Abstract
Background
Considering the new SARS-CoV-2 pandemic and the potential scarcity of material resources, the reuse of personal protective equipment such as filtering facepiece respirators (FFRs) for N95 filtering or higher is being discussed, mainly regarding the effectiveness and safety of cleaning, disinfection and sterilization processes.
Aim
To analyze the available evidence in the literature on the safety in processing FFRs.
Methods
A systematic review conducted by searching for studies in the following databases: PubMed, CINAHL, LILACS, CENTRAL, EMBASE, Web of Science, and Scopus.
Results
Forty studies were included in this review. The disinfectant/sterilizing agents most frequently tested at different concentrations and exposure periods were ultraviolet irradiation, vaporized hydrogen peroxide and steam sterilization. Microbial reduction was assessed in 21 (52.5%) studies. The only disinfectants/sterilizers that did not caused degradation of the material-integrity were alcohol, electric cooker, ethylene oxide, and peracetic acid fogging. Exposure to ultraviolet irradiation or microwave generated-steam resulted in a nonsignificant reduction in filter performance.
Conclusion
There is a complex relationship between the FFR raw materials and the cycle conditions of the decontamination methods, evidencing the need for validating FFRs by models and manufacturers, as well as the process. Some methods may require additional tests to demonstrate the safety of FFRs for use due to toxicity.
Key Words: Masks, Respiratory protective device, Disinfection, Sterilization, Decontamination, Equipment reuse
INTRODUCTION
Material resources during public health emergencies may be restricted and the processing of personal protective equipment such as filtering facepiece respirators (FFRs) for N95 filtering or higher may be placed on the agenda.1 Thus, health services have considered processing to reuse them in order to mitigate a possible shortage of respiratory protection devices.
In addition to the recommendation for prolonged use, decontamination followed by the reuse of FFR has been suggested as a contingency capacity strategy by the Centers for Disease Control and Prevention to conserve available supplies during a pandemic.2 , 3 This body emphasizes that FFRs must not be decontaminated to be reused as a standard procedure, as such practice is inconsistent with the approval of product use since it is not a requirement to support cleaning and disinfection; however, in a critical crisis situation it is an option to be considered.4 On the other hand, a recent publication by the US Food and Drug Administration (FDA) authorized the use of hydrogen peroxide-based equipment to sterilize N95 masks on an emergency basis, which would allow the reuse of each respirator up to 50 times.4
However, parameters such as biocidal efficacy, FFR functionality maintenance in relation to filtration performance and proper adjustment of the equipment to the face and presence of residual toxicity must be evaluated in order to consider the processing of the material a valid strategy to be implemented.3 , 5
Considering the new SARS-CoV-2 pandemic and the scarcity of data regarding the effectiveness of the cleaning, disinfection and sterilization processes of this equipment, the present study aims to analyze the evidence available in the literature on the safety of processing N95 or higher filtration masks.
METHODS
A systematic review following the recommendations of the PRISMA declaration (Preferred Reporting Items for Systematic Reviews and Meta-analyses) was used6 and the protocol of the project has been submitted on PROSPERO (Registration-No: CRD42020185605). The guiding question of the present systematic review was: Are disposable processed N95 or higher filtration FFRs safe for professional use regarding their integrity, filtration, and contamination?
Data sources and search strategy
The databases selected to search for the primary studies included in this review were: PubMed/Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS), Cochrane Central Register of Controlled Trials (CENTRAL), Excerpta Medica Database (EMBASE), Web of Science, and Scopus, from their conception until November 5, 2020. Controlled descriptors from each database were searched, as well as the following keywords: “masks,” “N95,” “n-95, ” “n95 filtering facepiece respirator,” “pff2,” “respiratory protective devices,” “respiratory protection device,” “disinfection,” “instrument sterilization,” “sterilizations,” “decontamination,” “medical device contamination,” “equipment reuse,” “reuse,” “reusable,” and “sanitization.”
For the location of publications, controlled descriptors, and keywords were delimited and combined (Supplementary Material 1). Gray literature (dissertations, government regulatory documents, and technical notes), including references cited in the included articles, published research reports, and preprint articles were also analyzed.
Study inclusion and exclusion criteria
Experimental studies, published in English, Spanish, or Portuguese, which evaluated decontamination and/or sterilization of FFRs were included with or without prior cleaning according to the outcomes of integrity, filtration and microbiological safety. Articles referring to respirators for industrial use or reusable respirators (dust respirators, plastic respirators, or elastomeric respirators), that submitted only fragments of FFRs to decontamination processes, letters to editor, research letters, and opinion articles not guided by scientific research were excluded.
Selection of studies
Two reviewers (authors C.S.L and J.R.G.) independently assessed the title and abstracts of potentially relevant studies using the selection criteria. A third author (V.B.P.) was consulted if it was unclear from the title and abstract whether a study met the inclusion criteria or if there was a disagreement over eligibility. The full text of articles considered as eligible were examined.
Data extraction
All data were extracted by 2 independent pairs of investigators (pair A: authors L.R.M. and G.A.A.M; pair B: authors R.A.O. and R.Q.S.) using a standardized form, and data were checked for integrity and accuracy by 2 other reviewers (authors C.S.L and J.R.G.). The data extracted from included studies were related to the following characteristics: title, journal of publication, year of publication, country of origin, language of publication; study design: type of study, fund source, mask type and model, manufacturer, pathogen load, and method of mask contamination, when available, and number of samples used; decontamination/sterilization procedures: Method of mask treatment, presence of cleaning process before the decontamination procedures, exposure time, frequency of exposure to the treatment, concentration/intensity of disinfectant agents; and the outcomes: penetration (absolute percentage, change relative to baseline), absolute and relative change in pathogen counting, observations of physical degradation and/or odor. When there were disagreements between investigators during data process extraction, another author was consulted (V.B.P).
Risk of bias and quality of evidence assessments
The risk of bias and the quality of evidence assessments were not evaluated due to the unavailability of validated tools for evaluating experimental laboratory studies.
Data synthesis
Due to the heterogeneity of the studies included in this SR, the synthesis of the included studies is presented in a narrative overview.
RESULTS
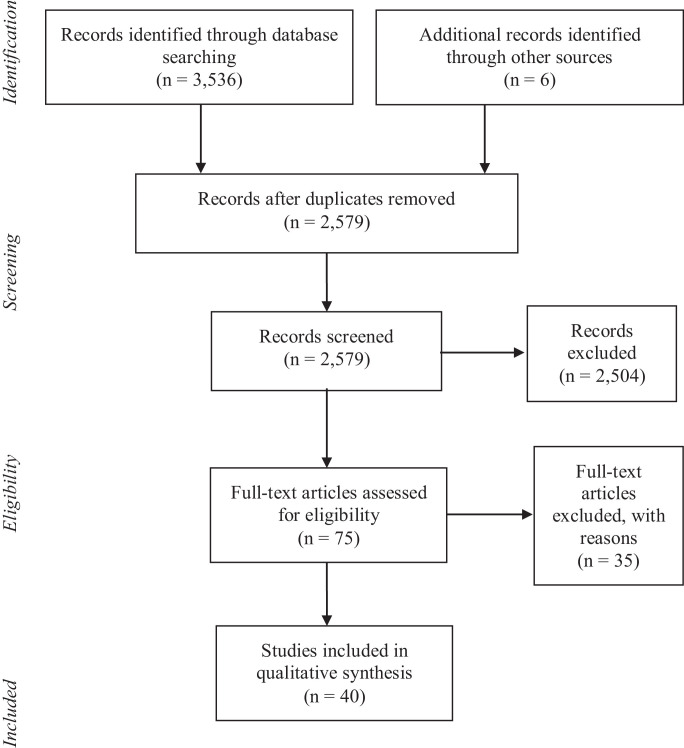
A total of 3,536 articles were identified during the database search, with 4 more articles found in additional searches. After removing duplicates, 2,579 articles were evaluated using titles and abstracts, of which 2,504 were excluded. The full texts of 75 articles were revised according to the inclusion and exclusion criteria, with 35 being excluded because they evaluated sterilization processes only with fragments of FFR, surgical masks, elastomeric masks, or were characterized as opinion articles, letters to the editor or recommendations by experts.
