Abstract
Traumatic brain injury (TBI) patients are reported to experience long-term sensorimotor dysfunction, with gait deficits evident up to 2 years after the initial brain trauma. Experimental TBI including rodent models of penetrating ballistic-like brain injury and severe controlled cortical impact (CCI) can induce impairments in static and dynamic gait parameters. It is reported that the majority of deficits in gait-related parameters occur during the acute phase post-injury, as functional outcomes return toward baseline levels at chronic time points. In the present study, we carried out a longitudinal analysis of static, temporal and dynamic gait patterns following moderate-level CCI in adult male C57Bl/6J mice using the automated gait analysis apparatus, CatWalk. For comparison, we also performed longitudinal assessment of fine-motor coordination and function in CCI mice using more traditional sensorimotor behavioral tasks such as the beamwalk and accelerating rotarod tasks. We determined that longitudinal CatWalk analysis did not detect TBI-induced deficits in static, temporal, or dynamic gait parameters at acute or chronic time points. In contrast, the rotarod and beamwalk tasks showed that CCI mice had significant motor function impairments as demonstrated by deficits in balance and fine-motor coordination through 28 days post-injury. Stereological analysis confirmed that CCI produced a significant lesion in the parietal cortex at 28 days post-injury. Overall, these findings demonstrate that CatWalk analysis of gait parameters is not useful for assessment of long-term sensorimotor dysfunction after CCI, and that more traditional neurobehavioral tests should be used to quantify acute and chronic deficits in sensorimotor function.
Keywords: beamwalk, CatWalk, gait, neurobehavioral tests, rotarod, sensorimotor, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a chronic debilitating disorder affecting >1,700,000 individuals annually.1,2 There is substantial evidence that patients with TBI exhibit sensorimotor dysfunction,3–5 with more than one third of patients showing impairments in balance up to 2 years following injury.3 Further, TBI patients have gait pattern characteristics of temporal asymmetry, increased double-limb support time, reduced stride length, and decreased walking speed.6,7 In the pre-clinical setting, controlled cortical impact (CCI) is a widely used and clinically relevant model of TBI.8 It produces a focal contusion injury that results in both primary and secondary injury mechanisms; the latter account for development of post-traumatic neurological deficits, including acute and chronic sensorimotor dysfunction.9,10 Previous work from our laboratory has shown that CCI induces chronic neurodegenerative processes,11 as well as long-term impairments in sensorimotor function.12–14
CatWalk, an automated gait analysis system, is reported to be a sensitive apparatus to measure parameters related to gait and sensorimotor function. It has been used extensively in experimental models of spinal cord injury (SCI),15 Parkinson's disease (PD),16 and ischemic stroke17 in rodents. Longitudinal analysis of sensorimotor function after experimental TBI routinely uses a beamwalk task, which assess fine-motor coordination,12–14 or an accelerating rotarod task, which measures gross motor function including balance and grip strength.18,19 However, a limitation of these manual tests is that they only measure a limited range of sensorimotor functions. In comparison, the CatWalk is a sensitive automated tool to measure gait and sensorimotor-function parameters simultaneously.15,16,20 It has been used in experimental models of TBI to measure static and dynamic gait parameters following penetrating ballistic-like brain injury (PBBI)20 and severe CCI.21,22 The primary aim of this neurobehavioral study was to conduct a longitudinal analysis of static and dynamic gait parameters in moderate-level CCI mice using the CatWalk apparatus, and compare them with more traditional sensorimotor tasks, including beamwalk and accelerating rotarod tasks.
Methods
Animals
Studies were performed using adult male C57Bl/6J mice (10–12 weeks old; Taconic Biosciences Inc., Germantown, NY). Mice were housed in shoebox cages (4–5 mice in each cage) at least 1 week prior to any procedures in a room (22–23°C) with a 12-h/12-h light–dark cycle. Food and water were provided ad libitum. The mice were handled and weighed daily for 3 days prior to surgery. Procedures were conducted from 10:00 am to 5:00 pm in a quiet room. All protocols involving the use of animals complied with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH) (DHEW publication NIH 85- 23-2985), and were approved by the University of Maryland Animal Use Committee.
Experimental design
C57Bl/6 mice (n = 11–12/group) underwent either CCI or sham surgery. Sensorimotor function was assessed using the beamwalk and rotarod tasks and gait analysis was performed using the CatWalk task on 1, 3, 7, 14, 21, and 28 days post-injury (dpi). For each task, mice were habituated to each behavioral room for 30 min prior to testing. The tests were completed in the following order: CatWalk, beamwalk, and rotarod, with an inter-trial interval (ITI) of 1 h and 30 min to allow for recovery. The rationale for preforming tests in this order was to reduce stress on mice and avoid motor practice effects in the CatWalk task (primary outcome). At 28 dpi, mice were anesthetized (100 mg/kg sodium pentobarbital, I.P.) and transcardially perfused with ice-cold 0.9% saline (100 mL), followed by 300 mL of 4% paraformaldehyde (PFA). Brains were removed and post-fixed in 4% PFA overnight, cryoprotected in 30% sucrose, and processed for histological outcome measures.
CCI
Our custom-designed CCI injury device consists of a microprocessor-controlled pneumatic impactor with a 3.5 mm diameter tip. Mice were anesthetized with isoflurane evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask (induction at 4% and maintenance at 2%). Depth of anesthesia was assessed by monitoring respiration rate and pedal withdrawal reflexes. Mice were placed on a heated pad, and core body temperature was maintained at 37°C. The head was mounted in a stereotaxic frame, and the surgical site was clipped and cleaned with Nolvasan and ethanol scrubs. A 10 mm midline incision was made over the skull, the skin and fascia were reflected, and a 5 mm craniotomy was made on the central aspect of the left temporoparietal bone, between bregma and lambda. The impounder tip of the injury device was then extended to its full stroke distance (44 mm), positioned to the surface of the exposed dura, and reset to impact the cortical surface. Moderate-level CCI (n = 11) was induced using an impactor velocity of 6 m/sec and a deformation depth of 2 mm as previously described.11–14 After injury, the incision was closed with interrupted 6-0 silk sutures, anesthesia was terminated, and the animal was placed into a heated cage to maintain normal core temperature for 45 min post-injury. The CCI model replicates several important secondary injury mechanisms after TBI, including apoptosis, edema, and secondary neuroinflammation,9 but does not produce acute intracranial hypertension. Sham mice (n = 12) underwent the same procedure as TBI mice except for the craniotomy and impact. All mice were monitored daily post-injury.
