Abstract
Heterogeneity of injury severity among children with traumatic brain injury (TBI) classified by the Glasgow Coma Scale (GCS) makes comparisons across research cohorts, enrollment in clinical trials, and clinical predictions of outcomes difficult. The present study uses latent class analysis (LCA) to distinguish severity subgroups from a prospective cohort of 433 children 2.5–15 years of age with TBI who were recruited from two level 1 pediatric trauma centers. Indicator variables available within 48 h post-injury including emergency department (ED) GCS, hospital motor GCS, Abbreviated Injury Score (AIS), Rotterdam Score, hypotension in the ED, and pre-hospital loss of consciousness, intubation, seizures, and sedation were evaluated to define subgroups. To understand whether latent class subgroups were predictive of clinically meaningful outcomes, the Pediatric Injury Functional Outcome Scale (PIFOS) at 6 and 12 months, and the Behavior Rating Inventory of Executive Function at 12 months, were compared across subgroups. Then, outcomes were examined by GCS (primary) and AIS (secondary) classification alone to assess whether LCA provided improved outcome prediction. LCA identified four distinct increasing severity subgroups (1–4). Unlike GCS classification, mean outcome differences on PIFOS at 6 months showed decreasing function across classes. PIFOS differences relative to the lowest latent class (LC1) were: LC2 2.27 (0.83, 3.72), LC3 3.99 (1.88, 6.10), and LC4 11.2 (7.04, 15.4). Differences in 12 month outcomes were seen between the most and least severely injured groups. Differences in outcomes in relation to AIS were restricted to the most and less severely injured at both time points. This study distinguished four latent classes that are clinically meaningful, distinguished a more homogenous severe injury group, and separated children by 6-month functional outcomes better than GCS alone. Systematic reporting of these variables would allow comparisons across research cohorts, potentially improve clinical predictions, and increase sensitivity to treatment effects in clinical trials.
Keywords: LCA, outcomes, pediatric, TBI
Introduction
Prediction of children's longer-term outcomes after traumatic brain injury (TBI) based on early clinical and radiographical information is difficult but important. For research, easily accessible ratings of severity would facilitate comparisons of children's outcomes across TBI study cohorts and enhance enrollment of homogenous patient groups in clinical trials. Clinically, scoring systems that predict 6-month outcomes would aid family counseling and planning for rehabilitation. The Glasgow Coma Scale (GCS) assesses the level of impaired consciousness in people with severe brain injury to allow rapid communication among clinicians in the field, emergency departments (EDs), and inpatient units about the patient's condition.1 The initial GCS obtained in the ED or on hospital admission is commonly used as the primary index of injury severity.2 The GCS was adapted for children to account for developmental differences in responses between young children and adults.3 The pediatric GCS is commonly used as the gold standard to judge injury severity in children with TBI, to enroll them in clinical trials, to guide treatment, and to predict outcomes.4,5 Although the acute GCS score is helpful in describing and tracking a brain-injured child's condition and probability of severe morbidity or mortality, it is of less help in forecasting the child's longer-term functional outcome,6,7 largely because of fluctuations in GCS scores related to factors such as post-ictal states and administration of sedatives and neuromuscular blockading agents.
Many studies have improved on the predictive ability of the GCS for severe disability or death by using it in conjunction with additional indicators of the child's status. Pupillary response increases the fit of mortality prediction models among children with a GCS ≤8;8,9 the Rotterdam score, which assigns points for findings on head computed tomography (CT), predicts mortality well among children with a GCS ≤12 and improves fit of prediction models with the GCS.10,11 The head Abbreviated Injury Scale (AIS), an anatomically based measure of injury severity, has been used alone or with the GCS to examine mortality after TBI, but is a poor predictor of longer term neurobehavioral outcomes in children.9,12–14 Investigators have studied whether the full GCS is necessary or if the motor GCS score is as predictive of poor versus good outcomes at ED admission15 or of mortality when measured within 24 h post-admission,16 with some advocating the use of motor component of the score alone. Prediction of longer-term outcomes with more granularity than death or severe disability has been studied using measures collected serially after injury such as duration of post-traumatic amnesia (the Children's Orientation and Amnesia Test [COAT])17 and time from injury to follow simple motor commands.7,18 Serial measurements may improve prediction of longer-term outcomes, as they permit characterization of variability in clinical course because of some patients deteriorating and others recovering rapidly.
Given the challenges of predicting children's longer-term outcomes, we applied latent class analysis (LCA) to a diverse, well-characterized cohort of children spanning a range of TBI severities who were followed for clinical outcomes. LCA is a statistical method that identifies unobserved, underlying, or latent subgroups using observed characteristics such as patterns of clinical signs and symptoms; subgroups are then externally validated to see whether they vary along other dimensions such as clinical features or outcomes.19 Our objectives were (1) to find whether a small group of variables available in the first 48 h post-injury could classify TBI into severity groups with clinical relevance, and (2) to compare the outcomes of children using the LCA classification system to outcomes using the classification by GCS, alone, and secondarily to outcomes based on the AIS. If a small group of routinely collected variables improves outcome prediction, they could be used to compare children's injury severity across research cohorts and potentially be used as clinical indicators of prognosis.
Methods
Participants
The analysis cohort consisted of 433 children between the ages of 31 months and 15 years of age who were enrolled in a longitudinal, prospective cohort recruited from two level 1 pediatric trauma centers: Primary Children's Hospital in Salt Lake City, Utah and the University of Texas Health Science Center between January 2013 and September 2016 (Fig. S1). Methods of recruitment and follow-up have been previously reported.20 In brief, families of children were approached in the ED or inpatient units of the two participating hospitals. Children were recruited according to age group and TBI severity strata defined by ED GCS score and imaging. Families completed a pre-injury survey as soon as possible after injury to establish children's baseline function and then completed 6- and 12-month interviews online or by telephone to establish outcomes. Online and phone surveys achieve similar results in pediatric TBI,21 as do interviews and self-administered questionnaires in adult TBI.22 Children who died in hospital were not included, as the goal of the study was to establish longer-term outcomes. The institutional review boards of both institutions approved the study.
