Abstract
Assessment of efficacy of therapeutic plasma exchange (TPE) following life-threatening COVID-19. This was an open-label, randomised clinical trial of ICU patients with life-threatening COVID-19 (positive RT-qPCR plus ARDS, sepsis, organ failure, hyperinflammation). Study was terminated after 87/120 patients enrolled. Standard treatment plus TPE (n = 43) versus standard treatment (n = 44), and stratified by PaO2/FiO2 ratio (>150 vs. ≤150), were compared. Primary outcomes were 35-day mortality and TPE safety. Secondary outcomes were association between TPE and mortality, improvement in SOFA score, change in inflammatory biomarkers, days on mechanical ventilation (MV), and ICU length of stay (LOS). Eighty-seven patients [median age 49 (IQR 34–63) years; 82.8% male] were randomised (44 standard care; 43 standard care plus TPE). Days on MV (P = 0.007) and ICU LOS (P = 0.02) were lower in the TPE group. 35-Day mortality was non-significantly lower in the TPE group (20.9% vs. 34.1%; Kaplan-Meier, P = 0.582). TPE was associated with increased lymphocytes and ADAMTS-13 activity and decreased serum lactate, lactate dehydrogenase, ferritin, d-dimers and interleukin-6. Multivariable regression analysis provided several predictors of 35-day mortality: PaO2/FiO2 ratio (HR, 0.98, 95% CI 0.96–1.00; P = 0.02]; ADAMTS-13 activity (HR, 0.89, 95% CI 0.82–0.98; P = 0.01); pulmonary embolism (HR, 3.57, 95% CI 1.43–8.92; P = 0.007). Post-hoc analysis revealed a significant reduction in SOFA score for TPE patients (P < 0.05). In critically-ill COVID-19 patients, addition of TPE to standard ICU therapy was associated with faster clinical recovery and no increased 35-day mortality.
Keywords: COVID-19, Acute respiratory distress syndrome, Cytokine release syndrome, Therapeutic plasma exchange, Intensive care unit, Thromboinflammation
1. Introduction
The novel disease COVID-19 (coronavirus disease 2019), caused by infection with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), emerged in 2019 in China and has spread worldwide [1], [2], [3], [4]. Whilst SARS-CoV-2 infection is mostly asymptomatic and/or self-limited, patients can become critically ill, as manifested by acute respiratory distress syndrome (ARDS), thromboemboli, hyperinflammation and multi-system organ failure (MSOF) [5], [6], [7], [8], [9], [10], [11], [12]. There is no treatment outside of dexamethasone, and the world is still awaiting widespread vaccination [13], [14], [15], [16], [17], [18]. Therefore, it is important to explore novel but rational therapies. Therapeutic plasma exchange (TPE) has been used for severe sepsis, MSOF and SARS-CoV infection [19], [20], [21]. Several groups, including the US Food and Drug Administration (FDA) [22], [23], [24], [25], [26], [27], [28], have also posited that rescue TPE might have a role in severe COVID-19, but to date it has been insufficiently studied. The rationale is to suppress cytokine release syndrome (CRS), ameliorate thrombosis and lessen MSOF [26,27]. In this randomised controlled clinical trial, we build upon our prior work [24], [25], [26], [27] by evaluating TPE (specifically its efficacy and adverse effects) when added to empirical intensive care unit (ICU) treatment for life-threatening COVID-19 and associated hyperinflammation.
2. Methods
This single-centre, open-label, randomised clinical study enrolled critically-ill COVID-19 patients admitted to the level III (300 bed) ICU of King Saud Medical City (Riyadh, Saudi Arabia) between 1 July 2020 and 1 October 2020. It was conducted according to the principles of the Declaration of Helsinki [29] and was approved by our Institutional Review Board. The study's protocol was registered at ISRCTN (ISRCTN21363594; doi: 10.1186/ISRCTN21363594) and is outlined elsewhere [30]. Written informed consent was obtained from patients or their legal representatives.
2.1. Inclusion criteria
The main inclusion criteria were: (i) age ≥18 years; (ii) intubation and ICU admission; and (iii) life-threatening COVID-19 [[2], [3], [4], [5], [6],[13], [14], [15],27,31] defined as (a) ARDS (according to the Berlin criteria) [32,33], (b) Acute Physiology and Chronic Health Evaluation (APACHE) II score ≥20 upon ICU admission [34], (c) presence of severe sepsis/septic shock and/or MSOF [35,36] and (d) one or more criteria for defining CRS [11,12,[37], [38], [39], [40]] (as previously described [27] and also presented in Supplementary e-Table 1). SARS-CoV-2 infection was confirmed by real-time reverse transcription PCR (RT-qPCR) assay using a QuantiNova Probe RT-PCR Kit (QIAGEN GmbH, Germany) in a LightCycler® 480 Real-Time PCR System (Roche, Basel, Switzerland) [[25], [26], [27],41,42]. Other inclusion criteria were: (i) positive RT-qPCR result within 48 h before randomisation; (ii) signed informed consent and acceptance of assignment to randomised treatment groups; (iii) randomisation within first 48 h of meeting the criteria for life-threatening COVID-19; and (iv) no participation in other clinical trials during the study period.
2.2. Exclusion criteria
Exclusion criteria were: (i) previous allergic reaction to plasma exchange or its ingredients (i.e. sodium citrate, plasma products); (ii) two consecutive negative RT-qPCR tests for SARS-CoV-2 taken ≥24 h apart; (iii) participation in other antiviral clinical trials for COVID-19 within 30 days prior to randomisation; and (iv) terminal illness and/or other contraindications (i.e. known immune suppression/deficiency status) as per the discretion of the attending physician.
2.3. Further screening and outcome measures
Primary outcomes were mortality at 35 days post ICU admission and the safety of TPE in life-threatening COVID-19. Secondary outcomes were improvement in Sequential Organ Function Assessment (SOFA) score [43], the effect of TPE on mortality, change in inflammatory biomarkers within 24 h of the final TPE session, days on mechanical ventilation (MV), and ICU length of stay [27,30]. Upon ICU admission, further screening was performed by computed tomography pulmonary angiograms (CT-PA) in subjects with a peripheral arterial oxygen saturation/fraction of inspired oxygen (PaO2/FiO2) ratio <80 for >24 h. Hence, pulmonary embolism (PE) was categorised, if discovered, as arising from main/lobar, segmental or subsegmental lung regions according to standard criteria [44].
