was lower in studies with mixed dosing of anticoagulation in ⩾ 50% of the population compared to studies with standard prophylactic dosing of anticoagulation in < 50% of the population. A retrospective study in more than 4000 hospitalized patients with COVID-19 showed anticoagulation therapy to be associated with lower mortality and intubation events.5 Current recommendations strongly support the use of thromboprophylaxis in all hospitalized patients with COVID-19, although this is based mainly on expert opinion and less so on high-quality evidence.6–10 Furthermore, important details such as the optimal dose-intensity of the anticoagulation therapy are lacking.
The findings of this review should be interpreted by considering several limitations. Most important is the heterogeneity among these studies and the lack of information regarding (i) the patients’ individual VTE risk and (ii) details on the anticoagulant therapy (time of initiation, modification, etc.), which might have influenced the outcome. In a sensitivity analysis for identification of publication bias, there was a trend for higher PE prevalence in smaller studies, but there was no apparent trend in DVT prevalence. Furthermore, in a significant proportion of the included studies, exclusive polymerase chain reaction-based diagnosis of COVID-19 was unclear or not reported. Other criteria for diagnosis, such as imaging or other laboratory tests, might have been used but these probably regarded only a minority of patients and not the whole study sample. Thus, the exposure might not have been measured in a strictly reliable way in a minority of patients in some of the studies but this also reflects real clinical practice. However, the outcome was measured in a valid and reliable way in most of the included studies, although, in some of these, adjustment for confounders would be needed for accurate assessment. In addition, meta-regression analysis examined the associations between outcome and several characteristics which were aggregate and summarized at the level of the study, which in turn introduces ecological bias. Last, most of the studies did not provide information on hemorrhagic complications.
Since the screening/assessment process for VTE diagnosis represents a significant source of heterogeneity among such studies, we included only studies that screened/assessed the total population. Limb ultrasonography is an easy test that can be performed massively in the context of a research protocol and can identify asymptomatic patients, which is not an uncommon finding. However, computed tomography pulmonary angiography is performed in selected patients upon clinical suspicion combined with the D-dimer value. By selecting studies that performed these assessments in the whole study population, a more realistic estimate of the DVT/PE prevalence among these patients can be calculated, which in turn determines the pre-test probability in such patients. The latter estimate is a major determinant in a Bayesian approach where the diagnostic strategy depends on the pre-test probability. Thus, the findings of this meta-analysis might provide answers regarding the prevalence of DVT in hospitalized patients with COVID-19, including both symptomatic and asymptomatic cases (the latter being quite common), as well as regarding the prevalence of PE in hospitalized patients with COVID-19 and high suspicion based on clinical characteristics and D-dimer values.
Conclusion
This systematic review of the evidence suggests that hospitalized patients with COVID-19, who are screened or assessed for VTE, present a pooled prevalence of DVT and PE at about 30% each, and despite thromboprophylaxis in most cases. The VTE risk appears to be considerably higher than in patients without COVID-19 admitted in the same ICUs. Further research is necessary to investigate the individualized VTE risk of patients with COVID-19, the underlying pathogenetic mechanisms, and the optimal preventive anticoagulant therapy.
Supplemental Material
Supplemental material, sj-pdf-1-vmj-10.1177_1358863X21995566 for Venous thromboembolism in COVID-19: A systematic review and meta-analysis by Anastasios Kollias, Konstantinos G Kyriakoulis, Styliani Lagou, Evangelos Kontopantelis, George S Stergiou and Konstantinos Syrigos in Vascular Medicine
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anastasios Kollias  https://orcid.org/0000-0002-2098-1665
https://orcid.org/0000-0002-2098-1665
Supplementary material: The supplementary material is available online with the article.
References
- 1.Kollias A, Kyriakoulis KG, Dimakakos E, et al. Throm-boembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol 2020; 189: 846–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kollias A, Kyriakoulis KG, Stergiou GS, et al. Heterogeneity in reporting venous thromboembolic phenotypes in COVID-19: Methodological issues and clinical implications. Br J Haematol 2020; 190: 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joly BS, Siguret V, Veyradier A.Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med 2020; 46: 1603–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76: 1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020; 18: 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020; 75: 2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Society of Hematology. COVID-19 and VTE/anticoagulation: Frequently asked questions, https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation (2021, accessed 10 October 2020).
- 9.Oudkerk M, Büller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology 2020; 297: E216–E222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerotziafas GT, Catalano M, Colgan MP, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: Position Paper from VAS–European Independent Foundation in Angiology/Vascular Medicine. Thromb Haemost 2020; 120: 1597–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Joanna Briggs Institute. Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Analytical Cross Sectional Studies, https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Analytical_Cross_Sectional_Studies2017_0.pdf (2017, accessed 20 October 2020).