Measures
Clinical variables
Three trained study coordinators abstracted data about children's clinical condition and history from the medical and transport records using a standardized form. Clinical variables included children's blood pressure (lowest measured in the ED), sedation at the time of GCS, whether they had a pre-hospital or ED seizure or loss of consciousness, and intubation status. Hypotension was classified as yes/no according to Pediatric Advanced Life Support (PALS) age-based guidelines.23
GCS
The lowest ED post-resuscitation pediatric GCS was abstracted from the medical record. If more than one GCS score was available, study coordinators used a pre-specified hierarchy to choose the score starting with the trauma attending. The GCS scores eye opening (1–4), verbal (1–5), and motor (1–6) responses separately and is summed for a total score that ranges from 3 to 15. Injury severity was originally defined based on ED GCS and was grouped as severe (GCS ≤8), moderate (GCS 9–12), and mild (GCS 13–15). Mild was subcategorized as complicated mild TBI if a child had a GCS of 13–15 and the presence of intracranial injury on CT scan. GCS was collected also at 24 and 48 h post-admission for hospitalized children and more frequently for children in the pediatric intensive care unit (PICU). Because prior studies have reported that the motor GCS is equally predictive as the total GCS,15,16 the time to return of the GCS motor score of 6 was recorded for hospitalized children and categorized into <12 h, 12 to <24 h, 24 to <48 h, and ≥48 h. GCS was assumed to be 15 (motor component of 6) at 12 h for children who were discharged home from the ED.
AIS
The AIS is an anatomically based injury scale that is scored for six body regions including the head. The scale ranges from 1 (minor injury) to 6 (maximal injury).12 The AIS score was assigned by trauma registrars at each institution. AIS scores of ≥3 are commonly grouped as severe injury.24 We grouped AIS scores in a more granular fashion (0–2, 3–4, 5–6) reflecting the comparison of AIS to GCS in a study by Rogers and Trickey.25
Rotterdam score
The Rotterdam score was chosen as an imaging measure because it has been validated as a predictive score in children.10,11 The Rotterdam score ranges from 1 to 6: elements include status of the basilar cisterns, presence/degree of midline shift, epidural mass lesion, and intraventricular or subarachnoid hemorrhage. Low Rotterdam scores predict better outcomes. The initial CT scan was read and scored by a neuroradiologist at each institution.
Family environment
The Social Capital Index measures people's connection to their community including perceptions of personal, family, neighborhood, and spiritual community support on a scale of 1–5, with higher scores indicating better social capital.26 Family function was assessed using the McMaster Family Assessment Device (FAD) – General Functioning Scale. 27 The FAD includes 12 items scored 1 to 4, with higher scores representing worse functioning. Families self-reported their income category by family size, from which their poverty level was calculated using federal norms. Family environment covariates are used for external validation of the LCA model.
Outcomes
Parent-reported outcomes, available for 376 children, included one functional outcome assessed at 6 and 12 months, and one behavioral measure assessed retrospectively at study entry to characterize pre-injury functioning, and prospectively at 12 months. Functional outcome was measured with the Pediatric Injury Functional Outcome Scale (PIFOS).28 The PIFOS, an injury-specific measure that quantifies motor, cognitive, communication, social-emotional, self-care, physical, and academic function among children 3–15 years of age, has been used in prior TBI studies.29 The PIFOS has good internal consistency (α = 0.90–0.93) and inter-rater reliability (α = 0.9), and is correlated significantly with multiple measures including the Glasgow Outcome Scale.30 Higher PIFOS scores represent worse function. The behavioral outcomes were the Behavior Rating Inventory of Executive Function (BRIEF) working memory and emotional control T-scores.31,32 The BRIEF is a parent rating scale of everyday executive skills involved in behavioral regulation and metacognition with high test–retest reliability (0.82–0.88). The BRIEF pre-school version (BRIEF-P) was used for children <5 years of age.33 T-scores are based on normative data for age and sex with higher T-scores representing more difficulties. BRIEF outcomes were evaluated as the difference in T-score from pre-injury to 12 months.
Statistical analysis
The goal of LCA is to identify clinically meaningful and distinct subgroups based on patterns in observed variables. Two sets of parameters are estimated from the LCA model: class membership probabilities (i.e., the proportion of a population expected to belong to each class) and item-response probabilities (i.e., the probability of an indicator variable being endorsed given the latent class). 34 We a priori identified key clinical indicators available in the first 48 h following injury to identify latent classes of TBI severity: pre-hospital loss of consciousness, lowest ED GCS score, Head AIS, Rotterdam score, and hours to motor GCS of 6. With this core set of variables, we considered models with up to five latent classes. The final number of latent classes, as well as appropriate cut-points for indicator variables, were selected based on clinical judgment and statistical measures of model fit. Measures of model fit included: Bayesian Information Criterion (BIC), entropy (summarizing how well the classes separate), and likelihood ratio tests comparing models with different numbers of classes. After the core model was finalized, we evaluated whether any of the following variables would improve separation between latent classes: intubation, sedation at time of GCS, seizure, hypotension, and other bodily injury (non-head AIS ≥3). Of these measures, only hypotension improved model fit and was retained. Latent class models were implemented in Mplus.35
We then assessed whether the latent class membership was predictive of clinically meaningful outcomes. Based on each individual's assigned latent class (defined as the class with the highest model-predicted probability of membership), we analyzed the association between latent class and outcomes at 6 and 12 months following injury. Mean outcomes and standard deviations were described by latent class, and, for comparison, by the original GCS assigned severity classification, and as a secondary comparison, by grouped AIS scores. Akaike Information Criteria (AIC) was used to compare model fit for the three severity measures. We also calculated pairwise differences in mean outcomes between groups with 95% confidence intervals. Standard errors were based on analysis of variance for BRIEF outcomes, and a mixed model framework allowing for different variances across groups for the PIFOS outcomes. Statistical significance of paired outcome differences was assessed using the Tukey–Kramer method with a familywise error rate of α = 0.05. These analyses were implemented in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Demographics
A description of the cohort is shown in Table 1. The original classification by GCS and imaging divided children into mild (n = 161, 37%), complicated mild (n = 144, 33%), moderate (n = 29, 7%), and severe TBI (n = 99, 23%). The cohort was diverse with 42% minority participants and 35% girls. The most frequent injury mechanism was fall, followed by motor vehicle crash. Most children did not have additional serious bodily injury. The highest AIS excluding head had a median of 1, and interquartile range (IQR) of 0, 2. Nearly one quarter of patients were discharged to home from the ED. The cohort for construction of the latent classes was compared with the group with available outcomes. The two groups were similar for all variables used to construct the latent classes, but differed on social capital, insurance type, and ethnicity (Table S1). The percent of children with available outcomes was similar across latent classes.