2.4. Randomisation
Patients were randomly assigned via computer-generated random numbering (1:1) to receive standard treatment plus TPE (intervention group) or standard treatment alone (control group). Eligible consented patients were further stratified into two groups based upon a PaO2/FiO2 ratio of >150 vs. ≤150. Randomisation occurred in variable block sizes of four to eight patients. We utilised a web-based randomisation service (randomize.net) to allocate patients to their respective stratification prior to intervention or control therapy. Given the difficulty in blinding TPE, the intervention was unblinded (open label); hence, no enrolment concealment was expedited [30]. We defined the time point of appropriateness for ICU transfer as a surrogate for the time of discharge from the ICU. This was chosen irrespective of treatment allocation, while recognising patients randomised to TPE could have improved to the point of being appropriate for a medical ward but would need to stay in the ICU to deliver the remaining TPE doses. To minimise further assessment bias, clinical outcomes were evaluated by an investigator who was blind to the study group allocation.
2.5. Control group
Patients in the control group received standard empirical therapy for COVID-19, which was based on the evolving Saudi Ministry of Health treatment protocol [45]. Empirical therapies included antivirals (ribavirin 400 mg every 12 h for 14 days), antibacterial medications, dexamethasone (6 mg/day for 7 days), anticoagulation and ICU supportive care [14,15,27,30,45].
2.6. Intervention (TPE) group
Patients in the intervention group received the standard empirical therapeutic regimen plus TPE without protective antibodies. TPE was initiated using a Spectra OptiaTM Apheresis System (Terumo BCT Inc., USA), which incorporates acid-citrate dextrose anticoagulant as per Kidney Disease: Improving Global Outcomes (KDIGO) 2019 guidelines [28,46]. TPE can remove significant proportions of interferon-gamma (IFNγ), interleukins IL-3, IL-10, IL-1B, IL-6 and IL-8, and tumour necrosis factor-alpha (TNFα) [12,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. A dose of 1.5 plasma volumes was used for the first daily treatment, then one plasma volume daily (sessions lasted for 4 h) to a maximum of five doses as per clinical case scenario. Intravenous calcium replacement and chlorpheniramine 10 mg were administered during TPE to reduce side effects [26]. Plasma was replaced with either fresh frozen plasma or artificial Octaplas LG® (Octapharma AG, USA), a fresh frozen pooled plasma product that has undergone viral/prion inactivation [47]. To evaluate the ability of TPE to remove the inflammatory mediators associated with COVID-19 [12,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]], we measured serum C-reactive protein (CRP), d-dimers, lactate dehydrogenase (LDH), ferritin and IL‐6 prior to initiation and within 24 h after the last TPE session. CRP was defined as elevated if it was >5.0 mg/L and IL‐6 if it was >7.0 pg/mL [48]. To determine thromboinflammation risk, we also measured the levels of ADAMTS-13 activity (TECHNOZYM®ELISA) at the same time points in all enrolled patients [26,49].
2.7. Treatment plan and safety monitoring
TPE sessions were discontinued if clinical improvement was determined by the attending physician as follows: (i) ability to wean from MV with PaO2/FiO2 ratio >300; (ii) haemodynamic stability defined as mean arterial pressure >65 mmHg without vasopressors; and (iii) absence of septic shock (defined as a lactate level ≤2 mmol/L) [26,27]. During the study period, all enrolled patients were monitored for extrapulmonary manifestations of COVID-19 (i.e. neurological symptoms) [25,26] and for putative complications of TPE (allergic reactions, coagulopathy, cardiac/renal failure), bacterial infections such as ventilator-associated pneumonia (defined as positive respiratory culture or respiratory PCR plus compatible clinical and radiological findings) [50], bloodstream infection (defined by one or more positive blood cultures associated with systemic signs of infection such as fever, chills and/or hypotension) and urinary tract infection (defined as one or more positive urinary culture associated with systemic signs of infection) [51]. ICU discharge criteria for all patients were normal vital signs, no need for supplemental oxygen, and two consecutive RT-qPCR test results from nasopharyngeal swabs ≥48 h apart (after which no more testing was performed) [30].
3. Statistical analysis
This was an open-label, randomised clinical study. The original sample size was determined to be 60 for each group, which would provide 80% power, with a two-sided significance level of P = 0.05, to detect a 20% reduction in 35-day mortality in the TPE arm from an estimated 40% mortality in the control arm based on previous studies of critically-ill COVID-19 patients [[3], [4], [5],13,15]. Analysis was performed according to the full data set, which is defined as the sum of all randomised patients who received at least one treatment. The primary efficacy analysis was conducted on the intention-to-treat population, defined as all patients who underwent randomisation and received TPE plus standard therapy or standard therapy alone before death or at 35 days after randomisation. Continuous data were summarised as the median and interquartile range (IQR) and were compared among groups using the Wilcoxon's rank-sum test. Discrete data were summarised as number (%) and were compared by χ2 or Fisher's exact test. Standard comparisons of changes in clinical and laboratory parameters were also performed by Tukey boxplots in the intervention and control groups. Pearson's correlation coefficient (r) measured the strength of association between studied markers of thromboinflammation such as d-dimers, IL-6 and ADAMTS-13 activity. The 35-day survival distributions between intervention (TPE) and control groups were described using proportions and Kaplan–Meier analysis. Crude differences were tested using a χ2 test and a log-rank test, respectively. A multivariable analysis of the primary 35-day survival outcome was conducted using a Cox proportional hazard model with 95% confidence intervals (CIs), where the main effect of TPE (intervention versus control) and relevant variables including age, admission PaO2/FiO2 ratio, laboratory parameters, admission APACHE II/SOFA scores, and high risk (>3 criteria; Supplementary e-Table 1) for developing CRS were entered as covariates. Variables that achieved a P-value of <0.10 in the univariate analysis were fit into a multivariable regression model predicting 35-day mortality. The final logistic model included variables that achieved a P-value of ≤0.05 on forward stepwise selection and after examination for collinearity. Moreover, a post-hoc analysis (with a Bonferroni correction of the P-values for repeated measures) was added to compare the progression of SOFA scores at Days 0, 7, 14 and 35 post ICU admission. All statistical tests were two tailed and were considered significant at a P-value of ≤0.05. IBM SPSS Statistics v.24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
4. Early study termination
Due to mitigation of the SARS-CoV-2 outbreak in Saudi Arabia, the number of COVID-19 cases decreased significantly by mid-September 2020. Our last patient was enrolled on 1 October 2020. Thereafter, we did not have any potential recruitment targets or expectation of a second COVID-19 wave. After preliminary data analysis and discussions that were held with our expert infectious diseases panel, institutional review board and the primary investigator on 30 October 2020, the study was provisionally terminated with 87 patients enrolled.