Thus, 40 studies for the qualitative synthesis of evidence remained in the review. The details of the study selection process are presented in the Figure 1 .
Fig 1.
PRISMA Flow diagram of the study selection process.
Studies’ characteristics
All identified publications developed experimental laboratory studies. All included studies analyzed N95 model FFRs.4 , 5 , 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 Additionally, 1 investigation employed FFR P100,7 another used PFF-3,20 and 7 also used surgical masks.5 , 16 , 21, 22, 23 , 31 , 41 More than half of the studies included in this review received funding.
Supplementary Material 2 presents the results of the studies according to the microbiological analysis conducted, the FFR integrity (including the facepiece and its components), filtering capacity, and residual presence of the disinfectant/sterilizing agent and funding source.
The most frequent disinfectant/sterilizing agents tested at different concentrations and exposure periods by the included studies were ultraviolet irradiation, vaporized hydrogen peroxide (VHP), steam sterilization (autoclave), sodium hypochlorite, microwave-generated steam, among others (Table 1 ). In addition to these, methods which are not traditionally used in environments aimed at disinfection or sterilization were also tested such as electric cookers and microwave ovens (Table 1).
Table 1.
Methods of disinfection/sterilization methods analyzed accordingly the included studies. Brazil, 2020
| Cleaning/disinfection/sterilization methods | Viscusi et al. (2007)7 | Vo et al. (2009)14 | Viscusi et al. (2009)19 | Bergman et al. (2010)16 | Salter et al (2010)15 | Fisher et al.,, 2010 24 | Fisher et al. (2011)17 | Viscusi et al. (2011)18 | Heimbuch et al. (2011)5 | Lore et al. (2012)8 | Heimbuch et al. (2014)9 | Lin et al. (2017)10 | Mills et al. (2018)11 | Lin et al. (2018)12 | Kumar et al. (2020)13 | Battelle et al. 2020)4 | Alijabo et al. (2020)25 | Andereg et al. (2020)26 | Bopp et al. (2020)23 | Cadnum et al. (2020)27 | Celina et al. (2020)28 | Czubryt et al. (2020)29 | Daeschler et al. (2020)31 | Grinshpun et al. (2020)31 | Harskamp et al. (2020)20 | Ibez-Cervantes, 2020)32 | Jatta et al. (2020)33 | Kim et al. (2020)34 | Lieu et al. (2020)35 | Lin et al. (2020)36 | Ludwig et al. (2020)37 | Ma et al. (2020)38 | Oh et al. (2020)39 | Ozog et al. (2020)40 | Pascoe et al. (2020)41 | Purschke et al. (2020)42 | Widmer et al. (2020)43 | Xiang et al. (2020)21 | Zhao et al. (2020)23 | Zulauf et al. (2020)44 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV-C/A | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 16 | ||||||||||||||||||||||||
| VHP | X | X | X | X | X | X | X | X | X | X | X | 11 | |||||||||||||||||||||||||||||
| Steam sterilization (Autoclave 121°C) | X | X | X | X | X | X | X | X | X | X | X | 11 | |||||||||||||||||||||||||||||
| Sodium hypochlorite | X | X | X | X | X | X | X | X | 8 | ||||||||||||||||||||||||||||||||
| Microwave-generated steam | X | X | X | X | X | X | X | X | 8 | ||||||||||||||||||||||||||||||||
| 70% Ethanol/70% Isopropyl/100% Isopropyl | X | X | X | X | X | X | 6 | ||||||||||||||||||||||||||||||||||
| Moist heat | X | X | X | X | X | X | 6 | ||||||||||||||||||||||||||||||||||
| Dry heat | X | X | X | X | X | 5 | |||||||||||||||||||||||||||||||||||
| EtO | X | X | X | X | X | 5 | |||||||||||||||||||||||||||||||||||
| HPGP | X | X | X | 3 | |||||||||||||||||||||||||||||||||||||
| Liquid hydrogen peroxide | X | X | X | 3 | |||||||||||||||||||||||||||||||||||||
| Electric cooker | X | X | X | 3 | |||||||||||||||||||||||||||||||||||||
| Microwave | X | X | 2 | ||||||||||||||||||||||||||||||||||||||
| DMDO | X | 1 | |||||||||||||||||||||||||||||||||||||||
| Pasteurization | X | 1 | |||||||||||||||||||||||||||||||||||||||
| Soap and water | X | 1 | |||||||||||||||||||||||||||||||||||||||
| Antiseptic wipes | X | 1 | |||||||||||||||||||||||||||||||||||||||
| Peracetic acid dry fogging system | X | 1 | |||||||||||||||||||||||||||||||||||||||
| High-level disinfection cabinet (Altapure, Mequon: peracetic acid, hydrogen peroxide, acetic acid) | X | 1 | |||||||||||||||||||||||||||||||||||||||
| 1% Pine-Sol and 1% benzalkonium chloride in Ethanol 70% | X | 1 | |||||||||||||||||||||||||||||||||||||||
| Gamma Irradiation | X | 1 |
HP = vaporized hydrogen peroxide; HPGP = hydrogen peroxide gas plasma.