Table 1.
Description of Cohort
| Overall (n = 433) | |
|---|---|
| Child and family characteristics | |
| Enrollment site: Utah | 240 (55%) |
| Rural residence | 94 (22%) |
| Age at injury | |
| 31 months to 5 years | 146 (34%) |
| 6-11 years | 145 (33%) |
| 12-15 years | 142 (33%) |
| Child sex: Female | 153 (35%) |
| Child race/ethnicity | |
| Hispanic or Latino | 115 (27%) |
| White, non-Hispanic | 247 (58%) |
| Black, non-Hispanic | 34 (8%) |
| Other, non-Hispanic | 31 (7%) |
| Insurance type | |
| None | 48 (11%) |
| Medicaid/CHIP | 157 (36%) |
| Commercial/Private/Military | 227 (53%) |
| Income at or below poverty level | 108 (27%) |
| Problematic family functioning | 61 (14%) |
| Social capital index: mean (SD) | 3.4 (1.1) |
| Injury and presenting clinical characteristics | |
| Injury mechanism | |
| Assault | 3 (1%) |
| Pedestrian or bicycle | 61 (14%) |
| Motorized vehicle | 152 (35%) |
| Fall | 159 (37%) |
| Struck by or against object | 32 (7%) |
| Organized sport | 17 (4%) |
| Other | 9 (2%) |
| Loss of consciousness pre-hospital | 214 (54%) |
| ED GCS Motor: median (IQR) | 6 (5, 6) |
| ED GCS Total: median (IQR) | 15 (10, 15) |
| Muscle relaxed (at time of ED GCS) | 80 (19%) |
| Sedated (at time of ED GCS) | 118 (27%) |
| Intubated prehospital or in the ED | 107 (25%) |
| Seizures prehospital or in the ED | 27 (6%) |
| Hypotension in ED | 15 (3%) |
| Head and neck AIS: median (IQR) | 3 (2, 3) |
| Max AIS excluding head: median (IQR) | 1 (0, 2) |
| Injury severity score: median (IQR) | 10 (5, 17) |
| Head imaging in ED | 412 (95%) |
| ED/OBS Destination | |
| Discharge to home | 91 (21%) |
| Operating room | 38 (9%) |
| Ward | 125 (29%) |
| PICU | 179 (41%) |
| Hospital admissiona | |
| Admission type | |
| ED/OBS only | 91 (21%) |
| Hospital but not PICU | 127 (29%) |
| PICU | 215 (50%) |
| First inpatient GCS - motor: median (IQR) | 6 (6, 6) |
| First inpatient GCS - total: median (IQR) | 15 (11, 15) |
| 24 h GCS - motor: median (IQR) | 6 (6, 6) |
| 24 h GCS - total: median (IQR) | 15 (15, 15) |
| Intracranial procedure(s) performed | 45 (10%) |
| Intracranial pressure monitor placed | 32 (7%) |
| n = 342 | |
| Hospital LOS (days): median (IQR) | 2 (1, 5) |
| Hospital discharge | n = 342 |
| Home | 297 (87%) |
| Inpatient rehabilitation | 42 (12%) |
| Skilled nursing or hospice | 3 (1%) |
For hospital variables, GCS values are indicated as normal (motor = 6, total = 15), procedures as “No,” and length of stay as 0 for all those discharged home from the ED.
ED, emergency department; GCS, Glasgow Coma Scale; AIS, Abbreviated Injury Scale; OBS, observation unit; PICU, pediatric intensive care unit; LOS, length of stay; SD: standard deviation; IQR, interquartile range.
Latent classes
Models with up to five latent classes were evaluated. Three or four latent classes were well supported based on statistical criteria. The four class solution was preferred over the three class solution because of face validity of the four class model. Latent classes were clinically distinguishable, with a reasonable distribution of individuals across the four classes. Entropy of the final model was 0.90 representing good separation of classes.
Key features of latent classes
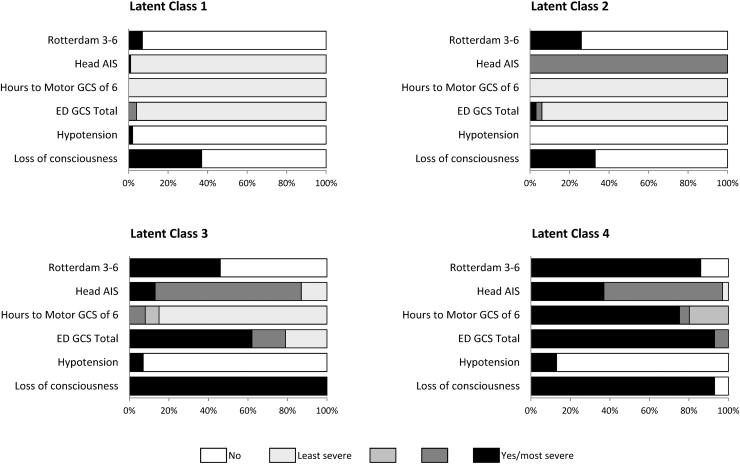
The indicator variables for each latent class are displayed in Figure 1, in which gradations of the indicators are represented from absent (no color), and least severe (light gray) to most severe (black). Latent class probabilities and item response probabilities are shown in Table 2. External validation of the classes was provided by evaluating whether groups differed with respect to the severity of other clinical indicators not included in the LCA. As seen in Table 3, although the demographic features of the cohort were similar across classes, injury characteristics differed substantively.
FIG. 1.
Indicator variables in each latent class.
Table 2.