5. Results
5.1. Study population
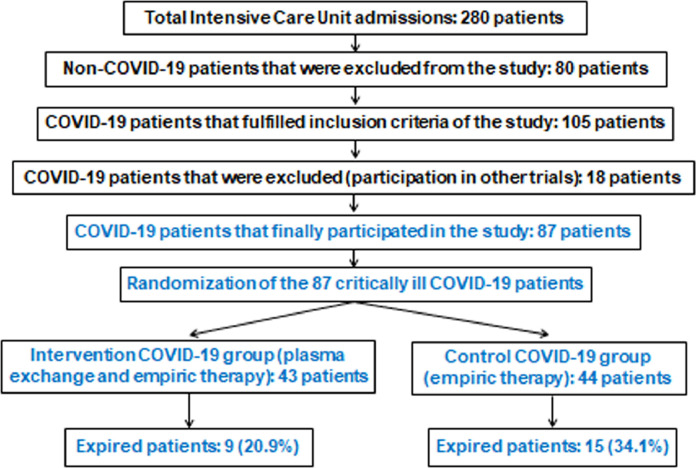
During the study period, a total of 280 critically-ill patients were admitted to the ICU, of whom 80 patients did not have COVID-19 and thus were excluded. Of the 200 COVID-19 ICU patients, 105 patients fulfilled the inclusion criteria. Of these, 18 were excluded due to participation in other trials. No protocol violations were observed. This left 87 critically-ill COVID-19 patients randomised and assigned to either the intervention (TPE) group or the control group in a 1:1 ratio as outlined in the CONSORT flow diagram (Fig. 1 ). This meant 43 patients in the intervention group and 44 patients in the control group. Baseline characteristics and outcome measures for the 87 COVID-19 patients are given in Table 1 . The median age was 49 years (IQR 34–63 years), 72 patients (82.8%) were male and the median body mass index (BMI) was 26 (IQR 21–31). Overall and within the PaO2/FiO2 ratio stratification groups, there were no significant differences in age, sex, BMI, prevalence of co-morbidities, duration of symptom onset to ICU admission, and APACHE II/SOFA scores between patients who underwent TPE versus controls. However, TPE patients did have a higher prevalence of PE and risk for developing CRS compared with controls (Table 1). No extrapulmonary manifestations related to the COVID-19 status were found. The duration of MV (P = 0.007) and ICU length of stay (P = 0.02) were significantly lower in the intervention group versus controls. Mortality on Day 35 post ICU admission was lower in the intervention group (20.9%) compared with the control group (34.1%), but did not reach statistical significance (P = 0.09). RT-qPCR for COVID-19 was negative in survivors after an average of 18 days post ICU admission for the TPE group (IQR 15–23 days) and 19 days (IQR 16–24 days) for the control group (P = 0.27). Following ICU admission, the median time to randomisation was 2 days (IQR 0.5–2.5 days) (Table 1). Baseline recorded clinical and laboratory parameters of the COVID-19 study population are presented in Table 2 . Upon ICU admission, 30 patients (68.2%) in the control group and 33 patients (76.7%) in the intervention group were receiving vasopressors. At baseline, there were no significant differences between the TPE and control groups for median arterial blood pressure, noradrenaline infusion dose, white blood cell and platelet counts, serum lactate and creatinine levels, and overall coagulation profile. However, patients in the TPE group had significantly lower baseline lymphocyte counts and ADAMTS-13 activity, and increased baseline levels of LDH, ferritin, d-dimers and IL-6 compared with controls (P < 0.05) (Table 2).
Fig. 1.
Flow diagram of the randomised clinical trial.
Table 1.
Baseline characteristics and outcomes for critically-ill COVID-19 patients
| Parameter | Total patients (n = 87) | Control group (n = 44) | Intervention (TPE) group (n = 43) | P-value |
|---|---|---|---|---|
| Age (years) | 49 (34–63) | 49 (33–63) | 48.3 (33.3–63.3) | 0.43 |
| Sex male [n (%)] | 72 (82.8) | 36 (81.8) | 36 (83.7) | 0.81 |
| BMI (kg/m2) | 26 (21–31) | 25.5 (19.5–32.5) | 27 (22–32) | 0.30 |
| Co-morbidities [n (%)] | 41 (47.1) | 19 (43.2) | 22 (51.2) | 0.46 |
| Diabetes mellitus [n (%)] | 18/41 (43.9) | 8/19 (42.1) | 10/22 (45.5) | 0.83 |
| Hypertension [n (%)] | 35/41 (85.4) | 16/19 (84.2) | 19/22 (86.4) | 0.85 |
| Coronary artery disease [n (%)] | 2/41 (4.9) | 1/19 (5.3) | 1/22 (4.5) | 0.92 |
| Symptoms onset to ICU admission (days) | 6 (3–9) | 7 (4–10) | 6 (3–9) | 0.19 |
| Parameters during hospitalisation | ||||
| APACHE II score upon ICU admission | 22 (20–24) | 22 (21–23) | 23 (21–25) | 0.25 |
| SOFA score upon ICU admission | 10 (7–13) | 9 (6–12) | 10 (8–13) | 0.07 |
| Acute kidney injury requiring CRRT [n (%)] | 12 (13.8) | 6 (13.6) | 6 (14.0) | 0.97 |
| Pulmonary embolism [n (%)] | 19 (21.8) | 6 (13.6) | 13 (30.2) | 0.05* |
| PaO2/FiO2 ratio at baseline | 125 (65–185) | 125 (75.5–174.5) | 135 (72–198) | 0.50 |
| PaO2/FiO2 ratio baseline stratification | ||||
| PaO2/FiO2 ratio ≤150 [n (%)] | 50 (57.5) | 27 (61.4) | 23 (53.5) | 0.52 |
| PaO2/FiO2 ratio >150 [n (%)] | 37 (42.5) | 17 (38.6) | 20 (46.5) | 0.49 |
| Cytokine release syndrome risk group | ||||
| Low-risk group (≤3 criteria) [n (%)] | 14 (16.1) | 14 (31.8) | 0 (0) | 0.001* |
| High-risk group (>3 criteria) [n (%)] | 73 (83.9) | 30 (68.2) | 43 (100) | 0.001* |
| Hospital-acquired infection [n (%)] | 13 (14.9) | 6 (13.6) | 7 (16.3) | 0.85 |
| No infections [n (%)] | 74 (85.1) | 38 (86.4) | 36 (83.7) | 0.89 |
| Ventilator-associated pneumonia [n (%)] | 8 (9.2) | 4 (9.1) | 4 (9.3) | 0.91 |
| Bloodstream infection [n (%)] | 5 (5.7) | 2 (4.5) | 3 (7.0) | 0.73 |
| Randomisation and TPE data | ||||
| Time to randomisation after ICU admission (days) | 2 (0.5–2.5) | – | – | - |
| Onset of TPE after ICU admission (days) | – | – | 2 (1–3) | - |
| Number of TPE sessions | – | – | 3 (1–5) | – |
| Outcome measures | ||||
| Duration of mechanical ventilation (days) | 17 (7–27) | 19 (7.7–30.3) | 15 (8–22) | 0.007* |
| ICU length of stay (days) | 22 (8–36) | 26 (11.5–31.5) | 19 (12–27) | 0.02* |
| Mortality on Day 35 [n (%)] | 24 (27.6) | 15 (34.1) | 9 (20.9) | 0.09 |
NOTE: Values are median and interquartile range unless otherwise stated.