Beamwalk
The beamwalk device consists of a narrow wooden beam 5 mm wide and 120 cm in length, which is suspended 1 m above the ground. The beamwalk test is a valuable predictor of fine-motor coordination differences between CCI and sham-operated mice, and was conducted as previously described.12–14 Briefly, the mouse was placed on the beamwalk and the number of foot faults for the right hindlimb was recorded over 50 steps. At the end of the beam there was an enclosed dark box, and mice remained there for ∼20 sec after beam crossing. This allowed mice to associate transvering the beam with the safety of the box. In order to achieve a baseline level of performance, each mouse underwent 3 days of training prior to injury, with an acceptance level of fewer than 10 foot faults per 50 steps. The test was performed at 0 (immediately before CCI/sham surgery), 1, 3, 7, 14, 21, and 28 dpi.
Rotarod
The rotarod test was used to assess motor function and balance as previously described.23 The mouse was placed on a five-lane rotarod device (IITC Life Science, Inc., Woodland Hills, CA), and the latency to fall of the accelerating rotarod was recorded. The rotarod accelerated from 1 rpm to 30 rpm over 90 sec, with each trial again lasting a maximum of 120 sec. Trials ended when mice either fell off the rod or clung to the rod as it made two complete rotations. In order to achieve a basal level of performance, each mouse underwent 3 days of training prior to injury, in which three trials per day cconducted, with a 10 min ITI between trials. The rotarod test was performed on 0 (immediately before CCI/sham surgery), 1, 3, 7, 14, 21, and 28 dpi. Individual scores from three trials were averaged and evaluated relative to their baseline latencies.
Gait analysis
Gait was assessed for 3 days prior to CCI/sham surgery, and at 0 (immediately before CCI surgery), 1, 3, 7, 14, 21, and 28 dpi, using the CatWalk Automated Gait Analysis System (Noldus Information Technology Inc., Leesburg, VA), as previously described.20,21 Briefly, in a dark room, mice were placed on the walkway and allowed to traverse from one end to a goal box at the other end. Direct contact between the paw and the glass surface generates light reflection in the form of illuminated footprints. Footprint images were video-recorded by a camera positioned under the walkway. All mice completed three consecutive runs. Runs with walk velocities >30% variation were excluded from analysis. The images from each trial were converted into digital signature and processed using CatWalk XT 9.1 software with a minimum threshold set at 80 (arbitrary units [a.u.] ranging from 0 to 225). Mean scores from three consecutive trials (per animal/time points) were analyzed for statistical significance. Following footprint identification and labeling, static, temporal, dynamic gait parameters and inter-limb coordination parameters were measured for the right and left hind paws (HP) in each trial. Gait analysis parameters included:
-
1.
Static gait parameters
Intensity: Describes the total floor area contacted by the paw during the stance phase and is expressed in square pixels.
Print area: Represents the complete print including all frames that makes up a stance.
Print width: Describes the width of the print area and is expressed in pixels.
Print length: Describes the length of the print area and is expressed in pixels.
-
2.
Temporal gait parameters
Stance: Describes the time duration during which the paw is in contact with the glass plate.
Swing: Describes the time interval between two consecutive paw placements of the same paw in which the paw is not in contact with the glass plate.
Step cycle duration: Is the duration between two consecutive surface contacts of the same paw.
Duty cycle: Is the percentage of the time spent in the stance phase relative to the entire step cycle.
-
3.
Dynamic gait parameters
Walk speed: Represents the distance that the mice walk per second. It is calculated as the sum of the stride length divided by Max Contact last step-Max Contact first step.
Stand Index: Measure for the speed at which the paw loses contact with the surface.
-
4.
Gait parameters relative to paw placement
Print position: This is the distance in cm between the position of the hind paw and the position of the previously placed front paw on the same side of the body (ipsilateral) and in the same step cycle. A positive value of the print position indicates that the hind paw is placed behind the front paw. A negative value of the print position indicates that the hind paw is placed in front of the front paw.
Base of support (BOS): Describes the distance between the mass-midpoints of two fore and hind prints at the max contact during each step cycle. The results from each step cycle are averaged and expressed in mm in each trial.
-
5.
Gait parameters of inter-limb coordination
Regularity index (RI; %): Is an index for the degree of inter-limb coordination during gait, as measured by the number of normal step sequence patterns (NSSP), multiplied by the number of paws and divided by the number of paw placements; RI = 100% × (NSSP X 4)/no. of paw placements.
% Support time: Describes the relative duration of contact with the glass plate of all combinations of number of paws. This combination range from no paw contact (zero), using a single paw, diagonal two paws (right front [RF] and left hind [LH] or left front [LF] and right hind [RH]), lateral two paws (RF and RH or LF and LH), girdle two paws (RF and LF or RH and LH), three paws, or four paws.
Lesion volume
Sixty micrometer coronal sections from mice were stained with cresyl violet (FD NeuroTechnologies, Baltimore, MD), dehydrated, and mounted for analysis (n = 10 per group). Lesion volume was quantified based on the Cavalieri method of unbiased stereology using Stereoinvestigator Software (MBF Biosciences, Williston, VT) as previously described.13,14 Briefly, the lesion volume was quantified by outlining the missing tissue on the injured hemisphere using the Cavalieri estimator with a grid spacing of 0.1 mm. Every eighth section from a total of 96 sections was analyzed beginning from a random start point.
