Abstract
Sport-related concussion (SRC) is common in contact sports, but there remains a lack of reliable, unbiased biomarkers of brain injury and recovery. Although the symptoms of SRC generally resolve over a period of days to weeks, the lack of a biomarker impairs detection and return-to-play decisions. To this date, the pathophysiological recovery profile and relationships between brain changes and symptoms remained unclear. In the current study, diffusion kurtosis imaging (DKI) was used to monitor the effects of SRC on the brain and the trajectory of recovery in concussed American football players (n = 96) at <48 h, and 8, 15, and 45 days post-injury, who were compared with a matched group of uninjured players (n = 82). The concussed group reported significantly higher symptoms within 48 h after injury than controls, which resolved by the 8-day follow-up. The concussed group also demonstrated poorer performance on balance testing at <48 h and 8 days than controls. There were no significant differences between the groups in the Standardized Assessment of Concussion (SAC), a cognitive screening measure. DKI data were acquired with 3 mm isotropic resolution, and analyzed using tract-based spatial statistics (TBSS). Additionally, voxel- and region of interest-based analyses were also conducted. At <48 h, the concussed group showed significantly higher axial kurtosis than the control group. These differences increased in extent and magnitude at 8 days, then receded at 15 days, and returned to the normal levels by 45 days. Kurtosis fractional anisotropy (FA) exhibited a delayed response, with a consistent increase by days 15 and 45. The results indicate that changes detected in the acute period appear to be prolonged compared with clinical recovery, but additional brain changes not observable acutely appear to progress. Although further studies are needed to understand the pathological features of DKI changes after SRC, these findings highlight a potential disparity between clinical symptoms and pathophysiological recovery after SRC.
Keywords: concussion management, concussion symptoms, diffusion tensor imaging, DKI, mild traumatic brain injury, SRC
Introduction
Sport-related concussion (SRC) is common in contact sports such as American football, with up to 5,000,000 incidents per year in high school and collegiate athletes.1 SRC is generally considered to be a mild injury, and the typical return-to-play (RTP) time is 1–2 weeks post-injury when physical symptoms resolve.2–5 However, animal studies6,7 and recent neuroimaging studies8–12 have shown that signs of physiological and anatomical abnormalities in the brain persist beyond this time frame, and potentially can persist up to several months after injury. The timeline of actual physiological recovery is critical because an early RTP before full recovery might render the athlete more susceptible to re-injury.2,5 Therefore, it is important to determine the effects of SRC on the brain and the time course of physiological recovery, to improve patient assessment and RTP guidelines.
White matter (WM) injury after SRC has been of particular interest because of the strain inflicted on axonal tracts.13–15 Diffusion magnetic resonance imaging (MRI) has been widely used in SRC research because of its capability to detect microscopic abnormalities that are invisible with conventional anatomical imaging at macroscopic scales. The majority of prior studies used diffusion tensor imaging (DTI), and investigated changes in fractional anisotropy (FA) and mean, axial, and radial diffusivity (MD, AD, RD).16–22 However, despite its high sensitivity, DTI has also considerable ambiguity. Both increases and decreases in DTI metrics have been reported, suggesting that direction of changes may depend on known or unknown factors, including injury mechanisms and severity. Additionally, the DTI metrics evolve over time after injury. A comprehensive review of DTI findings in SRC can be found in recently published review articles.23–25
The underlying physical and biological assumptions of DTI have furthered the investigation of alternative models. DTI assumes a Gaussian distribution of water diffusion behavior, and complex microenvironments such as neuronal tissue cause a deviation from this assumption.26,27 Diffusion kurtosis Imaging (DKI) is an alternative model that estimates both the Gaussian and non-Gaussian components of the diffusion signal by acquiring diffusion-weighted images at multiple diffusion weighting factors (b-values).26,27 Increased model accuracy afforded by DKI is expected to improve sensitivity and accuracy of DWI to the changes in WM induced by SRC. Our group and others studied the effects of SRC and mild traumatic brain injury (mTBI) on WM fiber tracts using DKI,8,9,28–31 and reported robust changes in the kurtosis parameters in injured subjects. Some of those studies conducted follow-up scans, demonstrating longitudinal changes from acute to subacute phases of injury.8,9,29,30 In addition, Grossman and coworkers reported correlations between cognitive impairment in subjects with concussion and mean kurtosis (MK) in major WM fiber tracts.28
In our previous work, we studied changes in DKI metrics in a group of 26 American football players with SRC and 26 matched controls during the acute (at 24 h), subacute ( 8 days),8 and chronic phases of injury (at 6 months).9 The concussed group exhibited decrease in MD and increased axial kurtosis in major fiber tracts during the acute phase, and the magnitude and extent of changes increased at 8 days. At the 6 month follow-up, the axial kurtosis measures returned to normal levels, but MD remained lower in athletes with high injury scores measured by Sport Concussion Assessment Tool (SCAT3).32
The objective of this study was to capture the trajectory of DKI changes during the acute and subacute phases of injury by scanning subjects at four time points over a period of 45 days. A larger cohort of American football players, independent from our original study, was studied, with 96 concussed athletes 82 matched control subjects. Based on our prior work, we hypothesized that concussed athletes would have elevated axial kurtosis at the acute and subacute phase with recovery toward normal control levels by later visits.
Methods
Participants
Participants were recruited for a prospective study of clinical recovery from SRC funded by the United States Department of Defense. American football athletes from four high schools and four colleges in southeastern Wisconsin were enrolled for this study between June 2015 and December 2017. Written informed consent was obtained from all adult athletes and parents of minor athletes prior to their first evaluation; minor participants completed assent. All testing procedures were approved by the institutional review board at the Medical College of Wisconsin.
A total of 1174 athletes were enrolled at pre-season baseline, and completed clinical assessments; 106 of those subjects sustained concussion and were scanned within the first 48 h (32.47 ± 14.19 h), and then at 8 days (8.20 ± 0.98 days), 15 days (15.42 ± 1.35 days), and 45 days (45.56 ± 3.77 days) after injury; 91 control subjects were matched on demographics and baseline neurocognitive test performance and were scanned and assessed at similar time points.33,34 Ten data sets from the concussed group and nine data sets from the control group were discarded after failing image quality controls, leaving a total of 96 athletes with SRC and 82 matched controls. Chi-square tests did not show a significant difference between the SRC and control groups in exclusions caused by subpar image quality (the χ2 statistic is 0.39, p = 0.53). Reason to exclude a data set were either too much head motion, operator error, equipment failure (e.g. radiofrequency [RF] coil channel failure), or a similar problem. Demographic information is shown in Table 1. Clinical diagnosis of concussion was determined by certified athletic trainers or team physicians trained in sports medicine at each institution; all injuries were screened to ensure compliance with study definition and requirements. The definition of concussion proposed by Centers for Disease Control and Prevention HEADS UP educational initiative was used in this study: “An injury resulting from a forceful bum, blow or jolt to the head that results in rapid movement of the head and causes a change in the athlete's behavior, thinking, physical functioning, or the following symptoms: headache, nausea, vomiting, dizziness/balance problems, fatigue, difficulty sleeping, drowsiness, sensitivity to light/noise, blurred vision, memory difficulty, and difficulty concentrating” (https://www.cdc.gov/headsup/basics/concussion_whatis.html). Participants were excluded from the parent study if their additional injuries precluded participation in study procedure, or if they reported their injuries outside the 48-h window, had a current diagnosis of psychotic disorder, had been using prescribed narcotics prior to injury, or had a history of moderate or severe TBI or other medical conditions known to cause cognitive dysfunction (e.g., epilepsy, stroke). Participants were also excluded from the current study if they were not cleared to undergo an MRI scan (e.g. claustrophobia, metal implant).