TPE, therapeutic plasma exchange; BMI, body mass index; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Function Assessment; CRRT, continuous renal replacement therapy; PaO2/FiO2, partial arterial pressure of oxygen/fractional inspired concentration of oxygen.
Comparisons between the two groups were considered significant at P ≤ 0.05.
Table 2.
Baseline clinical and laboratory parameters for critically-ill COVID-19 patients
| Parameter | Total patients (n = 87) | Control group (n = 44) | Intervention (TPE) group (n = 43) |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 94 (83–105) | 98.5 (85–113.5) | 92 (80–104) |
| Diastolic blood pressure (mmHg) | 50 (39–69) | 49 (39–69) | 52 (40–64) |
| Noradrenaline infusion (μg/kg/h) | 1 (0.8–1.2) | 1 (0.7–1.2) | 1.0 (0.8–1.2) |
| White blood cell count (cells/mm3) (normal range, 4000–10 000) | 12 000 (6000–18 000) | 12 000 (6200–17 800) | 12 000 (6000–18 000) |
| Lymphocytes (109/L) (normal range, 1.1–3.2) | 0.5 (0.2–0.8) | 0.6 (0.2–1) | 0.5 (0.2–0.7)* |
| Platelets (cells/mm3) (normal range, 150 000–450 000) | 128 000 (109 000–185 000) | 126 000 (103 000–196 000) | 129 000 (9700–176 000) |
| Prothrombin time (s) (normal range, 9–12) | 13 (11–15) | 13 (10.8–16.1) | 12 (10–14) |
| Partial thromboplastin time (s) (normal range, 26–36) | 43 (29–52) | 43 (28–51) | 43 (33–52) |
| International normalisation ratio (normal range, 0.8–1.2) | 1.10 (0.8–1.4) | 1.10 (0.8–1.4) | 1.10 (0.8–1.4) |
| ADAMTS-13 activity (%) (normal range, 53–205) | 19 (6–34) | 21 (7.7–35.3) | 17 (6–38)* |
| Creatinine (mg/dL) (normal range, 0.6–1.2) | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 1.1 (0.9–1.3) |
| Total bilirubin (μmol/L) (normal range, 0–26) | 33 (23–39) | 33.5 (20.2–38.8) | 33 (23–39) |
| Serum lactate (mmol/L) (normal range, 1.0–2.5) | 4 (1–7) | 4 (1–7) | 5 (2–8) |
| Alanine aminotransferase (U/L) (normal range, 9–50) | 56 (37–76) | 55.5 (40.2–68.8) | 56 (31–78) |
| Aspartate aminotransferase (U/L) (normal range, 15–40) | 45 (27–65) | 46.5 (20.2–66.8) | 43.0 (22.8–63.8) |
| C-reactive protein (mg/L) (normal range, 0–5) | 234 (112–355) | 234 (109–359) | 246 (157–356) |
| Lactate dehydrogenase (U/L) (normal range, 100–190) | 589 (109–1099) | 378 (100–656) | 876 (546–1206)* |
| Ferritin (ng/mL) (normal range, 23–336) | 569 (129–1161) | 320 (75–675) | 987 (319–1655)* |
| d-dimers (μg/mL) (normal values, <1) | 4 (1.4–7.6) | 2.5 (1.4–4.6) | 4.9 (2.9–7.9)* |
| Interleukin-6 (pg/mL) (normal range, 1–7) | 232 (55–809) | 122.5 (48.7–262.8) | 458 (225–1091)* |
NOTE: Values are the median and interquartile range.
TPE, therapeutic plasma exchange.
P ≤ 0.05 was considered statistically significant by Wilcoxon signed-rank test for non-parametric data between controls and patients who underwent TPE.