- 12.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67: 974–978. [DOI] [PubMed] [Google Scholar]
- 13.Kontopantelis E, Reeves D.Metaan: Random-effects meta-analysis. Stata J 2010; 10: 395–407. [Google Scholar]
- 14.Higgins JP, Thompson SG.Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 15.Kontopantelis E, Springate DA, Reeves D.A re-analysis of the Cochrane Library data: The dangers of unobserved heterogeneity in meta-analyses. PLoS One 2013; 8: e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petropoulou M, Mavridis D.A comparison of 20 heterogeneity variance estimators in statistical synthesis of results from studies: A simulation study. Stat Med 2017; 36: 4266–4280. [DOI] [PubMed] [Google Scholar]
- 17.MedCalc. Free statistical calculators. https://www.medcalc.org/calc/odds_ratio.php (2021, accessed 20 October 2020)
- 18.StatsToDo: Combine means and SDs into one group program. https://www.statstodo.com/CombineMeansSDs_Pgm.php (2020, accessed 20 October 2020)
- 19.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventura-Díaz S, Quintana-Pérez JV, Gil-Boronat A, et al. A higher D-dimer threshold for predicting pulmonary embolism in patients with COVID-19: A retrospective study. Emerg Radiol 2020; 27: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avruscio G, Camporese G, Campello E, et al. COVID-19 and venous thromboembolism in intensive care or medical ward. Clin Transl Sci 2020; 13: 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longhitano Y, Racca F, Zanza C, et al. Venous thrombo-embolism in hospitalized SARS-CoV-2 patients treated with three different anticoagulation protocols: Prospective observational study. Biology (Basel) 2020; 9: E310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavoni V, Gianesello L, Pazzi M, et al. Venous thromboembolism and bleeding in critically ill COVID-19 patients treated with higher than standard low molecular weight heparin doses and aspirin: A call to action. Thromb Res 2020; 196: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dujardin RWG, Hilderink BN, Haksteen WE, et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb Res 2020; 196: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espallargas I, Rodríguez Sevilla JJ, Rodríguez Chiaradía DA, et al. CT imaging of pulmonary embolism in patients with COVID-19 pneumonia: A retrospective analysis. Eur Radiol. 2021; 31: 1915–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller-Peltzer K, Krauss T, Benndorf M, et al. Pulmonary artery thrombi are co-located with opacifications in SARS-CoV2 induced ARDS. Respir Med 2020; 172: 106135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramadan L, Koziatek CA, Caldwell JR, et al. Pulmonary thromboembolism in COVID-19: Evaluating the role of D-dimer and computed tomography pulmonary angiography results. Am J Emerg Med. Epub ahead of print 5 Sep 2020. DOI: 10.1016/j.ajem.2020.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres-Machorro A, Anguiano-Álvarez VM, Grimaldo-Gómez FA, et al. Asymptomatic deep vein thrombosis in critically ill COVID-19 patients despite therapeutic levels of anti-Xa activity. Thromb Res 2020; 196: 268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez-Guiu X, Huici-Sánchez M, Romera-Villegas A, et al. Deep vein thrombosis in non-critically ill patients with coronavirus disease 2019 pneumonia: Deep vein thrombosis in non-intensive care unit patients. J Vasc Surg Venous Lymphat Disord. 2021; 9: 595–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouhat B, Besutti M, Bouiller K, et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J 2020; 56: 2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y, Tu J, Lei B, et al. Incidence and risk factors of deep vein thrombosis in hospitalized COVID-19 patients. Clin Appl Thromb Hemost 2020; 26: 1076029620953217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alonso-Fernández A, Toledo-Pons N, Cosío BG, et al. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: A prospective study. PLoS One 2020; 15: e0238216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgi-Pierfranceschi M, Paoletti O, Pan A, et al. Prevalence of asymptomatic deep vein thrombosis in patients hospitalized with SARS-CoV-2 pneumonia: A cross-sectional study. Intern Emerg Med 2020; 15: 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Jeune S, Suhl J, Benainous R, et al. High prevalence of early asymptomatic venous thromboembolism in anticoagulated COVID-19 patients hospitalized in general wards. J Thromb Thrombolysis. Epub ahead of print 18 August 2020. DOI: 10.1007/s11239-020-02246-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ierardi AM, Coppola A, Fusco S, et al. Early detection of deep vein thrombosis in patients with coronavirus disease 2019: Who to screen and who not to with Doppler ultrasound? J Ultrasound. 2021; 24: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alharthy A, Faqihi F, Abuhamdah M, et al. Prospective longitudinal evaluation of point-of-care lung ultrasound in critically ill patients with severe COVID-19 pneumonia. J Ultrasound Med 2021; 40: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzolo F, Rigoni AM, De Marchi S, et al. Deep vein thrombosis in SARS-CoV-2 pneumonia-affected patients within standard care units: Exploring a submerged portion of the iceberg. Thromb Res 2020; 194: 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho ES, McClelland PH, Cheng O, et al. Utility of D-dimer for diagnosis of deep vein thrombosis in coronavirus disease-19 infection. J Vasc Surg Venous Lymphat Disord 2021; 9: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monfardini L, Morassi M, Botti P, et al. Pulmonary thromboembolism in hospitalised COVID-19 patients at moderate to high risk by Wells score: A report from Lombardy, Italy. Br J Radiol 2020; 93: 20200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freund Y, Drogrey M, Miró Ò, et al. Association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: The PEPCOV International Retrospective Study. Acad Emerg Med 2020; 27: 811–820. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Wang X, Zhang S, et al. Characteristics of acute pulmonary embolism in patients with COVID-19 associated pneumonia from the City of Wuhan. Clin Appl Thromb Hemost 2020; 26: 1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longchamp A, Longchamp J, Manzocchi-Besson S, et al. Venous thromboembolism in critically Ill patients with COVID-19: Results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost 2020; 4: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whyte MB, Kelly PA, Gonzalez E, et al. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res 2020; 195: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marone EM, Bonalumi G, Curci R, et al. Characteristics of venous thromboembolism in COVID-19 patients: A multicenter experience from Northern Italy. Ann Vasc Surg 2020; 68: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: A French multicentre cohort study. Eur Heart J 2020; 41: 3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santoliquido A, Porfidia A, Nesci A, et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost 2020; 18: 2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med 2020; 48: e805–e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen K, Coolen-Allou N, Masse L, et al. Detection of pulmonary embolism in returning travelers with hypoxemic pneumonia due to COVID-19 in Reunion Island. Am J Trop Med Hyg 2020; 103: 844–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S, Zhang D, Zheng T, et al. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis 2021; 51: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koleilat I, Galen B, Choinski K, et al. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord 2021; 9: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bavaro DF, Poliseno M, Scardapane A, et al. Occurrence of acute pulmonary embolism in COVID-19 – A case series. Int J Infect Dis 2020; 98: 225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandmaison G, Andrey A, Périard D, et al. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open 2020; 4: e113–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzaccaro D, Giacomazzi F, Giannetta M, et al. Non-overt coagulopathy in non-ICU patients with mild to moderate COVID-19 pneumonia. J Clin Med 2020; 9: 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gervaise A, Bouzad C, Peroux E, et al. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol 2020; 30: 6170–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voicu S, Bonnin P, Stépanian A, et al. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol 2020; 76: 480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nahum J, Morichau-Beauchant T, Daviaud F, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020; 3: e2010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: Incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis 2020; 50: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: Prevalence, risk factors, and outcome. Circulation 2020; 142: 114–128. [DOI] [PubMed] [Google Scholar]
- 59.Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation 2020; 142: 181–183. [DOI] [PubMed] [Google Scholar]
- 60.Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID-19. Radiology 2020; 297: E335–E338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 2020; 192: 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J 2020; 56: 2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Criel M, Falter M, Jaeken J, et al. Venous thromboembolism in SARS-CoV-2 patients: Only a problem in ventilated ICU patients, or is there more to it? Eur Respir J 2020; 56: 2001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost 2020; 120: 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020; 18: 1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: Main characteristics, diagnosis and thromboprophylaxis. Crit Care 2015; 19: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danzi GB, Loffi M, Galeazzi G, et al. Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur Heart J 2020; 41: 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med 2020; 173: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID-19 patients: Awareness of an increased prevalence. Circulation 2020; 142: 184–186. [DOI] [PubMed] [Google Scholar]
- 73.Porfidia A, Valeriani E, Pola R, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res 2020; 196: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunutsor SK, Laukkanen JA.Incidence of venous and arterial thromboembolic complications in COVID-19: A systematic review and meta-analysis. Thromb Res 2020; 196: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Minno A, Ambrosino P, Calcaterra I, et al. COVID-19 and venous thromboembolism: A meta-analysis of literature studies. Semin Thromb Hemost 2020; 46: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu YF, Pan LY, Zhang WW, et al. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J Infect Dis 2020; 100: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi L, Xu J, Duan G, et al. The pooled prevalence of pulmonary embolism in patients with COVID-19. Intensive Care Med 2020; 46: 2089–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roncon L, Zuin M, Barco S, et al. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur J Intern Med 2020; 82: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vmj-10.1177_1358863X21995566 for Venous thromboembolism in COVID-19: A systematic review and meta-analysis by Anastasios Kollias, Konstantinos G Kyriakoulis, Styliani Lagou, Evangelos Kontopantelis, George S Stergiou and Konstantinos Syrigos in Vascular Medicine