Table 1.
Participant Characteristics
| |
Concussed |
Control |
|
|---|---|---|---|
| |
(n = 96) |
(n = 82) |
|
| mean (SD) | mean (SD) | p | |
| Demographics | |||
| Age (years) | 18.06 (1.5) | 18.37 (1.7) | 0.24 |
| Race (n, %) | |||
| White | 74(77%) | 63(76.8%) | |
| Black | 20(20.8%) | 18(21.9%) | |
| American Indian/Alaska Native | 2(2%) | 1(1.2%) | |
| Height (in.) | 71.7 (2.77) | 71.02 (2.4) | 0.08 |
| Weight (lb.) | 217.6 (50.8) | 206.4 (41.5) | 0.11 |
| ADHD diagnosis (n, %) | 9 (9.4%) | 6 (7.3%) | 0.62a |
| Learning disability (n, %) | 2 (2.1%) | 0.0 (0.0%) | 0.19a |
| Household SESb | 46.29 (11.1) | 45.63 (11.05) | 0.69 |
| Total number of previous concussionsc | 102 | 59 | 0.067 |
| Acute injury characteristics | |||
| Loss of consciousness | 2 (2.1%) | - | |
| Post-traumatic amnesia | 15 (15.6%) | - | |
| Retrograde amnesia | 4 (4.2%) | - |
Approximate significance using Cramer's V.
Hollingshead SES score.
History of previous concussions reported by the players.
SD, standard deviation; ADHD, attention-deficit/hyperactivity disorder; SES, socioeconomic status.
Baseline and post-injury clinical assessments
The clinical battery included the SCAT3, which evaluates somatic, cognitive, and emotional post-concussive symptoms,32 the Standardized Assessment of Concussion (SAC) as a cognitive screener,35,36 and the Balance Error Scoring System (BESS) to assess postural stability.37–39 All clinical assessments were administered before the season to establish a baseline, and then they were repeated in each follow-up visit. The number of symptomatic days and the length of RTP was also recorded at each follow-up visit.
MRI acquisition
All participants were scanned on a GE MR750 whole body 3T MRI scanner (Waukesha, WI) equipped with a 32-channel head coil. Diffusion weighted images for DKI were obtained using a single-shot spin-echo echo-planar imaging (EPI) sequence with 3 mm isotropic voxels and b-values of 1000 s/mm2 and 2000 s/mm2, with 30 diffusion directions for each b-value. The DKI acquisitions covered the whole brain, with an acquisition time of 5 min 30 sec. An additional non-diffusion weighted spin-echo EPI scan was acquired with the phase encode polarity reversed to correct for distortions caused by tissue magnetic susceptibility variations using post-processing software tools.
Processing of DKI data
Each diffusion weighted image set was visually inspected for artifacts before any processing was applied. Data sets that cleared quality inspection were further processed using an established pre-processing pipeline. First, spatial distortions caused by magnetic susceptibility variations were corrected using the TOPUP function40 of the FSL software (version 6.0.0, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Motion- and eddy current-related distortions were corrected using the EDDY function of FSL.41 After the pre-processing, images were inspected again to ensure that the processing did not introduce artifacts or distortions.
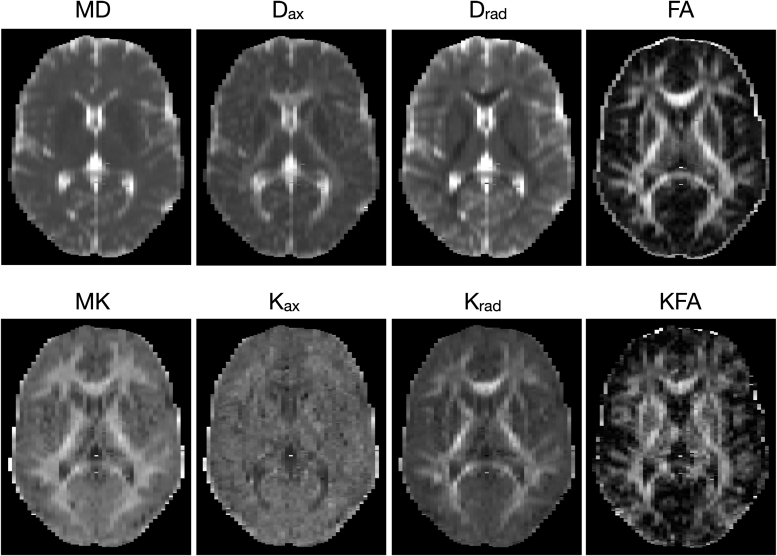
Diffusion and kurtosis tensors were estimated using DKE software (https://www.nitrc.org/projects/dke/)26,27 to derive maps of kurtosis fractional anisotropy (KFA), mean kurtosis (MK), and radial and axial kurtosis (Krad and Kax, respectively). Similarly, maps of FA, MD, and radial and axial diffusivity (Drad, and Dax) were calculated from the diffusion tensor estimation. The robust option was used, which detects and removes outliers using an iterative algorithm during the model fitting.42 A sample set of diffusion tensor and kurtosis maps from one of the participants is shown in Figure 1. The test–retest reliability of DKI maps was investigated at the beginning of our concussion studies using the concordance correlation coefficient (CCC)43 on repeated scans from control subjects. Average CCC was 0.91 for MD, 0.86 for Krad, and 0.85 for Kax in non-skeletonized DKI maps.
FIG. 1.
Sample diffusion kurtosis imaging (DKI) parameter maps from one of the participants.
A tract-based spatial statistics (TBSS) approach44 was used for analysis of group differences in major WM tracts. Pre-processing of DKI data for TBSS began with registering each subject's FA map to the FMRIB58_FA template (standard space FA map) using linear and non-linear transformations. Then, a WM skeleton was generated for the group. The local FA value was projected onto the skeleton. A threshold of FA >0.2 was applied to include all major WM tracts and exclude superficial WM and gray matter. The skeletonization was repeated for each of the other DTI and DKI metrics.
Statistical analysis
Demographic and clinical data
Group differences in demographic and clinical data were determined using the Statistical Package for Social Sciences (SPSS, version 24.0). Linear mixed effects models with visit as within-subject variable, group as between-subject factor, the group-by-visit interaction, and age and prior concussion included as covariates were used to determine changes in clinical measures across visits. Student's t tests were used for comparing group differences in demographic data while χ2 tests were used to examine group differences in categorical variables.
TBSS of the WM tracts
Group differences in major WM tracts were tested using the general linear model (GLM) analysis on skeletonized DKI maps obtained from the TBSS approach. Randomise function in FSL, which employs a non-parametric approach to evaluate the GLM using a large number of random permutations among groups to identify statistically significant effects, was used for voxelwise statistical tests.45,46 Randomise was run with 2000 permutations and the threshold-free cluster enhancement (TFCE) option.47 Each statistical test was corrected for multiple comparisons using a familywise error (FWE) rate, and results were thresholded using p < 0.05.
Group differences in each of the DTI and DKI metrics were compared at each time point. The concussed group had a higher number of prior concussions (Table 1) than the control group, and the number of prior concussions was included as a covariate. Likewise, although age was not different between groups, it was included as a covariate in all analyses, because age-dependent changes in diffusion MRI measures are well established.48–52
Voxel-based analysis (VBA) of the whole brain
A whole brain VBA was also conducted on each of the diffusion maps. Because TBSS excludes peripheral WM and gray matter regions, VBA was performed to complement the skeletonized WM analysis with TBSS. The spatially registered parameter maps were compared at a group level using the same GLM model and statistical comparisons using the prior TBSS description, including 1000 iterations and TFCE option. The images were smoothed with 3 mm Gaussian kernel prior to analysis.