Microbiological assessment
The microbiological survival was assessed in 21 studies.4 , 5 , 8 , 9 , 11, 12, 13, 14 , 17 , 21 , 24 , 25 , 27 , 31 , 32 , 37 , 39, 40, 41, 42 , 44 Seven studies proceeded the test using viruses such as bacteriophages,27 , 44 coronavirus,31 , 37 , 40 H1N1,21 adenovirus, and gastroenteritis virus.39 Five studies conducted the tests using only one bacterial specimens.4 , 9 , 32 , 41 , 42
From all 40 studies included in this review, only 6 evaluated 3 mainly parameters considered in this review, eaning microbial load, integrity, and filtration.4 , 21 , 25 , 39 , 41 , 44 A research report not published in a scientific journal that used Hydrogen Peroxide Vapor (HPV).4 The report recommends that further tests should be carried out with other models/brands of N95 FFR, because different respirators may have filter media which are affected in different ways by the sterilizing agent. In addition, they suggest tests to demonstrate the effectiveness of the HPV decontamination cycle against other microorganisms of interest in the community. In relation to the functionality tests, the amount of leakage in the FFR was measured during the light respiratory flow simulation (20 L/min) on a mannequin, having not been affected by up to 20 cycles.4
Other 5 studies used high temperature (heat). Steam sterilization resulted in successful inactivation of heat-resistant bacterial spores in 2 FFR models, and bacterial growth in samples of another model of FFR.25 The electric cooker method demonstrated higher inactivation efficacy of viruses inoculated on the hydrophilic surface (on the user's surface) compared to that on the hydrophobic surface (outside), and the final infectivity was below the detection limit of the plaque assay.39 Dry heat and microwave-generated steam were successful in achieving the target of >4 log10 reduction in S. aureus viability41 and killed 7 bacteria strains as well as inactivated the H1N1 virus.21 Also, in another study that used microwave generated steam a 6-log10 plaque-forming was detected.44
Integrity assessment
The preservation of material integrity was assessed in 22 studies (55.0%).4, 5 , 7 , 10 , 13 , 15 , 17 , 19, 20, 21, 22, 23 , 25, 26 , 28, 29, 30 , 33 , 35 , 39 , 41 , 44 It can be observed that the use of hydrogen peroxide,4 , 13 , 19 , 32 , 35 especially Hydrogen Peroxide Gas Plasma,4 , 19 and high-temperature methods10 , 15 , 16 , 19 , 23 , 25 , 26 , 28 , 41 were strongly associated with integrity degradation. Sodium hypochlorite also damaged the FFRs, causing stains, discoloration, dissolution of the nose seal comfort pad, oxidation, and stiffening of the filter and elastic7 , 10 , 15, 16 , 19 (Table 2 ).
Table 2.
Association between disinfection/sterilization methods analyzed by studies and damage to integrity and filtration, and the presence of chemical and microbial residues on FFR. Brazil, 2020
| Cleaning/disinfection/ sterilization method | Exposure time | Microbial residue | Damage integrity | Filtration damage | Chemical residue/Odor |
|---|---|---|---|---|---|
| Liquids (immersion) | |||||
| Alcohol 70% Ethanol10,12,31,34 70% Isopropyl7 100% Isopropyl10 Isopropyl (concentration not informed)36 |
1 sec7 1 min7 10 min10,12 120 min31 Overnight34 |
Survival of microorganisms up to 24 hours12 | Absence of structural damage10 | Filtration degradation:31 - between 17.02% to 21.6% (N95 or KF94) and 0.41% to 0.80% (P100 FFR);7,34 - N95: permeability for penetration of particles larger than 50 nm10 |
- |
| Soap and water 1g/L/ Tap water7 | 2min7 20 min7 |
- | - | Filtration degradation: - N95: between 38.8 and 34.9%7; - P100: 0.01%-0.14% with soap water; unchanged with tap water7 |
- |
| Sodium hypochlorite 0.525 and 5.25%7 0.54%, 2.7% or 5.4%12 0.60%15, 16 0.90%9 5.00%10 6.00%14,20 |
30 s910 min9,12 30 min7,15, 16,19 |
Absence of microbial survival12 or reduced microbial load14 Mucin removal was < a log10 reduction factor of 1; a log10 reduction factor of 3-59 |
Varied damage: - Stiffening of the filter and elastic strips7; - Stained metallic nasal bands and alteration of brightness, oxidation of metallic parts15,20; - Discoloration or dissolution of the nose seal comfort pad15,20 - unspecified damage10 |
Filtration degradation: - increased permeability for particle penetration below 5%9,10,15 |
Odor permanence15,16,20 8.25 mg/L: not detectable14 |
| Liquid hydrogen peroxide 3%7,166%7,15 |
30 min7,15, 16 | - | Varied damage: Oxidation of clamps to varying degrees15 |
Filtration degradation7 | Average amount of oxidants ranging between values below the detection limit at 0.70 mg16 |
| DMDO16 | 30 min16 | - | Oxidation of metal parts16 | - | Odor permanence16 |
| High temperature (heat) | |||||
| Steam sterilization (Autoclave) 115°C23 121°C 7,10,12, 13,20,23,25,29,31,34,36 130°C23 |
2 min23 4 min23 15 min7,10,12-13,34,36 17 min20 30 min7,23,25,29,31 60 min23 |
Absence of viable virus12,13 Bacterial growth in 1 of 4 models tested25 |
Varied damage: - Absence of damage in 10 autoclave cycles on models 3M 1870, VFlex 1804S and AO Safety 1054S13 - FFR-2: absence of damage20 - Deformed, shrunk and rigid outer layer10 - Significant variation was observed in the tensile force exerted by the straps of different FFR models25 - 1860 model did not pass fit testing under any of the decontamination conditions23 − Models1805 passed fit testing for up to 3 decontamination cycles, at both 115°C and 121.1°C. Models 1870/1870 passed fit testing for up to 3 decontamination cycles at both 115°C and 121.1°C but began to fail at 5 cycles at 121.1°C23 - PFF-3: showed deformities and failed the seal check2020 - FFR started failing after a second round of wear and sterilization29 |
Variable degradation results: - From little change(10) to increased particle permeability, between 18.7 to 34.4% (according to the exposure time)4,7,10,13,34 - After second autoclaving, the filtration efficiency decreased to 81.69 ± 17.28%34 - Drop in filtration, especially masks with protein31 - Maintained the minimum requirement of 95% filtration efficiency20,23,25,38 - FFR-3: Filtration degradation20 - Maintained the minimum requirement of 95% filtration efficiency in some models of FFR36 |
|
| Moist heat 65°C5,8,18,38 70°C30 70°C-85°C26 Temperature not informed38 |
20 min8,38 30 min18 40 min26 60 min30,38 120 min38 3 h5 |
Reduction of microbial load5,8 |
No signs of deterioration5,21,30 Modification of seal and fit; delamination of the nose bridge foam26 |
Non-significant reduction8,26 Significant reduction30 |
Odor permanence18 |
| Dry heat 60°C21 65°C28 70°C21,27,41 80°C 7,28 95°C28 102°C37 160°C7 |
30 min27 60 min7,21,37 5-90 min41 24 h28 |
Had limited effectiveness against bacteriophages MS2 and Phi6 versus S. aureus <1 log10 PFU versus >4 log10 CFU27 Reduction of microbial load21,37,41 |
