Summary:
Selective synaptic and axonal degeneration are critical aspects of both brain development and neurodegenerative disease. Inhibition of caspase signaling in neurons is a potential therapeutic strategy for neurodegenerative disease, but no neuron-specific modulators of caspase signaling have been described. Using a mass-spectrometry approach, we discovered that RUFY3, a neuronally-enriched protein, is essential for caspase-mediated degeneration of TrkA+ sensory axons in vitro and in vivo. Deletion of Rufy3 protects axons from degeneration even in the presence of activated CASP3 that is competent to cleave endogenous substrates. Dephosphorylation of RUFY3 at S34 appears required for axon degeneration, providing a potential mechanism for neurons to locally control caspase-driven degeneration. Neuronally-enriched RUFY3 thus provides an entry point for understanding non-apoptotic functions of CASP3, and a potential target to modulate caspase signaling specifically in neurons for neurodegenerative disease.
Introduction:
Nervous system development involves two phases: a generative phase, during which neurons differentiate and extend processes, and a regressive phase involving removal of surplus neurons and pruning of inappropriate connections, in some cases through activation of caspase-dependent apoptotic processes (Luo and O’Leary, 2005; Raff et al., 2002). Caspase activity has also been implicated in neuronal death in some neurodegenerative diseases (Friedlander, 2003; Hyman and Yuan, 2012), and application of caspase inhibitors can delay degeneration in models of stroke and neurodegeneration (Fink et al., 1998; Li et al., 2000). However, global inhibition of apoptosis can also lead to cancer (Ambrosini et al., 1997), so a neuron-specific approach to blocking apoptosis could be a more attractive strategy to target neurodegeneration.
Caspase-dependent degeneration of neuronal extensions has been observed across species, with caspase-3 (CASP3) as the major effector (Unsain and Barker, 2015). However, CASP3 activation also mediates non-degenerative processes in neurons, including regulation of synaptic function without morphological alteration in the adult (Cusack et al., 2013; Hollville and Deshmukh, 2018; Hyman and Yuan, 2012; Unsain and Barker, 2015; Yi and Yuan, 2009; Zhang et al., 2011). The finding that neuronal CASP3 activation results in degeneration in some settings but not others suggests the existence of specialized downstream effectors regulating pathway outcomes. This motivated us to search for such effectors, whether activators or inhibitors.
A powerful model of caspase-dependent axon degeneration is the elimination of Tropomyosin receptor kinase A positive (TRKA+) sensory axons driven by local competition for a limiting supply of the TRKA ligand nerve growth factor (NGF) (Saxena and Caroni, 2007). Trophic factor (NGF) deprivation (TD) of TRKA+ neurons initiates a cascade leading to activation of CASP3, which in turn triggers a further cascade leading to activation of terminal proteases of the calpain family and dismantling of cellular architecture (Schoenmann et al., 2010; Simon et al., 2012; Unsain et al., 2013; Yang et al., 2013). In other systems, proteins that are themselves cleaved by caspases during apoptosis (e.g., PARP or ROCK) participate in the apoptotic process (Boulares et al., 1999; Coleman et al., 2001). This prompted us to use mass spectrometry to identify proteolytic targets (Mahrus et al., 2008) in TRKA+ axons after TD, and to test them for involvement in degeneration.
Here we report a role for the neuronally-enriched protein RUFY3. Rufy3 loss of function dramatically delays axonal degeneration without blocking CASP3 activation, positioning RUFY3 as an effector of degeneration downstream of, or in parallel to, caspase activation in neurons.
Results
Identification of neuronal proteolytic targets after trophic deprivation
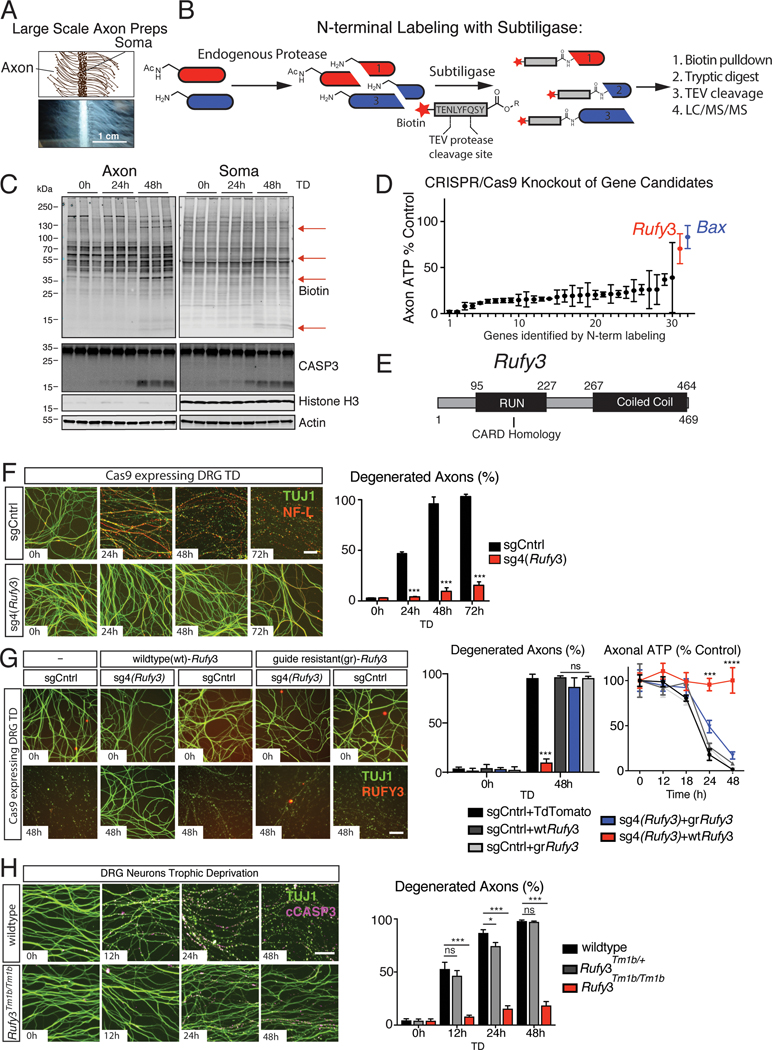
We identified newly cleaved proteins in TRKA+ dorsal root ganglion (DRG)-derived sensory axons after trophic deprivation (TD) by detecting de novo peptides via labeling of N-termini, followed by purification of labeled peptides and liquid chromatography mass spectrometry (LCMS) analysis (degradomics) (Mahrus et al., 2008). To obtain sufficient material, we developed a large-scale culture technique for isolation of milligram quantities of protein from both TRKA+ axon preparations (Histone H3 negative) and cell body (soma) preparations (Histone H3 positive) (Figure 1A–C and S1A–C). Using custom-made silicone dividers, we plated DRG cells from several mouse embryos (~2 million cells) in a linear array on 10 cm tissue culture dishes (Figure 1A). After a 14-day culture period with NGF, caspase-mediated degeneration was induced by withdrawal of NGF for 24 or 48 hours – timepoints when active CASP3 (cCASP3) can be detected by immunoblotting (Figure 1C and S1B). Newly cleaved proteins were labeled by subtiligase labeling of N-termini with a biotin containing peptide ester (Figure 1B), visualized by immunoblotting for biotin (Figure 1C and S1C), and isolated by streptavidin enrichment. Tobacco etch virus (TEV) protease was used to release purified peptides, and eluates were analyzed using tandem mass spectrometry (Detailed protocol in Data S1. Degradomics Protocol, related to Figure 1).
Figure 1 |. N-terminal labeling-mass spectrometry identifies RUFY3 as a gene required for caspase mediated axon degeneration.
A, Schematic (top) and micrograph (bottom) of large scale DRG preparations. (Scale bar=1 cm)
B, Flow chart of degradomics approach to identify newly cleaved proteins.
C, Large scale DRG cultures were deprived of NGF for the indicated times. Lysates were labeled with subtiligase and analyzed by blotting for biotin. Bands appearing following TD (red arrows) represent proteolytic targets.
D, CRISPR/Cas9 sgRNA-based screen of 30 candidates (see text). Each gene was targeted with a pool of 5 distinct sgRNAs. Targeting Rufy3 resulted in preservation of axonal ATP after TD to a similar extent as Bax, a control.
E, Schematic of RUFY3 showing its RUN domain (with structural CARD domain homology) and coiled-coil domain.
F, Introduction of sgRNA4 targeting Rufy3 (sg4(Rufy3) into Cas9 expressing DRG neurons results in significant protection of TUJ1+ axons following TD.
G Overexpression of guide resistant (gr) but not wildtype (wt) Rufy3 restores degeneration in presence of sg4(Rufy3) as evidenced by TUJ1 fragmentation and depletion of ATP levels.
H, DRG neurons derived from Rufy3Tm1b/tm1b mutant mice are strongly protected following TD.