Statistical analysis
Quantitative data were expressed as mean + standard errors of the mean (SEM). Normality testing was performed and data sets passed normality (D'Agostino and Pearson omnibus normality test), and therefore parametric statistical analysis was performed. Beamwalk, rotarod, and CatWalk were analyzed by one-way repeated measures analysis of variance (ANOVA) to determine the interactions of time and groups, followed by post-hoc adjustments using a Bonferroni's multiple comparison test. Stereological data were analyzed using an unpaired Student t test. Statistical analyses were performed using GraphPad Prism Program, Version 3.02 for Windows (GraphPad Software, San Diego, CA, USA). A p < 0.05 was considered statistically significant.
Results
CCI produces a significant cortical lesion
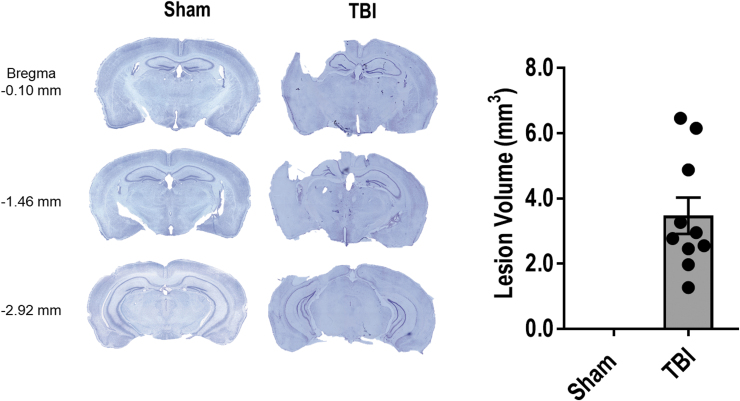
Unbiased stereological assessment of TBI-induced tissue loss at 28 dpi determined that CCI produced a cortical lesion of 3.47 ± 0.56 mm3 (Fig. 1). There was no observable lesion in sham operated mice.
FIG. 1.
Controlled cortical impact (CCI) is associated with a large lesion volume at 28 days post-injury. Data expressed as mean ± standard error of the mean (SEM), n = 10 per group. Color image is available online.
CCI induces long-term impairments in fine-motor coordination in the beamwalk test
Fine-motor coordination was assessed on 1, 3, 7, 14, 21, and 28 dpi using a well-characterized beamwalk test.12–14 All mice underwent training on the beamwalk for 3 consecutive days prior to sham or CCI surgery. A total of 50 steps were counted, and in order for a mouse to be enrolled in the study, it had to achieve fewer than 10 foot faults (FF) on the final day of training. A one-way repeated ANOVA revealed a significant effect of injury over time (F[6,105] = 7.435, p < 0.0001). When compared with sham mice, CCI resulted in fine-motor coordination impairments with 44.5 ± 3.1 FF (mean ± SEM; Fig. 2) at 1 dpi and persistent FF through 28 dpi (37.3 ± 3.4 FF). Post-hoc analysis revealed that CCI induced significant impairments in sensorimotor function at 1, 3, 7, 14, 21, and 28 dpi (p < 0.0001 vs. sham).
FIG. 2.
Controlled cortical impact (CCI) produces impairments in fine motor coordination lasting through 28 days post-injury. Moderate-level CCI increased the number of foot faults in the right hind paw at 1, 3, 7, 14, 21, and 28 days post-injury, when compared with sham-operated counterparts. ****p < 0.0001 versus sham-operated counterparts. Data expressed as mean ± standard error of the mean (SEM), n = 11–12 per group.
CCI induces long-term impairments in motor balance as assessed by the rotarod test
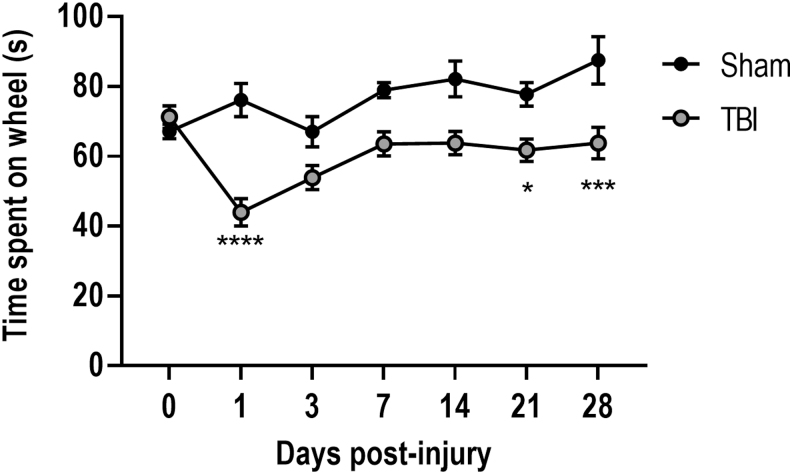
Balance and motor coordination in injured mice were assessed using a rotarod test at 1, 3, 7, 14, 21, and 28 dpi. Similar to the beamwalk task, a one-way repeated ANOVA revealed a significant interaction of injury over time (F[6,105] = 3.583, p = 0.003). Post-hoc analysis demonstrated that when compared with sham mice, CCI resulted in impairments in motor balance such that TBI mice spent significantly reduced time on the accelerating rotarod on 1 (p < 0.0001 vs. sham), 14 (p < 0.05 vs. sham), and 28 (p < 0.001 vs. sham) dpi (Fig. 3).
FIG. 3.
Controlled cortical impact (CCI) produces balance and coordination deficits lasting through 28 days post-injury. Moderate-level CCI leads to less time spent on the accelerating rotarod at 1, 21, and 28 days post-injury, when compared with sham-operated counterparts. ****p < 0.0001, ***p < 0.001, *p < 0.05 versus sham-operated counterparts. Data expressed as mean ± standard error of the mean (SEM), n = 11–12 per group.
CCI does not result in impairments in gait as assessed by CatWalk analysis
Clinical24 and pre-clinical studies20,21 indicate that TBI produces abnormality in gait and coordinated movements in injured subjects. We therefore used CatWalk, a sensitive automated analysis platform to measure gait- and sensorimotor-function parameters in sham and CCI mice through 28 dpi. All mice underwent training on the CatWalk apparatus for 3 consecutive days prior to sham or CCI surgery. Overall, we determined that when compared with performance levels in sham mice, CCI did not change critical parameters of gait function, including measures of static gait, temporal gait, or dynamic gait during the acute or chronic phases of recovery following TBI.