Some evidence for onset of material weaknesses after 80°C exposure (deformations at the chin seal)28 | Filtration degradation: between 0.84% and 0.008%7 Filtration performance was maintained21,28 |
- |
| Microwave7,19 | 2 min7,19 4 min7 |
Reduction of microbial load for some models19 | Varied damage: - Melting of the filtering material, internal foam sealing liner and elastic strips19 |
Filtration degraded by 1.77%7 Filtration was maintained in models where there was no melting19 |
- |
| Microwave-generated steam5,8,15,17,18,24,41,44 |
40 s24 60 s41 90 s17,41 2 min5,8,15,18,41 3 min44 |
Reduction of microbial load for some models;8,17 H1N1 virus detected after decontamination;5 The decontamination process was not affected by dirty24 Reduction on microbial load (> 6 log10 reductions on S. aureus41 Reduction on microbial load 6 log10 reduction on plaque forming unit of MS2 phage44 |
Varied damage: - The nose clip arced, with loss of adhesion between the nose clip and respirator41 - A slight separation of the nose seal comfort pad5 - Melting of the filtering material, internal foam sealing lining and elastic strips15 - Sparks in the microwave15,19 Absence of damage18,24,44 |
Non-significant reduction (penetration <5% by particles of 300 nm)8 or filtration maintained17 No detectable changes on bacterial filtration performance41 |
Odor permanence18 |
| Electric cooker 149°C -164°C10,12 120°C -170°C39 |
3 min10,12 50 min39 |
Absence of microbial survival12,39 | Absence of structural damage10,39 | Filtration degradation: - Particle penetration greater than 27.9 nm exceeded 5% and that of particles from 14.1 nm to 594 nm exceeded 8.6%10 - Maintained the minimum requirement of 95% filtration efficiency39 |
- |
| Pasteurization15 | 30 min15 | - | Damage to the nose seal comfort pad, Melting rubber bands15 |
- | - |
| Low temperature | |||||
| UV-C5,7,8,11,12,14,15,16,18,19,22,24,27,37,40,42 UV-A12 |
60-70 sec11,40 1 min27 4 min37 5 min42 20 min12,24 15 min5,8 30 min7,18, 45 min14, 15,18 60 min15, 16 2, 3, 4, or 5 h16 480 min7 Not informed22 |
Microbial load: a log10 reduction factor of 3-45,8,14,37 Survival of microorganisms after decontamination5,12 Difference in efficacy among the cycles of both the low and high soil load sample sets24 Log10 reduction was lower than 227 SARS-CoV-2 was below the limit of detection after the treatment.40 Microbial load: a 6-log bacteria spore (Bacillus pumilus) inactivation42 |
Absence of damage16 Varied damage: - Detachment of the cushion18 - No visible changes were observed5,15,19 - Optical microscopy: the morphological measurements suggest negligible changes to the mask materials at the UV doses applied22 |
Nonsignificant reduction (penetration <5% by particles of 300 nm)8,15; minor change in filtration performance7 Did not affect the filter aerosol penetration or filter airflow resistance of the FFRs19 Did not affect the filter airflow resistance of the FFRs15,19 The filtration test of an N95 FFR show no significant mask deterioration for up to 5 cycles of 1 J/cm222 |
Not detected16 The ozone levels were below the limit of detection of the sensor (0.001 ppm) and well below the minimum acceptable exposure levels42 |
| EtO 54%-55%7,14,16, 17,19 |
1 h (+4 h of aeration)7,19 1 h (+12 h of aeration) 3 h13,15,16 |
Absence of viral survival13 | Absence of structural damage7,15,19 | Filtration degradation: 1.29%7 | Several of the models and components treated with EO contained diacetone alcohol (4-hydroxy-4-methyl-2-pentanone) and traces of a contaminant identified as 2-hydroxyethyl acetate (HEA, ethylene glycol monoacetate)16 |
| Hydrogen Peroxide Gas Plasma (HPGP)7,13,15,19,32 | 28 min7 47 min13,32 55 min7,15,19 |
Absence of viral survival13 | Varied damage: - Stained metallic nasal bands and alteration of brightness19 - Structural damage from the second cycle13 |
Filtration degradation: - Between 4.64% and 8.76%15 minor change in filtration performance7 |
- |
| Vaporized Hydrogen Peroxide (VHP) 4,13,15,16,32,33,35,37,43 |
15 min15 20 min4 28 min33,35,37 30 min13 55 min16 60 min (± 15 min)37 Not informed43 |
Absence of viral survival,13 or a log10 reduction factor of 64 or viral load undetectable37 SARS-CoV-2; S. aureus and A. baumannii absent after disinfection32 |
Absence of structural damage4,13 Proportion of masks that failed fit testing after a single cycle of extended use and decontamination was 66% and varied according to model35 FFR reprocessed for 15 cycles were reported to be tight and uncomfortable on the face32 |
Did not cause any observable physical changes to the FFR4,32/ expected levels of filter aerosol penetration (< 5%) and filter airflow resistance15 The fit testing (followed the EN 149 - European standard for FFP respirators, which is similar to the NIOSH-42CFR84) was met for all the 10 test persons with both, new and reprocessed masks43 |
Average amount of oxidants ranging from 0.35 to 1.23 mg16 Residual H2O243: Packed FFRs: 1.5 ± 0.1 mg/mm3 Unpacked FFRs: 3.5 ± 1.5 mg/mm3 however it reduced to 0.2 ± 0.1 mg/mm3 after 24 h aeration |
| Others | |||||
| Cleaning wipes with benzalkonium chloride or 0.9% hypochlorite9 |
30 s9 | Reduction of microbial load9 | - | - | - |
| Peracetic acid dry fogging system13 | 1 h13 | No virus recovery post-decontamination13 | No loss of structural or functional integrity after 10 cycle13 | - | - |
| Multi-Purpose High-Level Disinfection Cabinet (Altapure, Mequon: peracetic acid, hydrogen peroxide, and acetic acid)27 | 21 min and with an extended 31 min cycle27 |
Reductions of >2.1, >3.6, and > 6 log10 CFU27 | - | - | - |
| Spraying 1% Pine-Sol and 1% benzalkonium chloride in Ethanol 70%28 | Not informed28 | - | - | No measurable consequences on filtration performance28 | - |
| Gamma irradiation36 | Not informed36 | - | - | Filtration degradation (violated the 5% penetration criteria)36 | - |
CFU = colony forming unit; h = hour; PFU = plaque forming unit; s = second.
In relation to heat methods, the absence or presence of damage caused by steam sterilization was associated with FFR model13 , 20 , 23 , 25 or number of decontamination cycles.23 , 29 The use of microwaves associated or not with the generation of steam obtained varied results. Some FFRs had part of their components melted after applying the technology15 , 19 or had damage to the nose seal comfort pad.15, 16 The risks represented by the use of this equipment also stand out, with the presence of sparks due to metallic pieces15 , 19 , 41 (Table 2); however, 3 studies18 , 24 , 44 did not report structural damage or sparks implementing this technology.
Regarding the UV method, most of the studies reported no visible changes,5 , 15, 16 , 19 , 22 and only 1 reported loss of product resistance and visible degradation.21 The methods which did not result in changes in the integrity of the FFR were ethylene oxide (EtO),13 , 15 , 19 electric cooker10 , 39 and Peracetic acid dry fogging system13 (Table 2).