Data presented as mean ± S.E.M, n=3 (D) or 6 (F,G,H); error bars represent SEM. Statistical analysis was two-way ANOVA * p < 0.05, *** p < 0.0001 Scale bar=100 μm
Application of this approach to axon and soma lysates yielded over 915 putative cleavage targets enriched after TD (Table S1. Axon proteolytic targets, Related to Figure 1 and Figure S1). To narrow the range of candidates, we focused on those that are putative caspase cleavage targets (i.e., in which cleavage occurs immediately following an aspartate residue) (p-1=D). Interestingly, in axons the most abundant de novo peptides were formed from cleavages immediately following an aspartate, but in soma we observed a more diverse set of cleavage patterns (Figure S1D,E). We identified 44 putative caspase targets in axons, including 30 detected consistently, i.e., each time in triplicate samples in two experiments (number in brackets indicates relative peptide counts): TBA1B (11); RUFY3 (6); AP180, AP2A2, BASP1, CLOCK, E9PYB0, MRP, NDRG1, PRUN2, UBE20 (5); CEND1, EIF5A, HUWE1, OSBL3, SQSTM1, TMTM30A, USP5 (4); and CAML, EPB41l3, PRS6A, PRS8, RIMS3, SEP11, STXB1, TPRGL, TRPM7, UBP14, UCHL1, VAPA (3). For a subset, we confirmed that full-length protein levels decreased upon TD (Figure S1F,G).
A cleavage target, RUFY3, is required for caspase-mediated axon degeneration
To assess the contribution of these 30 proteins to axonal degeneration, we first employed CRISPR/Cas9 technology to determine whether deleting their genes resulted in either acceleration or inhibition of degeneration. Each gene was targeted using 5 small guide RNAs (sgRNAs) (Morgens et al., 2016) delivered by lentiviral infection to TRKA+ sensory neurons isolated from Cas9 expressing mice (Table S2. shRNA and sgRNA sequences, Related to Figure 1 and Figure S1). As a control, we used sgRNAs targeting Bax, a known regulator of axon degeneration upstream of caspase signaling (Nikolaev et al., 2009), which protected axons fully, as assessed by axonal ATP levels (Figure 1D).
None of the targets tested, when knocked out, resulted in acceleration of degeneration (data not shown). However, knockout of one of them, RUN and FYVE domain containing protein 3 (Rufy3) (Wei et al., 2014), had a potent inhibitory effect, preserving axonal ATP levels to a similar extent as deletion of Bax (Figure 1D). RUFY3 contains a RUN and a Coiled-Coil domain (Figure 1E) and has been implicated in regulating neuronal polarity and axonal growth (Honda et al., 2017a,b; Mori et al., 2007; Wei et al., 2014). When sgRNAs were tested individually, Rufy3 sgRNA4 (sg4(Rufy3)) was the most strongly protective, as assessed by preservation of ATP levels and TUJ1 immunohistochemistry (IHC), and the protection was long-lasting (Figure 1F and data not shown). Similar protection was observed using Rufy3-specific shRNA (Figure S1H–J and Table S2. shRNA and sgRNA sequences, Related to Figure S1). To control for non-specific effects, we used a knockout-replacement strategy in neurons in which endogenous Rufy3 was eliminated with sg4(Rufy3) while simultaneously expressing a variant of Rufy3 made guide-resistant (gr) by modifying the NGG target of sg4 to include a silent mutation. Expression of gr-Rufy3 restored degeneration in presence of sg4(Rufy3) (Figure 1G), consistent with a specific effect of sg4.
Definitive confirmation of RUFY3 involvement was obtained using Rufy3 knockout mice (allele Tm1b, in which exon 5 is removed (Figure S1K)). Neurons from Rufy3Tm1b/Tm1b embryos, but not heterozygous littermates, were strongly resistant to TD-induced axon degeneration (Figure 1H). Importantly, Rufy3 knockout had no effect on degeneration induced by axotomy (i.e., Wallerian degeneration) (Figure S1L, M), which is caspase-independent (Simon et al., 2012; Whitmore et al., 2003). Collectively, these results position RUFY3 specifically in the caspase-dependent degeneration pathway.
RUFY3 functions downstream of CASP3
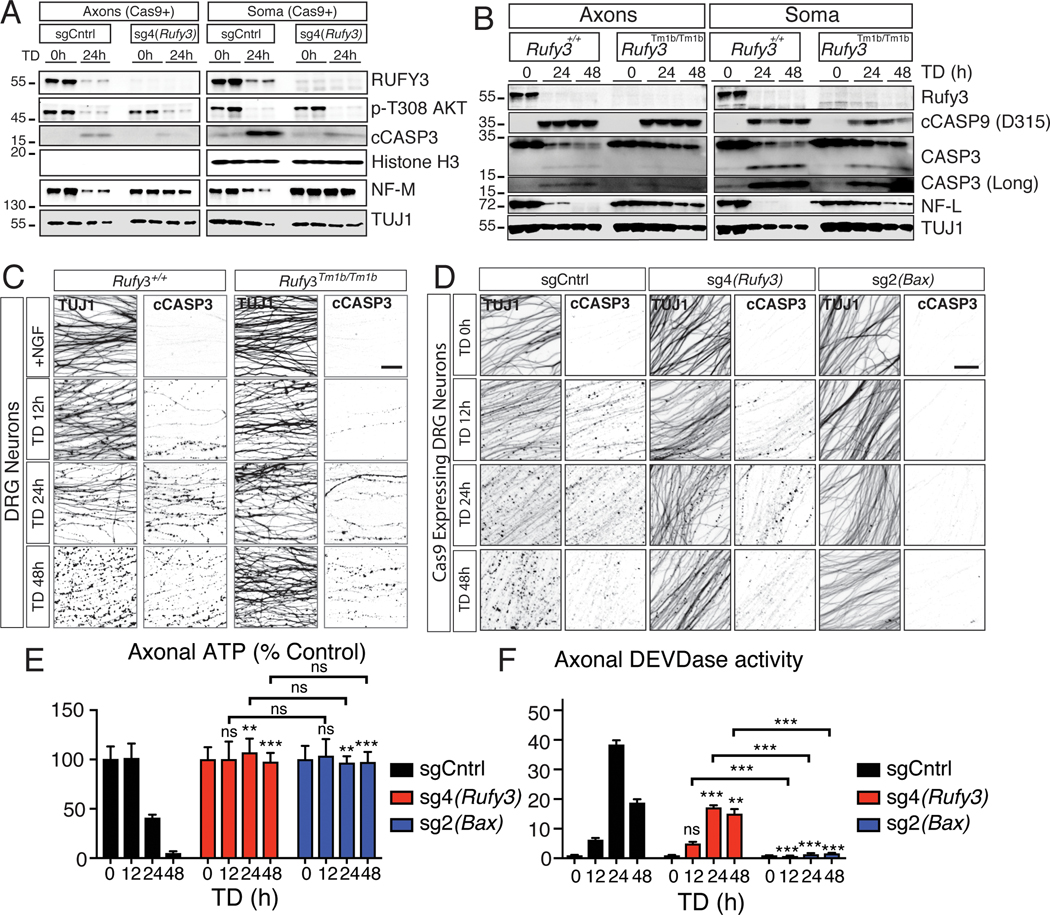
Analysis of extracts from axons and soma at various time points following NGF withdrawal showed that knockout of Rufy3 does not impact the known decrease in AKT phosphorylation after TD (Figure 2A and S2A), a proximal signaling event that occurs just downstream of TRKA (Simon et al., 2016). Downstream of AKT dephosphorylation, transcription of Puma activates a somatically derived pro-degenerative signal (Simon et al., 2016). Rufy3 knockout did not prevent robust expression of PUMA (Figure S2C, D). suggesting that RUFY3 also functions downstream of somatic PUMA.
Figure 2 |. RUFY3 functions downstream of, or in parallel to, CASP3.
A, Axon and soma lysates from Cas9-expressing DRGs infected with sg4(Rufy3) were analyzed by immunoblot. sg4(Rufy3) depletes RUFY3 protein. Following TD, dephosphorylation of AKT is unaffected, and CASP3 activation is reduced but not eliminated. Preservation of NF-M indicates protection.
B, Immunoblot analysis of Rufy3Tm1b/tm1b mutant axons and soma. RUFY3 is greatly reduced. Following TD, CASP9 activation is not markedly affected, and CASP3 activation is reduced but not eliminated. Preservation of NF-L indicates protection.
C, cCASP3-specific staining increases following TD in wildtype axons, concomitant with fragmentation of TUJ1. Rufy3Tm1b/Tm1b mutant axons are protected from degeneration yet display significant cCASP3 staining.
D, Control sg2(Bax) blocks CASP3 activation and TUJ1 fragmentation. sg4(Rufy3) reduces but does not eliminate cCASP3 staining, while protecting to a similar degree as sg2(Bax).
E,F, Effects of sgCntrl, sg4(Rufy3) and sg(Bax) on ATP levels (E) and DEVDase activity (F).