Effect of CCI on static gait parameters
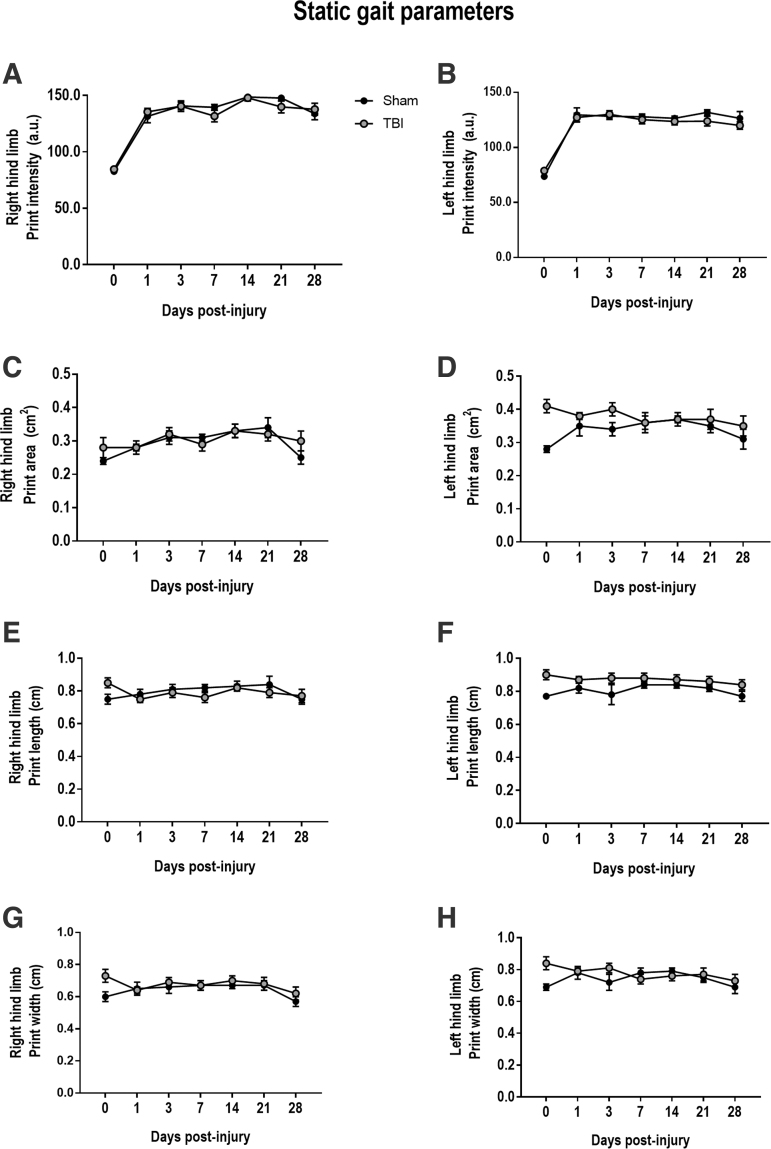
To assess static gait function, we measured print intensity, print area, print length, and print width parameters in sham and CCI mice. TBI-induced alterations in static parameters have previously been reported in PBBI20 and CCI21,22 models. However, in contrast to these prior findings, there were no TBI-induced alterations in right HP (RH) or left HP (LH) print intensity (RH: F[2,61] = 0.140, p = 0.869; LH: F[2,61] = 0.014, p = 0.985) or print area (RH: F[2,61] = 2.476, p = 0.092; LH: F[2,61] = 1.593, p = 0.211) in CCI mice at any time point post-injury (Fig. 4A–D), when compared with sham mice. Other static parameters including print length and print width were also examined. Similarly, there was no TBI-induced change in print length or width in either LH or RH of CCI mice thorough 28 dpi (Fig. 4E–H).
FIG. 4.
Controlled cortical impact (CCI) does not induce acute or chronic alterations in static gait parameters. Moderate-level CCI does not alter print intensity, print area, print length, or print width at any time point through 28 days post-injury, when compared with sham-operated counterparts. Data expressed as mean ± standard error of the mean (SEM), n = 11–12 per group. (A, B) print intensity, (C, D) print area, (E, F) print length, or (G, H) print width.