Item-Response Probabilities for Four Latent Classes of TBI Severity
| |
Latent class item-response probabilities |
|||
|---|---|---|---|---|
| Indicator | LC 1 |
LC 2 |
LC 3 |
LC 4 |
| (34.2%) | (36.0%) | (19.1%) | (10.7%) | |
| LOC pre-hospital | 0.37 | 0.33 | 1.00 | 0.93 |
| Hypotension | 0.02 | 0.00 | 0.07 | 0.13 |
| ED GCS total | ||||
| 3-8 | 0.00 | 0.03 | 0.62 | 0.93 |
| 9-12 | 0.04 | 0.03 | 0.17 | 0.07 |
| 13-15 | 0.96 | 0.94 | 0.21 | 0.00 |
| Hours to Motor GCS of 6 | ||||
| < 12 | 1.00 | 1.00 | 0.85 | 0.00 |
| 12-<24 | 0.00 | 0.00 | 0.07 | 0.20 |
| 24-<48 | 0.00 | 0.00 | 0.08 | 0.05 |
| ≥ 48 | 0.00 | 0.00 | 0.00 | 0.76 |
| Head AIS | ||||
| 0-2 | 0.99 | 0.00 | 0.13 | 0.03 |
| 3-4 | 0.00 | 1.00 | 0.74 | 0.60 |
| 5-6 | 0.01 | 0.00 | 0.13 | 0.37 |
| Rotterdam score 3-6 | 0.07 | 0.26 | 0.46 | 0.86 |
TBI, traumatic brain injury; LC, latent class; LOC, loss of consciousness; ED, emergency department; GCS, Glasgow Coma Scale; AIS, Abbreviated Injury Scale.
Table 3.
Child, Family, and Injury Characteristics by Latent Class
| Latent class (LC) |
|
||||
|---|---|---|---|---|---|
| LC 1 |
LC 2 |
LC 3 |
LC 4 |
pa | |
| (n = 150) | (n = 162) | (n = 75) | (n = 46) | ||
| Child and family characteristics | |||||
| Age at injury, n (%) | 0.78 | ||||
| 31 months to 5 years | 54 (36%) | 53 (33%) | 21 (28%) | 18 (39%) | |
| 6-11 years | 47 (31%) | 57 (35%) | 29 (39%) | 12 (26%) | |
| 12-15 years | 49 (33%) | 52 (32%) | 25 (33%) | 16 (35%) | |
| Child sex: Girl, n (%) | 64 (43%) | 52 (32%) | 24 (32%) | 13 (28%) | 0.13 |
| Hispanic ethnicity, n (%) | 37 (25%) | 37 (23%) | 26 (35%) | 15 (33%) | 0.18 |
| Income below poverty level, n (%) | 46 (33%) | 29 (19%) | 21 (31%) | 12 (32%) | 0.05 |
| Problematic family function, n (%) | 19 (13%) | 25 (15%) | 9 (12%) | 8 (17%) | 0.76 |
| Social capital index, mean (SD) | 3.4 (1.1) | 3.5 (1.1) | 3.3 (1.0) | 3.4 (1.0) | 0.67 |
| Injury characteristics | |||||
| Seizures pre-hospital or ED, n (%) | 4 (3%) | 8 (5%) | 8 (11%) | 7 (15%) | 0.01 |
| Sedated at time of ED GCS, n (%) | 13 (9%) | 18 (11%) | 50 (67%) | 37 (80%) | <0.001 |
| NMB at time of GCSb, n (%) | 0 (0%) | 3 (2%) | 43 (57%) | 34 (74%) | <0.001 |
| Max non-head AIS ≥3, n (%) | 20 (13%) | 14 (9%) | 19 (25%) | 18 (39%) | <0.001 |
| Mechanical ventilation (in hospital), n (%) | 4 (3%) | 4 (2%) | 57 (76%) | 45 (98%) | <0.001 |
| Any intracranial procedure, n (%) | 2 (1%) | 12 (7%) | 15 (20%) | 16 (35%) | <0.001 |
| ICP monitor placed, n (%) | 0 (0%) | 0 (0%) | 4 (5%) | 28 (61%) | <0.001 |
| Hospital LOS, median (IQR) | 0 (0, 2) | 2 (1, 3) | 5 (3, 9) | 17 (10, 21) | <0.001 |
p values evaluate the overall association between each characteristic and the latent class.
All children who received NMB at time of GCS also received sedation at time of GCS.
SD, standard deviation; ED, emergency department; GCS, Glasgow Coma Scale; NMB, neuromuscular blockade; AIS, Abbreviated Injury Scale; ICP, intracranial pressure monitoring; LOS, length of stay; IQR, interquartile range.
Latent class 1 (LC-1)
Approximately one third of the children were in the LC-1 group. Class membership for the LC-1 group was defined by high initial GCS (GCS 13–15), <12 h to motor GCS of 6, and low scores on the anatomical and imaging measures of injury. Regarding other clinical indicators, this group had a low proportion of children with seizure, severe other body injury, or a need for an intracranial procedure. Most children were discharged to home from the ED with a median hospital length of stay of 0 days (IQR: 0, 2). This group represents the lowest injury severity.
Latent Class 2 (LC-2)
This class had 37% of children. Class membership for the LC-2 group was differentiated from that for the LC-1 group primarily on anatomical and radiographical findings with higher probabilities of Head AIS 3–4 and Rotterdam score of 3–6. LC-2 had a 2 day median length of stay (IQR: 1, 3).
Latent class 3 (LC-3)
LC-3 had 17% of all children. Class membership in the LC-3 group was defined by 100% probability of loss of consciousness, a wider range of ED GCS scores with a 62% probability of a GCS 3–8, and higher Rotterdam scores. Interestingly, this group has a high probability (85%) of GCS motor score recovery within 12 h. Children in LC-3 were more likely to have received sedation in the ED, to have had a bodily injury in addition to their brain injury, and to require a neurosurgical intervention. Thus, this group is characterized by initial signs of high injury severity, but relatively quick recovery of the ability to follow commands or purposeful motor responses at the youngest ages. LC-3's median hospital length of stay was 5 days (IQR: 3, 9).
Latent class 4 (LC-4)
LC-4 was the smallest group with ∼10% of children. Class membership in the LC-4 group was defined by the highest item-response probabilities of the more severe indicator variables: low ED GCS scores (GCS 3–8), >48 h to motor GCS of 6, and Rotterdam score of 3–6. This group has the highest proportion of injury characteristics indicating severity including requiring sedation in the ED, other bodily injury, and neurosurgical procedures. This group represents those with highest injury severity and the longest hospital length of stay (median 17 days, IQR: 10, 21).