Data are presented as mean ± S.E.M n=4; error bars represent SEM, n=4. All comparisons were with sgCntrl or wildtype except where indicated. * p < 0.05,**P<0.001, *** p < 0.0001 Scale bar=100 μm
PUMA induction by TD leads to activation of a classical caspase cascade in the axon, starting with activation of proaptoptic Bax, leading to activation of CASP9, which, in turn, activates CASP3 (Simon et al., 2012). Interestingly, the crystal structure of the RUN domain of RUFY3 indicates that it has structural (but not sequence) homology with the caspase activation and recruitment domain (CARD) of APAF1 (Kukimoto-Niino et al., 2006), which functions in the caspase-cascade between Bax and CASP9 (Simon et al., 2016). To examine whether loss of Rufy3 alters activation of CASP9 or CASP3 following TD, we first probed lysates of soma and axons from Rufy3Tm1b/Tm1b neurons. Compared to wild-type, the levels of cleaved, active CASP9 (cCASP9) were not markedly altered, as assessed by immunoblot analysis (Figure 2B). Cleaved, active CASP3 (cCASP3) was also readily detected, albeit at a somewhat reduced level compared to controls (total CASP3 protein levels before TD were unaffected) (Figure 2A,B and S2E). Activation of cCASP3 in axons after TD was confirmed by IHC in Rufy3Tm1b/Tm1b neurons (Figure 2C) and in wild-type neurons infected with sg4(Rufy3) (Figure 2D). Bax sgRNA, a positive control, blocked cCASP3 appearance, as expected (Figure 2D). Both Rufy3 and Bax sgRNAs preserved axon morphology (Figure 2D) and ATP levels (Figure 2E). To directly quantify levels of CASP3 activity in axons, we utilized Caspase 3/7 glo, which measures DEVDase activity (and appears in these neurons to be due largely to cCASP3 (data not shown)). Consistent with the levels of cCASP3 seen by immunoblot, axonal lysates from Rufy3 sgRNA4-treated neurons after TD showed substantial DEVDase activity that was about half that seen in controls; Bax sgRNA fully abolished DEVDase induction, as expected (Figure 2F).
Consistent with activation of CASP3 in Rufy3 deficient neurons after TD, cleavage of the CASP3 targets alpha II Spectrin (SPTAN1) and Calpastatin (CSTN) was also observed, albeit at reduced levels – consistent with the reduction in cCASP3 (Figure S2E).
Collectively, these results show that loss of Rufy3 does not prevent activation of CASP3, which in turn acts on proteolytic substrates like SPTAN1 and CSTN, yet degeneration is blocked, as evidenced by preservation of ATP levels and of targets of terminal protease (calpain) activity, including neurofilament medium chain (NF-M) and light chain (NF-L) (Figure 2A,B,E and S2A,B). Loss of Rufy3 does cause a partial reduction (by about half) in the level of cCASP3 (but not cCASP9) after TD, which could reflect a direct or indirect effect (see Discussion), but this level is expected to be sufficient for degeneration (Simon et al., 2012; Unsain et al., 2013; Yang et al., 2013). Thus, RUFY3 is required for degeneration downstream of, or in parallel to, cCASP3.
Structure-function analysis of RUFY3
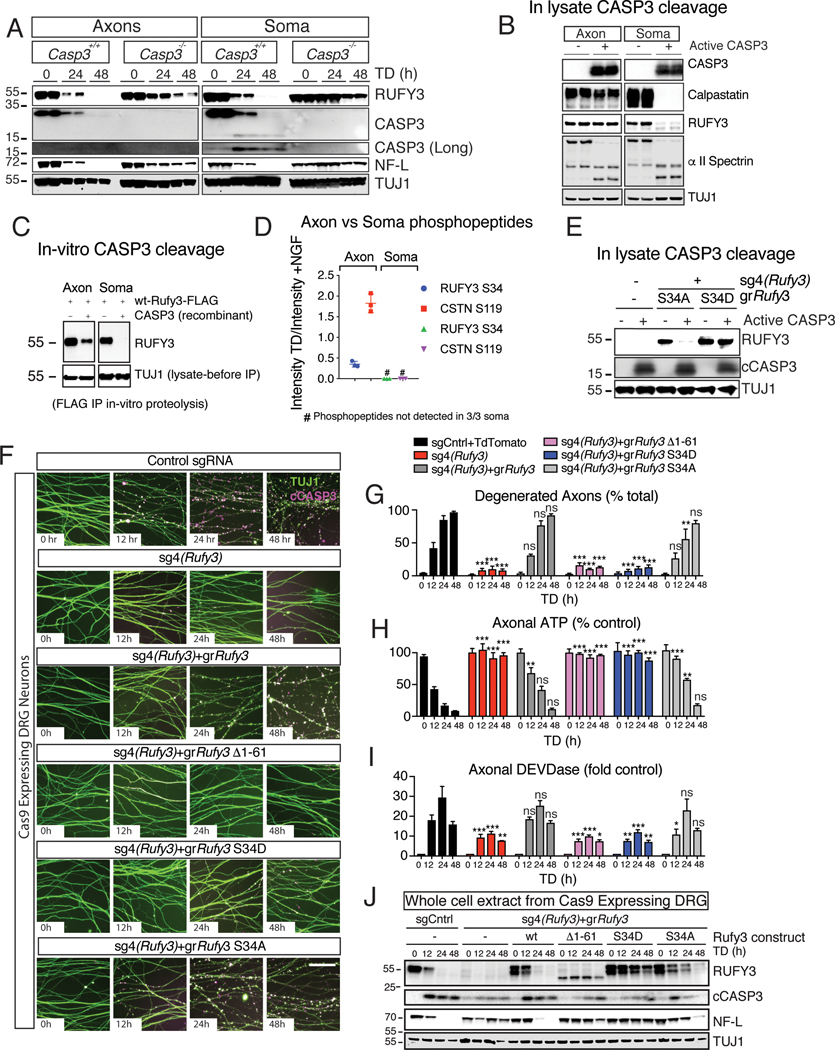
Since we identified RUFY3 in a screen for proteins cleaved after TD, and since the proteolytic site we identified is a putative caspase cleavage site, we examined whether loss of Casp3 affects its cleavage. In control cultures, the levels of intact RUFY3 and NF-L were significantly reduced at 24h and undetectable at 48h following TD in lysates from both soma and axons, whereas in cultures from Casp3−/− mice, loss of both was significantly delayed, though not completely blocked (Figure 3A and S3A). Thus, CASP3 is important for timely degradation of RUFY3, but there must be an additional protease(s) for RUFY3.
Figure 3 |. Phosphorylation at the N-terminus of RUFY3 regulates its cleavage and axon degeneration.
A, RUFY3 cleavage is strongly delayed in soma extracts from Casp3−/− DRGs and less strongly in axon derived extracts, suggesting an additional protease(s) for RUFY3.
B, RUFY3, CSTN and SPTAN in soma-derived lysates are sensitive to recombinant active CASP3. In axon-derived lysates, CSTN and RUFY3 are resistant while SPTAN is still cleaved.
C, In vitro proteolysis of FLAG-tagged immunoprecipitated RUFY3 shows sensitivity of soma- but not axonal-derived RUFY3 to active CASP3.
D, Mass spectrometry analysis reveals a site on RUFY3 that is phosphorylated in axon-derived but not soma-derived lysates.
E, The phospho-mimetic S34D mutation blocks proteolysis by active CASP3 of expressed RUFY3 from soma lysates, whereas the phospho-mutant S34A is permissive for degradation.
F-J In dissociated Cas9-expressing DRGs cultures, sg4(Rufy3) strongly protects axons. Expression of guide resistant (gr)Rufy3 restores degeneration, but deletion of the N-terminal 61 amino acids or phospho-mimetic mutation blocks cleavage and protects axons, whereas the phospho-mutant construct is both cleaved and permissive for degeneration. (F): micrographs showing TUJ1 and cCASP3 immunoreactivity. (G): quantification of degeneration. (H): axonal ATP levels. (I): Axonal DEVDase activity. (J) Western analysis of whole cell lysates.
Data are presented as mean ± S.E.M; error bars represent SEM, n=4. All comparisons were with sgCntrl or wildtype except where indicated; *p<0.05, ** p < 0.01, *** p < 0.0001, Statistical analysis was two-way ANOVA. Scale bar=100 μm
We next turned to cell lysates. Curiously, addition of purified cCASP3 caused complete loss of RUFY3 (and CSTN) in lysates derived from soma but not axons; in contrast, SPTAN1 was completely cleaved in both lysates, showing that added cCASP3 is active in both (Figure 3B). This suggested that RUFY3 (and CSTN) is differentially modified such that in soma but not axons it is cleavable by CASP3; alternatively, soma lysates could contain an activator needed for cleavage, or axonal lysates an inhibitor of cleavage. In support of differential modification, RUFY3 immunoprecipitated from soma but not axon lysates was degraded by cCASP3 (Figure 3C). Further support was obtained by mixing soma and axon lysates. Addition of cCASP3 to the mix gave an intermediate effect, with partial degradation of RUFY3 (and CSTN) that fits with the possibility that only RUFY3 (and CSTN) derived from soma, not axons, is susceptible to degradation (Figure S3B,C). Interestingly, when active CASP3 was added to purified recombinant RUFY3 generated in human embryonic kidney 293 cells (which are non-neuronal) or via in-vitro translation, it caused only a small shift in molecular weight (Figure S3D) suggesting that, unlike RUFY3 expressed in neurons, those forms of recombinant RUFY3 are not appropriately modified for full degradation by CASP3.