Region of interest (ROI) data
An ROI analysis was used for a subsequent investigation of the effects of time. ROIs were derived from the statistically significant TBSS group effects, which emerged for Kax, Krad, and KFA. ROIs were created from the largest cluster of voxels with statistically significant group differences, using Kax at the 48-h time point and Krad and KFA from the day-15 time point, because these time points showed the largest respective group effects. The mean values of each DKI metric for each subject were extracted and used for additional analysis. Linear mixed effects models with visit as within-subject variable, group as between-subject factor, the group-by-visit interaction, and age and prior concussion included as covariates were used to determine changes in metrics across visits. SPSS software version 24 (Armonk, NY USA) was used for the ROI statistics. Post-hoc tests were corrected for multiple comparisons using Bonferroni correction.
Results
Subject characteristics
All participants were male. There were no significant differences between concussed and control athletes in age, race, height, or weight (Table 1). Further, the groups did not differ regarding number of individuals diagnosed with attention-deficit/ hyperactivity disorder or a learning disability. Although not statistically significant, a trend existed for a higher number of prior SRCs in the concussed group (p = 0.067). In the concussed group, the average duration for resolution of symptoms was 9.5 days with a standard deviation of 8.8 days.
Clinical injury measures
Between-group differences
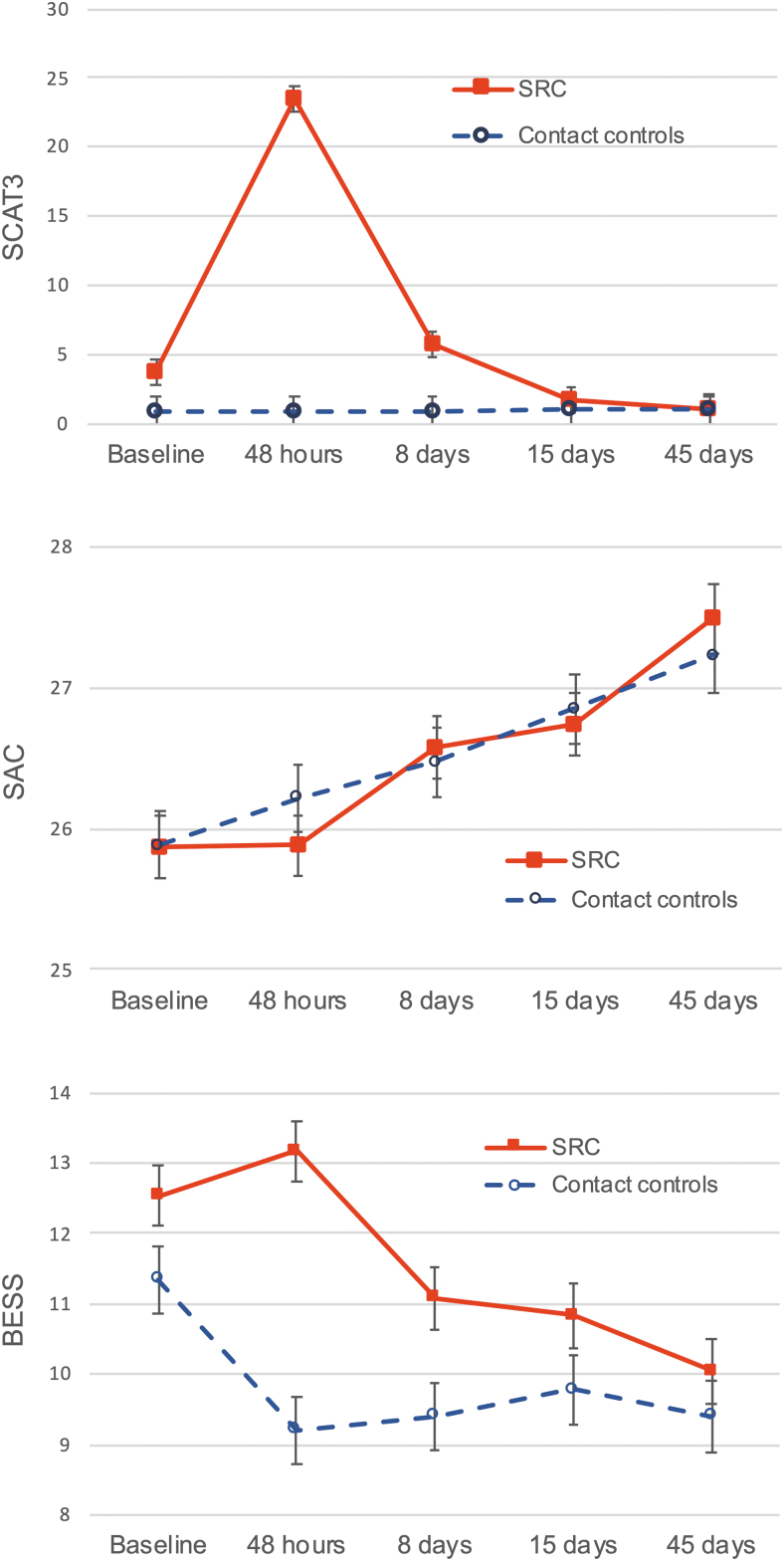
At pre-season baseline, there were no differences between the concussed group and controls on the SCAT3 symptom checklist, SAC, or BESS (Table 2). Within the first 48 h post-injury, the concussed group reported significantly more concussion symptoms on the SCAT3 than the control group (p < 0.001), but did not demonstrate a difference in cognitive performance on the SAC (p = 0.3). The groups differed on BESS balance testing at 48 h (p < 0.001) and at day 8 follow-up (p = 0.012). There were no significant differences between groups at day 15 and day 45 follow-ups in any of the clinical injury measures.
Table 2.
Clinical Assessment of Participants
| |
Concussed |
Control |
|
|---|---|---|---|
| |
(n = 96) |
(n = 82) |
|
| mean (SE) | mean (SE) | p | |
| SCAT3 Symptom Severity | |||
| Baseline | 3.7 (0.91) | 2.2 (1.02) | 0.267 |
| 48 h | 23.5 (0.92) | 1.7 (1.02) | < 0.001 |
| 8 days | 5.7 (0.94) | 1.1 (1.05) | 0.01 |
| 15 days | 1.7(0.97) | 0.93(1.07) | 0.58 |
| 45 days | 1.01(1.01) | 1.13(1.11) | 0.94 |
| SAC Total Score | |||
| Baseline | 25.87(0.22) | 25.88(0.24) | 0.96 |
| 48 h | 25.88(0.22) | 26.22(0.24) | 0.3 |
| 8 days | 26.58(0.22) | 26.47(0.25) | 0.75 |
| 15 days | 26.74(0.22) | 26.85(0.25) | 0.75 |
| 45 days | 27.49(0.24) | 27.23(0.26) | 0.55 |
| BESS Total Score | |||
| Baseline | 12.53 (0.43) | 11.35 (0.48) | 0.07 |
| 48 h | 13.17 (0.43) | 9.2 (0.48) | <0.001 |
| 8 days | 11.08 (0.44) | 9.4 (0.49) | 0.012 |
| 15 days | 10.83(0.45) | 9.78(0.5) | 0.12 |
| 45 days | 10.04(0.46) | 9.41(0.51) | 0.37 |
SE, standard error; SCAT3, Sport Concussion Assessment Tool, 3rd Edition; SAC, Standardized Assessment of Concussion; BESS, Balance Error Scoring System.
Significant differences are presented in boldface.