Effect of CCI on temporal gait parameters
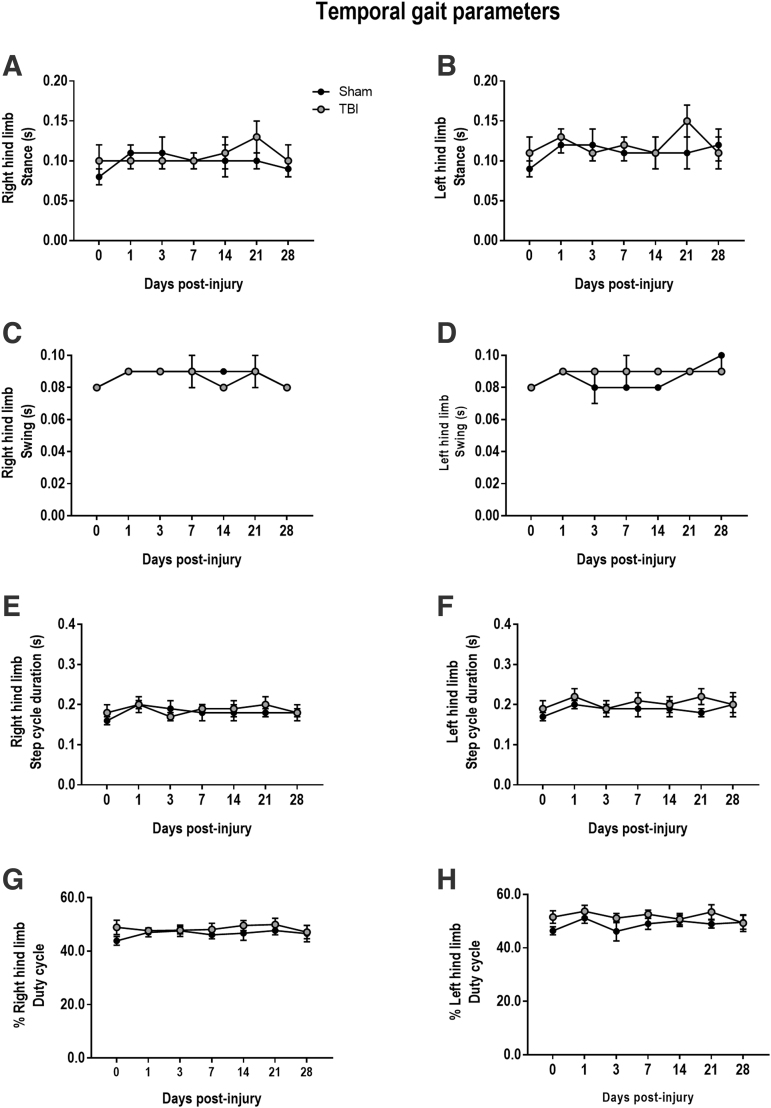
To assess temporal gait dynamics, we measured temporal parameters including stance and swing durations. Prior studies reported increased stance and swing durations in front and rear limbs of injured mice.21 However, in this study, there were no TBI-induced alterations in RH or LH stance (RH: F[2,61] = 0.198, p = 0.821; LH: (F[2,61] = 0.270, p = 0.764) and swing durations (RH: F[2,61] = 3.406, p = 0.06; LH: F[2,61] = 1.802, p = 0.174) in CCI mice at any time point post-injury (Fig. 5A-D), when compared with sham mice. Other temporal parameters, including step cycle duration and duty cycle, were analyzed, and there were no TBI-induced changes in step cycle duration (RH: F[2,61] = 0.641, p = 0.530; LH: F[2,61] = 0.252, p = 0.777) or duty cycle (RH: F[2,61] = 0.721, p = 0.492; LH: F[2,61] = 0.409, p = 0.665) of CCI mice (Fig. 5E–H).
FIG. 5.
Controlled cortical impact (CCI) does not induce acute or chronic alterations in temporal gait parameters. Moderate-level CCI does not alter stance, swing, step cycle duration, or duty cycle at any time point through 28 days post-injury, when compared with sham-operated counterparts. Data expressed as mean ± standard error of the mean (SEM), n = 11–12 per group. (A, B) stance, (C, D) swing, (E, F) step cycle duration, or (G, H) % duty cycle.
Effect of CCI on dynamic gait parameters
To assess dynamic gait parameters, we evaluated walk speed (cm/sec) and stance index for RH and LH. There were no significant differences between sham and TBI mice regarding RH or LH walk speed (RH: F[2,60] = 0.182, p = 0.833; LH: F[2,59] = 0.043, p = 0.958) or stance index (RH: F[2,60] = 0.972, p = 0.384); LH: F[2,60] = 0.949, p = 0.392) at baseline (immediately prior to sham or CCI surgery), 3 dpi or 28 dpi (Table 1). Additional parameters for paw position during locomotion such as print position and base of support were also examined. Consistent with analysis of other dynamic gait parameters, there were no observable differences in paw position (RH: F[2,61] = 1.267, p = 0.289; LH: F[2,61] = 0.768, p = 0.468) or base of support (BOS) (F[2,61] = 2.136, p = 0.126) between sham and CCI mice at any time point (Table 2).
Table 1.
Right and Left Hind Paw (HP) Gait Parameters
| Baseline | 3 dpi | 28 dpi | |||
|---|---|---|---|---|---|
| Walk speed (cm/sec) | Right HP | Sham | 95.52 ± 15.16 | 93.16 ± 18.75 | 84.40 ± 10.87 |
| TBI | 98.41 ± 8.81 | 83.54 ± 8.14 | 104.88 ± 9.38 | ||
| Left HP | Sham | 97.13 ± 8.53 | 94.54 ± 15.89 | 93.35 ± 7.52 | |
| TBI | 107.25 ± 10.19 | 94.16 ± 6.83 | 95.03 ± 8.65 | ||
| Stand index | Right HP | Sham | -34.08 ± 2.47 | -28.59 ± 4.08 | -24.82 ± 4.36 |
| TBI | -24.83 ± 1.79 | -17.67 ± 1.96 | -11.99 ± 2.33 | ||
| Left HP | Sham | -31.60 ± 2.79 | -26.44 ± 3.51 | -22.08 ± 3.64 | |
| TBI | -18.00 ± 1.79 | -16.01 ± 1.90 | -11.09 ± 2.11 | ||
dpi, days post-injury; TBI, traumatic brain injury.
Table 2.