To search for possible modifications of RUFY3, we again turned to mass spectrometry. Immobilized metal affinity chromatography (IMAC) coupled with stable isotope labeling of amino acids in cell culture (SILAC) (Yang et al., 2011) was used to identify differences in RUFY3 phosphorylation state between axons and soma. Interestingly, Serine 34 was phosphorylated in axons but not soma; after TD, the phosphorylation level in axons decreased substantially, whereas RUFY3 in soma remained unphosphorylated (Figure 3D and S3E).
To probe the effect of phosphorylation at S34, we expressed gr-Rufy3 phospho-mutants in the presence of sg4(Rufy3), and immunoprecipitated these mutants from soma and axon lysates. The phospho-resistant serine-to-alanine mutation at position 34 (S34A) was sensitive to addition of active CASP3, whereas a phospho-mimetic serine-to-aspartate mutant (S34D) was fully resistant (Figure 3E), suggesting that dephosphorylation of S34 is required for RUFY3 degradation triggered by active CASP3. Importantly, in presence of sg4(Rufy3) to remove endogenous Rufy3, both gr-RUFY3wt and gr-RUFY3S34A were able to rescue RUFY3 function as assessed by TUJ1 immunoreactivity, ATP depletion and DEVDase activity, whereas gr-RUFYS34D was not (Figure 3F–H), suggesting that dephosphorylation of RUFY3 at S34 is required for its function. We note that rescue by gr-Rufy3wt and gr-Rufy3S34A was very strong but not complete (Figure 3H,I), perhaps because of incomplete rescue of protein levels and/or slight impairment of function by the S34A mutation. The overexpression of gr-Rufy3 also led to a second, longer band (Figure 3J), suggesting two forms of RUFY3, one fully processed as with sg-Control, and another partially processed; both were dramatically reduced following TD. Finally, mutations to the other previously reported phosphorylation sites (T5, T12, S27 S49, T51, S53) (Huttlin et al., 2010; Sweet et al., 2009) did not affect the ability of active CASP3 to cause RUFY3 degradation or axon degeneration (data not shown).
We supplemented the phosphorylation analysis with deletion analysis. We expressed guide-resistant versions of either the N-terminal 254 amino acids (AA) (gr-Rufy31−254) or the C-terminal 215 AA (gr-Rufy3262−469) in combination with sg4(Rufy3) but found that neither alone was sufficient to drive degeneration (Figure S3F). Analysis of sequencing data from the Exome Aggregation Consortium (ExAC) (Lek et al., 2016) revealed a striking bias of deleterious mutations towards the C-terminal end of the human ortholog, RUFY3 (Figure S3G), raising the possibility that the N-terminal domain plays an important role in RUFY3’s function. To test this, we expressed gr-Rufy3Δ61, a fragment lacking the 61 most N-terminal AAs and found that this construct also was not able to rescue degeneration (Figure 3F–I and S3F). Interestingly, the two constructs that rescue function (gr-RUFY3wt and gr-RUFY3S34A) are degraded in lysates in response to TD, whereas the two that do not rescue (gr-RUFY3S34D and gr-RUFY3SΔ1−61) are not (Figure 3J), consistent with the possibility – but not proving – that degradation is required for RUFY3 function.
Finally, mutation of cleavage site D254, used initially to identify RUFY3, did not prevent RUFY3 degradation in axons or block degeneration (data not shown). Thus, this site, though helpful in identifying RUFY3, appears incidental to its function.
RUFY3 is neuronally enriched and essential for developmental neuronal degeneration
We next examined RUFY3 expression and function in vivo. We first assessed RUFY3 levels in the nervous system by immunostaining sections from embryonic day 12.5 mouse embryos (E12.5). Consistent with prior reports (Mori et al., 2007; Wei et al., 2014), RUFY3 immunoreactivity is widely observed in the central and peripheral nervous systems, including in both TRKA+ and TRKA- sensory neurons (Figure S4A,B). Utilizing immunolabeling-enabled three-dimensional imaging of solvent-cleared organs + (iDISCO+) (Renier et al., 2016) we confirmed localized expression of RUFY3 to the nervous system (Figure S4C). These findings are supported by adult gene-expression data showing Rufy3 transcripts enriched in tissues isolated from the nervous system, with high abundance in DRG (Figure S4D).
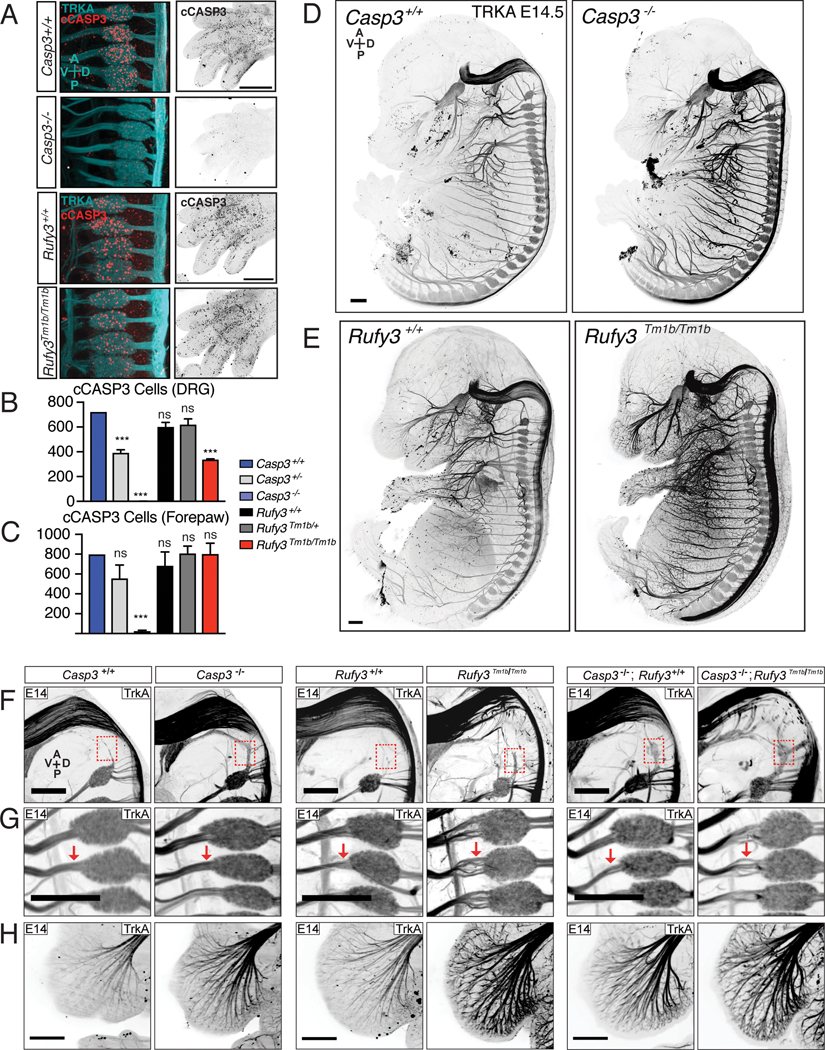
Next, we visualized TRKA and cCASP3 in embryos at E14.5, when cCASP3+ sensory neuron numbers peak before declining, due to apoptosis (Renier et al., 2014). Consistent with our findings in vitro, Rufy3Tm1b/Tm1b embryos still exhibited cCASP3 immunoreactivity in DRGs in vivo (Figure 4A). They did show a reduction in number of cCASP3 positive neurons by about half, which is similar to the reduction observed in Casp3 heterozygotes (Figure 4B) – a parallel that is consistent with the finding that loss of Rufy3 reduces CASP3 activation by about half in vitro. Interestingly, Rufy3Tm1b/Tm1b mutants showed no difference in cCASP3 immunoreactivity in the forepaw, where non-neuronal cells die back in the interdigital spaces (Figure 4A,C), consistent with a neuron-specific role for RUFY3.
Figure 4 |. Rufy3 regulates degeneration and caspase-3 activation in vivo.
A-C iDISCO+ analysis of cCASP immunoreactivity in E12.5 wild-type and mutant embryos. (A) Maximum projections from volume of DRGs (left; double-labelled for TRKA) and digits (right). (B, C) Quantification of cCASP3 cells in DRG (B) and forepaw (C). In Casp3−/− mutants, cCASP3 immunoreactivity is lost in both TRKA+ DRG and forepaw. In Rufy3Tm1b/Tm1b mutants, cCASP3 immunoreactivity is reduced by not eliminated in DRG, and is unaffected in the forepaw.
D,E, iDISCO+ analysis of TRKA immunoreactivity in control (left) or mutant (right: (D), Casp3−/− ; (E), Rufy3Tm1b/Tm1b) E14.5 embryos shows increased overall levels of TRKA in both types of knockout embryos, but a more severe defasciculation phenotype in the Rufy3Tm1b/Tm1b mutant than in the Casp3−/− mutant.