Filtration assessment
The filtration capacity was evaluated in 25 studies.4 , 7 , 8 , 10 , 15 , 17 , 19, 20, 21, 22, 23 , 25 , 26 , 28 , 30 , 31 , 33 , 34 , 36 , 38 , 39 , 41, 42, 43, 44 Changes with significant loss of recommended filtration efficiency were observed when using alcohols, sodium hypochlorite, water and soap and steam sterilization, dry heat, and moist heat7 , 9 , 10 , 13 , 15 , 20 , 30 , 31 , 34 (Table 2). The use of soap or alcohol resulted in increased filter penetration,10 and it is possible that these agents remove the electrostatic charge present in the FFR fibers.7
The Hydrogen Peroxide Gas Plasma method resulted in changes in filtration performance in 2 studies,7 , 15 although this result was not observed in another investigation of the method,19 even though the exposure time to the sterilizing agent was the same. The VHP method did not affect the filtration efficiency.15 , 32 , 43 Regarding exposure to ultraviolet irradiation (UV-C), studies have shown a nonsignificant reduction in filter performance7 , 8 , 22 or maintained filtration efficiency.15 , 19
Chemical residuals assessment
The presence of chemical residues was observed in 4 studies.14 , 16 , 19 , 42 A study evaluated the amount of residual chemicals created or deposited and found that 6 of the 7 methods evaluated did not deposit significant amounts of toxic waste in/on the FFRs.16 The presence of 2-hydroxyethyl acetate (HEA) was detected in the elastic strips of the FFR processed with EtO in 11 of the 15 samples and cyclohexanone in 2 samples. Another study identified that ozone levels were below the limit of detection of the sensor (0.001 ppm) and well below the minimum acceptable exposure levels.42 Odor presence was identified after decontamination with sodium hypochlorite and dimethyldioxirane (DMDO). Regarding DMDO, there was an accumulation of oxidant in the middle of the filter, probably due to its hydrophobic characteristic; however, there is no specific information about the toxicity of this agent.16 Residual H2O2 was detected after VHP method in packed FRR (1.5 ± 0.1 mg/mm3 and in unpacked FFR (3.5 ± 1.5 mg/mm3), however it reduced to 0.2 ± 0.1 mg/mm3 after 24 hour aeration.43
Still in relation to toxicity after applying sodium hypochlorite, tests conducted in triplicate demonstrated that neutralization with recovery medium on consecutive days confirmed that the recovery solution was adequate to neutralize the active components of the hypochlorite.14 Another investigation carried out measurements of chlorine gas elimination after processing with sodium hypochlorite after rehydration of the FFRs with deionized water and observed an increase in the measured gas release, thus simulating the release of moisture through breathing, allowing an individual to be exposed to low levels of chlorine (0.2 ppm).19
Studies which evaluated EtO, an agent known for its potential residual toxicity, did not perform tests for the presence of chemical residues.7 , 13 , 19 Only one of the studies evaluated the acceptance or perception of users after the masks were subjected to processing, which identified inadequate adjustment of the FFR and the presence of odor due to the residual presence of the agent.18
Design assessment
Of the 40 studies included, 244 , 7 , 8 , 10 , 11 , 13, 14, 15 , 16 , 17, 18, 19, 20 , 23 , 25, 26, 27, 28 , 30 , 33 , 35 , 36 , 38 , 40, 41, 42 had their FFR sample composed of different models and/or manufacturers. Three studies5 , 9 , 16 did not mention whether they worked with the same models or brands of FFR. Eleven studies worked with only 1 FFR model.12 , 14 , 21, 21 , 31, 32 , 34 , 37 , 39 , 43, 44
Variations in filtration efficiency and structural change seem to be associated with differences in structural conformation, such as the presence or absence of nose seal comfort pad, and the model which may have 3 or more filter layers, as experiments have shown different effects on the integrity of the FFRs submitted to the same disinfection/sterilization methods,7 , 19 or even different water absorption capacities, since the FFRs constructed with hydrophilic materials absorb more water depending on the brand.17
Some of the analyzed studies also observed differences in terms of filtration efficiency10 , 17 , 18 or integrity20 , 23 , 25 , 35 according to the tested FFR brand. Another difference noted between brands in the use of the same technology was related to differences in terms of microbial reduction between different brands in both the facepiece and the elastic.11 , 25
In this sense, although 1 investigation does not mention whether the tests were carried out with FFR of the same brand, it found that models with simple design (without a comfort pad for the nose) retained less oxidants than more complex models.16
In an overview, FFR previous cleaning, which is a recommended premise in terms of first step to the reuse of medical devices, was not adopted by the analyzed studies. In this way, FFR inspection to detect presence of soil and other residues like residual lipstick, make-up and others, or varied damage, was a criterion to discard this personal equipment before decontamination process.43 It should be considered in the practice of decontaminating FFR some parameters: integrity of used FFR before and after submitting to the decontamination process; choice of a method recognized as able to inactivate microbial load; being free of chemical residues or unpleasant odor on the FFR; amount of cycles that an FFR can be processed by the chosen method; and maintenance of filtration and design (fit-testing) performance after the process to assure the occupational health. The results show that no study included in this review evaluated all these points simultaneously.
DISCUSSION
Our analysis of the produced scientific literature showed great variability in the methods used as well as in the samples. The diversity in the raw materials used in the FFR, including elastics and pads to adapt to the face, responded in a nonuniform manner to the conditions used in each of the decontamination processes, which will be discussed below.
The scientific literature revealed a tendency to test automated sterilization methods. These are promising alternatives as they have several advantages over adapted or manual methods (eg, electric cookers),10 , 12 , 39 as the normative requirements of manufacturer, operation and qualification, as well as devices for monitoring provide additional security.
The routine use may expose FFRs to unreproduced laboratory conditions, as demonstrated by a study which quantified the damage inflicted on PFF-2 respirators over time and found that internal stains and folds were more frequent after 12-hour shifts when compared to 6-hour shifts.45 The reality of the services provided during the pandemic is prolonged use of masks, which can reach up to 12 hours.
A relevant aspect addressed by the studies is the possible number of reuses.4 , 13 , 15 , 17 , 20 , 23 , 24 , 29 , 31 , 35 , 39 However, different models of FFR presented different responses to decontamination whether in filtration efficiency, decomposition of components such as the elastic, and reduced efficiency of the sterilizing agent. Studies have found physical various types of damage soon after first and over 5 successive cycles of steam sterilization31 or altered filter capacity of FFRs after 1-3 steam sterilization cycles, with the results varying according to the manufacturer.20 According to Kim et al.,34 the Korean Ministry of Food and Drug Safety certified KF94 masks similar to regulations established by the National Institute of Occupational Safety and Health, can be reused after autoclaving up to 2 times without any significant decrease in the filtration efficiency.
A study that analyzed masks that were not used in healthcare assistance reported that samples can withstand up to 30 sterilization cycles in vaporized hydrogen peroxide.4 However, it is noted that studies that analyzed FFRs in real conditions of healthcare assistance reported a considerably lower number of reuses, as noted in studies that demonstrated that FFRs failed after a second round of wear and steam sterilization,29 66% fit testing failed after a single cycle of extended use and decontamination in vaporized hydrogen peroxide,35 and reduced filtration efficiency from 93.76% to 85.03% after 4 hours of use and steam sterilization.34 On the other hand, Ma et al.38 did not observe differences when comparing FFRs used for 7 days to new FFRs, after exposure to steam generated by boiling water, however without methodological detailing and temperature control during exposure. That said, the analysis of masks that were not exposed to the conditions of daily use can characterize an important limitation of the studies.
Considering that low temperature sterilization methods can become ineffective in the presence of organic matter,46 the sterilization safety of any medical devices without cleaning requires the use of samples which simulate the real conditions of use, including natural wear and tear, as well as a representative contamination challenge. For example, in the study conducted by Widmer and Richner,43 the used masks were collected in specific containers and sent to the Sterile Processing Department for inspection regarding surfaces, debris and visual changes, such as residual lipstick, make-up and other residuals. Among the 5,000 FFRs inspected, 10% were discarded due to the presence of soil.