5.2. Therapy, adverse events and mortality analysis
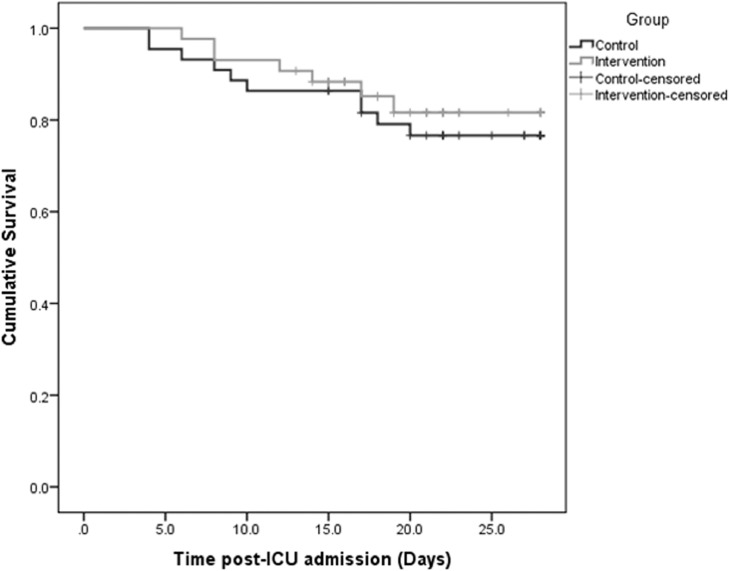
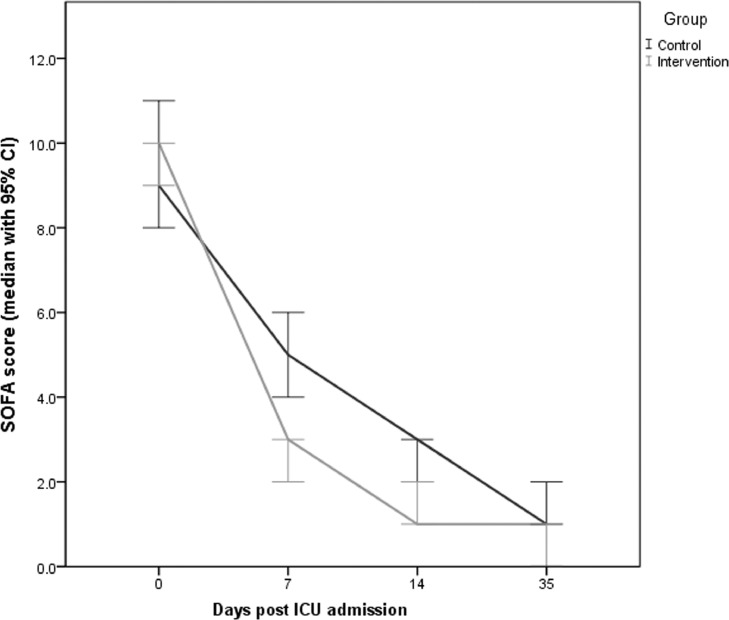
There were no adverse events recorded in either group. Specifically, TPE patients did not experience any allergic reactions, fever, coagulopathy, or cardiac and/or renal failure. The incidence of hospital-acquired infections was comparable between groups (Table 1). The baseline incidence of PE was higher in the TPE group (13 patients; 30.2%) versus the control group (6 patients; 13.6%). Of 13 PEs in the intervention group, 1 was massive, 10 were segmental and 2 were subsegmental. Of six PEs in the control group, four were segmental and two were subsegmental. Survivors from both groups had improved clinical and laboratory parameters after the completion of therapy (Table 3 ). Notably, TPE patients showed a marked and sustainable post-therapeutic increase in lymphocyte counts and ADAMTS-13 activity and a significant decrease of serum lactate, CRP, LDH, ferritin, d-dimers and IL-6 compared with baseline (Table 3). Temporal changes in the aforementioned parameters before and after therapy in COVID-19 patients are given in the Supplementary material (Supplementary e-fig.1 to e-fig.9). With respect to baseline thromboinflammatory markers in all 87 COVID-19 patients, ADAMTS-13 activity had an inverse linear association with IL-6 (r = –0.159, P = 0.14) and d-dimer levels (r = –0.317, P = 0.003) (Supplementary e-fig.10 and e-fig.11). Also, d-dimers had a positive linear association with IL-6 levels (r = 0.491, P = 0.001) (Supplementary e-fig.12). There was no significant difference in the mean survival distribution between patients in the TPE group versus the control group by Kaplan–Meier analysis (P = 0.582, log-rank test) (Fig. 2 ). The multivariable Cox proportional hazards model showed no significant effect of TPE on 35-day survival after adjustment for relevant confounders (Table 4 ). In the univariate model, predictors of 35-day mortality in COVID-19 patients were lower baseline PaO2/FiO2 ratio and ADAMTS-13 activity, higher SOFA score, increased levels of d-dimers and IL-6, high risk for developing CRS, and presence of PE. The multivariable Cox proportional hazards model revealed the following independent predictors of mortality: PaO2/FiO2 ratio [hazard ratio (HR) = 0.98, 95% CI 0.96–1.00; P = 0.02]; ADAMTS-13 activity (HR = 0.89, 95% CI 0.82–0.98; P = 0.01); and presence of PE (HR = 3.57, 95% CI 1.43–8.92; P = 0.007), (Table 4). Although the effect of TPE on survival did not reach statistical significance, TPE resulted in a significant decrease of SOFA scores in the intervention group compared with controls (Supplementary e-fig.13). Post-hoc (repeated measures) analysis of the SOFA scores for the two groups of COVID-19 patients over time is displayed in Fig. 3 and Table 5 . There was a significant reduction of SOFA score values, suggesting improved organ function, in the TPE group compared with the control group on Days 7 and 14 after the initiation of therapy.
Table 3.
Changes in clinical and laboratory parameters from baseline to end of therapy (EOT) between the two groups of COVID-19 patients
| Parameter | Control group | Intervention (TPE) group | ||
|---|---|---|---|---|
| Before therapy (n = 44) | EOT (n = 39) a | Before therapy (n = 43) | EOT (n = 34) a | |
| Systolic blood pressure (mmHg) | 98.5 (85–113.5) | 116 (105–127)* | 92 (80–104) | 118 (107–129)* |
| Diastolic blood pressure (mmHg) | 50 (39–69) | 66 (43–73)* | 52 (40–64) | 66.0 (50–82)* |
| Noradrenaline infusion dose (μg/kg/h) | 1 (0.7–1.2) | 0* | 1 (0.8–1.2) | 0* |
| PaO2/FiO2 ratio | 125 (75.5–174.5) | 255 (205–315)* | 135 (72–198) | 300 (220–380)* |
| SOFA score | 9 (6–12) | 4.5 (3.5–5.5)* | 10 (7–13) | 2 (1–3)* |
| Lymphocytes (109/L) (normal range, 1.1–3.2) | 0.6 (0.2–1) | 0.7 (0.3–1.1)* | 0.5 (0.2–0.7) | 1.0 (0.6–1.4)* |
| ADAMTS-13 activity (%) (normal range, 53–205) | 37 (26–57) | 32 (22–48)* | 17 (6–38) | 42 (29–56)* |
| Serum lactate (mmol/L) (normal range, 1.0–2.5) | 4 (1–7) | 1.65 (1.15–2.7)* | 5 (2–8) | 1.5 (1–2)* |
| Total bilirubin (μmol/L) (normal range, 0–26) | 33.5 (20.2–38.8) | 23 (14–29)* | 33 (23–39) | 22 (19–25)* |
| C-reactive protein (mg/L) (normal range, 0–5) | 234 (109–359) | 78 (31–135)* | 246 (157–356) | 45 (11–99)* |
| Lactate dehydrogenase (U/L) (normal range, 100–190) | 378 (100–656) | 343 (103–676)* | 876 (546–1206) | 236 (106–547)* |
| Ferritin (ng/mL) (normal range, 23–336) | 320 (75–675) | 287 (106–468) | 987 (319–1655) | 299 (146–655)* |
| d-dimers (μg/mL) (normal values, <1) | 2.5 (1.4–4.6) | 0.95 (0.6–3.2)* | 4.9 (2.9–7.9) | 0.9 (0.5–1.4)* |
| Interleukin-6 (pg/mL) (normal range, 1–7) | 122.5 (48.7–262.8) | 27.0 (17–144)* | 458 (225–1091 | 35 (18–112)* |
NOTE: Values are the median and interquartile range.