F-H, Neuronal defects in E14.5 Casp3−/− mutants, Rufy3Tm1b/Tm1b mutants, and Rufy3Tm1b/Tm1b;Casp3−/− double mutants, focusing on the hindbrain (F), DRG (G) and whisker pad (H). All three mutants show preservation of Froriep’s ganglion that has degenerated by E14.5 in wild-type (red box in (F)), as well as an increase in TRKA immunoreactivity, particularly evident in the whisker pad (H). Axonal disorganization and defasciculation is seen in hindbrain (F), DRG roots (G) and whisker pad (H) in both Rufy3Tm1b/Tm1b and Rufy3Tm1b/Tm1b;Casp3−/− mutants but not Casp3−/− mutants.
Data are presented as mean ± S.E.M; error bars represent SEM, n=4. Statistical analysis was two-way ANOVA *** p < 0.0001 Scale bar=500 μm
Consistent with previous reports (Kuida et al., 1996), we observed an increase in TRKA+ positive nerve bundles in E14.5 Casp3−/− embryos, likely reflecting a decrease in neuronal apoptosis (Figure 4D). Rufy3Tm1b/Tm1b E14.5 whole embryos similarly display an apparent increase in TRKA+ nerve bundles, as assessed by iDISCO+ (Figure 4E), highlighted here in the whisker pad (Figure 4H) and trigeminal nerve (Figure S4E), which could reflect a greater number of neurons (as in Casp3−/− embryos) and/or increased TRKA expression. We also found that a rostrally-located ganglion, Froriep’s ganglion, known to be eliminated by apoptotic cell death in wildtype mice by E11.5 (Geffen and Goldstein, 1996), was present in both Casp3−/− and the Rufy3Tm1bTm1b embryos at E14.5 (Figure 4F), consistent with Casp3 and Rufy3 functioning in overlapping pathways. Lastly, Rufy3Tm1b/Tm1b mutants display a pronounced defect in organization and fasciculation of TrkA+ axons in the hindbrain and proximal to the DRG, a phenotype not seen in Casp3 mutants (Figure 4F,G and S4F,G); the cause is uncertain, but may reflect the known role of RUFY3 in axonal growth and polarity (Honda et al., 2017a,b; Mori et al., 2007; Wei et al., 2014). Strikingly the Casp3;Rufy3 double knockout shows both preservation of Froriep’s and a similar defasciculation in the hindbrain and proximal to the DRG (Figure 4F,G and S4F,G), suggesting that RUFY3 participates in both CASP3-dependent neuronal degeneration and in an independent pathway that controls axon growth and/or fasciculation.
Discussion
The fact that CASP3, a canonical driver of cellular apoptosis, can have non-degenerative physiological effects in neurons, prompted us to search for effectors downstream of CASP3 using NGF deprivation of TRKA+ sensory neurons as a model of caspase-dependent axonal degeneration.
Degradomics of axons identifies RUFY3 as a regulator of CASP3 activity and function
To enrich for putative downstream effectors of CASP3, we used a mass spectrometry-based approach to identify axon and soma-derived de novo peptides produced during TD-induced degeneration. The resulting catalogue of several dozen putatively cleaved proteins in axons and soma (Table S1) should be helpful in analysis of apoptotic mechanisms. To narrow the range of candidates, we focused on proteins cleaved within axons at putative caspase cleavage sites, and tested which are necessary for degeneration through loss-of-function experiments.
We discovered that the product of the neuronally-enriched gene Rufy3, which is proteolytically cleaved during axonal degeneration, is essential for degeneration of TRKA+ sensory axons downstream of, or in parallel to, activated CASP3; whether it has this function in other neurons that express it remains to be explored. Importantly, loss of RUFY3 blocks apoptotic progression in vitro despite activation of CASP3 to levels that are biologically active, as evidenced by cleavage of downstream targets like SPTAN1. CASP3 activation in neurons lacking Rufy3 is about half that seen in wild-type embryos, which could either reflect a direct role for RUFY3 in CASP3 activation or be a secondary consequence of the failure of the axons to degenerate (if biochemical changes during degeneration normally feedback to further activate CASP3). Importantly, this level of activation is expected to be sufficient to trigger degeneration in wild-type neurons, since Casp3 heterozygous neurons, which have reduced CASP3 protein levels (Unsain et al., 2013) and reduced CASP3 activity (as evidenced both by greater sensitivity to inhibitors after TD in vitro (Simon et al., 2012; Unsain et al., 2013) and by reduced cCASP3+ neurons in vivo (this study)), show normal degeneration after TD (Unsain et al., 2013; Yang et al., 2013).
Thus, removal of neuronally-enriched RUFY3 appears to regulate whether activation of CASP3 does or does not result in degeneration, with potential implications for non-apoptotic actions of CASP3 in nervous system development, remodeling and function.
Structural determinants and mechanism of RUFY3 function
Phosphorylation at S34 appears to be an important site of regulation of RUFY3 function. In controls, phosphorylation is seen in axons but not soma, and TD causes a decrease in phosphorylation in axons without changing the level in soma. Our data using phospho-mimetic and phospho-mutant versions of RUFY3 suggest that dephosphorylation of S34 in axons following TD allows RUFY3 to become functionally active in degeneration. Dephosphorylation also appears necessary for RUFY3 degradation following TD, although is remains uncertain whether degradation is actually required for RUFY3 function. The apparent control of RUFY3 function by phosphorylation provides a potential mechanism for locally regulating whether activation of CASP3 results in degeneration. How TD triggers RUFY3 dephosphorylation remains to be determined.
How does RUFY3 regulate CASP3 function? RUFY3 or a proteolytic fragment could function as a scaffold protein allowing CASP3 to target some downstream targets required for axon degeneration (though not all, since some CASP3 targets are cleaved even in absence of RUFY3), or could directly contribute to activation of other downstream proteolytic effects. RUFY3 could also have a signaling function, since it is a regulator of GTPases that control neuronal polarity and axon growth (Honda et al., 2017a,b; Mori et al., 2007a; Wei et al., 2014), and its N-terminal RUN domain, in addition to having structural homology with APAF1 (Kukimoto-Niino et al., 2006), binds active RAB proteins as well as FASCIN, an actin binding protein (Wei et al., 2014). Our analysis of Rufy3 mutants supports an independent growth/polarity function by showing defects in axonal projection patterns in vivo that are observed on top of effects on CASP3 activation in DRGs and neuronal protection (the latter evidenced by preservation of Froriep’s ganglion).
Finally, beyond helping understand how activated CASP3 triggers degeneration in some settings and may have physiological effects without causing degeneration in others, RUFY3 may also provide a target to modulate disease-related degeneration in a neuron-specific manner. In this context, it is noteworthy that RUFY3 protein and mRNA levels are disrupted in the brain and spinal cord of Alzheimer’s Disease and Amyotrophic Lateral Sclerosis patients, and RUFY3 is a potential interactor with other proteins implicated in such disorders (Arosio et al., 2016; Zelaya et al., 2014).
STAR*METHODS
Detailed methods are provided in the online version of this paper and include the following:
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Marc Tessier-Lavigne (tessier3@stanford.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse husbandry:
Animals were bred and used according to IACUC protocols at The Rockefeller University and Stanford University. Embryos were harvested from pregnant female mice at stage E12.5-E14.5. Wild-type cultures were generated from CD1 mice. For mutant strains, comparisons between wild-type and mutant embryos derive from the same pregnant female. The following knockout strains were used for this study: Casp3Tm1Flv (Jax B6N.129S1), Constitutive Cas9 expressing (Jax B6J.129(Cg)-Gt(ROSA)26Sortm1.1(CAG-cas9*,-EGFP)Fezh/J) and Rufy3Tm1bTm1b (RBRC05780). EUCOMM, RIKEN BRC and the International Mouse Phenotyping Consortium were the original creator of the targeted mouse ES cells and the provider of the knockout mouse, respectively. Primers for Casp3Tm1Flv were those recommended by JAX. Rufy3Tm1bTm1b: LacZ F TTCACTGGCCGTCGTTTTACAACGTCGTGA, LacZ Reverse ATGTGAGCGAGTAACAAC CCGTCGGATTCT Rufy3 exon5 (deleted in KO) Forward ATGCCCTCATGATGGAAGAA, Reverse GCCTTGCAGAAACTTTGGAG.
Cell Culture:
Sensory neuron cultures were harvested from E12.5 embryos and grown in Neurobasal/B27 media supplemented with 50 ng/ml NGF as previously described (Simon et al., 2016). Briefly dorsal root ganglion cultures: 10 cm dishes or 24 well plates were coated overnight with PDL (100 μg/ml) and following a water wash were coated with laminin (1.33 mg/ml) for at least 3 hours at 37o C. DRGs were dissected from E12.5 mouse embryos and dissociated in 0.05% Trypsin-EDTA. Dissociated neurons were plated in Neurobasal supplemented with 2% B27, 0.45% D-glucose, 1% GlutaMAX, 100U/ml penicillin, and 100U/ml streptomycin. On DIV1, the cultures were fed with fresh Neurobasal/B-27 medium containing 5μM 5-fluoro-2’-deoxyuridine and 5μM uridine to inhibit the growth of non-neuronal cells. On DIV1, cells were transduced with lentivirus expressing sgRNAs targeting a safe genomic region (sgCntl) or indicated gene (sequences found in Table S2). On DIV 7–12 cells were treated with a complete growth medium lacking NGF and with a function blocking NGF antibody to induce trophic degeneration. For ATP measurements, neuronal cell bodies and proximal axons were removed, and axons were subjected to ATP measurement with Cell Titer Glo (Promega) or Caspase 3/7 Glo (Promega).