Latent class assignment
When latent class assignment was compared with the original GCS assignment, one can see that no child initially classified as having mild or complicated mild injury was classified as LC-4 and no child initially categorized as having severe injury moved to the LC-1 category (Table 4). Those initially classified as having mild or complicated mild injury were primarily assigned to LC-1 or LC-2, but there was shifting between groups. The small number of children originally classified as having moderate injury were spread across the four latent classes. Less than half of those originally classified as having severe TBI were assigned to LC-4.
Table 4.
Latent Class Assignment by Original GCS-Defined TBI Severity
| |
Latent class (LC) |
|||
|---|---|---|---|---|
| Original severity classification | LC 1 |
LC 2 |
LC 3 |
LC 4 |
| (n = 150) | (n = 162) | (n = 75) | (n = 46) | |
| Mild (n = 161) | 76.4% | 22.4% | 1.2% | 0.0% |
| Complicated mild (n = 144) | 13.9% | 83.3% | 2.8% | 0.0% |
| Moderate (n = 29) | 24.1% | 10.3% | 55.2% | 10.3% |
| Severe (n = 99) | 0.0% | 3.0% | 53.5% | 43.4% |
GCS, Glasgow Coma Scale; TBI, traumatic brain injury.
Latent class outcomes
Both 6 and 12-month mean PIFOS scores increased across latent classes 1–4 as expected for increasing severity. The change in the mean BRIEF T-scores from pre-injury to 12 months for the working memory subscale increased from LC-1 to LC-2 and from LC-3 to LC-4; however, LC-2 and LC-3 were similar. The BRIEF emotional control subscale T-scores increased across latent classes. For both 6- and 12-month outcomes, children in LC-4 had substantively higher scores than in the original severe classification, suggesting that LCA distinguished a more severely injured group who subsequently experienced worse functional and behavioral outcomes. (Table S2) . The AIS categories showed an increase in scores across severity for both the 6- and 12-month outcomes performing well for 6-month outcomes, but not distinguishing 12-month outcomes as well as LCA (Table S3).
The differences between mean outcome scores between latent classes were assessed to understand whether outcomes were differentiated by latent class as shown in Table 5. As comparators, the differences in outcomes for the original GCS severity classification and AIS are also displayed (Tables 5 and 6). Latent classes were well differentiated by outcome for the 6-month PIFOS: LC-1 was significantly different from all other latent classes; LC-2 and LC-3 were not significantly different from each other, but both differed significantly from LC-4. In the original severity classification, the 6-month PIFOS outcomes differed significantly only between mild and severe and complicated mild and severe. AIS scores separated 6-month outcomes on the PIFOS with significant differences in each category; however, the latent classes demonstrated larger separation among severity groups. Thus, children's 6-month functional outcomes appear to be better differentiated by the LCA grouping than by the original classification or the AIS categories. AIC criteria for model fit (Table S4) shows improved model fit for the latent classes compared with GCS and AIS at 6 months.
Table 5.
Mean Outcome Differences by Latent Class and Original GCS Severitya
| Latent class (LC) |
Original severity |
|||||
|---|---|---|---|---|---|---|
| LC 2 | LC 3 | LC 4 | cMild | Moderate | Severe | |
| PIFOS (6 month) n = 369 | ||||||
| LC 1/Mild | 2.27 (0.83, 3.72) | 3.99 (1.88, 6.10) | 11.2 (7.04, 15.4) | 0.10 (-1.41, 1.62) | 4.20 (0.78, 7.63) | 5.83 (3.15, 8.50) |
| LC 2/cMild | 1.71 (-0.54, 3.96) | 8.95 (4.69, 13.2) | 4.10 (0.67, 7.53) | 5.72 (3.04, 8.40) | ||
| LC 3/Moderate | 7.24 (2.71, 11.8) | 1.62 (-2.46, 5.70) | ||||
| PIFOS (12 month) n = 349 | ||||||
| LC 1/Mild | 0.51 (-1.18, 2.20) | 3.11 (0.54, 5.67) | 7.91 (4.30, 11.5) | 0.19 (-1.52, 1.90) | 4.09 (0.94, 7.24) | 4.77 (2.27, 7.26) |
| LC 2/cMild | 2.60 (0.14, 5.06) | 7.40 (3.87, 10.9) | 3.90 (0.70, 7.09) | 4.58 (2.03, 7.13) | ||
| LC 3/Moderate | 4.80 (0.78, 8.83) | 0.68 (-3.00, 4.35) | ||||
| BRIEF Working Memory T-scorebn = 347 | ||||||
| LC 1/Mild | 2.70 (-0.23, 5.62) | 2.58 (-1.15, 6.31) | 8.77 (4.54, 13.0) | 2.43 (-0.56, 5.41) | 2.81 (-2.68, 8.29) | 5.32 (2.03, 8.62) |
| LC 2/cMild | -0.11 (-3.76, 3.53) | 6.07 (1.92, 10.2) | 0.38 (-5.14, 5.89) | 2.90 (-0.46, 6.25) | ||
| LC 3/Moderate | 6.19 (1.43, 10.94) | 2.52 (-3.17, 8.21) | ||||
| BRIEF Emotional Control T-scorebn = 346 | ||||||
| LC 1/Mild | 3.39 (0.35, 6.44) | 4.21 (0.33, 8.10) | 7.67 (3.23, 12.12) | 1.50 (-1.60, 4.59) | 6.25 (0.57, 11.9) | 4.77 (1.34, 8.20) |
| LC 2/cMild | 0.82 (-2.98, 4.62) | 4.28 (-0.09, 8.65) | 4.75 (-0.97, 10.5) | 3.28 (-0.21, 6.76) | ||
| LC 3/Moderate | 3.46 (-1.53, 8.45) | -1.47 (-7.38, 4.43) | ||||
Example interpretation: At 6 months on the PIFOS, children in LC2 had a PIOFS score that was 2.27 points higher than those in LC1, on average.
Mean outcome differences with 95% confidence intervals (CI). Bolded intervals are significant at α = 0.05 using the Tukey–Kramer adjustment.
BRIEF scores reflect difference in the change from pre-injury to 12 month follow-up.
GCS, Glasglow Coma Scale; cMild, complicated Mild; PIFOS, Pediatric Injury Functional Outcome Scale; BRIEF, Behavior Rating Inventory of Executive Function.