Right and Left Gait Parameters Relative To Paw Position
| Baseline | 3 dpi | 28 dpi | |||
|---|---|---|---|---|---|
| Print position | Right HP | Sham | -0.12 ± 0.17 | 0.20 ± 0.10 | 0.36 ± 0.21 |
| TBI | 0.08 ± 0.13 | 0.30 ± 0.12 | 0.25 ± 0.13 | ||
| Left HP | Sham | -0.10 ± 0.20 | 0.30 ± 0.09 | 0.50 ± 0.10 | |
| TBI | 0.04 ± 0.13 | 0.30 ± 0.08 | 0.20 ± 0.21 | ||
| Base of support (BOS) (cm) | Right & left HP | Sham | 2.66 ± 0.12 | 2.60 ± 0.07 | 2.95 ± 0.06 |
| TBI | 2.73 ± 0.03 | 2.52 ± 0.05 | 2.53 ± 0.05 | ||
dpi, days post-injury; HP, hind paw; TBI, traumatic brain injury
Effect of CCI inter-limb coordination
To evaluate inter-limb coordination, we assessed step sequence patterns in sham and CCI mice. There are four main different step patterns described in rodents Supplementary Fig. S1 with the alternate b (Ab) pattern the most commonly used in uninjured mice.25 It has been reported that the cruciate b (Cb) step pattern significantly increases acutely after CCI.21 However, in the current study we did not observe changes in the Ca (F[2,61] = 1.370, p = 0.261), Cb (F[2,61] = 0.051, p = 0.946), Aa (F[2,61] = 1.332, p = 0.271) or the Ab (F[2,61] = 0.232, p = 0.787) step sequence pattern either acutely at 3 dpi or chronically at 28 dpi (Table 3). In order to assess inter-limb coordination, we evaluated regularity index (RI) and percent support time through 28 dpi (Table 4). RI is not a very sensitive measure of inter-limb coordination because “irregular step” is measured as loss of paw placement of one of multiple limbs; therefore, the likelihood of having 100% RI is high.21 Accordingly, in the current study, there was a minor decrease in RI in CCI mice compared with sham mice; however, this effect did not reach statistical significance (F[2,61] = 0.634, p = 0.533] (Table 4). We also assessed percent support time through 28 dpi. There was no difference between sham and CCI mice in zero (F[2,61] = 0.531, p = 0.591), single (F[2,61] = 0.576, p = 0.568), diagonal (F[2,61] = 0.652, p = 0.523), gridle (F[2,61] = 0.092, p = 0.912), lateral (F[2,61] = 0.171, p = 0.842), three (F[2,61] = 0.168, p = 0.845), or four (F[2,61] = 1.044, p = 0.356) support time parameters. We determined that diagonal support was the most commonly used support mechanism in both sham and CCI mice (Table 4).
Table 3.
Step Pattern (%)
| Baseline | 3 dpi | 28 dpi | ||
|---|---|---|---|---|
| Ca | Sham | 25.21 ± 4.42 | 17.42 ± 4.16 | 24.03 ± 4.57 |
| TBI | 21.67 ± 4.71 | 29.90 ± 5.83 | 28.05 ± 5.81 | |
| Cb | Sham | 23.02 ± 3.70 | 18.87 ± 3.18 | 18.06 ± 3.06 |
| TBI | 26.52 ± 6.57 | 18.72 ± 4.16 | 18.62 ± 5.45 | |
| Aa | Sham | 15.69 ± 5.65 | 36.71 ± 8.19 | 27.80 ± 8.27 |
| TBI | 30.45 ± 4.69 | 30.28 ± 5.20 | 34.80 ± 6.79 | |
| Ab | Sham | 32.74 ± 5.80 | 25.17 ± 6.92 | 27.20 ± 6.81 |
| TBI | 19.11 ± 5.09 | 19.62 ± 5.58 | 16.75 ± 4.84 | |
dpi, days post-injury; TBI, traumatic brain injury.
Table 4.
Parameters of Inter-Limb Coordination
| Regularity index (%) | Baseline | PID 3 | PID 28 | |
|---|---|---|---|---|
| Sham | 97.7 ± 2.25 | 93.9 ± 1.78 | 94.0 ± 5.16 | |
| TBI | 93.3±1.55 | 90.0 ± 2.29 | 89.53 ± 3.86 | |
| Support time (%) | Baseline | 3 dpi | 28 dpi | |
| Zero | Sham | 2.29 ± 0.48 | 1.36 ± 0.49 | 1.61 ± 0.47 |
| TBI | 2.90 ± 0.69 | 1.28 ± 0.45 | 1.17 ± 0.51 | |
| Single | Sham | 12.56 ± 2.62 | 13.69 ± 3.50 | 12.99 ± 2.99 |
| TBI | 12.94 ± 2.04 | 9.88 ± 2.09 | 15.22 ± 3.15 | |
| Diagonal | Sham | 61.65 ± 4.99 | 59.91 ± 4.85 | 63.66 ± 4.63 |
| TBI | 69.81 ± 4.67 | 68.59 ± 3.74 | 62.86 ± 4.21 | |
| Girdle | Sham | 4.20 ± 1.34 | 4.44 ± 1.95 | 3.89 ± 1.34 |
| TBI | 2.71 ± 0.66 | 3.22 ± 0.88 | 3.02 ± 1.41 | |
| Lateral | Sham | 0.86 ± 0.26 | 1.58 ± 0.38 | 1.60 ± 0.36 |
| TBI | 0.92 ± 0.31 | 1.40 ± 0.32 | 1.82 ± 0.37 | |
| Three | Sham | 8.42 ± 2.23 | 11.48 ± 2.94 | 13.03 ± 4.11 |
| TBI | 6.02 ± 3.26 | 11.55 ± 3.15 | 7.51 ± 3.37 | |
| Four | Sham | 10.02 ± 3.94 | 7.55 ± 3.11 | 4.47 ± 1.89 |
| TBI | 4.96 ± 2.68 | 4.08 ± 1.97 | 7.44 ± 3.40 |
PID, post-injury day; dpi, days post-injury; TBI, traumatic brain injury.
Discussion
In this pre-clinical study, we demonstrate that although moderate-level CCI in C57Bl/6J mice causes lasting deficits in sensorimotor function as shown by classically used tests, automated gait analysis fails to demonstrate such functional impairments. More specifically, we showed that CCI resulted in sustained impairments in fine motor coordination and balance through 28 dpi, as determined using beamwalk and rotarod tests, respectively. In contrast, using the CatWalk apparatus to perform detailed analysis of injury-induced alterations in gait, we found no effects of TBI on static, temporal, or dynamic gait parameters during either the acute or the chronic phase of recovery.