Cloning:
Gibson cloning was used to make all constructs. pL-CRISPR.EFS.GFP was a gift from Benjamin Ebert (Addgene plasmid # 57818) (Heckl et al., 2014). pL-CRISPR.EFS.GFP was modified by removal of the Cas9 P2A GFP and replacement with Td-Tomato or Rufy3 (wt or gr) P2A Td-Tomato to achieve sgRNA expression along with expression of guide resistant Rufy3 (gr-Rufy3). sgRNA RUFY3 #4 GATGTCATCCAGCTCCCGA and Bax sgRNA #2 GTTTCATCCAGGATCGAGCA (these and other relevant shRNA and sgRNA sequences and primers are found in supplemental table 1).
METHOD DETAILS
Lentivirus production:
Lentiviral constructs and the associated packaging plasmids (lentiviral system generation 2) psPAX2 and pMD2.G were transfected into 293FT cells using calcium phosphate. After 16 hours the media was switched to serum free viral production media: Ultraculture (Lonza) supplemented with 1% (v/v) Penn-Strep/L-glutamine, 1% (v/v) 100mM Sodium Pyruvate, 1% (v/v) 7.5% sodium bicarbonate, and 5 mM sodium butyrate. 293FT supernatants were harvested at the 46-hour time point, neutralized with 1M Tris-HCl (pH 7.5) to 50mM final concentration, and filtered through 0.45 μm polyethersulfone syringe filters. Lentiviral titer was determined using an ELISA-based quantification of p24 viral coat protein according to the manufacturer’s instruction (Clontech). Upon determining titer, the virus was concentrated using Lenti-X concentrator (Clontech) and re-suspended in Neurobasal/B27 DRG media containing 50ng/mL NGF. Lentivirus was added to DRG cultures 1–2 days after plating at a multiplicity of infection (MOI) of 10–500. Lentiviral shRNA were delivered using the pLKO.1 plasmid (Broad/TRC collection), whereas sgRNA constructs were delivered using the pL-CRISPR.EFS.GFP backbone. For each gene of interest, 4–5 shRNA or sgRNA constructs were tested in combination, for efficient target knockdown. Lentiviral shRNAs and sgRNAs were produced as above without further concentration. Lentiviruses delivering shRNA (onto wild-type neurons) or sgRNA (onto Cas9-expressing neurons) were added at 2 days post-plating at 5–10 MOI. In all cases where lentivirus was used, cultures were harvested 7–12 days post-infection.
Subtiligase and TEV protein purification:
Subtiligase and TEV protein purification was done by following published protocols (Agard et al., 2012). Briefly TEV protease was expressed in Rosetta2(DE3) pLysS cells by induction with 0.5 mM IPTG. Cells were lysed by sonication in 25 mM HEPES pH 7.5, 10% glycerol, 20 mM Imidazole, 400 mM NaCl with 1 mM BME, loaded on a NiNTA column using an FPLC (GE). Bound protein was washed with lysis buffer equivalent to 3 column volumes and eluted with lysis buffer including 400 mM Imidazole. Protein containing fractions were pooled and the resulting protein concentrated and desalted on a 26/10 column (GE) with ion exchange buffer A (IEXA) 25 mM HEPES, 10% Glycerol, 100 mM NaCl and 1 mM BME. Resulting protein was then loaded on a HiTrap Capto-S column, washed, and eluted with IEXA supplemented with 400 mM NaCl. Subtiligase was purified according to established protocols. Briefly, B. Subtilis containing pWO4 was grown in 2XYT supplemented with 10 μg/ml Chloramphenicol for 24 hours. Cells were removed and discarded. Ammonium sulfate (to 50% w/v) was slowly added to clarified growth medium to precipitate protein and collected by centrifugation (10,000×g). Subtiligase was then dissolved in 25 mM NaOAc pH 4.5, 5 mM DTT and ethanol was added to 75% final (v/v). Precipitate was collected and dissolved in 25 mM NaOAc pH 4.5, 5 mM DTT and dialyzed thoroughly. Dialyzed protein was then clarified by centrifugation and resulting supernatant was loaded on a HiTrap SP column, washed with 5 column volume of the same buffer and eluted with a shallow gradient of increasing NaCl concentration to 1 M. Protein containing fractions were pooled, concentrated and run on a Superdex S200 column in 100 mM Bicine pH 8.0, 5 mM DTT. Resulting protein containing fractions were concentrated to 300 uM (8.345 mg/ml) aliquoted and snap frozen in liquid Nitrogen.
Degradomics:
Detailed methods are provided in the supplemental subtiligase labeling document. Degradomics was performed in a similar manner to previous reports with several modifications to allow efficient analysis from 1 mg quantities of axonal protein. Briefly, axon or soma samples were lysed on ice in 100mM Bicine pH 8.0, 1mM EDTA, 5 mM EGTA 50mM NaF, 5mM (PO4)2 1mM Na3O4V, .27M Sucrose and 1% Triton supplemented with fresh E-64 (100 μM), Z-VAD-FMK (100 μM), AEBSF (1 mM), PMSF (1 mM). Following lysis, samples were clarified by centrifugation at 20,000×g for 20 min at 4oC and normalized following BCA quantification. Labeling was accomplished by adding 10% volume of 10 mM peptide ester (TEVest4b), DTT to 2 mM and Subtiligase to 10 μM final. Following labeling at 22oC for 1 hour protein was precipitated by addition to ice cold acetonitrile (final 95%) for 1 hour on ice. Following centrifugation at 20,000×g 1 min, supernatant was removed and pellet was dried for 5 minutes. Pellet was dissolved by boiling for 5 minutes in a minimum volume of 100 mM Bicine pH 8.0 containing 6M Guanidine HCl and 10 mM TCEP. After cooling, Iodoacetamide (10 mM) was added and reaction proceeded for 1 hour at 22oC. Samples were then diluted to 4M GnHCl with 100 mM Bicine pH 8.0 and quenched by addition of DTT to 15 mM. The entire reaction was then added to pre-equilibrated (100 mM Bicine pH 8) NeutrAvidin agarose beads and incubated overnight with rotation. Samples were then centrifuged at 500×g for 5 minutes and washed 2 times with 100 mM Bicine containing 4M GnHCl followed by 3 washes with trypsin buffer 100 mM NH4HCO3 pH 8.0, 100 mM NaCl, 5 mM CaCl2 1 M GnHCl. Following washes, proteins were cleaved by incubation with trypsin buffer containing 2 μg of both trypsin (Promega) and lys-c (Wako) overnight at 37oC. Samples were washed as before 2 times with 100 mM Bicine pH 8.0 4M GnHCl and 3 washes with TEV buffer (NH4HCO3 pH 8.0, 2 mM DTT, 1 mM EDTA). Peptides were then cleaved by 4 hour incubation with TEV buffer including 20 μg of TEV per sample. Supernatant was collected, acidified to 1% trifluoracetic acid (TFA), desalted using peptide tip C18 column and analyzed by tandem mass spectrometry.
Mass spectrometry:
Degradomics analysis:
Peptide samples, 5 time points, n=3, were desalted and concentrated (Rappsilber et al., 2007) prior to analysis by high resolution/high accuracy LC-MS/MS (Q-Exactive Plus, ThermoFisher). LC setup was operated with a trap column. Peptides were separated using a gradient delivered at 200nL/min, increasing from 1%B:99%A (B: 0.1% formic acid in acetonitrile, A: B: 0.1% formic acid in water) to 38%B:62%A in 111 minutes. For MS/MS acquisition, a resolution of 17,500 was used with Auto Gain Control of 1e6 and a maximum injection time of 100ms. Lowest mass was set to m/z 100. LC-MS/MS data were analyzed using ProteomeDiscoverer v.1.4.1.14 / MASCOT v. 2.2 combined with Percolator (Käll et al., 2007) and MaxQuant v. 1.3.0.5. For the MaxQuant analysis, match between runs were used. For all MS/MS data searches, a 1% False Discovery Rate was used. Tandem MS data were queried against Uniprot’s Mouse Complete Proteome (March 2016) concatenated with common contaminants. Semi-tryptic digestion constraints where used. All cysteines were treated as carbamidomethylated. Oxidation of methionine’s and peptide N-terminus modification with C4H7NO (Abu), the latter resulting in a mass increase of 85.052764 Da, were allowed as a variable modification. For analysis, peptide intensities were used. Data were processed using Perseus (Tyanova et al., 2016).