Within-group changes
The group × time interaction was statistically significant for SCAT3 symptom severity scores (p < 0.001), with the concussed group reporting significantly higher SCAT3 symptoms within the first 48 h post-injury than at baseline (p < 0.001). At 8 days post-injury, however, reported symptoms in the concussed group returned to baseline levels (p = 0.79) and remained at the same levels on day 15 and day 45 follow-ups (Fig. 2). For the control group, there were no significant differences between any time points for SCAT3.
FIG. 2.
Clinical injury scores for the two groups across the five time points. Error bars indicate standard error. Color image is available online.
SAC scores were not significantly different between the two groups at any time point. Also, the SAC scores in the concussed group did not differ from the baseline at 48 h post-injury (p = 1.00), but a significant monotonic increase was seen at 8 days (p = 0.022), 15 days (p = 0.003), and up to 45 days (p < 0.001). The control group also showed the same type of increase, with significant change from baseline at 15 days (p = 0.003) and 45 days (p < 0.001). Because higher SAC scores indicate better performance, this increase in performance could be the result of learning effects.
The group × time interaction was statistically significant for BESS (p < 0.001). BESS scores in both groups started with similar values (Fig. 2), which were within the normal values reported (12.03±7.34).39 Higher BESS scores indicate worse performance. Concussed athletes had higher BESS scores than controls at 48 h (p < 0.001) and 8 days (p = 0.012). Control athletes, but not athletes with SRC, had lower BESS scores at 48 h post-injury relative to baseline (p < 0.001).
DTI changes
There were no significant differences in FA, MD, RD, or AD between the two groups in TBSS or VBA analyses. There were also no significant change in these metrics with time within the groups.
DKI changes
Between-group differences in axial kurtosis, TBSS, and VBA
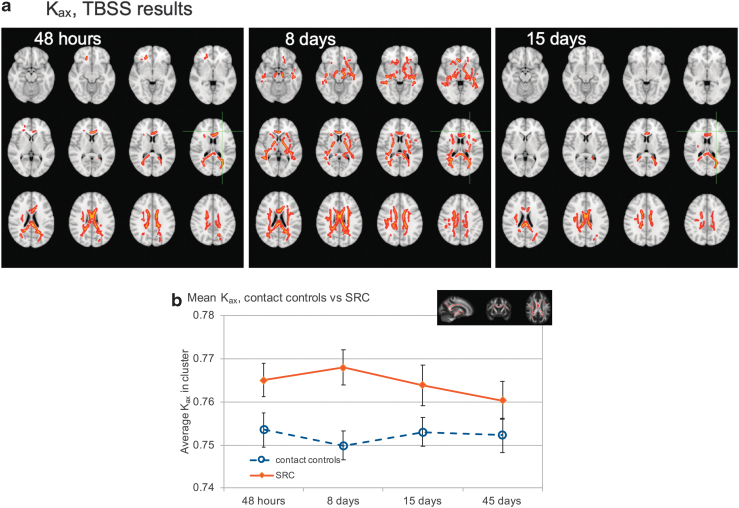
Kax was significantly higher in the concussed group than in the controls at 48 h, 8 days, and 15 days (p < 0.05, FWE corrected) (Fig. 3a). The affected areas were mostly confined to the corpus callosum, genu, and splenium in the 48-h scans. However, the effects became more widespread in the follow-up scans at 8 days. In addition to the corpus callosum, genu, and splenium, the majority of corticospinal tracts, superior longitudinal fasciculus, and inferior longitudinal fasciculus were affected bilaterally 1 week after the injury. Two weeks after the injury, the group differences were confined to the body of corpus callosum, and most of the other affected areas returned to levels comparable to those of controls. There were no significant differences in Kax by day 45.
FIG. 3.
(a) Results of tract-based spatial statistics (TBSS) white matter analysis for axial kurtosis. Voxels in red show regions of significant group differences (p < 0.05, familywise error [FEW] corrected). Significant voxels from the skeletonized TBSS data have been inflated using tbss_fill for viewing purposes, and are overlaid on the T1 weighted image in the Montreal Neurological Institute (MNI) space. Kax was higher in the concussed group than in controls in the first three visits. (b) Mean Kax values in the largest cluster of voxels in TBSS analysis that showed group differences in 48-h scans. Error bars show 95% confidence intervals. The inset shows the location and extent of the cluster (red voxels) from which these data are derived. Color image is available online.
There were no significant differences in Kax between the groups in the VBA.
Between-group differences in axial kurtosis, ROI analysis
Figure 3b shows mean Kax values for each time point for the concussed athletes and controls. The largest cluster of voxels that showed group differences in 48 h scans was used as a mask, and Kax values inside this mask were averaged across all subjects in each group for each time point. Mean values for the control group were ∼0.75 (unitless) and did not substantially differ over the evaluation period, whereas the concussed group had elevated levels across all time points. Kax values showed further increase on day 8, and then started to return to normal levels by day 45. The time × group interaction term was significant (p = 0.001), with the concussed group showing significant change with time (p < 0.001) and no significant changes in the control group (p = 0.136). In the concussed group, the Kax values were significantly elevated at 48 h (p = 0.009) and 8 days (p < 0.001) compared with at 45 days. In the control group, there were no statistically significant differences between any of the time points. The difference between the groups at each visit was also significant (p < 0.001 for 48 h, 8 days, and 15 days, and p = 0.004 for 45 days).
Between-group differences in Krad, TBSS, and VBA
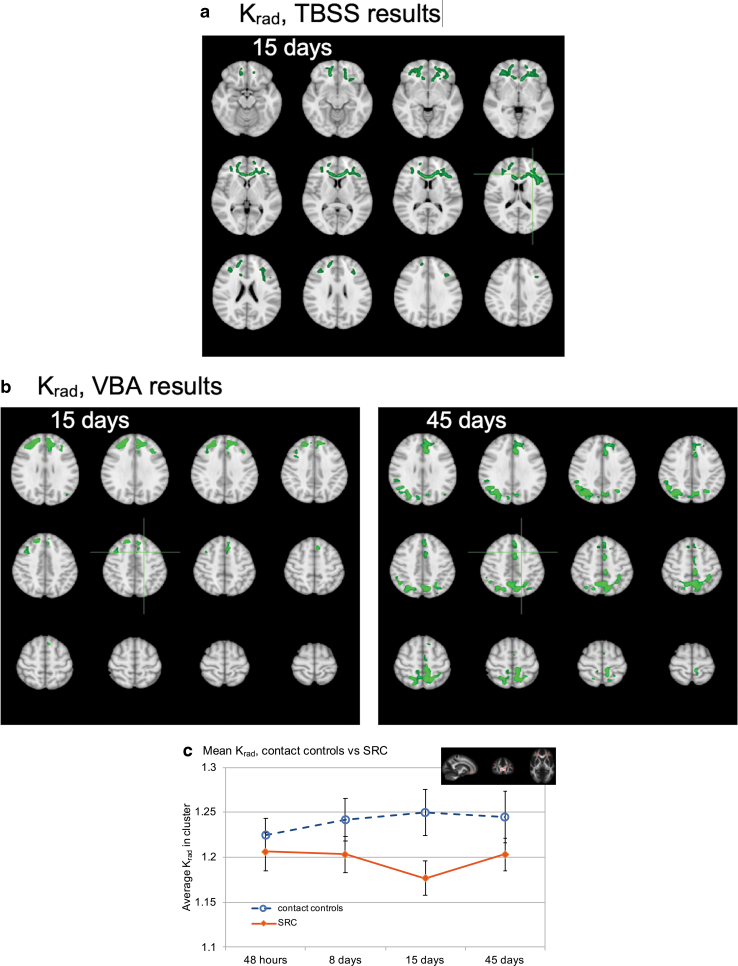
In the TBSS skeletonized analysis, Krad was lower in the concussed athletes than in the control athletes, and it showed a more delayed time course than Kax. There were significant group differences in Krad in frontal lobe WM at the day-15 visit, with lower Krad in athletes with SRC than in controls (Fig. 4a); (p < 0.05, FWE corrected).