Cleaning also implies some degree of mechanical action, temperature and use of solutions, which can cause additional stress on a surface which was not manufactured for this purpose. Only 1 study evaluated cleaning with water and soap (immersion for 20 minutes) as a potential method for decontamination and observed the degradation of the FRR filtration capacity,7 confirming that the product was not designed for dirt removal methods. The use of methods which involve “wipes” may not be the best alternative due to the possibility of mechanical action damaging the filter,9 as it is still a manual method subject to variables which may not have been predicted in the laboratory; this might include the physical strength of the performer, and functional tests to certify the maintenance of efficiency in the service routine which are not yet available.
In daily routine, professionals need to speak and cough during their daily activities which can lead to an increase in the amount of organic matter and can become cumulative due to the different sterilization cycles without cleaning.24 Some studies argue that organic matter may not be significant enough for the decontamination methods by UV11 , 24 or microwave-generated steam,17 , 24 as long as the effectiveness is proven. This information is based on the results of Fisher et al.24 which used 3 simulated reuses to show that the dirt had no effect on the steam generated by the microwave; however, it reduced the inactivation of viruses in the UV method. This reduction in effect could be compensated for by measuring the decrease in irradiation and by increasing the exposure time.24 At this point, the authors of this review emphasize that this finding should not be applied to all decontamination methods until evidence is produced.
The study by Heimbuch et al.9 discussed the difficulty of finding objective parameters to define a clean product. However, it should be kept in mind that residual organic matter, in any situation, must not prevent disinfection or sterilization. The impact of organic matter in successive decontamination cycles in other methods requires evidence, since the limitations of the effectiveness of sterility in the presence of organic matter in the physical-chemical methods (ethylene oxide - 100% and mixtures, hydrogen peroxide at low temperature and formaldehyde) have already been documented.46
There was no uniformity in the results regarding the UV method.5 , 8 , 11 , 18 , 19 , 22 , 23 , 27 , 37 , 40 , 42 According to recent systematic reviews, UV decontamination can be a promising alternative, at least for a single reuse. However, some aspects still require further investigation, such as the effectiveness of the method on different N95 FFR models, the impact of UV on mask fit, as well as the maximum number of UV cycles that can be safely applied to an FFR in the real-world setting.47 , 48
Lin et al.36 demonstrated that the quality of filtration in nonexpired and expired FFR models can be strongly affected by gamma irradiation. Since we did not find other studies using the method, we consider that further investigations on this method are necessary.
Regarding methods based on heat (wet or dry heat), not all components of the FFRs are thermo-resistant, which can restrict their use; in addition, there are variations in resistance in certain FFR models.13 , 20 , 23 , 29 , 31 The advantage of these methods is the possibility of validating specific cycles for FFRs, as long as they are made from raw materials which are compatible with the variables of the cycle used. It is important to highlight that the use of these methods requires qualification to ensure that the cycle conditions, which include the temperature necessary for microbial inactivation, are achieved on all sample surfaces of the same load.26
The data regarding the methods which used microwaves or steam generated by microwaves5 , 8 , 15 , 17 , 19 , 24 , 41 , 44 show divergent results and incompatibilities between the raw materials with the method; therefore, there are cycle adjustments which still need to be accomplished. Additionally, the use of new methods also requires proper characterization of the sterilizing agent and its routine monitoring and validation procedures49 so that its use can be ensured in the service routines. These observations are also valid for new technologies whose proposal is decontamination, such as methods which use fogging.13 That said, direct adaptation of methods originally not intended for decontamination is not recommended. The use of hydrogen peroxide deserves reservations with respect to the FFR integrity, since the results demonstrate that the strongly ionizing action of the agent can possibly neutralize the electrostatic charge of the filter due to trapping particles in it. Thus, changes in the mask integrity can result, especially after 2 sterilization cycles in equipment which uses plasma.13 There is also the interaction of some raw materials with the sterilizing agent,16 , 19 with evidence of aborting the cycles when more than 6 samples were placed in the chamber.17 The residuals also require control, since Widmer and Richner43 found 1.5 ± 0.1 mg/m3 hydrogen peroxide on individually packed FFP2 immediately after sterilization. The unpacked respirators showed 3.6 ± 1.5 mg/m3 immediately after sterilization but aeration for 24 hours led to 0.2 ± 0.1 mg/m3.
Another aspect that should be considered when using hydrogen peroxide, regardless of whether or not plasma is used, is the variability of cycles and equipment available on the market according to the results obtained. Based on the diversity of the materials used in mask manufacturing, it is prudent that any validations are carried out by type of FFR and that decision makers can clearly identify which FFR was used as the specimen in the tests, since there is no possibility of generalization. Thus, nonspecification of the samples constitutes an important limitation of the applicability of the results in practice. These reasons may explain the divergences evidenced in the results obtained through sterilization in hydrogen peroxide.4 , 7 , 15, 16 , 18 , 19 , 33 , 35 , 37 , 43
Ethylene oxide (EtO)12 , 13 , 15 , 16 , 19 is recognized for its penetrating power and has some advantages such as process automation and standardized procedures related to validation and monitoring, control and medical device release.50 However, it is toxic and requires care to ensure the safety of users of products which have been exposed to it.13 The materials can vary in the absorption and desorption rates of EtO and its by-products (ethylene glycol and ethylene chlorhydrin), requiring a demonstration that the residual quantities are within the permitted limits,13 , 51 characterizing an essential step in the studies which used using EtO in processing FFRs.
Thus, quantitative assessments of the exposure risk to EtO are necessary due to the proximity of the FFR to the user's face and breathing zone, and should not be underestimat ed.16 , 19 The presence of sterilizing agent residues in the filtering medium should be considered, since the larger the area of the filtering medium, the greater the exposure risk to the user.14 , 16 In addition, another aspect to be analyzed are designs with pads with thicker nasal tubes, which are more likely to retain residues.
There are proposals for using solutions for immersing FFRs.7 , 9 , 10 , 14 , 15 , 16 , 19 , 28 , 31 , 34 From the evaluation of the authors of this review this should be the last option due to the disadvantages of using these methods in practice, especially when used manually: the need for rigorous cleaning, drying, limited experiences on certain products, the need for controlled rinsing and sufficient to eliminate residues, since the solutions can be potentially irritating to eyes, skin and mucous membranes, difficulties in controlling and monitoring the process and fixative properties of some solutions.52
Due to uncertainties about the impact of decontamination on respirator performance, processed FFRs should not be used by healthcare professionals when performing or presenting an aerosol generation procedure, and it is further recommended that FFRs should be handled with care even after decontamination.3 Whatever the technology used, a decontamination cycle should not expose a product and its packaging (if used) to extreme cycle conditions, which could compromise its use.53 Based on the studies analyzed, further research should focus on the reuse of FFRs after routine use, validation of decontamination procedures specific to each model, maximum number of reuses, lack of cleanliness, tests for integrity control, and functionality in the Sterile Processing Department.