TPE, therapeutic plasma exchange; ICU, intensive care unit; PaO2/FiO2, partial arterial pressure of oxygen/fractional inspired concentration of oxygen; SOFA, Sequential Organ Function Assessment.
Survivors at EOT (Day 35 post ICU admission).
P ≤ 0.05 was considered statistically significant by Wilcoxon signed-rank test for non-parametric data within the two groups of patients before and after the completion of therapy.
Fig. 2.
Kaplan–Meier survival distributions in the intervention and control groups of critically-ill COVID-19 patients (log-rank test, P = 0.582; Cox regression model, HR = 0.81, 95% CI 0.35–1.87, P = 0.62). HR, hazard ratio; CI, confidence interval.
Table 4.
Cox proportional hazards model for 35-day mortality in the total COVID-19 patients (n = 87)
| Univariate model | Multivariable model | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Effect of TPE on mortality | 0.81 | 0.35 | 1.87 | 0.62 | – | – | – | – |
| Age (years) | 1.04 | 0.99 | 1.09 | 0.07 | – | – | – | – |
| PaO2/FiO2 ratio | 0.97 | 0.95 | 0.99 | 0.001 | 0.98 | 0.96 | 1.00 | 0.02 |
| APACHE II score | 1.23 | 0.93 | 1.63 | 0.16 | – | – | – | – |
| SOFA score | 1.33 | 1.04 | 1.69 | 0.02 | – | – | – | – |
| ADAMTS-13 activity (%) | 0.88 | 0.82 | 0.94 | 0.0001 | 0.89 | 0.82 | 0.98 | 0.01 |
| Interleukin-6 (pg/mL) | 1.1 | 1.00 | 1.2 | 0.08 | – | – | – | – |
| d-dimers (μg/mL) | 1.29 | 1.12 | 1.48 | 0.01 | – | – | – | – |
| Pulmonary embolism | 5.50 | 2.40 | 12.61 | 0.0001 | 3.57 | 1.43 | 8.92 | 0.007 |
| High risk (>3 criteria) for CRS | 6.07 | 0.82 | 45.19 | 0.08 | – | – | – | – |
HR, hazard ratio; CI, confidence interval; TPE, therapeutic plasma exchange; PaO2/FiO2, partial arterial pressure of oxygen/fractional inspired concentration of oxygen; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Function Assessment; CRS, cytokine release syndrome.
Fig. 3.
Post-hoc (repeated measures) analysis of Sequential Organ Function Assessment (SOFA) score (median values with 95% CI) over time (days post ICU admission) for the intervention and control groups of critically ill COVID-19 patients. CI, confidence interval; ICU, intensive care unit.
Table 5.
Post-hoc analysis of organ function over time (Days 0, 7, 14 and 35 post ICU admission) in critically-ill COVID-19 patients
| Day post ICU admission | SOFA score over time | P-value | ||
|---|---|---|---|---|
| Total patients (n = 87) | Control group (n = 44) | Intervention (TPE) group (n = 43) | ||
| Baseline (before therapy) | ||||
| Day 0 | 10 (7–13) | 9 (6–12) | 10 (8–13) | 0.07 |
| After therapy | ||||
| Day 7 | 4 (2–7) | 5 (2–8) | 3 (2–4) | 0.0001* |
| Day 14 | 2 (0–2) | 3 (2–4) | 1 (0.5–1.5) | 0.03* |
| Day 35 | 1 (0.5–1.5) | 1 (0.5–1.5) | 0.5 (0–1) | 0.07 |
ICU, intensive care unit; SOFA, Sequential Organ Function Assessment; TPE, therapeutic plasma exchange.
P ≤ 0.05 (with Bonferroni correction) was considered statistically significant.
6. Discussion
This randomised control clinical trial suggests that TPE could be a safe adjunct rescue therapy in critically-ill COVID-19 patients with ARDS, sepsis and CRS [27]. Whilst the addition of TPE to standard ICU treatment was associated with lower crude 35-day mortality (20.9% vs. 34.1%), the difference did not reach statistical significance nor was a mortality benefit seen after adjustment for important confounders [[3], [4], [5],13,15,[22], [23], [24], [25], [26], [27]]. This mirrors a randomised clinical trial on convalescent plasma transfusion (CPT) [13]. Both TPE (which does not include protective antibodies) and CPT (which does) are plausible immunomodulatory therapies for severe COVID-19 [13,27]. Both need to be rigorously studied, especially because there are pertinent differences. CPT relies on antibodies to neutralise the virus, but carries the putative risk of amplifying an antibody-mediated response. Also, CPT does not decrease thromboinflammation, a hallmark of severe COVID-19 [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15],[22], [23], [24], [25], [26], [27]]. TPE removes cytokines such as IFNγ, IL-3, IL-10, IL-1B, IL-6, IL-8 and TNFα [[19], [20], [21], [22], [23], [24], [25], [26], [27],52,53]. Our study has shown that TPE can reduce inflammatory biomarkers, improve oxygenation and ameliorate the clinical course of life-threatening COVID-19. TPE could conceivably cause immunosuppression [[19], [20], [21], [22], [23], [24], [25], [26], [27],[54], [55], [56]]. It is therefore relevant that we found no obvious side effects: no coagulopathy, no worsening renal or cardiac function, and no allergies. The incidence of hospital-acquired infections was also similar between the intervention and control groups. TPE has a cut-off of 1 000 000 Da and thus inflammatory mediators such as CRP (120 000 Da), ferritin (474 000 Da), LDH (144 000 Da), d-dimers (180 000 Da) and IL-6 (21 000 Da) should be removed. TPE may also remove immunoglobulins and complement components 3 and 4. Conceivably, this could mean immunoparalysis, which could exacerbate viral and bacterial infections [54], [55], [56]. Adding natural and artificial plasma products in the TPE regimen could mitigate immunoparalysis by replenishing immunoglobulins, hence mitigating acquired infections and coagulopathy [19], [20], [21], [22], [23], [24], [25], [26], [27]. For this reason, we used fresh frozen plasma and an artificial plasma product, both of which appeared to be safe and effective. Following TPE, we showed significant decreases in all inflammatory biomarkers and a marked sustained increase in lymphocyte count [22], [23], [24], [25], [26], [27]. We did not record any severe COVID-19-related coagulopathy or overlapping features of haemophagocytic syndrome and antiphospholipid antibodies [26,57]. We found suppressed levels of ADAMTS-13 activity, which were negatively correlated with increased levels of IL-6 and d-dimers. Also, IL-6 was positively correlated with d-dimers. These findings, recorded prior to therapy, may help explain the thromboinflammatory microangiopathy in severe COVID-19 [[7], [8], [9], [10], [11], [12],[22], [23], [24], [25], [26], [27],58,59]. We also showed that decreased ADAMTS-13 appears to portend a worse prognosis, which in turn could indicate more rapid progression towards MSOF [60]. Consistent with this speculation, after TPE we found that markers of COVID-19-associated thromboinflammation such as ADAMTS-13 activity, IL-6 and d-dimers were significantly corrected. Moreover, alongside these laboratory improvements, we demonstrated better organ function in the intervention group compared with controls as per our post-hoc SOFA scores on Days 7 and 14 following ICU admission. This clinical observation mirrors previous studies in septic patients who underwent TPE [21,61]. Overall, while TPE has not been shown to definitively save lives in the intervention group, prompt initiation following complex COVID-19 (characterised by ARDS, sepsis and thromboinflammation) does appear to be associated with better clinical recovery and less time on MV and ICU length of stay compared with controls [24], [25], [26], [27]. Our mortality analysis also confirms poor prognosticators in severe COVID-19, such as low PaO2/FiO2 ratio (HR = 0.98, 95% CI 0.96–1.00; P = 0.02), low ADAMTS-13 activity (HR = 0.89, 95% CI 0.82–0.98; P = 0.01) and presence of PE (HR = 3.57, 95% CI 1.43–8.92; P = 0.007) [[1], [2], [3], [4], [5],[62], [63], [64], [65], [66], [67], [68], [69], [70], [71]].