Table 6.
Mean Outcome Differences by AISa
| AIS 3-4 | AIS 5-6 | |
|---|---|---|
| PIFOS (6 month) n = 369 | ||
| AIS 0-2 | 3.01 (1.53, 4.50) | 9.50 (4.50, 14.5) |
| AIS 3-4 | 6.49 (1.46, 11.5) | |
| PIFOS (12 month) n = 349 | ||
| AIS 0-2 | 1.49 (-0.24, 3.22) | 5.84 (2.04, 9.64) |
| AIS 3-4 | 4.35 (0.63, 8.06) | |
| BRIEF Working Memory T-scorebn = 347 | ||
| AIS 0-2 | 3.18 (0.49, 5.87) | 4.06 (-0.91, 9.03) |
| AIS 3-4 | 0.87 (-3.92, 5.67) | |
| BRIEF Emotional Control T-scoreb n = 346 | ||
| AIS 0-2 | 4.32 (1.54, 7.10) | 4.44 (-0.68, 9.57) |
| AIS 3-4 | 0.12 (-4.82, 5.07) | |
Mean outcome differences with 95% confidence intervals (CI). Bolded intervals are significant at α = 0.05 using the Tukey–Kramer adjustment.
BRIEF scores reflect difference in the change from pre-injury to 12 month follow-up.
AIS, Abbreviated Injury Scale; PIFOS, Pediatric Injury Functional Outcome Scale; BRIEF, Behavior Rating Inventory of Executive Function.
Examining 12-month outcomes, on the PIFOS, the statistical differences among classes were similar between the LCA and the original severity classification; however, there were notably larger differences comparing LC-4 with other classes than when comparing the original severe classification to other injury groups. Both the LCA and original classification performed better than the AIS. A similar pattern is seen when examining the change from pre-injury to 12-month BRIEF working memory and emotional control T-scores: both the LCA and original classification schemas distinguished LC-1 or mild from LC-4 or severe but did not distinguish LC-3/moderate from severe injury at 12 months post-injury. AIS was unable to distinguish the severe group for either 12-month T-score. For both T-scores, score differences in the LC-4 group were higher than in the original severity group, suggesting a better differentiation of this group. Latent class model fit was better for 12-month PIFOS and BRIEF outcomes (Table S4).
Discussion
TBI severity can be difficult to quantify, as current classification using the GCS may group children in spite of considerable heterogeneity. Grouping children with heterogenous injury severity presents difficulties both in research and clinical applications. Without an accurate TBI severity classification, it is difficult to compare outcomes across observational cohorts. More precise classification of TBI severity may also enhance identification of treatment effects in interventional trials. Clinically, a classification system that predicts functional outcomes after hospital discharge as opposed to mortality would be useful in counseling families and forecasting rehabilitation needs. Findings from this study show that LCA distinguished four TBI latent classes or subgroups among children who presented to the hospital using routinely gathered clinical and imaging data early after injury. These four classes are clinically meaningful and separate children by 6-month functional outcomes better than GCS alone.
Six-month functional outcomes were separated well by the latent classes. In contrast to the original severity scoring of this cohort, the LCA classes showed a worsening of functional outcomes across classes as clinical and anatomical markers of severity increased. The LC-4 group, representing the most severely injured children, was especially well defined. Children in LC-4 had markedly worse functional outcome scores than children classified as severe by GCS alone, showing a good demarcation of this group. Separating children with mild and severe injury is not a clinical or research difficulty; however, separating children with moderate and severe TBI is especially challenging, as this group of children frequently require sedatives for transport and for imaging. Moderate TBI has been called the “gray zone,” as it typically includes both patients who recover quickly and patients who go on to die as illustrated by the wide dispersion across latent classes of children within this cohort who were originally classified as having moderate injury.36 The separation of LC-3 from LC-4 is an important distinction in terms of therapeutic decisions, enrollment in clinical trials, and comparison across research cohorts. Both the LC-3 and LC-4 classes have high markers of anatomic injury severity on admission, required a neurosurgical procedure at least 20% of the time, and frequently had received sedation at the time of ED GCS. The shared characteristics of these two groups make them difficult to distinguish on ED admission, but they had markedly different hospital length of stay and better 6-month outcomes than LC-4. Additionally, LCA was able to distinguish outcomes at 6 months among children with high ED GCS scores (LC-1 from LC-2) unlike the original severity groupings of mild and complicated mild TBI. AIS scores performed well in separating functional outcomes at 6 months post-injury. AIS does not group children as finely as the LCA; therefore, scores are moderated. The least injured category (AIS 0-2) had higher scores on 6 and 12 month outcomes and the most severe category had lower scores on 6 and 12 month outcomes compared with the LCA.
Latent classes separated the 12-month outcomes on the PIFOS and the BRIEF working memory and emotional control T-scores less well. Similar to the original classification, the LCA classes primarily showed differences between mild and severe injury outcomes. AIS scores performed poorly at distinguishing 12-month outcomes, especially in cognition, consistent with past research.14 Difficulties in forecasting 12-month outcomes is unsurprising. It is likely that children in the two mildest groups (LCA 1 and 2) had shown substantial recovery 1 year after injury, as has been noted for children with mild injury in other cohorts.37,38 Additionally, injury severity is only one of many factors contributing to 1-year outcomes for children with all severities of TBI. Non-injury factors appear to increase their influence on outcomes as time from injury increases; such factors include the child's pre-injury abilities and vulnerabilities, access to rehabilitation services, and family environment variables such as adjustment, social capital, and economic resources.20, 39–41
Some study limitations exist. Although this study drew from two large pediatric trauma centers, these results need to be replicated in other cohorts and with other 6-month outcomes to assure that results are robust and generalizable. A larger sample size would have increased our ability to distinguish latent class groups. Not all trauma centers use the Rotterdam score for classifying CT scans, which may make this variable less accessible. However, as children at risk for an intracranial lesion uniformly receive CT scans at trauma centers, adding Rotterdam scores is feasible. Infants and toddlers were not included in this cohort because of the different outcome measures used across age groups; therefore, this classification is not generalizable to children under 31 months of age. Not all children used to create the latent classes had outcomes; however, those with and without outcomes were similar across latent class variables. Children without outcomes were more likely to be Hispanic, lack health insurance, and have low social capital, reflecting the difficulty in following these groups. We do not know how complete follow-up would have changed the differences seen on outcomes among latent classes; however, missingness was similar across latent classes and overall follow-up was high. Therefore, we think differences would have been modest. Finally, because this study was designed to examine outcomes over time, children who died in the hospital were not included. Inclusion of children who died would likely have increased outcome differences observed between LC3 and LC4. Strengths of this study include the large, diverse cohort of well-characterized children across a wide range of injury severity, a granular, TBI-specific, functional outcome score, and the use of clinical variables that are routinely recorded in the analysis.