In summary, it is highlighted that the expected viral load during actual use in a healthcare environment is not known and will depend on several factors such as viral load eliminated by infected individuals, the amount of potential aerosol-generating procedures, system exhaust, and environmental pressurization. That said, managers must be clear that safety in the disinfection and sterilization processes is not limited to the microbiological efficacy results, as the objective is still to protect health professionals. Managers should really be decision-makers, and must consider that the reuse of masks implies substantial changes in processing-related activities, of which the following stand out:
-
•
Integration of these products with the existing traceability systems, since the same mask may or may not be shared by different professionals. In this topic, Jatta et al.33 presented a workflow in which an FFR is assigned to a single healthcare professional, and labeled with a permanent marker. The authors also presented an example of tracking for FFRs and control of the number of reuses. We also emphasize the need to control the number of reuses of each mask and the impact of this activity on the processing routine and managing health products;
-
•
Definition of parameters and training of personnel to inspect the integrity and functionality of the masks. Pascoe et al.41 proposed a workflow for FFP2/N95-type respirator reprocessing with decontamination method selection and criteria for disposal before and after decontamination. So far, there are no rapid tests which can objectively measure the filtration capacity in the operational routine of the Sterile Processing Department; therefore, this requirement would be limited to visual parameters, which may be insufficient to ensure the safety of professionals;
-
•
Construction of an evidence-based regulatory apparatus to legitimize and define the conditions necessary for the reuse of masks, whether temporary or permanent. One of the important regulatory issues concerns the responsibility for carrying out the processing, which could be done in each user facility under its own conditions or third parties which demonstrate the minimum technical capacity required. In any scenario, the conditions which must be met for reuse, including the production of safety evidence, must be clear.
Finally, when an FFR is submitted to a decontamination method in order to be reused, some aspects must be considered in addition to biocidal efficacy: the elimination of previous organic matter through cleaning, the natural tear related to routine use, the maximum number of safety reuses, and the preservation of the filtration quality. It also must be considered the adjustment/sealing factor of the FFR, which is noticeably affected by physical damage both in the respiratory part and in the rubber elastic, in addition to the residual toxicity resulting from the processing, which can represent an additional risk.
It is a limitation on this review that the authors decided to not provide a meta-analysis comparing the effects of disinfection and/or sterilization of FFRs on their usage. This is because there were substantial differences in the primary studies design, especially because the majority of studies did not evaluate the FFR after the conditions of daily use, procedures and assessment of the effect of disinfection and/or sterilization, which could result in an inaccurate estimative of effect. This could lead to mistaken interpretations of the systematic review results and ultimately put the healthcare personnel under unacceptable risks.
CONCLUSION
The results do not enable generalizations due to the diversity of tested products and methods. The analysis showed a complex relationship between the raw materials of the FFR and the cycle conditions of the decontamination methods, showing that the validations must be carried out for each FFR model, each manufacturer and each sterilization technology in the 3 evaluated outcomes. Some methods may require additional tests to demonstrate safety due to toxicity.
In any case, the questions related to the impact of natural wear of the masks during use associated with successive sterilizations performed without prior cleaning performed on the FFRs used in care practice have not yet been answered. This fact reveals an important limitation of the current evidence.
In emergency situations where reuse is inevitable for the continuity of patient care, automated sterilization methods are safer options due to the possibility of validating specific cycles which are compatible with each type of respirator, as long as they are intact and without any visible dirt. In addition, the maintenance of its functionality must be verified after processing, especially the facial sealing. Finally, the number of reuses must be controlled and incorporated into the Sterile Processing Department traceability (or tracking) systems.
Footnotes
Conflicts of interest: None to report.
PROSPERO Registration-No: CRD42020185605.
Funding: This research was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) [grant number 001, 2020]. The funder had no role in study design, data collection, data analysis and interpretation, or writing the report.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.11.022.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Brady TM, Strauch AL, Almaguer CM, et al. Transfer of bacteriophage MS2 and fluorescein from N95 filtering facepiece respirators to hands: measuring fomite potential. J Occup Env Hyg. 2017;14:898–906. doi: 10.1080/15459624.2017.1346799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . CDC; Atlanta. Available at: 2020. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings.https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html Accessed June 10, 2020. [Google Scholar]
- 3.Centers for Disease Control and Prevention . CDC; Atlanta. Available at: 2020. Decontamination and Reuse of Filtering Facepiece Respirators.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Accessed June 10, 2020. [Google Scholar]
- 4.United States Food and Drug Administration F. enforcement policy for face masks and respirators during the coronavirus disease (COVID-19) public health emergency (revised): guidance for industry and food and drug administration staff. 2020. p. 1–15. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health. Accessed June 10, 2020.
- 5.Heimbuch BK, Wallace WH, Kinney K, et al. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viscusi D, King W, Shaffer R. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Resp Prot. 2007;24:93–107. [Google Scholar]
- 8.Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 9.Heimbuch BK, Kinney K, Lumley AE, Harnish DA, Bergman M, Wander JD. Cleaning of filtering facepiece respirators contaminated with mucin and Staphylococcus aureus. Am J Infect Control. 2014;42:265–270. doi: 10.1016/j.ajic.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin T-H, Chen C-C, Huang S-H, Kuo C-W, Lai C-Y, Lin W-Y. Filter quality of electret masks in filtering 14.6-594 nm aerosol particles: effects of five decontamination methods. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills D, Harnish DA, Lawrence C, Sandoval-Powers M, Heimbuch BK. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin T-H, Tang F-C, Hung P-C, Hua Z-C, Lai C-Y. Relative survival of bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;28:754–762. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Kasloff SB, Leung A, et al. N95 mask decontamination using standard hospital sterilization technologies. medRxiv [preprint, 2020 [Google Scholar]
- 14.Vo E, Rengasamy S, Shaffer R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl Env Microbiol. 2009;75:7303–7309. doi: 10.1128/AEM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman M, Viscusi D, Heimbuch B, Wander J, Sambol A, Shaffer R. Evaluation of multiple (3-Cycle) decontamination processing for filtering facepiece respirators. J Eng Fiber Fabr. 2010;5:33–41. [Google Scholar]