Phosphorylation analysis:
In brief, between 600–1000 μg protein, per sample (n=3), in 8M Urea, was trypsinized and desalted. 20 ug of each digest was subjected to protein profiling while the remainder was subjected to titanium dioxide based phosphopeptide enrichment followed by quantitation similar to published results (Govek et al., 2018). All samples were analyzed by high resolution/high accuracy LC-MS/MS (Q-Exactive Plus, ThermoFisher). Nano-LC setup was operated with a trap column. For each sample type, only phosphosites measured in 2-of-3 replicates and with a localization probability better than 0.75 were considered. The two sample types combined, resulted in 6,072 phosphopeptides (80+ % enrichment efficiency). LC-MS/MS Analysis Data were analyzed using MaxQuant v. 1.6.0.13 combined with Perseus 1 and ProteomeDiscoverer v.1.4.1.14 / MASCOT v. 2.2 / PhosphoRS v.3.0 combined with Percolator. Tandem MS data were queried against Uniprot’s Mouse Complete Proteome (March 2016) concatenated with common contaminants.
Western Blotting:
Axons and cell bodies were independently harvested from sensory neurons cultured as reaggregated spots of 20,000 dissociated DRGs per spot in 24- well plates using either a biopsy punch needle or scalpel as previously described (Yang et al., 2013). Briefly, axon or cell body material from between 4 and 8 spots were pooled for each condition in 50 mM Tris-Cl (pH6.8), 8 M urea, 10 % (w/v) SDS, 10 mM sodium EDTA, and 50 mM DTT, and equal amounts of protein were subjected to SDS-PAGE using the Criterion Gel system (Bio-Rad), transferred to nitrocellulose membranes, and immunoblotted following standard protocols.
Immunohistochemistry:
Neuronal cultures were fixed in 4 % (w/v) paraformaldehyde/PBS at room temperature for 15 min, followed by permeabilization with 0.1 % Triton X-100/PBS for 30 min. After blocking for 1 hour with 5% bovine serum albumin (BSA) / 0.1 % Triton X-100 / PBS, axons were then stained with relevant primary antibodies diluted in PBS-X and 5% BSA (outlined in Antibodies and Reagents) and incubated in blocking buffer at 22oC overnight. After washing two times with 0.1% Triton X-100 / PBS, axons were labeled with the appropriate Alexa-dye conjugated secondary antibodies (Life Technologies) for 2 hours at room temperature, washed three times with 0.1 % Triton-X-100 / PBS, and mounted in Fluoromount-G. Images were acquired using either a Nikon Ti2 or Nikon Eclipse. Images were quantified using ImageJ (Fiji).
Measurement of Axonal DEVDase Activity and ATP levels:
Sensory neurons were cultured for 7 days as dissociated and re-aggregated spots (2–3×104 cells/spot) in 24-well plates. In both cases mitotic inhibitor was added the day after plating to remove proliferating cells. Following the indicated treatments, the cell body region was removed as previously described and outlined above (Yang et al., 2013). Immediately after removal of the cell bodies, half of the media was removed and replaced with an equal volume of Caspase-Glo 3/7 or Cell Titer Glo reagent (Promega). Following a 1-hr lysis at room temperature, the contents of each well was transferred to an opaque 96-well plate, and luminescence was determined using a plate reader (Molecular Devices). Statistical significance was assessed using two-way ANOVA with Bonferroni post-test Prism (v.7.0) (GraphPad).
In-vitro proteolysis
Axons and neuronal cell bodies were harvested as described above, Hank’s Buffered Salt Solution containing 2.5 mM sodium EDTA was added to each sample of either axon or soma material. Axons and soma were pooled from at least 2 plates. After centrifugation at 1,000 g for 5 min, the samples were either snap frozen in liquid nitrogen or the axons were homogenized on ice in 10 mM HEPES-Na (pH 7.2), 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EGTA, 0.1 mM DTT, including appropriately diluted HALT protease and phosphatase inhibitor cocktail. The lysate was centrifuged at 15,000 × g for 15 min, and the supernatant was adjusted to a protein concentration of 1 mg/ml. Each 30-μl proteolysis reaction was set up on ice containing 20 μg of axonal or soma protein, 50 mM HEPES-Na at pH 7.2 caspase-3 (0, 0.5, 1, 5, and 10 units). The mixtures were immediately incubated at 37oC for 30 min, and the reactions were stopped by the addition of 4x SDS containing gel loading buffer, boiled and analyzed by immunoblot for the indicated protein.
iDISCO+ Sample Processing and Imaging:
Modifications and continuous updates to the protocol can be found at http://www.idisco.info. Pregnant females were euthanized with a rising gradient of CO2 and embryos were harvested through manual dissection. All harvested samples were post-fixed overnight at 4C in 4% PFA in PBS. Embryos were processed with the iDISCO+ immunolabeling protocol, as detailed previously (Renier et al., 2016). Embryos were stained with the following primary antibodies: cleaved caspase-3 (Cell Signaling 9661) at 1:500 and TrkA (R&D AF1056) at 1:500. Cleared iDISCO+ samples were imaged on a light-sheet microscope (Ultramicroscope II, LaVision Biotec) equipped with a sCMOS camera (Andor Neo) and a 2x/0.5 objective lens (MVPLAPO 2x) equipped with a 6 mm working distance dipping cap. Version v285 of the Inspector Microscope controller software was used. The samples were scanned with a step-size of 3 mm using the continuous light sheet scanning method with the included contrast adaptive algorithm for the 640 nm channel (20 acquisitions per plane), and without horizontal scanning for the 568nm channel.
Image processing and analysis:
Cleaved caspase-3 positive cells were quantified using the ClearMap cell detection module (Renier et al., 2016). Maximum projections were performed using ImageJ (NIH, http://imagej.nih.gov/ij/).
QUANTIFICATION and STATISTICAL ANALYSIS
Statistics:
Data are expressed as average ± SEM. Statistical tests were performed using GraphPad Prism 7. Two or one-way ANOVA with multiple comparison (Bonferroni post-test) or t-tests were used as indicated. n values are indicated in the corresponding figure legends. Significance was placed at p<0.05 unless otherwise noted. Statistical methods were not used to determine sample size, but sample size was selected based on similar studies within the field.
Supplementary Material
Data S1 | Degradomics protocol
Detailed protocol for degradomics analysis of low protein samples. All reagents are listed as well as a step by step protocol to allow for enrichment of newly cleaved peptides from 1 mg or less of total protein.
Table S1 | Axon proteolytic targets
Putative cleavage targets identified after TD. Protein description is given as well as the previous 5 amino acids (predicted based on sequence) and full peptide sequence. The immediately previous and following amino acids are also given to assist characterization of the cleavage site. Peptide post translational modifications as well as the mass of the peptide identified are listed to assist future identification of these peptides.
Table S2 | sgRNA and shRNA sequences
ID of each shRNA plasmid (p.LKO1) as well as sequences of oligonucleotides used to construct sgRNA sequences are given.
KEY RESOURCES TABLE:
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| RIPX (RUFY3) Polyclonal Antibody | Thermo Fisher | PA5–31311 |
| Cleaved Caspase-3 (Asp175) Antibody | Cell Signaling Technology | 9661 |
| TUJ1 anti-Tubulin β 3 (TUBB3) | BioLegend | 801201 |
| Anti-Spectrin alpha chain (nonerythroid) Antibody, clone AA6 | Millipore | MAB1622 |
| Caspase-3 Antibody | Cell Signaling Technology | 9662 |
| Histone H3 (D1H2) XP® Rabbit mAb | Cell Signaling Technology | 4499 |
| Calpastatin Antibody | Cell Signaling Technology | 4146 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Nerve Growth Factor, 2.5S, Murine | Promega | G5141 |
| Critical Commercial Assays | ||
| Caspase-Glo® 3/7 Assay System | Promega | G8090 |
| CellTiter-Glo® Luminescent Cell Viability Assay | Promega | G7570 |
| Deposited Data | ||
| Table S1 List of peptide sequences identified in this paper along with previous AA and m/z ratio. | This paper | N/A |
| Table S2 List of full sgRNA and shRNA sequences. | This paper | N/A |
| Data S1 Detailed degradomics protocol. | This paper | N/A |
| Experimental Models: Cell Lines | ||
| Lenti-X™ 293T Cell Line | Clontech | 632180 |
| Experimental Models: Organisms/Strains | ||
| Mouse: B6J.129(Cg)-Gt(ROSA)26Sortm1.1(CAG-cas9*,-EGFP)Fezh/J | The Jackson Laboratory | JAX: 026179 |
| Mouse: RUFY3Tm1b/Tm1b (RBRC05780) | Riken | RBRC05780 |
| Mouse: Casp3Tm1Flv (Jax B6N.129S1) | The Jackson Laboratory | JAX: 006233 |
| Oligonucleotides | ||
| LacZ forward TTCACTGGCCGTCGTTTTACAACGTCGTGA | This paper | N/A |
| LacZ Reverse ATGTGAGCGAGTAACAAC CCGTCGGATTCT | This paper | N/A |
| RUFY3 Exon 5 forward ATGCCCTCATGATGGAAGAA | This paper | N/A |
| RUFY3 Exon 5 reverse GCCTTGCAGAAACTTTGGAG | This paper | N/A |
| sgRNA RUFY3 #4 GATGTCATCCAGCTCCCGA | This paper | N/A |
| Bax sgRNA #2 GTTTCATCCAGGATCGAGCA | This paper | N/A |
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| ImageStudio | N/A | https://www.licor.com/bio/image-studio-lite/ |
| Other | ||
ACKNOWLEDGEMENTS
We thank members of the Tessier-Lavigne, Barres, Bassik and Gitler labs for helpful discussions. Supported by NIH grant R01NS089786 to M.T.L. N.T.H was supported by that funding and a Helen Hay Whitney Fellowship. E.L.A. was supported by funding from the Stanford Bio-X Ph.D Fellowship program, and the William K. Bowes, Jr. Foundation. CWM and JAW were supported in part by R01 GM081051 and R01 CA191018 to JAW. We thank the Rockefeller Mass Spectrometry Facility. Mass Spectrometry raw data will be deposited with Molecular Cellular Proteomics. We thank Nona Velarde for assistance with mouse colony maintenance and Milica Tesic Mark for mass spectrometry analysis.