FIG. 4.
(a) Results of tract-based spatial statistics (TBSS) white matter analysis for radial kurtosis. Voxels in green show regions of significant group differences (p < 0.05, familywise error [FEW] corrected). Significant voxels from the skeletonized TBSS data have been inflated using tbss_fill for viewing purposes, and are overlaid on the T1 weighted image in the Montreal Neurological Institute (MNI) space. Krad was significantly lower in the concussed group than in controls only on day 15 scans. The effects were in the frontal lobe white matter, including the genu. (b) Results of voxel-based analysis (VBA). Krad was significantly lower in the concussed group than in controls on day 15 and day 45 scans (green voxels). (c) Mean Krad values in the largest cluster of voxels in TBSS analysis that showed group differences in day 15 scans. Error bars show 95% confidence intervals. The inset shows the location and extent of the cluster (red voxels) from which these data are derived. Color image is available online.
Whole brain voxel-based analysis showed group differences in Krad, mostly in peripheral WM and cortical gray matter regions (Fig. 4b). Similar to the findings in WM tracts, Krad was lower in the concussed group, and it showed the same delayed effect at day 15 scans. However, VBA analysis showed group differences in frontal, parietal, and occipital regions, unlike TBSS results, in which effects were only in the frontal WM.
Between-group differences in Krad, ROI analysis
Figure 4c shows the plots for means of Krad for the two groups across the four time points. The averages were calculated from the largest cluster from TBSS analysis on day 15. The time × group interaction term was significant (p = 0.006), and the concussed group showed significant change over time (p < 0.032). Although mean Krad values for the control group showed a monotonic increase over time, it was not significant (p = 0.059). The pairwise comparisons of Krad values between time points for the concussed group did not show statistically significantly differences, but there was a trend between 48 h and 15 days (p = 0.059). In the control group, there were no statistically significant differences between any of the time points. Additionally, concussed athletes had significantly lower Krad at 8 days (p = 0.005) and 15 days (p < 0.001) than controls.
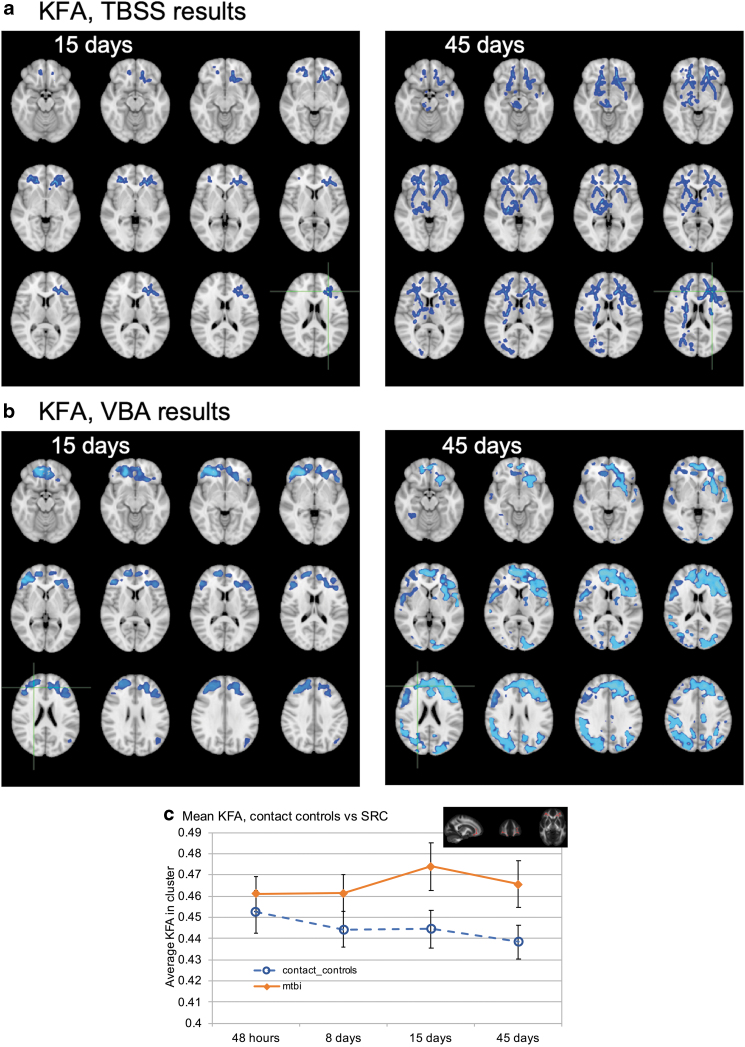
Between-group differences in KFA, TBSS, and VBA
KFA also showed a delayed effect, with the concussed group demonstrating higher KFA values in the frontal lobe WM (Fig. 5a). There were no significant differences in the 48 hour and day 8 scans. However, day 15 scans showed significant differences in the frontal WM, and the effects were more widespread by day 45 (p < 0.05, FWE corrected).
FIG. 5.
(a) Results of tract-based spatial statistics (TBSS) white matter analysis kurtosis fractional anisotropy (FA). Voxels in blue show regions of significant group differences (p < 0.05, familywise error [FEW] corrected). Significant voxels from the skeletonized TBSS data have been inflated using tbss_fill for viewing purposes, and are overlaid on the T1 weighted image in the Montreal Neurological Institute (MNI) space. Kurtosis fractional anisotropy (KFA) was higher in the concussed group than in controls in the day 15 and day 45 scans. (b) Results of voxel-based analysis (VBA). KFA was higher in the concussed group than in controls on the day 15 and day 45 scans (blue voxels). (c) Mean KFA changes in time measured in the largest cluster of voxels from TBSS analysis. Error bars show 95% confidence intervals. The inset shows the location and extent of the cluster (red voxels) from which these data are derived. Color image is available online.
KFA also followed the same trends in VBA, showing higher values in the concussed group than in controls on day 15 and day 45 scans (Fig. 5b).
Between-group differences in KFA, ROI analysis
Figure 5c shows mean KFA values in the largest cluster of voxels that showed group differences in day 15 scans. On the other hand, the concussed group had an elevated KFA across all four time points, but there was a notable increase in KFA on day 15. The group × time interaction was statistically significant for KFA (p = 0.029). The difference between 48 h and day 45 was significant in the control group (p = 0.044), because of the monotonic decrease in average KFA values over time. Other pairwise comparisons between time points did not show a significant difference for either group. On the other hand, pair-wise comparisons between the two groups showed significant differences at 8 days (p = 0.005), 15 days (p < 0.001), and 45 days (p < 0.001). Whereas Krad values had an increasing trend over time in the control group, KFA showed a decreasing trend, which might be expected given their mathematical relationship.
Associations among DKI metrics, symptom duration, symptom severity, and SCAT3
There were no significant associations between DKI metrics at any time point and 48-h SCAT3. Similarly, no associations were noted between DKI metrics at any time point and duration of symptom recovery or symptom severity.
Discussion
This study investigated changes in DKI measures in the brain during the acute, subacute, and recovery periods after SRC. These changes in DKI metrics demonstrate the progression of microscopic WM disruptions following concussion. The ability to capture the initial changes within the first 48 h after injury and at 8, 15, and 45 days provided a better understanding of the progression of tissue injury and recovery as evidenced by the DKI biomarkers. Notably, the different metrics exhibit different time courses and spatial patterns that might reflect their sensitivities to different pathological features.