Many TBI patients experience impairments in gait including loss of balance and decreased walking speeds, for several years following the initial injury.24 There are limited data describing changes in gait following experimental TBI in rodents. Moreover, published studies are conflicting, reporting either positive or negative findings. The CatWalk behavioral evaluation system is claimed to offer advantages for investigating sensorimotor function in rodents because it provides multivariate analysis of spatial, temporal, and dynamic gait parameters in individual limbs.20,21 Our findings are consistent with some CatWalk analyses in mice or rats that show no impairments in gait through 4–6 weeks post-CCI, including measurements of paw print area, stride length, stand duration, mean swing, swing speed, step cycle, base of support, speed, and cadence.26,27 However, another CCI study in mice reported alterations in several gait parameters including decreased paw pressure, area of paw contact, walk speed, and stride lengths at 3 dpi.21 Discrepancies between our study and that reported by Neumann and colleagues may be the result of differences in injury severity: moderate versus severe-level CCI, respectively. It is also important to note that the Neumann study only investigated acute injury responses. Therefore, it may be that whereas gait changes occur acutely after severe CCI, they may not persist more chronically. Consistent with this conclusion, it was reported that alterations in several static, temporal, and dynamic gait parameters induced following severe PBBI were evident during the acute phase after injury, but resolved at chronic time points.20
The absence of measurable gait deficits in our CatWalk analyses may reflect lack of damage to more critical regions involved in gait function, as the CatWalk assesses coordinated walking movements that are controlled by central pattern generators in the spinal cord. Therefore, central pattern generators located in the spinal cord regulate locomotion, whereas goal-directed actions such as grasping require conscious integration of spatial and proprioceptive cues provided by the motor cortex.28,29 Our CCI model causes unilateral contusion injury to the parietal cortex, resulting in a significant cortical lesion and damage to underlying structures, including the corpus callosum and hippocampus (Fig. 1). The neurobehavioral test used to measure fine-motor coordination (beamwalk test) requires mice to retain intact grasping functions when crossing the narrow wooden beam and the ability to integrate spatial and proprioceptive cues to reach the home cage platform. Therefore, damage to the motor cortex significantly limits the ability of mice to perform the beamwalk test. The cerebellum is another key region implicated in the coordination of gait.30,31 Clinical symptoms of cerebellar damage include gait ataxia and appendicular dysmetria.32–35 Therefore, lack of deficits in gait function suggest that our injury does not cause significant damage to the anterior lobe of the cerebellum.
Based on conflicting reports in the literature, the goal of our pre-clinical study was to longitudinally evaluate the utility of the automated CatWalk analysis of gait in a moderate-level CCI model in mice, and compare this to traditional sensorimotor tests. To peform a rigorous assessment of neurobehavior we factored in (1) behavioral test order, and (2) a lengthy rest time between tasks, to reduce potential confounds caused by practice effects and stress related to multiple tests in a single day, respectively. All mice in the study had the same ITI of 1 h and 30 min between each behavioral test, and underwent the same order of testing (CatWalk – Beam walk – Rotarod) each day. As the primary outcome measure in the analysis, the CatWalk was performed first each day to avoid motor practice effects arising from other behavioral tests. In addition, we chose to perform the rotarod last each test day because of the somewhat stressful nature and high physical demand on the mouse to maintain itself on an accelerating rod over time. Mice did not evidence signs of stress from the testing regimen, and beamwalk and rotarod outcome data showed that sham and CCI mice performances were consistent with previously published results from our laboratory.23,36 Therefore, the lack of TBI-induced deficits in gait functions that we report here are not likely the result of motor practice effects, as the CatWalk was the first test performed each test day, and there were no signs of over-testing in mice, because of our selected ITI.
Overall, the present findings and those of several previous reports20,26,27 indicate that a number of factors likely determine gait versus other sensorimotor dysfunction following experimental TBI; these include the injury model type, injury severity, and the time after injury. Based on our findings, we suggest that traditional neurobehavioral tests such as the beamwalk or rotarod, rather than more detailed automated gait analyses, are most appropriate for evaluating acute or chronic deficits in sensorimotor function in the mouse CCI model.
Funding Information
This work was supported by National Institutes of Health grants R01NS082308 (D.J.L), R01NS037313 (A.I.F), and R01NS110756 (D.J.L./A.I.F/B.A.S), a United States Veterans Affairs grant 1I01 RX001993 (B.A.S), and Science Foundation Ireland grant 17/FRL/4860 (D.J.L).
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Coronado, V.G., Xu, L., Basavaraju, S.V., McGuire, L.C., Wald, M.M., Faul, M.D., Guzman, B.R., Hemphill, J.D., and Centers for Disease Control and Prevention (2011). Surveillance for traumatic brain injury-related deaths–United States, 1997-2007. MMWR Surveill. Summ. 60, 1–32 [PubMed] [Google Scholar]
- 2. Faul, M., and Coronado, V. (2015). Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 127, 3–13 [DOI] [PubMed] [Google Scholar]
- 3. Basford, J.R., Chou, L.S., Kaufman, K.R., Brey, R.H., Walker, A., Malec, J.F., Moessner, A.M., and Brown, A.W. (2003). An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 84, 343–349 [DOI] [PubMed] [Google Scholar]
- 4. van Loo, M.A., Moseley, A.M., Bosman, J.M., de Bie, R.A., and Hassett, L. (2004). Test–re-test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain Inj. 18, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 5. Wiese, H., Stude, P., Nebel, K., Osenberg, D., Volzke, V., Ischebeck, W., Stolke, D., Diener, H.C., and Keidel, M. (2004). Impaired movement-related potentials in acute frontal traumatic brain injury. Clin. Neurophysiol. 115, 289–298 [DOI] [PubMed] [Google Scholar]