6.1. Study strengths and limitations
All patients received empirical and supportive ICU therapy including anticoagulation and dexamethasone [14,15]. Also, there were no differences in demographic, clinical and laboratory parameters between the groups of patients and within the PaO2/FiO2 ratio strata. The absence of protocol violations and the rapidity of TPE invitation should strengthen our conclusions [20,21,61]; however, there are limitations. For example, upon ICU admission, TPE patients had a significantly higher incidence of PE and more criteria for developing CRS compared with controls. The pivotal role of IL-6 in the development of CRS was outlined in previous studies using tocilizumab, a monoclonal antibody against IL-6 [16,[72], [73], [74], [75], [76], [77], [78], [79]]. Although we showed a decrease in IL-6 following TPE [19], [20], [21], [22], [23], [24], [25], [26], [27], inflammatory biomarkers also decreased over time in controls, as would be expected in any patient who recovers. Another potential limitation of our study is that the intervention was unblinded (open label); hence, no enrolment concealment was expedited. However, the lack of allocation concealment was mitigated by different measures described in Appendix 1.
This study was terminated early because of waning SARS-CoV-2 numbers. Accordingly, it may be underpowered to detect a survival benefit. This study was also open-label and single-centre, and physicians had some discretion [15,26,27,30,45]. Notwithstanding, our primary outcome (mortality) was not disputable, co-interventions were largely standardised, and we incorporated an independent blinded investigator. The definition of COVID-19-associated CRS remains up for debate [80]. Moreover, our work will be less generalisable if practices and laboratory methods differ elsewhere. Although this was a randomised control trial, the administration of dexamethasone might have meant additional anti-inflammation and altered viral clearance, even if the median time to negative RT-qPCR was comparable between groups [81], [82], [83]. In short, while our data are encouraging, TPE is no panacea. It is also costly and requires trained staff. TPE requires close monitoring, preferably in a high-dependency unit, and risks viral exposure [[19], [20], [21], [22], [23], [24], [25], [26], [27],52,53]. Finally, there was an imbalance between the groups in our study: the TPE group had lower lymphocyte counts and ADAMTS-13 activity and increased LDH, ferritin, d-dimers and IL-6 and a higher incidence of PE compared with controls. These values potentially indicate a more severely ill group of patients in the TPE group that can contradict the potential beneficial effect of the intervention. In addition, other unaccounted confounders might exist in the more severely ill group of patients.
Despite these limitations, we have shown that early TPE administration is feasible and is associated with biochemical and clinical recovery in profoundly sick COVID-19 patients with ARDS, sepsis, thromboinflammation and few therapeutic options. Our finding is in line with our previous report suggesting that applying TPE should be done early at the fulminant stage of COVID-19 infection, mainly because at this stage dysregulated immune system pathobiology is equally important as viral replication [84].
7. Conclusion
In patients with life-threatening COVID-19, TPE added to standard therapy compared with standard therapy alone resulted in clinical recovery but did not significantly affect 35-day mortality.
Acknowledgments
The authors wish to thank all healthcare workers involved in the diagnosis and treatment of COVID-19 patients in Riyadh, Saudi Arabia.
Funding: None.
Competing interests: None declared.
Ethical approval: This study was approved by the Institutional Review Board of King Saud Medical City (Riyadh, Kingdom of Saudi Arabia) [H-01-R-053, IORG0010374#, serial number: H1-R-20-00]. Written informed consent was obtained from all eligible patients or their legal representatives.
Editor: Dr S. Unal
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2021.106334.
Appendix. Supplementary materials
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 Mar 16 doi: 10.1093/cid/ciaa270. [Epub ahead of print]. [DOI] [Google Scholar]
- 7.Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24:275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;10:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 9.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 12.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Zhang W, Hu Y, Tong X, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramiro S, Mostard RLM, Magro-Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79:1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ader F, Team Discovery French Trial Management. Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beigel JH, Tomashek KM, Dodd LE, et al. ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 19.Patel P, Nandwani V, Vanchiere J, et al. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011;12:e87–e89. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaup H, Stahl K, Schmidt BMW, et al. Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care. 2018;22:285. doi: 10.1186/s13054-018-2220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keith PD, Wells AH, Hodges J, Fast SH, Adams A, Scott LK. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single-center experience. Crit Care. 2020;24:518. doi: 10.1186/s13054-020-03241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabibi S, Tabibi T, Conic RRZ, Banisaeed N, Streiff MB. Therapeutic plasma exchange: a potential management strategy for critically ill COVID-19 patients. J Intensive Care Med. 2020;35:827–835. doi: 10.1177/0885066620940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khamis F, Al-Zakwani I, Al Hashmi S, et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faqihi F, Alharthy A, Alshaya R, et al. Reverse takotsubo cardiomyopathy in fulminant COVID-19 associated with cytokine release syndrome and resolution following therapeutic plasma exchange: a case-report. BMC Cardiovasc Disord. 2020;20:389. doi: 10.1186/s12872-020-01665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faqihi F, Alharthy A, Memish ZA, et al. Peripheral neuropathy in severe COVID-19 resolved with therapeutic plasma exchange. Clin Case Rep. 2020;8:3234–3239. doi: 10.1002/ccr3.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alharthy A, Faqihi F, Balhamar A, Memish ZA, Karakitsos D. Life-threatening COVID-19 presenting as stroke with antiphospholipid antibodies and low ADAMTS-13 activity, and the role of therapeutic plasma exchange: a case series. SAGE Open Med Case Rep. 2020;8 doi: 10.1177/2050313X20964089. 2050313X20964089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faqihi A, Alharthy A, Alodat M, et al. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: a pilot study. J Crit Care. 2020;60:328–333. doi: 10.1016/j.jcrc.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration (FDA). Spectra Optia® Apheresis System Emergency Use Authorization (EUA). https://www.fda.gov/media/136834/download [accessed 14 July 2020].