The most pronounced differences between concussed athletes and matched contact control athletes were observed in Kax, which occurred during the first 2 weeks after injury. The differences were primarily confined to central WM tracts in the 48-h scans, which became more widespread at day 8. At day 15 follow-up, there were still persisting changes in the corpus callosum fibers, but these differences did not remain by day 45. However, ROI plots in Figure 3b show elevated Kax values in the injured group at all time points, including day 45, but it was not significant in the TBSS analysis. VBA did not yield statistically significant changes after multiple comparison corrections, although similar trends were seen in WM tracts. This type of variability might be expected between VBA and TBSS analyses, because partial volume effects and variability caused by imperfect registrations would affect the voxelwise statistics that TBSS explicitly accounts for.44
Conversely, Krad showed a delayed response with differences becoming evident at day 15 and increasing at day 45. These changes were mainly in the major frontal lobe WM tracts and peripheral WM and gray matter boundaries in the frontal and occipital areas. Based on the time plots from the ROI, Krad values were generally lower in the concussed group on days 8, 15, and 45. However, TBSS results were only significant on day 15, showing effects in the frontal lobe WM and genu. VBA analysis showed significant differences on day 15 and 45 scans, with group differences mainly in the pre-frontal and occipital areas.
KFA measurements showed a trend similar to Krad, where an increase in KFA was evident in the concussed group on day 15. There were no significant differences in the KFA values between the two groups during the acute and subacute phase (48 h and day8 scans). Significant group differences began to manifest in the frontal WM by day 15, which became more pronounced on day 45 scans. However, this increased effect on day 45 might be partially driven by the slight drop in KFA values in the control group at this time point. However, both groups showed a slight drop in average KFA values on day 45, maintaining the same magnitude of difference between the two groups at these two time points. Therefore, it might be concluded that the wider spread of effects might be actually related to some tissue changes during a long recovery period. The variations in the control group across the four time points were not significant, but they were significant in the concussed group. In addition, TBSS and VBA results complemented each other, demonstrating significant changes in major WM fiber tracts as well as peripheral WM regions in the concussed subjects. Further, the values did not return to normal levels even on day 45 follow-up. Considering the evidence from TBSS, VBA, and ROI analyses with KFA, it might be concluded that the concussed group had overall slightly elevated KFA values mainly in the frontal lobe across all four time points, but that group differences were only significant at the last two time points.
Overall, these findings support the notion that physiological changes caused by injury might extend beyond the resolution of clinical symptoms of SRC. Figure 2 shows that all three clinical injury measures (SCAT3, SAC, and BESS) returned to baseline levels within 8 days after injury. However, physiological changes detected by diffusion kurtosis metrics were evident beyond 8 days, some extending to 45 days. Further, there is a notable increase in the spatial extent of the injury-related WM changes on day 8, suggesting an evolving neuropathological process in concussed group. Importantly, these results are in accord with our earlier analysis of DKI changes in SRC in a smaller cohort of American football players (26 injured and 26 controls).8 Temporal and spatial changes in Kax showed identical characteristics in both data at 24–48 h and 8 days. In the earlier work, however, we did not have follow-up measurements at 15 and 45 days. Therefore, this study not only supported the validity of Kax changes in a larger and separate cohort of in injured athletes, but also provided a better trajectory of physiological changes. Additionally, we observed changes in Krad in this larger cohort, which we did not see in the earlier data. This could be because of increased statistical power with a larger sample. We did not calculate KFA in the earlier study; therefore, we do not have a comparison for that metric. On the other hand, we had observed reduced MD measurements in injured athletes in the earlier data, but we did not see it in this group. It is possible that the injured cohort in the earlier study had more severe injuries (mean SCAT3 = 27.6, mean SAC = 24.5) than this group (mean SCAT3 = 23.5, mean SAC = 25.9), which might explain some of these discrepancies, other than the cohort sizes. However, from both studies, Kax seems to be the most robust metric for measuring early changes after injury.
In addition to the conceptional advantages of DKI over DTI in characterizing diffusion in complex tissues, our result also demonstrated a greater sensitivity of DKI compared with DTI in detecting the effects of sports concussion in the same cohort. The results also shed light on possible pathophysiological effects of concussion, although noting that direct assignment of DKI or DTI changes to a single pathological feature is problematic. Nonetheless, because the kurtosis metrics exhibit different spatiotemporal patterns, insight into the putative pathophysiological effects is warranted. In this study, Kax was more sensitive to early responses to injury. Increased Kax is indicative of impeded diffusion along the axis of the WM fiber tracts, and this feature and its early effects after SRC would possibly be in agreement with axonal beading that occurs in consequence of acute injury.53 This axonal beading can be caused by ionic or osmotic effects, or driven by focal accumulation of organelles.54 The potential association between Kax and axonal beading was suggested by a DKI study of head impact exposure in high school American football players.55 They cited two DKI studies on stroke patients, which reported increased Kax after ischemic stroke.56,57 In those studies, the authors argued that tissue heterogeneity along the axial diffusion direction would lead to an increase in axial kurtosis. This view is also supported by an animal model of mTBI, which showed increased Kax and reduced Krad after a head impact.31 However, direct comparisons of injured axons after concussion and DKI measurements remain to be shown, and the pathophysiological implications need further external validation. On the other hand, delayed changes in KFA and Krad in frontal WM fibers and gray matter could possibly reflect ongoing or secondary effects. In animal models of contusion TBI, extensive neurodegeneration occurs outside of the primary site of injury.58 The spatial pattern and temporal effects of the Krad changes in this study are interesting, given the reported features of long-term pathological consequences of repetitive head trauma.57 However, in this relatively short-term longitudinal study after a single concussion by comparison with lifelong effect, these relationships are purely speculative, and further longitudinal studies and mechanistic animal studies are necessary to parse out these findings.
A strength of this study was the ability to capture acute changes within the first 48 h after injury. Including only male American football players with a narrow age range and matched for demographics allowed us to minimize various potential confounding factors that may affect concussion consequences such as sex, age, and the type of sports played. On the other hand, this limits the generalizability of these findings to female athletes, other sports, and other age groups, and to concussion in the general population. It is possible that the characteristics of injury-related changes could be different between males and females. Similarly, the brain undergoes significant changes throughout the lifespan,48,49,59 which might lead to different neurophysiological changes with similar type of injuries at different ages. In addition, the type of sports activity might lead to different type of brain injury characteristics, which in turn could manifest different neurophysiological changes after injury. Therefore, it would be beneficial to expand this type of research by including females, other age groups, and other contact sports.
Another limitation of the study was the spatial resolution of the DKI scans: 3 mm isotropic voxels allowed us to study WM tracts that were at the voxel resolution or larger. Future studies with higher resolution scans could help detect injury signatures in smaller fiber tracts.
Although the overarching goal is to establish diagnostic and prognostic biomarkers of SRC for individual athletes, the results presented here are just the first step in reaching that objective. Finding typical changes in DKI in injured athletes might help us focus on those particular metrics and explore their potential as a diagnostic or prognostic tool in future studies. In that respect, the findings reported here provide some indirect imaging evidence about persisting neurophysiological abnormalities in the brain beyond the time frame when clinical symptoms typically subside. If these DKI metrics can be used as a biomarker of tissue injury and recovery, an early RTP could be avoided, and the athlete could be protected from a potentially more severe re-injury. Although most of the injured athletes had reported symptom relief on day 8, DKI differences remained. Moreover, the magnitude and extent of the DKI changes appeared to have increased after a week compared with the acute period. Interestingly, some of the DKI metrics demonstrated delayed changes in tissue microstructure after 2 weeks, possibly indicating some latent effects after injury. Unfortunately, the full characteristics of brain tissue injury and recovery are not well understood at this time, with some studies reporting changes in brain structure and function ranging from a month up to a year after an mTBI.60–64 Considering that a history of multiple concussions within the window of increased cerebral vulnerability is associated with poorer recovery,2 it might be expected that the WM changes observed here could be accumulating and amplifying with multiple injuries during this vulnerable period and contributing to poor outcomes. Accumulating evidence from advanced imaging studies such as this and other objective clinical biomarkers might help inform more accurate RTP decisions that are in the best interest of athletes.