Footnotes
DECLARATION OF INTERESTS
N.T.H. is Chief Scientific Officer and a shareholder of Mitokinin Inc. M.T.L is a director, scientific advisor and shareholder of Denali Therapeutics and Regeneron Pharmaceuticals.
SUPPLEMENTAL INFORMATION
Supplemental information includes 4 figures (Figures S1–S4), 2 tables (Tables S1 and S2) and one Data file (Data S1). Table S1 contains peptides enriched in axons following degradomics analysis. Table S2 contains sgRNA and shRNA sequences used in this study. Data S1 contains a detailed degradomics protocol that was optimized for use with low protein amounts from axonal material.
REFERENCES:
- Agard NJ, Mahrus S, Trinidad JC, Lynn A, Burlingame AL, and Wells JA (2012). Global kinetic analysis of proteolysis via quantitative targeted proteomics. Proc Natl Acad Sci U S A 109, 1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, and Altieri DC (1997). A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med 3, 917–921. [DOI] [PubMed] [Google Scholar]
- Arosio A, Sala G, Rodriguez-Menendez V, Grana D, Gerardi F, Lunetta C, Ferrarese C, and Tremolizzo L. (2016). MEF2D and MEF2C pathways disruption in sporadic and familial ALS patients. Mol. Cell. Neurosci 74, 10–17. [DOI] [PubMed] [Google Scholar]
- Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, and Smulson M. (1999). Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem 274, 22932–22940. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, and Olson MF (2001). Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol 3, 339–345. [DOI] [PubMed] [Google Scholar]
- Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, and Deshmukh M. (2013). Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat. Commun 4, 1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Zhu J, Namura S, Shimizu-Sasamata M, Endres M, Ma J, Dalkara T, Yuan J, and Moskowitz MA (1998). Prolonged Therapeutic Window for Ischemic Brain Damage Caused by Delayed Caspase Activation. J. Cereb. Blood Flow Metab 18, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Friedlander RM (2003). Apoptosis and Caspases in Neurodegenerative Diseases. N. Engl. J. Med 348, 1365–1375. [DOI] [PubMed] [Google Scholar]
- Geffen R, and Goldstein RS (1996). Rescue of Sensory Ganglia That Are Programmed to Degenerate in Normal Development: Evidence That NGF Modulates Proliferation of DRG Cellsin Vivo. Dev. Biol 178, 51–62. [DOI] [PubMed] [Google Scholar]
- Govek E-E, Wu Z, Acehan D, Molina H, Rivera K, Zhu X, Fang Y, Tessier-Lavigne M, and Hatten ME (2018). Cdc42 Regulates Neuronal Polarity during Cerebellar Axon Formation and Glial-Guided Migration. IScience 1, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckl D, Kowalczyk MS, Yudovich D, Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A, and Ebert BL (2014). Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol 32, 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollville E, and Deshmukh M. (2018). Physiological functions of non-apoptotic caspase activity in the nervous system. Semin. Cell Dev. Biol 82, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Ito Y, Takahashi-Niki K, Matsushita N, Nozumi M, Tabata H, Takeuchi K, and Igarashi M. (2017a). Extracellular Signals Induce Glycoprotein M6a Clustering of Lipid Rafts and Associated Signaling Molecules. J. Neurosci 37, 4046–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Usui H, Sakimura K, and Igarashi M. (2017b). Rufy3 is an adapter protein for small GTPases that activates a Rac guanine nucleotide exchange factor to control neuronal polarity. J. Biol. Chem 292, 20936–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, and Gygi SP (2010). A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, and Yuan J. (2012). Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat. Rev. Neurosci 13, 395–406. [DOI] [PubMed] [Google Scholar]
- Käll L, Canterbury JD, Weston J, Noble WS, and MacCoss MJ (2007). Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, and Flavell RA (1996). Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384, 368–372. [DOI] [PubMed] [Google Scholar]
- Kukimoto-Niino M, Takagi T, Akasaka R, Murayama K, Uchikubo-Kamo T, Terada T, Inoue M, Watanabe S, Tanaka A, Hayashizaki Y, et al. (2006). Crystal structure of the RUN domain of the RAP2-interacting protein x. J. Biol. Chem 281, 31843–31853. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, et al. (2000). Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288, 335–339. [DOI] [PubMed] [Google Scholar]
- Luo L, and O’Leary DD (2005). Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28, 127–156. [DOI] [PubMed] [Google Scholar]
- Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, and Wells JA (2008). Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell 134, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgens DW, Deans RM, Li A, and Bassik MC (2016). Systematic comparison of CRISPR/Cas9 and RNAi screens for essential genes. Nat. Biotechnol 34, 634–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Wada T, Suzuki T, Kubota Y, and Inagaki N. (2007). Singar1, a novel RUN domain-containing protein, suppresses formation of surplus axons for neuronal polarity. J. Biol. Chem 282, 19884–19893. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DDM, and Tessier-Lavigne M. (2009). APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Raff MC, Whitmore AV, and Finn JT (2002). Axonal Self-Destruction and Neurodegeneration. Science (80-. ) 296. [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Mann M, and Ishihama Y. (2007). Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc 2, 1896–1906. [DOI] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, and Tessier-Lavigne M. (2014). iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910. [DOI] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, et al. (2016). Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell 165, 1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, and Caroni P. (2007). Mechanisms of axon degeneration: from development to disease. Prog Neurobiol 83, 174–191. [DOI] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, and Yaron A. (2010). Axonal Degeneration Is Regulated by the Apoptotic Machinery or a NAD+-Sensitive Pathway in Insects and Mammals. J. Neurosci 30, 6375–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O’Leary DDM, Hannoush RN, and Tessier-Lavigne M. (2012). A caspase cascade regulating developmental axon degeneration. J Neurosci 32, 17540–17553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, Tešić Mark M, Molina H, and Tessier-Lavigne M. (2016). Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell 164, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet SMM, Bailey CM, Cunningham DL, Heath JK, and Cooper HJ (2009). Large Scale Localization of Protein Phosphorylation by Use of Electron Capture Dissociation Mass Spectrometry. Mol. Cell. Proteomics 8, 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, and Cox J. (2016). The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740. [DOI] [PubMed] [Google Scholar]
- Unsain N, and Barker PA (2015). New Views on the Misconstrued: Executioner Caspases and Their Diverse Non-apoptotic Roles. Neuron 88, 461–474. [DOI] [PubMed] [Google Scholar]
- Unsain N, Higgins JM, Parker KN, Johnstone AD, and Barker PA (2013). XIAP Regulates Caspase Activity in Degenerating Axons. Cell Rep 4, 751–763. [DOI] [PubMed] [Google Scholar]
- Wei Z, Sun M, Liu X, Zhang J, and Jin Y. (2014). Rufy3, a protein specifically expressed in neurons, interacts with actin-bundling protein Fascin to control the growth of axons. J. Neurochem 130, 678–692. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, and Thompson CB (2003). The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 10, 260–261. [DOI] [PubMed] [Google Scholar]
- Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, and Tessier-Lavigne M. (2013). Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron 80, 1175–1189. [DOI] [PubMed] [Google Scholar]
- Yi CH, and Yuan J. (2009). The Jekyll and Hyde Functions of Caspases. Dev. Cell 16, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya MV, Perez-Valderrama E, de Morentin XM, Tunon T, Ferrer I, Luquin MR, Fernandez-Irigoyen J, and Santamaria E. (2014). Olfactory bulb proteome dynamics during the progression of sporadic Alzheimer’s disease: Identification of common and distinct olfactory targets across Alzheimer-related co-pathologies. Oncotarget 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Deinhardt K, Chao MV, and Neubert TA (2011). Study of neurotrophin-3 signaling in primary cultured neurons using multiplex stable isotope labeling with amino acids in cell culture. J Proteome Res 10, 2546–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 | Degradomics protocol
Detailed protocol for degradomics analysis of low protein samples. All reagents are listed as well as a step by step protocol to allow for enrichment of newly cleaved peptides from 1 mg or less of total protein.
Table S1 | Axon proteolytic targets
Putative cleavage targets identified after TD. Protein description is given as well as the previous 5 amino acids (predicted based on sequence) and full peptide sequence. The immediately previous and following amino acids are also given to assist characterization of the cleavage site. Peptide post translational modifications as well as the mass of the peptide identified are listed to assist future identification of these peptides.
Table S2 | sgRNA and shRNA sequences
ID of each shRNA plasmid (p.LKO1) as well as sequences of oligonucleotides used to construct sgRNA sequences are given.