Conclusion
LCA distinguished classes of injury severity well using commonly available clinical variables. The classes were associated with 6-month functional outcomes in an expected way. If this schema can be replicated across other pediatric cohorts, using these core variables may be a useful method to classify children into more homogeneous groups for clinical trials, predict clinical outcomes, and make comparisons across research cohorts.
Funding Information
This study was supported by The Centers for Disease Control (CDC) under Cooperative Agreement U01/CE002188. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. This study was supported also by the National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant number K24HD072984. Neither the NICHD or the CDC played a role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Teasdale, G., and Jennett, B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 2. King, J.T.Jr., Carlier, P.M., and Marion, D.W. (2005). Early Glasgow Outcome Scale scores predict long-term functional outcome in patients with severe traumatic brain injury. J. Neurotrauma 22, 947–954 [DOI] [PubMed] [Google Scholar]
- 3. Reilly, P.L., Simpson, D.A., Sprod, R., and Thomas, L. (1988). Assessing the conscious level in infants and young children: a paediatric version of the Glasgow Coma Scale. Childs Nerv. Syst. 4, 30–33 [DOI] [PubMed] [Google Scholar]
- 4. Kochanek, P.M., Tasker, R.C., Bell, M.J., Adelson, P.D., Carney, N., Vavilala, M.S., Selden, N.R., Bratton, S.L., Grant, G.A., Kissoon, N., Reuter-Rice, K.E., and Wainwright, M.S. (2019). Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatr. Crit. Care Med. 20, 269–279 [DOI] [PubMed] [Google Scholar]
- 5. Saatman, K.E., Duhaime, A.C., Bullock, R., Maas, A.I., Valadka, A., Manley, G.T., Workshop Scientific Team and Advisory Panel Members (2008). Classification of traumatic brain injury for targeted therapies. J, Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lieh-Lai, M.W., Theodorou, A.A., Sarnaik, A.P., Meert, K.L., Moylan, P.M., and Canady, A.I. (1992). Limitations of the Glasgow Coma Scale in predicting outcome in children with traumatic brain injury. J. Pediatr. 120, 195–199 [DOI] [PubMed] [Google Scholar]
- 7. Suskauer, S.J., Slomine, B.S., Inscore, A.B., Lewelt, A.J., Kirk, J.W., and Salorio, C.F. (2009). Injury severity variables as predictors of WeeFIM scores in pediatric TBI: time to follow commands is best. J. Pediatr. Rehabil. Med. 2, 297–307 [PMC free article] [PubMed] [Google Scholar]
- 8. Emami, P., Czorlich, P., Fritzsche, F.S., Westphal, M., Rueger, J.M., Lefering, R., and Hoffmann, M. (2017). Impact of Glasgow Coma Scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: a retrospective, multicenter cohort study. J. Neurosurg. 126, 760–767 [DOI] [PubMed] [Google Scholar]
- 9. Murphy, S., Thomas, N.J., Gertz, S.J., Beca, J., Luther, J.F., Bell, M.J., Wisniewski, S.R., Hartman, A.L., and Tasker, R.C. (2017). Tripartite stratification of the Glasgow Coma Scale in children with severe traumatic brain injury and mortality: an analysis from a multi-center comparative effectiveness study. J. Neurotrauma 34, 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liesemer, K., Riva-Cambrin, J., Bennett, K.S., Bratton, S.L., Tran, H., Metzger, R.R., and Bennett, T.D. (2014). Use of Rotterdam CT scores for mortality risk stratification in children with traumatic brain injury. Pediatr. Crit. Care Med. 15, 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maas, A.I., Hukkelhoven, C.W., Marshall, L.F., and Steyerberg, E.W. (2005). Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 12. Gennarelli, T.A., and Wodzin, E. (2008). Abbreviated Injury Scale 2005. Association for the Advancement of Automotive Medicine: Des Plaines, IL [Google Scholar]
- 13. Hubbard, M.E., Bin Zahid, A., Vonderhaar, K., Freeman, D., Nygaard, R.M., Kiragu, A., and Guillaume, D. (2019). Prediction of discharge destination after traumatic brain injury in children using the head abbreviated injury scale. Brain Inj. 33, 643–648 [DOI] [PubMed] [Google Scholar]
- 14. McDonald, C.M., Jaffe, K.M., Fay, G.C., Polissar, N.L., Martin, K.M., Liao, S., and Rivara, J.B. (1994). Comparison of indices of traumatic brain injury severity as predictors of neurobehavioral outcome in children. Arch. Phys. Med. Rehabil. 75, 328–337 [DOI] [PubMed] [Google Scholar]
- 15. Fortune, P.M., and Shann, F. (2010). The motor response to stimulation predicts outcome as well as the full Glasgow Coma Scale in children with severe head injury. Pediatr. Crit. Care Med. 11, 339–342 [DOI] [PubMed] [Google Scholar]
- 16. Gomez, D., Byrne, J.P., Alali, A.S., Xiong, W., Hoeft, C., Neal, M., Subacius, H., and Nathens, A.B. (2017). Inclusion of highest glasgow coma scale motor component score in mortality risk adjustment for benchmarking of trauma center performance. J. Am. Coll. Surg. 225, 755–762 [DOI] [PubMed] [Google Scholar]
- 17. Ewing-Cobbs, L., Levin, H.S., Fletcher, J.M., Miner, M.E. and Eisenberg, H.M. (1990). The Children's Orientation and Amnesia Test: relationship to severity of acute head injury and to recovery of memory. Neurosurgery 27, 683–691 [PubMed] [Google Scholar]
- 18. Davis, K.C., Slomine, B.S., Salorio, C.F., and Suskauer, S.J. (2016). Time to follow commands and duration of posttraumatic amnesia predict GOS-E Peds Scores 1 to 2 years after TBI in children requiring inpatient rehabilitation. J. Head Trauma Rehabil. 31, E39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rindskopf, D., and Rindskopf, W. (1986). The value of latent class analysis in medical diagnosis. Stat. Med. 5, 21–27 [DOI] [PubMed] [Google Scholar]