Conclusion
This study reported DKI changes in the brain after SRC, with different DKI measures showing group differences at different time points. These findings complemented and supported our earlier findings from a smaller and separate cohort with fewer follow-up visits.
Acknowledgment
The authors acknowledge the Research Computing Center at Medical College of Wisconsin (MCW) for providing cluster computing infrastructure that made it possible to process and analyze large amounts of imaging data.
Funding Information
This work was supported by the Defense Health Program under the Department of Defense Broad Agency Announcement for Extramural Medical Research through Award No. W81XWH-14-1-0561. The REDCap electronic database used for this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), award number UL1TR001436. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense or the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Nation, A.D., Nelson, N.G., Yard, E.E., Comstock, R.D., and McKenzie, L.B. (2011). Football-related injuries among 6- to 17-year-olds treated in US emergency departments, 1990-2007. Clin. Pediatr. (Phila), 50, 200–207 [DOI] [PubMed] [Google Scholar]
- 2. Guskiewicz, K.M., McCrea, M., Marshall, S.W., Cantu, R.C., Randolph, C., Barr, W., Onate, J.A., and Kelly, J.P. (2003). Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA, 290, 2549–2555 [DOI] [PubMed] [Google Scholar]
- 3. McCrea, M., Guskiewicz, K.M., Marshall, S.W., Barr, W., Randolph, C., Cantu, R.C., Onate, J.A., Yang, J., and Kelly, J.P. (2003). Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA, 290, 2556–2563 [DOI] [PubMed] [Google Scholar]
- 4. McCrea, M., Prichep, L., Powell, M.R., Chabot, R., and Barr, W.B. (2010). Acute effects and recovery after sport-related concussion: a neurocognitive and quantitative brain electrical activity study. J. Head Trauma Rehabil. 25, 283–292 [DOI] [PubMed] [Google Scholar]
- 5. McCrea, M., Broglio, S., McAllister, T., Zhou, W., Zhao, S., Katz, B., Kudela, M., Harezlak, J., Nelson, L., Meier, T., Marshall, S.W., Guskiewicz, K.M., and CARE Consortium Investigators (2020). Return to play and risk of repeat concussion in collegiate football players: comparative analysis from the NCAA Concussion Study (1999-2001) and CARE Consortium (2014-2017). Br. J. Sports Med. 54, 102–109 [DOI] [PubMed] [Google Scholar]
- 6. Giza, C.C., and Hovda, D.A. (2001). The neurometabolic cascade of concussion. J. Athl. Train.. 36, 228–235 [PMC free article] [PubMed] [Google Scholar]
- 7. Giza, C.C., and Hovda, D.A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75, Suppl. 4, S24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lancaster, M.A., Olson, D.V., McCrea, M.A., Nelson, L.D., LaRoche, A.A., Muftuler, L.T. (2016). Acute white matter changes following sport-related concussion: A serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum. Brain Mapp. 37, 3821–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lancaster, M.A., Meier, T.B., Olson, D.V., McCrea, M.A., Nelson, L.D., and Muftuler, L.T. (2018). Chronic differences in white matter integrity following sport-related concussion as measured by diffusion MRI: 6-Month follow-up. Hum. Brain Mapp. 39, 4276–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vagnozzi, R., Signoretti, S., Tavazzi, B., Floris, R., Ludovici, A., Marziali, S., Tarascio, G., Amorini, A.M., Di Pietro, V., Delfini, R., and Lazzarino, G. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes–part III. Neurosurgery 62, 1286–1295 [DOI] [PubMed] [Google Scholar]
- 11. Churchill, N.W., Hutchison, M.G., Richards, D., Leung, G., Graham, S.J., and Schweizer, T.A. (2017). Neuroimaging of sport concussion: persistent alterations in brain structure and function at medical clearance. Sci. Rep. 7, 8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier, T.B., Bergamino, M., Bellgowan, P.S., Teague, T.K., Ling, J.M., Jeromin, A., and Mayer, A.R. (2016). Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum. Brain Mapp. 37, 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blennow, K., Hardy, J., and Zetterberg, H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899 [DOI] [PubMed] [Google Scholar]
- 14. Peerless, S.J., and Rewcastle, N.B. (1967) Shear injuries of the brain. CMAJ 96, 577. [PMC free article] [PubMed] [Google Scholar]
- 15. Strich, S.J. (1956). Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J. Neurol. Neurosurg. Psychiatry 19, 163–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bazarian, J.J., Zhu, T., Blyth, B., Borrino, A., and Zhong, J. (2012). Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging, 30, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borich, M., Makan, N., Boyd, L., and Virji-Babul, N. (2013). Combining whole-brain voxel-wise analysis with in vivo tractography of diffusion behavior after sports-related concussion in adolescents: a preliminary report. J. Neurotrauma, 30, 1243–1249 [DOI] [PubMed] [Google Scholar]
- 18. Chamard, E., Lassonde, M., Henry, L., Tremblay, J., Boulanger, Y., De Beaumont, L., and Théoret, H. (2013). Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj. 27, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 19. Cubon, V.A., Putukian, M., Boyer, C., and Dettwiler, A. (2011). A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J. Neurotrauma, 28, 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henry, L.C., Tremblay, J., Tremblay, S., Lee, A., Brun, C., Lepore, N., Theoret, H., Ellemberg, D., and Lassonde, M. (2011). Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 28, 2049–2059 [DOI] [PubMed] [Google Scholar]
- 21. Murugavel, M., Cubon, V., Putukian, M., Echemendia, R., Cabrera, J., Osherson, D., and Dettwiler, A. (2014). A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. J. Neurotrauma, 31, 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virji-Babul, N., Borich, M.R., Makan, N., Moore, T., Frew, K., Emery, C.A., and Boyd, L.A. (2013). Diffusion tensor imaging of sports-related concussion in adolescents. Pediatr. Neurol. 48, 24–29 [DOI] [PubMed] [Google Scholar]
- 23. Chamard, E., and Lichtenstein, J.D. (2018). A systematic review of neuroimaging findings in children and adolescents with sports-related concussion. Brain Inj. 32, 816–831 [DOI] [PubMed] [Google Scholar]
- 24. Schneider, D.K., Galloway, R., Bazarian, J., Diekfuss, J.A., Dudley, J., Leach, J., Mannix, R., Talavage, T.M., Yuan, W., and Myer, G.D. (2019). Diffusion tensor imaging in athletes sustaining repetitive head impacts: a systematic review of prospective studies. J. Neurotrauma 36, 2831–2849 [DOI] [PubMed] [Google Scholar]
- 25. Borja, M.J., Chung, S., and Lui, Y.W. (2018). Diffusion MR imaging in mild traumatic brain injury. Neuroimaging Clin. N. Am. 28, 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen, J.H., Helpern, J.A., Ramani, A., Lu, H., and Kaczynski, K. (2005). Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 27. Jensen, J.H., and Helpern, J.A. (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 23, 698–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grossman, E.J., Ge, Y., Jensen, J.H., Babb, J.S., Miles, L., Reaume, J., Silver, J.M., Grossman, R.I., and Inglese, M. (2012). Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J. Neurotrauma 29, 2318–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grossman, E.J., Jensen, J.H., Babb, J.S., Chen, Q., Tabesh, A., Fieremans, E., Xia, D., Inglese, M., and Grossman, R.I. (2013). Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. AJNR Am. J. Neuroradiol. 34, 951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stokum, J.A., Sours, C., Zhuo, J., Kane, R., Shanmuganathan, K., and Gullapalli, R.P. (2015). A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Inj, 29, 47–57 [DOI] [PubMed] [Google Scholar]