- 29.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 30.Faqihi F, Alharthy A, Alodat M, et al. A pilot study of therapeutic plasma exchange for serious SARS CoV-2 disease (COVID-19): a structured summary of a randomized controlled trial study protocol. Trials. 2020;21:506. doi: 10.1186/s13063-020-04454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 34.Salluh JI, Soares M. ICU severity of illness scores: APACHE, SAPS and MPM. Curr Opin Crit Care. 2014;20:557–565. doi: 10.1097/MCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 36.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 37.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faqihi F, Alharthy A, Memish ZA, Karakitsos D. Comment on Hu et al: The cytokine storm and COVID-19. J Med Virol. 2021;93:631–633. doi: 10.1002/jmv.26396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faqihi F, Alharthy A, Karakitsos D. Therapeutic plasma exchange in life-threatening COVID-19 and associated cytokine release syndrome. J Formos Med Assoc. 2020;119:1888–1889. doi: 10.1016/j.jfma.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00310-20. e00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization (WHO) WHO; Geneva, Switzerland: 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance. https://www.who.int/publications/i/item/10665-331501. [accessed 9 April 2021] [Google Scholar]
- 43.Seymour CW, Liu VX, Iwashyna TJ. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel S, Kazerooni EA. Helical CT for the evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2005;185:135–149. doi: 10.2214/ajr.185.1.01850135. [DOI] [PubMed] [Google Scholar]
- 45.Saudi Ministry of Health . May 25th, 2020. Coronavirus diseases 19 (COVID-19) guidelines (revised version 1.7) https://covid19.moh.gov.sa. [accessed 9 April 2021] [Google Scholar]
- 46.Wang AY, Akizawa T, Bavanandan S, et al. 2017 Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) guideline update implementation: Asia Summit Conference Report. Kidney Int Rep. 2019;4:1523–1537. doi: 10.1016/j.ekir.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poullin P, Delmotte N, Sanderson F, Roche M, Gensollen S. Efficacy and safety of plasma exchange using a double viral inactivated and prion reduced solvent/detergent fresh frozen plasma for the treatment of thrombotic microangiopathy: the first French experience in a single center. Transfus Apher Sci. 2020;59 doi: 10.1016/j.transci.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubbard AR, Heath AB, Kremer Hovinga JA; Subcommittee on von Willebrand Factor Establishment of the WHO 1st International Standard ADAMTS13, plasma (12/252): communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:1151–1153. doi: 10.1111/jth.12881. [DOI] [PubMed] [Google Scholar]
- 50.Spalding MC, Cripps MW, Minshall CT. Ventilator-associated pneumonia: new definitions. Crit Care Clin. 2017;33:277–292. doi: 10.1016/j.ccc.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 52.Alharthy A, Faqihi F, Memish ZA, et al. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: a case-series. Artif Organs. 2020 Nov 15 doi: 10.1111/aor.13864. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dellinger RP, Bagshaw SM, Antonelli M, EUPHRATES Trial Investigators Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320:1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szczeklik W, Wawrzycka K, Włudarczyk A, et al. Complications in patients treated with plasmapheresis in the intensive care unit. Anaesthesiol Intensive Ther. 2013;45:7–13. doi: 10.5603/AIT.2013.0002. [DOI] [PubMed] [Google Scholar]
- 55.Rimmer E, Houston BL, Kumar A, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18:699. doi: 10.1186/s13054-014-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Honore PM, Barreto Gutierrez L, Kugener L, et al. Plasma exchange in critically ill COVID-19 patients improved inflammation, microcirculatory clot formation, and hypotension, thereby improving clinical outcomes: fact or fiction? Crit Care. 2020;24:551. doi: 10.1186/s13054-020-03262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deshpande C. Thromboembolic findings in COVID-19 autopsies: pulmonary thrombosis or embolism? Ann Intern Med. 2020;173:394–395. doi: 10.7326/M20-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aibar J, Castro P, Espinosa G, et al. ADAMTS-13 in critically ill patients with septic syndromes and noninfectious systemic inflammatory response syndrome. Shock. 2015;43:556–562. doi: 10.1097/SHK.000000000000034. [DOI] [PubMed] [Google Scholar]
- 61.Stahl K, Schmidt JJ, Seeliger B, et al. Effect of therapeutic plasma exchange on endothelial activation and coagulation-related parameters in septic shock. Crit Care. 2020;24:71. doi: 10.1186/s13054-020-2799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alharthy A, Aletreby W, Faqihi F, et al. Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: a retrospective study. J Epidemiol Glob Health. 2021;11:98–104. doi: 10.2991/jegh.k.200928.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;7:2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan. China. Crit Care. 2020;24:394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia. Italy. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 Jul 11 doi: 10.1093/cid/ciaa954. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mady A, Aletreby W, Abdulrahman B, et al. Tocilizumab in the treatment of rapidly evolving COVID-19 pneumonia and multifaceted critical illness: a retrospective case series. Ann Med Surg (Lond) 2020;60:417–424. doi: 10.1016/j.amsu.2020.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sinha P, Matthay MA, Calfee CS. Is a ‘cytokine storm’ relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 81.Lan L, Xu D, Ye G, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2021;181:450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Memish ZA, Faqihi F, Alharthy A, Alqahtani SA, Karakitsos D. Plasma exchange in the treatment of complex COVID-19-related critical illness: controversies and perspectives. Int J Antimicrob Agents. 2021;57 doi: 10.1016/j.ijantimicag.2020.106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.