- 20. Keenan, H.T., Clark, A.E., Holubkov, R., Cox, C.S., and Ewing–Cobbs, L. (2018). Psychosocial and executive function recovery trajectories one year after pediatric traumatic brain injury: the influence of age and injury severity. J. Neurotrauma 35, 286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rivara, F.P., Koepsell, T.D., Wang, J., Durbin, D., Jaffe, K.M., Vavilala, M., Dorsch, A., Roper-Caldbeck, M., Houseknecht, E. and Temk,in, N. (2011). Comparison of telephone with World Wide Web-based responses by parents and teens to a follow-up survey after injury. Health Serv. Res. 46, 964–981 [DOI] [PMC free article] [PubMed]
- 22. Kisala, P.A., Boulton, A.J., Cohen, M.L., Slavin, M.D., Jette, A.M., Charlifue, S., Hanks, R., Mulcahey, M.J., Cella, D., and Tulsky, D.S. (2019). Interviewer- versus self-administration of PROMIS(R) measures for adults with traumatic injury. Health Psychol. 38, 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. United Medical Education (2019). PALS Algorithm 2019. https://www.acls-pals-bls.com/algorithms/pals/ (last accessed February1, 2019)
- 24. Grote, S., Bocker, W., Mutschler, W., Bouillon, B., and Lefering, R. (2011). Diagnostic value of the Glasgow Coma Scale for traumatic brain injury in 18,002 patients with severe multiple injuries. J. Neurotrauma 28, 527–534 [DOI] [PubMed] [Google Scholar]
- 25. Rogers, S., and Trickey, A.W. (2017). Classification of traumatic brain injury severity using retrospective data. J. Nurs. Educ. Pract. 7, 23–29 [Google Scholar]
- 26. Runyan, D.K., Hunter, W.M., Socolar, R.R., Amaya-Jackson, L., English, D., Landsverk, J., Dubowitz, H., Browne, D.H., Bangdiwala, S.I., and Mathew, R.M. (1998). Children who prosper in unfavorable environments: the relationship to social capital. Pediatrics 101, 12–18 [DOI] [PubMed] [Google Scholar]
- 27. Miller, I.W., Bishop, D.S., Epstein, N.B., and Kietner, G.I. (1985). The McMaster Family Assessment Device: reliability and validity. J. Marital Fam. Ther. 11, 345–356 [Google Scholar]
- 28. Ewing-Cobbs, L., Bloom, D.R., Prasad, M.R., Waugh, J.K., Cox, C.S.Jr., and Swank, P.R. (2014). Assessing recovery and disability after physical trauma: the Pediatric Injury Functional Outcome Scale. J. Pediatr. Psychol. 39, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilkinson, A.A., Dennis, M., Simic, N., Taylor, M.J., Morgan, B.R., Frndova, H., Choong, K., Campbell, C., Fraser, D., Anderson, V., Guerguerian, A.M., Schachar, R., Hutchison, J., Canadian Critical Care Trials Group, and Canadian Critical Care Translational Biology Group (2017). Brain biomarkers and pre-injury cognition are associated with long-term cognitive outcome in children with traumatic brain injury. BMC Pediatr. 17, 173 [DOI] [PMC free article] [PubMed]
- 30. Jennett, B., and Bond, M. (1975). Assessment of outcome after severe brain damage. Lancet 1, 480–484 [DOI] [PubMed] [Google Scholar]
- 31. Gioa, G.A., Isquith, P.K., Guy, S.C., and Kenworthy, L. (2000). Behavior Rating Inventory of Executive Function. Psychological Assessment Resources: Odessa, Fl [Google Scholar]
- 32. Gioia, G.A., and Isquith, P.K. (2004). Ecological assessment of executive function in traumatic brain injury. Dev. Neuropsychol. 25, 135–158 [DOI] [PubMed] [Google Scholar]
- 33. Isquith, P.K., Gioia, G.A., and Espy, K.A. (2004). Executive function in preschool children: examination through everyday behavior. Dev. Neuropsychol. 26, 403–422 [DOI] [PubMed] [Google Scholar]
- 34. Kongsted, A., and Nielsen, A.M. (2017). Latent class analysis in health research. J. Physiother. 63, 55–58 [DOI] [PubMed] [Google Scholar]
- 35. Muthen, L.K., and Muthen, B.O. (2017). Mplus User's Guide, 8th ed. Muthen & Muthen: Los Angeles [Google Scholar]
- 36. Godoy, D.A., Rubiano, A., Rabinstein, A.A., Bullock, R., and Sahuquillo, J. (2016). Moderate traumatic brain injury: the grey zone of neurotrauma. Neurocrit. Care 25, 306–319 [DOI] [PubMed] [Google Scholar]
- 37. Maillard-Wermelinger, A., Yeates, K.O., Gerry Taylor, H., Rusin, J., Bangert, B., Dietrich, A., Nuss, K., and Wright, M. (2009). Mild traumatic brain injury and executive functions in school-aged children. Dev. Neurorehabil. 12, 330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rivara, F.P., Koepsell, T.D., Wang, J., Temkin, N., Dorsch, A., Vavilala, M.S., Durbin, D., and Jaffe, K.M. (2011). Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics 128, e1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jimenez, N., Symons, R.G., Wang, J., Ebel, B.H., Vavilala, M.S., Buchwald, D., Temkin, N., Jaffe, K.M., and Rivara, F.P. (2016). Outpatient rehabilitation for Medicaid-insured children hospitalized with traumatic brain injury. Pediatrics 137 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 40. Wade, S.L., Zhang, N., Yeates, K.O., Stancin, T., and Taylor, H.G. (2016). Social environmental moderators of long-term functional outcomes of early childhood brain injury. JAMA Pediatr. 170, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeates, K.O., Taylor, H.G., Drotar, D., Wade, S.L., Klein, S., Stancin, T., and Schatschneider, C. (1997). Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J. Int. Neuropsychol. Soc. 3, 617–630 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.