- 31. Zhuo, J., Xu, S., Proctor, J.L., Mullins, R.J., Simon, J.Z., Fiskum, G., and Gullapalli, R.P. (2012) Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 59, 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guskiewicz, K.M., Register-Mihalik, J., McCrory, P., McCrea, M., Johnston, K., Makdissi, M., Dvorák, J., Davis, G., and Meeuwisse, W. (2013). Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br. J. Sports Med. 47, 289–293 [DOI] [PubMed] [Google Scholar]
- 33. Kaushal, M., España, L.Y., Nencka, A.S., Wang, Y., Nelson, L.D., McCrea, M.A., and Meier, T.B. (2019) Resting-state functional connectivity after concussion is associated with clinical recovery. Hum. Brain Mapp. 40, 1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klein, A.P., Tetzlaff, J.E., Bonis, J.M., Nelson, L.D., Mayer, A.R., Huber, D.L., Harezlak, J., Mathews, V.P., Ulmer, J.L., Sinson, G.P., Nencka, A.S., Koch, K.M., Wu, Y.C., Saykin, A.J., DiFiori, J.P., Giza, C.C., Goldman, J., Guskiewicz, K.M., Mihalik, J.P., Duma, S.M., Rowson, S., Brooks, A., Broglio, S.P., McAllister, T., McRea, M.A., and Meier, T.B. (2019). Prevalence of potentially clinically significant magnetic resonance imaging findings in athletes with and without sport-related concussion. J. Neurotrauma 36, 1776–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCrea, M., Kelly, J.P., Kluge, B., and Randolph, C. (1997). Standardized assessment of concussion in football players. Neurology 48, 586–588 [DOI] [PubMed] [Google Scholar]
- 36. McCrea, M., Randolph, C., and Kelly, J.P. (2000). Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. CNS. Inc.: Waukesha, WI [Google Scholar]
- 37. Riemann, B.L., and Guskiewicz, K.M. (2000). Effects of mild head injury on postural stability as measured through clinical balance testing. J. Athl. Train. 35, 19–25 [PMC free article] [PubMed] [Google Scholar]
- 38. Riemann, B.L., Guskiewicz, K.M., and Shields, E.W. (1999). Relationship between clinical and forceplate measures of postural stability. J. Sport Rehabil. 8, 71–82 [Google Scholar]
- 39. Guskiewicz, K.M., Ross, S.E., and Marshall, S.W. (2001). Postural stability and neuropsychological deficits after concussion in collegiate athletes. J. Athl. Train. 36, 263–273 [PMC free article] [PubMed] [Google Scholar]
- 40. Andersson, J.L., Skare, S., and Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 [DOI] [PubMed] [Google Scholar]
- 41. Andersson, J.L., and Skare, S. (2002). A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage 16, 177–199 [DOI] [PubMed] [Google Scholar]
- 42. Tax, C.M., Otte, W.M., Viergever, M.A., Dijkhuizen, R.M., and Leemans, A. (2015). REKINDLE: robust extraction of kurtosis INDices with linear estimation. Magn. Reson. Med. 73, 794–808 [DOI] [PubMed] [Google Scholar]
- 43. Lin, L.I. (1989). A concordance correlation coefficient to evaluate reproducibility. Biometrics 45, 255–268 [PubMed] [Google Scholar]
- 44. Smith, S.M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T.E., Mackay, C.E., Watkins, K.E., Ciccarelli, O., Cader, M.Z., and Matthews, P.M. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 45. Nichols, T.E., and Holmes, A.P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Winkler, A.M., Ridgway, G.R., Webster, M.A., Smith, S.M., and Nichols, T.E. (2014). Permutation inference for the general linear model. Neuroimage 92, 381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith, S.M., and Nichols, T.E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 [DOI] [PubMed] [Google Scholar]
- 48. Barnea-Goraly, N., Menon, V., Eckert, M., Tamm, L., Bammer, R., Karchemskiy, A., Dant, C.C., and Reiss, A.L. (2005). White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex 15, 1848–1854 [DOI] [PubMed] [Google Scholar]
- 49. Lebel, C., Walker, L., Leemans, A., Phillips, L., and Beaulieu, C. (2008). Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 40, 1044–1055 [DOI] [PubMed] [Google Scholar]
- 50. Muftuler, L.T., Davis, E.P., Buss, C., Solodkin, A., Su, M.Y., Head, K.M., Hasso, A.N., and Sandman, C.A. (2012). Development of white matter pathways in typically developing preadolescent children. Brain Res. 1466, 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mukherjee, P., Miller, J.H., Shimony, J.S., Philip, J.V., Nehra, D., Snyder, A.Z., Conturo, T.E., Neil, J.J., and McKinstry, R.C. (2002). Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am. J. Neuroradiol. 23, 1445–1456 [PMC free article] [PubMed] [Google Scholar]
- 52. Schmithorst, V.J., and Yuan, W. (2010). White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 72, 16–25 [DOI] [PubMed] [Google Scholar]
- 53. Budde, M.D., and Skinner, N.P. (2018). Diffusion MRI in acute nervous system injury. J. Magn. Reson. 292, 137–148 [DOI] [PubMed] [Google Scholar]
- 54. Kilinc, D., Gallo, G., and Barbee, K.A. (2008). Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp. Neurol. 212, 422–430 [DOI] [PubMed] [Google Scholar]
- 55. Davenport, E.M., Apkarian, K., Whitlow, C.T., Urban, J.E., Jensen, J.H., Szuch, E., Espeland, M.A., Jung, Y., Rosenbaum, D.A., and Gioia, G.A. (2016). Abnormalities in diffusional kurtosis metrics related to head impact exposure in a season of high school varsity football. J. Neurotrauma, 33, 2133–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hui, E.S., Fieremans, E., Jensen, J.H., Tabesh, A., Feng, W., Bonilha, L., Spampinato, M.V., Adams, R., and Helpern, J.A. (2012). Stroke assessment with diffusional kurtosis imaging. Stroke 43, 2968–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jensen, J.H., Falangola, M.F., Hu, C., Tabesh, A., Rapalino, O., Lo, C., and Helpern, J.A. (2011). Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 24, 452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hall, E.D., Bryant, Y.D., Cho, W., and Sullivan, P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 59. Giedd, J.N., Lalonde, F.M., Celano, M.J., White, S.L., Wallace, G.L., Lee, N.R., and Lenroot, R.K. (2009). Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry, 48, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dimou, S., and Lagopoulos, J. (2014). Toward objective markers of concussion in sport: a review of white matter and neurometabolic changes in the brain after sports-related concussion. J. Neurotrauma, 31, 413–424 [DOI] [PubMed] [Google Scholar]
- 61. Eierud, C., Craddock, R.C., Fletcher, S., Aulakh, M., King-Casas, B., Kuehl, D., and LaConte, S.M. (2014). Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gardner, A., Kay-Lambkin, F., Stanwell, P., Donnelly, J., Williams, W.H., Hiles, A., Schofield, P., Levi, C., and Jones, D.K. (2012). A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma, 29, 2521–2538 [DOI] [PubMed] [Google Scholar]
- 63. Gardner, A., Iverson, G.L., and Stanwell, P. (2014). A systematic review of proton magnetic resonance spectroscopy findings in sport-related concussion. J. Neurotrauma 31, 1–18 [DOI] [PubMed] [Google Scholar]
- 64. Slobounov, S., Gay, M., Johnson, B., and Zhang, K. (2012). Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav. 6, 224–243 [DOI] [PubMed] [Google Scholar]