- 6. Johnk, K., Kuhtz-Buschbeck, J.P., Stolze, H., Serocki, G., Kalwa, S., Ritz, A., Benz, B., and Illert, M. (1999). Assessment of sensorimotor functions after traumatic brain injury (TBI) in childhood – Methodological aspects. Restor. Neurol. Neurosci. 14, 143–152 [PubMed] [Google Scholar]
- 7. Ochi, F., Esquenazi, A., Hirai, B., and Talaty, M. (1999). Temporal-spatial feature of gait after traumatic brain injury. J. Head Trauma Rehabil. 14, 105–115 [DOI] [PubMed] [Google Scholar]
- 8. Osier, N.D. and Dixon, C.E. (2016). The controlled cortical impact model: applications, considerations for researchers, and future directions. Front. Neurol. 7, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loane, D.J., Stoica, B.A., and Faden, A.I. (2015). Neuroprotection for traumatic brain injury. Handb. Clin. Neurol. 127, 343–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simon, D.W., McGeachy, M.J., Bayir, H., Clark, R.S., Loane, D.J., and Kochanek, P.M. (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loane, D.J., Kumar, A., Stoica, B.A., Cabatbat, R., and Faden, A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byrnes, K.R., Loane, D.J., Stoica, B.A., Zhang, J., and Faden, A.I. (2012). Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflammation 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henry, R.J., Doran, S.J., Barrett, J.P., Meadows, V.E., Sabirzhanov, B., Stoica, B.A., Loane, D.J., and Faden, A.I. (2019). Inhibition of miR-155 Limits neuroinflammation and improves functional recovery after experimental traumatic brain injury in mice. Neurotherapeutics 16, 216–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henry, R.J., Ritzel, R.M., Barrett, J.P., Doran, S.J., Jiao, Y., Leach, J.B., Szeto, G.L., Wu, J., Stoica, B.A., Faden, A.I., and Loane, D.J. (2020). Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J. Neurosci. 40, 2960–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamers, F.P., Koopmans, G.C., and Joosten, E.A. (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma 23, 537–548 [DOI] [PubMed] [Google Scholar]
- 16. Vandeputte, C., Taymans, J.M., Casteels, C., Coun, F., Ni, Y., Van Laere, K., and Baekelandt, V. (2010). Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci. 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Encarnacion, A., Horie, N., Keren-Gill, H., Bliss, T.M., Steinberg, G.K., and Shamloo, M. (2011). Long-term behavioral assessment of function in an experimental model for ischemic stroke. J. Neurosci. Methods 196, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peterson, T.C., Maass, W.R., Anderson, J.R., Anderson, G.D., and Hoane, M.R. (2015). A behavioral and histological comparison of fluid percussion injury and controlled cortical impact injury to the rat sensorimotor cortex. Behav. Brain Res. 294, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sackheim, A.M., Stockwell, D., Villalba, N., Haines, L., Scott, C.L., Russell, S., Hammack, S.E., and Freeman, K. (2017). Traumatic brain injury impairs sensorimotor function in mice. J. Surg. Res. 213, 100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mountney, A., Leung, L.Y., Pedersen, R., Shear, D., and Tortella, F. (2013). Longitudinal assessment of gait abnormalities following penetrating ballistic-like brain injury in rats. J. Neurosci. Methods 212, 1–16 [DOI] [PubMed] [Google Scholar]
- 21. Neumann, M., Wang, Y., Kim, S., Hong, S.M., Jeng, L., Bilgen, M., and Liu, J. (2009). Assessing gait impairment following experimental traumatic brain injury in mice. J. Neurosci. Methods 176, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang, G., Shi, Y., Jiang, X., Leak, R.K., Hu, X., Wu, Y., Pu, H., Li, W.W., Tang, B., Wang, Y., Gao, Y., Zheng, P., Bennett, M.V., and Chen, J. (2015). HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc. Natl. Acad. Sci. U. S. A. 112, 2853–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doran, S.J., Ritzel, R.M., Glaser, E.P., Henry, R.J., Faden, A.I., and Loane, D.J. (2019). Sex differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells. J. Neurotrauma 36, 1040–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker, W.C., and Pickett, T.C. (2007). Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J. Rehabil. Res. Dev. 44, 975–982 [DOI] [PubMed] [Google Scholar]
- 25. Cheng, H., Almstrom, S., Gimenez-Llort, L., Chang, R., Ove Ogren, S., Hoffer, B., and Olson, L. (1997). Gait analysis of adult paraplegic rats after spinal cord repair. Exp. Neurol. 148, 544–557 [DOI] [PubMed] [Google Scholar]
- 26. Neckel, N.D., Dai, H., and Burns, M.P. (2020). A novel multi-dimensional analysis of rodent gait reveals the compensation strategies used during spontaneous recovery from spinal cord and traumatic brain injury. J. Neurotrauma 37, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schonfeld, L.M., Jahanshahi, A., Lemmens, E., Schipper, S., Dooley, D., Joosten, E., Temel, Y., and Hendrix, S. (2017). Long-term motor deficits after controlled cortical impact in rats can be detected by fine motor skill tests but not by automated gait analysis. J. Neurotrauma 34, 505–516 [DOI] [PubMed] [Google Scholar]
- 28. Kiehn, O., Dougherty, K.J., Hagglund, M., Borgius, L., Talpalar, A., and Restrepo, C.E. (2010). Probing spinal circuits controlling walking in mammals. Biochem. Biophys. Res. Commun. 396, 11–18 [DOI] [PubMed] [Google Scholar]
- 29. Simone, L., Rozzi, S., Bimbi, M., and Fogassi, L. (2015). Movement-related activity during goal-directed hand actions in the monkey ventrolateral prefrontal cortex. Eur. J. Neurosci. 42, 2882–2894 [DOI] [PubMed] [Google Scholar]
- 30. Morton, S.M., and Bastian, A.J. (2004). Cerebellar control of balance and locomotion. Neuroscientist 10, 247–259 [DOI] [PubMed] [Google Scholar]
- 31. Morton, S.M., and Bastian, A.J. (2007). Mechanisms of cerebellar gait ataxia. Cerebellum 6, 79–86 [DOI] [PubMed] [Google Scholar]
- 32. Schmahmann, J.D., Macmore, J., and Vangel, M. (2009). Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience 162, 852–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoch, B., Dimitrova, A., Gizewski, E.R., and Timmann, D. (2006). Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage 30, 36–51 [DOI] [PubMed] [Google Scholar]
- 34. Stoodley, C.J., MacMore, J.P., Makris, N., Sherman, J.C., and Schmahmann, J.D. (2016). Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage Clin. 12, 765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Timmann, D., Brandauer, B., Hermsdorfer, J., Ilg, W., Konczak, J., Gerwig, M., Gizewski, E.R., and Schoch, B. (2008). Lesion-symptom mapping of the human cerebellum. Cerebellum 7, 602–606 [DOI] [PubMed] [Google Scholar]
- 36. Zhao, Z., Loane, D.J., Murray, M.G., 2nd, Stoica, B.A., and Faden, A.I. (2012). Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J. Neurotrauma 29, 2475–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.