- 16.Salter WB, Kinney K, Wallace WH, Lumley AE, Heimbuch BK, Wander JD. Analysis of residual chemicals on filtering facepiece respirators after decontamination. J Occup Env Hyg. 2010;7:437–445. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher EM, Williams JL, Shaffer RE. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS One. 2011;6:e18585. doi: 10.1371/journal.pone.0018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viscusi DJ, Bergman MS, Novak DA, et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Env Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 19.Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harskamp RE, Van Straten B, Bouman J, et al. Reprocessing filtering facepiece respirators in primary care using medical autoclave: prospective, bench-to-bedside, single-centre study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang Y, Song Q, Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am J Infect Control. 2020;48:880–882. doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Zhang Z, Lanzarini-Lopes M, et al. Germicidal ultraviolet light does not damage or impede performance of N95 Masks upon multiple uses. Environ Sci Technol Lett. 2020;7:600–605. doi: 10.1021/acs.estlett.0c00416. [DOI] [PubMed] [Google Scholar]
- 23.Bopp NE, Bouyer DH, Gibbs CM, Nichols JE, Ntiforo CA, Grimaldo MA. Multicycle autoclave decontamination of N95 filtering facepiece respirators. Appl Biosaf. 2020;25:150–156. doi: 10.1177/1535676020924171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher E, Williams J, Shaffer R. The effect of soil accumulation on multiple decontamination processing of N95 filtering facepiece respirator coupons using physical methods. J Int Soc Respir Prot. 2010;27:16–26. [Google Scholar]
- 25.Alijabo A, Mueller E, Azeez DA, Hore T, Jain A. Gravity steam reprocessing in healthcare facilities for the reuse of N95 respirators. J Hosp Infect. 2020;106:698–708. doi: 10.1016/j.jhin.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderegg L, Meisenhelder C, Ngooi CO, et al. A scalable method of applying heat and humidity for decontamination of n95 respirators during the covid-19 crisis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadnum JL, Li DF, Redmond SN, John AR, Pearlmutter B, Donskey CJ. Effectiveness of ultraviolet-c light and a high-level disinfection cabinet for decontamination of n95 respirators. Pathog Immun. 2020;5:52–67. doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celina MC, Martinez E, Omana MA, et al. Extended use of face masks during the COVID-19 pandemic - thermal conditioning and spray-on surface disinfection. Polym Degrad Stab. 2020;179 doi: 10.1016/j.polymdegradstab.2020.109251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czubryt MP, Stecy T, Popke E, et al. N95 mask reuse in a major urban hospital: COVID-19 response process and procedure. J Hosp Infect. 2020;106:277–282. doi: 10.1016/j.jhin.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daeschler SC, Manson N, Joachim K, et al. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ. 2020;192:E1189–E1197. doi: 10.1503/cmaj.201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinshpun SA, Yermakov M, Khodoun M. Autoclave sterilization and ethanol treatment of re-used surgical masks and N95 respirators during COVID-19: impact on their performance and integrity. J Hosp Infect. 2020;105:608–614. doi: 10.1016/j.jhin.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibáñez-Cervantes G, Bravata-Alcántara JC, Nájera-Cortés AS, et al. Disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria using hydrogen peroxide plasma: impact on the reutilization of disposable devices. Am J Infect Control. 2020;48:1037–1041. doi: 10.1016/j.ajic.2020.06.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jatta M, Kiefer C, Patolia H, et al. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. Am J Infect Control. 2020;20:30576–30579. doi: 10.1016/j.ajic.2020.06.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HP, Jo MS, Kim CH, Choi JS, Yu IJ. Re-use of health masks after autoclaving. NanoImpact. 2020;19 doi: 10.1016/j.impact.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieu A, Mah J, Zanichelli V, Exantus RC, Longtin Y. Impact of extended use and decontamination with vaporized hydrogen peroxide on N95 respirator fit. Am J Infect Control. 2020;48:1457. doi: 10.1016/j.ajic.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin T-H, Tseng C-C, Huang Y-L, Lin H-C, Lai C-Y, Lee S-A. Effectiveness of N95 facepiece respirators in filtering aerosol following storage and sterilization. Aerosol Air Qual Res. 2020;20:833–843. [Google Scholar]
- 37.Ludwig-Begall LF, Wielick C, Dams L, et al. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J Hosp Infect. 2020;106:577–584. doi: 10.1016/j.jhin.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma QX, Shan H, Zhang CM, et al. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: Experimental supports. J Med Virol. 2020;92:1971–1974. doi: 10.1002/jmv.25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh C, Araud E, Puthussery J V., et al. Dry heat as a decontamination method for n95 respirator reuse. Environ Sci Technol Lett. 2020;7:677–682. doi: 10.1021/acs.estlett.0c00534. [DOI] [PubMed] [Google Scholar]
- 40.Ozog DM, Sexton JZ, Narla S, et al. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int J Infect Dis. 2020;100:224–229. doi: 10.1016/j.ijid.2020.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascoe MJ, Robertson A, Crayford A, et al. Dry heat and microwave-generated steam protocols for the rapid decontamination of respiratory personal protective equipment in response to COVID-19-related shortages. J Hosp Infect. 2020;106:10–19. doi: 10.1016/j.jhin.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purschke M, Elsamaloty M, Wilde JP, et al. Construction and validation of UV-C decontamination cabinets for filtering facepiece respirators. Appl Opt. 2020;59:7585. doi: 10.1364/AO.401602. [DOI] [PubMed] [Google Scholar]
- 43.Widmer AF, Richner G. Proposal for a en 149 acceptable reprocessing method for FFP2 respirators in times of severe shortage. Antimicrob Resist Infect Control. 2020;9:88. doi: 10.1186/s13756-020-00744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zulauf KE, Green AB, Ba ANN, et al. Microwave-generated steam decontamination of N95 respirators utilizing universally accessible materials. MBio. 2020;11:1–9. doi: 10.1128/mBio.00997-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DuarteI LRP, Miola CE, Cavalcante NJF, Bammann RH. Maintenance status of N95 respiratormasks after use in a health care setting. Rev Esc Enferm USP. 2010;44:1011–1016. doi: 10.1590/s0080-62342010000400022. [DOI] [PubMed] [Google Scholar]
- 46.Alfa MJ, DeGagne P, Olson N, Puchalski T. Comparison of ion plasma, vaporized hydrogen peroxide, and 100% ethylene oxide sterilizers to the 12/88 ethylene oxide gas sterilizer. Infect Control Hosp Epidemiol. 1996;17:92–100. doi: 10.1086/647252. [DOI] [PubMed] [Google Scholar]
- 47.O'Hearn K, Gertsman S, Sampson M, et al. Decontaminating N95 and SN95 masks with ultraviolet germicidal irradiation does not impair mask efficacy and safety. J Hosp Infect. 2020;106:163–175. doi: 10.1016/j.jhin.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Martinez CE, Sossa-Briceño MP, Cortés JA. Decontamination and reuse of N95 filtering facemask respirators: a systematic review of the literature. Am J Infect Control. 2020;48:1520. doi: 10.1016/j.ajic.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Organization for Standardization. ISO 14937. Sterilization of health care products — General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices. 2009. Available at: https://www.iso.org/standard/44954.html. Accessed June 10, 2020.
- 50.International Organization for Standardization. ISO 11135. Sterilization of health-care products — ethylene oxide — requirements for the development, validation and routine control of a sterilization process for medical devices. 2014. Available at: https://www.iso.org/standard/56137.html. Accessed June 10, 2020.
- 51.Shintani H. Ethylene oxide gas sterilization of medical devices. Biocontrol Sci. 2017;22:1–16. doi: 10.4265/bio.22.1. [DOI] [PubMed] [Google Scholar]
- 52.Rutala WA, Weber DJ, Committee HICPA. Centers for Disease Control and Prevention; Atlanta: 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; pp. 1–163.https://stacks.cdc.gov/view/cdc/47378 Accessed June 10, 2020. [Google Scholar]
- 53.International Organization for Standardization. ISO 17665-1. Sterilization of health care products — moist heat — Part 1: requirements for the development, validation and routine control of a sterilization process for medical devices. 2006. Available at: https://www.iso.org/obp/ui/#iso:std:iso:17665:-1:en. Accessed June 10, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.